Abstract
Extensive research in the past decade has revealed cancer to be a multigenic disease caused by perturbation of multiple cell signalling pathways and dysregulation of numerous gene products, all of which have been linked to inflammation. It is also becoming evident that various lifestyle factors, such as tobacco and alcohol use, diet, environmental pollution, radiation and infections, can cause chronic inflammation and lead to tumourigenesis. Chronic diseases caused by ongoing inflammation therefore require chronic, not acute, treatment. Nutraceuticals, compounds derived from fruits, vegetables, spices and cereals, can be used chronically. This study discusses the molecular targets of some nutraceuticals that happen to be markers of chronic inflammation and how they can prevent or treat cancer. These naturally-occurring agents in the diet have great potential as anti-cancer drugs, thus proving Hippocrates, who proclaimed 25 centuries ago, ‘Let food be thy medicine and medicine be thy food’.
Keywords: Dietary agents, inflammation, cancer
Introduction
One of the earliest known examples of cancer is found in bone remains of Egyptian mummies, suggestive of the bone cancer osteosarcoma. The oldest description of cancer is also found in Egypt, in the Edwin Smith Papyrus, an ancient Egyptian textbook on trauma surgery that dates back to 1600 BCE. In that document, although the word ‘cancer’ is not used, a condition is described that is consistent with cancer: eight cases of tumours or ulcers of the breast, with the notation that there is no treatment for this condition and recommending cauterization. The word ‘cancer’ was first introduced by Greek physician Hippocrates (460–370 BCE), who used the term Karkinos (the Greek word for crab), which translates to ‘carcinos’ or ‘carcinoma’ in English, to describe ‘non-ulcer forming’ and ‘ulcer-forming tumours ’. The choice of the word crab stemmed from the similarity between blood vessels surrounding a tumour and the claws of a crab squeezing a malignant mass. Later, the Roman physician Celsus (28 -50 BCE) translated the Greek term into ‘cancer’, the Latin word for crab. Galen (130–200 CE), another Roman physician, used the word oncos (Greek for swelling) to describe tumours.
Today, cancer is considered a cluster of diseases involving alterations in the status and expression of multiple genes that confer a survival advantage and undiminished proliferative potential to somatic or germinal cells [1]. Only a minority of cancers are caused by germline mutations, whereas the rest (∼ 90%) are linked to somatic mutations and environmental factors [2]. There is increasing evidence to suggest that cancer is also driven by ‘epigenetic changes’, such as DNA methylation and altered patterns of histone modifications, which can lead to alterations in chromatin condensation status, thereby regulating the expression of a certain set of specific genes [3,4]. The mutations and epigenetic changes found in the cancer cell genome accumulate over the lifetime of cancer patients. The rate of mutation increases in the presence of exogenous mutagenic exposures, such as lifestyle factors, tobacco smoke, naturally occurring toxic chemicals or various forms of radiation, including ultraviolet light. Substantial exposure to these environmental and lifestyle factors is associated with increased rates of lung, liver and skin cancer [5]. Similar factors have been linked to inflammatory responses. This suggests a strong relationship between inflammation and cancer [5–7].
How lifestyle and environmental factors lead to tumourigenesis is becoming ever more clear. However, one of the major contributors to tumourigenesis caused by these factors is believed to be chronic inflammation [8]. Although acute inflammation exerts therapeutic potential, chronic inflammation is a low-level inflammation that can persist for long periods, eventually causing chronic diseases, including cancer. It is estimated that ∼ 15% of human cancers are associated with infections and inflammation [9]. The markers of chronic inflammation include pro-inflammatory cytokines (i.e. tumour necrosis factor [TNF] and interleukins [ILs]−1, −6 and −8) and chemokines, pro-inflammatory enzymes (cyclooxygenase-2 [COX-2], 5-lipoxygenase, matrix metalloproteinase [MMP] and urokinase plasminogen activator [uPA]), adhesion molecules (intercellular adhesion molecule-1 [ICAM-1], vascular cell adhesion molecule-1 and endothelial leukocyte adhesion molecule-1) and certain growth factors (such as epidermal growth factor [EGF] and platelet-derived growth factor [PDGF]) [8].
Although it is not yet possible to provide quantitative estimates of the overall risks, it has been estimated that 35% of cancer deaths may be related to dietary factors [10]. Diets that include fruits, vegetables and spices may provide substantial health benefits in terms of cancer prevention and treatment by suppressing the inflammatory processes that lead to transformation, hyperproliferation and the initiation of carcinogenesis. However, which component of these dietary agents is responsible for their anti-cancer effects and the mechanism by which they suppress cancer remain unknown. Dietary agents contain a variety of biologically active phytochemicals, many of which have been used in traditional medicine for thousands of years. Some phytochemicals are known to influence the transcription factors involved in carcinogenesis.
Transcription factors as a link between diet and cancer
Most oncogenes and tumour suppressor genes are transcription factors, which control gene expression and regulate numerous signalling pathways in cancer. Dysregulation of transcription factor activity is the result of numerous mechanisms, such as changes in gene expression, protein–protein interactions and post-translational modifications, leading to deregulation of gene products that are involved in both inflammation and carcinogenesis [11]. In this review, we will focus on selected transcription factors (e.g. nuclear factor –κB [NF-κB ], activator protein-1 [AP-1], signal transducer and activator of transcription-3 [STAT-3], nuclear factor erythroid 2-related factor [NRF2], peroxisome proliferator-activated receptor-γ [PPARγ], WNT/β-catenin, hypoxia inducible factor-1 [HIF-1] and hedgehog [Hh]) that are involved in tumour progression (Figure 1). In addition, we will review dietary nutraceuticals, including two powerful compounds, resveratrol and curcumin, that can modulate the activity of these transcription factors, playing a positive role not only in the prevention of various chronic diseases, including cancer, but also their treatment.
Figure 1.
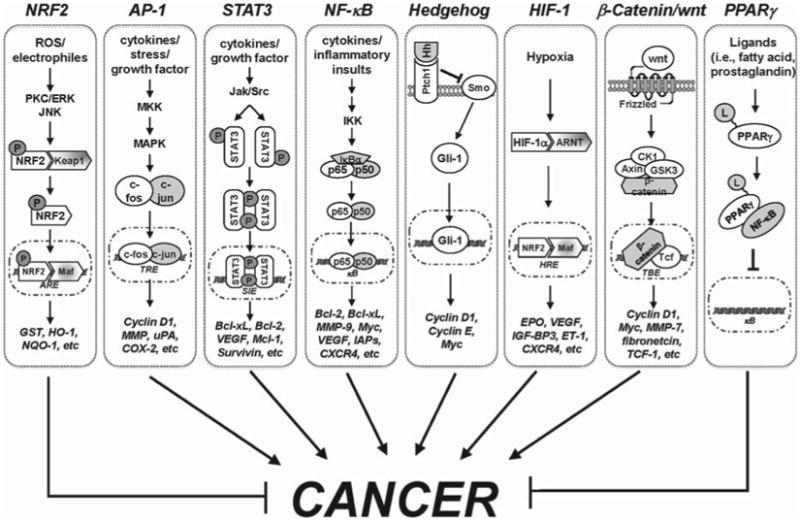
Molecular mechanism of selected transcription factors in cancer. AP-1, activator protein-1; ARE, antioxidant response element; ARNT, aryl hydrocarbon receptor nuclear translocator; CK1, casein kinase 1; COX-2, cyclooxygenase-2; CXCR4, C-X-C chemokine receptor type 4; EPO, erythropoietin; ERK, extracellular signal-regulated kinases; ET-1, endothelin-1; Gli-1 glioma-associated oncogene homologue 1; GSK3, glycogen synthase kinase 3; GST, glutathione-S-transferase; Hh, hedgehog; HIF-1, hypoxia inducible factor-1; HO-1, heme oxygenase-1; HRE, hypoxia responsive element; IAPs, inhibitor of apoptosis proteins; IGF-BP3, insulin-like growth factor-binding protein 3; IKK, inhibitor of κB (IκB) kinase; Jak, Janus kinase; JNK, c-Jun N-terminal kinases; L, ligands; MAPK, mitogen-activated protein kinase; MKK, MAPK kinases; MMP, matrix metallopeptidase; NF-κB, nuclear factor-κB; NQO-1, NAD(P)H:quinone oxidoreductase; NRF2, NF-E2-related factor-2; P, phosphorylation; PKC, protein kinase C; PPARγ, peroxisome proliferator-activated receptor; Ptch1, protein patched homologue 1; ROS, reactive oxygen species; Smo, smoothened; STAT3, signal transducer and activator of transcription 3; TBE, Tcf/β-catenin binding element; Tcf, T-cell factor; TRE, 12-O-tetradecanoylphorbol-13-acetate (TPA)-responsive element; uPA, urinary plasminogen activator; VEGF, vascular endothelial growth factor.
Nuclear factor-kappa B (NF-κB)
NF-κB was first discovered in 1986 in the nucleus of the B-cell as an enhancer of the κ immunoglobulin chain [12]. NF-κB consists of homodimers or heterodimers of its five family members and is activated in response to several stimuli, including pathogen-associated molecular patterns, TNF-α, IL-1 and others stimuli related to environmental and lifestyle factors [13]. Once these ligands are engaged with their respected receptors, signalling impinges on a common molecular target, the inhibitor of κB (IκB) kinase (IKK) complex, leading to phosphorylation, polyubiquitination and subsequent degradation of IκBα, thereby allowing NF-κB to translocate to the nucleus and to exert its functions as a transcriptional regulator. NF-κB has been implicated in inflammation, cell survival, proliferation, invasion and angiogenesis. During the past decade, the crucial role of the NF-κB signalling in connecting inflammation to cancer has been subjected to intense investigation by a number of laboratories in a variety of experimental systems and this connection has been well established. The intimate relationship between NF-κB and cancer has already been extensively reviewed [8,14–16].
Constitutively activated NF-κB is found in several human cancer cell lines, including lymphomas and carcinomas of the breast, prostate, lung, colon, pancreas, head and neck and oesophagus. In addition to cell lines, activated NF-κB has been noted in tissue samples from cancer patients [17]. Studies of cancer-associated mutations have also reported that mutations in the upstream signal component of NF-κB that could direct to constitutive NF-κB activation in multiple myeloma (MM) cell lines and patient samples [18,19]. Because of the role of NF-κB in human diseases, including cancer, this transcription factor is an ideal target for anti-cancer drug development.
Our research group and others have described more than 700 inhibitors of the NF-κB pathway, including nutraceuticals derived from dietary agents [13,20,21]. Active components of spices, such as curcumin [22], diosgenin [23], gambogic acid [24], capsaicin [25], ursolic acid [26], noscapine [27], sesamin [28], anethole [29] and eugenol [29] can inhibit IKK and p65 phosphorylation in vitro, leading to the suppression of NF-κB that has been activated by various carcinogens. This NF-κB suppression leads to the down-regulation of its regulated gene products, including COX-2, MMP-9 and cyclin D1. Nutraceuticals derived from various kinds of ginger, such as crotepoxide [30], zerumbone [31], 1′-acetoxychavicol acetate [32] and [6]-gingerol [33], have been reported to suppress the NF-κB signalling pathway and induce apoptosis in a variety of human cancer cell lines. Recently, zerumbone has been shown to suppress the receptor activator of NF-κB ligand (RANKL)-induced NF-κB activation and osteoclastogenesis induced by RANKL and tumour cells, thus decreasing osteolysis in MDA-MB-231 breast cancer tumour-bearing athymic nude mice [34]. Nutraceuticals such as plumbagin [35], caffeic acid phenethyl ester (CAPE) [36], picroliv [37] and xanthohumol [38] have been shown to suppress NF-κB activation through direct inhibition of p65 binding to DNA. Butein [39], nimbolide [40], berberine [41] and xanthohumol [38] have been reported to sensitize tumour cells to chemotherapeutic agents through direct interaction with IKK. A novel form of vitamin E, γ-tocotrienol (γ-T3), has been shown to suppress the activation of NF-κB induced by inflammatory and carcinogenic agents [42]. γ-T3 has been shown to inhibit the growth of human pancreatic tumours and sensitize them to gemcitabine by suppressing NF-κB-mediated inflammatory pathways that have been linked to tumourigenesis [43].
Other dietary agents, including escin [44], pinitol [45], anacardic acid [46], 3,4-dihydroxybenzalacetone [47], morin [48], fisetin [49], simvastatin [50], resveratrol [51], indole-3-carbinol (I3C) [52], evodiamine [53], betulinic acid [54], silymarin [55] and emodin [56], have been found to inhibit both inducible and constitutive NF-κB activation; suppress the activation of IKK that leads to abrogation of phosphorylation and degradation of IκBα and nuclear translocation of p65; and suppress NF-κB-dependent reporter gene expression. Curcumin and resveratrol in particular have been extensively studied in both pre-clinical and clinical settings for their anti-inflammatory and anti-cancer properties, which are mediated through a down-modulation of NF-κB activation [51,57,58]. Because it is one of the most thoroughly studied transcription factors, compounds that inhibit NF-κB, especially those derived from dietary agents, hold great promise in the treatment and prevention of cancer.
Ap-1
AP-1 was first identified as a transcriptional factor that binds to an essential cis-element of the human metallothionein lla promoter [59]. The activation of AP-1 is stimulated by a plethora of physiological stimuli and environmental factors, including redox status. AP-1 is considered to be one of the key players in tumourigenesis given its role in a wide range of cellular processes, including inflammation, cell proliferation, death, survival and differentiation. AP-1 exists as dimeric combinations of basic leucine zipper proteins from the Jun, Fos, Maf and ATF sub-families, which recognize either 12-O-tetradecanoylphorbol-13-acetate (TPA, also called PMA) response elements (TREs) or cAMP response elements [60].
AP-1 is also involved in inflammatory processes by regulating cytokine production, thus participating in the development of chronic inflammatory diseases such as inflammatory bowel disease, chronic obstructive pulmonary disease, rheumatoid arthritis, psoriasis and cancer. Indeed, this transcription factor has a critical role in the osteoclastogenesis activated by RANKL, thereby promoting bone resorption [61,62]. The role of AP-1 in tumour promotion was first discovered by Bernstein and Colburn [63]. They reported that transformation-resistant JB6 cells failed to activate AP-1 in response to tumour promoters such as TPA and EGF, whereas the AP-1 response was intact in transformation-sensitive JB6 cells. The requirement for AP-1 activation during tumour promotion in JB6 cells was later demonstrated by Brown et al. [64] by using a transactivation minus mutant of c-Jun (TAM67). Elevated AP-1 activity has been detected in a variety of cancers and tumour cell lines, suggesting a role for AP-1 in tumour progression [65]. Thus, AP-1 is a good target for both prevention and treatment for cancer.
Naturally occurring compounds such as curcumin, resveratrol, genistein, [6]-gingerol, capsaicin, epigal-locatechin gallate (EGCG), cyanidin-3-glucoside, nobiletin, 7,8-dihydroxyflavanone, theaflavin-3,3′-digallate, carnosol, silibinin, sulphoraphane, maslinic acid, ganoderic acid, lycopene, bavachin, kaempferol, berberine, momordin and caffeic acid phenethyl ester have been identified as inhibiting the activation of AP-1 [66–81].
Kundu et al. [82] demonstrated suppression by resveratrol of the TPA-stimulated activation of AP-1 in mouse skin in vivo. This stillbene, a component of grape and Japanese Knotweed, has also been shown to down-regulate PMA-induced IL-8 production and mRNA accumulation in U937 cells [83]. However, although resveratrol inhibited the PMA-stimulated DNA-binding activity of AP-1, it had little effect on PMA-induced NF-κB activation. These results suggest that suppression of IL-8 by resveratrol was, at least in part, due to the inhibition of AP-1 activation. Pterostilbene, a natural dimethylated analogue of resveratrol, is also known to suppress TPA-induced AP-1 activation, thereby inhibiting tumour invasion in HepG2 cells [84]. Takada et al. [85] found that a semi-synthetic flavone, flavopiridol, which was initially thought to be a specific inhibitor of cyclin-dependent kinases, abrogated the activation of AP-1 stimulated by TNF or various carcinogens and inflammatory stimuli. As a result, flavopiridol suppressed the expression of various anti-apoptotic proteins such as inhibitor of apoptosis (IAP)-1, IAP-2, X-linked IAP, Bcl-2 and Bcl-xL and tumourigenesis-mediated proteins such as ICAM-1, c-myc and c-Fos.
Curcumin, a polyphenol derived from turmeric, has been shown to inhibit TPA-induced AP-1 activation in HL-60 cells and Raji cells [86,87]. Curcumin treatment has also been shown to abrogate constitutive AP-1 activity in prostate cancer cell lines [88,89]. Inhibition of AP-1 activity by curcumin also correlated with inhibition of Lewis lung carcinoma invasion in an orthotopic mice model [90]. Curcumin has been reported to suppress lipopolysaccharide-induced COX-2 gene expression by inhibiting AP-1 DNA binding in BV2 microglial cells [91]. More recently, Prakobwong et al. [92] showed that suppression of AP-1 activation by curcumin led to the down-modulation of cell proliferative gene products such as cyclin D1 and c-myc in a cholangiocarcinoma hamster model. These results suggest that chemopreventive agents specifically targeting AP-1 or its activating kinases could be promising agents for the treatment of several cancers.
Signal transducer and activator of transcription (STAT)–3
STAT3 is one of the members of a family of STAT transcription factors. It was first identified as a DNA-binding factor that selectively binds to the IL-6-responsive element in the promoter of acute-phase genes from IL-6-stimulated hepatocytes [93]. The binding of growth factors (e.g. EGF and PDGF) or cytokines (e.g. IL-6) to their receptors results in the activation of their receptor tyrosine kinase or of receptor-associated tyrosine kinases that subsequently phosphorylate the cytoplasmic part of the receptor and provide docking sites for monomeric STAT3. Once recruited, STAT3 is phosphorylated on a specific tyrosine 705 residue, allowing its dimerization and translocation to the nucleus [94,95]. Emerging evidence suggests that STAT3 plays a crucial role in the inflammatory microenvironment, both at the initiation of tumourigenesis and during cancer progression [6,96–99]. Rebouissou et al. [100] showed that STAT3 is linked to inflammation-associated tumourigenesis, which is initiated by genetic alterations in tumourous hepatocytes. In addition, elevated STAT3 activity has been detected in a wide variety of cancers, including head and neck, breast, colorectal, ovarian, pancreatic, renal and prostate cell carcinoma; leukaemia; lymphoma; and MM [94].
STAT3 has been reported to interact with NF-κB at several levels. For instance, inflammatory factors regulated by NF-κ B, most notably IL-6, are important STAT3 activators [93,96]. Lee et al. [101] showed that STAT3 directly interacts with NF-κB Rel A and prolongs NF-κB nuclear retention through acetyl-transferase p300-mediated RelA acetylation, interfering with NF-κB nuclear export and thereby contributing to constitutive NF-κB activation in cancer. These two transcription factors, STAT3 and NF-κ B, co-regulate numerous oncogenic and inflammatory genes [102–104], so agents that can sever this alliance are of particular interest for the prevention and treatment of cancer.
Several nutraceuticals have been identified that can suppress STAT3 activation [94,95]. These include curcumin, resveratrol, caffeic acid, capsaicin, curcurbitacin, indirubin, piceatannol, parthenolide, flavopiridol, magnolol, guggulsterone, silibinin, ursolic acid, plumbagin, betulinic acid, γ-T3, butein and EGCG [94,105–108]. The mechanism of suppression of STAT3 varies depending on the compound. For example, guggulsterone, ursolic acid, betulinic acid, γ-T3 and butein transcriptionally up-regulate the expression of protein tyrosine phosphatase, which leads to inactivation of STAT3 [105–108]. By contrast, emodin suppresses IL-6-stimulated activation of Janus-activated kinase 2 and STAT-3; it also triggers the activation of caspases and down-regulation of Mcl-1. Ectopic expression of Mcl-1 has been shown to reverse emodin-induced apoptosis in human MM cells. This observation indicates that emodin could induce apoptosis in myeloid cells via down-regulation of Mcl-1 [109]. Capsaicin has been reported to induce apoptosis in MM cells by inactivating STAT3 and down-regulating STAT3-regulated gene expression of Bcl-2, Bcl-xL and survivin. In a xenotransplant murine model of human MM, capsaicin has been shown to inhibit the tumour growth correlated with the inhibition of STAT3 [110].
Adult T-cell leukaemia is an aggressive malignancy of peripheral T-cells infected with human T-cell leukaemia virus type 1 (HTLV-1). Deguelin has been shown to induce apoptosis in HTLV-1-transformed T-cells via inhibition of survivin expression and dephosphorylation of STAT3 through the ubiquitin/proteasome pathway [111].
Curcumin has been known to suppress STAT3 activation and induce apoptosis in a wide range of human cancer cell lines, including ovarian [112], pancreatic [113], head and neck [114] and endometrial cancer [112]; melanoma [115]; Hodgkin lymphoma [116]; primary effusion lymphoma [117]; T-cell leukaemia [118]; and MM cells [119]. However, the inhibitory mechanisms of curcumin on STAT3 vary— for example (i) activating Src homology 2 domain-containing protein tyrosine phosphatises-2 [120]; (ii) up-regulating protein inhibitors of activated STAT, which are associated with inhibition of the JAK/STAT pathway; and (iii) decreasing nuclear STAT3 without affecting its phosphorylation [121]. Curcumin has also been tested in patients and was shown to down-regulate STAT3 pathways [119,122,123].
Numerous evidences indicate that resveratrol inhibits STAT3 activation and induces apoptosis in various cancer cell lines [51,124–126]. A report from our group showed that resveratrol was able to inhibit nuclear translocation of STAT3 in CD138+ cells from patients with MM. Recently, Youn et al. [127] reported that oral administration of resveratrol (10 mg/kg body weight) for 7 constitutive days attenuated dextran sulphate sodium-induced inflammatory injury by suppressing inducible nitric oxide synthase expression and inhibiting NF-κB and STAT3 in a colitis mouse model. These results provide a rationale for testing resveratrol in patients with chronic inflammatory diseases, including cancer.
Nuclear factor erythroid 2–related factor (NRF2)
NRF2 is a transcription factor that regulates the expression of ∼ 100 cytoprotective genes, including glutathione S-transferases (GSTs), heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase 1 (NQO1), glutamate-cysteine ligase catalytic and modifier sub-units and peroxiredoxin 1, by binding to the DNA regulatory element, antioxidant response element (ARE). A negative regulator of NRF2, Keap1 (a 69-kDa protein), is located in the cytoplasm and is anchored to actin [128]. A site-directed mutagenesis study of Keap1 demonstrated that NRF2 is normally located in the cytoplasm by Keap1. Once NRF2 dissociates from Keap1, it translocates to the nucleus, heterodimerizes with small Maf and binds to ARE, resulting in responsive gene expression [129]. NRF2 also plays a major role as a central regulator of the adaptive response to oxidative stress, given that nrf2−/− mice have been shown to be sensitive to diverse oxidative stress conditions. When exposed to xenobiotics or chemicals that generate intracellular oxidative stress, nrf2−/− mice display increased tissue damage and prolonged inflammation; high amounts of DNA, lipid and protein oxidation; and increased incidence of cancer [130–132]. Numerous compounds that activate NRF2 are considered to be promising chemopreventive agents and some of these are now undergoing human trials of cancer chemoprevention. NRF2-activating substances include synthetic compounds (e.g. oltipraz), related dithiolthiones and novel triterpenoids, as well as natural products such as curcumin and sulphoraphane. The remarkable chemopreventive effects of these compounds are being tested in nrf2-knockout mouse models and gene deletion of NRF2 reversed the chemopreventive effects of these agents, indicating that their anti-cancer activities are mediated by the induction of NRF2 [133–136].
Among the natural compounds that exhibit chemopreventive activity through activation of NRF2, sulphur-containing and phenolic dietary phytochemicals have attracted a lot of attention. Sulphur-containing compounds, such as isothiocyanates (i.e. sulphoraphane, phenethyl isothiocyanate and allylisothiocyanate) and dithiolethiones, which are derived from cruciferous vegetables—including broccoli, watercress, Brussels sprouts, cabbage and cauliflower—have been extensively studied for their chemopreventive properties via evoking NRF2 [137]. The diallyl sulphides (i.e. diallyl sulphide, diallyl disulphide and diallyl trisulphide) are another class of potential chemopreventive agents that are found in the Allium family, including garlic, onion and chive. A study by Chen et al. [138] indicated the positive action of these diallyl sulphides on ARE-mediated gene expression and expression of NRF2, NQO1 and HO-1 proteins.
Phenolic compounds include various flavonoids, such as quercetin, rutin, genistein, tea polyphenols such as (–)-epigallocatechin and EGCG, black tea polyphenols and curcumin that can modulate the NRF2 signalling pathway [139]. Curcumin and CAPE disrupt the NRF2-Keap1 complex and lead to NRF2 binding to ARE [140,141]. Balogun et al. [140] have shown that both compounds stimulate the expression of NRF2, leading to a significant increase in the activity and expression of HO-1. Their findings indicate that p38 mitogen-activated protein kinase, which is upstream of NRF2, is involved in curcumin-induced HO-1 gene induction. In another study, curcumin increased the nuclear translocation of NRF2, ARE-DNA binding activity and glutamate-cysteine ligase expression [142].
Other natural chemopreventive agents that induce the NRF2/ARE pathway include indoles such as I3C and terpenoids. In HepG2 cells, I3C showed a weak induction of NRF2-reporter gene activity and NRF2 protein expression but had no effect on HO-1 protein expression [143]. Krajka-Kuźniak et al. [144] recently found that I3C significantly increased the activity of glutathione S-transferase (GST) and NQO1 in rat liver. This increase was correlated with nuclear translocation of NRF2. Treatment with I3C also significantly increased the activity of NQO1 in rat kidney. Therefore, the chemopreventive activity of I3C may occur through induction of the key detoxifying enzymes.
Among other examples, a mixture of coffee diter-penes, cafestol and kahweol palmitate has been shown to increase the enzymatic activities of NQO and GST in the small intestine of mice by 2-fold [145]. A sesquiterpene parthenolide derived from feverfew has been shown to stimulate ARE reporter-gene activity and to potently induce the expression of NRF2 and HO-1 proteins in HepG2 cells [143]. In addition, zerumbone was found to induce phase II enzymes, including GST-P1, γ-glutamyl cysteine synthetase, glutathione peroxidase and HO-1, through the NRF2 pathway and to reduce lipid peroxidation in hepatocytes [146]. These results suggest that nutraceuticals activating NRF2 may hold promise as a chemotherapeutic drug in the treatment of cancer and other diseases
Peroxisome proliferator-activated receptor (PPAR)–γ
PPARs, members of the nuclear receptor superfamily, are best understood as regulators of the lipid metabolism. There are three forms of PPAR: α, β/δ and γ [147]. These receptors also play an important role in modulation of the immune system through their ability to inhibit the expression of inflammatory cytokines by direct differentiation of immune cells toward anti-inflammatory sites. PPARs are involved in the regulation of cellular differentiation, development, metabolism and tumourigenesis [148]. In addition, they are known to directly down-regulate the expression of pro-inflammatory gene products in a ligand-dependent manner by antagonizing the activity of pro-inflammatory transcription factors such as NF-κB and AP-1 [149–151]. PPARγ (also known as the glitazone receptor) is one of the nuclear receptor proteins that act as transcription factors and control the expression of different genes. PPARγ is normally present in such diverse systems as adipocytes, skeletal muscle cells, osteoclasts, osteoblasts and several immune-type cells. Interestingly, somaticmutations in PPARγ have been found in sporadic colorectal carcinomas [152]. These findings suggest an important role for PPARγ as a tumour suppressor. However, several murine models have suggested that, under certain circumstances, PPARγ ligands may stimulate cancer formation [153].
The ligands of PPARγ have been shown to exert profound inhibitory effects on the growth and differentiation of human liposarcoma [154]. Indeed, accumulated evidence has indicated an anti-growth and/or pro-differentiation response of PPARγ ligands in a variety of cancer cells, including colon, lung, ovary, breast, thyroid and prostate cells [155]. Targeting of PPARγ is of great potential interest to the food industry, given that several natural dietary constituents act as PPARγ ligands and can activate it, including curcumin, resveratrol [156], 7-chloroarctinone-b [157], deoxyelephantopin [158], macelignan [159] and honokiol [160].
Liang et al. [161] have demonstrated the anti-inflammatory effects of apigenin, chrysin and kaempferol in murine macrophages. These flavonoids transactivate PPARγ as allosteric effectors rather than pure agonists. The flavonoids baicalin [162] and cyanidin [163] are also known to act as antagonists of PPARγ. In addition, capsaicin from hot peppers has been found to induce apoptosis of melanoma as well as colon and prostate cancer cells and, at least in the case of colon cancer, this occurred through the activation of PPARγ [164–166]. However, although capsaicin acts as a PPARγ ligand, its anti-cancer effect is controversial [167,168]. Terpenoids, including glycyrrhetinic acid [169], betulinic acid [170], farnesol, geranylgeraniol [171], auraptene, bixin, norbixin, geraniol and phytol (see [172]), have also been reported to activate PPARγ very efficiently.
Curcumin has been reported to inhibit the proliferation of non-adipocytes through the activation of PPARγ. The level of PPARγ is dramatically decreased along with activation of hepatic stellate cells (HSC). However, curcumin was able to induce the expression of PPARγ and thus inhibit cell proliferation in activated HSCs. In contrast, blocking the trans-activity of PPARγ by its antagonist markedly decreased the effects of curcumin on the inhibition of cell proliferation [173]. Chen and Xu [174] also reported that activation of PPARγ by curcumin in Moser cells inhibited growth and suppressed the gene expression of cyclin D1 and epidermal growth factor receptor (EGFR). Overall, the activation of PPARγ by dietary agents could be one of the mechanisms responsible for their cancer preventive and chemotherapeutic effects.
WNT/β-catenin
The mouse wnt1 gene (originally called ‘Int-1’) was first identified in 1982 by Nusse and Varmus [175] as a preferential integration site for the mouse mammary tumour virus in breast tumours. To date, 19 Wnt ligands have been identified in mammals that are known to activate β-catenin-dependent (canonical) and β-catenin-independent (non-canonical) signalling pathways on binding to the Frizzled-LRP co-receptor complex. Briefly, Wnt ligands bind to their receptors, proteins of the Frizzled family. These bindings result in the inactivation of a complex of cytoplasmic proteins (including adenomatous polyposis coli [APC] and axin) that promote the degradation of β-catenin, leading to cytoplasmic accumulation and nuclear localization. In the nucleus, β-catenin interacts with T-cell factor/lymphoid enhancer factors (TCF/LEF) to regulate the expression of target gene products. Wnt signalling is considered to be one of the fundamental mechanisms directing cell proliferation, cell polarity and cell fate determination during embryonic development and tissue homeostasis [176]. Thus, given the critical and pleiotropic roles of Wnt signalling, it is not surprising that perturbations/mutations in Wnt signalling have been implicated in a variety of human diseases, including cancer [177].
The nuclear accumulation of β-catenin, a hallmark of activated Wnt signalling, has been clearly observed in cancer cells [178]. Hyperactive β-catenin turns on a genetic programme sufficient to initiate the development of a multitude of different tumour types, primarily those of gastrointestinal origin. Loss-of-function mutations in components of the β-catenin degradation complex have been observed in many human cancers. For instance, >70% of colorectal cancers have been shown to bear mutations in APC, such that it fails to degrade β-catenin. Similarly, inactivating mutations in the Axin scaffold protein have been implicated in hepatocellular carcinomas [179].
Several compounds have been reported to regulate the β-catenin/Wnt signalling pathway. Silibinin from milk thistle suppresses the intestinal carcinogenesis in APCmin/+ mice as well as 1,2-dimethylhydrazine-induced colon cancer in male Wistar rats through inhibition of the β-catenin/wnt signalling pathway [180,181]. Pterostilbene, a natural dimethylated analogue of resveratrol found in blueberry and grapes, also inhibits colorectal aberrant crypt foci and colon carcinogenesis via suppression of multiple pathways, including WNT/β-catenin signalling, in azoxymethane-treated mice [182]. Several flavonoids—such as genistein, kaempferol, isorhamnetin and baicalein—have been shown to inhibit the transcriptional activity of β-catenin in HEK293 cells transiently transfected with a constitutively active mutant β-catenin gene [183]. In addition, a plant flavonoid, fisetin, has been shown to induce apoptosis and suppress the growth of colon cancer cells by inhibiting the COX-2 and Wnt/EGFR/NF-κB signalling pathways [184]. In a study from Tarapore et al. [185], the dietary triterpene lupeol was reported to decrease the transcriptional activity of β-catenin and the expression of Wnt target genes. Interestingly, lupeol inhibited the translocation of β-catenin from the cytoplasm to the nucleus in melanoma cells. Tea has also been reported to modulate β-catenin and its regulated proteins, cyclin D and c-Jun, in polyps in the APCmin mice model [186]. Tea polyphenols, including polymeric black tea polyphenol and EGCG, have been shown to be potent inhibitors of the WNT/β-catenin signalling pathway [187,188]. Moreover, EGCG suppresses Wnt signalling by inducing the high mobility group-box transcription factor-1 transcriptional repressor through an increase in HBP1 mRNA stability in invasive breast cancer cells [189]. In addition to these examples, the hydroxylated polymethoxy-flavones shikonin and decursin are also known to suppress β-catenin signalling [190–193].
Curcumin also appears to be an effective inhibitor of the β-catenin/Wnt pathway. A study by Jaiswal et al. [194] demonstrated that curcumin induces apoptosis in colorectal cancer cells. Curcumin treatment was found to induce caspase-3-mediated degradation of cell–cell adhesion proteins, including β-catenin, which was linked with apoptosis, and caspase-3 inhibitor prevented this degradation. Choi et al. [195] reported that curcumin suppressed the level of β-catenin, leading to down-regulation of its regulated gene products, such as cyclin D1 and c-myc, by inhibition of Akt/glycogen synthase kinase-3 β in human prostate cancer cells. Interestingly, curcumin was able to modulate the self-renewal of both normal and malignant breast stem cells [196]. Curcumin inhibited the formation of mammospheres and decreased the population of aldehyde dehydrogenase-positive cells in normal and malignant breast cells by inhibiting Wnt signalling, which further suggests the potential for curcumin as a cancer preventive agent.
Hypoxia inducible factor (HIF)–1
The microenvironment of solid tumours differs from that of normal tissues and is characterized by low levels of pO2 and low pH that are well below normal [197]. Hypoxia is a common feature of many cancers and it essentially occurs when the growth of the tumour outstrips the accompanying angiogenesis [198]. A major factor in the hypoxic response is HIF-1 [199], which mediates the adaptive responses to hypoxia by affecting the transcription of numerous hypoxia-inducible genes.
A growing body of evidence suggests that the up-regulation of HIF has a crucial role in the progression of a broad range of human malignancies [200–202]. Accumulation of HIF-1α has been associated with poor survival of patients with a variety of cancers, including cervical cancer, breast cancer, ovarian cancer, endometrial cancer and oropharyngeal squamous cell carcinoma [203]. HIF can directly up-regulate a number of angiogenic factors, including vascular endothelial growth factor (VEGF), VEGF receptors, plasminogen activator inhibitor-1, angiopoietins (ANG-1 and -2), platelet-derived growth factor B, the TIE-2 receptor and MMPs [204]. As a transcription factor, HIF-1 can also induce the expression of gene products encoding the glycolytic enzymes phosphoglycerate kinase-1 and lactate dehydrogenase A, which provide energy for cancer cells under hypoxic conditions [205]. Indeed, HIF-1 has been shown to be a potent inducer of metastatic genes including chemokine receptor 4, its ligands (stromal cell-derived fator-1; SDF-1) and lysyl oxidase in a broad range of tumour cells [206], as well as E-cadherin, a key factor governing metastatic probability in the majority of epithelial cancers [1].
Flavonoids (e.g. quercetin, baicalein, luteolin, fisetin, galangin, (–)-epicatechin-3-gallate, apigenin, vitexin and chrysin), which are derived from various fruits and vegetables, have been reported to inhibit HIF-1α [207]. In addition, some flavonoids (e.g. quercetin, luteolin and kaempferol) have been reported to down-regulate HIF-1α by suppressing nuclear accumulation and thus altering HIF-1 transcriptional activity [208]. Deguelin has been shown to sensitize lung cancer cell lines that are resistant to radiation, rendering them more susceptible to anticancer therapy [209]. This effect is mediated through the inhibition of Hsp90 function and subsequent suppression of the interaction between HIF-1α and Hsp90 and reduction in HIF-1α expression by deguelin in radioresistant cells. Deguelin caused profound inhibition of tumour growth and angiogenesis when combined with radiation in vivo. This effect of deguelin on HIF-1α expression is supported by results from another study in which the compound inhibited retinal vascularization [210]. These results indicate that deguelin can be used in combination with other anti-cancer therapy to inhibit neovascularization and enhance the overall effectiveness of therapy.
Resveratrol has been shown to significantly inhibit both basal levels and hypoxia-induced HIF-1α protein accumulation in cancer cells but to not affect HIF-1α mRNA levels. Resveratrol shortened the half-life of HIF-1α protein by enhancing the protein degradation induced by 26S proteasome [211]. Cao et al. [212] also demonstrated the inhibitory effects of resveratrol on the HIF pathway through several mechanisms: (i) inhibition of AKT and mitogen-activated protein kinase activation, which play a partial role in the down-regulation of HIF-1α expression; (ii) inhibition of insulin-like growth factor 1-induced HIF-1α expression by inhibiting protein translational regulators, including M(r) 70 000 ribosomal protein S6 kinase 1, S6 ribosomal protein, eukaryotic initiation factor 4E-binding protein 1 and eukaryotic initiation factor 4E; and (iii) induction of HIF-1α degradation through the proteasome pathway in human ovarian cancer cells.
Similarly, curcumin also exerts an effect on the HIF-1 pathway. This polyphenol has been shown to decrease hypoxia-induced HIF-17alpha; protein levels in HepG2 hepatocellular carcinoma cells. Moreover, curcumin suppressed the transcriptional activity of HIF-1 under hypoxia, leading to a decrease in the expression of VEGF [213]. Choi et al. [214] reported that, of the two HIF-1 sub-units, only ARNT was destabilized by curcumin and that ARNT expression rescued HIF-1 repression by curcumin. They also found that curcumin stimulated the proteasomal degradation of ARNT via oxidation and ubiquitination processes. In mice bearing Hep3B hepatoma, curcumin retarded tumour growth and suppressed ARNT, erythropoietin and VEGF in tumours [214]. These results suggest that chemopreventive agents specifically targeting HIF-1 expression or degradation could have potential in treatment of cancers.
Hedgehog (Hh)
The Hh signalling pathway was primarily identified in the development of the fruit fly, Drosophila melanogaster, as a segment polarity gene required for embryonic patterning [215]. The components of Hh signalling demonstrate high inter-species conservation. The mammalian Hh pathway has three family members, each named after their ligands: Sonic, Indian and Desert [216]. Hh signalling is involved in congenital development and cell growth; however, aberrant Hh signalling has been implicated in up to 25% of all cancers [217]. Dysregulation of the Hh signalling pathway can lead to an assortment of cancer types. Depending on the recipient cell of Hh signalling, a variety of cell-specific transcription factors may interfere with the developmental processes [216], including production of VEGF and ANG-1 and -2, which regulate angiogenesis [218]; cyclin D2, which regulates cell proliferation [219]; up-regulation of anti-apoptotic proteins that mediate cell survival [220]; transcription of SNAIL, which initiates the epithelial-mesenchymal transition in metastasis [221]; cell-cycle regulation; cell fate determination; and stem cell signalling [222]. Based on the importance of the Hh pathway in tumour development, the specific inhibitors that target Hh signalling could provide efficient therapy against a wide range of malignancies.
Hosoya et al. [223] identified zerumbone, isolated from Zingiber zerumbet, as an inhibitor of GLI-mediated transcription. In addition, they found that physalins F and B from the native gooseberry (Physalis minima) are potent inhibitors of the Hh pathway as well. These compounds also inhibited GLI2-mediated transactivation. By inhibiting the Hh pathway, zerumbone and physalins in turn down-regulated the expression of the anti-apoptotic protein Bcl-2 in human pancreatic PANC-1 cells. Similar results have been obtained in a study by Arai et al. [224] using the pentacyclic triterpenes colubrinic acid and betulinic acid, extracted from the Cambodian jujube (Zizyphus cambodiana). The inhibition of GLI-related protein expression with colubrinic or betulinic acid was observed in HaCaT cells with exogenous GLI1 and in human pancreatic cancer cells (PANC1), which express Hh/GLI components aberrantly. The expression of GLI-related proteins PTCH and Bcl-2 were clearly inhibited by both colubrinic and betulinic acid. Therefore, perturbing the Hh pathway may be one of the underlying mechanisms behind the anti-cancer activity of these compounds.
Curcumin is also a potential inhibitor of the Hh pathway. Curcumin induces apoptosis in medulloblastoma cells, presumably by suppressing Shh protein and its downstream targets, GLI1 and PTCH1. This response has been associated with a down-regulation of the anti-apoptotic protein Bcl-2 [225]. These results indicate that dietary agents targeting the Hh/GLI signalling pathway could be good leads for the development of new agents for the treatment of cancer.
Conclusions
Based on the descriptions above, it is clear that inflammation is tightly linked with cancer and that dietary agents can suppress chronic inflammation and thus help prevent and treat cancer.
This evidence regarding dietary nutraceuticals is fascinating and warrants greater attention. The recommendations made by the US National Cancer Institute are that a chemopreventive agent should be non-toxic to normal and healthy people, should have high efficacy against multiple sites, should be orally bio-available, should have a known mechanism of action, should be easily accessible and should be acceptable to most of the human population. Among the nutraceuticals, curcumin appears to meet most of these requirements. As described above, curcumin can modulate multiple transcription factors and their related cell-signalling pathways known to be involved in prevention and treatment of cancer. Cancer is a disease caused by dysregulation of multiple cell-signalling pathways. Because dietary agents such as curcumin can control several of these pathways, it has enormous potential for cancer prevention. However, use of nutraceuticals as part of cancer prevention or a treatment regimen in the clinic awaits the answer of several unresolved questions, including those about bioavailability and safety as well as the specific anti-cancer molecular targets of these dietary agents. Future studies are needed to accurately characterize these nutraceuticals and gain a better understanding of their molecular mechanisms of action, to determine their in vivo bioavailability and efficacy in proper animal models of cancer and to elucidate their safety and efficacy in clinical trials.
Acknowledgments
We thank Virginia M. Mohlere for carefully proofreading the manuscript and providing valuable comments. Dr Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research.
This work was supported by a grant from New Chapter, a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2) and a grant from the Center for Targeted Therapy of the University of Texas M.D. Anderson Cancer Center.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 3.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;(Suppl 33):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 4.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 5.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 11.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-kappaB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2010;30:1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 13.Ahn KS, Sethi G, Aggarwal BB. Nuclear factor-kappa B: from clone to clinic. Curr Mol Med. 2007;7:619–637. doi: 10.2174/156652407782564363. [DOI] [PubMed] [Google Scholar]
- 14.Prasad S, Ravindran J, Aggarwal BB. NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem. 336:25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends Immunol. 2005;26:318–325. doi: 10.1016/j.it.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: from bench to bedside. Exp Biol Med (Maywood) 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 18.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 1799:775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 23.Shishodia S, Aggarwal BB. Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, I kappa B kinase activation and NF-kappa B-regulated gene expression. Oncogene. 2006;25:1463–1473. doi: 10.1038/sj.onc.1209194. [DOI] [PubMed] [Google Scholar]
- 24.Pandey MK, Sung B, Ahn KS, Kunnumakkara AB, Chaturvedi MM, Aggarwal BB. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood. 2007;110:3517–3525. doi: 10.1182/blood-2007-03-079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S, Natarajan K, Aggarwal BB. Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a potent inhibitor of nuclear transcription factor-kappa B activation by diverse agents. J Immunol. 1996;157:4412–4420. [PubMed] [Google Scholar]
- 26.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63:4375–4383. [PubMed] [Google Scholar]
- 27.Sung B, Ahn KS, Aggarwal BB. Noscapine, a benzylisoquinoline alkaloid, sensitizes leukemic cells to chemotherapeutic agents and cytokines by modulating the NF-kappaB signaling pathway. Cancer Res. 70:3259–3268. doi: 10.1158/0008-5472.CAN-09-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harikumar KB, Sung B, Tharakan ST, Pandey MK, Joy B, Guha S, et al. Sesamin manifests chemopreventive effects through the suppression of NF-kappa B-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol Cancer Res. 2010;8:751–761. doi: 10.1158/1541-7786.MCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000;19:2943–2950. doi: 10.1038/sj.onc.1203614. [DOI] [PubMed] [Google Scholar]
- 30.Prasad S, Yadav VR, Sundaram C, Reuter S, Hema PS, Nair MS, et al. Crotepoxide chemosensitizes tumor cells through inhibition of expression of proliferation, invasion, and angiogenic proteins linked to proinflammatory pathway. J Biol Chem. 2010;285:26987–26997. doi: 10.1074/jbc.M110.121061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24:6957–6969. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]
- 32.Ichikawa H, Takada Y, Murakami A, Aggarwal BB. Identification of a novel blocker of I kappa B alpha kinase that enhances cellular apoptosis and inhibits cellular invasion through suppression of NF-kappa B-regulated gene products. J Immunol. 2005;174:7383–7392. doi: 10.4049/jimmunol.174.11.7383. [DOI] [PubMed] [Google Scholar]
- 33.Kim SO, Chun KS, Kundu JK, Surh YJ. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors. 2004;21:27–31. doi: 10.1002/biof.552210107. [DOI] [PubMed] [Google Scholar]
- 34.Sung B, Murakami A, Oyajobi BO, Aggarwal BB. Zerumbone abolishes RANKL-induced NF-kappaB activation, inhibits osteoclastogenesis, and suppresses human breast cancer-induced bone loss in athymic nude mice. Cancer Res. 2009;69:1477–1484. doi: 10.1158/0008-5472.CAN-08-3249. [DOI] [PubMed] [Google Scholar]
- 35.Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–17033. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- 36.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anand P, Kunnumakkara AB, Harikumar KB, Ahn KS, Badmaev V, Aggarwal BB. Modification of cysteine residue in p65 subunit of nuclear factor-kappaB (NF-kappaB) by picroliv suppresses NF-kappaB-regulated gene products and potentiates apoptosis. Cancer Res. 2008;68:8861–8870. doi: 10.1158/0008-5472.CAN-08-1902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Harikumar KB, Kunnumakkara AB, Ahn KS, Anand P, Krishnan S, Guha S, Aggarwal BB. Modification of the cysteine residues in IkappaBalpha kinase and NF-kappaB (p65) by xanthohumol leads to suppression of NF-kappaB-regulated gene products and potentiation of apoptosis in leukemia cells. Blood. 2009;113:2003–2013. doi: 10.1182/blood-2008-04-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandey MK, Sandur SK, Sung B, Sethi G, Kunnumakkara AB, Aggarwal BB. Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-kappaB and NF-kappaB-regulated gene expression through direct inhibition of IkappaBalpha kinase beta on cysteine 179 residue. J Biol Chem. 2007;282:17340–17350. doi: 10.1074/jbc.M700890200. [DOI] [PubMed] [Google Scholar]
- 40.Gupta SC, Prasad S, Reuter S, Kannappan R, Yadav VR, Ravindran J, et al. Modification of cysteine 179 of IkappaBalpha kinase by nimbolide leads to down-regulation of NF-kappaB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J Biol Chem. 2010;285:35406–35417. doi: 10.1074/jbc.M110.161984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey MK, Sung B, Kunnumakkara AB, Sethi G, Chaturvedi MM, Aggarwal BB. Berberine modifies cysteine 179 of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68:5370–5379. doi: 10.1158/0008-5472.CAN-08-0511. [DOI] [PubMed] [Google Scholar]
- 42.Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gammatocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J Biol Chem. 2007;282:809–820. doi: 10.1074/jbc.M610028200. [DOI] [PubMed] [Google Scholar]
- 43.Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, et al. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010;70:8695–8705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harikumar KB, Sung B, Pandey MK, Guha S, Krishnan S, Aggarwal BB. Escin, a pentacyclic triterpene, chemosensitizes human tumor cells through inhibition of nuclear factor-kappaB signaling pathway. Mol Pharmacol. 2010;77:818–827. doi: 10.1124/mol.109.062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sethi G, Ahn KS, Sung B, Aggarwal BB. Pinitol targets nuclear factor-kappaB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol Cancer Ther. 2008;7:1604–1614. doi: 10.1158/1535-7163.MCT-07-2424. [DOI] [PubMed] [Google Scholar]
- 46.Sung B, Pandey MK, Ahn KS, Yi T, Chaturvedi MM, Liu M, Aggarwal BB. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood. 2008;111:4880–4891. doi: 10.1182/blood-2007-10-117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sung B, Pandey MK, Nakajima Y, Nishida H, Konishi T, Chaturvedi MM, Aggarwal BB. Identification of a novel blocker of IkappaBalpha kinase activation that enhances apoptosis and inhibits proliferation and invasion by suppressing nuclear factor-kappaB. Mol Cancer Ther. 2008;7:191–201. doi: 10.1158/1535-7163.MCT-07-0406. [DOI] [PubMed] [Google Scholar]
- 48.Manna SK, Aggarwal RS, Sethi G, Aggarwal BB, Ramesh GT. Morin (3,5,7,2′,4′-Pentahydroxyflavone) abolishes nuclear factor-kappaB activation induced by various carcinogens and inflammatory stimuli, leading to suppression of nuclear factor-kappaB-regulated gene expression and up-regulation of apoptosis. Clin Cancer Res. 2007;13:2290–2297. doi: 10.1158/1078-0432.CCR-06-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 50.Ahn KS, Sethi G, Aggarwal BB. Simvastatin potentiates TNF-alpha-induced apoptosis through the down-regulation of NF-kappaB-dependent antiapoptotic gene products: role of IkappaBalpha kinase and TGF-beta-activated kinase-1. J Immunol. 2007;178:2507–2516. doi: 10.4049/jimmunol.178.4.2507. [DOI] [PubMed] [Google Scholar]
- 51.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 52.Takada Y, Andreeff M, Aggarwal BB. Indole-3-carbinol suppresses NF-kappaB and IkappaBalpha kinase activation, causing inhibition of expression of NF-kappaB-regulated antiapoptotic and metastatic gene products and enhancement of apoptosis in myeloid and leukemia cells. Blood. 2005;106:641–649. doi: 10.1182/blood-2004-12-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takada Y, Kobayashi Y, Aggarwal BB. Evodiamine abolishes constitutive and inducible NF-kappaB activation by inhibiting IkappaBalpha kinase activation, thereby suppressing NF-kappaB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J Biol Chem. 2005;280:17203–17212. doi: 10.1074/jbc.M500077200. [DOI] [PubMed] [Google Scholar]
- 54.Takada Y, Aggarwal BB. Betulinic acid suppresses carcinogen-induced NF-kappa B activation through inhibition of I kappa B alpha kinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J Immunol. 2003;171:3278–3286. doi: 10.4049/jimmunol.171.6.3278. [DOI] [PubMed] [Google Scholar]
- 55.Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163:6800–6809. [PubMed] [Google Scholar]
- 56.Kumar A, Dhawan S, Aggarwal BB. Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene. 1998;17:913–918. doi: 10.1038/sj.onc.1201998. [DOI] [PubMed] [Google Scholar]
- 57.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 58.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Lee W, Haslinger A, Karin M, Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987;325:368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- 60.Chinenov Y, Kerppola TK. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene. 2001;20:2438–2452. doi: 10.1038/sj.onc.1204385. [DOI] [PubMed] [Google Scholar]
- 61.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 62.Zenz R, Eferl R, Scheinecker C, Redlich K, Smolen J, Schonthaler HB, et al. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther. 2008;10:201. doi: 10.1186/ar2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernstein LR, Colburn NH. AP1/jun function is differentially induced in promotion-sensitive and resistant JB6 cells. Science. 1989;244:566–569. doi: 10.1126/science.2541502. [DOI] [PubMed] [Google Scholar]
- 64.Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993;8:877–886. [PubMed] [Google Scholar]
- 65.Young MR, Yang HS, Colburn NH. Promising molecular targets for cancer prevention: AP-1, NF-kappa B and Pdcd4. Trends Mol Med. 2003;9:36–41. doi: 10.1016/s1471-4914(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 66.Aravindaram K, Yang NS. Anti-inflammatory plant natural products for cancer therapy. Planta Med. 2010;76:1103–1117. doi: 10.1055/s-0030-1249859. [DOI] [PubMed] [Google Scholar]
- 67.Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135:2993S–3001S. doi: 10.1093/jn/135.12.2993S. [DOI] [PubMed] [Google Scholar]
- 68.Ding M, Feng R, Wang SY, Bowman L, Lu Y, Qian Y, et al. Cyanidin-3-glucoside, a natural product derived from blackBerry, exhibits chemopreventive and chemotherapeutic activity. J Biol Chem. 2006;281:17359–17368. doi: 10.1074/jbc.M600861200. [DOI] [PubMed] [Google Scholar]
- 69.Mizuno H, Cho YY, Zhu F, Ma WY, Bode AM, Yang CS, et al. Theaflavin-3, 3′-digallate induces epidermal growth factor receptor downregulation. Mol Carcinog. 2006;45:204–212. doi: 10.1002/mc.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol. 2005;69:221–232. doi: 10.1016/j.bcp.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 71.Kawabata K, Murakami A, Ohigashi H. Nobiletin, a citrus flavonoid, down-regulates matrix metalloproteinase-7 (mat-rilysin) expression in HT-29 human colorectal cancer cells. Biosci Biotechnol Biochem. 2005;69:307–314. doi: 10.1271/bbb.69.307. [DOI] [PubMed] [Google Scholar]
- 72.Hahm ER, Park S, Yang CH. 7, 8-dihydroxyflavanone as an inhibitor for Jun-Fos-DNA complex formation and its cytotoxic effect on cultured human cancer cells. Nat Prod Res. 2003;17:431–436. doi: 10.1080/1478641032000115322. [DOI] [PubMed] [Google Scholar]
- 73.Hsum YW, Yew WT, Hong PL, Soo KK, Hoon LS, Chieng YC, Mooi LY. Cancer chemopreventive activity of maslinic acid: suppression of COX-2 expression and inhibition of NF-kappaB and AP-1 activation in raji cells. Planta Med. 2011;77:152–157. doi: 10.1055/s-0030-1250203. [DOI] [PubMed] [Google Scholar]
- 74.Lee KM, Lee DE, Seo SK, Hwang MK, Heo YS, Lee KW, Lee HJ. Phosphatidylinositol 3-kinase, a novel target molecule for the inhibitory effects of kaempferol on neoplastic cell transformation. Carcinogenesis. 2010;31:1338–1343. doi: 10.1093/carcin/bgq102. [DOI] [PubMed] [Google Scholar]
- 75.Palozza P, Parrone N, Catalano A, Simone R. Tomato lycopene and inflammatory cascade: basic interactions and clinical implications. Curr Med Chem. 2010;17:2547–2563. doi: 10.2174/092986710791556041. [DOI] [PubMed] [Google Scholar]
- 76.Cheng CC, Chen YH, Chang WL, Yang SP, Chang DM, Lai JH, Ho LJ. Phytoestrogen bavachin mediates anti-inflammation targeting Ikappa B kinase-IkappaB alpha-NF-kappaB signaling pathway in chondrocytes in vitro. Eur J Pharmacol. 2010;636:181–188. doi: 10.1016/j.ejphar.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 77.Jiang J, Grieb B, Thyagarajan A, Sliva D. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling. Int J Mol Med. 2008;21:577–584. [PubMed] [Google Scholar]
- 78.Woo KJ, Kwon TK. Sulforaphane suppresses lipopolysaccharide-induced cyclooxygenase-2 (COX-2) expression through the modulation of multiple targets in COX-2 gene promoter. Int Immunopharmacol. 2007;7:1776–1783. doi: 10.1016/j.intimp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 79.Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC, Lu KH. Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis. 2007;28:977–987. doi: 10.1093/carcin/bgl221. [DOI] [PubMed] [Google Scholar]
- 80.Mitani N, Murakami K, Yamaura T, Ikeda T, Saiki I. Inhibitory effect of berberine on the mediastinal lymph node metastasis produced by orthotopic implantation of Lewis lung carcinoma. Cancer Lett. 2001;165:35–42. doi: 10.1016/s0304-3835(00)00710-2. [DOI] [PubMed] [Google Scholar]
- 81.Lee DK, Kim B, Lee SG, Gwon HJ, Moon EY, Hwang HS, et al. Momordins inhibit both AP-1 function and cell proliferation. Anticancer Res. 1998;18:119–124. [PubMed] [Google Scholar]
- 82.Kundu JK, Chun KS, Kim SO, Surh YJ. Resveratrol inhibits phorbol ester-induced cyclooxygenase-2 expression in mouse skin: MAPKs and AP-1 as potential molecular targets. Biofactors. 2004;21:33–39. doi: 10.1002/biof.552210108. [DOI] [PubMed] [Google Scholar]
- 83.Shen F, Chen SJ, Dong XJ, Zhong H, Li YT, Cheng GF. Suppression of IL-8 gene transcription by resveratrol in phorbol ester treated human monocytic cells. J Asian Nat Prod Res. 2003;5:151–157. doi: 10.1080/1028602031000066852. [DOI] [PubMed] [Google Scholar]
- 84.Pan MH, Chiou YS, Chen WJ, Wang JM, Badmaev V, Ho CT. Pterostilbene inhibited tumor invasion via suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Carcinogenesis. 2009;30:1234–1242. doi: 10.1093/carcin/bgp121. [DOI] [PubMed] [Google Scholar]
- 85.Takada Y, Sethi G, Sung B, Aggarwal BB. Flavopiridol suppresses tumor necrosis factor-induced activation of activator protein-1, c-Jun N-terminal kinase, p38 mitogen-activated protein kinase (MAPK), p44/p42 MAPK, and Akt, inhibits expression of antiapoptotic gene products, and enhances apoptosis through cytochrome c release and caspase activation in human myeloid cells. Mol Pharmacol. 2008;73:1549–1557. doi: 10.1124/mol.107.041350. [DOI] [PubMed] [Google Scholar]
- 86.Han SS, Keum YS, Seo HJ, Surh YJ. Curcumin suppresses activation of NF-kappaB and AP-1 induced by phorbol ester in cultured human promyelocytic leukemia cells. J Biochem Mol Biol. 2002;35:337–342. doi: 10.5483/bmbrep.2002.35.3.337. [DOI] [PubMed] [Google Scholar]
- 87.Hergenhahn M, Soto U, Weninger A, Polack A, Hsu CH, Cheng AL, Rosl F. The chemopreventive compound curcumin is an efficient inhibitor of Epstein-Barr virus BZLF1 transcription in Raji DR-LUC cells. Mol Carcinog. 2002;33:137–145. doi: 10.1002/mc.10029. [DOI] [PubMed] [Google Scholar]
- 88.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 89.Nakamura K, Yasunaga Y, Segawa T, Ko D, Moul JW, Srivastava S, Rhim JS. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol. 2002;21:825–830. [PubMed] [Google Scholar]
- 90.Ichiki K, Mitani N, Doki Y, Hara H, Misaki T, Saiki I. Regulation of activator protein-1 activity in the mediastinal lymph node metastasis of lung cancer. Clin Exp Metastasis. 2000;18:539–545. doi: 10.1023/a:1011980313237. [DOI] [PubMed] [Google Scholar]
- 91.Kang G, Kong PJ, Yuh YJ, Lim SY, Yim SV, Chun W, Kim SS. Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor kappab bindings in BV2 microglial cells. J Pharmacol Sci. 2004;94:325–328. doi: 10.1254/jphs.94.325. [DOI] [PubMed] [Google Scholar]
- 92.Prakobwong S, Khoontawad J, Yongvanit P, Pairojkul C, Hiraku Y, Sithithaworn P, et al. Curcumin decreases cholangiocarcinogenesis in hamsters by suppressing inflammation-mediated molecular events related to multistep carcinogenesis. Int J Cancer. 2011;129:88–100. doi: 10.1002/ijc.25656. [DOI] [PubMed] [Google Scholar]
- 93.Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, et al. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 94.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, et al. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 96.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 97.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angio-genesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bollrath J, Greten FR. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009;10:1314–1319. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kannappan R, Yadav VR, Aggarwal BB. {Gamma}-tocotrienol but not {gamma}-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents. J Biol Chem. 2010;285:33520–33528. doi: 10.1074/jbc.M110.158378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Sandur SK, Pandey MK, Sung B, Aggarwal BB. 5-hydroxy-2-methyl-1,4-naphthoquinone, a vitamin K3 analogue, suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase, SHP-1: potential role in chemosensitization. Mol Cancer Res. 2010;8:107–118. doi: 10.1158/1541-7786.MCR-09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pandey MK, Sung B, Aggarwal BB. Betulinic acid suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase SHP-1 in human multiple myeloma cells. Int J Cancer. 2010;127:282–292. doi: 10.1002/ijc.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pandey MK, Sung B, Ahn KS, Aggarwal BB. Butein suppresses constitutive and inducible signal transducer and activator of transcription (STAT) 3 activation and STAT3-regulated gene products through the induction of a protein tyrosine phosphatase SHP-1. Mol Pharmacol. 2009;75:525–533. doi: 10.1124/mol.108.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muto A, Hori M, Sasaki Y, Saitoh A, Yasuda I, Maekawa T, et al. Emodin has a cytotoxic activity against human multiple myeloma as a Janus-activated kinase 2 inhibitor. Mol Cancer Ther. 2007;6:987–994. doi: 10.1158/1535-7163.MCT-06-0605. [DOI] [PubMed] [Google Scholar]
- 110.Bhutani M, Pathak AK, Nair AS, Kunnumakkara AB, Guha S, Sethi G, Aggarwal BB. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res. 2007;13:3024–3032. doi: 10.1158/1078-0432.CCR-06-2575. [DOI] [PubMed] [Google Scholar]
- 111.Ito S, Oyake T, Murai K, Ishida Y. Deguelin suppresses cell proliferation via the inhibition of survivin expression and STAT3 phosphorylation in HTLV-1-transformed T cells. Leuk Res. 2010;34:352–357. doi: 10.1016/j.leukres.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 112.Saydmohammed M, Joseph D, Syed V. Curcumin suppresses constitutive activation of STAT-3 by up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in ovarian and endometrial cancer cells. J Cell Biochem. 2010;110:447–456. doi: 10.1002/jcb.22558. [DOI] [PubMed] [Google Scholar]
- 113.Glienke W, Maute L, Wicht J, Bergmann L. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and downregulation of survivin/BIRC5 gene expression. Cancer Invest. 2010;28:166–171. doi: 10.3109/07357900903287006. [DOI] [PubMed] [Google Scholar]
- 114.Chakravarti N, Myers JN, Aggarwal BB. Targeting constitutive and interleukin-6-inducible signal transducers and activators of transcription 3 pathway in head and neck squamous cell carcinoma cells by curcumin (diferuloylmethane) Int J Cancer. 2006;119:1268–1275. doi: 10.1002/ijc.21967. [DOI] [PubMed] [Google Scholar]
- 115.Bill MA, Bakan C, Benson DM, Jr, Fuchs J, Young G, Lesinski GB. Curcumin induces proapoptotic effects against human melanoma cells and modulates the cellular response to immunotherapeutic cytokines. Mol Cancer Ther. 2009;8:2726–2735. doi: 10.1158/1535-7163.MCT-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mackenzie GG, Queisser N, Wolfson ML, Fraga CG, Adamo AM, Oteiza PI. Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF-kappaB and STAT3 pathways in Hodgkin's lymphoma cells. Int J Cancer. 2008;123:56–65. doi: 10.1002/ijc.23477. [DOI] [PubMed] [Google Scholar]
- 117.Uddin S, Hussain AR, Manogaran PS, Al-Hussein K, Platanias LC, Gutierrez MI, Bhatia KG. Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene. 2005;24:7022–7030. doi: 10.1038/sj.onc.1208864. [DOI] [PubMed] [Google Scholar]
- 118.Rajasingh J, Raikwar HP, Muthian G, Johnson C, Bright JJ. Curcumin induces growth-arrest and apoptosis in association with the inhibition of constitutively active JAK-STAT pathway in T cell leukemia. Biochem Biophys Res Commun. 2006;340:359–368. doi: 10.1016/j.bbrc.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 119.Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–3184. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 120.Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–6079. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- 121.Blasius R, Reuter S, Henry E, Dicato M, Diederich M. Curcumin regulates signal transducer and activator of transcription (STAT) expression in K562 cells. Biochem Pharmacol. 2006;72:1547–1554. doi: 10.1016/j.bcp.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 122.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 123.Zhang C, Li B, Zhang X, Hazarika P, Aggarwal BB, Duvic M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients' PBMCs: potential role for STAT-3 and NF-kappaB signaling. J Invest Dermatol. 2010;130:2110–2119. doi: 10.1038/jid.2010.86. [DOI] [PubMed] [Google Scholar]
- 124.Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, et al. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther. 2006;5:621–629. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- 125.Li T, Wang W. Regulatory effect of resveratrol on JAK1/STAT3 signal transduction pathway in leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16:772–776. [PubMed] [Google Scholar]
- 126.Li T, Wang W, Chen H, Ye L. Evaluation of anti-leukemia effect of resveratrol by modulating STAT3 signaling. Int Immunopharmacol. 2010;10:18–25. doi: 10.1016/j.intimp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 127.Youn J, Lee JS, Na HK, Kundu JK, Surh YJ. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr Cancer. 2009;61:847–854. doi: 10.1080/01635580903285072. [DOI] [PubMed] [Google Scholar]
- 128.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis. 2003;24:461–467. doi: 10.1093/carcin/24.3.461. [DOI] [PubMed] [Google Scholar]
- 132.Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 133.Shen G, Xu C, Hu R, Jain MR, Gopalkrishnan A, Nair S, et al. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]
- 134.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu C, Huang MT, Shen G, Yuan X, Lin W, Khor TO, et al. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 136.Yates MS, Kwak MK, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-, 12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- 137.Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- 138.Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, et al. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med. 2004;37:1578–1590. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 139.Zhao CR, Gao ZH, Qu XJ. Nrf2-ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol. 2010;34:523–533. doi: 10.1016/j.canep.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 140.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jaiswal AK, Venugopal R, Mucha J, Carothers AM, Grunberger D. Caffeic acid phenethyl ester stimulates human antioxidant response element-mediated expression of the NAD(P)H:quinone oxidoreductase (NQO1) gene. Cancer Res. 1997;57:440–446. [PubMed] [Google Scholar]
- 142.Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003;17:473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 143.Jeong WS, Keum YS, Chen C, Jain MR, Shen G, Kim JH, et al. Differential expression and stability of endogenous nuclear factor E2-related factor 2 (Nrf2) by natural chemopreventive compounds in HepG2 human hepatoma cells. J Biochem Mol Biol. 2005;38:167–176. doi: 10.5483/bmbrep.2005.38.2.167. [DOI] [PubMed] [Google Scholar]
- 144.Krajka-Kuzniak V, Szaefer H, Bartoszek A, Baer-Dubowska W. Modulation of rat hepatic and kidney phase II enzymes by cabbage juices: comparison with the effects of indole-3-carbinol and phenethyl isothiocyanate. Br J Nutr. 2011;105:816–826. doi: 10.1017/S0007114510004526. [DOI] [PubMed] [Google Scholar]
- 145.McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, et al. The Cap‘n’ Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- 146.Nakamura Y, Yoshida C, Murakami A, Ohigashi H, Osawa T, Uchida K. Zerumbone, a tropical ginger sesquiterpene, activates phase II drug metabolizing enzymes. FEBS Lett. 2004;572:245–250. doi: 10.1016/j.febslet.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 147.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Belfiore A, Genua M, Malaguarnera R. PPAR-gamma agonists and their effects on IGF-I receptor signaling: implications for cancer. PPAR Res. 2009;2009:830501. doi: 10.1155/2009/830501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 150.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 151.Marx N, Schonbeck U, Lazar MA, Libby P, Plutzky J. Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res. 1998;83:1097–1103. doi: 10.1161/01.res.83.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 153.Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res. 2003;9:1–9. [PubMed] [Google Scholar]
- 154.Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CD, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci USA. 1997;94:237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Han S, Roman J. Peroxisome proliferator-activated receptor gamma: a novel target for cancer therapeutics? Anticancer Drugs. 2007;18:237–244. doi: 10.1097/CAD.0b013e328011e67d. [DOI] [PubMed] [Google Scholar]
- 156.Ge H, Zhang JF, Guo BS, He Q, Wang BY, He B, Wang CQ. Resveratrol inhibits macrophage expression of EMMPRIN by activating PPARgamma. Vascul Pharmacol. 2007;46:114–121. doi: 10.1016/j.vph.2006.08.412. [DOI] [PubMed] [Google Scholar]
- 157.Li YT, Li L, Chen J, Hu TC, Huang J, Guo YW, et al. 7-Chloroarctinone-b as a new selective PPARgamma antagonist potently blocks adipocyte differentiation. Acta Pharmacol Sin. 2009;30:1351–1358. doi: 10.1038/aps.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zou G, Gao Z, Wang J, Zhang Y, Ding H, Huang J, et al. Deoxyelephantopin inhibits cancer cell proliferation and functions as a selective partial agonist against PPARgamma. Biochem Pharmacol. 2008;75:1381–1392. doi: 10.1016/j.bcp.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 159.Han KL, Choi JS, Lee JY, Song J, Joe MK, Jung MH, Hwang JK. Therapeutic potential of peroxisome proliferators–activated receptor-alpha/gamma dual agonist with alleviation of endoplasmic reticulum stress for the treatment of diabetes. Diabetes. 2008;57:737–745. doi: 10.2337/db07-0972. [DOI] [PubMed] [Google Scholar]
- 160.Liu SH, Shen CC, Yi YC, Tsai JJ, Wang CC, Chueh JT, et al. Honokiol inhibits gastric tumourigenesis by activation of 15-lipoxygenase-1 and consequent inhibition of peroxisome proliferator-activated receptor-gamma and COX-2-dependent signals. Br J Pharmacol. 2010;160:1963–1972. doi: 10.1111/j.1476-5381.2010.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liang YC, Tsai SH, Tsai DC, Lin-Shiau SY, Lin JK. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001;496:12–18. doi: 10.1016/s0014-5793(01)02393-6. [DOI] [PubMed] [Google Scholar]
- 162.Lee H, Kang R, Hahn Y, Yang Y, Kim SS, Cho SH, et al. Antiobesity effect of baicalin involves the modulations of proadipogenic and antiadipogenic regulators of the adipogenesis pathway. Phytother Res. 2009;23:1615–1623. doi: 10.1002/ptr.2937. [DOI] [PubMed] [Google Scholar]
- 163.Munoz-Espada AC, Watkins BA. Cyanidin attenuates PGE2 production and cyclooxygenase-2 expression in LNCaP human prostate cancer cells. J Nutr Biochem. 2006;17:589–596. doi: 10.1016/j.jnutbio.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 164.Kim CS, Park WH, Park JY, Kang JH, Kim MO, Kawada T, et al. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor gamma in HT-29 human colon cancer cells. J Med Food. 2004;7:267–273. doi: 10.1089/jmf.2004.7.267. [DOI] [PubMed] [Google Scholar]
- 165.Mori A, Lehmann S, O'Kelly J, Kumagai T, Desmond JC, Pervan M, et al. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006;66:3222–3229. doi: 10.1158/0008-5472.CAN-05-0087. [DOI] [PubMed] [Google Scholar]
- 166.Jun HS, Park T, Lee CK, Kang MK, Park MS, Kang HI, et al. Capsaicin induced apoptosis of B16-F10 melanoma cells through down-regulation of Bcl-2. Food Chem Toxicol. 2007;45:708–715. doi: 10.1016/j.fct.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 167.Surh YJ, Lee SS. Capsaicin in hot chili pepper: carcinogen, co-carcinogen or anticarcinogen? Food Chem Toxicol. 1996;34:313–316. doi: 10.1016/0278-6915(95)00108-5. [DOI] [PubMed] [Google Scholar]
- 168.Yoshitani SI, Tanaka T, Kohno H, Takashima S. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by dietary capsaicin and rotenone. Int J Oncol. 2001;19:929–939. doi: 10.3892/ijo.19.5.929. [DOI] [PubMed] [Google Scholar]
- 169.Chintharlapalli S, Papineni S, Jutooru I, McAlees A, Safe S. Structure-dependent activity of glycyrrhetinic acid derivatives as peroxisome proliferator-activated receptor {gamma} agonists in colon cancer cells. Mol Cancer Ther. 2007;6:1588–1598. doi: 10.1158/1535-7163.MCT-07-0022. [DOI] [PubMed] [Google Scholar]
- 170.Chintharlapalli S, Papineni S, Liu S, Jutooru I, Chadalapaka G, Cho SD, et al. 2-cyano-lup-1-en-3-oxo-20-oic acid, a cyano derivative of betulinic acid, activates peroxisome proliferator-activated receptor gamma in colon and pancreatic cancer cells. Carcinogenesis. 2007;28:2337–2346. doi: 10.1093/carcin/bgm189. [DOI] [PubMed] [Google Scholar]
- 171.Au-Yeung KK, Liu PL, Chan C, Wu WY, Lee SS, Ko JK. Herbal isoprenols induce apoptosis in human colon cancer cells through transcriptional activation of PPARgamma. Cancer Invest. 2008;26:708–717. doi: 10.1080/07357900801898656. [DOI] [PubMed] [Google Scholar]
- 172.Goto T, Takahashi N, Hirai S, Kawada T. Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res. 2010;2010:483958. doi: 10.1155/2010/483958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:20–30. doi: 10.1152/ajpgi.00474.2002. [DOI] [PubMed] [Google Scholar]
- 174.Chen A, Xu J. Activation of PPAR{gamma} by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol. 2005;288:447–456. doi: 10.1152/ajpgi.00209.2004. [DOI] [PubMed] [Google Scholar]
- 175.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 176.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 177.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 178.Bienz M, Clevers H. Armadillo/beta-catenin signals in the nucleus-proof beyond a reasonable doubt? Nat Cell Biol. 2003;5:179–182. doi: 10.1038/ncb0303-179. [DOI] [PubMed] [Google Scholar]
- 179.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 180.Sangeetha N, Aranganathan S, Panneerselvam J, Shanthi P, Rama G, Nalini N. Oral supplementation of silibinin prevents colon carcinogenesis in a long term preclinical model. Eur J Pharmacol. 2010;643:93–100. doi: 10.1016/j.ejphar.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 181.Rajamanickam S, Kaur M, Velmurugan B, Singh RP, Agarwal R. Silibinin suppresses spontaneous tumorigenesis in APC min/+ mouse model by modulating beta-catenin pathway. Pharm Res. 2009;26:2558–2567. doi: 10.1007/s11095-009-9968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chiou YS, Tsai ML, Wang YJ, Cheng AC, Lai WM, Badmaev V, et al. Pterostilbene inhibits colorectal aberrant crypt foci (ACF) and colon carcinogenesis via suppression of multiple signal transduction pathways in azoxymethane-treated mice. J Agric Food Chem. 2010;58:8833–8841. doi: 10.1021/jf101571z. [DOI] [PubMed] [Google Scholar]
- 183.Park S, Choi J. Inhibition of beta-catenin/Tcf signaling by flavonoids. J Cell Biochem. 2010;110:1376–1385. doi: 10.1002/jcb.22654. [DOI] [PubMed] [Google Scholar]
- 184.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30:300–307. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tarapore RS, Siddiqui IA, Saleem M, Adhami VM, Spiegelman VS, Mukhtar H. Specific targeting of Wnt/beta-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis. 2010;31:1844–1853. doi: 10.1093/carcin/bgq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Orner GA, Dashwood WM, Blum CA, Diaz GD, Li Q, Dashwood RH. Suppression of tumorigenesis in the Apc(min) mouse: down-regulation of beta-catenin signaling by a combination of tea plus sulindac. Carcinogenesis. 2003;24:263–267. doi: 10.1093/carcin/24.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Patel R, Ingle A, Maru GB. Polymeric black tea polyphenols inhibit 1,2-dimethylhydrazine induced colorectal carcinogenesis by inhibiting cell proliferation via Wnt/beta-catenin pathway. Toxicol Appl Pharmacol. 2008;227:136–146. doi: 10.1016/j.taap.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 188.Dashwood WM, Orner GA, Dashwood RH. Inhibition of beta-catenin/Tcf activity by white tea, green tea, and epigal-locatechin-3-gallate (EGCG): minor contribution of H(2) O(2) at physiologically relevant EGCG concentrations. Biochem Biophys Res Commun. 2002;296:584–588. doi: 10.1016/s0006-291x(02)00914-2. [DOI] [PubMed] [Google Scholar]
- 189.Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. Suppression of Wnt signaling by the green tea compound (-)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–10875. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 190.Lai CS, Tsai ML, Cheng AC, Li S, Lo CY, Wang Y, et al. Chemoprevention of colonic tumorigenesis by dietary hydroxylated polymethoxyflavones in azoxymethane-treated mice. Mol Nutr Food Res. 2011;55:32–45. doi: 10.1002/mnfr.201000224. [DOI] [PubMed] [Google Scholar]
- 191.Lee H, Bae S, Kim K, Kim W, Chung SI, Yang Y, Yoon Y. Shikonin inhibits adipogenesis by modulation of the WNT/beta-catenin pathway. Life Sci. 2011;88:294–301. doi: 10.1016/j.lfs.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 192.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–2590. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Song GY, Lee JH, Cho M, Park BS, Kim DE, Oh S. Decursin suppresses human androgen-independent PC3 prostate cancer cell proliferation by promoting the degradation of beta-catenin. Mol Pharmacol. 2007;72:1599–1606. doi: 10.1124/mol.107.040253. [DOI] [PubMed] [Google Scholar]
- 194.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 195.Choi HY, Lim JE, Hong JH. Curcumin interrupts the interaction between the androgen receptor and Wnt/beta-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010;13:343–349. doi: 10.1038/pcan.2010.26. [DOI] [PubMed] [Google Scholar]
- 196.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 198.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 199.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 200.Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Curr Opin Genet Dev. 2001;11:293–299. doi: 10.1016/s0959-437x(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 201.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 202.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- 203.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hickey MM, Simon MC. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev Biol. 2006;76:217–257. doi: 10.1016/S0070-2153(06)76007-0. [DOI] [PubMed] [Google Scholar]
- 205.Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci USA. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Arya M, Ahmed H, Silhi N, Williamson M, Patel HR. Clinical importance and therapeutic implications of the pivotal CXCL12-CXCR4 (chemokine ligand-receptor) interaction in cancer cell migration. Tumour Biol. 2007;28:123–131. doi: 10.1159/000102979. [DOI] [PubMed] [Google Scholar]
- 207.Manolescu B, Oprea E, Busu C, Cercasov C. Natural compounds and the hypoxia-inducible factor (HIF) signalling pathway. Biochimie. 2009;91:1347–1358. doi: 10.1016/j.biochi.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 208.Triantafyllou A, Mylonis I, Simos G, Bonanou S, Tsakalof A. Flavonoids induce HIF-1alpha but impair its nuclear accumulation and activity. Free Radic Biol Med. 2008;44:657–670. doi: 10.1016/j.freeradbiomed.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 209.Kim WY, Oh SH, Woo JK, Hong WK, Lee HY. Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor-1alpha. Cancer Res. 2009;69:1624–1632. doi: 10.1158/0008-5472.CAN-08-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim JH, Yu YS, Shin JY, Lee HY, Kim KW. Deguelin inhibits retinal neovascularization by down-regulation of HIF-1alpha in oxygen-induced retinopathy. J Cell Mol Med. 2008;12:2407–2415. doi: 10.1111/j.1582-4934.2008.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J, Le AD. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol Cancer Ther. 2005;4:1465–1474. doi: 10.1158/1535-7163.MCT-05-0198. [DOI] [PubMed] [Google Scholar]
- 212.Cao Z, Fang J, Xia C, Shi X, Jiang BH. trans-3,4,5′-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res. 2004;10:5253–5263. doi: 10.1158/1078-0432.CCR-03-0588. [DOI] [PubMed] [Google Scholar]
- 213.Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, Kim SR, et al. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15:1557–1562. [PubMed] [Google Scholar]
- 214.Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol. 2006;70:1664–1671. doi: 10.1124/mol.106.025817. [DOI] [PubMed] [Google Scholar]
- 215.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 216.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 217.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 218.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 219.Yoon JW, Kita Y, Frank DJ, Majewski RR, Konicek BA, Nobrega MA, et al. Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem. 2002;277:5548–5555. doi: 10.1074/jbc.M105708200. [DOI] [PubMed] [Google Scholar]
- 220.Bigelow RL, Chari NS, Unden AB, Spurgers KB, Lee S, Roop DR, et al. Transcriptional regulation of bcl-2 mediated by the sonic hedgehog signaling pathway through gli-1. J Biol Chem. 2004;279:1197–1205. doi: 10.1074/jbc.M310589200. [DOI] [PubMed] [Google Scholar]
- 221.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Katoh Y, Katoh M. Hedgehog signaling, epithelial-tomesenchymal transition and miRNA (review) Int J Mol Med. 2008;22:271–275. [PubMed] [Google Scholar]
- 223.Hosoya T, Arai MA, Koyano T, Kowithayakorn T, Ishibashi M. Naturally occurring small-molecule inhibitors of hedgehog/GLI-mediated transcription. Chembiochem. 2008;9:1082–1092. doi: 10.1002/cbic.200700511. [DOI] [PubMed] [Google Scholar]
- 224.Arai MA, Tateno C, Hosoya T, Koyano T, Kowithayakorn T, Ishibashi M. Hedgehog/GLI-mediated transcriptional inhibitors from Zizyphus cambodiana. Bioorg Med Chem. 2008;16:9420–9424. doi: 10.1016/j.bmc.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 225.Elamin MH, Shinwari Z, Hendrayani SF, Al-Hindi H, Al-Shail E, Khafaga Y, et al. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog. 2010;49:302–314. doi: 10.1002/mc.20604. [DOI] [PubMed] [Google Scholar]


