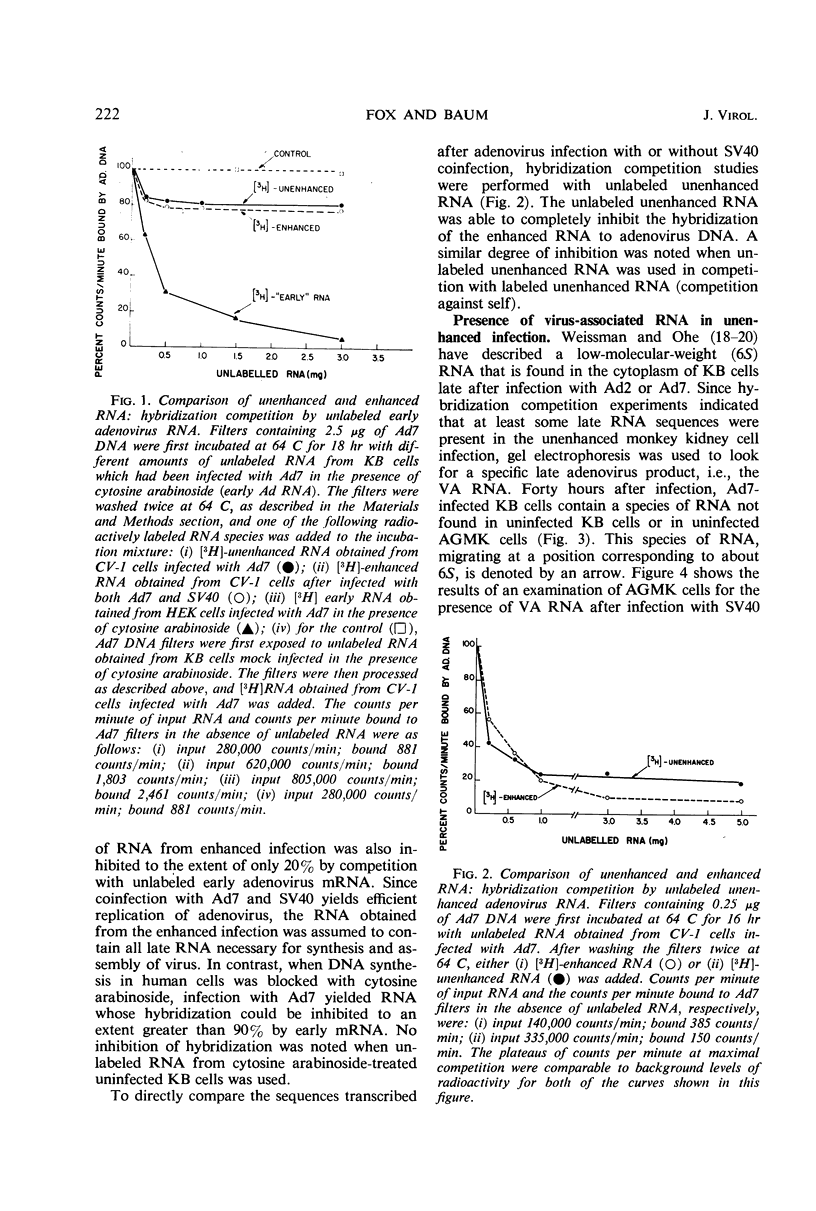
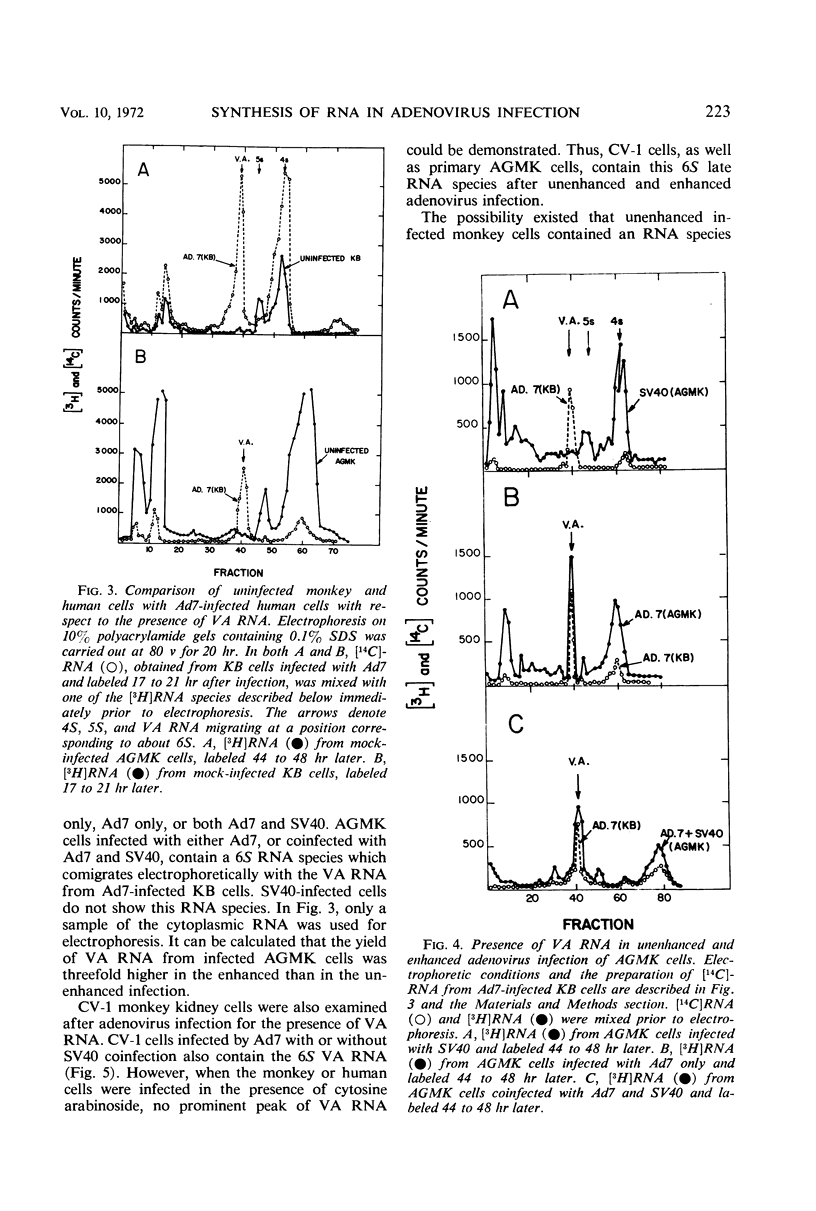
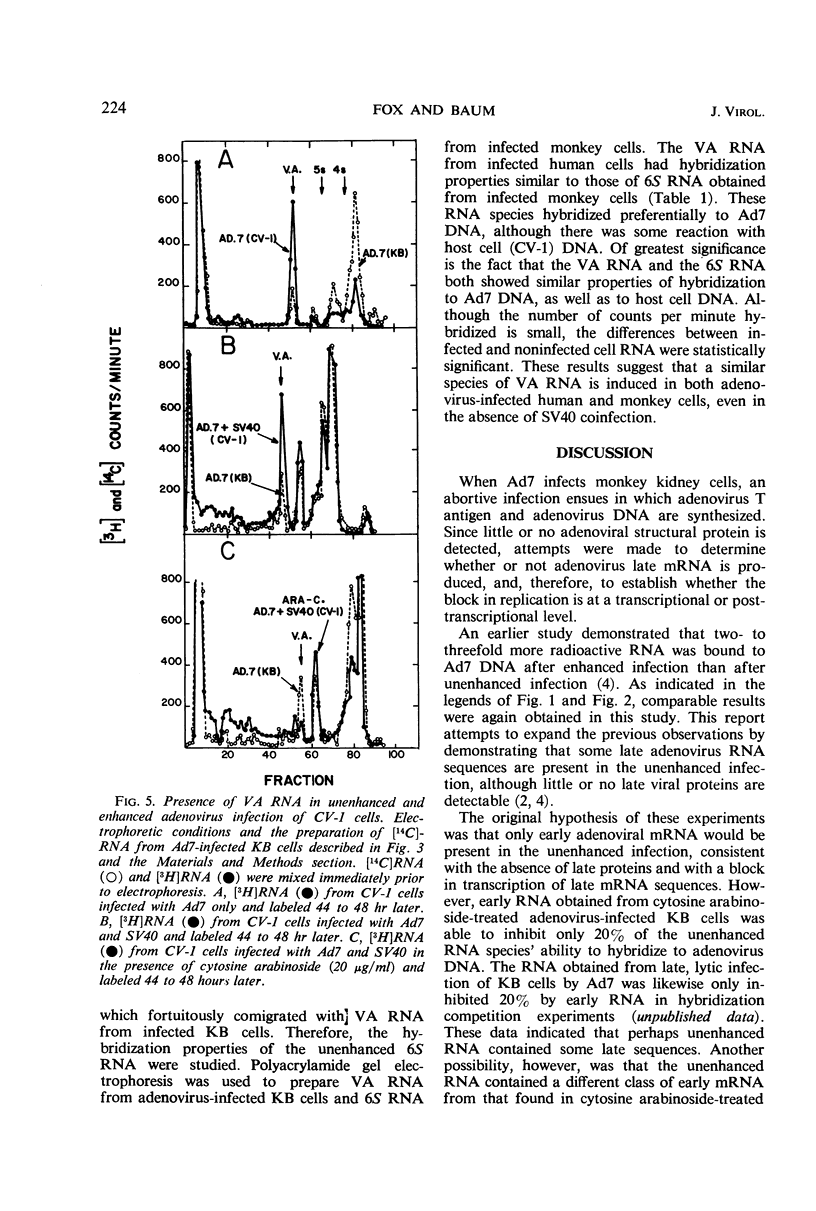
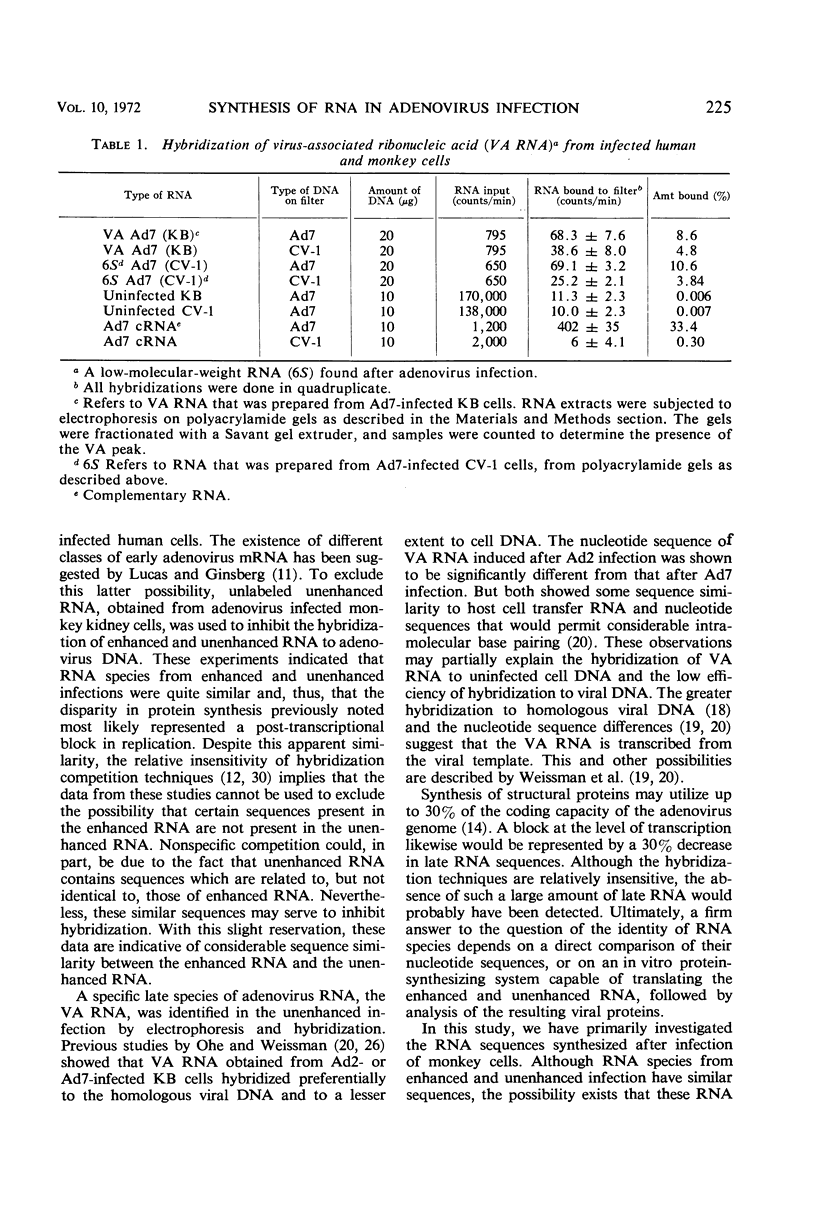
Abstract
The mechanism by which simian virus 40 converts the abortive adenovirus type 7 infection of monkey cells into an efficient lytic infection has been investigated. Analysis of ribonucleic acid (RNA) synthesis during unenhanced and enhanced infection of monkey cells has shown that adenovirus RNA synthesized in the abortive infection contains both “early” and “late” sequences. In hybridization competition experiments, early adenovirus RNA from human cells prevented the hybridization of only 20% of the adenovirus RNA transcribed in unenhanced infection. Further, the RNA from unenhanced cells was able to completely block the hybridization of RNA synthesized during enhanced infection. Finally, virus-associated RNA, which is a late RNA transcribed in lytic adenovirus infection, is also produced in the unenhanced infection. An accompanying paper describes a marked deficiency in adenoviral capsid protein synthesis in the unenhanced infection. We conclude that RNA sequences, which are sufficient to code for the synthesis and assembly of structural proteins of adenovirus, are transcribed but are not efficiently translated in the unenhanced adenovirus infection of monkey cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK P. H., ROWE W. P. VIRAL STUDIES OF SV40 TUMORIGENESIS IN HAMSTERS. J Natl Cancer Inst. 1964 Jan;32:253–265. [PubMed] [Google Scholar]
- Baum S. G., Fox R. I. Components of adenovirus-simian virus 40 hybrid viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1525–1529. doi: 10.1073/pnas.68.7.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Reich P. R., Hybner C. J., Rowe W. P., Weissman S. M. Biophysical evidence for linkage of adenovirus and SV40 DNA's in adenovirus 7-SV40 hybrid particles. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1509–1515. doi: 10.1073/pnas.56.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Wiese W. H., Reich P. R. Studies on the mechanism of enhancement of adenovirus 7 infection in African green monkey cells by simian virus 40: formation of adenovirus-specific RNA. Virology. 1968 Feb;34(2):373–376. doi: 10.1016/0042-6822(68)90253-5. [DOI] [PubMed] [Google Scholar]
- Feldman L. A., Butel J. S., Rapp F. Interaction of a simian papovavirus and adenoviruses. I. Induction of adenovirus tumor antigen during abortive infection of simian cells. J Bacteriol. 1966 Feb;91(2):813–818. doi: 10.1128/jb.91.2.813-818.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. P., Lyons M. J., Ginsberg H. S. Biochemical consequences of type 2 adenovirus and Simian virus 40 double infections of African green monkey kidney cells. J Virol. 1970 May;5(5):586–597. doi: 10.1128/jvi.5.5.586-597.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. VII. Viral genes transcribed in adenovirus type 2 infected and transformed cells. Proc Natl Acad Sci U S A. 1970 Feb;65(2):375–382. doi: 10.1073/pnas.65.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge L. D., Robbins E., Scharff M. D. Persistence of messenger RNA through mitosis in HeLa cells. J Cell Biol. 1969 Feb;40(2):497–507. doi: 10.1083/jcb.40.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J., Church R. B. The specificity of molecular hybridization reactions. Annu Rev Biochem. 1970;39:131–150. doi: 10.1146/annurev.bi.39.070170.001023. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- O'CONOR G. T., RABSON A. S., MALMGREN R. A., BEREZESKY I. K., PAUL F. J. MORPHOLOGIC OBSERVATIONS OF GREEN MONKEY KIDNEY CELLS AFTER SINGLE AND DOUBLE INFECTION WITH ADENOVIRUS 12 AND SIMIAN VIRUS 40. J Natl Cancer Inst. 1965 May;34:679–693. [PubMed] [Google Scholar]
- Ohe K. Virus-coded origin of a low molecular weight RNA from KB cells infected with adenovirus 2. Virology. 1972 Mar;47(3):726–733. doi: 10.1016/0042-6822(72)90562-4. [DOI] [PubMed] [Google Scholar]
- Ohe K., Weissman S. M., Cooke N. R. Studies on the origin of a low molecular weight ribonucleic acid from human cells infected with adenoviruses. J Biol Chem. 1969 Oct 10;244(19):5320–5332. [PubMed] [Google Scholar]
- Ohe K., Weissman S. M. Nucleotide sequence of an RNA from cells infected with adenovirus 2. Science. 1970 Feb 6;167(3919):879–881. doi: 10.1126/science.167.3919.879. [DOI] [PubMed] [Google Scholar]
- Okubo C. K., Raskas H. J. Thermosensitive events in the replication of adenovirus type 2 at 42 degrees. Virology. 1971 Nov;46(2):175–182. doi: 10.1016/0042-6822(71)90020-1. [DOI] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., BAUM S. G. EVIDENCE FOR A POSSIBLE GENETIC HYBRID BETWEEN ADENOVIRUS TYPE 7 AND SV40 VIRUSES. Proc Natl Acad Sci U S A. 1964 Dec;52:1340–1347. doi: 10.1073/pnas.52.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F. Defective DNA animal viruses. Annu Rev Microbiol. 1969;23:293–316. doi: 10.1146/annurev.mi.23.100169.001453. [DOI] [PubMed] [Google Scholar]
- Rapp F., Feldman L. A., Mandel M. Synthesis of virus deoxyribonucleic acid during abortive infection of simian cells by human adenoviruses. J Bacteriol. 1966 Oct;92(4):931–936. doi: 10.1128/jb.92.4.931-936.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich P. R., Baum S. G., Rose J. A., Rowe W. P., Weissman S. M. Nucleic acid homology studies of adenovirus type 7-SV40 interactions. Proc Natl Acad Sci U S A. 1966 Feb;55(2):336–341. doi: 10.1073/pnas.55.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich P. R., Forget B. G., Weissman S. M. RNA of low molecular weight in KB cells infected with adenovirus type 2. J Mol Biol. 1966 Jun;17(2):428–439. doi: 10.1016/s0022-2836(66)80153-5. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Bau S. G. Studies of adenovirus SV40 hybrid viruses. II. Defectiveness of the hybrid particles. J Exp Med. 1965 Nov 1;122(5):955–966. doi: 10.1084/jem.122.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. Competition hybridization by "pre-saturation" of HeLa cell DNA. J Mol Biol. 1969 Sep 28;44(3):551–562. doi: 10.1016/0022-2836(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Jakes K. Phage T7 lysozyme mRNA transcription and translation in vivo and in vitro. Biochem Biophys Res Commun. 1971 Oct 15;45(2):315–320. doi: 10.1016/0006-291x(71)90820-5. [DOI] [PubMed] [Google Scholar]
- Warocquier R., Samaille J., Green M. Biochemical studies on adenovirus multiplication. XVI. Transcription of the adenovirus genome during abortive infection of elevated temperatures. J Virol. 1969 Oct;4(4):423–428. doi: 10.1128/jvi.4.4.423-428.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson B., Joklik W. K. The inhibition of vaccinia virus multiplication by isatin-beta-thiosemicarbazone. Proc Natl Acad Sci U S A. 1965 Sep;54(3):946–953. doi: 10.1073/pnas.54.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]



