Abstract
Persistent inflammation promotes internalization of synaptic GluR2-containing Ca2+-impermeable AMPA receptors (AMPARs) and insertion of GluR1-containing Ca2+-permeable AMPARs at extrasynaptic sites in dorsal horn neurons. Previously we have shown that internalization of synaptic GluR2-containing AMPARs requires an activation of spinal cord protein kinase C alpha (PKCα), but molecular mechanisms that underlie altered trafficking of extrasynaptic AMPARs are still unclear. By utilizing the antisence oligodeoxynucleotides that specifically knockdown PKCα, we have found that a decrease in dorsal horn PKCα expression prevents complete Freund’s adjuvant (CFA)-induced increase in a functional expression of extrasynaptic Ca2+-permeable AMPARs in substantia gelatinosa (SG) neurons of the rat spinal cord. This was manifested as an abolishment of augmented AMPA-induced currents and associated [Ca2+]i transients, and as a reverse of the current rectification 1 d post-CFA. These changes were observed specifically in SG neurons characterized by intrinsic tonic firing properties, but not in those exhibiting strong adaptation. Finally, dorsal horn PKCα knockdown produced anti-nociceptive effect on CFA-induced thermal and mechanical hypersensitivity during the maintenance period of inflammatory pain, indicating a role for PKCα in persistent inflammatory pain maintenance. Altogether, our results indicate that inflammation-induced trafficking of extrasynaptic Ca2+-permeable AMPARs in tonically firing SG neurons depends on PKCα, and suggest that this PKCα-dependent trafficking may contribute to the persistent inflammatory pain maintenance.
Keywords: Extrasynaptic AMPA receptors, PKCα, AMPARs trafficking, GluR1 and GluR2 subunits, inflammatory pain, substantia gelatinosa neurons
1. Introduction
Persistent or chronic pain, which may result from inflammation, infection, tissue or nerve injury, is a major public health problem worldwide. Its treatment success is limited due to our incomplete understanding of the molecular mechanisms that underlie the transmission and perception of chronic pain. It is generally believed that spinal cord dorsal horn central sensitization contributes to the induction and maintenance of chronic pain.20
Activity-dependent trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), which mediate fast excitatory transmission, has emerged as a key mechanism underlying synaptic plasticity in the brain24,30,31 and central sensitization (a specific form of plasticity) in spinal cord dorsal horn.14,20,25,33 This activity-dependent trafficking represents a regulated functional expression of the receptor subunits GluR1-GluR4, which composition determines the functional properties of AMPARs.32 Recent evidence indicates that altered AMPAR trafficking in dorsal horn neurons is causally linked to peripheral inflammatory pain maintenance.14,17,18,25 It has been shown that complete Freund’s adjuvant (CFA)-induced inflammation activates PKCα in dorsal horn that is required for phosphorylation of GluR2 at S880 residue.3,25,37 This phosphorylation disrupts interaction of GluR2 with its synaptic anchoring protein ABP/GRIP and promotes GluR2 endocytosis from the postsynaptic membrane of dorsal horn neurons.3,25,37 As the result, GluR2-containing AMPARs switch to GluR2-lacking AMPARs and Ca2+ permeability of synaptic AMPARs increases in dorsal horn neurons.14,17,25,34
Our recent studies of extrasynaptic AMPAR trafficking in dorsal horn neurons have demonstrated that CFA-induced inflammation substantially increases functional expression of GluR2-lacking GluR1-containing Ca2+-permeable AMPARs in the extrasynaptic plasma membrane of substantia gelatinosa (SG) neurons during the maintenance period of inflammatory pain.18 In spite of a significant role of extrasynaptic Ca2+-permeable AMPARs in synaptic plasticity7,16 and nociception,12,18,21 molecular mechanisms that underlie extrasynaptic AMPARs trafficking during the maintenance period of inflammatory pain have not been studied yet. Recently it has been shown that phosphorylation of GluR1 by PKC promotes activity-dependent GluR1 membrane insertion in hippocampal neurons to maintain extrasynaptic pool of GluR1-containing AMPARs for their recruitment to postsynaptic densities during sustain synaptic potentiation.6,22 All these findings suggest that inflammation-induced activation of PKCα3,25,37 may be involved in functional expression of extrasynaptic Ca2+-permeable AMPARs18 and contribute to inflammatory pain maintenance.
In this work the PKCα antisence (AS) oligodeoxynucleotide (ODN) knockdown strategy was used for a selective PKCα inhibition locally in a spinal cord. Using this approach we have demonstrated a significant role of PKCα in inflammatory-induced upregulation of extrasynaptic Ca2+-permeabile AMPAR in dorsal horn neurons as well as in the maintenance of persistent inflammatory pain.
2. Materials and Methods
2.1 Animal preparation
Male rats (18–30 days old) were housed in cages on a standard 12:12 h light/dark cycle, with water and food available ad libitum. The animals were used in accordance with protocols that were approved by the Animal Care and Use Committee at the Bogomoletz Institute of Physiology and Johns Hopkins University and were consistent with the ethical guidelines of the National Institutes of Health and the International Association for the Study of Pain. All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2 Experimental drugs
CFA was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Fura-2 was obtained from Invitrogen (Carlsbad, CA, USA); tetrodotoxin (TTX) – from Alomone Labs Ltd. (Israel). APV, AMPA, cyclothiazide (CTZ), bicuculline, strychnine, and 1-trimethylammonio-5-(1-adamantane-methyl-ammoniopentane dibromide) (IEM-1460) were purchased from Tocris Bioscience (Ellisville, MO). Antisense (AS) and missense (MS) oligodeoxynucleotides (ODN) were obtained from ISIS Pharmaceuticals Inc. (Carlsbad, CA).
2.3 Induction of peripheral inflammation
To produce unilateral peripheral inflammation and nociceptive hypersensitivity, 100 µl of CFA (Mycobacterium tuberculosis) suspended in an oil-saline (1:1) emulsion was injected subcutaneously into the plantar side of one hind paw of the rats. Because studies from our laboratory and those of others showed that CFA injection produces a significant change in synaptic AMPAR trafficking in dorsal horn neurons 24 h post-CFA injection,25,26 we focused on this time point. Saline (0.9%; 100 µl) injection was used as a control.
2.4 Intrathecal administration of PKCα ODN
To specifically and selectively knock down the expression of PKC subtype α, AS ODN and MS ODN were designed and synthesized with followed sequences: AS, 5'-GACATCCCTTTCCCCCTCGG-3' and MS, 5'-CGTCCTCAGTCGTCCCTCAC-3' as described previously10. ODNs were dissolved in saline, and stored at −20°C.
For chronic local delivery of ODNs to L4–5 spinal cord region, a polyethylene (PE-10) tube was inserted into the subarachnoid space at the rostral level of the spinal cord lumbar enlargement segment through an incision at the atlanto-occipital membrane, as described previously.26,38 The rats were allowed to recover at least for 3–5 days before being used experimentally. Rats showing any neurological deficits postoperatively were discarded. The position of the catheter was confirmed in each animal after behavioral experiment and prior to an electrophysiological recording.
The rats received intrathecal injection with saline (10 µl; control), AS ODN (10 µg/10 µl), or MS ODN (10 µg/10 µl) followed by an injection of 10 µl of saline to flush the catheter daily for 4 days. On the third day after intrathecal ODN or saline injection, the rats received intraplantar injection of CFA or saline as described above. Western blot analysis or electrophysiological recording was carried out 24 h post-CFA or post-saline.
2.5. Western blot analysis
Soluble proteins were prepared according to procedures described previously.25,26 In brief, after the rats (n = 4/group) were euthanized by an overdose of isoflurane, the tissues from the dorsal portions of lumbar spinal cord were dissected and rapidly frozen in liquid nitrogen. The tissues were then homogenized in the homogenization buffer [50 mM Tris-HCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 µM leupeptin, 2 µM pepstatin A]. The crude homogenate was centrifuged at 4°C for 15 min at 1,000g, and the supernatants were collected. After measurement of protein concentration, the protein was heated for 5 min at 98°C and loaded onto 4% stacking/7.5% separating SDS-polyacrylamide gels. After separation, the protein was electrophoretically transferred onto a nitrocellulose membrane. The membrane was blocked with 3% non-fat dry milk and subsequently incubated for 2 h with polyclonal rabbit primary antibodies for PKCα (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA) or PKCγ (1:1,000, 000, Santa Cruz Biotechnology), and with monoclonal mouse primary antibody for β-actin (1:2,000, Santa Cruz Biotechnology). The proteins were detected by horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies and visualized with chemiluminescence reagents provided with the ECL kit (Amersham Pharmacia Biotech, Piscataway, NJ) and exposure to film. The blot density from naïve rats was set as 100%. The relative density values from other groups were determined by dividing the optical density values from these groups by the value from naïve rats after they were normalized to the corresponding β-actin.
2.6 Behavioral testing
Animals were acclimatized to the experimental setup before the testing. The experimenters were blinded to the treatment groups in the behavioral testing.
Paw withdrawal responses to thermal stimuli were measured in rats using Hargreaves technique. To measure paw withdrawal response to noxious heat stimuli, the animal was placed in a Plexiglas chamber on a glass plate located above a light box. Radiant heat was applied by focused infrared beam through a hole in the light box through the glass plate to the middle of the plantar surface of each hind paw. When the animal lifted its foot, the light beam was automatically turned off. The length of time between the start of the beam and the foot lift was defined as the paw withdrawal latency (PWL). Each trial was repeated five times at 5-min intervals for each paw. A cut-off time of 30 s was used to avoid tissue damage. Behavioral tests were performed before ODN injection and at 1, 2 and 3 days after OND injection, and then at 1, 2, 3, 4, 5, 6 and 7 days after CFA injection.
To measure paw withdrawal responses to repeated mechanical stimuli, von Frey method was employed. A rat was placed in a Plexiglas chamber on an elevated mesh screen and each von Frey monofilament (Bioseb) was applied to the hind paw for approximately 1–2 s. Each trial was repeated 10 times to both hind paws at 1-min intervals on each hind paw. The occurrence of paw withdrawal in each of these trials was expressed as a percentage response frequency.
2.7 Spinal cord slice preparation
Spinal cord slices were prepared from 18–30-days-old male rats as described previously.18,35 Briefly, after rats were deeply anesthetized with an overdose of isoflurane, the L4–5 spinal segments were removed. Transverse slices (300 µm thick) were cut on a vibratome in an ice-cold solution containing (in mM) 250 sucrose, 2 KCl, 1.2 NaH2PO4, 0.5 CaCl2, 7 MgCl2, 26 NaHCO3, 11 glucose (pH 7.4) and continuously bubbled with 95% O2, 5% CO2. Slices were maintained at room temperature in a physiologic Krebs bicarbonate solution that contained (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 10 glucose (pH 7.4, osmolarity 310–320 mOsM) and was equilibrated with 95% O2, 5% CO2.
2.8 Simultaneous Ca2+ imaging and patch-clamp recording
Simultaneous Ca2+ imaging and whole-cell electrophysiological recordings were obtained from the lamina II (Substantia Gelatinosa, SG) neurons of the spinal L4–5 dorsal horn as described previously.18 Briefly, the neurons were visually identified with a video microscopy system (Olympus, Japan). The patch pipettes with resistance of 6–10 MΩ were filled with an internal solution containing (in mM) 133 K-gluconate, 5 NaCl, 0.5 MgCl2, 10 HEPES-Na, 2 MgATP, 0.1 GTP-Na, and 0.2 fura-2 pentapotassium salt (pH 7.2, osmolarity 290 mOsM). The membrane potential of SG neurons was held at −60 mV. Only data from neurons that exhibited a resting membrane potential negative to less −60 mV were included in the analysis.
All SG neurons were categorized according to their discharge pattern in response to the series of 1-s current pulses, as described previously.18 SG neurons were predominantly divided into “tonic” and “transient” groups. Tonic neurons were defined as those able to support continued discharge of action potentials during 1-s depolarizing inward current and an increased frequency of discharge with increasing current intensity (Fig. 3A). Transient neurons were those that exhibited a strong adaptation by generating short bursts of spikes or just a single spike regardless of depolarizing current intensity.18
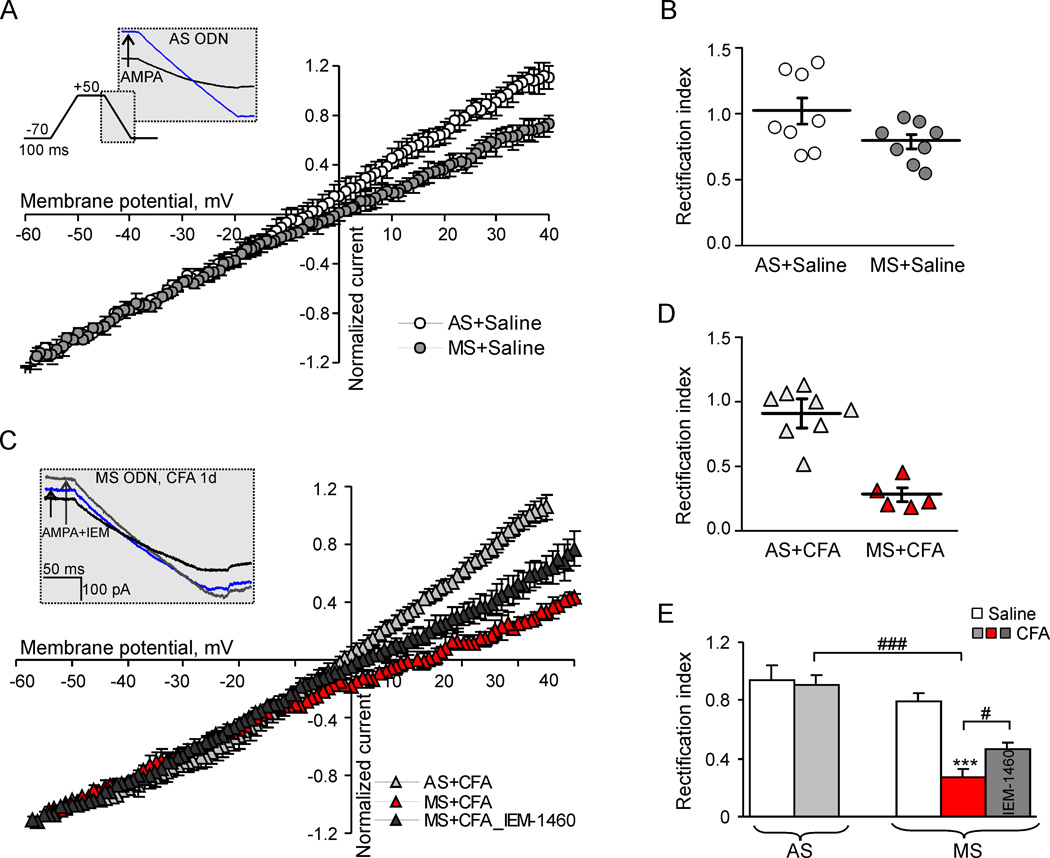
Fig.3. Effect of dorsal horn PKCα knockdown on the I-V relationship of AMPARs-mediated currents in tonically firing SG neurons.
(A) I-V curves obtained in tonic SG neurons of the AS- and MS ODNs-treated groups 1d post-saline. Insert illustrates the protocol for reconstruction of the I-V relationship from ramp recordings. (B) The scatter plot illustrates a spread in rectification index (RI = I+30mV/I−50mV) in tonic SG neurons after AS ODN or MS ODN treatment for 4 days. (C–D) I-V curves (C) and a scatter plot of RI (D) in tonic SG neurons from the AS- and MS ODN-treated groups 1d after CFA injection. (E) A statistical summary for RI in tonic neurons from the AS ODN- and MS ODN-treated groups 1 d post-saline and post-CFA before (black graph) and after (grey) an application of selective blocker of Ca2+-permeable AMPARs, IEM-1460 (40 µM). *** p < 0.001 versus the saline-injected group, #p < 0.05, ### p < 0.001 versus the CFA-injected group.
To isolate AMPAR-mediated current and associated [Ca2+]i increase, recordings were made in the continuous presence of APV (50 µM), bicuculline (5 µM), and strychnine (2 µM) to block NMDA, GABAA, and glycine receptors, respectively. In addition, TTX (0.5 µM) and cadmium chloride (100 µM) were added to Krebs bicarbonate solution to block corresponding voltage-activated sodium and calcium channels. To prevent a desensitization of AMPARs during bath application of the agonist, AMPA was applied in the continuous presence of CTZ (20 µM). Typically, one neuron was studied per slice.
Simultaneous fura-2 fluorescence was measured by using a 60× water-immersion objective and a 12-bit cooled CCD camera and capturing board (Sensicam, PCO, Germany). Fluorescent signals from SG neurons were collected between 50 and 100 µm below the surface of the slice. Calcium changes were detected by a PolyChrome IV monochromator (Till Photonics, Germany) as the change in fura-2 fluorescence measured at wavelengths > 510 nm when excitation light was 380 nm and 340 nm using Imaging Workbench software (INDEC System, USA). The changes in soma and dendrites were measured simultaneously and expressed as changes in the ratio of fura-2 fluorescence at 340 and 380 nm, which is proportional to [Ca2+]i. The amplitude of AMPA-induced [Ca2+]i signal was estimated as the difference between the fura-2 fluorescence ratio prior to AMPA application and that at the maximum [Ca2+]i rise after AMPA stimulation.
To study the current-voltage (I-V) relationship, we used an internal solution that contained (in mM) 130 Cs-methylsulphonate, 10 NaCl, 0.5 EGTA, 10 HEPES, 0.2 spermine tetrahydrochloride, 2 Mg-ATP, and 0.1 Na-GTP (pH 7.2, osmolarity 290 mOsM). I-V curves were constructed by holding neurons at −70 mV in a voltage clamp and ramping for 100–300 ms every 5 s initially to +50 mV and then to −70 mV. Short hyperpolarizing voltage step to −75 mV was applied before every ramp to monitor input and access resistance. To inhibit K+-channel currents, the AMPA-induced currents were recorded after at least 10 min perfusion of patched neurons with an internal solution. To isolate AMPA-induced current, we subtracted the ramp currents recorded before AMPA application from the currents recorded during the bath AMPA application at each membrane potential. The rectification index (RI) of the AMPA-induced current was determined by dividing the current amplitude at +30 mV by the current amplitude at −50 mV. The RI was also estimated by dividing the AMPAR conductance calculated at positive and negative potentials (+30 mV and −50 mV, respectively) with an accounting for the corresponding reversal potential for each I-V curve. Clampfit 8.0 software (Molecular Devices) was used to analyze the currents.
2.9 Statistical analysis
All data are presented as mean ± SEM with n referring to the number of cells analyzed. Student’s t-tests were used to determine statistically significant differences. A p value of less than 0.05 was considered as statistically significant.
3. Results
3.1 Effect of intrathecal PKCα AS ODN on spinal cord PKCα protein expression
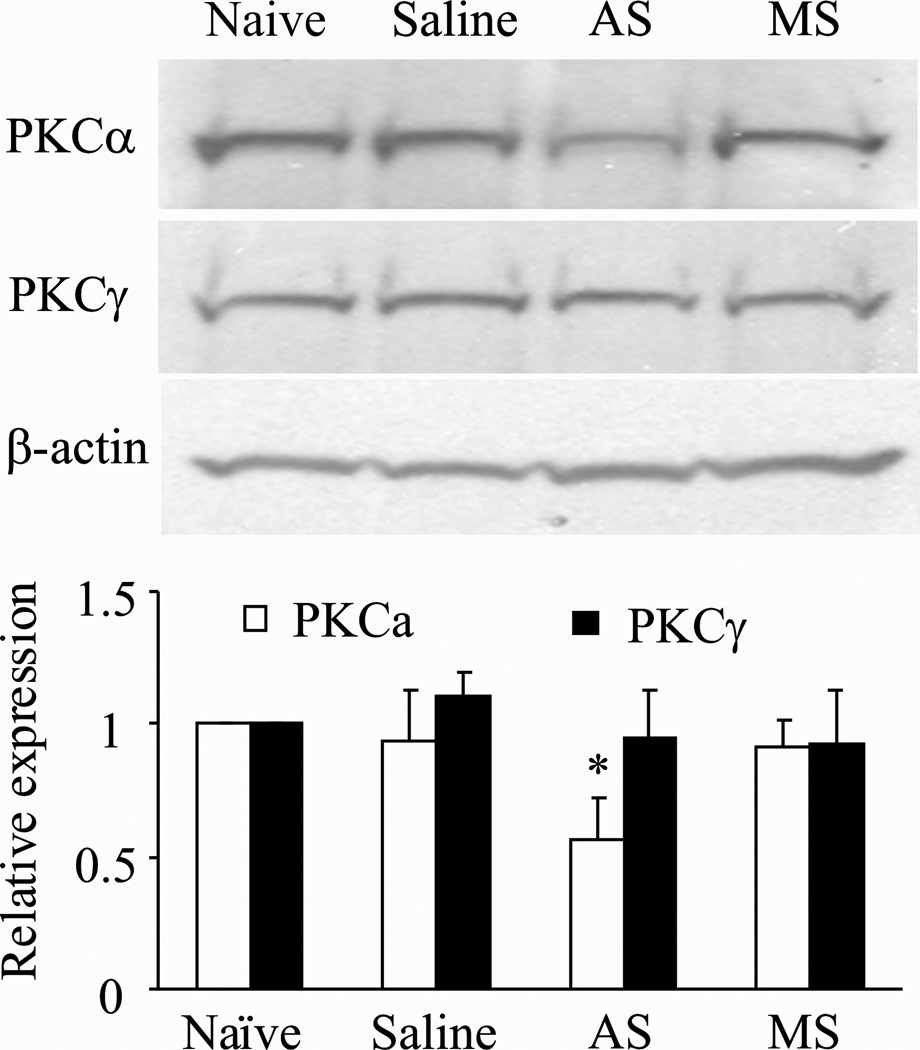
We first examined whether intrathecal administration of PKCα AS ODN selectively and specifically altered dorsal horn PKCα expression. MS ODN was used as a control. Consistent with the previous report,10 daily intrathecal injection of PKCα AS ODN, but not MS ODN, for 4 days significantly reduced PKCα protein expression in the dorsal horn of spinal cord lumbar enlargement segment (Fig 1). The levels of PKCα in the saline-treated (n = 4), AS ODN-treated (n = 4), and MS ODN-treated (n = 4) groups were reduced by 7% (P > 0.05), 43% (P < 0.05), and 9% (P > 0.05), respectively, compared to the value of the naïve group (n = 4; Fig. 1). Neither AS ODN nor MS ODN affected the expression of PKCγ (Fig. 1).
Fig. 1. Effect of PKCα AS ODN on PKCα protein expression in spinal cord.
Intrathecal injection of 10 µg PKCα AS ODN, but not saline or 10 µg MS ODN, significantly reduced expression of PKCα, but not PKCγ, in the dorsal horn of spinal cord lumbar enlargement segments compared to that in naïve rats. Top: Representative Western blots. Bottom: Statistical summary of the densitometric analysis expressed relative to naïve rats after normalization to corresponding β-actin.
3.2 Effect of intrathecal PKCα AS ODN on CFA-induced augmentation of extrasynaptic AMPAR-mediated currents and [Ca2+]i transients in tonicaly firing SG neurons
Previously we have shown that CFA-induced peripheral inflammation substantially increases a functional expression of Ca2+-permeable AMPARs at extrasynaptic plasma membrane of tonically firing SG neurons.18 In this work, we investigated whether down-regulation of dorsal horn PKCα by AS ODN affected this increase. Simultaneous recording of AMPA-induced transmembrane currents and associated changes of [Ca2+]i in soma and dendrites of SG neurons were performed. Consistent with previous reports,18,35 bath administration of AMPA (5 µM, 60 s) to the spinal cord slices evoked an inward current in all SG neurons at a holding potential of −60 mV, that was characterized by a slow rising phase and reached a plateau level. Although bath applied AMPA activates a total pool of plasma membrane AMPARs, the AMPA-induced current is predominantly contributed by the extrasynaptic AMPARs due to their relative abundance in the neuronal plasma membrane compared to synaptic ones.2 Besides, the substantial differences in functional properties of the total AMPARs versus synaptic ones (e.g. rectification properties of the current in dorsal horn and hippocampal neurons and sensitivity to polyamine derivatives)13,18,25 additionally confirm the major contribution of extrasynaptic AMPARs to AMPA-induced current.
We found that an acute transient knockdown of dorsal horn PKCα was sufficient to significantly attenuate CFA-induced augmentation of AMPAR-mediated current in tonically firing SG neurons (Fig. 2A, 2B). Consistent with our previous study,18 CFA injection markedly increased the amplitude of AMPA-induced currents in the saline-treated group (−460 ± 48 pA; n = 20 vs −204 ± 18 pA; n = 35 in control; p < 0.001; Fig. 2A), indicating a dramatic upregulation of extrasynaptic AMPARs during CFA-induced inflammation. Contrary to the extrasynaptic receptors, CFA-induced inflammatory insult did not alter the amplitude of evoked AMPAR-mediated excitatory post-synaptic currents (eEPSCs) in dorsal horn neurons as has been demonstrated by our previous work25 and those of others,17,34 additionally supporting the major contribution of extrasynaptic AMPARs to the AMPA-induced currents.
Fig. 2. Knockdown of dorsal horn PKCα attenuates CFA-induced augmentation of AMPA-induced current and [Ca2+]i transients in tonically firing SG neurons.
(A) Top: a fluorescent image of SG neuron loaded with fura-2 (200 µM); scale bar = 20 µm. Bottom: typical firing pattern for tonic neurons in response to sustained depolarizing current. (B) Representative examples of a somatic membrane current (bottom trace) and associated [Ca2+]i transients (upper traces), recorded from the soma (black trace) and dendrites (blue trace), in tonic neurons during AMPA bath application (5 µM, 60 s) in the AS- (right) and MS ODNs-treated (left) groups 1 d post-CFA. (C) Statistical summary of the amplitudes of AMPA-induced current (left graph) and associated [Ca2+]i transients in soma and dendrites (right graph) of tonic SG neurons from the saline-, AS- and MS ODNs-treated groups 1 d after saline or CFA injection. * p < 0.05, *** p < 0.001. AS, antisense; MS, missense.
In contrast, after daily treatment with AS ODN for 4 days, the average amplitude of AMPA-induced currents was −191 ± 32 pA (n = 9) in neurons from the CFA-treated group of animals, that was close to the value obtained in the saline-treated non-inflamed animals (−204 ± 18 pA; n = 35; p > 0.7; Fig. 2C). As expected, MS ODN did not affect the CFA-induced increase in the current amplitude (MS ODN: −454 ± 44 pA, n = 13; p > 0.4 compared to the saline-treated CFA-inflamed animals). There was no significant difference between the AS- and MS ODN-treated groups 1 day post-CFA in a resting membrane potential of tonic neurons (AS ODN: −52 ± 2 mV, n = 13; MS ODN: −54 ± 2 mV, n = 23. p = 0.5), an input resistance (AS ODN: 709 ± 77 MΩ, n = 26; MS ODN: 528 ± 85 MΩ, n = 23. p > 0.1), membrane capacitance (AS ODN: 26 ± 2 pF, n = 26; MS ODN: 25 ± 1 pF, n = 25. p > 0.7), and series resistance (AS ODN: 30 ± 2 MΩ, n = 26; MS ODN: 34 ± 2 MΩ, n = 23. p > 0.2).
The AMPA-induced current was associated with a synchronous rise in [Ca2+]i, observed both in soma and dendrites of tonic neurons. This rise in [Ca2+]i consisted of initial fast rise followed by a slow decay to the baseline within a few minutes. In line with our previous results,18,35 CFA-induced inflammatory insult markedly increased the amplitude of AMPA-induced [Ca2+]i transients in soma and dendrites of tonically firing SG neurons (by 92 ± 11% and 96 ± 17%, n = 11; p < 0.05, respectively), indicating a dramatic upregulation of extrasynaptic Ca2+-permeable AMPARs during CFA-induced inflammation. Similar to the AMPA-induced currents, knockdown of dorsal horn PKCα also significantly reversed augmented [Ca2+]i transients 1 d after CFA injection. The average amplitudes of AMPA-induced [Ca2+]i transients were 0.22 ± 0.05 (n = 10) vs. 0.91 ± 0.10 (n = 11; p < 0.05) for soma and 0.32 ± 0.06 (n = 14) vs. 1.17 ± 0.21 (n = 11; p < 0.05; Fig. 2C) for dendrites in the neurons from the AS ODN-treated and saline-treated CFA-inflamed groups, respectively. It is interesting to note that the average amplitude of AMPA-induced [Ca2+]i transients in the AS ODN-treated CFA-inflamed group was lower than one in the saline-treated non-inflamed group (in soma: 0.22 ± 0.05; n = 10 vs. 0.47 ± 0.10; n = 21; p < 0.05; in dendrites: 0.32 ± 0.06; n = 14 vs. 0.60 ± 0.12; n = 14; p < 0.05; Fig. 2C, respectively). No significant difference was observed in the amplitudes of somatic and dendritic [Ca2+]i transients between the MS ODN- and saline-treated groups 1 d post-CFA (in soma: 0.91 ± 0.10, n = 11 vs. 0.75 ± 0.09, n = 9; p > 0.4; in dendrites: 1.17 ± 0.21, n = 11 vs. 0.93 ± 0.12, n = 9; p > 0.3, respectively; Fig. 2C).
Taken together, our findings demonstrate that knockdown of dorsal horn PKCα efficiently abolished CFA-induced augmentation of extrasynaptic AMPAR-mediated current and associated [Ca2+]i transients in tonically firing SG neurons, indicating that CFA-promoted upregulation of extrasynaptic Ca2+-permeable AMPAR in these neurons is PKCα dependent.
It should be noted that knockdown of dorsal horn PKCα did not affect either AMPAR-mediated current or somatic and dendritic [Ca2+]i transients in SG neurons exhibiting a strong adaptation to sustained membrane depolarization by generating short bursts of spikes or just a single spike regardless of depolarizing current intensity (transient type of neurons). No significant difference was observed in the average amplitudes of AMPA-induced currents and [Ca2+]i transients among saline-treated (n = 28), AS ODN-treated (n = 7), and MS ODN-treated (n = 13) groups 1 d after injection of CFA or saline. Average amplitudes of AMPA-induced currents were −173 ± 19 pA (n = 28) vs −150 ± 17 pA (n = 21; p > 0.37) for the saline- and CFA- treated groups, respectively, and −180 ± 56 pA (n = 7) vs −196 ± 41 pA (n = 13; p > 0.8) for the AS ODN- and MS ODN-treated groups 1 d post-CFA, respectively. Average amplitudes of [Ca2+]i transients in soma were: 0.48 ± 0.09 vs. 0.47 ± 0.08 for the saline- and CFA-treated groups (p > 0.9), respectively, and 0.46 ± 0.09 vs. 0.29 ± 0.08 for the AS ODN- and MS ODN-treated groups 1 d post-CFA (p > 0.2), respectively. Dendritic [Ca2+]i transients were: 0.52 ± 0.09 vs. 0.59 ± 0.10 for the saline- and CFA- treated groups (p > 0.6), respectively, and 0.52 ± 0.10 vs. 0.40 ± 0.10 for AS ODN- and MS ODN-treated groups (p > 0.4), respectively. There was no significant difference between the transient SG neurons from the AS- and MS ODN-treated groups 1 day post-CFA in electrophysiological properties such as input resistance (AS ODN: 1096 ± 215 MΩ, n = 21; MS ODN: 932 ± 170 MΩ, n = 31; p > 0.6), membrane capacitance (AS ODN: 18 ± 3 pF, n = 21; MS ODN: 22 ± 1 pF, n = 31. p > 0.2), and series resistance (AS ODN: 30 ± 2 MΩ, n = 21; MS ODN: 32 ± 2 MΩ, n = 31. p > 0.4). These results demonstrate that functional expression of extrasynaptic AMPAR in SG neurons, exhibiting a strong adaptation, is not altered during the maintenance of peripheral inflammation and PKCα does not play a significant role for AMPAR functioning in this type of SG neurons.
3.3 Effect of intrathecal PKCα AS ODN on CFA-induced increase in the proportion of Ca2+-permeable AMPARs in the total pool of extrasynaptic AMPARs
Persistent inflammation increases the proportion of Ca2+-permeable AMPARs within the total extrasynaptic AMPARs pool in tonically firing SG neurons during the maintenance period of CFA-induced inflammatory pain.18 To examine whether the knockdown of dorsal horn PKCα affects this increase, we compared I-V relationship of the AMPA-induced currents in SG neurons 1 day after saline or CFA injection among saline-, AS ODN-, and MS ODN-treated groups, since the relative proportion of Ca2+-permeable AMPARs can be estimated based on the prominent inwardly rectifying properties of Ca2+-permeable AMPARs.9 To obtain I-V curves, we used a previously described protocol,18 in which a neuron was held at −70 mV and ramped every 5 s initially to +50 mV, and then to −70 mV before and during the bath application of AMPA (Fig. 3A). AMPAR-mediated component of the recorded current was isolated by subtracting the ramp currents recorded before and during the agonist application (see Methods for details).
I-V curves obtained from the tonic neurons in the AS ODN-treated group was linear and did not show any rectification at positive membrane potentials on day 4 after intrathecal infusion of AS ODN (Fig. 3A). In contrast, extrasynaptic AMPAR-mediated currents displayed a weak inward rectification in the MS ODN-treated group as well as in the saline-treated animals (Fig. 3A). Rectification of AMPAR-mediated currents was quantitatively expressed as a rectification index (RI+30/−50). RI values were 1.02 ± 0.10 (n = 8) in the AS ODN-treated group and 0.79 ± 0.05 (n = 8; p = 0.07; Fig. 3B and 3C) in the MS ODN-treated group. At the same time, in the saline-treated rats, RI was 0.74 ± 0.07 (n = 11)18 that was significantly lower compared to that in the AS ODN-treated group (by 38 ± 4%; p < 0.05). Given that an increase in RI value after dorsal horn PKCα knockdown indicates a decrease in the proportion of extrasynaptic Ca2+-permeable AMPARs, our findings suggest that PKCα participates in trafficking of extrasynaptic Ca2+-permeable AMPARs in tonically firing SG neurons under normal conditions.
Consistent with our previous report,18 CFA-induced peripheral inflammation led to a strong inward rectification of the AMPA-induced currents. On day 1 after CFA injection, RI was 0.28 ± 0.05 (n = 5; p < 0.001 compared to control; Fig. 3C and 3D) and 0.27 ± 0.05 (n = 12; p < 0.001 compared to control) in the MS ODN-treated and saline-treated groups, respectively. CFA-induced inward rectification of AMPAR-mediated currents could be reversed by a selective blocker of Ca2+-permeable AMPARs, polyamine derivative, IEM-1460.8 In the MS ODN-treated group, IEM-1460 (40 µM) increased RI value by 67 ± 7% (n = 3; p < 0.05; Fig. 3C and 3E). However, we did not observe a significant inward rectification of AMPA-induced currents in tonic SG neurons from the AS ODN-treated group 1 d post-CFA (Fig. 4C). RI value was 0.90 ± 0.07 (n = 8; p < 0.001) that did not significantly differ from those in the AS ODN-treated non-inflamed animals (p > 0.35) or saline-treated non-inflamed rats (p > 0.1). Taking into account that a reversal potential of the AMPA-induced currents could be potentially right-shifted in the neurons of inflamed animals due to the greater contribution of Ca2+ influx in the total current, we also performed RI calculation based on the measurements of AMPAR conductance (not currents) at positive and negative potentials (+30 mV and −50 mV, respectively). The RI values, obtained in this way (see the Methods for details), were also significantly different in the AS ODN- and MS ODN-treated groups 1 d post-CFA (1.61 ± 0.23, n = 8 vs 0.59 ± 0.13, n = 6; p < 0.01), while no significant changes were found between the AS ODN- and MS ODN-treated groups 1 d post-saline (1.18 ± 0.13, n = 8 vs 1.09 ± 0.15, n = 9; p = 0.64). These results indicate that dorsal horn PKCα knockdown was sufficient to significantly abolish CFA-induced increase in the proportion of extrasynaptic Ca2+-permeable AMPARs in tonic SG neurons.
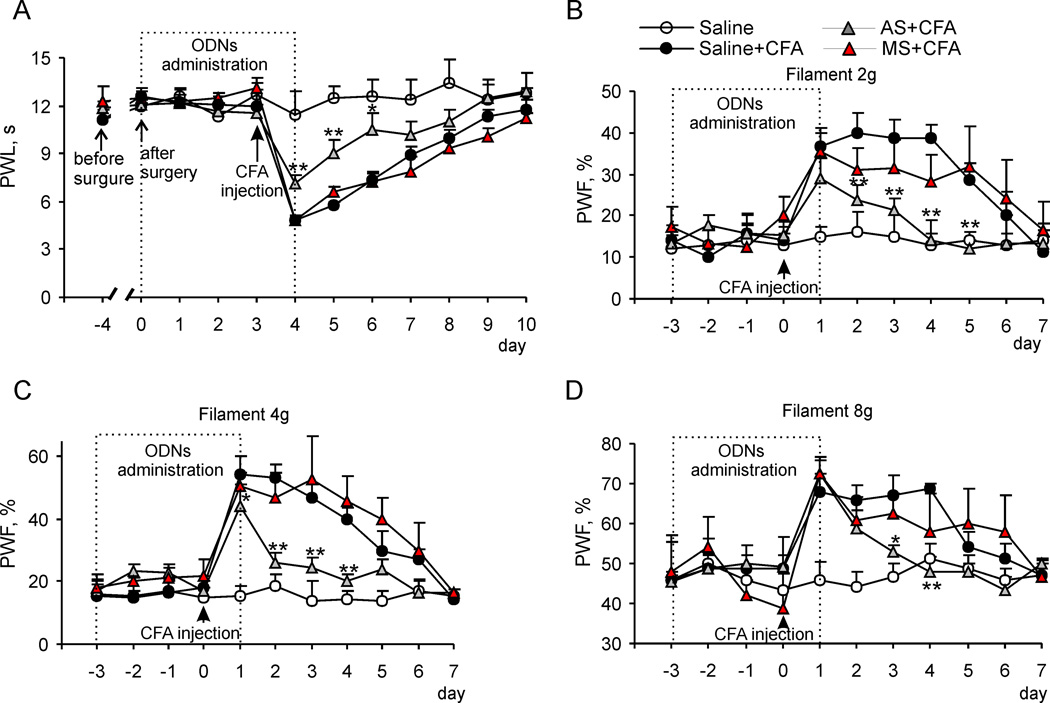
Fig. 4. Dorsal horn PKCα knockdown reduces CFA-induced peripheral thermal and mechanical hypersensitivities and maintenance of inflammatory pain.
(A) Intrathecal injection of 10 µg PKCα AS ODN, but not saline or 10 µg MS ODN, significantly attenuated the CFA-induced decrease in paw withdrawal latency (PWL) in response to thermal stimulation. (B–D) Effect of PKCα AS ODN or MS ODN treatment on CFA-induced increase in the paw withdrawal frequency (PWF) in response to 2 g (B), 4 g (C), and 8 g (D) von Frey filaments at different time points after CFA injection. * p < 0.05, ** p < 0.01 vs the corresponding time point in the saline-treated CFA-inflamed group. AS, antisense; MS, missense.
3.4 Effect of intrathecal PKCα AS ODN on CFA-induced inflammatory pain maintenance
So far, our findings indicate that spinal cord PKCα is required for inflammation-induced increase in Ca2+-permeable AMPARs at extrasynaptic sites of dorsal horn neurons. Finally, we examined whether spinal cord PKCα was also required for the maintenance of CFA-induced inflammatory pain. Therefore, we evaluated the effect of knock down of spinal cord PKCα on CFA-induced thermal and mechanical pain hypersensitivity. Consistent with previous reports,25,26 subcutaneous injection of CFA into a hind paw of rat led to a development of long-term thermal pain hypersensitivity on the ipsilateral (but not on the contralateral) side, which reached a peak level on day 1 and persisted for at least 5 d after CFA injection, in the saline-treated group (n = 10; Fig. 4). Inthrathecal AS ODN significantly alleviated CFA-induced thermal pain hypersensitivity 1 d post-CFA injection. Paw withdrawal latency reduced by CFA injection was increased by 47 ± 3% (n = 18; p < 0.001; Fig. 4A) in the AS ODN-treated group compared to the saline-treated group. In addition, the maintenance period of CFA-induced thermal pain hypersensitivity was markedly reduced in the AS ODN-treated group (Fig. 4A). The increases in paw withdrawal latency on days 2 and 3 after CFA injection (that is, on days 2 and 3 after the final AS OND injection) were 57 ± 5% (n = 11; p < 0.01) and 42 ± 2% (n = 7; p < 0.05), respectively, compared to those in the saline-treated group (Fig. 4A). In contrast, MS ODN given intrathecally (n = 14) did not affect the CFA-induced thermal pain hypersensitivity during the observation period (Fig. 4A). AS ODN intrathecally given alone did not change a basal response to thermal stimulation (Fig. 4A).
The effect of intrathecal PKCα AS ODN on CFA-induced mechanical pain hypersensitivity was also observed. Consistent with previous studies,3,25,26 CFA injection led to mechanical pain hypersensitivity manifested as a marked increase in paw withdrawal frequencies in response to von Frey filaments applied to the injected hind paw (Fig. 4B, 4C, 4D). The basal paw withdrawal frequencies in response to 2 g, 4 g, and 8 g von Frey filaments were similar among the saline-treated, AS ODN-treated, and MS ODN-treated groups (Fig. 4B, 4C, 4D), indicating no effect of intrathecal ODNs on a basal response to mechanical stimuli. CFA-induced increases in paw withdrawal frequencies in the AS ODN-treated group were significantly decreased compared to those in the saline-treated group 1 d after CFA injection (for 2 g von Frey filament: by 29 ± 5%, n = 10; for 4 g von Frey filament: by 19 ± 1%, n = 10; p < 0.05, Fig. 4B, 4C, 4D). In addition, the values of response frequencies to 2 g von Frey filament were reduced in the AS ODN-treated group (n = 10) by 47 ± 7%, 52 ± 7%, 64 ± 23 % and 58 ± 10% (p < 0.01, Fig. 4B) on day 2, 3, 4 and 5 post-CFA injection, respectively, compared to those in the saline-treated group. The values of response frequencies to 4 g von Frey filament were reduced in the AS ODN-treated group (n = 10) by 51 ± 14%, 48 ± 6% and 50 ± 8% (p < 0.01; Fig. 4C) on day 2, 3 and 4 post-CFA injection, respectively, compared to those in the saline-treated group. In the case of 8 g filament, the response frequencies was reduced significantly on day 3 and 4 (by 21 ± 1%, p < 0.05 and 30 ± 2%, p < 0.01, respectively; Fig. 4D).
Together, these findings indicate a functional role of PKCα in the maintenance of CFA-induced inflammatory pain.
4. Discussion
Activity-dependent trafficking of Ca2+-permeable AMPA receptors at synaptic and extrasynaptic plasma membranes plays a critical role in synaptic plasticity and spinal central sensitization associated with development and maintenance of pain.14,20,25,33 However, the molecular mechanisms that underlie the regulation of extrasynaptic Ca2+-permeable AMPAR trafficking in persistent pain are still elusive. Utilizing gene-silencing approach we have demonstrated in this study that spinal cord PKCα is required for the upregulation of Ca2+-permeable AMPARs at extrasynaptic sites of tonically firing dorsal horn SG neurons during the persistent inflammatory pain. This altered PKCα-dependent AMPAR trafficking in dorsal horn neurons is associated with the inflammation-induced pain hypersensitivity and might be involved in the maintenance of persistent inflammatory pain.
The data from the present study and those of others10 showed that gene-silencing approach based on local intrathecal administration of AS ODN specific for alpha isoform of PKC enzyme family significantly and selectively downregulated PKCα protein expression in the dorsal horn of spinal cord lumbar enlargement segment. This downregulation of PKCα was able to significantly attenuate the upregulation of extrasynaptic Ca2+-permeable AMPARs in SG neurons, produced by CFA-evoked peripheral inflammation. A high level of extrasynaptic AMPAR expression was detected in the dorsal horn26,27 and functional properties of extrasynaptic AMPARs have been characterized, demonstrating their substantial differences from synaptic ones (e.g. sensitivity to polyamine blockers and rectification index of the current).18,25,26 At the same time, normally most dorsal horn AMPARs are Ca2+ impermeable both at postsynaptic membrane17,25,34 and at extrasynaptic sites18. Using patch-clamp recording combined with Ca2+ imaging, we demonstrated a dramatic upregulation of Ca2+-permeable AMPARs in the extrasynaptic membrane of SG neurons 1 d post-CFA that is consistent with previous reports.18,26 An increase in the currents induced by bath applied AMPA in the present study is attributed to upregulation of extrasynaptic pool of Ca2+-permeable AMPARs, since there were no significant changes in the amplitude of synaptically evoked AMPAR-mediated eEPSCs in dorsal horn neurons 1d post-CFA, that was shown in our previous work25 and those of others.17,34 Besides, our previous EM and biochemical data further support this conclusion demonstrating an inflammation-induced increase in GluR1-containing Ca2+-permeable AMPARs in extrasynaptic membranes of dorsal horn neurons.18,26 In the present work, we have demonstrated that PKCα is required for this inflammation-induced receptors upregulation since knockdown of spinal PKCα significantly attenuated CFA-induced augmentation of the amplitudes of both AMPA-induced current and [Ca2+]i transients. Besides, PKCα knockdown significantly reversed an inward rectification of AMPA-induced currents, indicating that PKCα-dependent upregulation of extrasynaptic Ca2+-permeable AMPAR is due to altered AMPAR trafficking during persistent inflammation.
The changes in AMPAR-mediated currents and [Ca2+] transients could be also potentially mediated by the alternative mechanisms such as PKCα-dependent modulation of single channel conductance, channel gating or their affinity to agonists. However, GluR2-containing Ca2+-impermeable AMPARs are mainly contributed to AMPA-induced currents in control18 and changes in their affinity to AMPA and/or conductance during the inflammation could hardly affect their Ca2+ permeability. At the same time, the increased Ca2+ permeability of AMPARs was demonstrated by an increase in the amplitudes of [Ca2+]i transients (Fig.2). Moreover, the statistically significant changes in rectification of AMPA-induced currents (Fig. 3) and their increased sensitivity to polyamines18, both of which are the prominent features of GluR2-lacking Ca2+-permeable AMPARs9, directly indicate that altered AMPAR trafficking takes place during the persistent inflammation and should be the main contributor to the inflammatory-induced upregulation of Ca2+ permeability of AMPARs.
Inflammation-induced PKCα-dependent trafficking of extrasynaptic AMPARs specifically occurs in SG neurons characterized by intrinsic tonically firing properties. Neither CFA-induced increase in functional expression of Ca2+-permeable AMPARs (this work and 18) nor PKCα-dependent extrasynaptic AMPARs trafficking have been observed in neurons exhibiting strong adaptation. At the same time, similar values of AMPA-induced currents and associated [Ca2+]i transients recorded in tonic and transient SG neurons under normal conditions (this work and 18) suggest a similar pattern of functional expression of extrasynaptic AMPARs in both neuronal types. Thus, PKCα plays an essential role in inflammatory-induced AMPAR trafficking only in tonically firing SG neurons. Knockdown of dorsal horn PKCα did not significantly change electrophysiological properties of both tonic and transient SG neurons suggesting that PKCα silencing does not substantially modulate neuronal excitability. Since AMPAR trafficking is required for the maintenance of persistent inflammatory pain,17,25 our data suggest that the transient SG neurons might not contribute to the maintenance of persistent pain. Taking into account that the overwhelming majority of tonic SG interneurons are excitatory,28 our results suggest that spinal cord PKCα activation in tonically firing excitatory dorsal horn SG neurons is specifically required for inflammation-induced upregulation of extrasynaptic Ca2+-permeable AMPARs during the maintenance period of inflammatory pain.
Dorsal horn PKCα may also play a functional role in Ca2+-permeable AMPARs trafficking in SG neurons under physiological conditions. E.g. the amplitudes of somatic and dendritic AMPA-induced [Ca2+]i transients in the AS ODN-treated CFA-inflamed group were lower compared to those in the saline-treated non-inflamed group. This observation indicates that knockdown of PKCα is sufficient to suppress Ca2+-permeability of extrasynaptic AMPARs to the level, which was even lower than in control and suggests a decreased pool of extrasynaptic Ca2+-permeable AMPARs upon PKCα knockdown under normal conditions. Moreover, in AS ODN-treated non-inflamed rats, the I-V relationship of the AMPA-induced currents was linear (RI=1.02) contrary to slightly rectified I-V curves either in the MS ODN-treated (RI=0.79) or saline-treated groups (RI=0.74). Therefore, it is likely that under normal conditions PKCα might regulate trafficking of Ca2+-permeable AMPARs in tonic SG neurons.
Phosphorylation of GluR1 by PKCα may underlie the observed CFA-induced trafficking of extrasynaptic Ca2+-permeable AMPAR in dorsal horn neurons during inflammatory pain maintenance. As we have demonstrated previously, CFA-induced peripheral inflammatory insult promotes insertion of GluR1 into extrasynaptic membrane of SG neurons.18 A few serine residue sites on the GluR1 have been identified in hippocampal neurons to be phosphorylated by PKC: S831, S818 and S816.4,6,15,22 S831 is a substrate for both PKC and CaMKII and undergoes phosphorylation during long-term potentiation,5 increasing the single channel conductance of synaptic AMPARs23 but does not influence membrane insertion.15 In contrast, phosphorylation of GluR1 at S818 by PKC promotes synaptic incorporation of GluR1-containing AMPARs and facilitates their interaction with a protein 4.1N, a downstream actin-binding protein, stabilizing GluR1.6,29 More recent studies also demonstrated that protein 4.1N is required for activity dependent GluR1 insertion at extrasynaptic sites by the mechanism of PKC-dependent phosphorylation of GluR1 S816 and S818, which in turn enhances 4.1N binding to GluR1 and facilitates GluR1-containing AMPARs insertion in extrasynaptic plasma membrane.22 Thus, it is likely that an inflammation-induced increase in extrasynaptic Ca2+-permeable AMPARs is due to PKCα-dependent phosphorylation of GluR1 S816 and S818 and subsequent promoted insertion of GluR1-containing AMPARs in extrasynaptic plasma membrane in dorsal horn neurons during the maintenance of inflammation. This conclusion could be further confirmed if specific and selective antibodies against phosphorylation sites of GluR1 S816 and S818 were available.
PKCα-dependent trafficking of extrasynaptic Ca2+-permeable AMPARs in dorsal horn SG neurons might be involved in the maintenance of CFA-induced persistent inflammatory pain. Our results demonstrate that acute down-regulation of dorsal horn PKCα significantly attenuated thermal and mechanical pain hypersensitivity during the CFA-induced inflammatory pain maintenance. Moreover, a significant shortening of the maintenance period of inflammatory pain was also observed. At the same time, AS OND alone did not produce any significant effects on basal responses to thermal and mechanical stimuli. Our behavioral results are in contrast with the report of Zhao et al. that PKCβ, γ and δ, but not PKCα, are involved in the CFA-induced thermal hyperalgesia and that none of PKC isoforms are required in the development of CFA-induced mechanical allodynia in knockout mice.39 The reason for these differences may include distinct species used as well as an aging difference. Besides, a gene knockout approach is limited by a development of multiple compensatory mechanisms leaving knockdown strategies to be more reliable alternative to perform inducible and localized knockouts in nociceptive pathways.
The observed anti-hyperalgesic effect of dorsal horn PKCα knockdown might be apparently related to preventing PKCα-dependent extrasynaptic Ca2+-permeable AMPAR insertion, as replenishing the extrasynaptic AMPAR pool is required for synaptic insertion of Ca2+-permeable AMPARs11 observed during the maintenance of inflammatory pain.25,34 Besides, in the conditions of strong primary afferent inputs during injures or inflammation, an excessive glutamate release occurs from presynaptic neurons and glia producing glutamate spillover from nearby primary afferent terminals1,19,36 that activates extensive population of extrasynaptic AMPARs thus strengthening glutamatergic transmission. Promoted membrane insertion of GluA1 causally links to increased density of Ca2+-permeable AMPARs firstly at extrasynaptic sites and then in synapses (two-step delivery mechanism of AMPARs), increasing Ca2+ influx and potentiating irreversible chronic damage of calcium homeostasis. Such events might contribute to the maintenance of inflammatory pain. It should be noted that other mechanism is also involved in the observed anti-hyperalgesic effect of dorsal horn PKCα knockdown. Internalization of synaptic GluR2-containing Ca2+-impermeable AMPARs in dorsal horn neurons17,25,34 also involves an activation of PKCα, as an upstream trigger, with subsequent GluR2 phosphorylation at S880, disruption of GluR2 binding to its synaptic anchoring protein, and GluR2 internalization.25 Targeted mutation of the GluR2 PKCα phosphorylation at S880 prevents GluR2 internalization and facilitates CFA-induced thermal and mechanical pain hypersensitivity in the animals during the maintenance period of inflammatory pain.14,25 Thus, PKCα-dependent phosphorylation of GluR1 and GluR2 AMPAR subunits in tonically firing excitatory SG neurons participate in long-lasting changes of functional expression of synaptic and extrasynaptic AMPARs and might be a possible mechanism contributing to the maintenance of persistent inflammatory pain.
In conclusion, our study demonstrates that dorsal horn PKCα knockdown significantly attenuates the inflammatory-induced upregulation of extrasynaptic Ca2+-permeable AMPARs in tonically firing SG neurons during the maintenance period of inflammatory pain. This attenuation is associated with a substantial anti-nociceptive effect of dorsal horn PKCα knockdown during the persistent peripheral inflammation. Our findings reveal a functional role of PKCα as an intracellular trigger for trafficking of extrasynaptic Ca2+-permeable AMPARs in dorsal horn neurons and may inspire the possible implications of PKCα gene-silencing therapy for the treatment of persistent inflammatory pain.
Perspective.
The present study shows that PKCα knockdown blocks inflammatory-induced upregulation of extrasynaptic Ca2+-permeable AMPARs in dorsal horn neurons and produces anti-nociceptive effect during the maintenance period of inflammatory pain. These findings may inspire the possible implications of PKCα gene-silencing therapy for preventing and/or treating persistent inflammatory pain.
Acknowledgments
Disclosures: This work was supported by NASU Biotechnology, STCU #5510, DFFD F46.2/001 Grants (N.V.), and NASU Grant for Young Scientists (O.K.), by Mr. David Koch and the Patrick C. Walsh Prostate Cancer Research Fund, the Blaustein Pain Research Fund, Rita Allen Foundation, and NIH Grants NS 058886 and NS072206 (Y.X.T) and by NIH Biobehavioral Pain Research Fellowship (F.E.A.).
Footnotes
The authors declare no conflict of interests.
References
- 1.Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- 2.Arendt KL, Royo M, Fernandez-Monreal M, Knafo S, Petrok CN, Martens JR, Esteban JA. PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane. Nat Neurosci. 2010;13:36–44. doi: 10.1038/nn.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atianjoh FE, Yaster M, Zhao X, Takamiya K, Xia J, Gauda EB, Huganir RL, Tao YX. Spinal cord protein interacting with C kinase 1 is required for the maintenance of complete Freund's adjuvant-induced inflammatory pain but not for incision-induced post-operative pain. Pain. 2010;151:226–234. doi: 10.1016/j.pain.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- 5.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 6.Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- 8.Buldakova SL, Kim KK, Tikhonov DB, Magazanik LG. Selective blockade of Ca2+ permeable AMPA receptors in CA1 area of rat hippocampus. Neuroscience. 2007;144:88–99. doi: 10.1016/j.neuroscience.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 10.Butler M, Hayes CS, Chappell A, Murray SF, Yaksh TL, Hua XY. Spinal distribution and metabolism of 2'-O-(2-methoxyethyl)-modified oligonucleotides after intrathecal administration in rats. Neuroscience. 2005;131:705–715. doi: 10.1016/j.neuroscience.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 11.Choquet D, Triller A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci. 2003;4:251–265. doi: 10.1038/nrn1077. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson AR, Christensen RN, Gensel JC, Miller BA, Sun F, Beattie EC, Bresnahan JC, Beattie MS. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J Neurosci. 2008;28:11391–11400. doi: 10.1523/JNEUROSCI.3708-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guire ES, Oh MC, Soderling TR, Derkach VA. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci. 2008;28:6000–6009. doi: 10.1523/JNEUROSCI.0384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann B, Ahmadi S, Heppenstall PA, Lewin GR, Schott C, Borchardt T, Seeburg PH, Zeilhofer HU, Sprengel R, Kuner R. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron. 2004;44:637–650. doi: 10.1016/j.neuron.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 16.Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katano T, Furue H, Okuda-Ashitaka E, Tagaya M, Watanabe M, Yoshimura M, Ito S. N-ethylmaleimide-sensitive fusion protein (NSF) is involved in central sensitization in the spinal cord through GluR2 subunit composition switch after inflammation. Eur J Neurosci. 2008;27:3161–3170. doi: 10.1111/j.1460-9568.2008.06293.x. [DOI] [PubMed] [Google Scholar]
- 18.Kopach O, Kao SC, Petralia RS, Belan P, Tao YX, Voitenko N. Inflammation alters trafficking of extrasynaptic AMPA receptors in tonically firing lamina II neurons of the rat spinal dorsal horn. Pain. 2011;152:912–923. doi: 10.1016/j.pain.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kullmann DM. Spillover and synaptic cross talk mediated by glutamate and GABA in the mammalian brain. Prog Brain Res. 2000;125:339–351. doi: 10.1016/S0079-6123(00)25023-1. [DOI] [PubMed] [Google Scholar]
- 20.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonoudakis D, Zhao P, Beattie EC. Rapid Tumor Necrosis Factor {alpha}-Induced Exocytosis of Glutamate Receptor 2-Lacking AMPA Receptors to Extrasynaptic Plasma Membrane Potentiates Excitotoxicity. J Neurosci. 2008;28:2119–2130. doi: 10.1523/JNEUROSCI.5159-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luthi A, Wikstrom MA, Palmer MJ, Matthews P, Benke TA, Isaac JT, Collingridge GL. Bi-directional modulation of AMPA receptor unitary conductance by synaptic activity. BMC Neurosci. 2004;5:44. doi: 10.1186/1471-2202-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 25.Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009;29:3206–3219. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JS, Yaster M, Guan X, Xu JT, Shih MH, Guan Y, Raja SN, Tao YX. Role of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund's adjuvant-induced inflammatory pain. Mol Pain. 2008;4:67. doi: 10.1186/1744-8069-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petralia RS, Wang YX, Mayat E, Wenthold RJ. Glutamate receptor subunit 2-selective antibody shows a differential distribution of calcium-impermeable AMPA receptors among populations of neurons. J Comp Neurol. 1997;385:456–476. doi: 10.1002/(sici)1096-9861(19970901)385:3<456::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Santos SF, Rebelo S, Derkach VA, Safronov BV. Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol. 2007;581:241–254. doi: 10.1113/jphysiol.2006.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 41N-linked actin cytoskeletal association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 31.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 32.Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao YX. Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain. Anesthesiology. 2010;112:1259–1265. doi: 10.1097/ALN.0b013e3181d3e1ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vikman KS, Rycroft BK, Christie MJ. Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol. 2008;586:515–527. doi: 10.1113/jphysiol.2007.145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voitenko N, Gerber G, Youn D, Randic M. Peripheral inflamation-induced increase of AMPA-mediated currents and Ca2+ transients in the presence of cyclothiazide in the rat substantia gelatinosa neurons. Cell Calcium. 2004;35:461–469. doi: 10.1016/j.ceca.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Weng HR, Chen JH, Cata JP. Inhibition of glutamate uptake in the spinal cord induces hyperalgesia and increased responses of spinal dorsal horn neurons to peripheral afferent stimulation. Neuroscience. 2006;138:1351–1360. doi: 10.1016/j.neuroscience.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 37.Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Tao F, Liaw WJ, Bredt DS, Johns RA, Tao YX. Effect of knock down of spinal cord PSD-93/chapsin-110 on persistent pain induced by complete Freund's adjuvant and peripheral nerve injury. Pain. 2003;106:187–196. doi: 10.1016/j.pain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Zhao C, Leitges M, Gereau RW. Isozyme-specific effects of protein kinase C in pain modulation. Anesthesiology. 2011;115:1261–1270. doi: 10.1097/ALN.0b013e3182390788. [DOI] [PMC free article] [PubMed] [Google Scholar]