Abstract
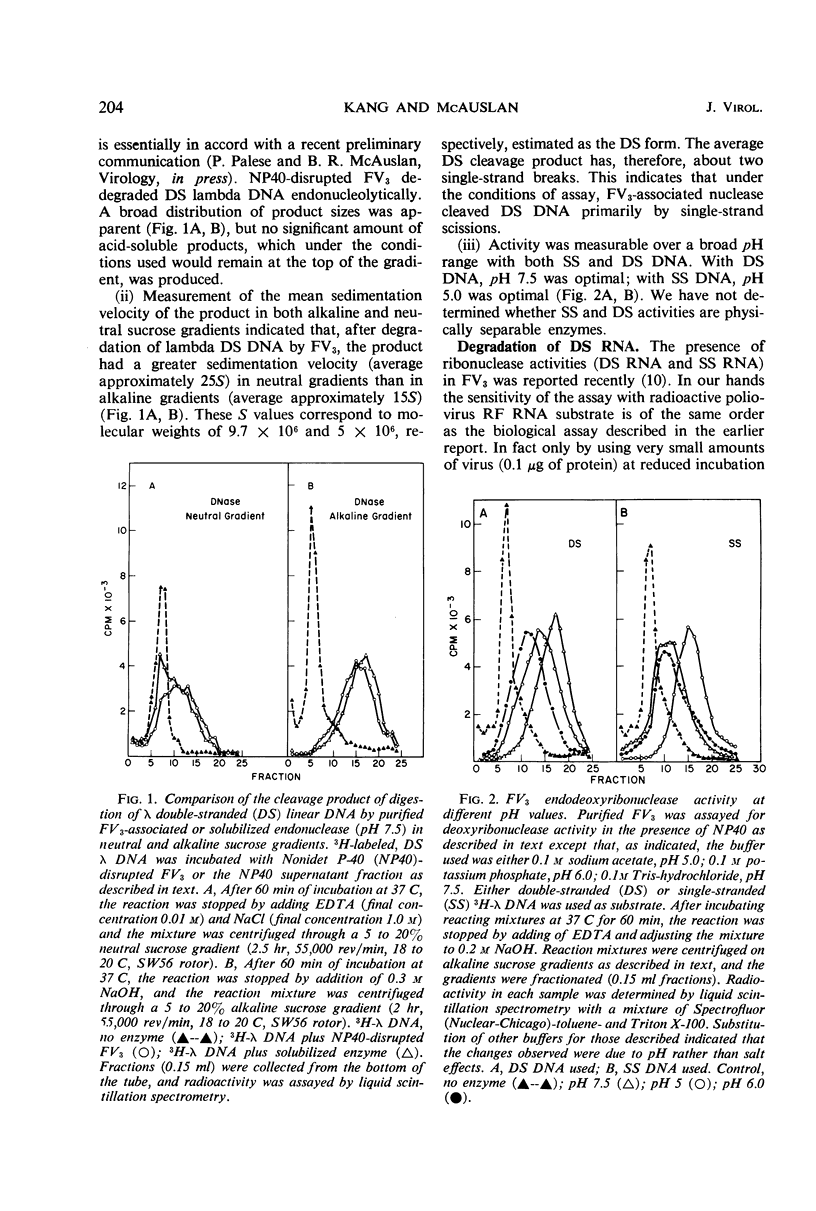
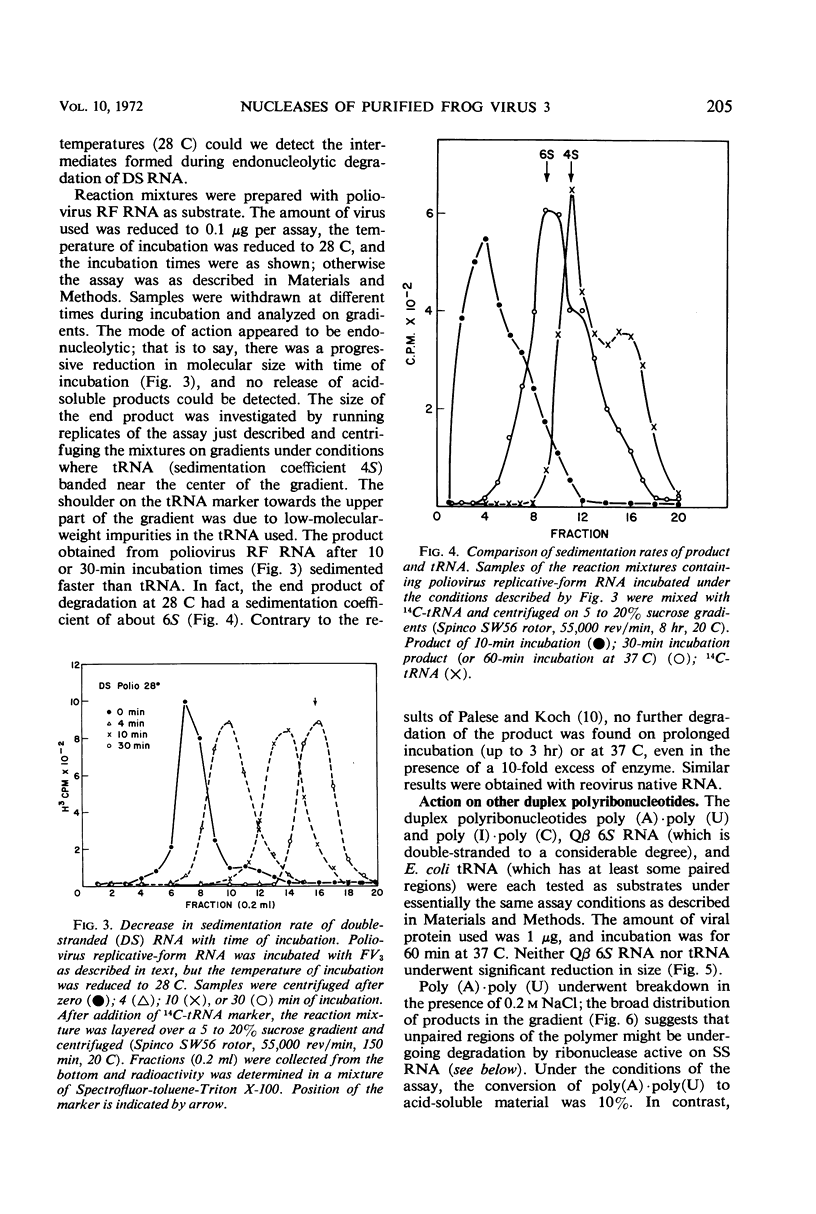
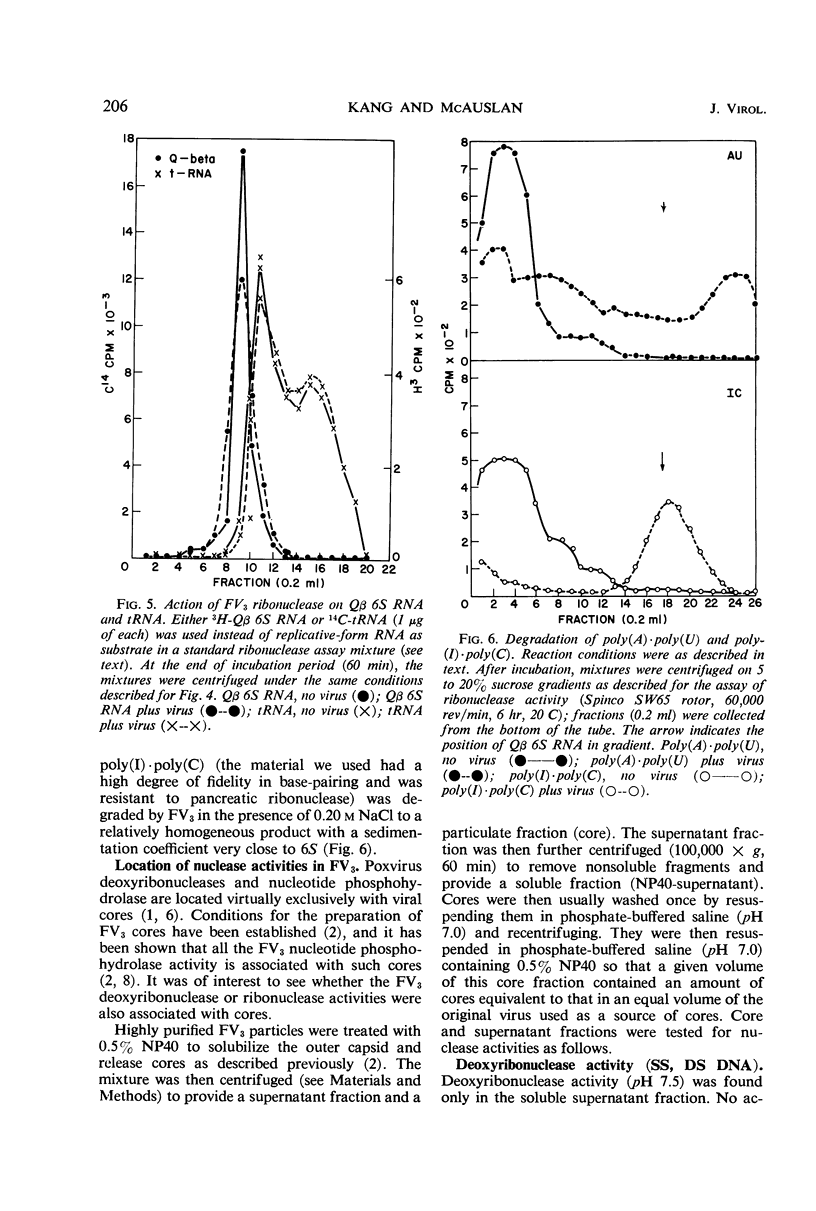
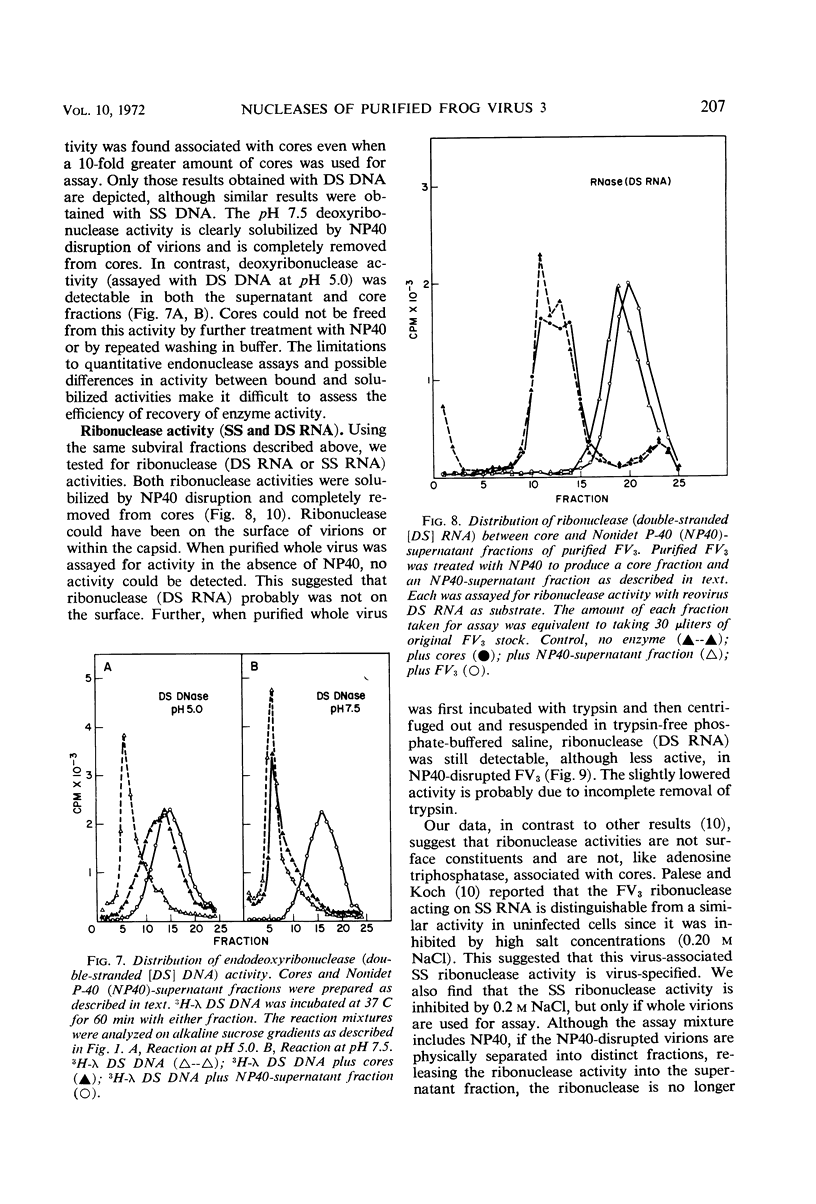
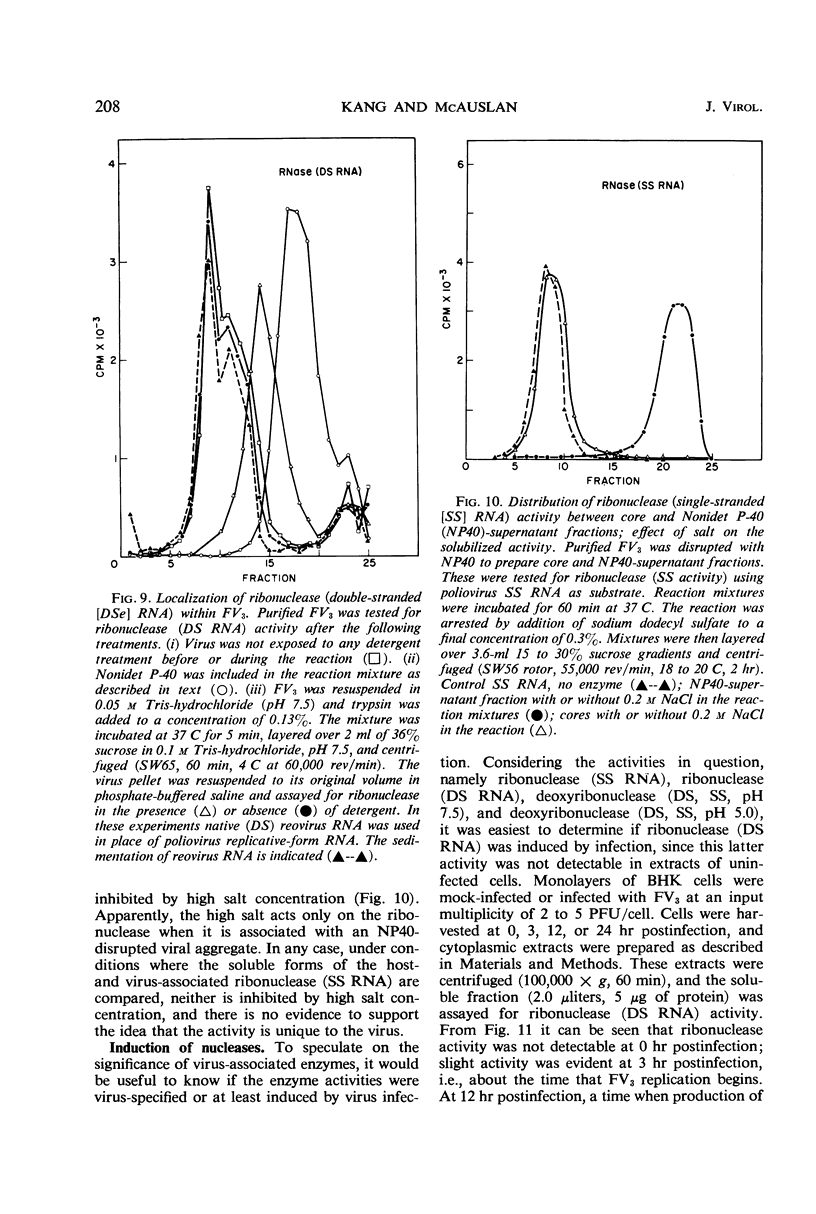
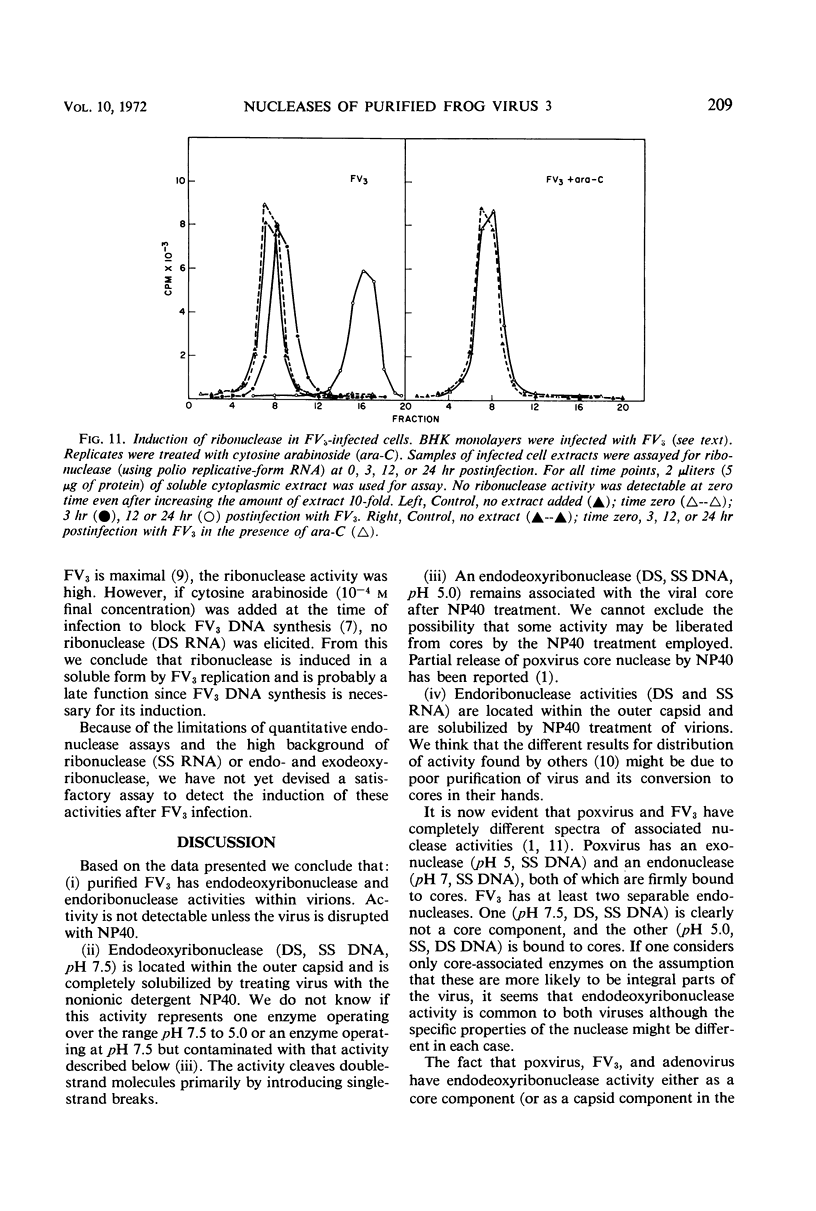
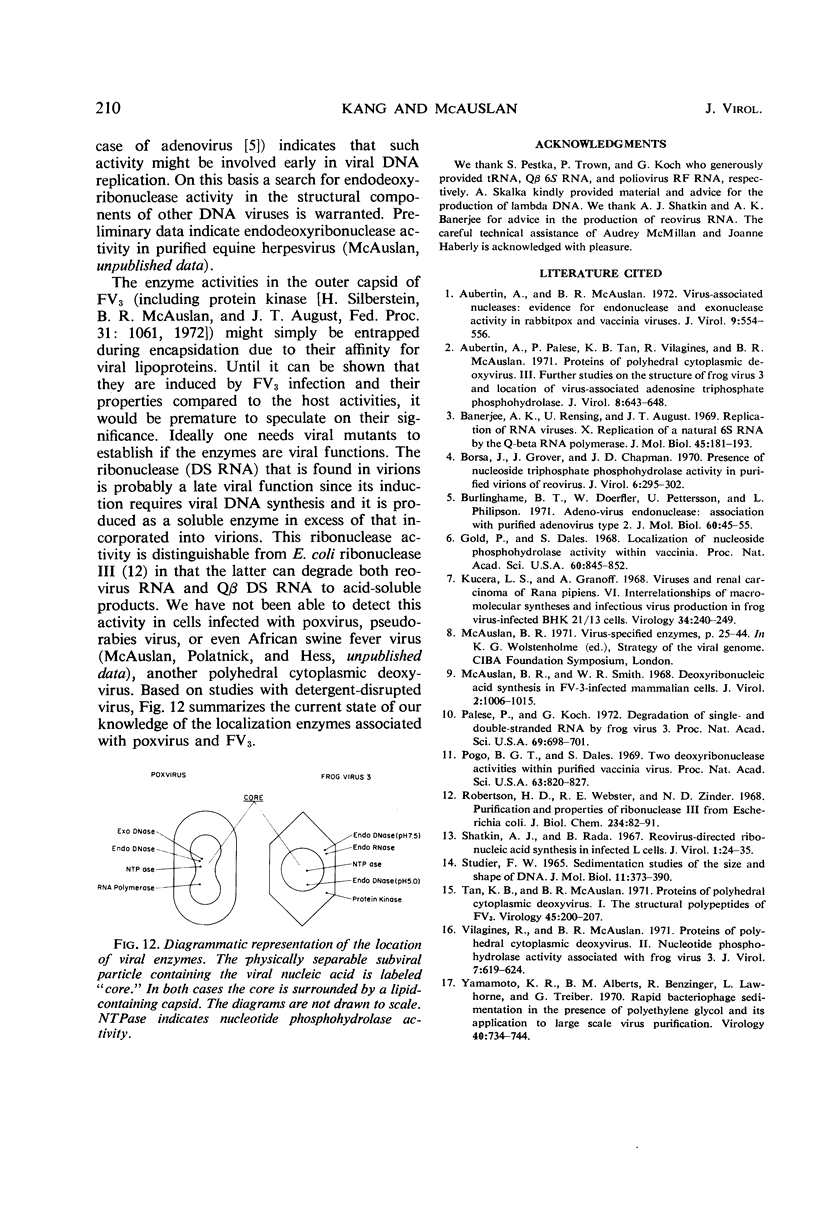
At least three nuclease activities are associated with purified frog virus 3. These activities are endodeoxyribonuclease (pH 7.5, double-stranded [DS] and single-stranded [SS] deoxyribonucleic acid [DNA]); endodeoxyribonuclease (pH 5.0, DS and SS DNA); endoribonuclease (DS and SS ribonucleic acid [RNA], pH 7.5). These activities are not adsorbed to the surface of the virion but are within the viral capsid and require detergent disruption of virions to unmask enzyme activity. Only one activity, deoxyribonuclease (pH 5.0, SS and DS DNA) appears to be core-associated after detergent disruption of virions. The ribonuclease degrades poliovirus replicative-form RNA, reovirus native RNA, and poly(I) poly(C) to a product with a sedimentation coefficient of about 6S. Qβ 6S DS RNA and 4S transfer RNA are not degraded. The ribonuclease appears to be a late function of the virus and is elicited in a soluble form as well as a virus-associated form.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubertin A. M., McAuslan B. R. Virus-associated nucleases: evidence for endonuclease and exonuclease activity in rabbitpox and vaccinia viruses. J Virol. 1972 Mar;9(3):554–556. doi: 10.1128/jvi.9.3.554-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubertin A., Palese P., Tan K. B., Vilagines R., McAuslan B. R. Proteins of a polyhedral cytoplasmic deoxyvirus. 3. Structure of frog virus 3 and location ov virus-associated adenosine triphosphate phosphohydrolase. J Virol. 1971 Nov;8(5):643–648. doi: 10.1128/jvi.8.5.643-648.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K., Rensing U., August J. T. Replication of RNA viruses. X. Replication of a natural 6 s RNA by the Q-beta RNA polymerase. J Mol Biol. 1969 Oct 28;45(2):181–193. doi: 10.1016/0022-2836(69)90098-9. [DOI] [PubMed] [Google Scholar]
- Borsa J., Grover J., Chapman J. D. Presence of nucleoside triphosphate phosphohydrolase activity in purified virions of reovirus. J Virol. 1970 Sep;6(3):295–302. doi: 10.1128/jvi.6.3.295-302.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W., Pettersson U., Philipson L. Adenovirus endonuclease: association with the penton of adenovirus type 2. J Mol Biol. 1971 Aug 28;60(1):45–64. doi: 10.1016/0022-2836(71)90446-3. [DOI] [PubMed] [Google Scholar]
- Gold P. H., Dales S. Localization of nucleotide phosphohydrolase activity within vaccinia. Proc Natl Acad Sci U S A. 1968 Jul;60(3):845–852. doi: 10.1073/pnas.60.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera L. S., Granoff A. Viruses and renal carcinoma of Rana pipiens. VI. Interrelationships of macromolecular synthesis and infectious virus production in frog virus 3-infected BHK 21/13 cells. Virology. 1968 Feb;34(2):240–249. doi: 10.1016/0042-6822(68)90233-x. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R. Enzymes specified by DNA-containing animal viruses. In: strategy of the viral genome. Ciba Found Symp. 1971:25–44. doi: 10.1002/9780470719824.ch3. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Smith W. R. Deoxyribonucleic acid synthesis in FV-3-infected mammalian cells. J Virol. 1968 Oct;2(10):1006–1015. doi: 10.1128/jvi.2.10.1006-1015.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Koch G. Degradation of single- and double-stranded RNA by frog virus 3. Proc Natl Acad Sci U S A. 1972 Mar;69(3):698–701. doi: 10.1073/pnas.69.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Rada B. Reovirus-directed ribonucleic acid synthesis in infected L cells. J Virol. 1967 Feb;1(1):24–35. doi: 10.1128/jvi.1.1.24-35.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., McAuslan B. R. Proteins of polyhedral cytoplasmic deoxyviruses. I. The structural polypeptides of FV 3 . Virology. 1971 Jul;45(1):200–207. doi: 10.1016/0042-6822(71)90127-9. [DOI] [PubMed] [Google Scholar]
- Vilagines R., McAuslan B. R. Proteins of polyhedal cytoplasmic deoxyvirus. II. Nucleotide phosphohydrolase activity associated with frog virus 3. J Virol. 1971 May;7(5):619–624. doi: 10.1128/jvi.7.5.619-624.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



