Abstract
We have previously found that neonatal treatment with clomipramine (CLI) induced a decrease in brain orexins during the juvenile period and that these changes were reversed at adulthood. This study investigated the effect of CLI on the orexinergic component and sleep/wake states. Two groups of adult male rats were conducted for 48-h polysomnographic recording. One group of rats was treated with CLI (20 mg/kg every 12 h), and a second group was treated with equivolume of saline (SAL) simultaneously after the first 24 h of polysomnographic recording. Rats were killed 2 h after the third dose of treatment. Brain tissues were collected for radioimmunoassay quantification of orexins and real-time PCR analysis of prepro-orexin and orexin receptor mRNA. The CLI group had significantly shorter rapid eye movement (REM) sleep and longer REM latency compared with both the baseline day and the SAL group and had significantly less active wake and more quiet wake. Compared with the control rats, the CLI rats had significantly higher mRNA expression of prepro-orexin in the hypothalamus and the frontal cortex, but not in the hippocampus. The CLI rats also had significantly less orexin B in the hypothalamus than the control rats. These results suggest that suppression of active wake and orexin B by CLI may be a factor responsible for CLI-induced depression and that the increase of prepro-orexin mRNA may be a sign of increased brain orexins found in this model.
Keywords: antidepressant, clomipramine, orexin, orexin receptors, rats, sleep, wake
Introduction
Clomipramine (CLI) is a tricyclic agent with both antidepressant and antiobsessional properties (Judd, et al., 1991). Clomipramine inhibits norepinephrine (Crews and Smith, 1980) and serotonin uptake (Henderson, 1983) and increases their release in central nerve terminals, possibly by blocking the membrane-pump of neurons. This action appears to enhance the release of transmitter monoamines at receptor sites per impulse (Sangdee and Franz, 1979; Romero, et al., 1996) from desensitisation of the terminal autoreceptor (Maudhuit, et al., 1995) because blocking 5-HT1A receptor abolished the increase of serotonin (5-HT) (Auerbach, et al., 1995). Although the actual neurochemical mechanism is unknown, the capacity of CLI to inhibit serotonin reuptake is thought to be an important mechanism underlying its effects. Clomipramine is also a powerful rapid eye movement (REM) sleep suppressant (Mirmiran, et al., 1981; Mirmiran, et al., 1983). We have previously reported that CLI strongly suppresses REM sleep without significantly altering wake percentage when administered to rats from the age of 2 to 3 weeks (Feng and Ma, 2002).
Despite CLI’s powerful antidepressant effect in adult human, many studies have shown that administering CLI in rat during the neonatal period results in multiple behavioural, neuronal and molecular changes in adulthood that resemble human depression (Mirmiran, et al., 1981; Mirmiran, et al., 1983; Vogel, et al., 1990b; Kinney, et al., 1997). The adult CLI rat has been well evaluated as a model of depression (Vogel, et al., 1990b; Vogel, 1999). Recently, we reported that 2 weeks of treatment with CLI in rat during the neonatal period induced a substantial decrease in orexin A and orexin B at 5 weeks of age. The affected regions include the hypothalamus, hippocampus and frontal cortex. However, in adult CLI rats, these changes were reversed; orexins were found to be increased in this model (Feng, et al., 2007). Other studies showed a decrease in the number or the size of hypothalamic hypocretin neurons in Wistar-Kyoto (WKY) rats compared with the control of Wistar (WIS) rats (Allard, et al., 2004); decreased cerebrospinal fluid levels of orexin A in suicidal patients (Brundin, et al., 2007b) and patients with major depressive disorder (Brundin, et al., 2007a) were also reported. These findings suggest that CLI affects both REM sleep and orexinergic regulation in the neonatal period and that orexinergic alteration may be involved in the development of depressive pathogenesis.
Orexins, including orexin A and orexin B (also called hypocretin 1 and 2) isolated from the hypothalamus (De Lecea, et al., 1998; Sakurai, et al., 1998), were originally described as part of the hypothalamic network for energy homeostasis and are known to promote wakefulness and suppress sleep (Trivedi, et al., 1998; Espana, et al., 2001). Orexins are synthesised in neurons of the perifornical region and the lateral hypothalamus. These neurons receive inputs from diverse sensory and limbic systems and innervate, via their fibres, most brain regions including the brain stem and basal forebrain, cortex and spinal cord (Peyron, et al., 1998; Zhang, et al., 2001; Mignot, et al., 2002; Sutcliffe and de Lecea, 2002). Local application of orexin A in the basal forebrain (Thakkar, et al., 2001) or in the laterodorsal and pedunculopontine tegmentum (LDT/ PPT) (Xi, et al., 2001) increases wakefulness dramatically. Intracerebroventricular administration of orexin A promotes both quiet wake and active wake, but orexin B promotes active wake only in the normal adult rat. Orexin A promotes quiet wake only if administered into the medial preoptic area, medial septal area and substantia innominata (Espana, et al., 2001). Brain injection of orexin A in the hypothalamic paraventricular nucleus, rostral lateral hypothalamic area and substantia nigra pars compacta significantly increases time spent rearing and ambulating (Kotz, et al., 2006).
Although the orexinergic neuronal system has been shown to be a key component of wake/sleep regulation, energy metabolism and the pathology of narcolepsy, recent evidence suggests that suppression of this system may be involved in the pathogenesis of depression. First, orexin A levels have been shown to be decreased in the cerebrospinal fluid in human depression (Salomon, et al., 2003; Brundin, et al., 2007a). Second, the number and size of orexin A–immunostained neurons were decreased in a genetic rat model of depression (Allard, et al., 2004). As mentioned earlier, our own research has shown that the hypothalamic levels of both orexin A and B were significantly increased in a rat model of depression (Feng, et al., 2008). We also found that orexin A levels were increased in a second rat model of depression with additional features of stress and insomnia (Feng, et al., 2008). At the neuronal level, orexin excites serotonin (5-HT) neurons and increases 5-HT release (Brown, et al., 2002; Liu, et al., 2002), which has been widely acknowledged to play a role in depression. In turn, orexins are inhibited by 5-HTnergic neurons (Muraki, et al., 2004; Li and van den Pol, 2005). This closed feedback circuit for the neurobiological regulation of orexin-5–HT activity may provide new insights into the understanding of the pathogenesis of depression.
In the following study, we hypothesise that treatment with CLI will have an immediate biological effect on orexinergic components and sleep/wake states. We report the effect of CLI on orexinergic reactions and wake/sleep states in normal adult rats.
Materials and methods
Animal and sleep study
Adult Long Evans male rats were used throughout the study. All procedures were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University and the Louis Stokes Cleveland Veterans Affairs Medical Center or by the Institutional Animal Care and Use Committee of Zhengzhou University. Twenty-three adult rats (3- to 5-month old) were surgically implanted with electrodes for polysomnographic recording of electroencephalogram (EEG) and electro-myogram (EMG) under pentobarbital anaesthesia. After 7 days of post surgical recovery and 3 days of adaptation to recording chambers, baseline polysomnographic recording was conducted. After baseline recording, rats were injected a total of three times (i.p.) with either saline (SAL, n = 11) or CLI (20 mg/kg in double distilled water, n = 12) at 12-h intervals (8:00 a.m. and 8:00 p.m.).
Polysomnographic data were scored as active wake, quiet wake, rapid eye movement (REM) sleep and non REM (NREM) sleep in 30-s epochs by a computer program and subsequently confirmed visually according to our established method (Feng and Ma, 2003; Feng, et al., 2008).
Ribonucleic acid (RNA) and peptide extraction
Sixteen rats were used for quantification of orexins and mRNA of prepro-orexin, orexin 1 receptor (OX1R) and orexin 2 receptor (OX2R). Three injections of either CLI (n = 8) or SAL (n = 8) were administered as described above, without surgery or polysomnographic recording. Animals were killed 2 h after the last injection. Rats were immediately decapitated after 2 min of CO2 exposure. Brain tissues were dissected immediately and tissue blocks of the hypothalamus, frontal cortex and hippocampus were divided into two sets. One set was processed for peptide extraction according to our previous publication (Feng, et al., 2008), and the other set was processed for RNA extraction in the following method.
Total RNA was extracted from rat brain tissue using TRI-zol reagent as specified by the manufacturer (Invitrogen, Carlsbad, California, USA). Briefly, each tissue blocks were immersed in 2 mL TRIzol reagent and homogenised immediately following collection. After the addition of 0.2 mL chloroform, the samples were centrifuged at 12,000 × g for 15 min at 4 °C to obtain phase separation. The upper aqueous phase containing RNA was transferred to a clean set of microcentrifuge tubes and diluted in 0.5 mL of 100% isopropyl. The tubes were centrifuged at 12,000 × g for 10 min at 4 °C to obtain a solid pellet. The RNA pellet was then washed in 75% ethanol and recentrifuged. After discarding the supernatant and air-drying the RNA pellet for 10 min under a hood, the RNA was dissolved in diethylpyrocarbonate-treated water and stored at −80 °C for real-time PCR.
Real-time PCR quantification of mRNA of orexin-1 receptor (OX1R), orexin-2 receptor (OX2R) and prepro-orexin
Total RNA of the hypothalamus, hippocampus and frontal cortex from eight rats in each group were analysed by the Gene Expression and Genotyping Facility of Case Western Reserve University, Ohio, USA. Integrity of the isolated RNA was verified with Bioanalyzer 2100 (Agilent Technologies Inc., Santa Clara, CA) before further analysis. Three micrograms of RNA from each sample were reverse transcribed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) to generate rat cDNA (100 µL in total) for amplification. Rat-specific Taq- Man probe and primer set (Applied Biosystems, Foster City, CA) were used for real-time PCR amplification of preproorexin, OX1R, OX2R and β-actin as an endogenous control mRNA. Amplifications were conducted according to the manufacturer’s protocol on an ABI PRISM 9700 sequence system (Applied Biosystems, Foster City, CA). The manufacturer’s standard thermal cycler program used was 50 °C for 2 min, 95 °C for 10 min, then 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Results were generated using ABI SDS 2.0 software (SDS 2.2 software, Applied Biosystems, Foster City, CA) and are presented as relative fold changes versus a designated calibrator sample, which was the mean of the control group.
The quantitation of mRNA from each sample was first standardised by the mRNA of β-actin and then calibrated by the mean of the control group. We present the relative quantitation (RQ) calculated by dividing the value of each individual quantitation (Q) with the mean quantitation (MQ) of the control group. The RQ of OX1R was calculated as the following:
where Q, quantitation; QA, quantitation of one individual sample. RQ values for each gene were calculated from each sample.
We present the relative quantitation (RQ) calculated by dividing each actual value with the mean of control group. Thus, each actual value would have one relative value. Results include 95% confidence limits.
Radioimmunoassay (RIA) quantification of orexin A and orexin B
Orexin peptides were measured by RIA using standard RIA kits for detecting orexin A (#RK-003-30) and orexin B (#RK-003-32) purchased from Phoenix Pharmaceuticals, Inc., (Belmont, California, USA) according to the standard protocol provided with the kits (Feng, et al., 2007, 2008). Orexin levels were calculated as the value of picogram per milligram of wet tissue.
Data analysis and statistics
Two-way (Day × Treatment; Region × Treatment and Treatment × Gene) ANOVAs were used to evaluate sleep states, brain peptide levels and mRNA expression, respectively. All Pairwise Multiple Comparison Procedures (Bonferroni t-test) were used for further analysis of test-specific and region-specific differences. Student’s t-test was used to analyse a variable that was not qualified for two-way ANOVA evaluation. To minimise the probability that the large variation in peptide levels between brain regions would mask differences because of treatment, brain regions that were found to have very high levels of orexins were analysed apart from those with lower levels. All data are presented as mean ± standard error.
Results
Wake/sleep states
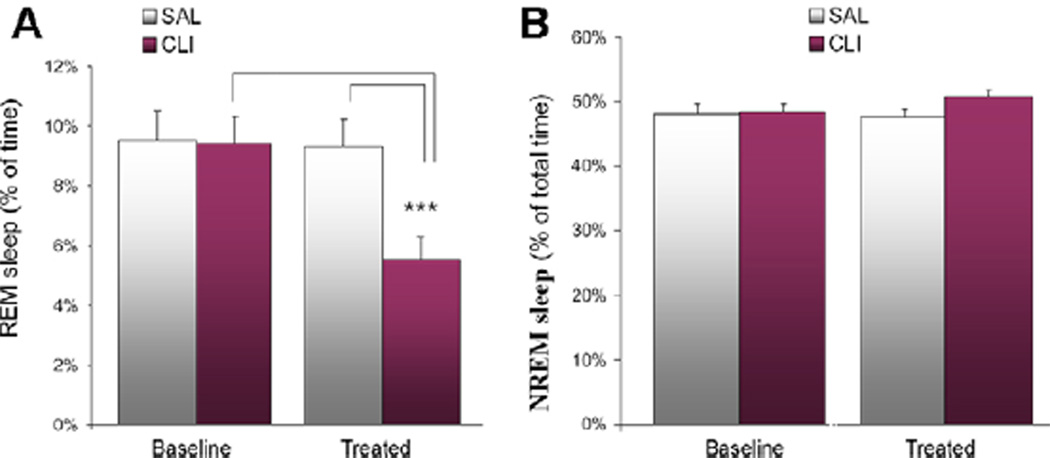
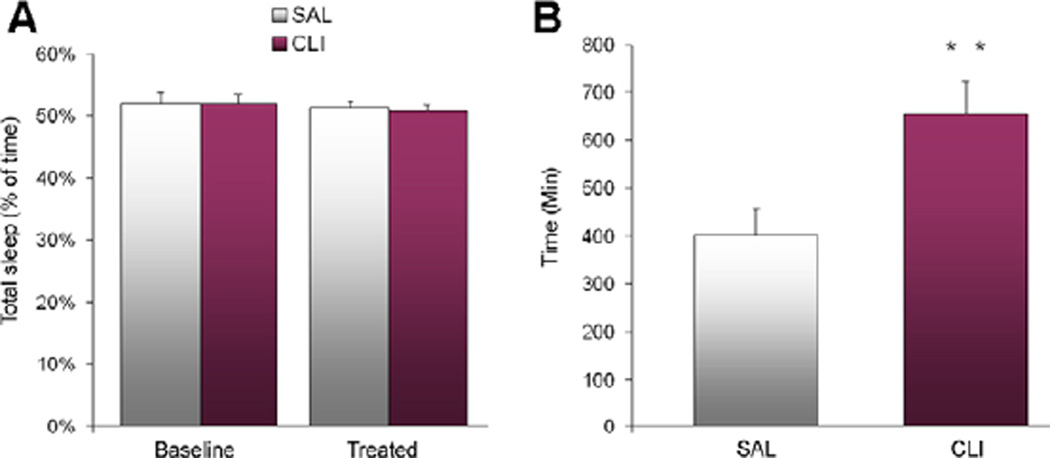
Twenty-four hours of polysomnographic recording was conducted before and after treatment with CLI or saline (SAL group). The baseline wake/sleep states were similar between the CLI and SAL groups. However, after treatment, the CLI rats had 41.39% less REM sleep than the SAL group and 40.57% less than the baseline. A two-way (Day × Treatment) ANOVA found significant differences in REM sleep (F = 44.496, P < 0.001). All Pairwise Multiple Comparisons (Bonferroni t-test) showed that the CLI group had significantly less REM sleep on the treatment day than the SAL group (t = 9.650, P < 0.001) and the baseline (t = 9.942, P < 0.001) (Figure 1A). No differences were found in the comparisons of NREM sleep (Figure 1B) or total sleep (Figure 2A). However, analysis of sleep latency found that latency to REM sleep was increased by 63.16% in the CLI group compared with the SAL group. A Student’s t-test found that this difference was significant (t = 3.411, n = 21, P = 0.003) (Figure 2B).
Figure 1.
REM and NREM sleep over 48 h (24 h before and after treatment with saline (SAL) or clomipramine (CLI) in rats). (A) REM sleep after treatment was significantly reduced in the CLI group compared with both the SAL group (P < 0.001) and the baseline (P < 0.001). (B) No significant differences were seen in NREM sleep either between groups or between treatments.
Figure 2.
Total sleep and sleep latency over 48 h (24 h before and after treatment with saline (SAL) or clomipramine (CLI) in rats). (A) No differences were found in comparisons of total sleep. (B) REM sleep latency after treatment was significantly increased in the CLI group compared with the SAL group (P = 0.003).
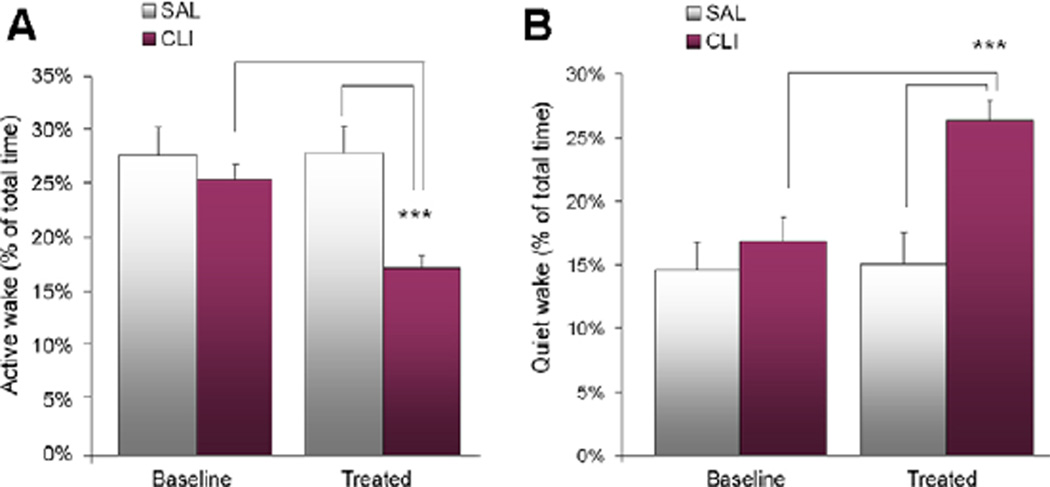
Wake was scored as active wake and quiet wake using our established method (Feng, et al., 2008). After treatment, active wake in the CLI group was 38.11% lower than the SAL group and 32.11% lower than the baseline (Figure 3A). The differences were found to be significant by a two-way (Day × Treatment) ANOVA (F = 50.470, P < 0.001) and post-hoc comparisons. That is, the CLI group had significantly less active wake after treatment than the SAL group (t = 5.937, P < 0.001) and the baseline (t = 9.617, P < 0.001). On the contrary, quiet wake in the CLI group increased 75.19% after treatment compared with the SAL group and 56.37% compared with the baseline of the same group (Figure 3B). These differences were found to be significant by a two-way (Day × Treatment) ANOVA (F = 50.470, P < 0.001) and post-hoc comparisons. Effectively, the CLI group had significantly more quiet wake after treatment than the SAL group (t = 14.301, P < 0.001) and the baseline (t = 19.179, P < 0.001).
Figure 3.
Active wake (AW) and quiet wake (QW) over 48 h (24 h before and after treatment with saline (SAL) or clomipramine (CLI) in rats). (A) There was no significant difference in the baseline percentage between groups in AW. However, AW after treatment was significantly reduced in the CLI group compared with both the SAL group (P < 0.001) and the baseline (P < 0.001). (B) There were no significant differences in the baseline percentage between groups in QW. However, QW was significantly increased in the group treated with CLI compared with the group treated with SAL. This increase was also significant compared with the baseline (P < 0.001).
Gene expression
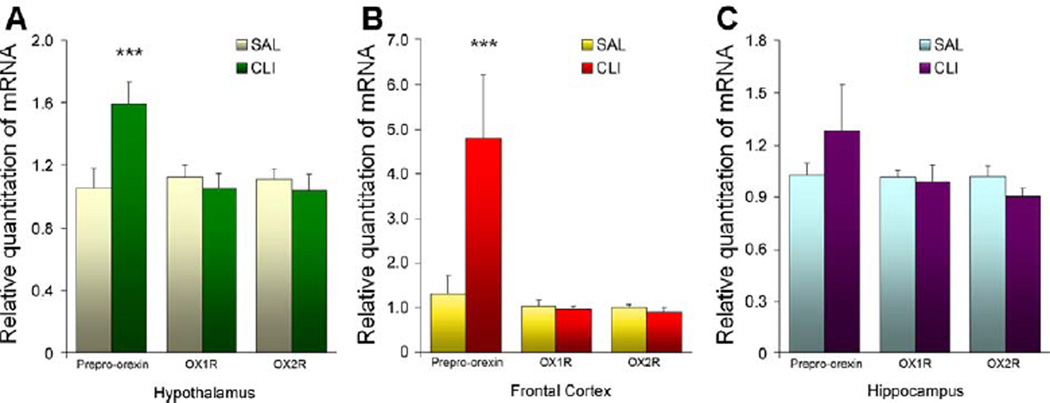
Expression of mRNA of OX1R, OX2R and prepro-orexin in the hypothalamus, frontal cortex and hippocampus was quantified by real-time PCR. As shown in Figure 4, there were no significant differences in the expression of OX1R or OX2R (presented by the RQ) in any of the tested brain regions. However, significant differences of prepro-orexin mRNA were found in both the hypothalamus and frontal cortex. In the hypothalamus, the RQ of prepro-orexin mRNA was 50.80% higher in the CLI group than in the SAL group. A two-way (Treatment × Gene) ANOVA (F = 5.276, P = 0.010) and post-hoc Bonferroni t-test (t = 3.521 and P = 0.001) found that this difference was highly significant. In the frontal cortex, the disparity was much greater. Compared with the SAL group, the mean RQ was 262.65% higher in the CLI group. A two-way (Treatment × Gene) ANOVA (F = 5.753, P = 0.006) and post-hoc comparisons (t = 4.047, P < 0.001) confirmed that this difference was also significant. A similar trend was found in the hippocampus. In the CLI group, the mean RQ of preproorexin mRNA was 25.38% greater than in the SAL group. However, a two-way (Treatment × Gene) ANOVA (F = 1.249, P = 0.297) found that this difference was not significant.
Figure 4.
Gene expression of prepro-orexin, orexin-1 receptor (OX1R) and orexin-2 receptor (OX2R) in the hypothalamus in rats treated with saline (SAL) or clomipramine (CLI). There is no difference between groups in the expression of OX1R and OX2R mRNA in any of the tested brain regions. However, the expression of prepro-orexin mRNA was significantly increased in rats treated with CLI in the hypothalamus (t = 3.521, P = 0.001) and frontal cortex (t = 4.047, P < 0.001), but not in the hippocampus.
Brain levels of orexin A and orexin B
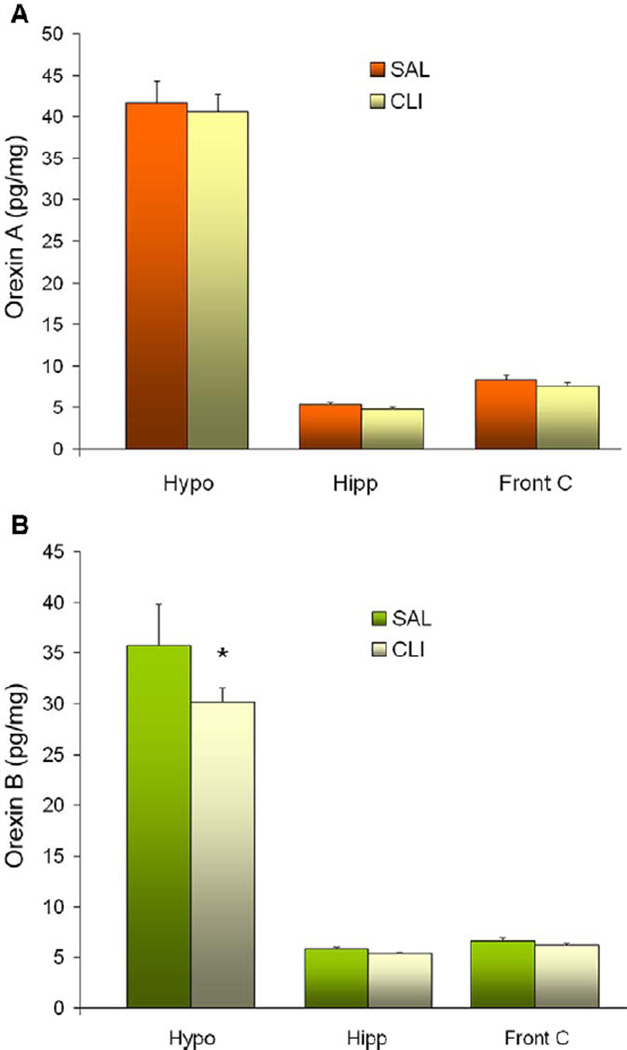
Brain levels of orexins were quantified by RIA in the hypothalamus, hippocampus and frontal cortex. The mean levels of orexin A in the hypothalamus were 40.53 ± 2.10 pg/mg of tissue in the CLI rats and 41.168 ± 2.65 pg/mg in the SAL rats. These means were 4.80 ± 0.28 pg/mg (CLI) and 5.35 ± 0.28 pg/mg (SAL) in the hippocampus and 7.54 ± 0.41 pg/mg (CLI) and 8.38 ± 0.564 pg/mg (SAL) in the frontal cortex (Figure 5A). A two-way (Region × Treatment) ANOVA found no differences between groups in any brain regions (F = 0.0555 and P = 0.954).
Figure 5.
Orexin peptide levels of three brain regions in rats treated with saline (SAL) or clomipramine (CLI). (A) Orexin A levels in the hypothalamus (Hypo), hippocampus (Hipp) and frontal cortex (Front C). No significant difference between treatments groups was seen in any of these brain regions. (B) Orexin B levels in the same brain regions. Hypothalamic orexin B was significantly (t = 2.391, P = 0.021) decreased after three doses of treatment with CLI.
Orexin B levels were similarly quantified in the same brain regions. The mean levels of orexin B were 30.15 ± 1.38 pg/mg (CLI) and 35.78 ± 3.99 pg/mg (SAL) in the hypothalamus, 5.36 ± 0.16 pg/mg (CLI) and 5.84 ± 0.15 pg/mg (SAL) in the hippocampus and 6.18 ± 0.23 pg/mg (CLI) and 6.69 ± 0.24 pg/mg (SAL) in the frontal cortex (Figure 5B). A two-way (Region × Treatment) ANOVA found a significant difference in the hypothalamus (t = 2.391, P = 0.021). Specifically, the CLI group had significantly less orexin B (a decrease of 15.76%) compared with the SAL group.
Discussion
Our study produced three major findings. First, treatment with CLI induced several wake/sleep alterations. CLI rats were found to have significantly less REM sleep, significantly increased REM sleep latency, significantly less active wake and significantly more quiet wake. Second, real-time PCR analysis of mRNA found that CLI rats had increased mRNA expression of prepro-orexin in the hypothalamus and frontal cortex, but not in the hippocampus. Furthermore, mRNA expression of OX1R and OX2R were not different between groups in any of the brain regions measured. Finally, RIA analysis of orexin peptides found that hypothalamic tissue levels of orexin B, but not of orexin A, were significantly decreased in the CLI rats. No significant differences were detected in the other brain regions tested. The above finding that CLI, a multiple aminergic neurotransmitter reuptake inhibitor prescribed for the treatment of depression, alters brain orexin levels lends additional support to the supposition that orexin alteration is exclusively involved in the pathogenesis of depression with regard to neonatal treatment with CLI-induced depression and the likely antidepressive effect of CLI used in human adults.
The finding that CLI treatment suppressed REM sleep (without change of total sleep) is consistent with the literature, which reports that CLI is a REM sleep suppressant (Mirmiran, et al., 1981; Vogel, et al., 1990a; Feng and Ma, 2002). CLI is a tricyclic drug, which inhibits both norepinephrine and serotonin uptake, possibly by blocking the membrane-pump of neurons, thus increasing the concentration of transmitter monoamines at receptor sites. In clinical observations, CLI appears to have a mild sedative effect (Persson, 1980; Ogren, et al., 1981), which may be helpful in alleviating the anxiety component that often accompanies depression. This is consistent with an observed decrease in the active wake of rats treated with CLI compared with both the saline-treated control group and the pretreatment baseline in this study. However, the increase in quiet wake rather than sleep indicates that this drug does not improve total sleep. Rather, it suppresses REM sleep as indicated by decreased total REM sleep and increased REM latency, which is a necessary component of CLI’s antidepressive effect (Vogel, et al., 1990a; Riemann, et al., 2001). The fact that the CLI-induced reduction in active wake was compensated by increased quiet wake without a change in total wake time suggests that the actual change during the wake period was a reduction of motor activity. Thus, the effect of CLI may be simplified as suppression of both motor activity during the wake period and REM sleep during sleep. The findings that voluntary exercise increases REM sleep immediately (Blanco-Centurion and Shiromani, 2006) suggest that a decrease in REM sleep in the CLI-treated rat could be the result of a reduction of motor activity during previous wakefulness. The fact that REM sleep deprivation increases swim activity (Asakura, et al., 1993, 1994) and voluntary movement (Andrade, et al., 1987) does not suggest that REM sleep suppression after CLI treatment reduces locomotor activity in the subsequent wake period, that is, active wake. Rather, this evidence suggests that a reduction in REM sleep may construct a feedback circuit that modulates locomotor activity during the wake period. In summary, these data support our hypothesis at the behavioural level that CLI directly suppresses motor activity during wake and subsequently reduces REM sleep. This finding also implies that suppression of motor activity in the neonatal period may be a causal factor in the adult depression induced by CLI. Reduction of daytime physical activity is a risk factor for mood disorders (Morgan, 2003), and regular physical activity negatively correlates with depression (Goodwin, 2003; Dunn, et al., 2005).
At the molecular level, we quantified mRNA of OX1R, OX2R and prepro-orexin. Three administrations of CLI did not affect the expression of orexin receptor mRNA. This result indicates that CLI does not directly act on orexin receptors. However, highly increased expression of prepro-orexin mRNA, which leads to the production of the prepro-orexin protein, was found in both the hypothalamus and the frontal cortex. Prepro-orexin is a 130–131 amino acid precursor of both orexin A and orexin B. After detachment of the N-terminal 33-amino acid residue signal peptide, prepro-orexin (now pro-orexin) is cleaved by prohormone convertases to yield one molecule each of orexin A and orexin B (Sakurai, et al., 1999). This procedure is believed to occur primarily in the regions that produce orexins, that is, the perifornical region and the lateral hypothalamus. The fact that the mRNA expression was highly increased in both the frontal cortex and hypothalamus after CLI treatment suggests several hypotheses. First, we do not have sufficient evidence to rule out a direct effect of CLI on the expression of prepro-orexin mRNA. However, conventional understanding of this drug’s effect on aminergic neurotransmitters, that is, inhibiting reuptake of the neurotransmitters 5-HT and norepinephrine and increasing 5-HT and norepinephrine levels (Sangdee and Franz, 1979; Romero, et al., 1996), does not support this view. This is because that 5-HT hyperpolarises hypothalamic orexin neurons (Muraki, et al., 2004). Till now, there is no data showing whether three doses of CLI induce a decrease in 5-HT. Second, increased brain levels of prepro-orexin mRNA imply an increase in prepro-orexin protein according to common understanding of molecular biology. Actual levels of prepro-orexin remain to be experimentally verified; however, as an effective tool for the quantification of prepro-orexin has yet to be developed. The mechanism that mediates the alteration of preproorexin gene expression also remains to be understood. Available evidence indicates that serotonin hyperpolarises orexin neurons in a concentration-dependent manner. A previous study found that this effect is inhibited by the 5-HT1A receptor antagonist WAY100635 (Muraki, et al., 2004), indicating that hyperpolarisation occurs via this receptor. Given that 5-HT has a negative effect on the activation of orexinergic neurons as reviewed earlier, it is unclear how CLI, which enhances the effect of serotonin by inhibiting reuptake, stimulates the expression of prepro-orexin mRNA, a likely product of orexinergic neuronal activity. The third implication is that the post-treatment reaction of genes that direct the production of prepro-orexin is not limited to the hypothalamus. We found an increase in prepro-orexin mRNA in the frontal cortex in addition to the hypothalamus. This finding is surprising in that the current concept of orexin production presumes that this process occurs only in the hypothalamus. However, prepro-orexin mRNA was recently found to be expressed in other brain regions in female Sprague Dawley rats (Silveyra, et al., 2007), as well as in peripheral organs (Randeva, et al., 2001). Additional testing is necessary to determine where CLI and other antidepressants stimulate the production of preproorexin outside of the hypothalamus.
Orexin A and orexin B are 33- and 28-amino acid peptides, respectively. As mentioned above, both of them are derived from the post-translational cleavage of prepro-orexin (Kastin and Akerstrom, 1999). Because the development of radioimmunoassay (RIA) for the measurement of orexins in 2000 (Mitsuma, et al., 2000a,b), RIA kits have been used substantially to quantify orexin levels in various types of samples. In our study, analysis of orexin A and orexin B peptides after CLI treatment found no increase in the tissue of any measured brain region despite that prepro-orexin mRNA was over-expressed in this group. In fact, hypothalamic orexin B was actually significantly decreased after CLI treatment. There is still insufficient evidence to explain these apparently contradictory results, that is, CLI increased prepro-orexin mRNA expression but decreased orexin B. Several possibilities include (1) the biological reactive chain from the expression of prepro-orexin mRNA to the synthesis of prepro-orexin and to the dehydration of this protein into orexin A and orexin B did not occur according to the understood process; (2) the stability of orexins was modified by CLI via an unknown mechanism and (3) the tissue levels of orexins do not indicate the actual levels of extracellular orexin. The decrease of hypothalamic orexin B, however, was consistent with the evidence that (1) CLI has a sedative effect behaviourally; (2) polysomnographic recording found that CLI rats had decreased active wake and (3) orexinergic neurons are motor activity dependent (Torterolo, et al., 2003; Martins, et al., 2004). This evidence suggests that the decrease of orexin B could be a result of the decreased behavioural activity. We speculate that the expression of prepro-orexin is regulated by the levels of orexin B at least partially and that the dehydration and cleavage of prepro-orexin into orexin peptides are regulated by motor or physical activity. Further empirical testing is needed.
In summary, this study provided evidence that short-term treatment with CLI, a conventional antidepressant, reduces wake time motor activity, that is, active wake, suppresses REM sleep and hypothalamic tissue levels of orexin B and simultaneously increases quiet wake and the expression of prepro-orexin mRNA in both the hypothalamus and frontal cortex. Findings that CLI suppresses active wake and orexin B add to our understanding of the pathogenesis of adult depression induced by the neonatal treatment of CLI. Furthermore, increased mRNA of prepro-orexins could be a precursor of the development of increased brain levels of both orexin A and orexin B found in adult rats neonatally treated with CLI.
Acknowledgements
This work was supported by the NIMH Grant RO1 MH 069854 to Feng, Cleveland VA Research Service and by the Award of Program for Innovative Scientist in Medical Science of Henan Province, Department of Health, Henan Province, China.
Footnotes
Declaration of conflict of interest
This was not an industry-supported study. None of the authors have any financial conflicts of interest.
Contributor Information
P Feng, Department of Physiology, School of Medicine, Zhengzhou University, Zhengzhou, China; Pulmonary and Critical Care Division, Department of Medicine, Case Western Reserve University, Cleveland, Ohio, USA; Department of Psychiatry, Case Western Reserve University, Cleveland, Ohio, USA.
Y Hu, Pulmonary and Critical Care Division, Department of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
D Li, Department of Physiology, School of Medicine, Zhengzhou University, Zhengzhou, China.
D Vurbic, Pulmonary and Critical Care Division, Department of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
H Fan, Department of Physiology, School of Medicine, Zhengzhou University, Zhengzhou, China.
S Wang, Department of Physiology, School of Medicine, Zhengzhou University, Zhengzhou, China.
KP Strohl, Pulmonary and Critical Care Division, Department of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
References
- Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38:311–315. doi: 10.1016/j.npep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Andrade LA, Lima JG, Tufik S, Bertolucci PH, Carlini EA. Rem sleep deprivation in an experimental model of Parkinson’s disease. Arq Neuropsiquiatr. 1987;45:217–223. doi: 10.1590/s0004-282x1987000300001. [DOI] [PubMed] [Google Scholar]
- Auerbach SB, Lundberg JF, Hjorth S. Differential inhibition of serotonin release by 5-HT and NA reuptake blockers after systemic administration. Neuropharmacology. 1995;34:89–96. doi: 10.1016/0028-3908(94)00137-h. [DOI] [PubMed] [Google Scholar]
- Asakura W, Matsumoto K, Ohta H, Watanabe H. Effect of alpha 2-adrenergic drugs on REM sleep deprivation-induced increase in swimming activity. Pharmacol Biochem Behav. 1993;46:111–115. doi: 10.1016/0091-3057(93)90325-n. [DOI] [PubMed] [Google Scholar]
- Asakura W, Matsumoto K, Ohta H, Watanabe H. Involvement of dopamine D2 receptor mechanism in the REM sleep deprivation-induced increase in swimming activity in the forced swimming test. Pharmacol Biochem Behav. 1994;48:43–46. doi: 10.1016/0091-3057(94)90495-2. [DOI] [PubMed] [Google Scholar]
- Blanco-Centurion CA, Shiromani PJ. Beneficial effects of regular exercise on sleep in old F344 rats. Neurobiol Aging. 2006;27:1859–1869. doi: 10.1016/j.neurobiolaging.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin L, Bjorkqvist M, Petersen A, Traskman-Bendz L. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur Neuropsychopharmacol. 2007a;17:573–579. doi: 10.1016/j.euroneuro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Brundin L, Petersen A, Bjorkqvist M, Traskman-Bendz L. Orexin and psychiatric symptoms in suicide attempters. J Affect Disord. 2007b;100:259–263. doi: 10.1016/j.jad.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Crews FT, Smith CB. Potentiation of responses to adrenergic nerve stimulation in isolated rat atria during chronic tricyclic antidepressant administration. J Pharmacol Exp Ther. 1980;215:143–149. [PubMed] [Google Scholar]
- De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Feng P, Ma Y. Clomipramine suppresses postnatal REM sleep without increasing wakefulness: implications for the production of depressive behaviors. Sleep. 2002;25:177–184. doi: 10.1093/sleep/25.2.177. [DOI] [PubMed] [Google Scholar]
- Feng P, Ma Y. Instrumental REM sleep deprivation in neonates leads to adult depression-like behaviors in rats. Sleep. 2003;26:990–996. doi: 10.1093/sleep/26.8.990. [DOI] [PubMed] [Google Scholar]
- Feng P, Vurbic D, Wu Z, Hu Y, Strohl KP. Changes in brain orexin levels in a rat model of depression induced by neonatal administration of clomipramine. J Psychopharmacol. 2008 doi: 10.1177/0269881106082899. (in press doi: 10.1177/0269881107082899). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Vurbic D, Wu Z, Strohl KP. Brain orexins and wake regulation in rats exposed to maternal deprivation. Brain Res. 2007;1154C:163–172. doi: 10.1016/j.brainres.2007.03.077. [DOI] [PubMed] [Google Scholar]
- Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med. 2003;36:698–703. doi: 10.1016/s0091-7435(03)00042-2. [DOI] [PubMed] [Google Scholar]
- Henderson LP. The role of 5-hydroxytryptamine as a transmitter between identified leech neurones in culture. J Physiol. 1983;339:309–324. doi: 10.1113/jphysiol.1983.sp014718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd FK, Chua P, Lynch C, Norman T. Fenfluramine augmentation of clomipramine treatment of obsessive compulsive disorder. Aust N Z J Psychiatry. 1991;25:412–414. doi: 10.3109/00048679109062643. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289:219–223. [PubMed] [Google Scholar]
- Kinney GG, Vogel GW, Feng P. Decreased dorsal raphe nucleus neuronal activity in adult chloral hydrate anesthetized rats following neonatal clomipramine treatment: implications for endogenous depression. Brain Res. 1997;756:68–75. doi: 10.1016/s0006-8993(97)00119-4. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, et al. Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience. 2006;142:29–36. doi: 10.1016/j.neuroscience.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Direct and indirect inhibition by cate-cholamines of hypocretin/orexin neurons. J Neurosci. 2005;25:173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins PJ, D’Almeida V, Pedrazzoli M, Lin L, Mignot E, Tufik S. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul Pept. 2004;117:155–158. doi: 10.1016/j.regpep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Maudhuit C, Hamon M, Adrien J. Electrophysiological activity of raphe dorsalis serotoninergic neurones in a possible model of endogenous depression. Neuroreport. 1995;6:681–684. doi: 10.1097/00001756-199503000-00024. [DOI] [PubMed] [Google Scholar]
- Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5(Suppl.):1071–1075. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Scholtens J, van de Poll NE, Uylings HB, van der Gugten J, Boer GJ. Effects of experimental suppression of active (REM) sleep during early development upon adult brain and behavior in the rat. Brain Res. 1983;283:277–286. doi: 10.1016/0165-3806(83)90184-0. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, van de Poll NE, Corner MA, van Oyen HG, Bour HL. Suppression of active sleep by chronic treatment with chlorimipramine during early postnatal development: effects upon adult sleep and behavior in the rat. Brain Res. 1981;204:129–146. doi: 10.1016/0006-8993(81)90657-0. [DOI] [PubMed] [Google Scholar]
- Mitsuma T, Hirooka Y, Kayma M, Mori Y, Yokoi Y, Izumi M, et al. Radioimmunoassay for orexin A. Life Sci. 2000a;66:897–904. doi: 10.1016/s0024-3205(99)00673-6. [DOI] [PubMed] [Google Scholar]
- Mitsuma T, Hirooka Y, Kayama M, Mori Y, Yokoi Y, Rhue N, et al. Radioimmunoassay for hypocretin-2. Endocr Regul. 2000b;34:23–27. [PubMed] [Google Scholar]
- Morgan K. Daytime activity and risk factors for late-life insomnia. J Sleep Res. 2003;12:231–238. doi: 10.1046/j.1365-2869.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J Neurosci. 2004;24:7159–7166. doi: 10.1523/JNEUROSCI.1027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren SO, Cott JM, Hall H. Sedative/anxiolytic effects of antidepressants in animals. Acta Psychiatr Scand. 1981;290(Suppl.):277–288. doi: 10.1111/j.1600-0447.1981.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Persson SA. The effects of chlorimipramine and protriptyline on tube running activity in mice. Pharmacol Biochem Behav. 1980;12:255–258. doi: 10.1016/0091-3057(80)90365-2. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW. Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab. 2001;86:4808–4813. doi: 10.1210/jcem.86.10.7921. [DOI] [PubMed] [Google Scholar]
- Riemann D, Berger M, Voderholzer U. Sleep and depression – results from psychobiological studies: an overview. Biol Psychol. 2001;57:67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- Romero L, Hervas I, Artigas F. The 5-HT1A antagonist WAY-100635 selectively potentiates the presynaptic effects of serotonergic antidepressants in rat brain. Neurosci Lett. 1996;219:123–126. doi: 10.1016/s0304-3940(96)13199-2. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1–696. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Moriguchi T, Furuya K, Kajiwara N, Nakamura T, Yanagisawa M, et al. Structure and function of human prepro-orexin gene. J Biol Chem. 1999;274:17771–17776. doi: 10.1074/jbc.274.25.17771. [DOI] [PubMed] [Google Scholar]
- Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, et al. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol Psychiatry. 2003;54:96–104. doi: 10.1016/s0006-3223(02)01740-7. [DOI] [PubMed] [Google Scholar]
- Sangdee C, Franz DN. Enhancement of central norepinephrine and 5-hydroxytryptamine transmission by tricyclic antidepressants. A comparison. Psychopharmacology. 1979;62:9–16. doi: 10.1007/BF00426028. [DOI] [PubMed] [Google Scholar]
- Silveyra P, Catalano PN, Lux-Lantos V, Libertun C. Impact of proestrous milieu on expression of orexin receptors and preproorexin in rat hypothalamus and hypophysis: actions of cetrorelix and nembutal. Am J Physiol Endocrinol Metab. 2007;292:E820–E828. doi: 10.1152/ajpendo.00467.2006. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep. 2003;26:25–28. [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Vogel GW. REM sleep deprivation and behavioral changes. In: Mallick BN, Inoué S, editors. Rapid Eye Movement Sleep. London: Narasa Publishing House; 1999. pp. 355–366. [Google Scholar]
- Vogel G, Neill D, Hagler M, Kors D. A new animal model of endogenous depression: a summary of present findings. Neurosci Biobehav Rev. 1990a;14:85–91. doi: 10.1016/s0149-7634(05)80164-2. [DOI] [PubMed] [Google Scholar]
- Vogel GW, Buffenstein A, Minter K, Hennessey A. Drug effects on REM sleep and on endogenous depression. Neurosci Biobehav Rev. 1990b;14:49–63. doi: 10.1016/s0149-7634(05)80159-9. [DOI] [PubMed] [Google Scholar]
- Xi MC, Morales FR, Chase MH. Effects on sleep and wakefulness of the injection of hypocretin-1 (orexin-A) into the laterodorsal tegmental nucleus of the cat. Brain Res. 2001;901:259–264. doi: 10.1016/s0006-8993(01)02317-4. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Sampogna S, Morales FR, Chase MH. Orexin (hypocretin)-like immunoreactivity in the cat hypothalamus: a light and electron microscopic study. Sleep. 2001;24:67–76. doi: 10.1093/sleep/24.1.67. [DOI] [PubMed] [Google Scholar]