Abstract
In this study the hypothesis was tested that chronic infusion of ANG II attenuates acute volume expansion (VE)-induced inhibition of renal sympathetic nerve activity (SNA). Rats received intravenous infusion of either vehicle or ANG II (12 ng·kg−1 ·min−1) for 7 days. ANG II-infused animals displayed an increased contribution of SNA to the maintenance of mean arterial pressure (MAP) as indicated by ganglionic blockade, which produced a significantly (P < 0.01) greater decrease in MAP (75 ± 3 mmHg) than was observed in vehicle-infused (47 ± 8 mmHg) controls. Rats were then anesthetized, and changes in MAP, mean right atrial pressure (MRAP), heart rate (HR), and renal SNA were recorded in response to right atrial infusion of isotonic saline (20% estimated blood volume in 5 min). Baseline MAP, HR, and hematocrit were not different between groups. Likewise, MAP was unchanged by acute VE in vehicle-infused animals, whereas VE induced a significant bradycardia (P < 0.05) and increase in MRAP (P < 0.05). MAP, MRAP, and HR responses to VE were not statistically different between animals infused with vehicle vs. ANG II. In contrast, VE significantly (P < 0.001) reduced renal SNA by 33.5 ± 8% in vehicle-infused animals but was without effect on renal SNA in those infused chronically with ANG II. Acutely administered losartan (3 mg/kg iv) restored VEinduced inhibition of renal SNA (P < 0.001) in rats chronically infused with ANG II. In contrast, this treatment had no effect in the vehicle-infused group. Therefore, it appears that chronic infusion of ANG II can attenuate VE-induced renal sympathoinhibition through a mechanism requiring AT1 receptor activation. The attenuated sympathoinhibitory response to VE in ANG II-infused animals remained after arterial barodenervation and systemic vasopressin V1 receptor antagonism and appeared to depend on ANG II being chronically increased because ANG II given acutely had no effect on VE-induced renal sympathoinhibition.
Keywords: sympathetic nerve activity, cardiopulmonary reflex, body fluid balance, arterial baroreceptor reflex
Numerous studies have investigated interactions between circulating ANG II and the arterial baroreceptor reflex. Whereas recent studies indicate that an acute increase in circulating ANG II can attenuate the increase in sympathetic nerve activity (SNA) that follows arterial baroreceptor unloading (34), chronic increases of ANG II have been repeatedly demonstrated to reset arterial baroreflex control of SNA (4, 6). The latter effect may permit SNA to rise relative to the prevailing level of arterial pressure. In addition to the arterial baroreflex, the cardiopulmonary baroreflex is also an important determinant of sympathetic outflow as well as sodium and water balance. However, the extent to which chronic increases of ANG II might interact with this reflex to modify the SNA response to volume expansion (VE) is not known. This is surprising given that normal VE-induced inhibition of renal SNA is attenuated in animals with established congestive heart failure (CHF) (5, 11, 12), which is subsequently improved after AT1 receptor blockade (12). These results indicate that ANG II may alter the cardiopulmonary- renal reflex (12, 42), potentially contributing to the sympathoexcitation in disease states such as CHF in which extracellular fluid (ECF) volume becomes progressively increased in the presence of elevated circulating ANG II.
In this study, to characterize the extent to which ANG II can influence the cardiopulmonary-renal reflex, experiments were performed to test the hypothesis that inhibition of renal SNA by acute VE is attenuated when circulating ANG II is chronically infused. The role of AT1 receptors in mediating this effect was tested by giving losartan acutely, just before VE. To avoid potential interactions between the cardiopulmonary and arterial baroreflexes, experiments were also performed on rats subjected to sinoaortic denervation (SAD).
METHODS
Experiments were performed in male Sprague-Dawley rats (250–350 g). Animals were housed in a temperature-and humidity-controlled room, maintained on a 14:10-h light-dark cycle, and allowed free access to rat chow and water. All procedures were approved by the Institutional Animal Care and Use Committee and were carried out in accordance with guidelines and principles set forth by the National Institutes of Health and the American Physiological Society (2).
VE Protocol
In each of the following experiments, mean arterial pressure (MAP), heart rate (HR), mean right atrial pressure (MRAP), and renal SNA responses to acute VE were determined. VE was performed by infusing isotonic saline into the right atriocaval junction at a rate of 20% of the estimated blood volume in a period of 5 min. Blood volume was estimated to be equivalent to 7% of body weight (vol/wt) (21).
Chronic Infusion of ANG II
Eleven days before experimentation, rats were anesthetized with pentobarbital sodium (40 mg/kg ip). Through an abdominal incision, telemetry probes were placed in the abdominal aorta to continuously record blood pressure before and during chronic infusions. In addition, a catheter was placed in the abdominal vena cava for intravenous infusions. Its distal end was exteriorized between the scapulae, passed through a stainless steel spring, and connected to a syringe pump using a hydraulic swivel to allow the animals to move freely throughout the study. A solution containing 5% dextrose and ampicillin (270 µg/24 h) was infused continuously (24 h/day) throughout the study at a rate of 5.7 ml/24 h. After 5 days, ANG II (12 ng·kg−1 ·min−1, Sigma) was added to the infusion solution, and fresh ANG II was added to the infusate daily. A control group received vehicle solution throughout the infusion protocol.
In a separate group of conscious animals, the contribution of parasympathetic nerve activity to the control of HR was determined both 3 days before and at completion of the 7-day ANG II (n = 4) or vehicle (n = 4) infusion protocol by blocking muscarinic ACh receptors with atropine methylbromide (2 mg/kg iv). After muscarinic receptor blockade, the contribution of SNA to the maintenance of blood pressure was determined by measuring the fall in MAP produced by interrupting ganglionic transmission with the nicotinic ACh receptor antagonist chlorisondamine (2.5 mg/kg iv). The completeness of ganglionic blockade was verified by the absence of an HR response to baroreceptor unloading with nitroprusside (1 µg/kg iv). Animals that received atropine and chlorisdondamine were not used in any other protocols.
To assess the effect of chronically infused ANG II on plasma volume, blood was collected from the femoral vein in anesthetized animals, and hematocrit was determined in a subset of animals (n = 6) before acute VE. On completion of these procedures, effects of chronic infusion of ANG II (7 days) on MAP, HR, MRAP, and renal SNA responses to acute VE were examined. To control for potential effects associated with chronic surgical instrumentation, acute VE was also performed in untreated rats, which were not subjected to prior interventions.
Experimental Procedures
After 7 days of continuous ANG II (or vehicle) infusion, and without interrupting infusion, rats were anesthetized with a mixture (200 µl) of α-chloralose (70 mg/kg ip) and urethane (70 mg/kg ip). Catheters (PE-50) were then inserted in the right femoral vein and artery to infuse drugs and to measure arterial pressure, respectively. Two additional catheters were inserted into the right atrium via the right jugular vein: one for infusion of isotonic saline and the other for continuous measurement of MRAP. After tracheal cannulation, rats were ventilated with oxygen-enriched room air. End-tidal Pco2 was maintained within normal limits (35–40 mmHg) by adjusting ventilation rate (80–100 breaths/min) and/or tidal volume (2.0–3.0 ml). Body temperature was maintained at 37 ± 1°C by using a ventrally placed water-circulating pad. The depth of anesthesia was monitored by the stability of MAP and by the lack of a withdrawal response to noxious mechanical stimulation of a hindpaw. When necessary, a supplemental dose of anesthetic (10% initial dose, 100 µl ip) was administered and at least 20 min was allowed for recorded variables to stabilize.
Through a left flank incision, a renal sympathetic nerve bundle was isolated from surrounding tissue and mounted on a bipolar stainless steel wire electrode (A-M systems, 0.127 mm OD). The electrode and nerve were insulated and secured in place with a silicon impression material (Coltene, light body, Switzerland). Nerve signals were obtained using a high-impedance probe connected to an AC amplifier (P5 series, Grass Instruments). Signals were amplified (×10,000–20,000), filtered (0.1–1.0 kHz), rectified, and integrated (200–ms time constant). At the end of each experiment, an overdose of anesthetic was given and the contribution of noise to the integrated voltage was determined 30 min after death. The noise level was subtracted from all recorded renal SNA values.
Forty-five minutes after surgical instrumentation, monitoring of MAP, HR, MRAP, and renal SNA began. After a 5-min control period, the VE protocol was initiated (see above) and recordings were continued at least 5 min after the infusion was complete.
Determining Effects of AT1 Receptor Blockade
In separate groups of animals infused chronically with either ANG II (n = 6) or vehicle (n = 6), losartan (3 mg/kg) was administered intravenously 30 min before initiation of acute VE. Due to the depressor response that ensued, two additional groups were studied. One group (n = 6) chronically infused with ANG II received losartan, and MAP was restored to the level recorded just before losartan administration by infusing the α-adrenoceptor agonist phenylephrine (PE) (1–3 µg/min iv). The other group (n = 6) was chronically infused with vehicle, received losartan, and again MAP was restored with PE. In all animals, normalization of MAP was maintained for at least 10 min before initiation of VE and continued throughout the infusion protocol. The effectiveness of AT1 receptor blockade was confirmed in these experiments by comparing blood pressure responses to ANG II (8 ng iv) recorded before and 45 min after losartan administration.
Determining Effects of Acute ANG II Treatment
To determine if effects of chronically administered ANG II on VE-induced responses could be elicited acutely, the ANG II infusion was begun 20 min before initiating the VE protocol. Two groups of animals were used: one received ANG II at the same rate as used chronically (12 ng·kg−1 ·min−1, n = 6), and the other received ANG II at the rate (12 ng/min, n = 3) used by Veelken et al. (42). The latter dose was shown to attenuate VE-induced renal sympathoinhibition (42). Experiments were carried out in animals chronically infused with vehicle only. Control experiments were performed by infusing saline alone for 20 min in place of ANG II.
Determining Effects of Sinoaortic Baroreceptor Afferent Denervation
Two weeks before instrumentation, SAD was performed in a group of 8 rats. The procedure was performed according to standard methods (20). The superior laryngeal and aortic nerves were transected, and the cervical sympathetic ganglia were removed. Each carotid bifurcation was stripped of connective tissue, and a 10% phenol in ethanol solution was painted on each bifurcation to destroy any remaining nerve tissue. As with baroreceptor afferent-intact animals, blood pressure telemetry probes and abdominal vena cava catheters were implanted after 2 wk of recovery from SAD surgery. Telemeters allowed the completeness of each SAD to be determined before the start of the chronic infusion protocol. SAD was verified in conscious rats by the absence of an HR change during a 25-mmHg increase in MAP induced by PE (5–10 µg/kg iv). SAD rats were separated into two groups that received either chronically administered vehicle or ANG II. After chronic infusion, rats were subjected to acute VE as outlined above.
Determining Effects of Vasopressin Receptor Blockade
To determine the role of vasopressin in the ANG II-induced attenuation of VE responses, an arginine vasopressin (AVP) V1 receptor antagonist (Manning compound, 50 µg) was administered intravenously 30 min before initiation of the VE protocol. On completion of VE, V1 receptor blockade was confirmed by the lack of a pressor response to AVP (50 ng iv).
Analysis
Average values of baseline MAP, HR, and renal SNA were measured over a 60-s period immediately before acute VE. Responses to VE were measured each minute during volume infusion by taking the average of a 20-s period and included the period of maximum renal SNA inhibition. When plasma VE was continued for up to 20 min, no further decreases in renal SNA were observed past 5 min; therefore, data in this study are shown over a 5-min period. Baseline renal SNA values were taken as 100%, and values recorded 30 min after euthanasia are considered to represent background noise (0% nerve activity), which was subtracted from all renal SNA values. The effect of VE on renal SNA is expressed as a percent change from baseline.
The unpaired Student’s t-test was used to compare hematocrit, HR responses to PE in SAD rats, and MAP responses to ganglionic blockade. Two-way ANOVA with repeated measures was used to determine effects of VE in all groups. When a significant interaction was obtained, Tukey’s post hoc test was applied to assess pairwise differences. Statistical significance is defined as P < 0.05. All values in the text and figures are expressed as means ± SE.
RESULTS
Effects of Chronic ANG II Infusion
Ganglionic blockade
HR responses to atropine and chlorisondamine did not differ between groups both before and after chronic infusion of either ANG II or vehicle (Fig. 1A). Similarly, MAP responses to atropine were not different before or after chronic ANG II or vehicle infusion (Fig. 1B). In contrast, ganglionic block-ade with chlorisondamine produced a significantly greater decrease (P < 0.01) in MAP in ANG II-infused (116 ± 2 to 40 ± 2 mmHg; Δ = 75 ± 3 mmHg, n = 4) compared with vehicle-infused (85 ± 5 to 36 ± 6 mmHg; Δ = 47 ± 8 mmHg, n = 4) animals (Fig. 1B). These data indicate that the contribution of SNA in maintaining arterial pressure was significantly increased in animals chronically infused with ANG II. Differences in the magnitude of the depressor response did not appear to involve differences in HR control because tachycardic responses to atropine were not different between ANG II-infused (80 ± 16 beats/min, n = 4) and vehicle-infused (86 ± 16 beats/min, n = 4) animals (Fig. 1A). These data suggest that, in this model, chronic ANG II treatment significantly increases ongoing SNA.
Fig. 1.
Effect of atropine (2 mg/kg iv) and ganglionic blockade with chlorisondamine (2.5 mg/kg iv) on heart rate (HR; A) and mean arterial pressure (MAP; B) in rats both before and after chronic vehicle (n = 4) or ANG II (n = 4) infusion. *Significant difference (P < 0.05) compared with vehicle-infused group. Data indicate changes (Δ) from baseline values. bpm, Beats/min.
Hematocrit
Baseline hematocrit in vehicle-infused (43.8 ± 2%) and ANG II-infused (45.4 ± 0.9%) animals was not significantly different (Table 1), suggesting that ECF volume was not significantly different between groups.
Table 1.
Effect of 20% VE on MAP, HR, MRAP, RSNA, and hematocrit in rats receiving chronic infusions
| Vehicle-Infused Rats |
ANG II-Infused Rats |
|||
|---|---|---|---|---|
| Baseline | 20% VE | Baseline | 20% VE | |
| MAP, mmHg | 131 ± 5.7 | 128 ± 4 | 124 ± 6 | 123 ± 5 |
| HR, beats/min | 408 ± 11 | 357 ± 16* | 419 ± 9 | 384 ± 9* |
| MRAP, mmHg | −0.9 ± 0.27 | 0.25 ± 5* | −0.13 ± 0.17† | 1.04 ± 8* |
| ΔMRAP, mmHg | 0.99 ± 0.1 | 1.0 ± 0.2 | ||
| RSNA, % | 100 | 66.5 ± 8* | 100 | 97.5 ± 2.4‡ |
| Hematocrit, % | 43.8 ± 2 | 45.4 ± 0.9 | ||
Values are means ± SE; n = 6 in all groups. MAP, mean arterial pressure; HR, heart rate; MRAP; mean right atrial pressure; ΔMRAP, change in MRAP; RSNA, renal sympathetic nerve activity.
P < 0.05 vs. baseline;
P < 0.05 vs. vehicle-infused baseline;
P < 0.001 vs. 20% volume expansion (VE) in vehicle-infused rats.
Effect of Chronically Infused ANG II
VE significantly reduced renal SNA in vehicle-infused rats (66.5 ± 8%, n = 6, P < 0.001) but had no effect in rats chronically infused with ANG II (97.5 ± 2%, n = 6) (Fig. 2). The difference between these two responses did not appear to depend on the level of resting MAP or HR because baseline values in both groups were not different (Table 1). Similarly, MAP and HR responses to acute VE did not differ between groups (Fig. 2, Table 1). Although baseline MRAP was greater in ANG II-infused (−0.13 ± 0.17 mmHg, P < 0.05) vs. vehicle-infused animals (−0.9 ± 0.27 mmHg), the change in MRAP in response to VE was not different (Table 1). Overall, these data indicate that chronic ANG II treatment results in an attenuated renal SNA response to acute VE.
Fig. 2.
ΔMAP, ΔHR, change in mean right atrial pressure (ΔMRAP), and renal sympathetic nerve activity (RSNA) (% of baseline) in response to acute volume expansion (20% estimated blood volume in 5 min). Chronic vehicle infusion, n = 6; chronic ANG II infusion, n = 6. *Significant difference (P < 0.05) from baseline; †P < 0.05, ANG II infused vs. vehicle infused. All data are changes from baseline values.
Effect of Acutely Administered Losartan
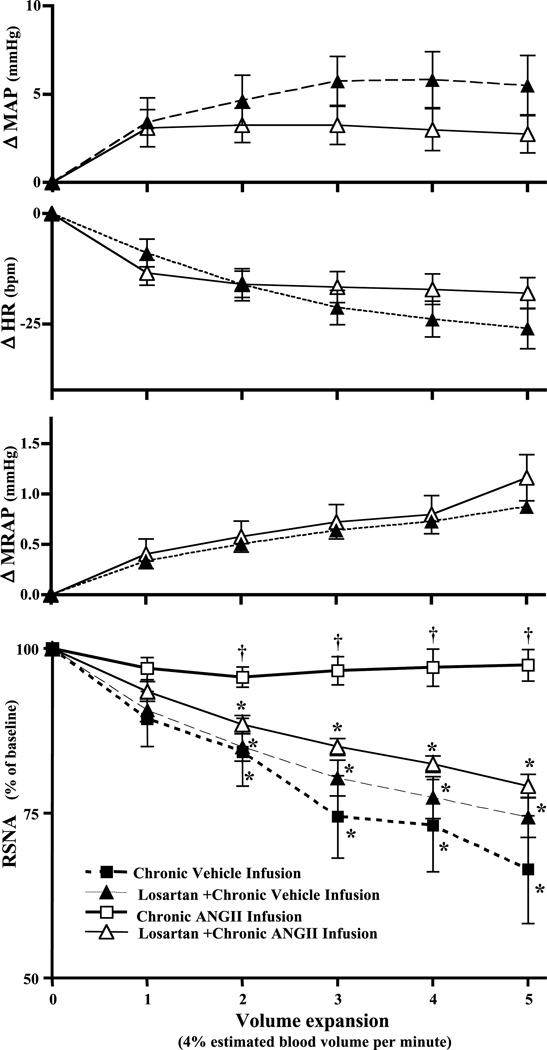
To determine the role of ongoing AT1 receptor activation in the attenuated VE-induced renal SNA response after chronic ANG II treatment, the AT1 selective antagonist losartan was given 20 min before initiation of VE in a separate group of animals receiving either chronic vehicle (n = 12) or ANG II (n = 12) infusion. In both vehicle- and ANG II-infused animals, losartan produced similar changes in MAP (101.8 ± 4 to 77.8 ± 3.6 and 95 ± 4 to 77.6 ± 3 mmHg, respectively), HR (343 ± 10 to 347 ± 11 and 375 ± 16 to 372 ± 16 beats/min, respectively), and MRAP (−0.91 ± 0.21 to −1.25 ± 0.25 and −0.6 ± 0.37 to −0.97 ± 0.4 mmHg, respectively). To eliminate the possibility that the starting level of MAP plays a significant role in the renal SNA response to acute VE in the presence of losartan, PE was used to restore MAP to the pre-losartan level at least 10 min before and throughout acute VE in 6 animals from each group of 12 described above. The remaining six animals from each group were subjected to acute VE without prior normalization of MAP. Because normalization of MAP in six animals from each group did not significantly alter the MAP, HR, MRAP, and renal SNA responses to acute VE compared with animals in which the depressor responses to losartan were not normalized, data were combined for statistical analysis and are presented in the subsequent paragraph and graphically (Fig. 3, Table 2) as two groups: vehicle-infused + losartan (n = 12) and ANG II-infused + losartan (n = 12).
Fig. 3.
Effect of acutely administered losartan (3 mg/kg iv) on ΔMAP, ΔHR, ΔMRAP, and RSNA responses to acute volume expansion (20% estimated blood volume in 5 min). Chronic vehicle infusion, n = 6; losartan + chronic vehicle infusion, n = 12; chronic ANG II infusion, n = 6; losartan + chronic ANG II infusion, n = 12. *Significant difference (P < 0.05) from baseline; †significant difference (P < 0.05) compared with both losartan-treated groups. Data indicate changes from baseline values.
Table 2.
Effect of 20% VE on MAP, HR, MRAP, and RSNA after intravenous losartan in rats receiving chronic infusions
| Vehicle-Infused Rats |
ANG II-Infused Rats |
|||
|---|---|---|---|---|
| Baseline | 20% VE | Baseline | 20% VE | |
| MAP mmHg | 88.5 ± 4 | 94 ± 4 | 89.7 ± 5 | 92 ± 5 |
| HR, beats/min | 342 ± 11 | 316 ± 12 | 372 ± 16 | 355 ± 15 |
| MRAP, mmHg | − 1.23 ± 0.23 | − 0.19 ± 0.2* | −0.81 ± 0.3 | 0.51 ± 0.3* |
| ΔMRAP, mmHg | 0.9 ± 0.07 | 1.1 ± 0.2 | ||
| RSNA, % | 100 | 74 ± 3* | 100 | 79 ± 2* |
Values are means ± SE; n = 12 in both groups.
P < 0.05 vs. baseline.
Results indicate that AT1 receptor blockade effectively restored the inhibitory renal SNA response to VE (79.2 ± 2%, n = 12, P < 0.001) (Fig. 3, Table 2). In contrast, losartan had no effect on VE-induced sympathoinhibition in vehicle-infused animals (74.4 ± 3%, n = 12) (Fig. 3, Table 2). Baseline MAP, HR, and MRAP values in both groups were not different (Table 2). Similarly, MAP, HR, and MRAP responses to acute VE did not differ between groups (Fig. 3, Table 2). Overall, losartan pretreatment restored VE-induced inhibition of renal SNA in rats chronically treated with ANG II but remained ineffective in altering the response in vehicle-infused controls. These data suggest that acute interruption of AT1 receptor activation is sufficient to prevent the attenuated renal SNA response to VE in animals chronically infused with ANG II.
Effect of Acutely Administered ANG II
In these experiments ANG II was acutely infused at either the same rate as used chronically (12 ng·kg−1 ·min−1, n = 6) or at the rate used by Veelken et al. (42) (12 ng/min, n = 3). Renal sympathoinhibition to VE in these two groups [55 ± 5% (Fig. 4) and 59.3 ± 7%, respectively] was not different from that observed in untreated rats (64.6 ± 5.4%, n = 7) (Fig. 4, Table 3). Likewise, the renal SNA response to VE in animals infused acutely with ANG II did not differ from that observed in animals infused either acutely (64.2 ± 6%, Fig. 4, n = 5) or chronically (66.5 ± 8%, Fig. 2, n = 6) with vehicle alone. However, the VE-induced renal SNA decrease observed during acute ANG II was significantly (P < 0.001) greater than that observed when ANG II was infused chronically (97.5 ± 2%, n = 6). Baseline MAP and HR values were not different between groups (Table 3), and MAP and HR responses to acute VE did not differ between groups (Fig. 4). These results indicate that ANG II-induced attenuation of the renal SNA response to VE requires chronic but not acute ANG II treatment.
Fig. 4.
ΔMAP, ΔHR, and RSNA in response to acute volume expansion (20% estimated blood volume in 5 min) during acute infusion of ANG II (12 ng·kg−1 ·min−1). Untreated rats, n = 7; acute vehicle infusion, n = 5; acute ANG II infusion, n = 6; chronic ANG II infusion, n = 6. *Significant difference (P < 0.05) from baseline; †significant difference (P < 0.05) from untreated rats and those acutely treated with ANG II. Data indicate changes from baseline values.
Table 3.
Effect of 20% VE on MAP, HR, and RSNA after acute intravenous ANG II infusion, acute intravenous vehicle infusion, and in untreated rats
| Acute ANG II Infusion |
Acute Vehicle Infusion |
Untreated Rats |
||||
|---|---|---|---|---|---|---|
| Baseline | 20% VE | Baseline | 20% VE | Baseline | 20% VE | |
| MAP, mmHg | 115 ± 3 | 118 ± 4 | 106 ± 2 | 110 ± 3 | 112 ± 4 | 115 ± 4 |
| HR, beats/min | 407 ± 23 | 393 ± 22 | 328 ± 24 | 305 ± 30 | 373 ± 14 | 343 ± 14 |
| RSNA, % | 100 | 55 ± 5* | 100 | 64 ± 2* | 100 | 64 ± 6* |
Values are means ± SE; n = 6 in acute ANG II (12 ng·kg−1 ·min−1) infusion group, n = 5 in acute vehicle infusion group, and n = 7 in untreated group.
P < 0.05 vs. baseline.
Effect of SAD and AVP V1 Receptor Antagonism
SAD did not significantly alter the ability of chronically administered ANG II to attenuate the renal sympathoinhibitory response to VE (96.8 ± 3%, n = 4). Likewise, SAD had no effect on VE-induced renal sympathoinhibition in vehicle-infused rats (77.5 ± 4%, n = 4) (Fig. 5, Table 4). Removal of arterial baroafferents appeared complete because increases in MAP (25 mmHg) produced by PE elicited no significant change in HR (−7.3 ± 3 beats/min). Baseline MAP, HR, and MRAP values did not differ between groups (Table 4), and MAP, HR, and MRAP responses to acute VE were not different between groups (Fig. 5). These results suggest that ANG II-induced attenuation is not dependent on intact arterial baroreceptor afferents.
Fig. 5.
Effect of sinoaortic denervation (SAD) on ΔMAP, ΔHR, ΔMRAP, and RSNA (% of baseline) responses to acute volume expansion (20% estimated blood volume in 5 min). Barointact + chronic vehicle infusion, n = 6; SAD + chronic vehicle infusion, n = 6; barointact + chronic ANG II infusion, n = 6; SAD + chronic ANG II infusion, n = 4. *Significant difference (P < 0.05) from baseline; †significant difference (P < 0.05) between ANG II- and vehicle-infused rats. Data indicate changes from baseline values.
Table 4.
Effect of 20% VE on MAP, HR, MRAP, and RSNA in SAD rats receiving chronic infusions
| Vehicle-Infused Rats |
ANG II-Infused Rats |
|||
|---|---|---|---|---|
| Baseline | 20% VE | Baseline | 20% VE | |
| MAP, mmHg | 97 ± 10 | 115 ± 9 | 110 ± 4 | 133 ± 6 |
| HR, beats/min | 339 ± 24 | 326 ± 17 | 415 ± 10 | 405 ± 5 |
| MRAP, mmHg | −0.39 ± 0.18 | 0.62 ± 0.2* | −0.53 ± 0.3 | 0.31 ± 0.3* |
| ΔMRAP mmHg | 1.01 ± 0.1 | 0.83 ± 0.06 | ||
| RSNA % | 100 | 77.5 ± 4* | 100 | 96.8 ± 3 |
Values are means ± SE; n = 4 in both groups. SAD, sinoaortic denervation.
P < 0.05 vs. baseline.
Additionally, the renal SNA response to acute VE was not affected by AVP V1 receptor antagonism in animals chronically infused with either vehicle or ANG II (61.8 ± 3%, n = 4 and 91.5 ± 3%, n = 6, respectively) (Fig. 6), suggesting that the effect of chronically infused ANG II to attenuate VE-induced renal sympathoinhibition is not due to an AVP V1 receptor-mediated mechanism. Baseline MAP, HR, and MRAP values were not different between groups (Table 5). Similarly, MAP, HR, and MRAP responses to acute VE in both groups were similar (Fig. 6).
Fig. 6.
Effect of acutely administered AVP V1 receptor antagonist (50 ng iv) on ΔMAP, ΔHR, ΔMRAP, and RSNA (% of baseline) responses to acute volume expansion (20% estimated blood volume in 5 min). Chronic vehicle infusion, n = 6; AVP V1 receptor blockade + chronic vehicle infusion, n = 5; chronic ANG II infusion, n = 6; AVP V1 receptor blockade + chronic ANG II infusion, n = 5. *Significant difference (P < 0.05) from baseline; †significant difference (P < 0.05) between ANG II- and vehicle-infused rats. Data indicate changes from baseline values.
Table 5.
Effect of 20% VE on MAP, HR, MRAP, and RSNA after AVP V1 receptor blockade in rats receiving chronic infusions
| Vehicle-Infused Rats |
ANG II-Infused Rats |
|||
|---|---|---|---|---|
| Baseline | 20% VE | Baseline | 20% VE | |
| MAP, mmHg | 97 ± 10 | 104 ± 7 | 98 ± 5 | 104 ± 5 |
| HR, beats/min | 342 ± 18 | 317 ± 19 | 372 ± 32 | 350 ± 34 |
| MRAP, mmHg | −0.16 ± 0.8 | 0.76 ± 0.8 | −0.53 ± 0.6 | 0.53 ± 0.7 |
| ΔMRAP mmHg | 0.92 ± 0.14 | 1.0 ± 0.11 | ||
| RSNA % | 100 | 61.8 ± 3* | 100 | 91.5 ± 3† |
Values are means ± SE; n = 6 in both groups. AVP, arginine vasopressin.
P < 0.05 vs. baseline;
P < 0.001 vs. 20% VE in vehicle-infused rats.
DISCUSSION
The present study was designed to assess the effect of elevated circulating ANG II on the inhibition of renal SNA by acute VE. Our data provide evidence that the renal sympathoinhibitory response to VE is effectively abolished by chronically, but not acutely, infused ANG II. VE-induced inhibition of renal SNA was effectively restored by acute administration of losartan, indicating involvement of an AT1 receptor mechanism.
The potential for antinatriuretic and antidiuretic actions of ANG II to increase ECF volume was an initial concern as this could potentially lead to chronic atrial distension. Such an effect, if present, could alter the sensitivity of cardiopulmonary receptors to acute VE and contribute to the attenuated sympathoinhibitory response observed. Based on our data showing that hematocrit was not different, it seems unlikely that an increase in ECF volume occurred in our model. This result appears consistent with those of other studies showing no change in sodium and water balance or ECF volume in animals chronically infused with ANG II (7, 18). Overall, the conclusion that chronic ANG II infusion did not significantly affect ongoing atrial compliance is supported by evidence that MRAP responses during VE did not differ between animals that received chronic ANG II compared with those infused with vehicle alone.
We are aware of only one previous study in which effects of exogenously administered ANG II on the renal SNA response to acute VE were determined. Veelken et al. (42) reported that acutely administered ANG II blunted, but did not abolish, the renal sympathoinhibitory response to VE (42). Our data, however, do not confirm this finding. Although the dose of ANG II used was the same in both studies, we observed a slight pressor (+10 mmHg) effect, whereas the same dose was reported to be “subpressor” in their study. What seems clear is that the pressor effect we observed does not appear to account for the discrepancy between the two studies. This is indicated by the fact that MAP just before initiation of the VE protocol was not significantly different among animals infused with ANG II (acutely or chronically) and those chronically infused with vehicle. Although a discrepancy does exist concerning the effects of acutely administered ANG II on the sympathoinhibitory response to VE, it seems that additional studies will be necessary to explain these differences.
ANG II is known to stimulate the synthesis of AVP and its release from the posterior pituitary (35). To eliminate any potential contribution of AVP to the attenuated renal SNA response to VE we observed in ANG II-infused animals, we blocked AVP V1 receptors. Acute V1 receptor blockade did not alter the renal sympathoinhibitory effect of VE in either vehicle- or ANG II-infused rats. This finding is perhaps not unexpected because Cowley et al. (9) reported that effects of ANG II on AVP release appear to be transient.
Studies demonstrate that mitogenic effects of long-term infusions of ANG II can cause significant tissue hypertrophy and hyperplasia (17). Such processes could ultimately influence mechanical coupling of cardiopulmonary stretch receptors to their tissues. As a result, atrial stretch and cardiopulmonary receptor afferent activity could potentially be reduced. Overall, such an effect may be expected to contribute to the attenuated renal sympathoinhibitory response to VE observed in the present study. However, this seems unlikely because acutely administered losartan rapidly restored the sympathetic nerve response to VE.
In this study, the ability of chronic infusion of ANG II to attenuate the renal SNA response to VE does not appear to depend on the resting level of arterial pressure because baseline values did not differ between ANG II- and vehicle-infused groups. Additionally, results from this study demonstrate that the effect of peripherally administered losartan to effectively restore the renal SNA response to VE in rats chronically infused with ANG II is not dependent on the starting level of MAP because the depressor response to losartan was similar in both groups. Furthermore, the losartan treatment restored VE-induced sympathoinhibition in animals where MAP was either normalized with PE or not.
It is well documented that arterial baroreceptors are not required for the decrease in renal SNA elicited by acute VE (8, 33). Our study confirms this finding because SAD did not alter the sympathoinhibitory response to acute VE. Unlike numerous studies indicating that chronically infused ANG II can alter arterial baroreflex function (4, 6), the effect of chronic infusion of ANG II on the cardiopulmonary reflex has not been previously studied. The present findings demonstrate that during chronic infusion of ANG II, the VE-induced decrease in renal SNA is abolished even after baroreceptor afferent denervation, suggesting that ANG II-induced attenuation is not dependent on ongoing baroreceptor afferent input.
There is abundant evidence that ANG II can elicit a centrally mediated pressor response that depends largely on activation of sympathetic outflow (38). Results from this and previous studies from our laboratory (10) support this conclusion because the depressor response to ganglionic blockade was significantly greater in rats chronically infused with ANG II compared with those infused with vehicle only. These results suggest that sympathetic activity was increased and illustrate the potential for ANG II to influence autonomic control systems through its interactions in the central nervous system (CNS) (10, 32).
Although additional studies are required to specifically define the CNS circuitry involved in our observed attenuated VE-induced renal SNA responses in the presence of chronic increases of ANG II, several studies demonstrate that the paraventricular nucleus of the hypothalamus (PVN) is one particular site in the CNS that is important for receiving and processing ANG II-sensitive inputs from forebrain circumventricular organs (CVOs) (3, 14, 22, 40). Neurons in the CVOs, notably the subfornical organ (SFO) and the organum vasculosum of the lamina terminalis (OVLT), not only sense circulating peptides like ANG II (3, 14) but also send ANG II-immunoreactive efferent projections to the PVN where neurons express a high concentration of ANG II AT1 receptors (23, 29). Electrophysiological studies demonstrate that ANG II can increase PVN unit discharge when applied either directly within the SFO or when given systemically (1, 15). Together, available evidence indicates that ANG II is a likely neurotransmitter released from SFO terminals within the PVN (22, 41). Furthermore, parvocellular neurons of the PVN project to regions of the CNS that control sympathetic outflow (36), including the rostral ventrolateral medulla, the dorsomedial medulla, and the spinal intermediolateral cell column (26, 36, 39). Numerous studies support the concept that neuronal projections from autonomic neurons in the PVN can modulate the activity of sympathetic nerves (27, 28). Such data demonstrate that the PVN is an important central site where information concerning the level of blood-borne ANG II is processed and may play a significant role in mediating the attenuated renal SNA response to acute VE when circulating ANG II is chronically infused.
Recent studies have shown that the PVN is not only important for processing ANG II-sensitive inputs but also for producing the renal SNA response to volume expansion (19, 24, 25). Normally, expansion of plasma volume activates cardiopulmonary receptors that produce profound reflex inhibition of SNA (33). However, selective destruction of the parvocellular PVN neurons significantly attenuates the renal sympathoinhibitory response to VE (19, 25), suggesting that the PVN is a site necessary for the normal integration of blood volume information. Overall, evidence indicates that ANG II-induced sympathoexcitation as well as VE-induced sympathoinhibition depend on autonomic neurons in the PVN that influence brain stem and spinal cord regions controlling sympathetic outflow.
It is perhaps not surprising that acute and chronic ANG II treatment produces distinct effects on VE-induced renal SNA responses, given that differential effects of acute vs. chronic ANG II treatment have also been demonstrated in studies of arterial baroreflex control of SNA (4, 6, 30). These findings are consistent with increasing evidence that distinct intracellullar signaling pathways can be activated downstream from the AT1 receptor by acute vs. chronic stimulation. For example, acute effects include membrane depolarization mediated through protein kinase C-dependent inhibition of K+ and activation of Ca2+ currents (37). On the other hand, chronic ANG II treatment can stimulate slower neuromodulatory pathways (Ras/Raf/MAP kinase and Jak/STAT) that increase synthesis and expression of proteins involved in vesicular trafficking and exocytosis (16). Thus time-dependent effects of ANG II on physiological responses such as those reported here may well depend on specific cellular processes that remain to be fully elucidated.
In conclusion, the present study demonstrates that elevated circulating ANG II attenuates the renal sympathoinhibitory response to acute VE. Observed responses appear to require chronic ANG II treatment and AT1 receptor activation. Further experiments are necessary to define the potential role of central vs. peripheral AT1 receptors in mediating this response.
Perspectives
An increase in sodium and water intake is associated with expansion of ECF volume. Through activation of the cardiopulmonary reflex, such an increase in ECF volume normally results in an increase in sodium and water excretion that is mediated by neural and humoral effectors. Ultimately, through this feedback system, ECF volume is restored toward normal. However, this is not the case in certain disease states such as CHF (12) and diabetes (31), in which data suggest that the cardiopulmonary baroreflex is blunted. Evidence indicates that, at least in part, ANG II AT1 receptors play a role in mediating the depressed renal SNA response to volume expansion in CHF rats (12), leading to the suggestion that ANG II, which is elevated during CHF, might contribute to the attenuated renal SNA response to VE in CHF rats.
ANG II is known to influence the arterial baroreceptor reflex by resetting control of SNA and permitting sympathetic activity to rise relative to the prevailing level of arterial pressure. Numerous studies have focused on this effect and its potential contribution to arterial hypertension. Although it has been shown that the renal SNA response to acute VE is augmented in rats receiving a low-sodium vs. high-sodium diet (13), the volume state of these animals introduces another component that warrants further study. To date, little is known concerning the influence of circulating ANG II on other visceral afferent reflexes, in particular the cardiopulmonary reflex in animal models that display either normal or elevated ECF volume. Our results provide evidence that the neural component of the cardiopulmonary reflex is altered in the presence of chronic increases of ANG II and further provides a potential integral mechanism by which ANG II contributes to sympathoexcitation in disease states such as nephrotic syndrome, some forms of arterial hypertension, and CHF in which ECF volume becomes progressively increased in the presence of elevated circulating ANG II.
Acknowledgments
We gratefully acknowledge Dr. Q. H. Chen for expert guidance in performing surgical sinoaortic barodenervation. We also thank R. Guler and K. Rees for excellent technical assistance and S. Garner for assistance with preparing this manuscript.
This project was supported by National Heart, Lung, and Blood Institute Grant HL-59326.
REFERENCES
- 1.Akaishi T, Negoro H, Kobayasi S. Electrophysiological evidence for multiple sites of action of angiotensin II for stimulating paraventricular neurosecretory cells in rat. Brain Res. 1984;220:386–390. doi: 10.1016/0006-8993(81)91230-0. [DOI] [PubMed] [Google Scholar]
- 2.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283:R281–R283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]
- 3.Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–229. doi: 10.1016/0006-8993(92)90395-p. [DOI] [PubMed] [Google Scholar]
- 4.Bishop VS, Ryuzaki M, Cai Y, Nishida Y, Cox BF. Angiotensin II-dependent hypertension and the arterial barore-flex. Clin Exp Hypertens. 1995;17:29–38. doi: 10.3109/10641969509087052. [DOI] [PubMed] [Google Scholar]
- 5.Brandle M, Wang W, Zucker IH. Ventricular mechanoreflex and chemoreflex alterations in chronic heart failure. Circ Res. 1994;74:262–270. doi: 10.1161/01.res.74.2.262. [DOI] [PubMed] [Google Scholar]
- 6.Brooks VL, Ell KR, Wright RM. Pressure-independent baroreflex resetting produced by chronic infusions of angiotensin II in rabbits. Am J Physiol Heart Circ Physiol. 1993;265:H1275–H1282. doi: 10.1152/ajpheart.1993.265.4.H1275. [DOI] [PubMed] [Google Scholar]
- 7.Brown AJ, Casals-Stenzel J, Goffard S, Lever AF, Morton JJ. Comparison of fast and slow pressor effects of angiotensin II in the conscious rat. Am J Physiol Heart Circ Physiol. 1981;241:H381–H388. doi: 10.1152/ajpheart.1981.241.3.H381. [DOI] [PubMed] [Google Scholar]
- 8.Clement DL, Pelletier CL, Shepherd JT. Role of vagal afferents in the control of renal sympathetic nerve activity in the rabbit. Circ Res. 1972;31:824–830. doi: 10.1161/01.res.31.6.824. [DOI] [PubMed] [Google Scholar]
- 9.Cowley AW, Jr, Switzer SJ, Skelton MM. Vasopressin, fluid, and electrolyte response to chronic angiotensin II infusion. Am J Physiol Regul Integr Comp Physiol. 1981;240:R130–R138. doi: 10.1152/ajpregu.1981.240.3.R130. [DOI] [PubMed] [Google Scholar]
- 10.Cox BF, Bishop VS. Neural and humoral mechanisms of angiotensin-dependent hypertension. Am J Physiol Heart Circ Physiol. 1991;261:H1284–H1291. doi: 10.1152/ajpheart.1991.261.4.H1284. [DOI] [PubMed] [Google Scholar]
- 11.Dibner-Dunlap ME, Thames MD. Control of sympathetic nerve activity by vagal mechanoreflexes is blunted in heart failure. Circulation. 1992;86:1929–1934. doi: 10.1161/01.cir.86.6.1929. [DOI] [PubMed] [Google Scholar]
- 12.DiBona GF, Jones SY, Sawin LL. Angiotensin receptor antagonist improves cardiac reflex control of renal sodium handling in heart failure. Am J Physiol Heart Circ Physiol. 1998;274:H636–H641. doi: 10.1152/ajpheart.1998.274.2.H636. [DOI] [PubMed] [Google Scholar]
- 13.Dibona GF, Sawin LL. Renal nerve activity in conscious rats during volume expansion and depletion. Am J Physiol Renal Fluid Electrolyte Physiol. 1985;248:F15–F23. doi: 10.1152/ajprenal.1985.248.1.F15. [DOI] [PubMed] [Google Scholar]
- 14.Felix D, Akert K. The effect of angiotensin II on neurones of the cat subfornical organ. Brain Res. 1974;76:350–353. doi: 10.1016/0006-8993(74)90468-5. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson AV. Systemic angiotensin acts at the subfornical organ to control the activity of paraventricular nucleus neurons with identified projections to the median eminence. Neuroendocrinology. 1988;47:489–497. doi: 10.1159/000124960. [DOI] [PubMed] [Google Scholar]
- 16.Gallinat S, Busche S, Yang H, Raizada MK, Sumners C. Gene expression profiling or rat brain neurons reveals angiotensin II-induced regulation of calmodulin and synapsin I: possible role in neuromodulation. Endocrinology. 2001;142:1009–1016. doi: 10.1210/endo.142.3.8016. [DOI] [PubMed] [Google Scholar]
- 17.Griffin SA, Brown WCB, MacPherson F, McGrath JC, Wilson VG, Korsgaard N, Mulvany MJ, Lever AF. Angiotensin II causes vascular hypertrophy in part by a nonpressor mechanism. Hypertension. 1991;17:626–635. doi: 10.1161/01.hyp.17.5.626. [DOI] [PubMed] [Google Scholar]
- 18.Hall JE, Guyton AC, Salgado HC, McCaa RE, Balfe JW. Renal hemodynamics in acute and chronic angiotensin II hypertension. Am J Physiol Renal Fluid Electrolyte Physiol. 1978;235:F174–F179. doi: 10.1152/ajprenal.1978.235.3.F174. [DOI] [PubMed] [Google Scholar]
- 19.Haselton JR, Goering J, Kaushik PP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Auton Nerv Syst. 1994;50:1–11. doi: 10.1016/0165-1838(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 20.Krieger EM. Neurogenic hypertension in the rat. Circ Res. 1964;15:511–521. doi: 10.1161/01.res.15.6.511. [DOI] [PubMed] [Google Scholar]
- 21.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;25:72–76. [PubMed] [Google Scholar]
- 22.Li Z, Ferguson AV. Subfornical organ efferents to the paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol Regul Integr Comp Physiol. 1993;265:R302–R309. doi: 10.1152/ajpregu.1993.265.2.R302. [DOI] [PubMed] [Google Scholar]
- 23.Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. Neuroendocrinology. 1985;40:2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- 24.Lovick TA, Coote JH. Effects of volume loading on para-ventriculo-spinal neurones in the rat. J Auton Nerv Syst. 1988;25:135–140. doi: 10.1016/0165-1838(88)90018-5. [DOI] [PubMed] [Google Scholar]
- 25.Lovick TA, Malpas S, Mahony MT. Renal vasodilatation in response to acute volume load is attenuated following lesions of parvocellular neurones in the paraventricular nucleus in rats. J Auton Nerv Syst. 1993;43:247–256. doi: 10.1016/0165-1838(93)90331-n. [DOI] [PubMed] [Google Scholar]
- 26.Luiten PGM, Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- 27.Martin DS, Haywood JR. Hemodynamic responses to paraventricular nucleus disinhibition with bicuculline in conscious rats. Am J Physiol Heart Circ Physiol. 1993;265:H1727–H1733. doi: 10.1152/ajpheart.1993.265.5.H1727. [DOI] [PubMed] [Google Scholar]
- 28.Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res. 1992;577:261–267. doi: 10.1016/0006-8993(92)90282-e. [DOI] [PubMed] [Google Scholar]
- 29.Mendelsohn FAO, Quirion R, Saavedra JM, Aguilera G, Catt KJ. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci USA. 1984;81:1575–1579. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida Y, Ryan KL, Bishop VS. Angiotensin II modulates arterial baroreflex function via a central α1-adrenoceptor mechanism in rabbits. Am J Physiol Regul Integr Comp Physiol. 1995;269:R1009–R1916. doi: 10.1152/ajpregu.1995.269.5.R1009. [DOI] [PubMed] [Google Scholar]
- 31.Patel MB, Zhang PL. Reduced renal sympathoinhibition in response to acute volume expansion in diabetic rats. Am J Physiol Regul Integr Comp Physiol. 1994;267:R372–R379. doi: 10.1152/ajpregu.1994.267.2.R372. [DOI] [PubMed] [Google Scholar]
- 32.Reid IA. Actions of angiotensin II on the brain: mechanisms and physiologic role. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;246:F533–F543. doi: 10.1152/ajprenal.1984.246.5.F533. [DOI] [PubMed] [Google Scholar]
- 33.Ricksten SE, Noresson E, Thoren P. Inhibition of renal sympathetic nerve traffic from cardiac receptors in normotensive and spontaneously hypertensive rats. Acta Physiol Scand. 1979;106:17–22. doi: 10.1111/j.1748-1716.1979.tb06364.x. [DOI] [PubMed] [Google Scholar]
- 34.Sanderford MG, Bishop VS. Angiotensin II acutely attenuates range of arterial baroreflex control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2000;279:H1804–H1812. doi: 10.1152/ajpheart.2000.279.4.H1804. [DOI] [PubMed] [Google Scholar]
- 35.Sladek CD, Armstrong WE. Effect of Neurotransmitters and Neuropeptides on Vasopressin Release: Properties and Principles. New York: Plenum; 1987. pp. 275–316. [Google Scholar]
- 36.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491:274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- 37.Sumners C, Zhu M, Gelband CH, Posner P. Angiotensin II type 1 receptor modulation of neuronal K+ and Ca2+ currents: intracellular mechanisms. Am J Physiol Cell Physiol. 1996;271:C154–C163. doi: 10.1152/ajpcell.1996.271.1.C154. [DOI] [PubMed] [Google Scholar]
- 38.Suter C, Coote JH. Intrathecally administered angiotensin II increases sympathetic activity in the rat. J Auton Nerv Syst. 1987;19:31–37. doi: 10.1016/0165-1838(87)90142-1. [DOI] [PubMed] [Google Scholar]
- 39.Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka J, Kaba H, Saito H, Seto K. Electrophysiological evidence that circulating angiotensin II sensitive neurons in the subfornical organ alter the activity of hypothalamic paraventricular neurohypophyseal neurons in the rat. Brain Res. 1985;342:361–365. doi: 10.1016/0006-8993(85)91137-0. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka J, Kaba H, Saito H, Seto K. Subfornical organ neurons with efferent projections to the hypothalamic paraventricular nucleus: an electrophysiological study in the rat. Brain Res. 1985;346:151–154. doi: 10.1016/0006-8993(85)91106-0. [DOI] [PubMed] [Google Scholar]
- 42.Veelken R, Hilgers KF, Scrogin KE, Mann JFE, Schmieder RE. Endogenous angiotensin II and the reflex response to stimulation of cardiopulmonary serotonin 5HT3 receptors. Br J Pharmacol. 1998;125:1761–1767. doi: 10.1038/sj.bjp.0702259. [DOI] [PMC free article] [PubMed] [Google Scholar]