Summary
Neonatal alloimmune thrombocytopenia (NAIT) is caused by fetomaternal platelet incompatibility with maternal antibodies crossing the placenta and destroying fetal platelets. Antibodies against human platelet antigen-1a (HPA-1a) and HPA-5b are responsible for the majority of NAIT cases. We observed a suspected NAIT in a newborn with a platelet count of 25 G/l and petechial haemorrhages. Serological analysis of maternal serum revealed an immunisation against αIIbβ3 on paternal platelets only, indicating the presence of an antibody against a new rare alloantigen (Seca) residing on αIIbβ3. The location of Seca on αIIbβ3 was confirmed by immunoprecipitation. Nucleotide sequence analysis of paternal β3 revealed a single nucleotide exchange (G1818T) in exon 11 of the β3 gene (ITGB3), changing Lys580 (wild-type) to Asn580 (Seca). Two additional members of the family Sec were typed Seca positive, but none of 300 blood donors. Chinese hamster ovary cells expressing Asn580, but not Lys580 αIIbβ3, bound anti-Seca, which was corroborated by immunoprecipitation. Adhesion of transfected cells onto immobilised fibrinogen showed reduced binding of the Asn580 variant compared to wild-type αIIbβ3. Analysis of transfected cells with anti-LIBS and PAC-1 antibody showed reduced binding when compared to the wild-type. No such effects were observed with Seca positive platelets, which, however, are heterozygous for the Lys580Asn mutation. In this study, we describe a NAIT case caused by maternal alloimmunisation against a new antigen on αIIbβ3. Analysis with mutant transfected cells showed that the Lys580Asn mutation responsible for the formation of the Seca antigenic determinant affects αIIbβ3 receptor function.
Keywords: NAIT, HPA, thrombocytopenia, GP IIb/IIIa
Introduction
Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is a severe bleeding disorder of the fetus and the newborn which is caused by destruction of platelets by maternal alloantibodies during the pregnancy and after birth. The alloantibodies are directed against fetal platelet-specific antigens which are inherited from the father. The most common human platelet antigens (HPAs) responsible for the maternal immunisation in the Caucasian populations are HPA-1 (~70%) and HPA-5 (~20%) (1). An increasing number of private or rare HPAs associated with FNAIT have been reported during the last two decades. Meanwhile, 15 low-frequency HPAs (HPA-6bw to –14bw and HPA-16bw to –21bw) have been assigned. Most of them reside on the αIIbβ3 integrin (n = 12), one (HPA-12bw) resides on glycoprotein (GP) GPIbα and two (HPA-13bw, –18bw) on the α2β1 integrin (for further information see http://www.ebi.ac.uk/ipd/hpa/). The αIIbβ3 integrin is the major integral platelet glycoprotein which functions as a receptor for divalent fibrinogen, multivalent von Willebrand factor and other ligands such as vitronectin, fibronectin and thrombospondin. Ligand binding to αIIbβ3 integrin is controlled by inside-out signals that modulate receptor conformation and clustering. In turn, ligand binding triggers outside-in signals through αIIbβ3 (2). Crystal structure analysis revealed a complex domain structure that rearranges when the integrin switches from a resting to an active form (3). The αIIb subunit consists of an amino-terminal β-propeller domain followed by a thigh domain and two calf domains. The β3 subunit has eight domains: an amino-terminal PSI domain, an Ig-like hybrid domain that contains the ligand-binding αA-hybrid domain, four EGF-like domains, and the β-tail domain. By the identification of these domains, point mutations responsible for HPAs could be localised precisely. No preferential domain was observed for HPAs, and all HPA- related polymorphisms on GP IIb/IIIa described so far did not impair the receptor function.
In this study, we describe a case of FNAIT caused by maternal alloimmunisation against a previously unreported, low frequency polymorphism (Lys580Asn) on the β3 integrin subunit, termed Seca. This mutation is located within the EGF4 domain and alters the adhesion of αIIbβ3 to fibrinogen. Thus, the Seca alloantigen represents the first low-frequency polymorphism on β3 integrin which influences the receptor’s function.
Materials and methods
Case report
A 35-year-old female (Sec) with a history of miscarriages (Gravida III/Para 0) at gestational weeks 10 and 21, respectively, received dalteparin during her third pregnancy. She delivered a full-term boy in the 39th week of gestation with facial petechiae and cephalic haematoma, but no intracranial bleeding. Neonatal platelet count was 25 G/l. An initial therapy with intravenous immunoglobulins (1 g/kg bodyweight) resulted in a rapid increase of the platelet count (160 G/l), and the newborn was discharged without any signs of sequelae. While antibody testing in MAIPA using random donor platelets revealed negative results, a cross-match analysis between maternal serum and paternal platelets in a glycoprotein-specific assay showed positive reactions with αIIbβ3, indicating an alloimmunisation against a new low-frequency antigen residing on the αIIbβ3 heterodimer.
Antibodies
Alloantibodies against HPA-1a were obtained from a mother who gave birth to a child with NAIT (4). Control serum was obtained from a healthy male blood donor. Monoclonal antibodies (mab) Gi5, Gi9 against αIIbβ3 and α2β1, respectively, were produced and characterised in our laboratory (5). Mab FMC25 against GPIb/IX complex was purchased from AbD Serotec (Oxford, UK). The mab D3 against ligand-induced binding site (LIBS) on β3 was kindly provided by Dr. Lisa Jennings (Memphis, TN, USA). Mab PAC-1 against activated αIIbβ3 heterodimer was purchased from Becton Dickinson (Heidelberg, Germany).
Characterisation of platelets alloantibodies by antigen capture assay
Platelets from the father and known HPA phenotyped healthy blood donors were isolated from EDTA-anticoagulated blood by differential centrifugation and stored at 4°C in isotonic saline containing 0.1% NaN3. Antibody detection was performed using antigen capture assay, MAIPA (monoclonal antibody-specific immobilisation of platelet antigens) and a panel of mabs (see above), as previously described (6).
Immunoprecipitation
Platelets and Chinese hamster ovary (CHO) stably transfected cells (see below) were surface labelled with 5 mM NHS-LC-Biotin (Pierce, Rockford, IL, USA) and precipitated as previously described (7). Labelled cell lysates (100–300 µl) were incubated with 50 µl serum or mab (20 µg/ml) overnight at 4°C in the presence of 100 µl protein G beads (Pierce). After washings with immunoprecipitation buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100), bound proteins were eluted by adding SDS buffer for 5 minutes (min) at 100°C. Eluates were analysed on 7.5% SDS-PAGE under reducing conditions. Separated proteins were transferred onto nitrocellulose membranes and developed with peroxidase-labeled streptavidin and a chemiluminescence system (ECL, Amersham Biosciences, Freiburg, Germany).
Nucleotide sequencing analysis
Full-length sequencing of αIIb and β3 was carried out as described previously (8). Briefly, αIIb and β3 coding regions of paternal genomic DNA were PCR amplified with primers corresponding to intronic sequence surrounding all exons of αIIb and β3. PCR was carried out using a Fast-Start High Fidelity PCR system (Roche Diagnostic Corp., Indianapolis, IN, USA). Prior to sequence analysis, PCR products (ranged from 500–1200 bp) were purified with a QIAquick PCR purification kit (Qiagen Sciences, Valencia, CA, USA). Automated sequence analysis was performed in both directions on a genetic analyzer (ABI 3100, Applied Biosystems, Foster City, CA, USA) as described (9). Nucleotide sequences of PCR primers, sequencing, and reaction conditions are available upon request.
Genotyping by TaqMan
Genomic DNA was extracted from peripheral blood leukocytes derived from 5 ml EDTA anticoagulated by the use of DNeasy Blood & Tissue Kit (Qiagen, Duesseldorf, Germany). One hundred nanograms of genomic DNA were amplified using forward primer (nt 52382.547–565) and reverse primer (nt 52383.067–047), 2.5 µl dNTP (1.25 mM each nucleotide) and 1.25 U Taq Gold polymerase (Perkin Elmer, Waltham, MA, USA) in the presence of a VIC-probe (5’-CGGCAAGTGTGAATG-3’) and a FAM-probe (5’-CGGCAATTGTGAATG-3’) in a total volume of 15 µl. Thirty-two cycles of denaturation at 95°C for 1 min, annealing at 58°C for 1 min, and extension at 72°C for 1 min were performed. Prior to PCR cycle samples were denatured at 95°C for 5 min.
Construction of β3 allelic expression vector
A full-length β3 cDNA in the mammalian vector pMPSV encoding for β3 Glu580 isoform (Seca) was produced by site-directed mutagenesis using Quick Change Mutagenesis Kit (Strategene, Heidelberg, Germany) as previously described (7). For PCR amplification, site directed mutagenesis primers encompassing nucleotides 1795–1831 of β3 cDNA were constructed. After denaturation for 30 seconds (sec) at 95°C, plasmid (20 ng) was amplified for 12 cycles (denaturation at 95°C for 30 sec, annealing at 55°C for 1 min, and extension at 65°C for 12 min). PCR products were digested with Dpn endonuclease for 1 hour (h) at 37°C and transformed into DH5α high efficiency competent E. coli bacteria (Invitrogen, Carlsbad, CA, USA). Plasmid DNA from positive clones was verified by nucleotide sequencing as described above.
Stable transfection of Seca alloantigen in CHO cells
CHO (American Type Tissue Collection, Rockville, MD, USA) cells were grown and were transfected with allele specific β3 constructs encoding for Lys580 or Asn580 isoform together with αIIb construct as previously described (7). Stably expressing cells were selected with Genicitin (G418; final concentration 1 mg/ml; GIBCO BRL) and were subcloned by limited dilution method. Four clones were isolated and were analysed for αIIbβ3 surface expression in flow cytometry.
Flow cytometric analysis of stably transfected CHO cells
The expression of recombinant αIIbβ3 complex on the cell surface of transfected cells was measured by flow cytometry (FACSCalibur, Becton Dickinson) as previously described (7). Cells were incubated with mab Gi5 specific for αIIbβ3 complex and then labelled with fluorescein isothiocyanate (FITC)-conjugated secondary antibody. For the analysis of LIBS, 180 µl of cell suspension (~106 cells) in phosphate-buffered saline (PBS) supplemented with 2% bovine serum albumin (BSA) were incubated with 20 µl RGDW or RGEW peptide (10 mM) for 7 min at room temperature prior to incubation with 20 µl mab D3 (20 µg/ml). After washings cells were incubated with FITC-labelled anti-mouse IgG (dilution 1:50), washed and measured as described above. PAC-1 binding was analysed as described (7). Aliquots of 200 µl cell suspension (~106 cells) in Tyrode’s buffer (TB; 137 mM NaCl, 2.8 mM KCl, 12 mM NaHCO3, 10 mM Hepes, pH 7.4) were treated with 10 mM dithiothreitol (DTT) or buffer for 20 min at room temperature. After washings, 100 µl of cell suspension were stained with 20 µl of FITC-conjugated mab PAC-1 (1 µg/ml) in the presence of 10 mM MgCl2 and 1 mM CaCl2 for 30 min at room temperature. Cells were washed and resuspended in 500 µl TB containing MgCl2 and CaCl2 for FACS analysis.
Platelet adhesion assay
Resting platelets were isolated from ACD anticoagulated blood and washed with TB. An aliquot of 1 ml platelet suspension (106 cells) was labelled with 2.5 µM Calcein (Invitrogen) for 15 min at room temperature in the presence of 10 µl PGE-1 (3.5 µg/ml; Sigma, Dreieich, Germany). Labelled platelets were washed twice and were adjusted to a concentration of 2 × 108/ml with TB. For adhesion, microtitre wells were coated overnight with different fibrinogen concentrations (50 µg/ml; Sigma), BSA (10 mg/ml; Sigma) or mab Gi5 (10 µg/ml), washed three times with 200 µl PBS and blocked with 200 µl 1% BSA in PBS for 1 h at 37°C. Aliquots of 100 µl labelled platelets were added in triplicate to wells coated either with BSA, fibrinogen or mab Gi5, and were permitted to adhere at 37°C for 30 min. Non-adherent cells were removed by gently aspiration and by washing of the wells two times. Bound cells were measured on a fluorescence microplate reader (Flx-800; Biotek, Neufahrn, Germany).
Adhesion of stably transfected CHO cells onto fibrinogen
One ml of transfected CHO cells (5 × 106 cells) in α-MEM medium (PAA, Marburg, Germany) were labelled with 35 µl BCEFC (2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein; Molecular Probes; Invitrogen) for 30 min at 37°C. Labelled cells were then washed twice with 5 ml α-MEM medium and adjusted to a concentration of 1 × 106/ml. Aliquots of 300 µl cell suspensions were untreated or treated with 150 µl AB serum, 150 µl anti-Seca, or 30 µl mab Gi5 (30 µg) for 30 min at 37°C. For adhesion test, microtiter wells were coated overnight with different fibrinogen concentration (50 µg/ml; Sigma), BSA (10 mg/ml; Sigma) or mab Gi5 (10 µg/ml), washed three times with 200 µl PBS and blocked with 200 µl 1% BSA in PBS for 1 h at 37°C. The adhesion test was performed as described above.
Results
Serological and immunochemical analysis
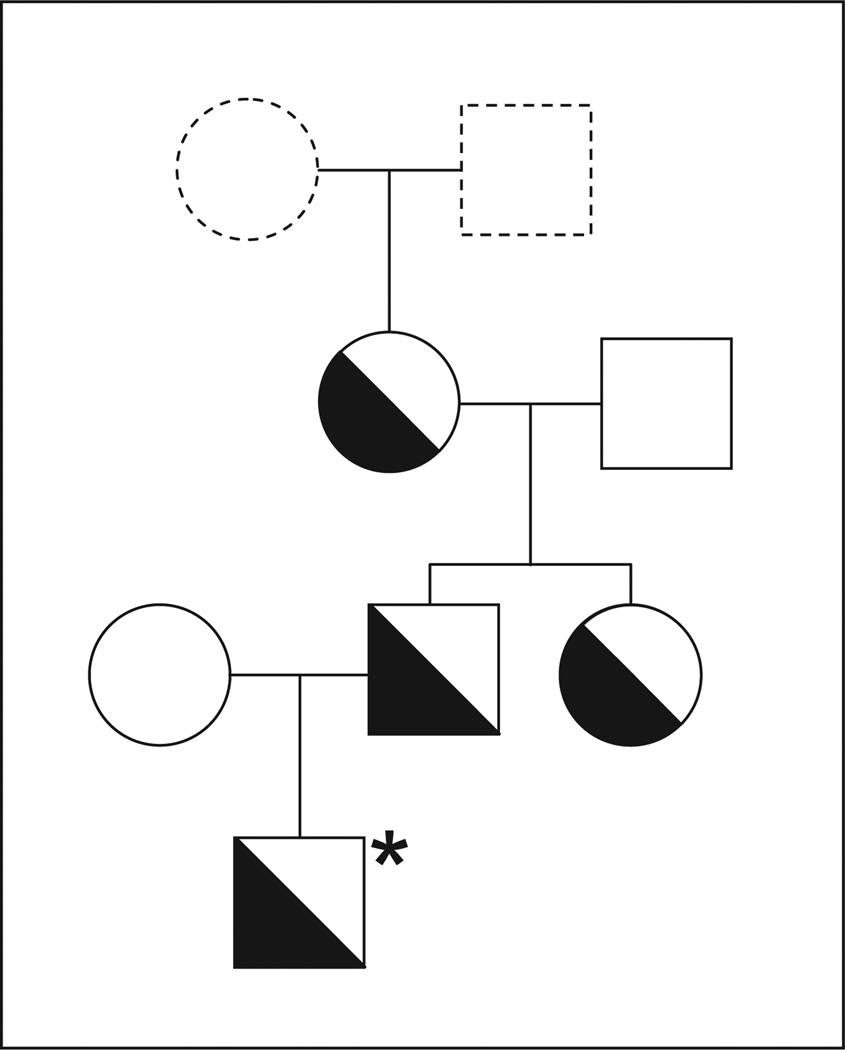
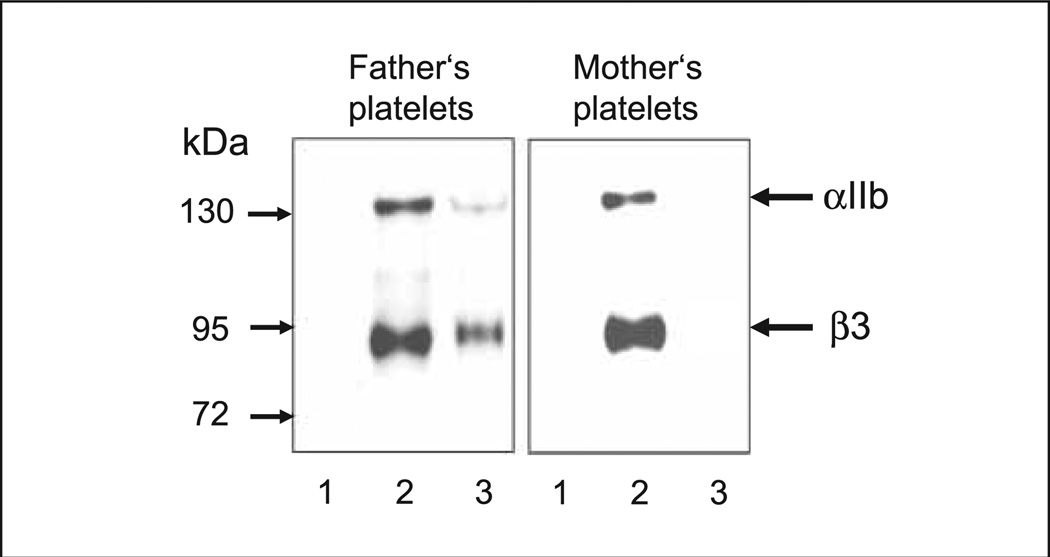
The crossmatch analysis between maternal serum and paternal platelets in the MAIPA assay showed strong reactions when mab against αIIbβ3 integrin was used as a capture antibody, but not with mabs against GPIb/IX, α2β1 (GPIa/IIa) or CD109. When maternal serum was tested with a panel of HPA phenotyped platelets (12 different platelet suspensions), no reaction was observed (data not shown). Extended genotyping of αIIb and β3 polymorphism ruled out the presence of rare HPAs on paternal platelets. These results indicated that the maternal serum contained an alloantibody against a new low-frequency platelet alloantigen on αIIbβ3 heterodimer, which we termed Seca. Within the family of the father, two further Seca-positive individuals were identified by the MAIPA assay (Fig. 1). When immunoprecipitation analysis of biotin-labelled platelets was performed with paternal platelets, maternal serum precipitated the αIIbβ3 complex (Fig. 2). In the control experiment, anti-Seca antibody failed to precipitate αIIbβ3 from maternal platelets.
Figure 1. The pedigree of the index family Seca.
Open symbols represent Seca (−), half-solid symbol represent Seca (+) individuals. All individuals are Caucasian. * indicates the affected child.
Figure 2. Immunoprecipitation analysis of anti-Seca.
Paternal and maternal platelets were surface labelled with biotin and lysed. Labelled platelet lysates were precipitated with control serum (lanes 1), anti-HPA-1a antibodies (lanes 2) and maternal serum (lanes 3). Immunoprecipitates were run on 7.5% SDS-PAGE under non-reducing conditions, transferred onto nitrocellululose membrane and visualised by streptavidin-chemiluminescence system.
Genetic analysis
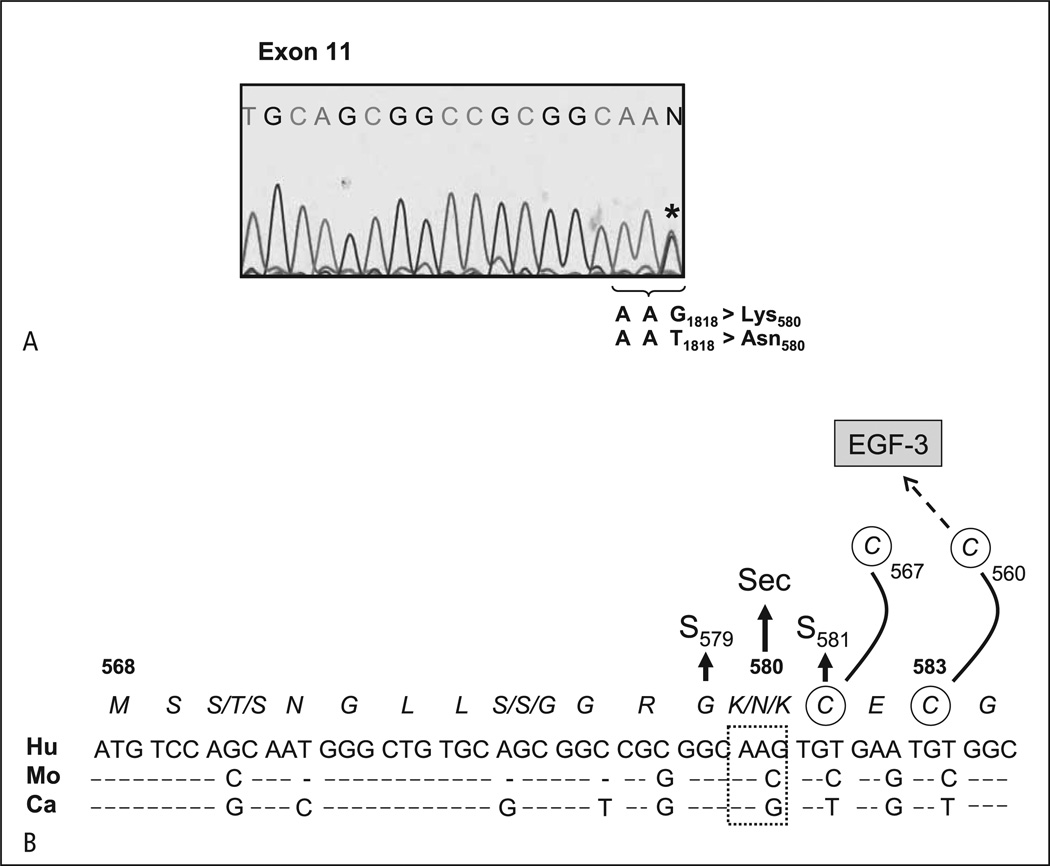
To ascertain the molecular genetic basis underlying the Seca antigen, paternal genomic DNA corresponding to the coding regions of αIIb and β3 was sequentially amplified by PCR using 28 sets of primers. Nucleotide sequencing of β3 gene encompassing nucleotides 1 to 2367 (nucleotide 1 refers to A of the ATG translation start codon on human β3) showed one nucleotide substitution G>T at nt 1818 located on exon 11 of the β3 gene (Fig. 3A). This mutation predicted the amino acid Lys (AAG) at position 580 in Seca-negative and Asn (AAT) in Seca-positive individuals. This result was confirmed by nucleotide sequencing analysis of the child (Fig. 3A) and other Seca-positive family members (data not shown). Alignment analysis between human, mouse and canine genes showed that this mutation occurred in the EGF4 conserved region of β3, which is adjacent to the Cys residue at position 581 (Fig. 3B).
Figure 3. Nucleotide sequencing analysis of amplified β3 DNA.
A) Genomic DNA derived from paternal platelets was amplified by PCR. The analysis of PCR product encompassing nucleotides 1801 – 1818 is presented. The wild-type β3 isoform Asn580 (AAT) and the mutated β3 isoform Lys580 (AAG) are indicated (*). B) Nucleotide sequence alignment of β3 integrin (residues 568 – 584) from human (Hu), monkey (Mo) and canine (Ca) genes. The position associated with the Seca phenotype is indicated. Two adjacent point mutations, S579 and S581, associated with functional defects of the αIIbβ3 receptor are shown.
To study the frequency of Seca, genotyping based on TaqMan approach was established. Among 300 unrelated Caucasian blood donors, no Seca-positive individual was identified.
Expression study on mammalian cells
Allele specific constructs encoding wild-type β3 (Lys580) or mutant β3 (Asn580) were transfected into CHO cells together with αIIb construct to prove the impact of Lys580Asn mutation on the formation of Seca alloantigen. As shown in Figure 4A, transfected cells expressing wild-type β3 did not show any reaction with anti-Sec alloantibody in flow cytometry. In contrast, anti-Seca recognised the mutant β3. These results could be confirmed by immunoprecipitation analysis (Fig. 4B).
Figure 4. Analysis of CHO cells transfected with β3 allelic constructs and αIIb expression vector.
A) Flow cytometry analysis of stably transfected cells expressing wild-type and mutant β3 isoforms with normal serum (Ctrl), mab Gi5 against αIIbβ3, and anti-Seca antibody. The expression of αIIbβ3 was comparable between the two cell lines (mean fluorescence intensity (MFI; mean ±SD) for Asn580 = 557 ±89 and MFI for Lys580 = 702 ±74). Bound antibody was detected with fluorescein-labelled secondary antibodies. B) Immunoprecipitation analysis of stably transfected cells expressing wild-type (lanes 1) and two different clones (clone 2 and clone 13) expressing mutant β3 isoform (lanes 2 and 3) with normal serum (Ctrl), anti-HPA-1a antibody and anti-Seca antibody as indicated.
Effect of the Lys580Asn on cell function
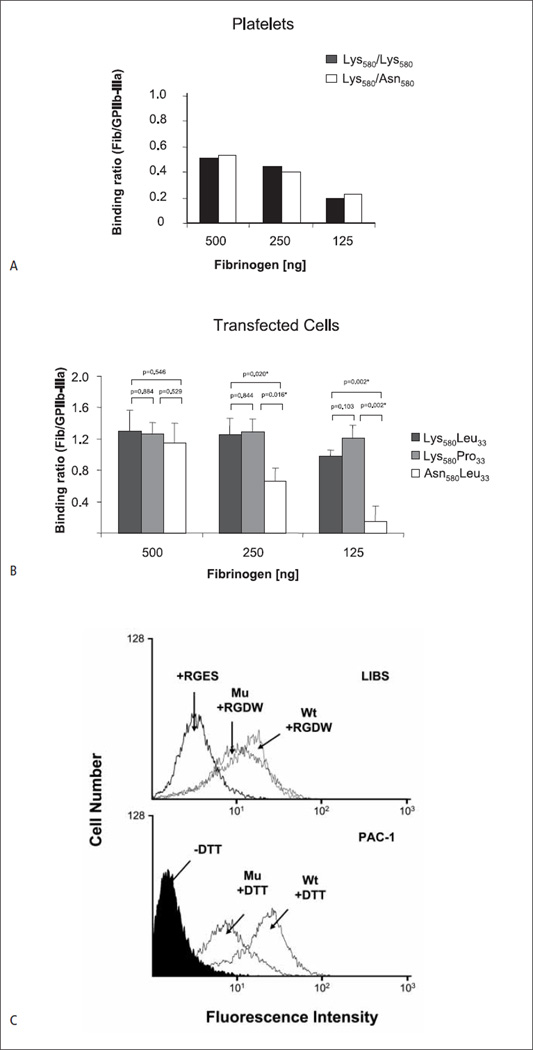
To determine possible effects of the Lys580Asn substitution on platelet function, aggregation studies with Seca-phenotyped individuals were performed. Anti-Seca alloantibody was not able to inhibit platelet aggregation induced by ADP (5–25 µM), and the platelet aggregation response of Seca-positive and -negative individuals was not different (data not shown). Unfortunately, all Seca positive platelets identified so far are heterozygous, expressing both variants (Lys580 and Asn508) of β3 integrin on their cell surface. Comparing the adhesion of platelets expressing homozygous Lys580/Lys580 (wild type) and heterozygous Lys580/Asn580 β3 integrin onto immobilised fibrinogen did not reveal a significant difference (Fig. 5A). To exclude a possible impact of HPA-1 on platelet adhesion, homozygous (HPA-1aa) platelets were selected for these experiments.
Figure 5. Effects of the Lys580Asn substitution on αIIbβ3 function.
A) Adhesion of homozygous Seca-negative (Lys580/Lys580) and heterozygous Seca-positive (Lys580/Asn580) platelets onto different concentrations of immobilised fibrinogen. Both platelets are typed as homozygous for HPA-1a (Leu33/Leu33). Fluorescence labelled platelets was allowed to adhere to microtitre wells coated either with BSA, mab Gi5 or fibrinogen. After washings, bound cells were measured on a fluorescence microtiter reader. Binding was calculated as ratio between the cells bound to fibrinogen and to mab Gi5 (n = 2). B) Adhesion of CHO cells expressing Leu33Lys580 (wild-type; HPA-1a), Pro33Lys580 (mutant; HPA-1b) and Leu33Asn580 (Seca) β3 isoforms on immobilised fibrinogen. Cell adhesion was performed as described above (n=5). C) Flow cytometry analysis of stably transfected cells expressing wild-type and mutant β3. The expression of αIIbβ3 was comparable between the two cell lines (mean fluorescence intensity (MFI; mean ±SD) for Asn580 = 557±89 and MFI for Lys580 = 702 ±74). Upper panel shows the binding of LIBS antibody after treatment with RGDW or RGEW peptide (as control); lower panel the binding of PAC-1 antibody with and without activation by DTT. Bound antibody was detected with fluorescein-labelled secondary antibody.
When transfected cells expressing the αIIbβ3 Asn580 isoform (homozygous) were tested in the adhesion assay, reduced adhesion capacity was observed in comparison to wild-type cells, either Leu33Lys580 (HPA-1a) or Pro33Lys580 (HPA-1b) (Fig. 5B). This phenomenon, however, depends on the concentration of immobilised fibrinogen; no significant difference was observed when high fibrinogen concentrations (> 500 ng) were applied. Increased binding of HPA-1b transfected cells was observed in comparison to HPA-1a cells at the low fibrinogen concentration (<125 ng) (Fig. 5B). This difference, however, was not statistically significant.
To examine whether the β3 Asn580 isoform can undergo conformational changes for ligand binding, we compared the binding of anti-LIBS to both mutant and wild-type cells in the presence of RGDW or RGES peptide (as control). Decreased binding of anti-LIBS was observed with the mutant (Asn580) isoform (Fig. 5C). Furthermore, analysis of the function of the ligand binding domain with the ligand mimetic mab PAC-1 to DTT-activated cells showed a significantly decreased binding of PAC-1 antibody to mutant in comparison to wild-type cells. These results indicated that the Lys580Asn mutation affects αIIbβ3 receptor-ligand binding.
Discussion
In this study, we report on a new rare alloantigen, Seca, located on platelet β3, which was involved in a case of FNAIT. In a population study, none of 300 unrelated donors was found to carry the Seca alloantigen. Examination of the nucleotide sequence of the β3 gene derived from the Seca-positive father showed one nucleotide substitution G>T at position 1818 in heterozygous state located in exon 11. This mutation predicted the amino acid Lys (AAG) at position 580 in Seca-negative and Asn (AAT) in Seca-positive individuals. Analysis of recombinant allele-specific αIIbβ3 in mammalian cells showed that the single amino acid substitution Lys580Asn is directly responsible for the formation of Seca alloantigenic determinant(s). Functional studies of paternal platelets expressing the Seca alloantigen in heterozygous state showed no influence of the Lys580Asn dimorphism on platelet function. Interestingly, the adhesion onto immobilised fibrinogen of transfected cells expressing the Seca alloantigen in a homozygous state is reduced when compared with the wild-type cells. Further analysis showed that Lys580Asn substitution affects ligand as well as post-ligand events of αIIbβ3 receptor in these cells.
The Lys580Asn mutation occurred in the EGF4 conserved region of β3, which is adjacent to the Cys residues at position 581 (see Fig. 3B). Interestingly, αIIbβ3 integrin is bent under resting conditions, with the 3rd and the 4th EGF-like β3 domains inserted into a crevice formed by the upper β3 leg on one side, and the αIIb leg on the other side (10). Kamata et al. reported that clustering of disulphide bonds in EGF domains is important for the regulation of αIIbβ3 integrin function (11). They found that disruption of a single disulphide bond in the EGF domains was enough to activate αIIbβ3 fully. These results indicate that intact disulphide bonds in the EGF domains are important for the preservation the αIIbβ3 resting state. Recently, Mor-Cohen et al. demonstrated that disruption of the Cys567-Cys581 disulphide bond in the 4th EGF-like domain sustained the inactive state of αIIbβ3 integrin, even after exposure to αIIbβ3 activating antibodies, indicating that this disulphide bond is important for integrin activation (12). We speculate that the Lys580Asn mutation responsible for the formation of Seca epitopes impairs the adjacent Cys567-Cys581 and/or Cys583-Cys560 disulphide bonds, altering the activation capability of αIIbβ3 integrin; a phenomenon, which we observed with our transfected cells expressing the β3 Asn580 isoform.
The role of one polymorphism residing on β3, Leu33Pro (HPA-1a and HPA-1b), has been studied intensively as genetic risk factor for arterial thrombosis in the last decade. Contradictory results were observed (13). Vijayan et al. showed that CHO and human kidney embryonal (HEK) 293 cells overexpressing the HPA-1b (Pro33 isoform) bound significantly more in comparison to HPA-1a (Leu33 isoform) transfected cells (14). In our study, however, no difference in the adhesion capacity onto fibrinogen was observed between HPA-1a and HPA-1b transfected cells, although both cells had comparable αIIbβ3 surface expression (data not shown). Recent data indicated that glutathione may regulate αIIbβ3-mediated cell adhesion under flow conditions (15); enhanced adhesion of HPA-1b transfected cells was only observed in the absence of reduced and oxidised glutathione (GSH or GSSH). This observation indicates that not only the HPA-1b phenotype, but also the redox state of platelet αIIbβ3 may play a role in the regulation of this important platelet fibrinogen receptor (16). The direct effect of the Leu33Pro dimorphismus on the behaviour of the integrin disulphide bonds, however, is currently not known.
Point mutations and deletions responsible for the formation of HPAs on β3 subunit were found in different domains of the molecule; HPA-1 in the PSI-domain; HPA-4, –16, –17, –19 and Mat in the βA-domain; HPA-7, –10 and Hit in the hybrid domain, and HPA-8, –11, –14 and –2 in the β-tail domain (see Table 1). Several functional studies have been performed for most of these HPAs, but none of these mutations appears to alter αIIbβ3 function.
Table 1. Synopsis of human platelet alloantigens (HPAs) located on β3 integrin.
Nucleotide/amino acid numbers, nucleotide/amino acid exchanges are given according to the guidelines of nomenclature of human platelet antigens from the International Society of Blood Transfusion (ISBT) and the International Society on Thrombosis and Haemostasis (ISTH) (17). n.a. = not assigned.
| HPA | Synonym | Nucleotide | Amino acid | Domain | Reference |
|---|---|---|---|---|---|
| 1a 1b |
Zwa, PlA1 Zwb, PlA2 |
T176 C176 |
Leu33 Pro33 |
PSI | 17 |
| 4a 4b |
Yukb, Pena Yuka, Penb |
G506 A506 |
Arg143 Gln143 |
βA | 17 |
| 6bW | Caa, Tua | 1544G>A | Arg489Gln | EGF1–2 | 17 |
| 7bW | Moa | 1297C>G | Pro407Ala | Hybrid | 17 |
| 8bW | Sra | 1984C>T | Arg636Cys | β–TD | 17 |
| 10bW | Laa | 263G>A | Arg62Gln | Hybrid | 17 |
| 11bW | Groa | 1976G>A | Arg633His | β–TD | 17 |
| 14bW | Oea | 1909–1911 del AAG | Lys611del | β–TD | 17 |
| 16bW | Duva | 497C>T | Thr140Ile | βA | 17 |
| 17bW | Vaa | 662C>T | Thr195Met | βA | 18 |
| 19bW | Staa | 487A>C | Lys137Gln | βA | 8 |
| 21bW | Nosa | 1960G>A | Glu628Lys | β−TD | 8 |
| n.a. | Hita | 1297C>T | Pro407Ser | Hybrid | 19 |
| n.a. | Mata | 520A>G | Gln141Arg | βA | 20 |
| n.a. | Seca | 1818G>T | Lys580Asn | EGF4 | this study |
In this study, we identified a polymorphism, Lys480Asn, located on the fourth EGF4 domain of β3, which is responsible for the formation of a new rare platelet alloantigen Seca, and which affects αIIbβ3 function.
What is known about this topic?
Fetal/neonatal alloimmune thrombocytopenia is a severe bleeding disorder of the fetus and newborn.
Rare mutations of glycoprotein (GP) IIb/IIIa can induce the alloantigen against which the mother becomes immunised during pregnancy.
What does this paper add?
A new rare human platelet alloantigen (HPA) on GP IIb/IIIa, Seca, (Lys580Asn) is described.
This alloantigen is the first HPA to affect ligand and post-ligand events of GP IIb/IIIa.
Acknowledgements
Our gratitude is extended to the Sec family for their cooperation in this study. This work was supported by research grants from the University Medical Center Giessen and Marburg (UKGM; to U.J.S. and T.B.), by a research grant from the National Heart, Lung and Blood Institute (HL-13629; to R.H.A.), and the Deutsche Forschungsgemeinschaft (Excellence Cluster Cardiopulmonary System; to S.S.).
Footnotes
Conflict of interest
None declared.
References
- 1.Mueller-Eckhart C, Kiefel V, Grubert A, et al. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet. 1989;1:363–366. doi: 10.1016/s0140-6736(89)91733-9. [DOI] [PubMed] [Google Scholar]
- 2.Bennett JS. Structure and function of the platelet integrin alphaIIbbeta3. J Clin Invest. 2005;115:3363–3369. doi: 10.1172/JCI26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 4.Bakchoul T, Kubiak S, Krautwurst A, et al. Low-avidity anti-HPA-1a alloantibodies are capable of antigen-positive platelet destruction in the NOD/SCID mouse model of alloimmune thrombocytopenia. Transfusion. 2011;51:2455–2461. doi: 10.1111/j.1537-2995.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- 5.Santoso S, Kalb R, Walka M, et al. The human platelet alloantigens Bra and Brb are associated with a single amino acid polymorphism on glycoprotein Ia (integrin subunit a2) J Clin Invest. 1993;92:2427–2432. doi: 10.1172/JCI116849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiefel V, Santoso S, Weisheit M, et al. Monoclonal antibody-specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet reactive antibodies. Blood. 1987;70:1722–1766. [PubMed] [Google Scholar]
- 7.Santoso S, Kiefel V, Richter IG, et al. A functional platelet fibrinogen receptor with a deletion in the cysteine-rich repeat region of the β3 integrin: the Oea alloantigen in neonatal alloimmune thrombocytopenia. Blood. 2002;99:1205–1214. doi: 10.1182/blood.v99.4.1205. [DOI] [PubMed] [Google Scholar]
- 8.Peterson JA, Gitter ML, Kanack A, et al. New low-frequency platelet glycoprotein polymorphisms associated with neonatal alloimmune thrombocytopenia. Transfusion. 2010;50:324–333. doi: 10.1111/j.1537-2995.2009.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson JA, Balthazor SM, Curtis BR, et al. Maternal alloimmunization against the rare platelet-specific antigen HPA-9b (Max a) is important cause of neonatal alloimmune thrombocytopenia. Transfusion. 2005;45:1487–1495. doi: 10.1111/j.1537-2995.2005.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Luo BH, Xiao T, et al. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell. 2008;32:849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamata T, Ambo H, Puzon-McLaughlin W, et al. Critical cysteine residues for the regulation of integrin αIIbb3 are clustered in the epidermal growth factor domains of the β3 subunit. Biochem J. 2004;378:1079–1082. doi: 10.1042/BJ20031701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mor-Cohen R, Rosenberg N, Landau M, et al. Specific cysteine in β3 are involved in disulphide bond exchange-dependent and -independent activation of αIIbβ3. J Biol Chem. 2008;283:19235–19244. doi: 10.1074/jbc.M802399200. [DOI] [PubMed] [Google Scholar]
- 13.Jones CI, Garner SF Angement et al., on behalf of the BLOODOMICC consortium. Mapping the platelet profile for functional genomic studies and the effect size of the GPVI locus. J Throm Haemost. 2007;5:1756–1765. doi: 10.1111/j.1538-7836.2007.02632.x. [DOI] [PubMed] [Google Scholar]
- 14.Vijayan KV, Goldschmidt-Clermont PJ, Roos C, et al. The Pl(A2) polymorphism of integrin beta(3) enhances outside-in signaling and adhesive functions. J Clin Invest. 2000;105:793–802. doi: 10.1172/JCI6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball C, Vijayan KV, Nguyen T, et al. Glutathione regulates integrin αIIbβ3-mediated cell adhesion under flow conditions. Thromb Haemost. 2008;100:857–863. [PubMed] [Google Scholar]
- 16.Goodall AH. A radical explanation for the effect of the HPA-1b polymorphism in platelet αIIbβ3-integrin? Thromb Haemost. 2008;100:731–732. [PubMed] [Google Scholar]
- 17.Metcalfe P, Watkins NA, Ouwehand WH, et al. Nomenclature of human platelet antigens. Vox Sang. 2003;85:240–245. doi: 10.1046/j.1423-0410.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- 18.Stafford P, Garner SF, Rankin A, et al. A single-nucleotide polymorphism in the human ITGB3 gene is associated with the platelet-specific alloantigen Va (HPA-17bw) involved in fetal maternal alloimmune thrombocytopenia. Transfusion. 2008;48:1432–1438. doi: 10.1111/j.1537-2995.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 19.Koh Y, Taniue A, Ishii H, et al. Neonatal alloimmune thrombocytopenia caused by an antibody specific for a newly identified allele of human platelet antigen-7. Transfusion. 2010;50:1276–1284. doi: 10.1111/j.1537-2995.2009.02557.x. [DOI] [PubMed] [Google Scholar]
- 20.Nogues N, Esteban R, Garcia M, et al. A new polymorphism in the platelet glycoprotein GP IIIa associated with severe neonatal alloimmune thrombocytopenia. Vox Sang. 2007;93(Suppl 1):18–19. [Google Scholar]