Abstract
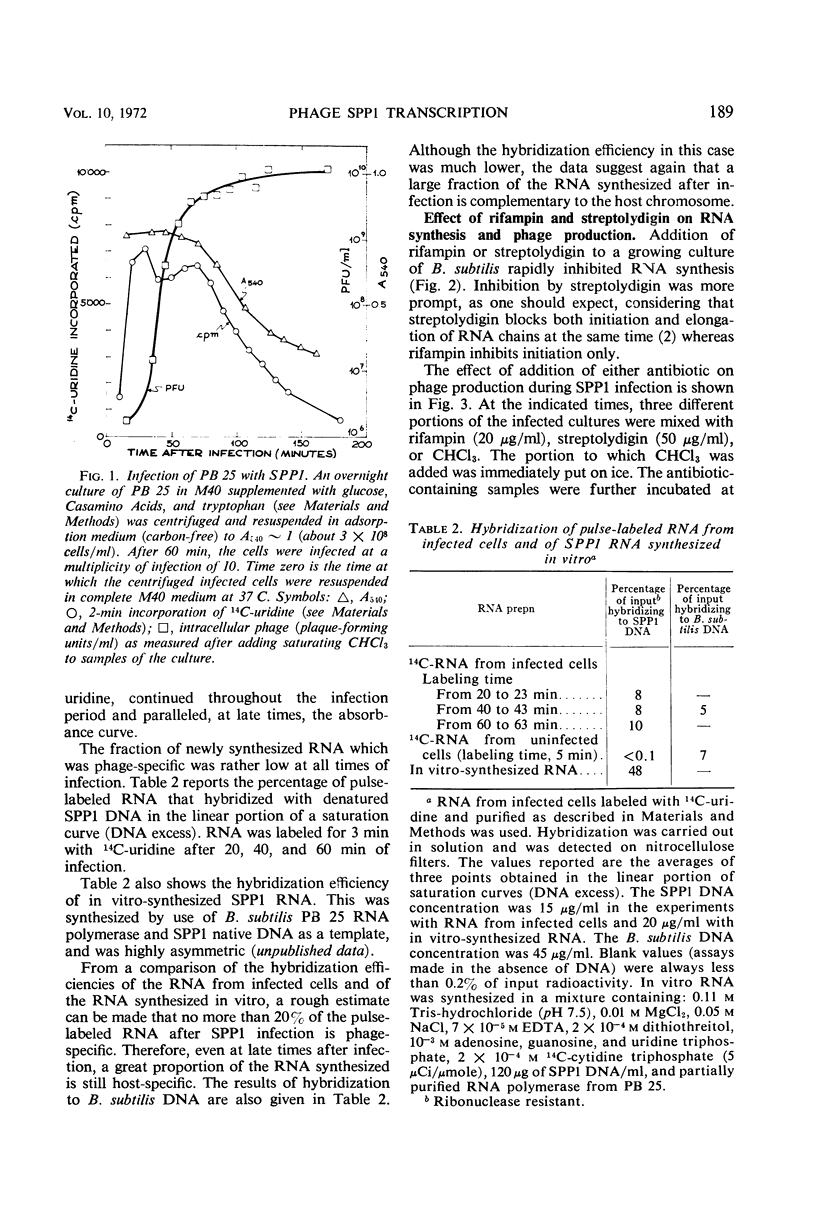
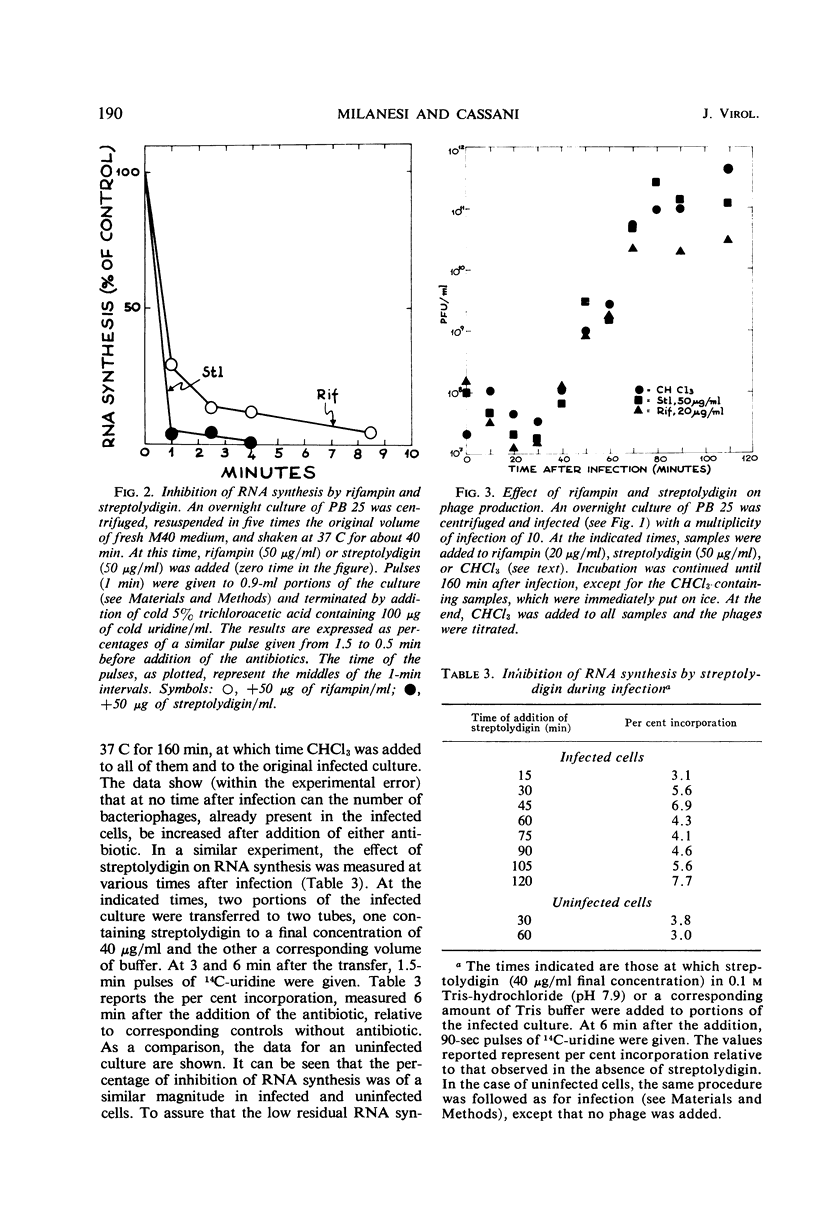
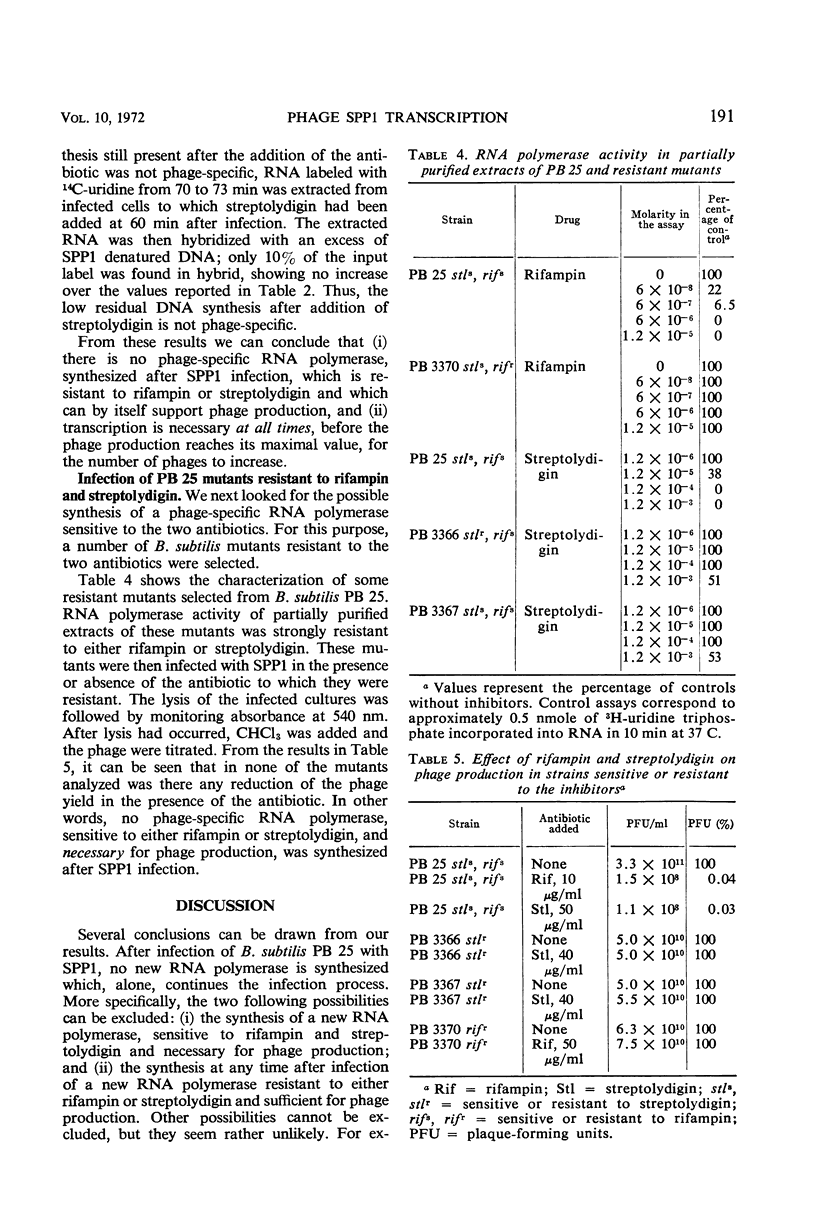
The role of the host polymerase in Bacillus subtilis infected with phage SPP1 was studied in vivo with regard to production of phage-specific and host-specific ribonucleic acid (RNA) and to phage yield. Evidence is presented that the subunit(s) of B. subtilis RNA polymerase which is sensitive to rifampin and streptolydigin is necessary at all times during infection for phage production. The synthesis of phage RNA and the phage yield in strains resistant to either antibiotic were unaffected by the drug. Host RNA synthesis continued throughout infection; phage-specific RNA never accounted for more than 20% of pulse-labeled RNA at any time during infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Cassani G., Burgess R. R., Goodman H. M., Gold L. Inhibition of RNA polymerase by streptolydigin. Nat New Biol. 1971 Apr 14;230(15):197–200. doi: 10.1038/newbio230197a0. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Bautz F. A., Bautz E. K. Different template specificities of phage T3 and T7 RNA polymerases. Nat New Biol. 1971 Mar 17;230(11):94–96. doi: 10.1038/newbio230094a0. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Sklar J. Continual requirement for a host RNA polymerase component in a bacteriophage development. Nature. 1969 Mar 1;221(5183):833–836. doi: 10.1038/221833a0. [DOI] [PubMed] [Google Scholar]
- Klotz G., Spatz H. C. A biological assay for intracellular SPP1 DNA. Mol Gen Genet. 1971;110(4):367–373. doi: 10.1007/BF00438279. [DOI] [PubMed] [Google Scholar]
- Maitra U. Induction of a new RNA polymerase in Escherichia coli infected with bacteriophage T3. Biochem Biophys Res Commun. 1971 Apr 16;43(2):443–450. doi: 10.1016/0006-291x(71)90773-x. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. A method for the detection of RNA-DNA complexes. Biochem Biophys Res Commun. 1963 Jul 18;12:98–104. doi: 10.1016/0006-291x(63)90242-0. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L., ALBERTSSON P. A., FRICK G. The purification and concentration of viruses by aqueous polymerphase systems. Virology. 1960 Jul;11:553–571. doi: 10.1016/0042-6822(60)90100-8. [DOI] [PubMed] [Google Scholar]
- Polsinelli M., Beretta M. Genetic Recombination in Crosses Between Streptomyces aureofaciens and Streptomyces rimosus. J Bacteriol. 1966 Jan;91(1):63–68. doi: 10.1128/jb.91.1.63-68.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsinelli M., Milanesi G., Ganesan A. T. Short fragments from both complementary strands in the newly replicated DNA of bacteriophage SPP-1. Science. 1969 Oct 10;166(3902):243–245. doi: 10.1126/science.166.3902.243. [DOI] [PubMed] [Google Scholar]
- ROMIG W. R. Infection of Bacillus subtilis with phenol-extracted bacteriophages. Virology. 1962 Apr;16:452–459. doi: 10.1016/0042-6822(62)90226-x. [DOI] [PubMed] [Google Scholar]
- Riva S. C. Asymmetric transcription of B. subtilis phage SPP1 DNA in vitro. Biochem Biophys Res Commun. 1969 Mar 31;34(6):824–830. doi: 10.1016/0006-291x(69)90254-x. [DOI] [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- Schachner M., Seifert W., Zillig W. A correlation of changes in host and T 4 bacteriophage specific RNA synthesis with changes of DNA-dependent RNA polymerase in Escherichia coli infected with bacteriophage T 4 . Eur J Biochem. 1971 Oct 26;22(4):520–528. doi: 10.1111/j.1432-1033.1971.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Spatz H. C., Trautner T. A. One way to do experiments on gene conversion? Transfection with heteroduplex SPP1 DNA. Mol Gen Genet. 1970;109(1):84–106. doi: 10.1007/BF00334048. [DOI] [PubMed] [Google Scholar]
- Spatz H. C., Trautner T. A. The role of recombination in transfection of B. subtilis. Mol Gen Genet. 1971;113(2):174–190. doi: 10.1007/BF00333191. [DOI] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Marino P., Colvill A. J. Mutant of E. coli containing an altered DNA-dependent RNA polymerase. Nature. 1968 Oct 19;220(5164):275–276. doi: 10.1038/220275a0. [DOI] [PubMed] [Google Scholar]
- Travers A. Control of transcription in bacteria. Nat New Biol. 1971 Jan 20;229(3):69–74. doi: 10.1038/newbio229069a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



