Abstract
Route of administration of chemicals in adults is an important factor in pharmacokinetics of chemicals such as bisphenol A (BPA), the monomer with estrogenic activity used to make polycarbonate plastic products and to line food and beverage cans. Based on findings in adults it has been proposed (CERHR, 2007) that non-oral routes of administration in newborn rodents would also lead to high exposure relative to oral administration. However, in fetuses and neonates, the enzyme that conjugates BPA (UDP-glucuronosyltransferase) is expressed at low levels, suggesting that there may be no differences in pharmacokinetics between oral and non-oral dosing. We thus conducted an analysis of plasma concentrations of unconjugated 3H-BPA after HPLC separation in postnatal day 3 female mice throughout the 24 hr after administering 3H-BPA orally or via subcutaneous injection at doses above and below the current EPA reference dose. We found no significant difference in plasma BPA based on route of administration in neonatal mice at either dose. However, compared to data from other studies conducted with adults, there was a markedly higher plasma BPA level after oral administration of BPA in newborn mice. This finding sets aside the belief that non-oral administration of BPA renders data as not suitable for consideration of the hazard posed by low-dose exposure to BPA during neonatal life. Therefore the large numbers of BPA studies that used non-oral administration at very low doses during the neonatal period should not be dismissed by scientists or the regulatory community based on route of administration.
Keywords: bisphenol A, neonatal metabolism, pharmacokinetics, UDP-glucuronosyltransferase
1. Introduction
There is now a large published literature reporting a wide range of adverse effects due to exposure during both prenatal and early postnatal development to doses of the estrogenic chemical bisphenol A (BPA) that are far below doses identified by regulatory agencies as being safe for daily human exposure, and this literature was recently reviewed [1–6]. Human exposure to BPA occurs via many routes, but oral exposure is typically considered to be the most significant route. Oral exposure occurs due to BPA leaching from polycarbonate food and beverage containers as well as from the plastic lining of cans containing food and beverages. However, BPA is detected in indoor air primarily associated with dust, indicating exposure can occur by inhalation. BPA is also found in streams and rivers, and leaches from landfill, suggesting that BPA is a common contaminant in water used for drinking and bathing. Since BPA can be absorbed through the skin during bathing, trans-dermal exposure also has be to considered in assessing all potential routes that might account for the relatively high levels detected in human blood, urine and tissues by a variety of analytical techniques [4].
In the USA findings published by the CDC from the 2003–2004 National Health and Nutrition Examination Survey (NHANES) showed that 93% of people had detectable levels of BPA in their urine, with levels in children being significantly higher than levels in adolescents, who had higher levels than adults [7]. The relative contribution of different routes of exposure to the unexpectedly high (ng/ml) circulating levels of unconjugated BPA measured in human blood, which are very similar to the values for total BPA detected in urine, has never been determined. In fact, a consensus conclusion of a panel of 38 expert scientists at a NIH/EPA-sponsored conference on BPA was that no current exposure models accurately predict the levels of BPA found in people in studies conducted in Europe, Asia and the USA [4, 5].
In prior studies we have examined various aspects of pharmacokinetics of BPA and other chemicals with estrogenic activity including: 1) the intrinsic estrogenic activity of the molecule in interaction with ERs in the nucleus of the cell [8], 2) how the compound is carried in blood, and 3) what fraction is delivered free (unconjugated and unbound to plasma proteins) to receptors in target cells [9]. To understand pharmacokinetics it is also necessary to understand how the compound partitions between the circulation and body lipid, and its absorption and metabolism relative to the route of exposure. Our focus here on the impact of route of exposure on plasma unconjugated BPA during the early neonatal period of life in mice, which is a critical period in development during which BPA can permanently alter organogenesis resulting in subsequent disease [3].
It is well known that route of administration of BPA and other chemicals impacts pharmacokinetics in adults, since the first-pass metabolism that occurs with oral administration does not occur if a chemical is administered via injection or via a subcutaneously (sc) implanted capsule [10–12]. It has been mistakenly considered in some published studies that first-pass metabolism results in complete clearance of BPA, even though many studies have shown aglycone (unconjugated, biologically active) BPA for at least 24 hr in plasma after a single oral dose; however, this finding requires that the sensitivity of the assayed employed is adequately matched to the dose [11]. In the case of BPA the liver enzyme that is responsible for conjugation is UDP-glucuronosyltransferase (UGT2B1), which is also the enzyme that metabolizes the estrogenic drug diethylstilbestrol (DES) [13]. BPA is excreted primarily in the urine in adult humans and feces in adult rodents [4].
The maxim in pediatric medicine is that “babies are not little adults”. It is thus well known that the fetal and neonatal rodent liver does not have the adult capability of metabolizing BPA, other chemicals or drugs. For example, Matsumoto and colleagues [14] were unable to detect either the UGT2B1 mRNA or protein in the fetal rat liver. Between birth and weaning in rodents, there is typically about a 10-fold increase in the rate at which chemicals such as BPA and DES are metabolized to inactive conjugates [14, 15]. A study claiming nearly complete clearance of BPA only a few hours after a relatively high oral dose in neonatal rats [16] is likely explained by the use of a relatively insensitive assay [17].
We conducted this study because a panel formed by the Center for the Evaluation of Risks to Human Reproduction (CERHR) within the US National Toxicology Program (NTP) to evaluate the human health risks of BPA made the decision that all developmental studies of BPA in experimental animals that did not involve oral administration would be considered of “limited utility” with regard to assessing the potential for BPA to impact human health. This determination by the CERHR BPA panel was a major contributing factor in not accepting as useful the extensive literature showing that developmental exposure to very low doses of BPA causes prostate interepithelial neoplastia (PIN) in male rats, damage to chromosomes in mouse oocytes, and a wide range of other adverse effects on the male and female reproductive system [18]. Postnatal day (PND) 3 was selected for this study, since administration of BPA to neonatal female mice by sc injection resulting in adverse effects on the reproductive system occurred between PND 1 – 5 [19].
While, as described above, the low liver enzyme activity in fetuses and neonates in rodents is known to impact blood levels of chemicals including BPA, a study using methods with appropriate sensitivity to determine the impact of route of administration of BPA on the amount of bioavailable BPA in plasma throughout the 24 hr after administration has not been conducted. We thus designed this experiment to directly examine the impact of route of exposure on the plasma levels of unconjugated BPA in CD-1 female mice on PND 3. We chose to conduct this experiment with females, since on PND 3 the ovaries do not secrete gonadal steroids, while in males, testosterone is secreted at low levels by the testes. Testosterone reduces the metabolism of BPA in rats [20, 21], and in response to a relatively high dose of BPA after oral administration in neonatal rats, males had higher plasma levels of BPA than females [16]. The female neonatal mouse thus provided a gonadal steroid-free background against which to examine the pharmacokinetics of BPA. Also, a number of studies reporting adverse effects of BPA in female mice have involved exposure via sc injection of BPA during development. Our prediction, based on the low activity of the UGT2B1 enzyme activity in neonates, was that rapid first pass metabolism following oral administration in adults, and thus lower plasma levels of BPA after oral administration relative to administration by sc injection in adults, would not occur in neonates.
We report here that no significant difference in the 24-hr clearance rate (area under the curve) or any other measured parameter was observed for unconjugated BPA in plasma in response to doses of BPA below and above the current US EPA reference dose of 50 μg/kg/day that is considered to be safe for human daily exposure [22].
2. General Methods
2.1 Experimental animals
Adult female CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA) and paired with males one week after arrival at the University of Missouri. After 14 days the males were removed, and the pregnant females were singly housed. Animals were housed in standard polypropylene cages on corncob bedding, with food and water available ad libitum. Glass water bottles were used, and water was purified by reverse osmosis and carbon filtration. Rooms were kept at 23°C and maintained on a 12 h light and 12 h dark cycle. Prior to pregnancy all animals were fed Purina 5001 rodent diet; pregnant and lactating females were fed Purina 5008 rodent diet. All animal procedures were approved by the University of Missouri Animal Care and Use Committee and conformed to the NIH Guide. Parturition dates were recorded, but litters were not disturbed until day 3 postpartum.
2.2 Chemicals and standards
Tritiated BPA (3H-BPA; specific activity 7.3 Ci/mmol) was obtained from Moravek Biochemicals (Brea, CA), and unlabeled BPA (>99%) pure was obtained from Aldrich (Milwaukee, WI). Tocopherol-stripped corn oil was from MP Biomedicals (Solon, OH). Methanol and tert-butyl methyl ether were HPLC grade and obtained from Fisher Scientific (Pittsburgh, PA).
2.3 Doses and administration procedures
3H-BPA was mixed with unlabeled BPA and dissolved in tocopherol-stripped corn oil. Two stock solutions were made; the final concentrations were 16 and 189 μg BPA/ml and each contained 2.5 μCi BPA per administered dose. We administered these two different doses in tocopherol-stripped corn oil to PND 3 female pups (average weight was 2.4 g). The two solutions of BPA (16 and 189 μg BPA/ml) were administered orally (po) or subcutaneously (sc) and resulted in pups being treated on PND 3 at 1000 hr with a single dose of BPA at 35 μg/kg (low dose) or 395 μg/kg body weight (high dose). Samples of each solution were kept as reference to measure the actual radioactivity used in each dose, from which the final specific activities for each dose were calculated. The specific activity of the low dose was 7.1 Ci/mmol, and the specific activity of the high dose was 0.61 Ci/mmol.
For oral administration of pups, feeding consisted of gently picking up the pup and placing the tip of a manual micropipetter into the pup’s mouth. The pups readily drink the solution, and the procedure is far less stressful than gavage. The sc injection was administered in the dorsal region with a micro-fine insulin syringe, and liquid bandage was then applied to the puncture site. While the same doses were administered either orally or by sc injection, for sc administration the dosing solution was mixed with one volume of oil and administered at 10 μl to increase the accuracy of injection. As described below, injection volume of 5 μl or 10 μl did not influence the results. In order to achieve the same dose/kg body weight, all pups were weighed, and the exact volume administered was adjusted to achieve the same dose per body weight.
Treated pups were returned to the mother and sacrificed at 0.5, 1, 2, 4, 6 or 24 hr after BPA administration by cervical dislocation. Up to fourteen animals were sacrificed at each time point, and care was taken to distribute the female pups from each litter across multiple collection points to control for litter effects. Blood was collected by decapitation into heparinized capillary tubes, and the plasma was separated by centrifugation and frozen at −20°C until analysis.
2.4 Sample preparation
The volume of plasma that can be obtained from this age mouse pup is small, and plasma from two pups (obtained from different litters) was pooled for each analysis point (typically 50–100 μl plasma); analysis of 6 samples thus represented the levels of BPA in 12 pups. BPA was extracted from plasma twice with 2 ml tert-butyl methyl ether, a method that extracts the unconjugated protein-bound and free BPA but not the water-soluble conjugates (if present). The ether extracts were dried under nitrogen, reconstituted in 80 μl methanol, and brought to a final concentration of 50% methanol by the addition of distilled water. Positive control samples (described below), consisting of untreated mouse pup plasma spiked with 3H-BPA, were included in each set of extractions to monitor extraction efficiency.
2.5 Plasma BPA quantification after HPLC separation
The reconstituted samples were separated by high pressure liquid chromatography (HPLC). The running time for BPA was verified at regular intervals using the positive control extracts spiked with 3H-BPA that were included to also monitor the percent recovery of BPA after extraction and HPLC separation (Figure 1). BPA was quantified by summing the radioactivity in the fractions eluting at the same time points as authentic BPA. Counts per minute (cpm) were converted to mass by referencing the specific activity of the original administered oil sample. The sensitivity of the assay, calculated as two-fold above background cpm, was 0.75 pg BPA/ml plasma at the low dose and 8.7 pg BPA/ml plasma at the high dose.
Figure 1.
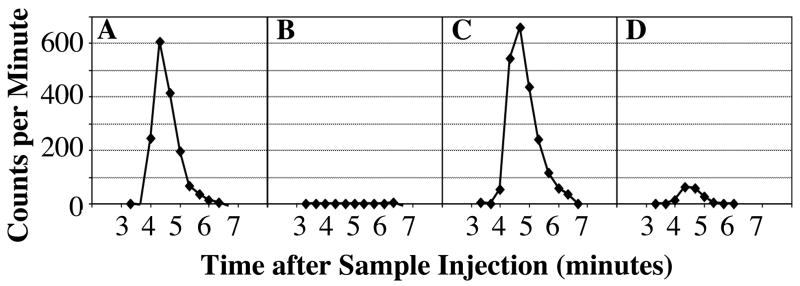
Representative chromatograms of radioactivity across the collection window in samples separated by HPLC. Panel A depicts the chromatogram for recovery from plasma spiked with 54 pg 3H-BPA. Panel B shows the solvent blank that was separated immediately after collection of the fractions in Panel A. For comparison, Panel C shows a separation of a 0.5-hour high dose pup plasma sample, and Panel D shows a separation of a 24-hour high dose pup plasma sample; in each case the plasma sample was from pups administered BPA orally. The similarity in the 3H-BPA elution times for the spiked and experimental samples shows that the radioactivity was associated with unconjugated BPA.
Chromatographic separations were accomplished on a reverse phase Hypersil C18 (4.6 × 100 mm) column (Phenomenex), using a mobile phase of 65% methanol at a flow rate of 0.82 ml/min. Sample injection volume was 160 μl in 50% methanol. Elution of separated components was monitored by UV absorbance at 260 nm on a Perkin-Elmer LC-90 spectrophotometric detector, and also with a ®RAM in-line scintillation counter (IN/US Systems, FL) to monitor radioactivity. The elution time of BPA was initially established using authentic 3H-BPA. The positive control samples, which consisted of untreated pup plasma containing 3H-BPA (see below), were used as standards to establish the expected elution time, which under these conditions was approximately 4.5 minutes. Fractions from injected samples were collected at 20-second intervals across a window spanning the expected BPA elution time (Figure 1). Radioactivity per fraction was counted on a scintillation counter for 10 minutes/sample. Data from the 10-min count of these fractions were used for quantification rather than the data from the in-line scintillation counter.
2.6 Recovery of BPA from HPLC-separated plasma samples
Plasma from untreated mouse pups was spiked with 3H-BPA (~1600 cpm per 100 μl), and 100 μl aliquots were extracted and separated by HPLC as described above for use as positive controls. The recovery of the added 3H-BPA, determined by comparing the sum of the radioactivity measured in the HPLC fractions to radioactivity in spiked plasma that had not been extracted, averaged (±SEM) 100.7±2.54% across 13 positive control sample runs. Negative controls (n = 7) were run directly after some of these spiked plasma controls; these negative controls consisted of the injection solvent only and monitored for possible between-run contamination. Background counts in fractions of the spiked samples collected before and after the elution of 3H-BPA as well as from the negative control samples were similar, averaging 14.9 and 10.5 cpm, respectively. In all of our samples BPA-associated radioactivity was more than 4-fold above background. Because the recoveries of 3H-BPA in the control samples averaged 100%, no adjustment of the pup sample data for extraction efficiency was made.
2.7 Statistical methods
The following parameters shown in Table I were measured from the plasma concentrations of BPA after oral administration and subcutaneous injection. The maximum concentration in plasma (Cmax) for all four groups was the first data point collected at 0.5 hr after administration. The decision to use 0.5 hr as the first time of collection was based on the fact that in most prior studies, this was the reported as the time (Tmax) at which the maximum concentration was reached [4]. Our initial rate constant (Kinit) was calculated from the slope of the natural log of concentration versus sample collection time. The Kinit was taken as the steepest rate of decay from the steepest two of the initial 3 collection time points (0.5, 1 and 2 hr). This was the first two time points (between 0.5 and 1 hr) for oral and injected low dose and the oral high dose, while for the injected high dose, this occurred between the second and third time points (between 1 and 2 hr).
Table I.
Pharmacokinetic parameters measured after administration of two doses of bisphenol A by oral and sc injection routes.
| Dose | 35 μg/kg | 395 μg/kg | ||||
|---|---|---|---|---|---|---|
| Oral | Injection | Oral | Injection | |||
| Cmax (ng/ml) | 1.78 | 2.60 | NS | 14.82 | 13.10 | NS |
| Standard error | 0.97 | 0.51 | 2.12 | 3.43 | ||
| Tmax (h) [first time point] | 0.5 | 0.5 | 0.5 | 0.5 | ||
| Initial slope (/h) | −1.15 | −1.70 | NS | −1.42 | −1.20 | NS |
| Standard Error | 0.90 | 0.70 | 0.49 | 0.23 | ||
| Initial t ½ (h) | 0.60 | 0.41 | 0.49 | 0.58 | ||
| Terminal slope (/h) | −0.074 | −0.039 | NS | −0.074 | −0.062 | NS |
| Standard Error | 0.021 | 0.022 | 0.014 | 0.031 | ||
| Terminal t ½ (h) | 9.41 | 17.63 | 9.35 | 11.21 | ||
| AUC 0->24h (ng h/ml) | 5.87 | 5.53 | NS | 66.7 | 63.6 | NS |
| AUC 0 -> ∞ (ng h/ml) | 6.78 | 7.58 | 77.2 | 74.9 | ||
| AUC 0 -> ∞ Scaled to Reference Dose of 50 μg/kg) | 9.69 | 10.83 | 9.78 | 9.48 | ||
NS = not statistically different
The terminal phase elimination rate constant (Kterm) was taken from the last two time points (between 6 and 24 hr). Half-lives (T1/2) were calculated as the natural log of 0.5 divided by the rate constant. Area under the curve (AUC) for the first 24 hr after dosing (AUC0-24) was calculated by using the linear trapezoidal rule and the assumption that 3H-BPA in plasma at the time just prior to administration (Time 0) was zero. The AUC extrapolated to infinity (AUC0→∞) was calculated by dividing the concentration at 24 hr, the last time point, by the terminal rate constant and adding this term to the AUC0-24. Finally, the AUC data obtained at the low dose (35 μg/kg) and the high dose (395 μg/kg) for both oral and injected routes of administration were scaled to the US-EPA’s current reference dose of 50 μg/kg/day by dividing the AUC values by the associated dose in μg/kg and then multiplying the result by 50. The data were analyzed using the PROC GLM ANOVA procedure, and planned comparisons were made using LS means in SAS. Differences were considered to be statistically significant at P < 0.05.
3. Results
For every calculated parameter (Cmax, Kinit, Kterm, AUC0-24, AUC0→∞) there was no significant difference after injected or oral administration of BPA for both the low (35 μg/kg) and high (395 μg/kg) dose (Table I). Chromatograms showing recovery of 3H-BPA by HPLC separation are shown in Figure 1 and reveal that 3H-BPA eluted as a defined peak. The in-line scintillation counter served as a real-time guide for determining when to stop collection; this also allowed monitoring of radioactivity eluting early in the separation where any polar compounds, such as conjugated 3H-BPA, would elute. This demonstrated the absence of conjugated 3H-BPA in the sample extract injected into the HPLC.
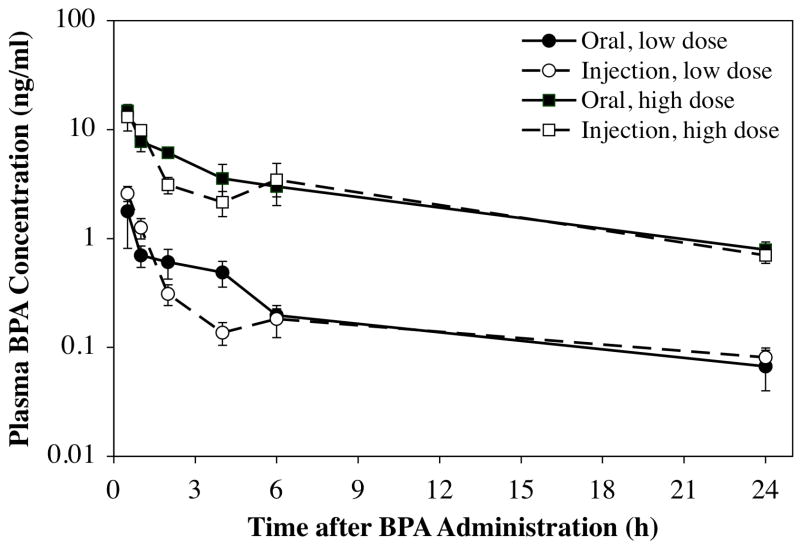
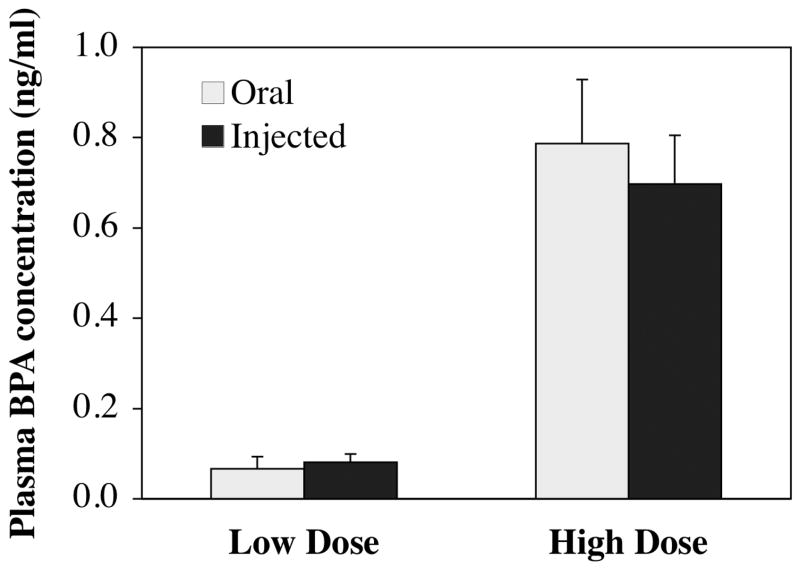
The group means (±SEM) for BPA (expressed as ng/ml plasma) at each time point calculated from the recovered 3H-BPA (and specific activity of each dose) are presented in Figure 2. The results show that throughout the 24 h after administration, the curve for low and high dose was parallel, and there were no significant differences based on route of administration at either dose. The mean (±SEM) plasma BPA for each group at 24 hr after administration is shown in Figure 3, revealing that at this time point the high dose of 395 μg/kg/day resulted in a 10.0-fold higher plasma BPA concentration relative to the low dose of 35 μg/kg/day, with no significant difference in plasma BPA concentration based on route of administration.
Figure 2.
Concentrations (in ng/ml) of unconjugated BPA in plasma in female mouse pups throughout the 24 hr after a single dose, administered either orally (solid line) or by subcutaneous injection (dashed line). BPA was administered at either 35 μg/kg (low dose, circles) or 395 μg/kg (high dose, squares). Values represent mean plasma values at each time point (±SEM). Note the log scale for the Y axis.
Figure 3.
Plasma unconjugated BPA concentrations at 24 hours after a single dose, BPA was administered at either 35 μg/kg (low dose) or 395 μg/kg (high dose). Values represent mean plasma values at each time point (±SEM).
Throughout the 24 hr after administration of a low or high dose of BPA by oral or injected routes, the overall exposure to bioavailable, unconjugated BPA was not significantly different, and when scaled to the reference dose of 50 μg/kg/day as described in the methods section, the AUC data did not differ based on route of administration or dose (Table I). The 10-fold difference in plasma BPA between the low and high dose pups (Figure 3), together with the similarity in the scaled data for the low and high doses (Table 1) and parallel clearance curves over the 24 hr after administration (Figure 2), indicates that over the 24 hr after administration, the initial dose administered was linearly related to the amount of BPA recovered in plasma for both the orally administered or sc injected routes.
While the clearance curves for unconjugated BPA in plasma were parallel for both the low and high doses whether injected or orally administered (Figure 2), at 2 and 4 hr after administration of the low or high dose, plasma levels of BPA were slightly higher after oral administration relative to injection. In addition, the low and high dose that was orally administered resulted in a continuous decline throughout the 24 hr after administration, but for both the low and high injected dose, there was a slight increase in plasma unconjugated BPA at 6 hr relative to 4 hr after administration, although the means at 4 and 6 hr did not differ significantly. Finally, the volume of oil used to inject BPA sc did not significantly influence the amount of unconjugated BPA in plasma when measured at 2 hr after administration of the 395 μg/kg dose (mean±SEM; 5 μl injection = 3.79±0.48 ng/ml BPA (n = 5); 10 μl injection = 3.08±0.39 ng/ml BPA (n = 6; P > 0.2).
4. Discussion
The findings here demonstrate that the very marked difference in pharmacokinetics when BPA is administered to adult rodents orally versus by injection [11, 12, 23] is not observed in neonatal mice. Specifically, the AUC measured after BPA was injected in adult rats in different studies ranged from 7-fold to 18-fold higher than the AUC following oral administration [23, 24], and in one study injected BPA was reported to result in an AUC over 300-fold higher than the AUC after oral administration [11]. We view these prior findings as providing conclusive evidence that in the adult rodent, route of administration of BPA has a substantial effect on the proportion of the BPA dose available to reach receptors in target tissues due to differences in pharmacokinetics. The issue of statistical power required to demonstrate this effect of route of administration of BPA in the adult is important, since our finding is that there is no statistical difference between oral administration and sc injection. Studies in the adult were able to detect differences in pharmacokinetics of BPA based on route of administration with 3–5 animals per group [23], while we analyzed plasma collected from 12–14 pups per group and controlled for potential litter effects. If route of administration in the adult was predictive of findings in neonates, our sample size should have been sufficient to detect a significant effect.
In sharp contrast, our results show that the findings from adult rodents do not apply to neonates. An important aspect of our finding that route of administration did not influence plasma levels of unconjugated BPA in newborn mice is that our results were predicted by prior studies showing that during early life in rodents, there is very low activity of the UGT2B1 enzyme that metabolizes BPA and numerous other xenobiotics, including DES [14, 15]. Thus, we had predicted that the relatively rapid metabolism and clearance of plasma unconjugated BPA after oral administration in the adult due to direct vascular transport to the liver from the intestines [14] would not occur in the neonatal female mouse.
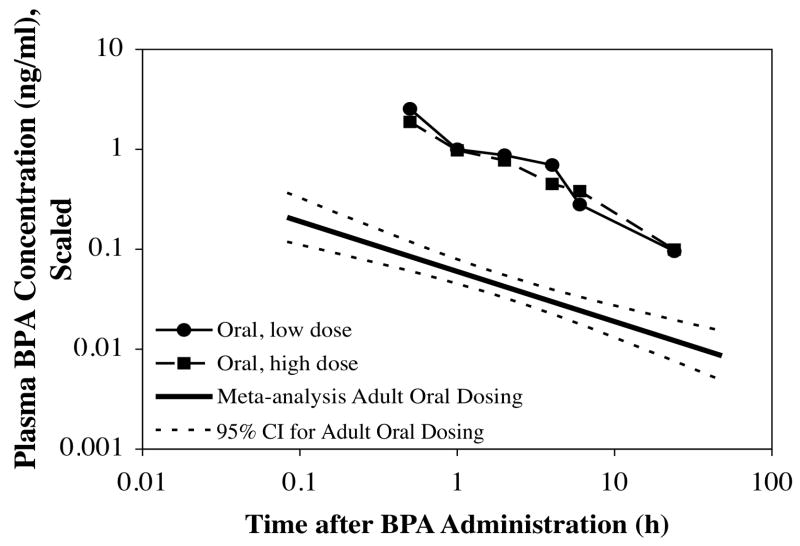
We observed a linear relationship between administered dose and amount of unconjugated BPA in plasma throughout the 24 hr after administration at doses below and above the current reference dose. This finding is consistent with prior results, and was also determined based on a meta-analysis of published studies where BPA was administered to adult rodents [4]. Specifically, based on a review of the pharmacokinetic literature, Vandenberg et al. [4] proposed that there was a linear relationship between administered dose and plasma unconjugated BPA. This suggests that it is possible to calculate the amount of unconjugated BPA that would be predicted to occur in plasma regardless of the dose administered. In Figure 4 we used this approach (a scaling procedure) to reference all administered doses to 50 μg/kg (the current US EPA reference dose) and compared the plasma concentrations of unconjugated BPA after oral administration or injection in neonatal mice with data obtained from 18 data sets in 11 published studies involving oral administration of BPA in adult rats; the findings from adults have been previously reviewed [4]. The findings presented in Figure 4 show that oral administration of BPA in neonatal mice results in dramatically higher (by about 10 fold) blood levels of BPA over the 24 hr after administration relative to data obtained from adults. This is consistent with studies in rats showing an approximately 10-fold change in the activity between birth and adulthood in liver UGT2B1 [14, 15]. In the newborn mouse the lack of a difference in plasma levels of BPA between the oral and sc injected routes thus appears to be due to a marked decrease in the rate of metabolism after oral administration in the neonate relative to the adult.
Figure 4.
A log-log plot of plasma BPA in neonatal mice after oral administration of both high and low doses of BPA. A log-log plot is required when the data being depicted cover a wide range of values on both axes. The data for plasma unconjugated BPA for both low and high dose orally administered BPA from our study was compared with a meta-analysis of 18 data sets from 11 studies of unconjugated BPA in adult rodent blood that have been previously reviewed [4]. All data were scaled to the US-EPA’s current reference dose of 50 μg/kg/day [22] by dividing the plasma unconjugated BPA values by the associated administered dose in μg/kg and then multiplying the result by 50; this is based on the assumption that there is a linear relationship between administered oral dose and plasma unconjugated BPA, as previously reviewed [4]. The comparison shows a marked decrease in the rate of metabolism in newborn mice relative to data from adults, consistent with prior findings [16]. 95% confidence intervals for the meta-analysis regression data are shown (dashed lines). For a more thorough discussion of the adult meta-analysis data see Figure 1 in Vandenberg et al [4].
The sensitivity of the assays in prior studies that used 14C-BPA is thousands of times less than the sensitivity we achieved using 3H-BPA. Specifically, the assay sensitivity reported by Domoradzki and colleagues [16] when administering a 1 mg/kg oral dose of BPA to neonatal rats was 6–10 ng/g blood, while at the 10-fold higher dose of 10 mg/kg, the reported sensitivity was 14 – 48 ng/g blood. In dramatic contrast, the sensitivity of our assay (calculated as two-fold above background counts) for the low dose was 0.75 pg/ml plasma, while for the high dose the sensitivity was 8.7 pg/ml plasma (due to differences in specific activity of the administered dose). The importance of this comparison is that in prior experiments that used insensitive assays [16] to determine the pharmacokinetics of BPA after oral administration in newborn rats, within a short time after administration, the 14C-BPA was not detectable, and the authors concluded that rapid (and nearly complete) clearance of BPA had occurred. While our data from a study that used much more sensitive assay techniques show that this conclusion is incorrect, other data presented by Domoradzki et al. [16] were consistent with our findings in Figure 4; specifically, plasma levels of BPA after oral administration to neonatal rats were significantly higher than in adults, which is consistent with low UGT2B1 activity in neonatal rats relative to adults [14, 15]. Our findings here thus likely apply to neonatal rats as well as mice.
The initial half life of unconjugated BPA in our study for the two dose groups combined (since they did not differ significantly) was 0.52±0.04 hr (mean±SEM), while the terminal half life of BPA for all of the groups combined was 12±2 hr. This latter finding reveals substantial persistence of the remaining biologically active, unconjugated BPA in plasma after the initial period of more rapid clearance, regardless of route of administration or dose. Importantly, an assay such as ours, with adequate sensitivity to measure plasma BPA throughout the entire 24 hr after administration (or longer), is required for an accurate estimation of terminal half life.
An interesting finding is that after oral administration in PND 3 mice there was a steady decrease in plasma BPA throughout the 24 h after administration, but after sc injection, between 4 and 6 hr after injection, at both the low and high dose there was a transient (albeit small and not statistically significant) increase in plasma BPA. This is similar to findings in the adult where a similar transient increase in plasma BPA was observed at 6 hr after oral administration [11, 12]. The suggestion from adult studies was that the transient increase in plasma BPA at 6 hr might be due to enterohepatic recirculation. The basis for our finding a similar increase after the sc injection in neonates is not clear.
Our findings are important in that they add to a large literature showing that the maxim in pediatric medicine that “babies are not little adults” has to be recognized by scientists and regulators who are making determinations about the potential for chemicals in the environment to adversely impact the health of fetuses, infants and children. A key factor in categorizing developmental studies as being of “limited utility” in assessing concern for human health by the CERHR BPA panel was administration of BPA by sc injection. A recently published study showed a number of adverse effects of BPA on the female mouse reproductive system [19] and another study (reviewed by the CERHR panel) showed prostate interepithelial neoplasia (PIN) lesions in male rats [25]; these reported adverse effects were due to exposure during the first 5 days after birth via sc injection, and in each case the dose of BPA was 10 μg/kg/day. This dose is 5,000 times lower than the 50 mg/kg/day dose used by the US EPA to estimate the “safe” daily human exposure dose of 50 μg/kg/day. Other “limited utility” studies involved continuous release of extremely low doses of BPA (0.025 – 25 μg/kg/day) from sc-implanted capsules (for example: [26, 27]). Importantly, published studies reviewed above suggest that the expected difference based on route of administration in an adult would only be 10–20 fold, while in the neonate our data show that there is no difference at all. The prediction of the CERHR panel was that sc administration during development: “would produce irrelevantly high internal doses of the active parent compound” (CERHR, 2007; p 122), and this prediction is not supported by our findings or any other published data. Evidence that humans are most likely continuously exposed to BPA, which is best approximated by use of continuous-release capsules, has been previously reviewed [4]. Future research should take into account the evidence from biomonitoring studies suggesting the virtually continuous exposure of people to BPA, and experimental studies that examine pharmacokinetics of BPA after continuous exposure or multiple exposures at different life stages need to be conducted.
In summary, findings here strongly indicate that during the neonatal period in mice oral and non-oral administration of BPA give the same internal active dose, and this is also likely to apply to rats. Furthermore, the evidence from the extensive research conducted with the estrogenic drug diethylstilbestrol (DES) shows that injecting sc pregnant mice or rats with DES results in virtually the identical suite of diseases that have been observed in the offspring of pregnant women administered this drug orally over approximately 25 years during which physicians were unaware of the devastating long-latency adverse effects that were being programmed into the fetuses’ differentiating cells [29], including most recently a significant increase in risk of breast cancer over four decades later [30]. The massive experimental animal and epidemiological literature on DES thus shows that sc injection in pregnant mice directly predicted the adverse effects of maternal orally administered DES on human fetuses. We view this as strong evidence that route of administration is irrelevant in pregnant mice and rats as well as in neonatal mice and rats. The DES literature is particularly relevant to concerns about the health effects of BPA, since there are numerous studies showing that developmental exposure to BPA and DES produce similar effects [31]. Taken together, a review of prior findings as well as our findings here indicate that animal studies that examined the effects of extremely low doses of BPA administered by non-oral routes during early development are as valid for assessing the concern regarding the potential for BPA to impact human health as are studies that involved oral administration. Thus, scientists and regulatory bodies can no longer dismiss developmental studies in rats and mice as not relevant solely based on route of administration of BPA.
Acknowledgments
Support was provided during the preparation of this manuscript by grants to FvS and WVW from NIEHS (ES11283) and funding from the University of Missouri Food for the 21st Century to WVW (VMFC0018). The authors declare they have no competing financial interests.
Financial Support: Support was provided during the preparation of this manuscript by grants to FvS and WVW from NIEHS (ES11283) and funding from the University of Missouri Food for the 21st Century to WVW (VMFC0018). The authors declare they have no competing financial interests.
Abbreviations used
- AUC0-24
area under the curve for the 24 hr after administration
- AUC0→∞
area under the curve extrapolated to infinity
- BPA
bisphenol A
- DES
diethylstilbestrol
- Cmax
maximum concentration in plasma
- Kinit
initial rate constant
- Kterm
terminal phase elimination rate constant
- PND
postnatal day
- SC
subcutaneous
- Tmax
Time at which the maximum concentration was reached
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, et al. An ecological assessment of bisphenol A: Evidence from comparative biology. Reprod Toxicol. 2007;24:225–39. doi: 10.1016/j.reprotox.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Keri RA, Ho S-M, Hunt PA, Knudsen KE, Soto AM, Prins GS. An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod Toxicol. 2007;24:240–52. doi: 10.1016/j.reprotox.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–77. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 5.vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, et al. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24(2):131–8. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetherill YB, Akingbemi B, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, et al. In Vitro Molecular Mechanisms of Bisphenol A Action. Reprod Toxicol. 2007;24:178–98. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Exposure of the U.S. Population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Environ Health Perspect. 2007 doi: 10.1289/ehp.10753. Online 24 October 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111(8):994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagel SC, vom Saal FS, Welshons WV. Developmental effects of estrogenic chemicals are predicted by an in vitro assay incorporating modification of cell uptake by serum. J Steroid Biochem Molec Biol. 1999;69(1–6):343–57. doi: 10.1016/s0960-0760(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi O, Oishi S. Testicular toxicity of dietarily or parenterally administered bisphenol A in rats and mice. Food Chem Toxicol. 2003;41(7):1035–44. doi: 10.1016/s0278-6915(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 11.Tominaga T, Negishi T, Hirooka H, Miyachi A, Inoue A, Hayasaka I, et al. Toxicokinetics of bisphenol A in rats, monkeys and chimpanzees by the LC-MS/MS method. Toxicol. 2006;226(2–3):208–17. doi: 10.1016/j.tox.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Upmeier A, Degen GH, Diel P, Michna H, Bolt HM. Toxicokinetics of bisphenol A in female DA/Han rats after a single i.v. and oral administration. Arch Toxicol. 2000;74(8):431–6. doi: 10.1007/s002040000144. [DOI] [PubMed] [Google Scholar]
- 13.Yokota H, Iwano H, Endo M, Kobayashi T, Inoue H, Ikushiro S, et al. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem J. 1999;340(Pt 2):405–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto J, Yokota H, Yuasa A. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect. 2002;110(2):193–6. doi: 10.1289/ehp.02110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer LJ, Weissinger JL. Development in the newborn rat of the conjugation and de-conjugation processes involved in the enterohepatic circulation of diethylstilboestrol. Xenobiotica. 1972;2(4):399–412. doi: 10.3109/00498257209111067. [DOI] [PubMed] [Google Scholar]
- 16.Domoradzki JY, Thornton CM, Pottenger LH, Hansen SC, Card TL, Markham DA, et al. Age and dose dependency of the pharmacokinetics and metabolism of bisphenol A in neonatal sprague-dawley rats following oral administration. Toxicol Sci. 2004;77(2):230–42. doi: 10.1093/toxsci/kfh054. [DOI] [PubMed] [Google Scholar]
- 17.Zalko D, Soto AM, Dolo L, Dorio C, Rathahao E, Debrauwer L, et al. Biotransformations of bisphenol A in a mammalian model: answers and new questions raised by low-dose metabolic fate studies in pregnant CD-1 mice. Environ Health Perspect. 2002;111:309–19. doi: 10.1289/ehp.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CERHR. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Center for the Evaluation of Risks to Human Reproduction (CERHR), US National Toxicology Program (NTP) 2007. [accessed December 5, 2007]. http://cerhr.niehs.nih.gov/chemicals/bisphenol/BPAFinalEPVF112607.pdf.
- 19.Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 2007;24(2):253–8. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata N, Matsumoto J, Nakada K, Yuasa A, Yokota H. Male-specific suppression of hepatic microsomal UDP-glucuronosyl transferase activities toward sex hormones in the adult male rat administered bisphenol A. Biochem J. 2002;368(Pt 3):783–8. doi: 10.1042/BJ20020804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeuchi T, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Fujiwara T, et al. Elevated Serum Bisphenol A Levels under Hyperandrogenic Conditions May Be Caused by Decreased UDP-Glucuronosyltransferase Activity. Endocr J. 2006;53:485–91. doi: 10.1507/endocrj.k06-032. [DOI] [PubMed] [Google Scholar]
- 22.IRIS. Bisphenol A. (CASRN 80-05-7) US-EPA Integrated Risk Information System Substance file. 1988. [accessed November 25, 2007]. http://www.epa.gov/iris/subst/0356.htm.
- 23.Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM. The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci. 2000;54(1):3–18. doi: 10.1093/toxsci/54.1.3. [DOI] [PubMed] [Google Scholar]
- 24.Negishi T, Tominaga T, Ishii Y, Kyuwa S, Hayasaka I, Kuroda Y, et al. Comparative study on toxicokinetics of bisphenol A in F344 rats, monkeys (Macaca fascicularis), and chimpanzees (Pan troglodytes) Exp Anim. 2004;53(4):391–4. doi: 10.1538/expanim.53.391. [DOI] [PubMed] [Google Scholar]
- 25.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinol. 2006;147(8):3681–91. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 27.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3(1):63–70. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]