Abstract
The field of tissue engineering and regenerative medicine (TERM) has exploded in the last decade. In this Year (or so) in Review, we highlight some of the high impact advances within the field over the past several years. Using the past as our guide and starting with an objective premise, we attempt so to identify recent “hot topics” and transformative publications within the field. Through this process, several key themes emerged: (1) tissue engineering: grafts and materials, (2) regenerative medicine: scaffolds and factors that control endogenous tissue formation, (3) clinical trials, and (4) novel cell sources: induced pluripotent stem cells. Within these focus areas, we summarize the highly impactful articles that emerged from our objective analysis and review additional recent publications to augment and expand upon these key themes. Finally, we discuss where the TERM field may be headed and how to monitor such a broad-based and ever-expanding community.
Scope and Aim of This Review
The objective of this “Year in Review” article is to identify and recount significant recent events in the broad discipline of tissue engineering and regenerative medicine (TERM). This builds from the foundation established by Dr. Michael Lysaght, whose data-driven publications first defined our field and the early challenges and opportunities in it, identified for us when the “end of the beginning” had occurred, and detailed the ups and the downs in our translational and commercial aspirations.1–5 In constructing this review, some of the questions that we asked were “What constitutes the TERM field?” and “How would one write a review that both captures the events of today and presages the events of tomorrow?” Moreover, since reviews abound, we wished to write a review that could add to the literature instead of duplicating it. Indeed, we recognized from the outset that we are by definition limited by our own small world-view and specialization (both of the authors being orthopedically inclined tissue engineers), and that this might limit our ability to capture (and subsequently detail) the incredibly diversity of our field.
To answer these questions and to capture the breadth of the TERM field, we started with an objective premise. Specifically, we developed a framework to identify the occurrence of a “transformative event” (i.e., publication) in the TERM domain. As will be detailed below, the sheer number of publications in this area is overwhelming, and it is oftentimes very difficult to separate the “wheat from the chaff,” particularly in publications that are outside one's own small area of expertise. While there are of course certain key signals that may indicate that an event of significance has occurred—for example, publication in a high impact factor journal and/or dissemination in the lay press—quite often, the most impactful publications in our field arise from the primordial TERM soup without much fanfare, but are quickly taken up as a new path forward by the field. In order to establish objective metrics by which to identify such important contributions, we first gathered numerical data on the history of TERM publications, and from this, identified specific criteria that could provide an early reading on the “impact trajectory” for any work, even very soon after its original publication. We then applied these metrics to the literature of the last few years, and used the outcomes of this analysis to define and populate the substance of this review article. Our methods of analysis are detailed in the following section, and the content we identified is then parsed into specific categories and reviewed with respect to ongoing work.
Methods of Review
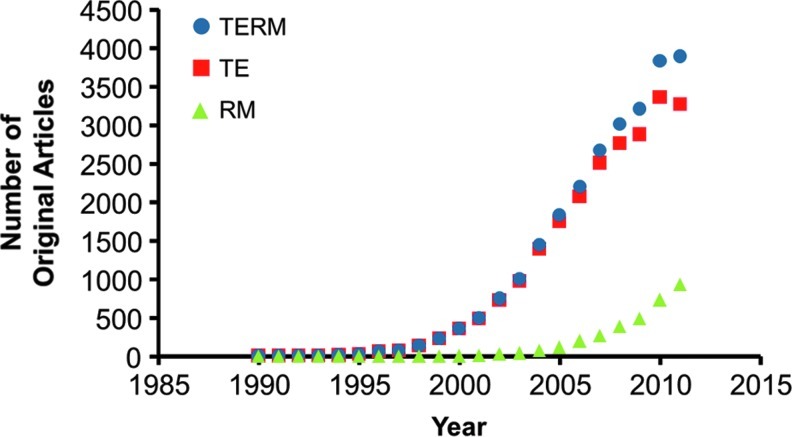
The first challenge in conducting this review was the sheer number of recent publications in the TERM field. The origins of the terms “tissue engineering” and “regenerative medicine” have been previously discussed in this journal,1 with the former coming into common parlance in the mid-to-late 1980s and the latter gaining momentum around the turn of the 21st century. Since their definition, the TERM field has grown substantially. A simple search for “tissue engineering” or “regenerative medicine” within a commonly used database (Scopus®; Elsevier) reveals over 40,000 hits to date, with more than 28,000 original articles and 6000 review articles. The number of TERM articles continues to rise (Fig. 1) with nearly 4000 original articles published in 2010, compared to a mere 360 a decade earlier. This can be partially attributed to the increasing use of the same common terminology, particularly for the more recent “regenerative medicine.” Still, there is no doubt that our field is expanding and capturing a larger portion of the work done across the biomedical sciences. More importantly, it makes a brute force review of the field impossible.
FIG. 1.
The rise of tissue engineering and regenerative medicine (TERM). (Results obtained via Scopus® search using key words “tissue engineering” OR “regenerative medicine”). Color images available online at www.liebertpub.com/teb
This brings us to our second challenge, namely, how to objectively decide which recent articles to include in this review. The most objective measure of an article's impact (in our un-objective opinion) is the number of times it has been cited. However, each impactful article must be read by the field, digested, and inspire new experiments (and possibly obtain funding for said experiments), which then need to be completed and published. This process creates a “citation lag time” of 1–3 years. Indicators which allow a more immediate sense of an article's impact include a journal's impact factor, “buzz” within the field, online views or downloads, and press releases or newspaper/periodical stories, but lack somewhat in objectivity.
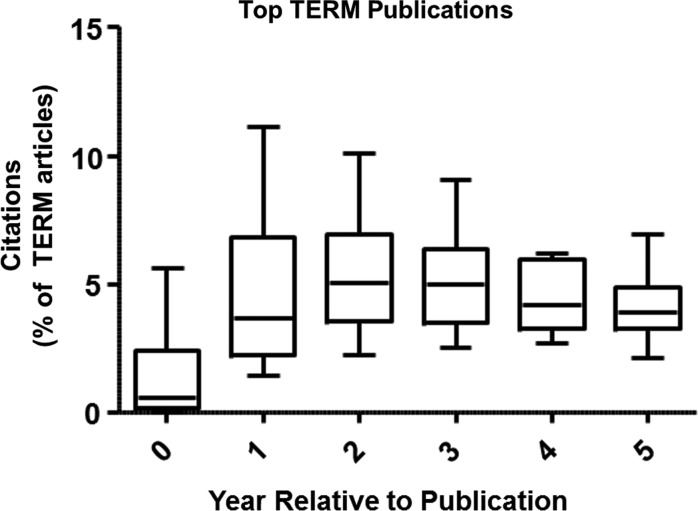
Our challenge then was to develop a framework in which to organize recent advances in the TERM field given both the abundance of literature and the need for objective metrics to evaluate impact. To do so, we first examined impactful articles from previous years to determine if there were early and common trends among these articles. We limited our initial TERM search to original articles (excluding reviews) and organized these by the number of times cited. Examining the top 20 most cited articles in the TERM field (Table 1),6–25 a few trends become clear. First, a high number of citations early (within 2 years) generally indicated that the article would be impactful later as well (5 years). A typical example is the article by Zuk et al.7 published in Tissue Engineering in 2001, which first described the isolation and characterization of adipose-derived progenitor cells. Second, in the year of publication, there is substantial variability due to articles being published early or later in the year (Fig. 2). By 1–2 years postpublication, this variability sharply decreases. Thus, even though the number of citations varies widely early on, the numbers tend to converge for impactful articles. This means however, that there may be impactful articles published in 2012, which we cannot isolate at this time. As such, we have expanded our review to include years 2010 and 2011, since we feel we can better identify key publications in this more expansive time frame using citations as an objective measure.
Table 1.
Top Cited Tissue Engineering and Regenerative Medicine Articles
| |
|
|
|
|
Citations postpublication [number (% of TERM articles)] |
||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Title | Year | Journal | Total | In Y0 | In Y1 | In Y2 | In Y5 | |
| 1 | Langer, R., and Vacanti, J.P. | Tissue engineering | 1993 | Science | 4029 | n/a (n/a) | n/a (n/a) | n/a (n/a) | 64 (220.7) |
| 2 | Zuk, P.A., et al. | Multilineage cells from human adipose tissue: Implications for cell-based therapies | 2001 | Tissue Engineering | 2206 | 10 (2.0) | 51 (6.8) | 81 (8.1) | 153 (7.0) |
| 3 | Hartgerink, J.D., et al. | Self-assembly and mineralization of peptide-amphiphile nanofibers | 2001 | Science | 1307 | 0 (0.0) | 22 (2.9) | 53 (5.3) | 10 (4.9) |
| 4 | Willert, K., et al. | Wnt proteins are lipid-modified and can act as stem cell growth factors | 2003 | Nature | 954 | 24 (2.4) | 110 (7.6) | 98 (5.4) | 137 (4.6) |
| 5 | Nakagawa, M., et al. | Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts | 2008 | Nature Biotechnology | 945 | 148 (4.9) | 204 (6.4) | 215 (5.6) | n/a (n/a) |
| 6 | Sato, N., et al. | Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor | 2004 | Nature Medicine | 900 | 40 (2.8) | 93 (5.1) | 92 (4.2) | 114 (3.6) |
| 7 | Li, W.-J., et al. | Electrospun nanofibrous structure: a novel scaffold for tissue engineering | 2002 | Journal of Biomedical Materials Research | 889 | 2 (0.3) | 22 (2.2) | 54 (3.7) | 105 (3.9) |
| 8 | Niklason, L.E., et al. | Functional arteries grown in vitro | 1999 | Science | 884 | 13 (5.6) | 40 (11.1) | 50 (10.1) | 78 (5.4) |
| 9 | Altman, G.H., et al. | Silk-based biomaterials | 2003 | Biomaterials | 874 | 9 (0.9) | 30 (2.1) | 41 (2.2) | 117 (3.9) |
| 10 | Kehat, I., et al. | Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes | 2001 | Journal of Clinical Investigation | 855 | 4 (0.8) | 51 (6.8) | 76 (7.6) | 85 (3.9) |
| 11 | Matthews, J.A., et al. | Electrospinning of collagen nanofibers | 2002 | Biomacromolecules | 840 | 3 (0.4) | 21 (2.1) | 51 (3.5) | 88 (3.3) |
| 12 | Yamashita, J., et al. | Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors | 2000 | Nature | 815 | 2 (0.6) | 43 (8.7) | 62 (8.3) | 84 (4.6) |
| 13 | Liechty, K.W., et al. | Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep | 2000 | Nature Medicine | 742 | 0 (0.0) | 27 (5.5) | 52 (6.9) | 59 (3.2) |
| 14 | Yoshimoto, H., et al. | A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering | 2003 | Biomaterials | 735 | 2 (0.2) | 46 (3.2) | 64 (3.5) | 92 (3.1) |
| 15 | Okita, K., et al. | Generation of mouse induced pluripotent stem cells without viral vectors | 2008 | Science | 692 | 3 (0.1) | 173 (5.4) | 184 (4.8) | n/a (n/a) |
| 16 | Meissner, A., et al. | Genome-scale DNA methylation maps of pluripotent and differentiated cells | 2008 | Nature | 682 | 16 (0.5) | 117 (3.7) | 193 (5.1) | n/a (n/a) |
| 17 | Kokubo, T., et al. | Novel bioactive materials with different mechanical properties | 2003 | Biomaterials | 681 | 3 (0.3) | 21 (1.5) | 59 (3.2) | 110 (3.7) |
| 18 | Madihally, S.V., and Matthew, H.W.T. | Porous chitosan scaffolds for tissue engineering | 1999 | Biomaterials | 625 | 0 (0.0) | 8 (2.2) | 15 (3.0) | 31 (2.1) |
| 19 | Boyan, B.D., et al. | Role of material surfaces in regulating bone and cartilage cell response | 1996 | Biomaterials | 616 | 3 (4.7) | 2 (2.7) | 9 (6.7) | 27 (5.5) |
| 20 | Le Blanc, K., et al. | Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study | 2008 | The Lancet | 603 | 28 (0.9) | 110 (3.4) | 155 (4.1) | n/a (n/a) |
| ** | Takahashi, K., and Yamanaka, S. | Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors | 2006 | Cell | 4518 | 24 (1.1) | 156 (5.8) | 449 (14.9) | 1210 (31.2) |
Search conducted on 12/3/2012 using Scopus®. Excludes Reviews as defined in Scopus®. Citation numbers are given as number of citations within a given year postpublication. Percentages represent number of citations divided by the number of TERM publications in that year. Article by Takahashi and Yamanaka85 did not appear within search parameters, but is provided given its seminal nature.
n/a, data unavailable; TERM, tissue engineering and regenerative medicine.
FIG. 2.
Number of citations each year postpublication (as a percentage of total TERM articles in that year) for the top 20 TERM publications. Data presented as box and whiskers plot featuring median, interquartile range, and minimum/maximum (Langer and Vacanti6 excluded as an outlier).
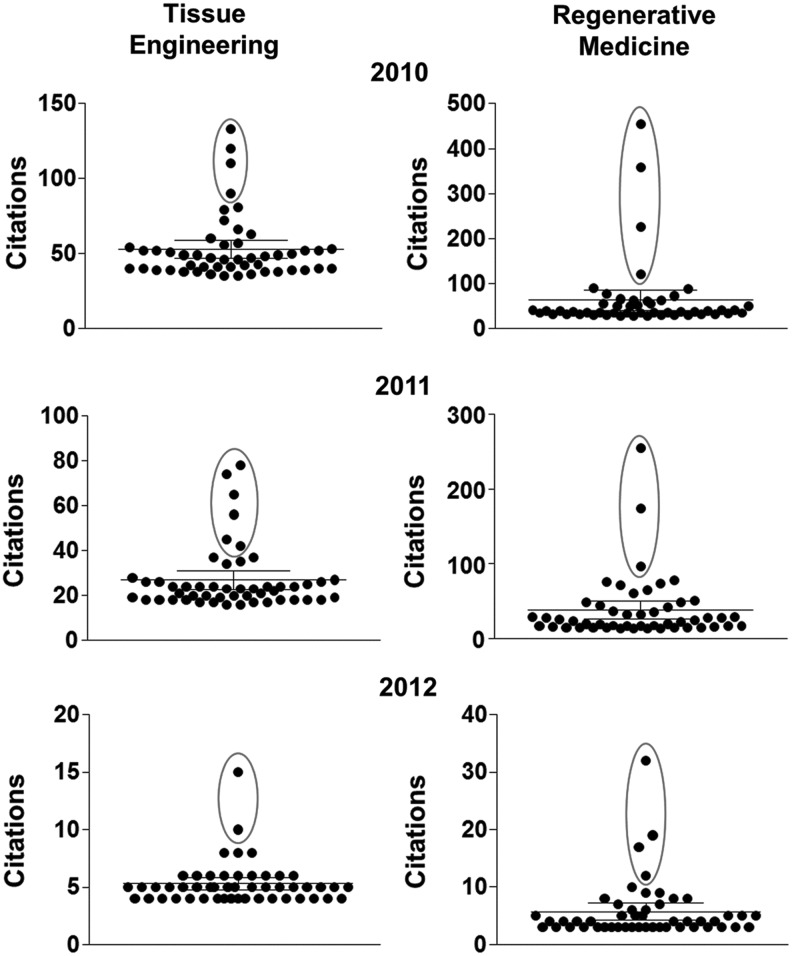
We then applied this analysis to TERM articles published in 2010–2012. Examining the top 50 publications in terms of citations (See Fig. 3 for a scatter plot in each year and domain), one can appreciate the distribution in times cited. Using the Grubbs' test for outliers (circled on these plots), one can then clearly identify publications that have separated from the pack and represent a study that has had broad impact on the TERM field in a short period of time. Within this set of studies, we identified several key themes in the TERM literature. These are: Tissue Engineering: Grafts and Materials, Regenerative Medicine: Scaffolds and Factors that Control Endogenous Tissue Formation, Clinical Trials, and Novel Cell Sources: Induced Pluripotent Stem Cells. These categories will form the basis for the next sections. In each area, one or more highly impactful articles will be highlighted, and a discussion of recent publications will be included to augment and expand upon these key themes that are clearly emerging in the TERM field. Finally, we will conclude with a discussion on where the TERM field may be headed and how to monitor such an ever-expanding community.
FIG. 3.
Top 50 TE and RM publications for 2010–2012 with the outliers circled. These publications formed the basis of this review and defined discussion categories.
Recent Advances in TERM
Tissue engineering: grafts and materials
When first conceived, many engineers considered that tissue engineering would involve the de novo engineering of new tissues for implantations, using starting materials and methods drawn from chemical engineering, biomaterials science, and mechanical engineering principles. However, some of the most high profile recent studies on tissue engineering have focused on translating relatively simple approaches into preclinical and clinical studies. For example, in 2009 the field was stimulated by the first report of an engineered airway based on a cadaveric decellularized implant in a human patient,26 one of the first instances of a tissue engineering approach saving a life. Since then, replacements based on this idea have increased in complexity, including engineered lungs, livers, and vascular grafts. A common theme in many of these approaches is the use of decellularized extracellular matrix (ECM) to serve as a scaffold for cell seeding. The rationale is that a decellularized ECM maintains a niche, which can serve to maintain cell phenotype and encourage production of tissue specific matrix and functional properties. This type of approach is not radical, as decellularized tissues have been used as allografts in surgical replacement procedures for decades, and pioneering work by Badylak and colleagues27,28 has defined the regenerative potential of these ECM-based implants.
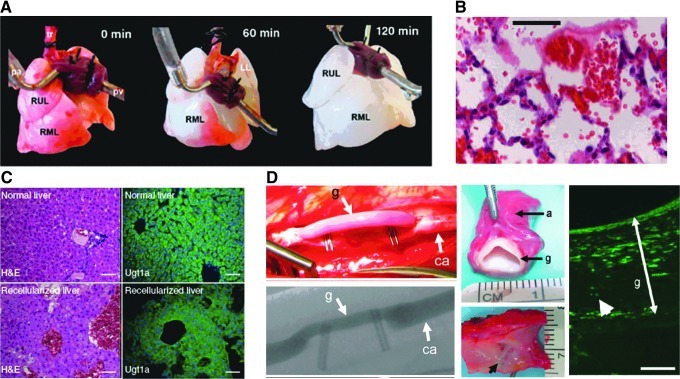
Two recent notable examples in this area that emerged from our objective analysis were studies by Petersen et al.29 and Ott et al.30 Both of these studies reported data on the use of decellularized lung tissue as a scaffold for viable lung replacement (Fig. 4A). In general, cell seeding of decellularized ECM from donor animals and culture allowing both media and air exchange maintained cell viability and allowed the production of lung-specific matrix (Fig. 4B) and other molecules, including surfactant protein precursors. When used for short-term replacement (several hours) of the left lung in adult rats, blood perfusion and ventilation were achieved with the tissue-engineered graft. Petersen et al.29 reported lower oxygen levels compared to normal; however, complete hemoglobin saturation was achieved. Ott et al.30 further noted the presence of pulmonary secretions, suggesting functionality. Yet, the transplanted lungs required higher pressure to be fully inflated and had fluid buildup, suggestive of pulmonary edema. While additional preclinical studies are necessary to show the longer-term viability and efficacy of such approaches, these results are unquestionably promising with regard to ECM-based scaffolds for lung replacement.
FIG. 4.
Use of decellularized tissue matrix for TE applications. Whole lungs can be readily decellularized through perfusion techniques [right upper lobe (RUL), right middle lobe (RML)] (A). When implanted in vivo, such tissues are perfused by red blood cells and maintain partial function in the short-term (B). Recellularized liver matrix also maintains similar structure and cellular viability and phenotype (C). Scale bar=100 μm. Decellularized tissue-engineered vascular grafts maintain patency when implanted and allow repopulation with native cells [graft (g), carotid artery (ca), adventitia (a)] (D). White arrowhead points out alpha-smooth muscle actin-positive cell. Scale bars=100 μm. (Adapted from Petersen et al.,29 Ott et al.,30 Uygun et al.,31 and Dahl et al.,33 [with permission from AAAS and MacMillan Publishers, Ltd]).
These same principles have also been applied to liver regeneration, for example in a recent publication by Uygun et al.,31 which also emerged from our objective screen. In this study, rat liver tissue was decellularized and perfused with primary rat hepatocytes. Cell viability was maintained over 5 days in culture, and albumin and urea production increased with time, with albumin production reaching 20% of normal. Vascularized grafts were then implanted and perfused in vivo, following a nephrectomy in recipient rats. After 8 h, cells maintained viability at preoperative levels (∼80% viability) and remained histologically similar to the native tissue (Fig. 4C). Here too, additional preclinical work is required to fully investigate the potential of such ECM-based cell-seeded grafts, but this early work is quite promising.
While vascular autografts are quite common, some have considered devitalized allografts (from cadaveric tissue) as an alternative source, and have shown good mechanics and biocompatibility through this decellularization process.32 As an alternative to removing cells from native tissue, Dahl et al.33 recently took an interesting tissue engineering approach to create vascular grafts. Here, the authors isolated human smooth muscle cells and cultured them on tubular polyglycolic acid scaffolds (Fig. 4D). Over a period of several months, the grafts possessed suture retention strength, burst pressure, and compliance similar to native tissues. These “engineered” grafts were then decellularized and stored for up to 1 year without an appreciable loss in mechanical function. In a model of arteriovenous bypass in baboons, the grafts remained functional for up to 6 months, with little fibrosis, calcification, or thickening, and 88% of the grafts remained patent and mechanically stable. In a canine model of carotid or coronary bypass, these grafts preseeded with endothelial cells remained 83% patent at 1 month with only one occlusion. Although little immunogenicity was noted and all grafts remodeled to possess a biochemical composition more similar to native tissue, longer-term studies and direct comparison to the clinical gold standard will be needed to fully validate this approach. This work is quite interesting, however, as it replaces traditional cadaveric grafts with those generated through “traditional” TE approaches (cells coupled to biomaterials and grown in vitro), which have several potential advantages. It will be interesting to see whether this novel concept is adopted across the spectrum of organ/tissue replacement in TERM.
Stemming from the publications noted above that met our objective criteria for inclusion in this review, considerable work has been carried out to expand this exciting new TERM focus area. For instance, recent studies have examined how other cell types, such as mesenchymal stem cells, can colonize and integrate within decellularized lung tissues.34 In cardiac applications, methods have been developed to enhance decellularization while preserving vascular beds in thick tissue slices.35 Similarly, bone allograft processing techniques have recently been optimized to support mesenchymal cell attachment and mineral deposition.36 Investigators have also begun to explore how such tissues can be re-enervated, for example in skeletal muscle preparations, guiding both vascular health as well as neural connectivity to the host.37 These and other publications support the growing interest and increasing complexity in organ/native ECM-based tissue replacement approaches.
In addition to these ECM-derived approaches, there of course remains active development of novel biomaterial scaffolds. Progress in the past year has included electrospun materials that foster cellular colonization38,39 and can direct depth dependent and anatomic reconstitution of cell and matrix organization.40,41 Other studies have built off of the acellular concept, imbuing acellular materials with molecules that attract progenitor cells to the wound interface.42 Still other studies have developed materials that can optimally deliver and sequester cells within a repair environment, based on nano-scale surface topography.43 In terms of vascularization, progress has been made in both engineered artificial vascular networks44 as well as via the development of advanced materials that allow endothelial cells to remodel and build their own vascular network.45 Finally, while quite a lot of work focuses on decellularized matrices and cell-seeded biomaterials, significant progress continues in the formation of almost completely cell based tissue constructs, even for load bearing structures.46 This activity, premised upon both “traditional” and emerging themes, points to continued innovation and expansion of TERM applications for the repair and replacement of ever more complex systems.
Regenerative medicine: scaffolds and factors that control endogenous tissue formation
Over the past decade, it is clear that regenerative medicine approaches have increased in popularity for a variety of reasons. Cellular therapies remain a large focus, and the efficacy of several cell types have been evaluated in vivo. In addition, considerable efforts in regenerative biology have focused on the developmental origins of stem and progenitor cells, and on elucidating how they persist (or don't) in adult organisms. Another major focus has been on the development of biomaterials to either release bioactive factors to aid in the healing response and/or provide a scaffold that can promote appropriate tissue formation. This generally acellular approach seeks to foster repair by optimizing the response of endogenous progenitor cell pools, rather than cell delivery itself.
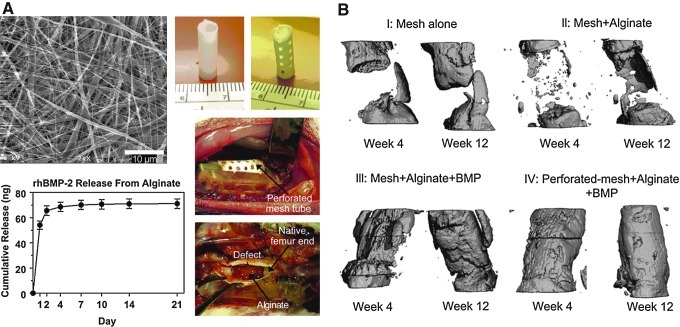
For example, one interesting recent study that emerged from our objective analysis highlighted the multiple roles that a scaffold may play in promoting the regenerative response in the case of bone healing in a rat model. This study, by Kolambkar et al.,47 employed a combined scaffold using a tubular mesh made from electrospun poly(ɛ-caprolactone) nanofibers that wrapped around the bone defect and helped to localize a peptide-modified alginate hydrogel injected to fill the defect and deliver recombinant bone morphogenetic protein-2 (rhBMP-2) (Fig. 5). With delivery of rhBMP-2, bony bridging occurred consistently (as assessed by micro-computed tomography), but did not occur with the scaffolds alone, showing the importance of growth factor delivery. In addition, macroscopic perforations in the nanofiber mesh seemed to accelerate repair, resulting in a twofold increase in the torsional stiffness of the healing bone. Revascularization of the defects was not increased, and the authors suggest that the perforations allowed endogenous progenitor cells to infiltrate and positively influence repair. Thus, when designing scaffolds for regenerative medicine applications, there are many factors to consider which may ultimately impact their success, even for relatively simple tissues such as ligaments and tendons.48
FIG. 5.
Bioscaffolds for bone regeneration. Nanofibers with macroscopic perforations and alginate releasing bone morphogenetic protein-2 (BMP-2) were placed within a critically sized bone defect (A). Micro-computed tomography images showing increased bone formation with scaffold treatment (B). (Adapted from Kolambkar et al.47 [with permission from Elsevier]).
For methods such as that noted above to be successful, the endogenous progenitor pool must be both local to the defect site and of sufficient number/capacity as to mount a repair response. In a recent study by Jaskelioff et al.49 (which also emerged from our objective analysis), the authors investigated how stem cell depletion is related to genome damage. Specifically, they tested the hypothesis that declining organ function may be eliminated or even reversed by engineering progenitor cells to reactivate endogenous telomerase activity. Their findings showed a marked reversal of degenerative neuronal and sensory phenotypes in adult mice, supporting the notion that maintenance of progenitor cell pools, or rescuing progenitor cell activity, can lead to wide scale tissue and organ rejuvenation.
Along a similar line, investigators have queried normal regenerative processes in amphibians and mammals to define the role of endogenous stem cell populations in regeneration. For instance, our objective screen of the literature picked up a recent publication by Porrello et al.,50 who studied cardiac regeneration in mice. These investigators found, quite strikingly, that while cardiac regeneration can occur early (1 day after birth), this capacity is lost by 7 days after birth. This early healing was characterized as “regenerative,” with a lack of fibrosis and normal heart function achieved after 2 months. Conversely, just a week later, the response to the same injury was characterized by a repair response, with scar tissue formation, much like one would see in mature animals. This disjunction between fetal and adult regenerative response may offer new insight into regenerative therapies across a range of diseases and injuries. More generally, the study of injury mechanisms, and how they interface and activate endogenous progenitor cell populations, is an active and growing area of inquiry,51,52 with a particular focus on elucidating how progenitor cells interact with cells of the immune system.53–55
In addition to this age dependent change in regenerative capacity, considerable work has focused on identifying precisely which pool of stem cells actively participates in endogenous repair/regeneration processes. Work by Rinkevich et al.56 showed via lineage tracing that those cells that reconstitute the mouse digit are lineage restricted. That is, the stem cell pool that forms cartilage and bone for instance is different than the cell population that forms other adjacent tissues (muscle and skin). Understanding how, when, and where these different progenitor cell populations are operative will inform ongoing TERM studies aimed at recruiting and manipulating these endogenous regenerative cell sources. Studying atypical resolution of injury and/or repair in other mammalian species may shed additional light on regenerative processes. For example, the recent report on “skin shedding” and regeneration in an African spiny mouse,57 while certainly atypical, may provide new directions for TERM investigators. While these models may sometimes be far afield from the actual application of regenerative strategies (arising as they often do from developmental biology studies), they build a basic science framework from which novel technologies may grow. Moreover, they define the theoretical and basic science limits on what will be possible with endogenous tissue engineering/regenerative medicine.
This area of regenerative medicine is continually expanding. For instance, in a high profile article from 2010, Lee et al. showed that whole joint cartilage repair could be achieved in a rabbit model in which a polymeric joint prosthesis was implanted, with endogenous cells recruited to via inclusion of transforming growth factor beta (TGF-β).58 Along these same lines, Shah et al. reported the use of nanofibers made from peptide amphiphile molecules which have high binding affinity for TGF-β and showed the potential for cartilage regeneration in rabbits.59 Still others have turned to novel methods, such as control through magnetic fields, to permit superior release of factors in vivo.60 In some cases, rather than delivering a specific growth factor, investigators have utilized the broader paracrine activities of injected cells, particularly stem cells, to advance regeneration. Indeed, this paracrine effect is thought by many to be one of the dominant mechanisms by which injected stem cells offer a therapeutic benefit.61–64 It is important to point out that these factors, whether they derive from the scaffold or are released by implanted cells, can influence the regenerative environment in nonintuitive ways. For instance, a recent report detailed how growth factors themselves can bind to pro-inflammatory cytokines to prevent arthritis in mice,65 allowing for the possibility of simultaneous anabolic and anti-catabolic functionality.
In the biomaterials domain of regenerative medicine, much attention has been paid to the advancement of material design through the promotion of endogenous stem cell differentiation toward a specific phenotype of interest by modulating physical attributes of the scaffold (including stiffness, topography, and porosity).66–70 Others have suggested that stem cells with a specific propensity towards a defined lineage may have different physical properties71 and have used these differences to select for a specific cell phenotype from a heterogeneous population prior to implantation.72,73 Thus advanced materials, which both select refined populations and direct their differentiation, may provide a mechanism to achieve improved in vivo regeneration.
Clinical trials
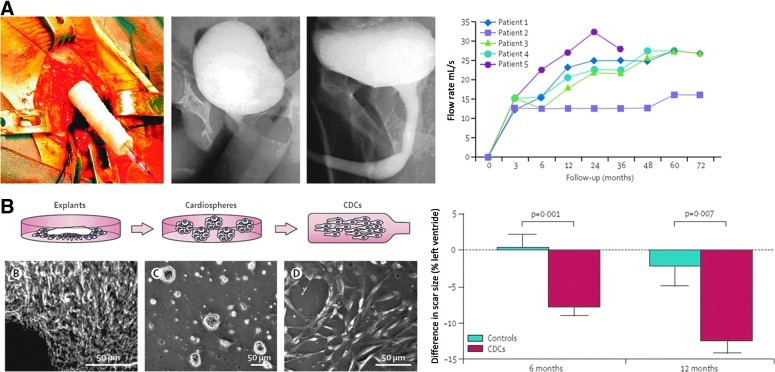
TERM approaches are also making their way into clinical practice, with a number of clinical studies published over the past few years. For instance, a tissue engineering approach was used to restore function in a small cohort of boys with large urethral defects.74 Biopsies of bladder tissue were obtained, and smooth muscle and epithelial cells were isolated and cultured. The cells were then seeded onto tubular poly (glycolic acid)-poly (lactic-co-glycolic acid) scaffolds (Fig. 6A). Following culture for 1 week to ensure cell viability and matrix production, grafts were used for urethral reconstruction. The authors report that all five patients maintained functional flow rates at 36–72 months postimplantation and that biopsies confirmed tissue organization similar to the native tissue. This approach shows promise for traumatic or congenital injuries to the urethra where simple repair or on-lay grafts are insufficient.
FIG. 6.
Recent clinical applications of TERM approaches. A tissue-engineered urethra can maintain function in patients in the long-term (A). Injection of cardiosphere-derived stem cells can reduce scar size in patients postinfarction (B). (Adapted from Raya-Rivera et al.74 and Makkar et al.75 [with permission from Elsevier]). CDC, cardiosphere-derived cells.
In addition to tissue replacement, much interest has focused on the perfusion of cells into the heart for the treatment of myocardial infarction, and several clinical studies have recently reported initial results. One phase I prospective, randomized, controlled trial (RCT) aimed to treat infarction with progenitor cells (called cardiosphere-derived autologous stem cells).75 Autologous endomyocardial cells were obtained and cultured using a specific culture method, including culture in nonadherent plates to form three-dimensional (3D) cardiospheres in order to obtain a population positive for CD105 and negative for CD45 (Fig. 6B). Although some adverse events were reported with implantation of these cells, by 6 months, a significant reduction in scar mass and increase in viable heart mass and wall thickness was found. However, no differences in functional measurements, such as ejection fraction could be determined at this early time point. Nevertheless, the reduction in scar tissue and increase of viable tissue with this treatment supported movement on to a phase 2 study. Other recent studies injecting bone marrow-derived progenitor cells also lend support to the injection of stem cells to enhance function following myocardial infarction and failure of coronary stents.76
In other applications, autologous stem cell therapy has been used to reverse corneal destruction due to burns. In one study,77 autologous limbal stem cells were cultured on fibrin to treat corneal damage in over 100 patients. At 10 years follow-up, more than 75% of patients had a restored corneal epithelium layer. Clinical success was correlated to the percentage of functional stem cells (holoclone-forming) observed in culture. Specifically, if cultures contained more than 3% holoclone-forming cells, clinical success was found in 78% of patients. On the other hand, if less than 3% holoclone-forming cells were found, success was seen in only 11% of patients. This study shows the potential for limbal stem cells for corneal repair; however, additional randomized controlled clinical trials are needed.
Another quite popular focus of clinical trials in recent years has revolved around the use of platelet-rich plasma (PRP) to treat orthopedic sports injuries, with both considerable interest and criticism abounding in the literature. Several randomized control trials are underway, and early results have been recently published. Peerbooms et al. first reported the beneficial effects of PRP over corticosteroid injections for the treatment of lateral epicondylitis in a double-blind RCT.78 At 1 year, marked improvements were seen in both patient reported outcomes as well as functional scores, and more recent data suggests these results persist up to 2 years.79
Others have reported on the limited impact of PRP or platelet-rich fibrin matrix (PRFM) for other types of injuries, such as rotator cuff repairs or Achilles tendinopathy.80–82 In addition to these studies, another recent study showed that blood elements (including platelets) can induce a pro-inflammatory response.83 Thus, while such platelet-based treatments may offer some promise, much research, both clinical and basic science, is still needed to evaluate the impact of PRP and PRFM. Complications include variable patient demographics, injury types and previous treatment, and potential differences between the many PRP and PRFM products currently on the market. Together, these clinical and preclinical studies may soon resolve these issues, and may provide a valuable adjuvant to repair in certain clinical scenarios.
In the coming years, it is likely that there will be a dramatic increase in the number of published clinical studies that utilize TERM approaches. Indeed, the number of registered clinical trials in the U.S. featuring either “tissue engineering” and/or “regenerative medicine” has increased from 38 in 2007 to 83 in 2011. These translational studies will inform the field as to the true efficacy of TERM approaches in clinical practice and will help to refine work across the research space, from in vitro development to preclinical models.
Novel cell sources: induced pluripotent stem cells
While the work above quite often involves the delivery of adult stem cells, significant interest remains in the development and application of newly emergent progenitor cell sources. For example, research into the use of embryonic stem cells (ESCs) for TERM applications continues unabated. A recent study by Marolt et al. showed, for example, that culture of human ESCs on decellularized bone matrix in a perfusion bioreactor84 resulted in formation of a stable bone-like tissue. Quite interestingly, when these tissue constructs were implanted subcutaneously in immunodeficient mice, teratomas formed with implantation of undifferentiated human ESCs but not with implantation of ESC-derived bone. This work illustrates the continued development of ESC-based therapies in TERM, as well as the potential limitations in such work when differentiation is not adequately controlled.
The past several years have also borne witness to a truly impactful (if not the most impactful) development in TERM. That is, the discovery and characterization of induced pluripotent stem cells (iPSCs), first defined by Takahashi and Yamanaka in 2006,85 describing how differentiated adult cells could be reprogrammed back to an embryonic state through the presence of only four transcription factors (Oct-4, Sox-2, c-Myc, and Klf4). The original article detailing this technology has already been cited over 4400 times to date! The impact of this work cannot be overstated, as was evidenced by the awarding of the Nobel Prize in Medicine to Dr. Yamanaka this past year, only 6 years after publication of his seminal article. Research surrounding the use of iPSCs has seen a rapid and exponential growth, and interested readers are directed to a number of fine reviews on this topic.86–89 Instead, we will highlight some of the most highly impactful articles found through our objective analysis to provide a general sense of the immense progress in this area.
While the original iPSC “cocktail” was quite simple, recent work has focused on further refining and simplifying this process. Instead of ectopic expression of several transcription factors, an alternative approach is to express specific microRNAs (miRNAs). In a recent study, expression of the miR302/367 cluster via viral transfection was able to reprogram both mouse embryonic fibroblasts and human foreskin and dermal fibroblasts with a several fold increase in efficiency relative to the typical transcription factors.90 Another major issue limiting translation of iPSC technology has been the need for viral infection to introduce the necessary transcription factors for reprogramming, which raises regulatory concerns due to genomic integration or mutagenesis and is relatively inefficient. Alternatively, synthetic mRNAs can be used directly to reprogram differentiated cells into iPSCs.91 This approach was much more efficient (two orders of magnitude higher than viral transfection). Due to the short activity span of such treatments, however, multiple injections would be needed clinically. The authors also note that RNA-derived iPSCs had global transcriptional patterns that were more similar to human ESCs, suggesting that this reprogramming process may yield cells of higher quality. Direct transfection of miRNAs without a viral carrier can also allow reprogramming of mouse and human somatic cells into iPSCs.92 Another way to create iPSCs is to create heterokaryons (fused cells) from mouse ESCs and human fibroblasts, which allows reprogramming with 70% efficiency within 1 day and allows study of the molecular mechanisms of reprogramming.93
Others have tested the hypothesis that differentiated cells can be reprogrammed in situ, abrogating the need for in vitro manipulation. For example, Qian et al. injected a retrovirus expressing the Yamanaka transcription factors to reprogram adult resident nonmyocytes in the murine heart into iPSCs.94 Analysis of single cells revealed ventricular cardiomyocyte-like action potentials, beating upon electrical stimulation, and evidence of electrical coupling. In an injury model (coronary ligation), retroviral treatment modestly attenuated cardiac dysfunction for up to 3 months. Although iPSC induction did not fully restore function following injury, this study shows that in vivo reprogramming could have therapeutic potential.
Along these lines, there has been considerable interest in direct reprogramming of one differentiated cell into another. For example, it has recently been shown that retroviral expression of a set of transcription factors can reprogram cardiac fibroblasts into myocyte-like cells both in vitro and in vivo in a mouse model.95 Following induction of myocardial infarction, injected retroviruses led to a substantial improvement in functional outcomes, such as ejection fraction, for up to 12 weeks relative to controls. More recently, cardiac fibroblasts have been reprogrammed into myocytes via expression of specific miRNAs.96 Huang et al. directly induced mouse tail-tip fibroblasts into hepatocyte-like (iHep) cells.97 When transplanted ectopically in vivo in a model of liver failure, these iHep cells significantly improved survivability (∼40%) at 8 weeks relative to fibroblasts (0%); however, survivability was still less than true hepatocytes (∼90%). Similarly, expression of a combination of three genes has been shown to convert mouse embryonic and postnatal fibroblasts into neurons in vitro.98 These studies, and many others like them, indicate the very rapid developments and innovations that are occurring in this sphere of regenerative medicine.
As iPSCs become more widely available and adopted by the TERM community, the transition to translation of this cell type has already started. However, some caution must be taken in this process, as there remain several issues that could limit their eventual clinical potential. For instance, while some studies have focused in the in vivo applications of these cells in situations of organ repair, issues have arisen related to the immunogenicity of autologous cell-derived iPSCs that are reprogrammed in vitro and reimplanted. In an inbred mouse model, “autologous” ESCs failed to illicit an immune response, while “autologous” embryonic fibroblasts reprogrammed into iPSCs were mostly rejected by the host and featured T cell infiltration.99 The authors of this study attributed these findings to abnormal gene expression in a portion of the iPSCs. While this is certainly a potential reason, additional work is required to fully understand this behavior.
An additional persistent concern with iPSCs is the variability in their differentiation pathways, most likely arising from incomplete or aberrant reprogramming of the parent population.100 In response, Boulting et al. have recently suggested the use of a set of validated cell lines to expedite research findings and to better standardize results.101 Other investigators have developed novel processing methods to isolate “pure” populations of iPSC for particular applications. For instance, work by Diekman et al.102 showed that passage through a chondrogenic “filter,” that is, selecting subpopulations that originally differentiate towards the chondrogenic lineage most robustly, enabled the isolation of sub-populations of iPSCs that had improved cartilage formation when placed back into 3D culture conditions (Fig. 7). These differentiation-based selection criteria for iPSC will undoubtedly become more complex and refined in future work. For example, such sorting could be coupled with concepts demonstrated in the work of Gilbert et al.,103 who showed that the matrix elasticity on which progenitor cells are plated can influence the retention or loss of stemness. Similarly, soluble factors, sometimes derived from high throughput screening of chemical libraries, could be used to further refine these cell populations, as well as enforce their differentiation trajectories in vitro and in vivo.104,105 These and other microenvironmental inputs will likely be used to tune iPSC differentiation specificity, efficacy, and fidelity over the coming years to forward specific TERM applications and translation of this cell type on to preclinical and clinical applications.
FIG. 7.
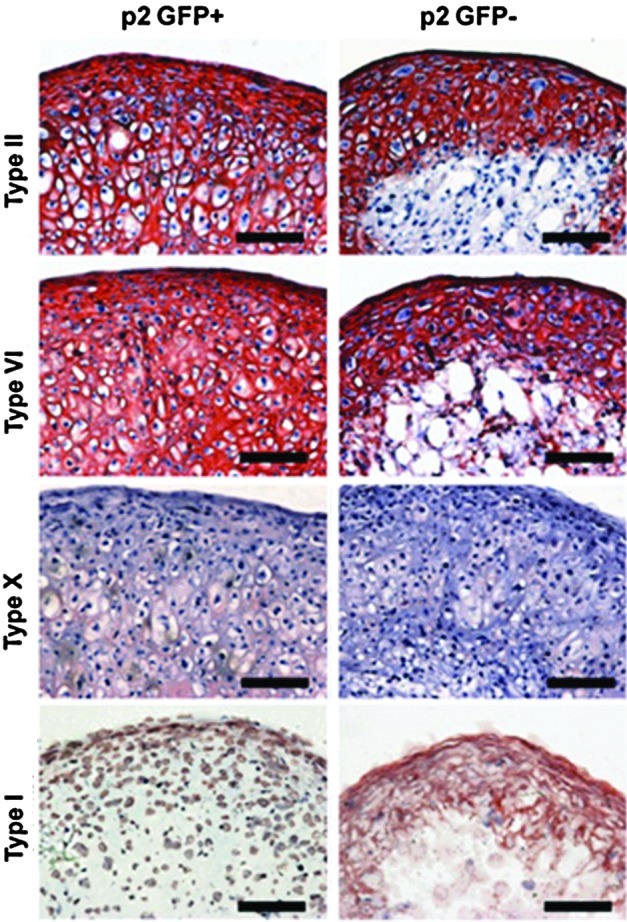
Induced pluripotent stem cells (iPSCs) can produce cartilage-like matrix. Collagen immunohistochemistry for matrix made by passage 2 iPSCs sorted via expression of type II collagen (GFP+). (Adapted from Diekman et al.102 [with permission from the National Academy of Sciences]).
Concluding Remarks and Discussion
Writing a Year in Review that captures the present state and future directions of any field of inquiry is no easy task, especially when that field is as diverse and ever-changing as TERM. In fact, since its inception, the TERM field has been characterized in some sense by its lack of definition and its incredible breadth. Indeed, it is precisely this breadth and diversity that drew so many of us into the field in the first place. Many seemingly discordant lines of research have now become intertwined in TERM and constitute the fabric of our field, with these concepts arising from the blurring of boundaries between traditional disciplines. While this point is sometimes easy to forget, much of what we now consider commonplace in TERM was only a short time ago separated by barriers of dogma and discipline. As these lines continue to blur, and multi-disciplinary research becomes more the rule than the exception, our field is experiencing tremendous growth. The unlimited potential of this work has captured the attention of the lay public (as evidenced by the recent 3 day, front-page New York Times profile on the TERM field106). Moreover, in almost every aspect of the field as evidenced in this review, we are beginning to witness the critical stage of transition to translation.
While quite exciting for all in involved, rapid growth of any field is not without its drawbacks. Specifically, the pace of growth is now so fast that it impossible for most of us to keep up with the field as a whole, or even a small subset of it. For example, a TERM search specific to “cartilage” returns more than 450 articles published in 2011 alone, meaning that one would need to read more than one article per day just to stay abreast of this small portion of the TERM terrain. It is difficult (amongst all this noise) to recognize and separate the truly important contributions from those that are less so. In addition to the sheer numbers of physical publications in the TERM field, the number of outlets through which research findings are being disseminated is also expanding. The scientific community is increasingly exploiting new mechanisms to achieve faster publication (i.e., all electronic journals). In the absence of other, faster objective metrics, the increased use of editorials, reviews, letters to the editor, etc. might provide a way to increase credibility and interest quickly in emerging themes in TERM, as well as mitigate the “citation lag time” commonly found, even for important articles.
In this review, we attempted to both engage the diversity and to encapsulate the ever-expanding TERM field by “letting the numbers speak for themselves,” using the impact trajectories of the last several years to provide a framework for discussion. In using these objective criteria to guide us, we found considerable innovation in a number of traditional TERM fields, but also new ideas that are beginning to take hold in emerging focal areas. For instance, in the realm of tissue replacement, we are now seeing not just scaffolds of ever-increasing complexity derived from standard engineering methods, but also complex scaffolds predicated on natural designs (and native tissues themselves, once decellularized). In the broader field of regenerative medicine, we are seeing developmental biology begin to address not just the formation of tissues, but the specific role that endogenous stem cells play in both generative and regenerative processes. Integrating these basic science findings with novel materials that specifically recruit endogenous populations may provide a next wave in smart biomaterials for tissue repair. Likewise, new cell sources, most prominently iPSCs, have come to the fore, making autologous cell-based therapies for any tissue a real possibility. Finally, our objective screen showed that ours is truly a translational field, and that TERM advances are being reduced to clinical practice at an ever-increasing rate. Furthermore, as we are by and large quantitative scientists, both the quantitative nature of these outcome measures and levels of evidence in support of these applications are advancing as well. Together, these advances are now beginning to change the lives of small subsets of the population, and in the future, these novel approaches will be able to address a host of diseases and instances of tissue degeneration that were heretofore untreatable. Here's hoping that the next Year in Review will continue to find such exciting activity, and further illustrate the growing impact of TERM technologies on improving human health.
Acknowledgments
We gratefully acknowledge support from the National Institutes of Health (R01EB02425, R01EB008722, R01AR056624), the Veterans' Administration (I01RX000174, I01RX000700), the Musculoskeletal Transplant Foundation (Junior Investigator Grant), the Orthopedic Research and Education Foundation, the Penn Center for Musculoskeletal Disorders (P30AR050950), and the Penn Institute for Regenerative Medicine.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lysaght M.J. Crager J. Origins. Tissue Eng Part A. 2009;15:1449. doi: 10.1089/ten.tea.2007.0412. [DOI] [PubMed] [Google Scholar]
- 2.Lysaght M.J. Hazlehurst A.L. Tissue engineering: the end of the beginning. Tissue Eng. 2004;10:309. doi: 10.1089/107632704322791943. [DOI] [PubMed] [Google Scholar]
- 3.Lysaght M.J. Jaklenec A. Deweerd E. Great expectations: private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng Part A. 2008;14:305. doi: 10.1089/tea.2007.0267. [DOI] [PubMed] [Google Scholar]
- 4.Lysaght M.J. Reyes J. The growth of tissue engineering. Tissue Eng. 2001;7:485. doi: 10.1089/107632701753213110. [DOI] [PubMed] [Google Scholar]
- 5.Lysaght M.J. Product development in tissue engineering. Tissue Eng. 1995;1:221. doi: 10.1089/ten.1995.1.221. [DOI] [PubMed] [Google Scholar]
- 6.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 7.Zuk P.A., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 8.Hartgerink J.D. Beniash E. Stupp S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 9.Willert K., et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa M., et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 11.Sato N., et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 12.Li W.J., et al. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 13.Niklason L.E., et al. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 14.Altman G.H., et al. Silk-based biomaterials. Biomaterials. 2003;24:401. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 15.Kehat I., et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews J.A., et al. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita J., et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 18.Liechty K.W., et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimoto H., et al. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 20.Okita K., et al. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 21.Meissner A., et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kokubo T. Kim H.M. Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24:2161. doi: 10.1016/s0142-9612(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 23.Madihally S.V. Matthew H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials. 1999;20:1133. doi: 10.1016/s0142-9612(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 24.Boyan B.D., et al. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17:137. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 25.Le Blanc K., et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 26.Macchiarini P., et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 27.Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 28.Badylak S.F. Freytes D.O. Gilbert T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Petersen T.H., et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ott H.C., et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 31.Uygun B.E., et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilshaw S.P., et al. Development and characterization of acellular allogeneic arterial matrices. Tissue Eng Part A. 2012;18:471. doi: 10.1089/ten.tea.2011.0287. [DOI] [PubMed] [Google Scholar]
- 33.Dahl S.L., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 34.Daly A.B., et al. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 2012;18:1. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarig U., et al. Thick acellular heart extracellular matrix with inherent vasculature: a potential platform for myocardial tissue regeneration. Tissue Eng Part A. 2012;18:2125. doi: 10.1089/ten.tea.2011.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coquelin L., et al. In vivo and in vitro comparison of three different allografts vitalized with human mesenchymal stromal cells. Tissue Eng Part A. 2012;18:1921. doi: 10.1089/ten.TEA.2011.0645. [DOI] [PubMed] [Google Scholar]
- 37.Kang S.B., et al. Functional recovery of completely denervated muscle: implications for innervation of tissue-engineered muscle. Tissue Eng Part A. 2012;18:1912. doi: 10.1089/ten.tea.2011.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker B.M., et al. Sacrificial nanofibrous composites provide instruction without impediment and enable functional tissue formation. Proc Natl Acad Sci U S A. 2012;109:14176. doi: 10.1073/pnas.1206962109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coburn J.M., et al. Bioinspired nanofibers support chondrogenesis for articular cartilage repair. Proc Natl Acad Sci U S A. 2012;109:10012. doi: 10.1073/pnas.1121605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCullen S.D., et al. Anisotropic fibrous scaffolds for articular cartilage regeneration. Tissue Eng Part A. 2012;18:2073. doi: 10.1089/ten.tea.2011.0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher M.B., et al. Organized nanofibrous scaffolds that mimic the macroscopic and microscopic architecture of the knee meniscus. Acta Biomater. 2012;9:4496. doi: 10.1016/j.actbio.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sukegawa A., et al. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng Part A. 2012;18:934. doi: 10.1089/ten.TEA.2011.0380. [DOI] [PubMed] [Google Scholar]
- 43.Liu X. Jin X. Ma P.X. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat Mater. 2011;10:398. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller J.S., et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanjaya-Putra D., et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood. 2011;118:804. doi: 10.1182/blood-2010-12-327338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma J., et al. Three-dimensional engineered bone-ligament-bone constructs for anterior cruciate ligament replacement. Tissue Eng Part A. 2012;18:103. doi: 10.1089/ten.tea.2011.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolambkar Y.M., et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32:65. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher M.B., et al. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surg Sports Traumatol Arthrosc. 2012;20:1357. doi: 10.1007/s00167-011-1800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaskelioff M., et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porrello E.R., et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joe A.W., et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smart N., et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee S., et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sindrilaru A., et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown B.N., et al. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rinkevich Y., et al. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476:409. doi: 10.1038/nature10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seifert A.W., et al. Skin shedding and tissue regeneration in African spiny mice (Acomys) Nature. 2012;489:561. doi: 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee C.H., et al. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah R.N., et al. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci U S A. 2010;107:3293. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao X., et al. Active scaffolds for on-demand drug and cell delivery. Proc Natl Acad Sci U S A. 2011;108:67. doi: 10.1073/pnas.1007862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doorn J., et al. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev. 2012;18:101. doi: 10.1089/ten.TEB.2011.0488. [DOI] [PubMed] [Google Scholar]
- 62.Lai R.C., et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Chimenti I., et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loffredo F.S., et al. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang W., et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Visage C., et al. Mesenchymal stem cell delivery into rat infarcted myocardium using a porous polysaccharide-based scaffold: a quantitative comparison with endocardial injection. Tissue Eng Part A. 2012;18:35. doi: 10.1089/ten.TEA.2011.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marklein R.A. Burdick J.A. Controlling stem cell fate with material design. Adv Mater. 2010;22:175. doi: 10.1002/adma.200901055. [DOI] [PubMed] [Google Scholar]
- 68.Kloxin A.M. Benton J.A. Anseth K.S. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials. 2010;31:1. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin Z., et al. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 70.Yuan H., et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci U S A. 2010;107:13614. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonzalez-Cruz R.D. Fonseca V.C. Darling E.M. Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proc Natl Acad Sci U S A. 2012;109:E1523. doi: 10.1073/pnas.1120349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hur S.C., et al. Deformability-based cell classification and enrichment using inertial microfluidics. Lab Chip. 2011;11:912. doi: 10.1039/c0lc00595a. [DOI] [PubMed] [Google Scholar]
- 73.Schriebl K., et al. Selective removal of undifferentiated human embryonic stem cells using magnetic activated cell sorting followed by a cytotoxic antibody. Tissue Eng Part A. 2012;18:899. doi: 10.1089/ten.TEA.2011.0311. [DOI] [PubMed] [Google Scholar]
- 74.Raya-Rivera A., et al. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Makkar R.R., et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bolli R., et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Rama P., et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 78.Peerbooms J.C., et al. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38:255. doi: 10.1177/0363546509355445. [DOI] [PubMed] [Google Scholar]
- 79.Gosens T., et al. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 80.Castricini R., et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39:258. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 81.Rodeo S.A., et al. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40:1234. doi: 10.1177/0363546512442924. [DOI] [PubMed] [Google Scholar]
- 82.de Vos R.J., et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 83.Boilard E., et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marolt D., et al. Engineering bone tissue from human embryonic stem cells. Proc Natl Acad Sci U S A. 2012;109:8705. doi: 10.1073/pnas.1201830109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 86.Yamanaka S. Blau H.M. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stadtfeld M. Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amabile G. Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 89.Sommer C.A. Mostoslavsky G. The evolving field of induced pluripotency: Recent progress and future challenges. J Cell Physiol. 2013;228:267. doi: 10.1002/jcp.24155. [DOI] [PubMed] [Google Scholar]
- 90.Anokye-Danso F., et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Warren L., et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miyoshi N., et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Bhutani N., et al. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qian L., et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song K., et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jayawardena T.M., et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang P., et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 98.Vierbuchen T., et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao T., et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 100.Lister R., et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boulting G.L., et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:279. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diekman B.O., et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:19172. doi: 10.1073/pnas.1210422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gilbert P.M., et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnson K., et al. A stem cell-based approach to cartilage repair. Science. 2012;336:717. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- 105.Shimono K., et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med. 2011;17:454. doi: 10.1038/nm.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fountain H. New York Times; Sep 16, 2012. A first: organs tailor-made with body's own cells. [Google Scholar]