Abstract
BACKGROUND
Maternal immunization against low-frequency, platelet (PLT)-specific antigens is being recognized with increasing frequency as a cause of neonatal alloimmune thrombocytopenia (NAIT).
STUDY DESIGN AND METHODS
Serologic and molecular studies were performed on PLTs and DNA from two families in which an infant was born with severe thrombocytopenia not attributable to maternal immunization against known PLT-specific alloantigens.
RESULTS
Antibodies reactive only with paternal PLTs were identified in each mother using flow cytometry and solid-phase assays. Unique mutations encoding amino acid substitutions K164T in glycoprotein (GP)IIb (Case 1) and R622W in GPIIIa (Case 2) were identified in paternal DNA and in DNA from the affected infants. Each maternal antibody recognized recombinant GPIIb/IIIa mutated to contain the polymorphisms identified in the corresponding father. None of 100 unselected normal subjects possessed these paternal mutations.
CONCLUSIONS
Severe NAIT observed in the affected infants was caused by maternal immunization against previously unrecognized, low-frequency antigens created by amino acid substitutions in GPIIb/IIIa (αIIb/β3 integrin). A search should be conducted for novel paternal antigens in cases of apparent NAIT not explained on the basis of maternal-fetal incompatibility for known human PLT antigens.
Neonatal alloimmune thrombocytopenia (NAIT), caused by maternal antibodies produced against fetal platelet (PLT) alloantigens inherited from the father of an affected infant, occurs about once in 1000 live births and can result in intracranial hemorrhage leading to death or disability.1–4 It is important that a serologic diagnosis be made even in mild cases of NAIT because infants subsequently born to the same mother can be more severely affected.1,5 The most common antigen implicated as a trigger for NAIT in Western countries is human PLT antigen (HPA)-1a, determined by a leucine/proline polymorphism at Position 33 in the β-subunit of the PLT glycoprotein (GP)IIb/IIIa complex (αIIbβ3 integrin).6 However, multiple cases of NAIT caused by antibodies reactive with antigens designated HPA-2a/b carried on GPIb, HPA-3a/b on GPIIb, HPA-4a/b on GPIIIa, HPA-5a/b on GPIa, and HPA-15a/b on CD109 have been described.7 In recent years, sporadic reports have appeared of infants with NAIT caused by maternal immunization against a group of low-frequency PLT antigens created by amino acid polymorphisms on PLT GP complexes IIb/IIIa, Ib/IX, and Ia/IIa.7–10 In this report, we describe two recently encountered cases of severe NAIT caused by maternal immunization against novel, low-frequency PLT-specific antigens, one carried on PLT GPIIb and the other on GPIIIa.
CASE REPORTS
Case 1 (HPA-22bw, Sey)
The first child, a girl, born to a 31-year-old mother by a spontaneous vaginal delivery, was found to have scattered petechial hemorrhages at birth. The PLT count was 13 × 109/L. Other hematologic indices were normal. A random-donor PLT transfusion and intravenous immune globulin (IVIG) 1.0 g/kg body weight were administered, after which the PLT count increased to 80 × 109/L. During the next 11 days, the child received two additional PLT transfusions and two IVIG infusions. After each transfusion, the PLT count increased to the range of 50 × 109 to 80 × 109/L but subsequently declined. On Day 9, the PLT count decreased to 22 × 109/L and bloody stools were observed. A PLT transfusion and IVIG were again administered. PLTs rose to 65 × 109/L and increased steadily thereafter. The child was discharged on Day 15 with a normal PLT count.
Case 2 (HPA-23bw, Hug)
The second child, a boy, born to a 22-year-old woman by spontaneous vaginal delivery developed widespread petechial and subconjunctival hemorrhages soon after birth and was found to have a PLT level of 13 × 109/L. Other hematologic findings were unremarkable except for a weakly positive direct antiglobulin test thought to be a consequence of maternal-fetal incompatibility for blood group B. At 1 day of age, the infant was transferred to Vanderbilt University Medical Center where it was found to have resolving petechial hemorrhages and a PLT level 27 × 109/L. IVIG, 1 g/kg body weight, was administered. The PLT counts were 38 × 109/L the next day and 102 × 109/L 2 days later. The child was discharged on Day 5.
MATERIALS AND METHODS
Antibodies
Monoclonal antibodies (MoAb) AP2 specific for the GPIIb/IIIa complex and AP3 specific for GPIIIa were described previously.10 MoAbs 314.5, 142.11, and 202.2 specific for the GPIIb calf-2 domain, GPIb/IX, and GPIa/IIa, respectively, were produced in the Monoclonal Antibody Laboratory of the Blood Research Institute. MoAb Tab specific for GPIIb calf-2 was a gift from R. McEver (University of Oklahoma, Oklahoma City, OK). Alloantibodies specific for HPA-1a (PlA1) and HPA-3a (Baka) were from the Platelet and Neutrophil Immunology Laboratory of the Blood-Center of Wisconsin.
Serologic studies
Maternal samples from suspected NAIT cases were tested against paternal PLTs and PLTs from group O donors of known phenotypes using flow cytometry as previously described.10–12 Antibodies reactive with GPIIb/IIIa, GPIb/IX, GPIa/IIa, and GPIV were detected by direct and modified antigen-capture enzyme-linked immunosorbent assay (ELISA, ACE, and MACE).10
Genotyping and frequency analysis
PLT genotyping for HPA-1-6a/b, -9a/b, and -15a/b was performed as described previously.10 Genotyping for known single-nucleotide polymorphisms (SNPs) encoding low-frequency antigens of the HPA-7 to -14 and -16 to -21, Swi13 and Cab214 was performed using a custom-designed genotyping system (Taqman OpenArray, Applied Biosystems, Foster City, CA).15–17 Full-length sequencing of GPIIb and GPIIIa was performed as described previously.10 Genotyping of 100 normal donor DNA samples for newly identified mutations was performed with an allelic discrimination assay using quantitative polymerase chain reaction (PerfeCTa, qPCR SuperMix, UNG, Low ROX, Quanta Biosciences, Gaithersburg, MD) and a real-time PCR system (Model 7500, Applied Biosystems).
Chinese hamster ovary cell lines expressing mutant versions of GPIIb/IIIa
Throughout this report, nucleotide (nt) 1 refers to A of the ATG translation start codon of human GPIIb or GPIIIa. Point mutations were generated using a site-directed mutagenesis kit (QuickChange IIXL, Stratagene, La Jolla, CA) and were verified by direct sequencing. Full-length human ITGA2B (GPIIb) cDNA in mammalian expression vector pCDNA3.1 Neo (Invitrogen, Carlsbad, CA) was modified at nt 584 from an “A” to a “C” to encode GPIIb K164T. Full-length ITGB3 (GPIIIa) in mammalian expression vector pcDNA3.1 Zeo (Invitrogen) was mutated at nt 1942 from a “C” to a “T” resulting in GPIIIa R622W. GPIIb K164T with GPIIIa, GPIIb with GPIIIa R622W, and GPIIb with GPIIIa plasmids were cotransfected into Chinese hamster ovary (CHO)-K1 cells as previously described.18 Stable cell lines expressing recombinant forms of GPIIb and GPIIIa were identified after multiple sorts (FACStar Plus, Becton Dickinson, Franklin Lakes, NJ) for high integrin expression using MoAb AP210 and cloning at limiting dilution. Stable CHO-K1 cell lines were maintained as described.10
Human research
Patient samples were from suspected NAIT cases referred for routine testing to the Platelet & Neutrophil Immunology Laboratory, BloodCenter of Wisconsin. All studies involving human subjects were approved by the institutional research review board of BloodCenter of Wisconsin.
RESULTS
Serologic findings
Each mother had an IgG antibody that reacted strongly with paternal PLTs in flow cytometry but not with PLTs from normal group O donors carrying the HPA alloantigens HPA-1a/b, -2a/b, 3a/b, 4a, -5a/b, -6a, and -15a/b. Neither serum contained Class I HLA antibodies. When tested in MACE using monoclonal AP2 for capture, each mother was found to have an antibody that recognized GPIIb/IIIa on paternal PLTs. Blood types of fathers were A1 (Sey) and A2B (Hug). Because high-titer IgG maternal antibodies specific for blood groups A or B can cause neonatal thrombocytopenia in infants who possess a genetic trait that causes PLTs to express many times the normal levels of A or B antigens,12,19 the maternal sera were absorbed with washed group A1 and B red blood cells. This treatment had no effect on the reactions with paternal PLTs. All serologic findings are summarized in Table 1.
TABLE 1.
Summary of findings
| Molecular and serologic characterization | Case 1 (HPA-22 [Sey]) | Case 2 (HPA-23 [Hug]) |
|---|---|---|
| Maternal serum versus healthy donor GPIIb/IIIa (HPA-1a/a, -3a/a) | ||
| Flow cytometric analysis | IgG−/IgM− | IgG−/IgM− |
| MACE (AP2 capture of GPIIb/IIIa) | − | − |
| Maternal serum versus healthy donor GPIIb/IIIa (HPA-1b/b, -2a/a, -3b/b, -5a/b, -15a/b) | ||
| Flow cytometric analysis | IgG−/IgM− | IgG−/IgM− |
| MACE (AP2 capture of GPIIb/IIIa) | − | − |
| Maternal serum versus paternal GPIIb/IIIa | ||
| Flow cytometric analysis | IgG+/IgM+ | IgG+/IgM− |
| (AP2 capture of GPIIb/IIIa) | − | − |
| MACE (AP2 capture of GPIIb/IIIa) | + | + |
| Mother/Father blood type | O/A1 | O/A2B |
| MACE reactivity after absorption of anti-A or anti-B | + | + |
| Molecular characterization | ||
| Exon/nucleotide change | 5/A584C | 12/C1942T |
| Amino acid change | K164T | R622W |
| Protein | GPIIb | GPIIIa |
| NCBI dsSNP accession number | rs142811900 | rs139166528 |
| Maternal serum vs. recombinant GPIIb/IIIa mutated to express paternal SNP | ||
| Flow cytometric analysis with transfected CHO cell lines | + | + |
| MACE with mutated GPIIb/IIIa (AP2/Tab capture) | + | + |
PLT typing
Genotyping of parental DNA for antigens of the HPA-1-6, -9, and -15 systems showed that there was potential maternal-fetal incompatibility for HPA-1b in Case 1 and HPA-2b, -3b, and -15a in Case 2. However, reactions of maternal sera with normal PLTs carrying one or more of these markers were completely negative (Table 1). DNA from each father was also typed for the recognized low-frequency alleles HPA-4b, 6-14bw, 16-21bw, Swi,13 and Cab2,14 but none were detected.
Mutations predicting novel amino acid changes in GPIIb (HPA-22bw, Sey) and GPIIIa (HPA-23bw, Hug) were identified in DNA from the fathers and the affected infants
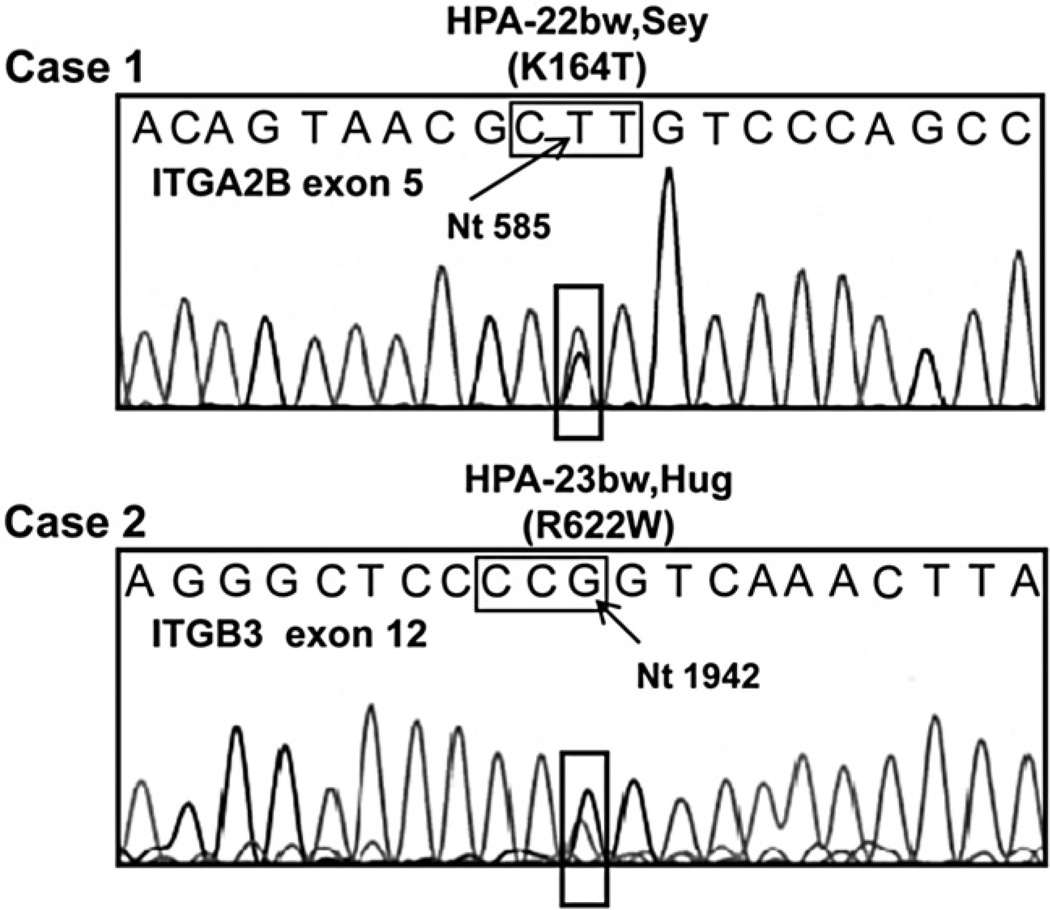
The strong reactions of maternal serum with paternal GPIIb/IIIa together with failure to identify one of the known common or low-frequency HPA antigens as a cause of maternal immunization suggested that the maternal antibodies might be specific for previously non-described PLT-specific antigens. Therefore, paternal DNA encoding the extracellular domains of GPIIb and GPIIIa was sequenced to detect mutations predicting amino acid polymorphisms in these proteins. In Case 1 (Sey), sequencing of paternal ITGA2b (GPIIb) Exon 5 revealed an A- to C- SNP at nt 584 that predicted a lysine-to-threonine switch at Position 164 (Fig. 1). In Case 2 (Hug), sequencing of paternal ITGb3 (GPIIIa) Exon 12 revealed a C-to-T SNP at nt 1942 that predicted an arginine-to-tryptophan switch at Position 622 (Fig. 1). The same novel mutations were found in DNA obtained by buccal swab from the affected infants but not in DNA from their mothers (data not shown). Neither mutation was detected in any of 100 unselected normal individuals.
Fig. 1.
Novel mutations predicting amino acid substitutions in GPIIb and GPIIIa. Case 1—Sequencing of GPIIb revealed an A-to-C substitution at nt 584 resulting in a lysine-to-threonine switch at Position 164. Case 2—A C-to-T substitution at nt 1942 predicting an arginine-to-tryptophan switch at Position 622 in GPIIIa was identified. Results shown were obtained with antisense primers. Corresponding results were obtained with sense primers (data not shown). Nucleotides coding for the mutant amino acid are boxed.
Maternal antibodies recognized GPIIb/IIIa mutated to contain the appropriate paternal mutation
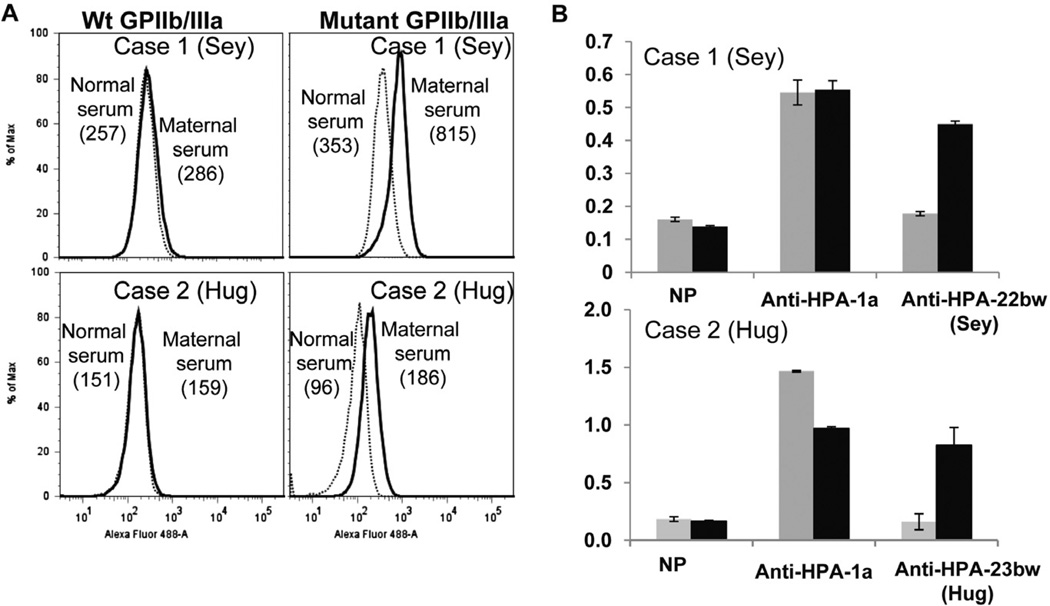
To confirm that the maternal antibodies were specific for the mutations identified in the two fathers, stable CHO cell lines expressing GPIIb/IIIa mutated to contain threonine 164 in GPIIb (Sey) and tryptophan 622 in GPIIIa (Hug) were generated and used as targets for maternal antibody. As shown in Fig. 2, each antibody recognized the appropriate paternal mutant, but not nonmutated GPIIb/IIIa using both flow cytometric and MACE assays for antibody detection.
Fig. 2.
Reactions of maternal sera with CHO cell lines stably expressing recombinant wild type (Wt) GPIIb/IIIa and recombinant mutated GPIIb/IIIa. (A) Reactions of Case 1 and Case 2 maternal sera with transfected CHO cell lines (flow cytometry). Serum from Case 1 (Sey), top, and Case 2 (Hug), bottom, recognized GPIIb/IIIa mutated to contain the amino acid change identified in paternal DNA (right) but not wild-type GPIIb/IIIa (left). Numbers shown are median fluorescence intensity values. (B) Reactions of maternal sera with transfected CHO cell lines (MACE).Maternal antibody from Case 1 (top) and Case 2 (bottom) precipitated GPIIb/IIIa containing the mutation identified in paternal DNA ( ) but not wild-type GPIIb/IIIa (
) but not wild-type GPIIb/IIIa ( ). Anti-HPA-1a recognized both constructs and normal serum (NP) recognized neither. Brackets denote mean of triplicate determinations ±1 SD.
). Anti-HPA-1a recognized both constructs and normal serum (NP) recognized neither. Brackets denote mean of triplicate determinations ±1 SD.
DISCUSSION
These observations demonstrate novel mutations predicting amino acid substitutions in GPIIb (K164T) and GPIIIa (R622W) in fathers of Cases 1 and 2, respectively, and in the affected infants. In each case, maternal antibodies were detected that reacted only with paternal PLTs and with recombinant GPIIb/IIIa mutated to contain the identified paternal mutation. Together, the findings indicate that maternal immunization against the newly identified antigens was the cause of severe NAIT experienced by each of the infants.
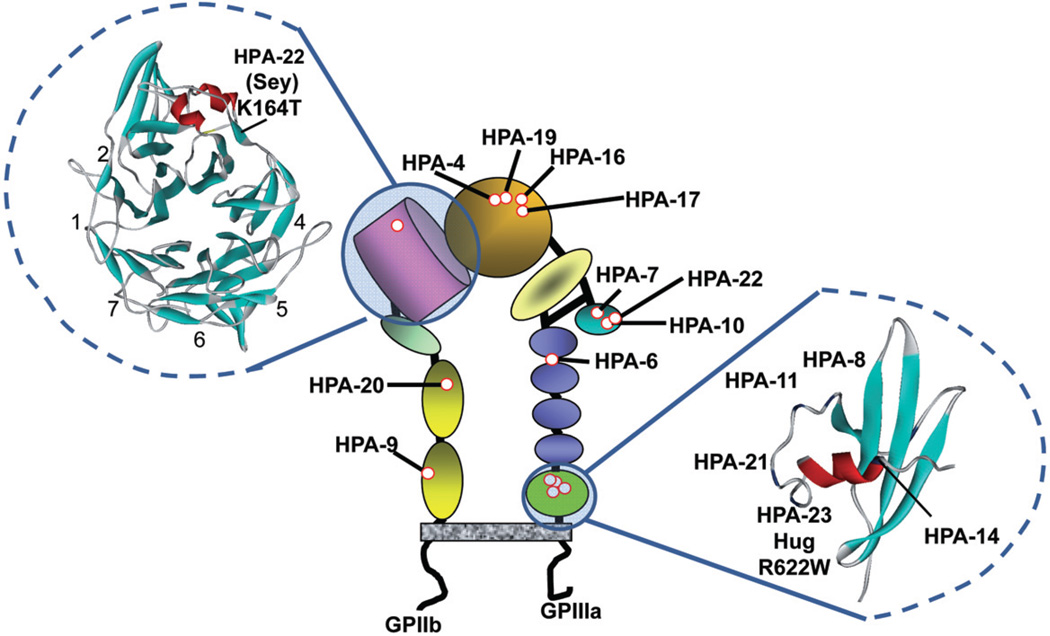
Location of the HPA-22bw (Sey) and HPA-23bw (Hug) antigens in the PLT GPIIb/IIIa complex is illustrated in Fig. 3. HPA-22bw is the first alloantigen to be localized to the β propeller domain of GPIIb; HPA-23bw is the fifth antigen identified in the GPIIIa cystatin domain, located immediately adjacent to the cell membrane. Locations of other recognized low-frequency antigens produced by amino acid polymorphisms on GPIIb/IIIa are also shown in Fig. 3. It is apparent that there is clustering of mutations in the βA and cystatin domains of GPIIIa. Recent structural studies show that, in its resting state, GPIIb/IIIa (αIIb/β3 integrin) is folded in such a way that the βA and cystatin domains are adjacent to each other,20 bringing all 9 of the sites where these mutations occur into close proximity. As would be expected, each of the sites is predicted to be solvent exposed.20 The HPA-22bw mutation in the GPIIb β propeller domain is very close to the RGD recognition site that is critical for binding of fibrinogen to activated GPIIb/IIIa.21–23 It is conceivable that interference of the HPA-22bw antibody with this function was in part responsible for the severe bleeding experienced by the infant although, in a heterozygote, half of the GPIIb/IIIa receptors would be unaffected by antibody and its father had no history of spontaneous bleeding.
Fig. 3.
Location of HPA-22bw (Sey), HPA-23bw (Hug), and other low-frequency HPA antigens in the GPIIb/IIIa complex. Ribbon diagrams of the β propeller domain of GPIIb (left) and the β3 cystatin domain (right) were generated using crystal coordinates reported by Zhu et al.20
Low-frequency PLT alloantigens shown to cause maternal immunization leading to NAIT include those now designated HPA-4b;24,25 HPA-6bw;26,27 HPA-7bw;28 HPA-8bw;29 HPA-9bw;30 HPA-10bw;31 HPA-11bw;32 HPA-12bw;33 HPA-13bw;34 HPA-14bw;35 HPA-16bw;36 HPA-17bw;37 HPA-18bw;38 HPA-19bw, -20bw, and -21bw;10 HPA-22bw;39 Swi;13 and Cab2.14 The two new antigens described here bring the total to 21. The most important of these antigens from a clinical standpoint appears to be HPA-9bw, found on PLT GPIIb of approximately 1 in 400 persons of Northern European ancestry.18,30,40 Fourteen cases of NAIT caused by HPA-9bw antibodies have now been reported, many of which were severe and five of which were complicated by intracranial hemorrhage.18,30,40 Other reports of NAIT caused by maternal immunization against low-frequency antigens involved single cases with the exception of HPA-4b,24,25 HPA-6bw,26,27,41 HPA-8bw,29,41 HPA-10bw,31,41 HPA-11bw,9,32 and HPA-13bw.9,34,42
Between 6511 and 80%41 of suspected NAIT cases are unresolved on the basis of maternal-fetal incompatibility for “common” antigens of the HPA-1, -2, -3, and -5 systems. The number of low-frequency PLT antigens that appears to be capable of triggering NAIT is steadily growing and it is important to define the extent to which maternal immunization against these markers contributes to the mix of NAIT cases. Ghevaert and coworkers41 typed about 1000 paternal DNA samples from fathers (mainly Caucasians) of suspected NAIT cases unresolved by standard testing for 11 low-frequency antigens. They found eight fathers who possessed the antigens HPA-6b, -10bw, -11bw, or -12bw and concluded that NAIT caused by sensitization against low-frequency HPA antigens is rare in Caucasian populations. However, typing for HPA-9bw, clearly an important trigger for NAIT, and other, more recently described antigens, was not performed. Recent studies indicate that two low-frequency antigens, HPA-4b and HPA-6bw, are much more prevalent in Asian populations than in Caucasians.43,44 Further studies are needed to determine whether other low-frequency antigens are relatively common in certain racial groups and may therefore be important causes of NAIT in those populations.
ACKNOWLEDGMENTS
JAP, BC, JM, and RHA each contributed to research design, oversight of laboratory studies, interpretation of results, and manuscript preparation. SP, MG, and AK performed laboratory studies, contributed to interpretation of findings made, and helped with manuscript preparation. JR and VK identified the affected patients, provided clinical details, and obtained blood and buccal swab samples.
This work was supported by Grant HL-13629 (RHA) from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS
- CHO
Chinese hamster ovary
- GP
glycoprotein
- MACE
modified antigen-capture enzyme-linked immunosorbent assay
- NAIT
neonatal alloimmune thrombocytopenia
- nt
nucleotide (when followed by a number)
Footnotes
CONFLICT OF INTEREST
The authors have no disclaimers to make or conflicts to disclose.
REFERENCES
- 1.Kaplan C. Neonatal alloimmune thrombocytopenia. Haematologica. 2008;93:805–807. doi: 10.3324/haematol.13160. [DOI] [PubMed] [Google Scholar]
- 2.Bussel JB, Primiani A. Fetal and neonatal alloimmune thrombocytopenia: progress and ongoing debates. Blood Rev. 2008;22:33–52. doi: 10.1016/j.blre.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Williamson LM, Hackett G, Rennie J, Palmer CR, Maciver C, Hadfield R, Hughes D, Jobson S, Ouwehand WH. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92:2280–2287. [PubMed] [Google Scholar]
- 4.Kjeldsen-Kragh J, Killie MK, Tomter G, Golebiowska E, Randen I, Hauge R, Aune B, Øian P, Dahl LB, Pirhonen J, Lindeman R, Husby H, Haugen G, Grønn M, Skogen B, Husebekk A. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopenia. Blood. 2007;110:833–839. doi: 10.1182/blood-2006-08-040121. [DOI] [PubMed] [Google Scholar]
- 5.Bussel JB, Sola-Visner M. Current approaches to the evaluation and management of the fetus and neonate with immune thrombocytopenia. Semin Perinatol. 2009;33:35–42. doi: 10.1053/j.semperi.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Newman PJ, Derbes RS, Aster RH. The human platelet alloantigens, PlA1 and PlA2, are associated with a leucine33/proline33 amino acid polymorphism in membrane glycoprotein IIIa, and are distinguishable by DNA typing. J Clin Invest. 1989;83:1778–1781. doi: 10.1172/JCI114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metcalfe P, Watkins NA, Ouwehand WH, Kaplan C, Newman P, Kekomaki R, De Haas M, Aster R, Shibata Y, Smith J, Kiefel V, Santoso S. Nomenclature of human platelet antigens. Vox Sang. 2003;85:240–245. doi: 10.1046/j.1423-0410.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan C. The role of the low-frequency antigens in neonatal alloimmune thrombocytopenia. ISBT Sci Ser. 2007;2:106–110. [Google Scholar]
- 9.Kroll H, Yates J, Santoso S. Immunization against a low-frequency human platelet alloantigen in fetal alloimmune thrombocytopenia is not a single event: characterization by the combined use of reference DNA and novel allele-specific cell lines expressing recombinant antigens. Transfusion. 2005;45:353–358. doi: 10.1111/j.1537-2995.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 10.Peterson JA, Gitter ML, Kanack A, Curtis B, McFarland J, Bougie D, Aster R. New low-frequency platelet glycoprotein polymorphisms associated with neonatal alloimmune thrombocytopenia. Transfusion. 2010;50:324–333. doi: 10.1111/j.1537-2995.2009.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davoren A, Curtis BR, Aster RH, McFarland JG. Human platelet antigen-specific alloantibodies implicated in 1162 cases of neonatal alloimmune thrombocytopenia. Transfusion. 2004;44:1220–1225. doi: 10.1111/j.1537-2995.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 12.Curtis BR, Edwards JT, Hessner MJ, Klein JP, Aster RH. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood. 2000;96:1574–1581. [PubMed] [Google Scholar]
- 13.Kroll H, Feldmann K, Zwingel C, Hoch J, Bald R, Bein G, Bayat B, Santoso S. A new platelet alloantigen, Swi(a), located on glycoprotein Ia identified in a family with fetal and neonatal alloimmune thrombocytopenia. Transfusion. 2011;51:1745–1754. doi: 10.1111/j.1537-2995.2010.03038.x. [DOI] [PubMed] [Google Scholar]
- 14.Jallu V, Dusseaux M, Kaplan C. A new Ser472Asn (Cab2[a+]) polymorphism localized within the alphaIIb “thigh” domain is involved in neonatal thrombocytopenia. Transfusion. 2011;51:393–400. doi: 10.1111/j.1537-2995.2010.02815.x. [DOI] [PubMed] [Google Scholar]
- 15.Roberts DG, Morrison TB, Liu-Cordero SN, Cho J, Garcia J, Kanigan TS, Munnelly K, Brenan CJ. A nanoliter fluidic platform for large-scale single nucleotide polymorphism genotyping. Biotechniques. 2009;46(Suppl):ix–xiii. doi: 10.2144/000112887. [DOI] [PubMed] [Google Scholar]
- 16.Brenan CJ, Roberts D, Hurley J. Nanoliter high-throughput PCR for DNA and RNA profiling. Methods Mol Biol. 2009;496:161–174. doi: 10.1007/978-1-59745-553-4_12. [DOI] [PubMed] [Google Scholar]
- 17.Hopp K, Weber K, Bellissimo D, Johnson ST, Pietz B. High-throughput red blood cell antigen genotyping using a nanofluidic real-time polymerase chain reaction platform. Transfusion. 2010;50:40–46. doi: 10.1111/j.1537-2995.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- 18.Peterson JA, Balthazor SM, Curtis BR, McFarland JG, Aster RH. Maternal alloimmunization against the rare platelet-specific antigen HPA-9b (Max a) is an important cause of neonatal alloimmune thrombocytopenia. Transfusion. 2005;45:1487–1495. doi: 10.1111/j.1537-2995.2005.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis BR, Fick A, Lochowicz AJ, McFarland JG, Ball RH, Peterson J, Aster RH. Neonatal alloimmune thrombocytopenia associated with maternal-fetal incompatibility for blood group B. Transfusion. 2008;48:358–364. doi: 10.1111/j.1537-2995.2007.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J, Negri A, Provasi D, Filizola M, Coller BS, Springer TA. Closed headpiece of integrin alphaIIbbeta3 and its complex with an alphaIIbbeta3-specific antagonist that does not induce opening. Blood. 2010;116:5050–5059. doi: 10.1182/blood-2010-04-281154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Artoni A, Li J, Mitchell B, Ruan J, Takagi J, Springer TA, French DL, Coller BS. Integrin beta3 regions controlling binding of murine mAb 7E3: implications for the mechanism of integrin alphaIIbbeta3 activation. Proc Natl Acad Sci U S A. 2004;101:13114–13120. doi: 10.1073/pnas.0404201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 24.Friedman JM, Aster RH. Neonatal alloimmune thrombocytopenic purpura and congenital porencephaly in two siblings associated with a “new” maternal antiplatelet antibody. Blood. 1985;65:1412–1415. [PubMed] [Google Scholar]
- 25.Shibata Y, Matsuda I, Miyaji T, Ichikawa Y. Yuka, a new platelet antigen involved in two cases of neonatal alloimmune thrombocytopenia. Vox Sang. 1986;50:177–180. doi: 10.1111/j.1423-0410.1986.tb04874.x. [DOI] [PubMed] [Google Scholar]
- 26.Kekomaki R, Jouhikainen T, Ollikainen J, Westman P, Laes M. A new platelet alloantigen, Tua, on glycoprotein IIIa associated with neonatal alloimmune thrombocytopenia in two families. Br J Haematol. 1993;83:306–310. doi: 10.1111/j.1365-2141.1993.tb08286.x. [DOI] [PubMed] [Google Scholar]
- 27.McFarland JG, Blanchette V, Collins J, Newman PJ, Wang R, Aster RH. Neonatal alloimmune thrombocytopenia due to a new platelet-specific alloantibody. Blood. 1993;81:3318–3323. [PubMed] [Google Scholar]
- 28.Kuijpers RW, Simsek S, Faber NM, Goldschmeding R, van Wermerkerken RK, dem Borne AE. Single point mutation in human glycoprotein IIIa is associated with a new platelet-specific alloantigen (Mo) involved in neonatal alloimmune thrombocytopenia. Blood. 1993;81:70–76. [PubMed] [Google Scholar]
- 29.Santoso S, Kalb R, Kroll H, Walka M, Kiefel V, Mueller-Eckhardt C, Newman PJ. A point mutation leads to an unpaired cysteine residue and a molecular weight polymorphism of a functional platelet beta 3 integrin subunit. The Sra alloantigen system of GPIIIa. J Biol Chem. 1994;269:8439–8444. [PubMed] [Google Scholar]
- 30.Noris P, Simsek S, Bruijne-Admiraal LG, Porcelijn L, Huiskes E, van der Vlist GJ, van Leeuwen EF, van der Schoot CE, von dem Borne AE. Max(a), a new low-frequency platelet-specific antigen localized on glycoprotein IIb, is associated with neonatal alloimmune thrombocytopenia. Blood. 1995;86:1019–1026. [PubMed] [Google Scholar]
- 31.Peyruchaud O, Bourre F, Morel-Kopp MC, Reviron D, Mercier P, Nurden A, Kaplan C. HPA-10w(b) (La[a]): genetic determination of a new platelet-specific alloantigen on glycoprotein IIIa and its expression in COS-7 cells. Blood. 1997;89:2422–2428. [PubMed] [Google Scholar]
- 32.Simsek S, Folman C, van der Schoot CE, dem Borne AE. The Arg633His substitution responsible for the private platelet antigen Gro(a) unravelled by SSCP analysis and direct sequencing. Br J Haematol. 1997;97:330–335. doi: 10.1046/j.1365-2141.1997.502696.x. [DOI] [PubMed] [Google Scholar]
- 33.Sachs UJ, Kiefel V, Bohringer M, Afshar-Kharghan V, Kroll H, Santoso S. Single amino acid substitution in human platelet glycoprotein Ibbeta is responsible for the formation of the platelet-specific alloantigen Iy(a) Blood. 2000;95:1849–1855. [PubMed] [Google Scholar]
- 34.Santoso S, Amrhein J, Hofmann HA, Sachs UJ, Walka MM, Kroll H, Kiefel V. A point mutation Thr(799)Met on the alpha(2) integrin leads to the formation of new human platelet alloantigen Sit(a) and affects collagen-induced aggregation. Blood. 1999;94:4103–4111. [PubMed] [Google Scholar]
- 35.Santoso S, Kiefel V, Richter IG, Sachs UJ, Rahman A, Carl B, Kroll H. A functional platelet fibrinogen receptor with a deletion in the cysteine-rich repeat region of the beta(3) integrin: the Oe(a) alloantigen in neonatal alloimmune thrombocytopenia. Blood. 2002;99:1205–1214. doi: 10.1182/blood.v99.4.1205. [DOI] [PubMed] [Google Scholar]
- 36.Jallu V, Meunier M, Brement M, Kaplan C. A new platelet polymorphism Duv(a+), localized within the RGD binding domain of glycoprotein IIIa, is associated with neonatal thrombocytopenia. Blood. 2002;99:4449–4456. doi: 10.1182/blood.v99.12.4449. [DOI] [PubMed] [Google Scholar]
- 37.Stafford P, Garner SF, Rankin A, Kekomaki R, Watkins NA, Ouwehand WH. A single-nucleotide polymorphism in the human ITGB3 gene is associated with the platelet-specific alloantigen Va (HPA-17bw) involved in fetal maternal alloimmune thrombocytopenia. Transfusion. 2008;48:1432–1438. doi: 10.1111/j.1537-2995.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 38.Bertrand G, Jallu V, Saillant D, Kervran D, Martageix C, Kaplan C. The new platelet alloantigen Cab a: a single point mutation Gln 716 His on the alpha 2 integrin. Transfusion. 2009;49:2076–2083. doi: 10.1111/j.1537-2995.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 39.Koh Y, Taniue A, Ishii H, Matsuyama N, Amakishi E, Hayashi T, Furuta RA, Fukumori Y, Hirayama F, Yoshimura K, Nagamine T, Tamai S, Nakano S. Neonatal alloimmune thrombocytopenia caused by an antibody specific for a newly identified allele of human platelet antigen-7. Transfusion. 2010;50:1276–1284. doi: 10.1111/j.1537-2995.2009.02557.x. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan C, Porcelijn L, Vanlieferinghen P, Julien E, Bianchi F, Martageix C, Bertrand G, Jallu V. Anti-HPA-9bw (Maxa) fetomaternal alloimmunization, a clinically severe neonatal thrombocytopenia: difficulties in diagnosis and therapy and report on eight families. Transfusion. 2005;45:1799–1803. doi: 10.1111/j.1537-2995.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 41.Ghevaert C, Rankin A, Huiskes E, Porcelijn L, Javela K, Kekomaki R, Bakchoul T, Santoso S, Nutland S, Smyth DJ, Smith GA, McBride S, Watkins NA, Ouwehand WH. Alloantibodies against low-frequency human platelet antigens do not account for a significant proportion of cases of fetomaternal alloimmune thrombocytopenia: evidence from 1054 cases. Transfusion. 2009;49:2084–2089. doi: 10.1111/j.1537-2995.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- 42.Bertrand G, Bianchi F, Alexandre M, Quesne J, Chenet C, Martageix C, Jallu V, Kaplan C. HPA-13bw neonatal alloimmune thrombocytopenia and low frequency alloantigens: case report and review of the literature. Transfusion. 2007;47:1510–1513. doi: 10.1111/j.1537-2995.2007.01291.x. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka S, Ohnoki S, Shibata H, Okubo Y, Yamaguchi H, Shibata Y. Gene frequencies of human platelet antigens on glycoprotein IIIa in Japanese. Transfusion. 1996;36:813–817. doi: 10.1046/j.1537-2995.1996.36996420760.x. [DOI] [PubMed] [Google Scholar]
- 44.Ohto H, Miura S, Ariga H, Ishii T, Fujimori K, Morita S. The natural history of maternal immunization against foetal platelet alloantigens. Transfus Med. 2004;14:399–408. doi: 10.1111/j.1365-3148.2004.00535.x. [DOI] [PubMed] [Google Scholar]