Abstract
A number of synthetic peptides are significant commercial or pharmaceutical products, ranging from the dipeptide sugar-substitute aspartame to clinically used hormones, such as oxytocin, adrenocorticotropic hormone, and calcitonin. This unit provides an overview of the field of synthetic peptides and proteins. It discusses selecting the solid support and common coupling reagents. Additional information is provided regarding common side reactions and synthesizing modified residues.
Keywords: peptide, protein, solid-phase peptide synthesis, coupling reagent, chemoselective ligation, Fmoc-amino acid
DEVELOPMENT OF SOLID-PHASE PEPTIDE-SYNTHESIS METHODOLOGY
A number of synthetic peptides are significant commercial or pharmaceutical products, ranging from the dipeptide sugar substitute aspartame to clinically used hormones such as oxytocin, adrenocorticotropic hormone, and calcitonin (Pontiroli, 1998). In the year 2008, the peptide therapeutics market reached the multi-billion dollar level (Saladin et al., 2009). More than 400 peptides have entered clinical studies so far. Rapid, efficient, and reliable methodology for the chemical synthesis of these molecules is therefore of utmost interest. The stepwise assembly of peptides from amino acid precursors has been described for nearly a century. The concept is a straightforward one, whereby peptide elongation proceeds via a coupling reaction between amino acids, followed by removal of a reversible protecting group. The first peptide synthesis, as well as the creation of the term “peptide,” was reported by Fischer and Fourneau (Fischer and Fourneau, 1901). Bergmann and Zervas created the first reversible Nα-protecting group for peptide synthesis, the carbobenzoxy (Cbz) group (Bergmann and Zervas, 1932). DuVigneaud successfully applied early “classical” strategies to construct a peptide with oxytocin-like activity (Vigneaud et al., 1953). Classical, or solution-phase methods for peptide synthesis have an elegant history and have been well chronicled. Solution synthesis continues to be especially valuable for large-scale manufacturing and for specialized laboratory applications.
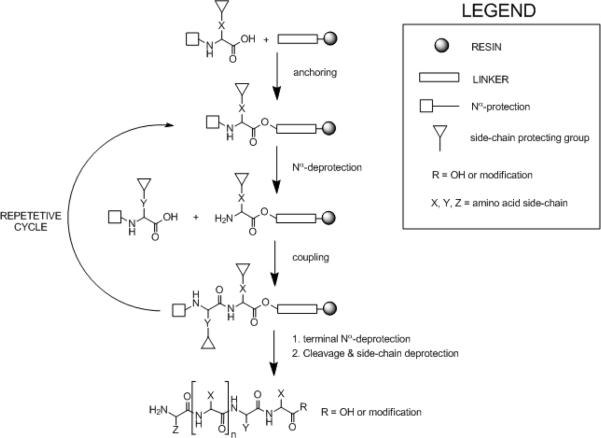
Peptide synthesis became a more practical part of present-day scientific research following the advent of solid-phase techniques. The concept of solid-phase peptide synthesis (SPPS) is to retain chemistry that has been proven in solution but to add a covalent attachment step that links the nascent peptide chain to an insoluble polymeric support (resin). Subsequently, the anchored peptide is extended by a series of addition cycles (Fig. 18.1.1). It is the essence of the solid-phase approach that reactions are driven to completion by the use of excess soluble reagents, which can be removed by simple filtration and washing without manipulative losses. Once chain elongation has been completed, the crude peptide is released from the support.
Figure 18.1.1.
[*Gwen: legend same as original fig]
Generalized approach to solid-phase peptide synthesis.
In the early 1960s, Merrifield proposed the use of a polystyrene-based solid support for peptide synthesis. Peptides could be assembled stepwise from the C to N terminus using Nα-protected amino acids. SPPS of a tetrapeptide was achieved by using Cbz as an α-amino-protecting group, coupling with N,N'-dicyclohexylcarbodiimide (DCC), and liberating the peptide from the support by saponification or by use of HBr (Merrifield, 1963). SPPS was later modified to use the t-butyloxycarbonyl (Boc) group for Nαprotection (Merrifield, 1967) and hydrogen fluoride (HF) as the reagent for removal of the peptide from the resin (Sakakibara et al., 1967). SPPS was thus based on “relative acidolysis,” where the Nα-protecting group (Boc) was labile in the presence of moderate acid (trifluoroacetic acid; TFA), while side-chain-protecting benzyl (Bzl)-based groups and the peptide/resin linkage were stable in the presence of moderate acid and labile in the presence of strong acid (HF). The peptide bonds of the assembled chain were stable to these manipulations. The first instrument for automated synthesis of peptides, based on Boc SPPS, was built by Merrifield, Stewart, and Jernberg (Merrifield et al., 1966). From the 1960s through the 1980s, Boc-based SPPS was fine-tuned (Merrifield, 1986). This strategy has been utilized for synthesis of proteins such as interleukin-3 and active enzymes including ribonuclease A and all-l and all-d forms of HIV-1 aspartyl protease.
In 1970, Carpino introduced the 9-fluorenylmethoxycarbonyl (Fmoc) group for Nα protection (Carpino and Han, 1970). The Fmoc group requires moderate base for removal, and thus offered a chemically mild alternative to the acid-labile Boc group. In the late 1970s, the Fmoc group was adopted for solid-phase applications. Fmoc-based strategies utilized t-butyl (tBu)-based side-chain protection and hydroxymethylphenoxy-based linkers for peptide attachment to the resin. This was thus an “orthogonal” scheme requiring base for removal of the Nα-protecting group and acid for removal of the side-chain protecting groups and liberation of the peptide from the resin. The milder conditions of Fmoc chemistry as compared to Boc chemistry, which include elimination of repetitive moderate acidolysis steps and the final strong acidolysis step, were envisioned as being more compatible with the synthesis of peptides that are susceptible to acid-catalyzed side reactions. In particular, the modification of the indole ring of Trp was viewed as a particular problem during Boc-based peptide synthesis (Barany and Merrifield, 1979), which could be alleviated using Fmoc chemistry. One example of the potential advantage of Fmoc chemistry for the synthesis of multiple-Trp-containing peptides was in the synthesis of gramicidin A. Gramicidin A, a pentadecapeptide containing four Trp residues, had been synthesized previously in low yields (5% to 24%) using Boc chemistry. The mild conditions of Fmoc chemistry dramatically improved the yields of gramicidin A, in some cases up to 87% (Fields et al., 1989; Fields et al., 1990). A second multiple-Trp-containing peptide, indolicidin, was successfully assembled in high yield by Fmoc chemistry (King et al., 1990). Thus, the mild conditions of Fmoc chemistry appeared to be advantageous for certain peptides, as compared with Boc chemistry.
One of the subsequent challenges for practitioners of Fmoc chemistry was to refine the technique to allow for construction of proteins, in similar fashion to that which had been achieved with Boc chemistry. Fmoc chemistry had its own set of unique problems, including suboptimum solvation of the peptide/resin, slow coupling kinetics, and base-catalyzed side reactions. Improvements in these areas of Fmoc chemistry (Atherton and Sheppard, 1987; Fields et al., 2001; Fields and Noble, 1990) allowed for the synthesis of proteins such as bovine pancreatic trypsin inhibitor analogs, ubiquitin, yeast actin-binding protein 539–588, human β-chorionic gonadotropin 1–74, mini-collagens, HIV-1 Tat protein, HIV-1 nucleocapsid protein NCp7, and active HIV-1 protease.
The milder conditions of Fmoc chemistry, along with improvements in the basic chemistry, have led to a shift in the chemistry employed by peptide laboratories. This trend is best exemplified by a series of studies (Angeletti et al., 1997) carried out by the Peptide Synthesis Research Committee (PSRC) of the Association of Biomolecular Resource Facilities (ABRF). The PSRC was formed to evaluate the quality of the synthetic methods utilized in its member laboratories for peptide synthesis. The PSRC designed a series of studies from 1991 to 1996 to examine synthetic methods and analytical techniques. A strong shift in the chemistry utilized in core facilities was observed during this time period, i.e., the more senior Boc methodology was replaced by Fmoc chemistry. For example, in 1991 50% of the participating laboratories used Fmoc chemistry, while 50% used Boc-based methods. By 1994, 98% of participating laboratories were using Fmoc chemistry. This percentage remained constant in 1995 and 1996. In addition, the overall quality of the peptides synthesized improved greatly from 1991 to 1994. Possible reasons for the improved results were any combination of the following (Angeletti et al., 1997):
The greater percentage of peptides synthesized by Fmoc chemistry, where cleavage conditions are less harsh;
The use of different side-chain protecting group strategies that help reduce side reactions during cleavage;
The use of cleavage protocols designed to minimize side reactions;
More rigor and care in laboratory techniques.
The present level of refinement of solid-phase methodology has led to numerous commercially available instruments for peptide synthesis (Table 18.1.1).
Table 18.1.1.
Instruments for Solid-Phase Peptide Synthesis currently available on the market.
| Vendor | Model | Fmoc | Boc | Batch | Flow | Monitoring | Scale (mmol)a | Max number of reaction vessels |
|---|---|---|---|---|---|---|---|---|
| Peptide Machines Inc. | Discovery-12 | Y | N | Y | N | N | 0.05–1 | 12 |
| CS Bio Co | CS 136XT | Y | Y | Y | N | N | 0.2–5 | 1 |
| CS 336X | Y | Y | Y | N | N | 0.05–0.5 | 1 | |
| CS 536XT | Y | Y | Y | Y | 0.2–10 | 1 | ||
| Protein Technologies | PS3 | Y | Y | Y | N | N | 0.005–1.5 | 3 |
| Tribute | Y | Y | Y | N | option | 0.005–2 | 2 | |
| Prelude | Y | Y | Y | N | N | 0.005–5.6 | 6 | |
| Symphony Quartet | Y | Y | Y | N | N | 0.005–2.5 | 4 | |
| Symphony Quartet | Y | Y | Y | N | N | 0.005–7.5 | 12 | |
| Sonata | Y | Y | Y | N | N | 0.25–100 | 1 | |
| Sonata XT | Y | Y | Y | N | N | 0.25–200 | 1 | |
| Overture | Y | Y | Y | N | N | 0.001–24 | 96 | |
| AAPTec | Apex 396 | Y | Y | Y | N | N | NAb | 96 |
| Apogee | Y | Y | Y | N | N | NAb | 1 | |
| Endeavor 90 | Y | Y | Y | N | N | 1.00–10 | 2 | |
| Focus XC | Y | Y | Y | N | N | 0.05–5 | 24 | |
| LabMate | Y | Y | Y | N | N | NAb | 24 | |
| Matrix 384 | Y | Y | Y | N | N | NAb | 384 | |
| Titan 357 | Y | Y | Y | N | N | multigram | 36 | |
| Vantage | Y | Y | Y | N | N | NAb | 96 | |
| Activotec | Activo-P11 | Y | Y | Y | N | Y | 0.05–2 | 1 |
| Activo-P14 | Y | Y | Y | N | Y | 0.05–5 | 1 | |
| Activo-LS55 | Y | Y | Y | N | Y | 0.5–5 | 1 | |
| Applied Biosystems | spare parts available/no new instruments being offered | |||||||
| Biotage | Initiator+ SP Wave | Y | Y | Y | N | N | 0.005–0.3 | 1 |
| Syro Wave | Y | Y | Y | N | N | 0.001–0.3 | 24/48 | |
| Syro I | Y | Y | Y | N | N | 0.005–0.3 | 24/48 | |
| Syro II | Y | Y | Y | N | N | 0.005–0.3 | 24/48 | |
| SAM | Y | Y | Y | N | N | 0.05–3 | 24 | |
| SAP | Y | Y | Y | N | N | 0.5–15 | 1 | |
| CEM | Liberty | Y | Y | Y | N | option | 0.025–5 | 12 |
| Liberty1 | Y | N | Y | N | option | 0.05–5 | 1 | |
| Discover SPS | Y | Y | Y | N | N | 0.025–1 | 1 | |
| Intavis | MultiPep RS | Y | N | Y | N | N | 0.001–0.01 or 0.025–0.1 based on configuration |
72/384 |
| ResPep SL | Y | N | Y | N | N | 0.001–0.01 or 0.025–0.1 based on configuration |
3/96 | |
| Peptide Scientific, Inc. | PSI 200 | Y | Y | Y | N | N | 100mg/10g | 2–6 |
| PSI 300 | Y | Y | Y | N | N | 10mg/8g | 1 | |
| PSI 400 | Y | Y | Y | N | N | 0.5s–50s | 1 | |
| PSI 500 | Y | Y | Y | N | N | 1g–1kg | 1 |
when applicable
NA = information not available on website.
The next step in the development of solid-phase techniques includes applications for peptides containing non-native amino acids, post-translationally modified amino acids, and pseudoamino acids, as well as for organic molecules in general. Several areas of solid-phase synthesis need to be refined to allow for the successful construction of this next generation of biomolecules. The solid support must be versatile so that a great variety of solvents can be used, particularly for organic-molecule applications. Coupling reagents must be sufficiently rapid so that sterically hindered amino acids can be incorporated. Construction of peptides that contain amino acids bearing post-translational modifications should take advantage of the solid-phase approach. Finally, appropriate analytical techniques are needed to assure the proper composition of products.
THE SOLID SUPPORT
Successful SPPS depends upon the choice of the solid support, linker (between the solid support and the synthesized peptide), appropriately protected amino acids, coupling methodology, and protocol for cleaving the peptide from the solid support (Fields, 1997). Choosing the right solid support is often paramount for successful, non-problematic synthesis of the desired peptide. Currently, there are a vast number of commercially available resins, suitable for complex peptide synthesis. It has to be noted that effective solvation of the peptide/resin is perhaps the most crucial condition for efficient chain assembly during solid-phase synthesis. Swollen resin beads may be reacted and washed batch-wise with agitation, then filtered either with suction or under positive nitrogen pressure. Alternatively, they may be packed in columns and utilized in a continuous-flow mode by pumping reagents and solvents through the resin (Lukas et al., 1981). 1H, 2H, 13C, and 19F nuclear magnetic resonance (NMR) experiments have shown that, under proper solvation conditions, the linear polystyrene chains of copoly(styrene-1%-divinylbenzene) resin (PS) are nearly as accessible to reagents as if free in solution (Albericio et al., 1989; Ford and Balakrishnan, 1981; Live and Kent, 1982; Ludwick et al., 1986; Manatt et al., 1980). 13C and 19F NMR studies of Pepsyn (copolymerized dimethylacrylamide, N,N'-bisacryloylethylenediamine, and acryloylsarcosine methyl ester) have shown similar mobilities at resin-reactive sites as PS. Additional supports created by grafting polyethylene glycol (polyoxyethylene) onto PS [either by controlled anionic polymerization of ethylene oxide on tetraethylene glycol–PS (POE-PS) or by coupling Nω-Boc– or Fmoc–polyethylene glycol acid or –polyethylene glycol diacid to amino-functionalized PS (PEG-PS)] combine the advantages of liquid-phase synthesis (i.e., a homogeneous reaction environment) and solid-phase synthesis (an insoluble support). 13C NMR measurements of POE-PS showed the polyoxyethylene chains to be more mobile than the PS matrix, with the highest T1 spin-lattice relaxation times observed with POE of molecular weight 2000 to 3000. Other supports that show improved solvation properties and/or are applicable to organic synthesis include polyethylene glycol polyacrylamide (PEGA), cross-linked acrylate ethoxylate resin (CLEAR), and augmented surface polyethylene prepared by chemical transformation (ASPECT). As the solid-phase method has expanded to include organic-molecule and library syntheses, the diversity of supports will enhance the efficiency of these new applications.
Successful syntheses of problematic sequences can be achieved by manipulation of the solid support. In general, the longer the synthesis, the more polar the peptide/resin will become (Sarin et al., 1980). One can alter the solvent environment and enhance coupling efficiencies by adding polar solvents and/or chaotropic agents (Fields and Fields, 1994). Also, using a lower substitution level of resin to avoid interchain crowding can improve the synthesis (Tam and Lu, 1995). During difficult syntheses, deprotection of the Fmoc group can proceed slowly. By spectrophotometrically monitoring deprotection as the synthesis proceeds, one can detect problems and extend base-deprotection times and/or alter solvation conditions as necessary.
Solid phase peptide synthesis is traditionally carried out in the C → N direction. The majority of peptides are being synthesized as C-terminal acids or amides. For synthesis of C-terminal modified peptides one can take advantage of many linkers that are available (Guillier et al., 2000). The use of linkers provides control and flexibility of the synthetic process, e.g., functionalization of the C-terminal amino acid, loading of the C-terminal amino acid, and/or cleavage conditions utilized for liberation of the peptide following synthesis.
COUPLING REAGENTS
The term coupling refers to formation of a peptide bond between two adjacent amino acids. Coupling involves attack of the amino group of one residue at the carbonyl group of the carboxy-containing component that has been activated by an electron withdrawing group. The activated form of the amino acid can be a shelf-stable reagent, compound of intermediate stability, or a transient intermediate which is neither isolable nor detectable (El-Faham and Albericio, 2011).
The classical examples of in situ coupling reagents are N,N'-dicyclohexylcarbodiimide (DCC) and the related N,N'-diisopropylcarbodiimide (Rich and Singh, 1979). The generality of carbodiimide-mediated couplings is extended significantly by the use of either 1-hydroxybenzotriazole (HOBt) or 1-hydroxy-7-azabenzotriazole (HOAt) as an additive, either of which accelerates carbodiimide-mediated couplings, suppresses racemization, and inhibits dehydration of the carboxamide side chains of Asn and Gln to the corresponding nitriles. Protocols involving benzotriazol-1-yl-oxy-tris(dimethylamino)phosphonium hexafluorophosphate (BOP), benzotriazol-1-yl-oxy-tris(pyrrolidino)phosphonium hexafluorophosphate (PyBOP), 7-azabenzotriazol-1-yl-oxytris(pyrrolidino)phosphonium hexafluorophosphate (PyAOP), O-benzotriazol-1-yl-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU), O-(7-azabenzotriazol-1-yl)-N,N,N'N'-tetramethyluronium hexafluorophosphate (HATU), O-(6-Chlorobenzotriazol-1-yl)-N, N,N',N'-tetramethyluronium hexafluorophosphate (HCTU), and O-benzotriazol-1-yl-N,N,N',N'-tetramethyluronium tetrafluoroborate (TBTU) result in coupling kinetics even more rapid than that obtained with carbodiimides. Amino acid halides have also been applied to SPPS. Nα-protected amino acid chlorides have a long history of use in solution synthesis. Fmoc–amino acid chlorides and fluorides react rapidly under SPPS conditions in the presence of HOBt/N,N-diisopropylethylamine (DIEA) and DIEA, respectively, with very low levels of racemization. For convenience, tetramethylfluoroformamidinium hexafluorophosphate (TFFH) can be used for automated preparation of Fmoc–amino acid fluorides. Amino acid fluorides have been found to be especially useful for the preparation of peptides containing sterically hindered amino acids, such as peptaibols. Other coupling agents that result in low levels of epimerization, and thus are particularly useful for head-to-tail peptide cyclizations and fragment condensations, include O-(3,4-dihydro-4-oxo-1,2,3-benzotriazine-3-yl)-N,N, N',N'-tetramethyluronium tetrafluoroborate (TDBTU) and 3-(diethylphosphoryloxy)-1,2,3-benzotriazin-4(3H)-one (DEPBT). All of the coupling reagents and additives discussed here are commercially available (Table 18.1.2).
Table 18.1.2.
Peptide synthesis reagents suppliers list. [*Gwen: remove boxes so it's just a simple list.]
| 1 | AAPPTec |
| 2 | Acros Organics |
| 4 | Advanced ChemTech |
| 5 | AGTC Bioproducts |
| 6 | Anaspec |
| 7 | Applied Biosystems |
| 8 | Auspep |
| 9 | Bachem |
| 10 | Biopeptek |
| 12 | CBL Biopharma |
| 13 | Chem-Impex |
| 14 | ChemPep |
| 15 | CHI Scientific |
| 16 | CS Bio |
| 17 | CSPS Pharmaceuticals |
| 18 | EMD Chemicals |
| 19 | Fluka |
| 20 | GL Biochem |
| 21 | INBIOS S.r.l. |
| 24 | Luxembourg Bio Technologies |
| 25 | Midwest Bio-Tech |
| 26 | Mimotopes |
| 27 | Neuland Laboratories |
| 28 | New England Peptides |
| 29 | Omegachem |
| 30 | ORPEGEN |
| 31 | Pentabiotech |
| 33 | Peptides International |
| 34 | Polymer Laboratories |
| 35 | Polypeptide Laboratories |
| 36 | Rapp Polymere |
| 37 | Reanal Finechemical Private |
| 38 | RS Synthesis |
| 39 | Ryss Laboratory |
| 40 | Senn Chemicals |
| 41 | Sigma-Aldrich |
| 42 | Sussex Research |
| 43 | Syd Labs |
| 44 | Synthetech |
| 45 | TCI America |
| 46 | Toronto Research Chemicals |
SYNTHESIS OF MODIFIED RESIDUES AND STRUCTURES
Peptides of biological interest often include structural elements beyond the 20 genetically encoded amino acids. Particular emphasis has been placed on peptides containing phosphorylated or glycosylated residues or disulfide bridges. Incorporation of side-chain-phosphorylated Ser and Thr by SPPS is especially challenging, as the phosphate group is decomposed by strong acid and lost with base in a β-elimination process. Boc-Ser(PO3phenyl2) and Boc-Thr(PO3phenyl2) have been found to be useful derivatives, where hydrogen fluoride (HF) or hydrogenolysis cleaves the peptide/resin and hydrogenolysis removes the phenyl groups. Fmoc-Ser(PO3Bzl,H) and Fmoc-Thr(PO3Bzl,H) can be used in conjunction with Fmoc chemistry with some care (Perich et al., 1999; Wakamiya et al., 1994). Alternatively, peptide/resins that were built up by Fmoc chemistry to include unprotected Ser or Thr side chains may be subject to “global” or post-assembly phosphorylation (Otvös et al., 1989a). Side-chain-phosphorylated Tyr is less susceptible to strong-acid decomposition and is not at all base-labile. Thus, SPPS has been used to incorporate directly Fmoc-Tyr(PO3methyl2) (Kitas et al., 1989), Fmoc-Tyr(PO3tBu2) (Perich and Reynolds, 1991), Fmoc-Tyr(PO3H2) (Ottinger et al., 1993), and Boc-Tyr(PO3H2) (Zardeneta et al., 1990). Phosphorylation may also be accomplished on-line, directly after incorporation of the Tyr, Ser, or Thr residue but prior to assembly of the whole peptide (Perich, 1997).
Methodology for site-specific incorporation of carbohydrates during chemical synthesis of peptides has developed rapidly. The mild conditions of Fmoc chemistry are more suited for glycopeptide syntheses than Boc chemistry, as repetitive acid treatments can be detrimental to sugar linkages. Fmoc-Ser, -Thr, -5-hydroxylysine (-Hyl), -4-hydroxyproline (-Hyp), and -Asn have all been incorporated successfully with glycosylated side chains (Cudic and Burstein, 2008). The side-chain glycosyl is usually hydroxyl-protected by either benzoyl or acetyl groups, although some SPPSs have been successful with no protection of glycosyl hydroxyl groups (Otvös et al., 1989b). Deacetylation and debenzylation are performed with hydrazine/methanol prior to glycopeptide/resin cleavage or in solution with catalytic methoxide in methanol (Sjölin et al., 1996).
Disulfide-bond formation has been achieved on the solid-phase by air, K3Fe(CN)6, dithiobis(2-nitrobenzoic acid), or diiodoethane oxidation of free sulfhydryls, by direct deprotection/oxidation of Cys(acetamidomethyl) residues using thallium trifluoroacetate or I2, by direct conversion of Cys(9-fluorenylmethyl) residues using piperidine, and by nucleophilic attack by a free sulfhydryl on either Cys(3-nitro-2-pyridinesulfenyl) or Cys(S-carboxymethylsulfenyl). The most generally applicable and efficient of these methods is direct conversion of Cys(acetamidomethyl) residues by thallium trifluoroacetate. In solution, disulfide formation may be mediated by a lengthy catalogue of reagents, the most straightforward of which are molecular O2 (from air) and DMSO (Tam et al., 1991).
Intra-chain lactams are formed between the side-chains of Lys or Orn and Asp or Glu to conformationally restrain synthetic peptides, with the goal of increasing biological potency and/or specificity. Lactams can also be formed via side-chain-to-head, side-chain-to-tail, or head-to-tail cyclization (Kates et al., 1994). The residues used to form intra-chain lactams must be selectively side-chain deprotected, while all side-chain protecting groups of other residues remain intact. Selective deprotection is best achieved by using orthogonal side-chain protection, such as allyloxycarbonyl or 1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl protection for Lys and allyl or N-[1-(4,4,-dimethyl-2,6-dioxocyclohexylidene)-3-methyl butyl]aminobenzyl protection for Asp/Glu in combination with an Fmoc/tBu strategy. Cyclization is carried out most efficiently with BOP in the presence of DIEA while the peptide is still attached to the resin (Felix et al., 1988; Plaue, 1990).
The three-dimensional orthogonal protection scheme of Fmoc/tBu/allyl protecting groups is the strategy of choice for head-to-tail cyclizations. An amide linker is used for side-chain attachment of a C-terminal Asp/Glu (which are converted to Asn/Gln) and the α-carboxyl group is protected as an allyl ester (Stawikowski and Cudic, 2006). For side-chain-to-head cyclizations, the N-terminal amino acid (head) can simply be introduced as an Nα-Fmoc derivative while the peptide-resin linkage and the other side-chain protecting groups are stable to dilute acid or carry a third dimension of orthogonality.
PROTEIN SYNTHESIS
There are three general chemical approaches for constructing proteins. First is stepwise synthesis, in which the entire protein is synthesized one amino acid at a time. Second is “fragment assembly,” in which individual peptide strands are initially constructed stepwise, purified, and finally covalently linked to create the desired protein. Fragment assembly can be divided into two distinct approaches: (1) convergent synthesis of fully protected fragments, and (2) chemoselective ligation of unprotected fragments. Third is “directed assembly,” in which individual peptide strands are constructed stepwise, purified, and then noncovalently driven to associate into protein-like structures. Combinations of the three general chemical approaches may also be employed for protein construction.
Convergent synthesis utilizes protected peptide fragments for protein construction (Albericio et al., 1997). The advantage of convergent protein synthesis is that fragments of the desired protein are first synthesized, purified, and characterized, ensuring that each fragment is of high integrity; these fragments are then assembled into the complete protein. Thus, cumulative effects of stepwise synthetic errors are minimized. Convergent synthesis requires ready access to pure, partially protected peptide segments, which are needed as building blocks. The application of solid-phase synthesis to prepare the requisite intermediates depends on several levels of selectively cleavable protecting groups and linkers. Methods for subsequent solubilization and purification of the protected segments are nontrivial. Individual rates for coupling segments are substantially lower than for activated amino acid species by stepwise synthesis, and there is always a risk of racemization at the C-terminus of each segment. Careful attention to synthetic design and execution may minimize these problems.
As an alternative to the segment condensation approach, methods have been developed by which unprotected peptide fragments may be linked. “Native chemical ligation” results in an amide bond being generated between peptide fragments (see UNIT 18.4) (Muir et al., 1997). A peptide bearing a C-terminal thioacid is converted to a 5-thio-2-nitrobenzoic acid ester and then reacted with a peptide bearing an N-terminal Cys residue (Dawson et al., 1994). The initial thioester ligation product undergoes spontaneous rearrangement, leading to an amide bond and regeneration of the free sulfhydryl on Cys. The method was later refined so that a relatively unreactive thioester can be used in the ligation reaction (Ayers et al., 1999; Dawson et al., 1997). “Safety-catch” linkers are used in conjunction with Fmoc chemistry to produce the necessary peptide thioester (Shin et al., 1999). Safety-catch linkers anchor the nascent peptide to the resin and are stable throughout the synthesis. These linkers then allow the release of a C-terminally modified peptide from the solid support under mild conditions following an additional activation step.
SIDE-REACTIONS
The free Nα-amino group of an anchored dipeptide is poised for a base-catalyzed intramolecular attack of the C-terminal carbonyl. Base deprotection of the Fmoc group can thus release a cyclic diketopiperazine while a hydroxymethyl-handle leaving group remains on the resin. With residues that can form cis peptide bonds, e.g., Gly, Pro, N-methylamino acids, or d-amino acids, in either the first or second position of the (C → N) synthesis, diketopiperazine formation can be substantial. The steric hindrance of the 2-chlorotrityl linker may minimize diketopiperazine formation of susceptible sequences during Fmoc chemistry.
The conversion of side-chain protected Asp residues to aspartimide residues can occur by repetitive base treatments. The cyclic aspartimide can then react with piperidine to form the α- or β-piperidide or α- or β-peptide. Aspartimide formation can be rapid, and is dependent upon the Asp side-chain protecting group. Sequence dependence studies of Asp(OtBu)-X peptides revealed that piperidine could induce aspartimide formation when X = Arg(2,2,5,7,8-pentamethylchroman-6-sulfonyl; Pmc), Asn(triphenylmethyl; Trt), Asp(OtBu), Cys(Acm), Gly, Ser, Thr, and Thr(tBu) (Lauer et al., 1995). Aspartimide formation can also be conformation-dependent. This side-reaction can be minimized by including 0.1 M HOBt in the piperidine solution (Lauer et al., 1995), or by using an amide backbone protecting group (i.e., 2-hydroxy-4-methoxybenzyl) for the residue in the X position of an Asp-X sequence (Quibell et al., 1994).
Cys residues are racemized by repeated piperidine deprotection treatments during Fmoc SPPS. Racemization of esterified (C-terminal) Cys can be reduced by using 1% 1,8-diazabicyclo[5.4.0]undec-7-ene in N,N-dimethylformamide (DMF). Additionally, the steric hindrance of the 2-chlorotrityl linker minimizes racemization of C-terminal Cys residues. When applying protocols for Cys internal (not C-terminal) incorporation which include phosphonium and aminium salts as coupling agents, as well as preactivation in the presence of suitable additives and tertiary amine bases, significant racemization is observed. Racemization is generally reduced by avoiding preactivation, using a weaker base (such as collidine), and switching to the solvent mixture DMF-dichloromethane (DCM) (1:1). Alternatively, the pentafluorophenyl ester of a suitable Fmoc-Cys derivative can be used.
The combination of side-chain protecting groups and anchoring linkages commonly used in Fmoc chemistry are simultaneously deprotected and cleaved by TFA. Cleavage of these groups and linkers results in liberation of reactive species that can modify susceptible residues, such as Trp, Tyr, and Met. Modifications can be minimized during TFA cleavage by utilizing effective scavengers. Three efficient cleavage “cocktails” quenching reactive species and preserving amino acid integrity, are (1) TFA-phenolthioanisole-1,2-ethanedithiol-H2O (82.5:5:5:2.5:5) (reagent K) (King et al., 1990), (2) TFA-thioanisole-1,2-ethanedithiol-anisole (90:5:3:2) (reagent R) (Albericio et al., 1990), and (3) TFA-phenol-H2O-triisopropylsilane (88:5:5:2) (reagent B) (Solé and Barany, 1992). The use of Boc side-chain protection of Trp also significantly reduces alkylation by Pmc or 2,2,4,6,7-pentamethyldihydro-benzofuran-5-sulfonyl (Pbf) groups. For a list of common problems encountered during peptide synthesis refer to Table 18.1.3.
Table 18.1.3.
Common problems encountered during peptide synthesis.
| Problem | Solutions | Reference |
|---|---|---|
| Diketopiperazine formation | • Use of 2-chlorotrityl linker | (Barlos et al., 1989a; Barlos et al., 1989b) |
|
| ||
| Aspartamide formation | • May be minimized by addition of 0.1 M HOBt in the piperidine solution | (Lauer et al., 1995) |
| • Use of amide backbone protecting group | (Quibell et al., 1994) | |
|
| ||
| Racemization of C-terminal Cys | • Use of 2-chlorotrityl linker | (Wade et al., 1991) |
| • Use 1% DBU in DMF | ||
|
| ||
| Modification of amino acids during cleavage | ||
| If present: Cys(Trt), Met, Trp(Boc) | • Use Reagent K: TFA/phenol/water/thioanisole/EDT (82.5/5/5/5/2.5) | (King et al., 1990) |
| • Do not use when Cys(Acm) is present | ||
|
|
||
| If present: Arg(Mtr/Pmc), Arg(Pbf), Asn(Mbh/Tmob), Gln(Mbh/Tmob), Trp(any), Met, Cys(any), His(any) | • Use Reagent R: TFA/thioanisole/EDT/anisole (90/5/3/2) or |
(Albericio et al., 1990) |
|
| ||
| • Use Reagent B: TFA/phenol/water/triisopropylsilane (88/5/5/2) | (Solé and Barany, 1992) | |
PURIFICATION AND ANALYSIS OF SYNTHETIC PEPTIDES
Each synthetic procedure has limitations, and even in the hands of highly experienced workers, certain sequences defy facile preparation. The maturation of high-performance liquid chromatography (HPLC) has been a major boon to modern peptide synthesis, because the resolving power of this technique facilitates removal of many of the systematic low-level by-products that accrue during chain assembly and upon cleavage. Peptide purification is most commonly achieved by reversed-phase HPLC (RP-HPLC; UNIT 11.6). Either alternatively to or in tandem with RP-HPLC, ion-exchange HPLC (UNIT 8.2) and gel-filtration HPLC (UNIT 8.3) can be used for isolation of desired peptide products. The progress of peptide purification can be monitored rapidly by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS; UNIT 16.2 & 16.3) or ion-trap electrospray MS (UNIT 16.8).
The homogeneity of synthetic materials should be checked by at least two chromatographic or electrophoretic techniques, e.g., RP-HPLC (UNIT 11.6), ion-exchange HPLC (UNIT 8.2), and capillary zone electrophoresis (UNIT 10.9). Also, determination of a molecular ion by MS (see Chapter 16) using a mild ionization method is important for proof of structure. Synthetic peptides must be checked routinely for the proper amino acid composition, and in some cases sequencing data are helpful. The PSRC studies (see discussion of Development of Solid-Phase Peptide Synthesis Methodology) have allowed for a side-by-side comparison of a variety of analytical techniques. Efficient characterization of synthetic peptides best been obtained by a combination of RP-HPLC and MS, with sequencing by either Edman degradation sequence analysis or tandem MS (UNIT 16.1) being used to identify the positions of modifications and deletions. Proper peptide characterization by multiple techniques is essential.
LITERATURE CITED
- Albericio F, et al. Preparation and application of the 5-(4-(9-fluorenylmethyloxycarbonyl)aminomethyl-3,5-dimethoxyphenoxy)valeric acid (PAL) handle for the solid-phase synthesis of C-terminal peptide amides under mild conditions. J. Org. Chem. 1990;55:3730–3743. [Google Scholar]
- Albericio F, Lloyd-Williams P, Giralt E. Convergent solid-phase peptide synthesis. Methods Enzymol. 1997;289:313–36. doi: 10.1016/s0076-6879(97)89054-4. [DOI] [PubMed] [Google Scholar]
- Albericio F, Pons M, Pedroso E, Giralt E. Comparative study of supports for solid-phase coupling of protected-peptide segments. The Journal of Organic Chemistry. 1989;54:360–366. [Google Scholar]
- Angeletti RH, Bonewald LF, Fields GB. Six-year study of peptide synthesis. Methods Enzymol. 1997;289:697–717. doi: 10.1016/s0076-6879(97)89071-4. [DOI] [PubMed] [Google Scholar]
- Atherton E, Sheppard RC. The fluorenylmethoxycarbonyl amino protecting group. In: Udenfriend S, Meienhofer J, editors. The Peptides. Academic Press; New York: 1987. pp. 1–38. [Google Scholar]
- Ayers B, et al. Introduction of unnatural amino acids into proteins using expressed protein ligation. Peptide Science. 1999;51:343–354. doi: 10.1002/(SICI)1097-0282(1999)51:5<343::AID-BIP4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Barany G, Merrifield RB. Solid-phase peptide synthesis. In: Gross E, Meienhofer J, editors. The Peptides. Vol. 2. Academic Press; New York: 1979. pp. 1–284. [Google Scholar]
- Barlos K, et al. Darstellung neuer säureempfindlicker harze vom sek.-alkohol-typ und ihre anwendung zur synthese von peptiden. Liebigs Ann. Chem. 1989a:951–955. [Google Scholar]
- Barlos K, et al. Darstellung geschützter peptid-fragmente unter einsatz substituierter triphenylmethyl-harze. Tetrahedron Lett. 1989b;30:3943–3946. [Google Scholar]
- Bergmann M, Zervas L. Über ein allgemeines Verfahren der Peptid-Synthese. Berichte der deutschen chemischen Gesellschaft (A and B Series) 1932;65:1192–1201. [Google Scholar]
- Carpino LA, Han GY. 9-Fluorenylmethoxycarbonyl function, a new base-sensitive amino-protecting group. J Am Chem Soc. 1970;92:5748–5749. [Google Scholar]
- Cudic M, Burstein GD. In: Preparation of Glycosylated Amino Acids Suitable for Fmoc Solid-Phase Assembly Peptide-Based Drug Design. Otvos L, editor. Humana Press; 2008. pp. 187–208. [DOI] [PubMed] [Google Scholar]
- Dawson PE, Churchill MJ, Ghadiri MR, Kent SBH. Modulation of reactivity in native chemical ligation through the use of thiol additives. J. Am. Chem. Soc. 1997;119:4325–4329. [Google Scholar]
- Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- El-Faham A, Albericio F. Peptide Coupling Reagents, More than a Letter Soup. Chemical Reviews. 2011;111:6557–6602. doi: 10.1021/cr100048w. [DOI] [PubMed] [Google Scholar]
- Felix AM, Wang C-T, Heimer EP, Fournier A. Applications of BOP reagent in solid phase synthesis. International Journal of Peptide and Protein Research. 1988;31:231–238. doi: 10.1111/j.1399-3011.1988.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Fields CG, Fields GB. Solvents for solid-phase peptide synthesis. In: Pennington MW, Dunn BM, editors. Methods in Molecular Biology, Vol. 35: Peptide Synthesis Protocols. Humana Press, Inc.; Totowa, NJ: 1994. pp. 29–40. [DOI] [PubMed] [Google Scholar]
- Fields CG, Fields GB, Noble RL, Cross TA. Solid phase peptide synthesis of 15N-gramicidins A, B, and C and high performance liquid chromatographic purification. Int J Pept Protein Res. 1989;33:298–303. doi: 10.1111/j.1399-3011.1989.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Fields GB, editor. Methods In Enzymology. Academic Press; Orlando, FL: 1997. Solid-phase peptide synthesis; p. 289. [Google Scholar]
- Fields GB, Lauer-Fields JL, Liu R.-q., Barany G. Principles and Practice of Solid-Phase Peptide Synthesis. In: Grant GA, editor. Synthetic Peptides: A User's Guide. 2nd Edition W.H. Freeman & Co.; New York: 2001. pp. 93–219. [Google Scholar]
- Fields GB, Noble RL. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Fields GB, Otteson KM, Fields CG, Noble RL. The versatility of solid phase peptide synthesis. In: Epton R, editor. Innovation and Perspectives in Solid Phase Synthesis. Solid Phase Conference Coordination, Ltd.; Birmingham, U.K.: 1990. pp. 241–260. [Google Scholar]
- Fischer E, Fourneau E. Ueber einige Derivate des Glykocolls. Berichte der deutschen chemischen Gesellschaft. 1901;34:2868–2877. [Google Scholar]
- Ford WT, Balakrishnan T. (13)C-NMR Spectra of Cross-Linked Poly(Styrene-co-Chloromethylstyrene) Gel. Macromolecules. 1981:14. [Google Scholar]
- Guillier F, Orain D, Bradley M. Linkers and Cleavage Strategies in Solid-Phase Organic Synthesis and Combinatorial Chemistry. Chemical Reviews. 2000;100:2091–2158. doi: 10.1021/cr980040+. [DOI] [PubMed] [Google Scholar]
- Kates SA, Solé NA, Albericio F, Barany G. Solid-phase synthesis of cyclic peptides. In: Basava C, Anantharamaiah GM, editors. Peptides: Design, Synthesis and Biological Activity. Birkhauser; Boston: 1994. pp. 39–57. [Google Scholar]
- King DS, Fields CG, Fields GB. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int J Pept Protein Res. 1990;36:255–66. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Kitas EA, Perich JW, Wade JD, Johns RB, Tregear GW. FMOC-polyamide solid phase synthesis of an O-phosphotyrosine-containing tridecapeptide. Tetrahedron Letters. 1989;30:6229–6232. [Google Scholar]
- Lauer JL, Fields CG, Fields GB. Sequence dependence of aspartimide formation during 9-fluorenylmethoxycarbonyl solid-phase synthesis. Lett. Peptide Sci. 1995;1:197–205. [Google Scholar]
- Live D, Kent SBH. Elastomers and Rubber Elasticity. American Chemical Society; Washington, DC: 1982. Fundamental Aspects of the Chemical Applications of Cross-linked Polymers; pp. 501–515. [Google Scholar]
- Ludwick AG, Jelinski LW, Live D, Kintanar A, Dumais JJ. Association of peptide chains during Merrifield solid-phase peptide synthesis. A deuterium NMR study. Journal of the American Chemical Society. 1986;108:6493–6496. [Google Scholar]
- Lukas TJ, Prystowsky MB, Erickson BW. Solid-phase peptide synthesis under continuous-flow conditions. Proceedings of the National Academy of Sciences. 1981;78:2791–2795. doi: 10.1073/pnas.78.5.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manatt SL, et al. A fluorine-19 NMR approach for studying merrifield solid-phase peptide syntheses. Tetrahedron Letters. 1980;21:1397–1400. [Google Scholar]
- Merrifield B. Solid phase synthesis. Science. 1986;232:341–347. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- Merrifield RB. Solid phase peptide synthesis I: Synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963;85:2149–2154. [Google Scholar]
- Merrifield RB. New approaches to the chemical synthesis of peptides. Recent Prog Horm Res. 1967;23:451–82. doi: 10.1016/b978-1-4831-9826-2.50013-1. [DOI] [PubMed] [Google Scholar]
- Merrifield RB, Stewart JM, Jernberg N. Instrument for automated synthesis of peptides. Anal Chem. 1966;38:1905–14. doi: 10.1021/ac50155a057. [DOI] [PubMed] [Google Scholar]
- Muir TW, Dawson PE, Kent SBH. Protein synthesis by chemical ligation of unprotected peptides in aqueous solution. Meth. Enzymol. 1997;289:266–298. doi: 10.1016/s0076-6879(97)89052-0. [DOI] [PubMed] [Google Scholar]
- Ottinger EA, Shekels LL, Bernlohr DA, Barany G. Synthesis of phosphotyrosine-containing peptides and their use as substrates for protein tyrosine phosphorylation. Biochemistry. 1993;32:4354–4361. doi: 10.1021/bi00067a027. [DOI] [PubMed] [Google Scholar]
- Otvös L, Elekes I, Lee VMY. Solid-phase synthesis of phosphopeptides. International Journal of Peptide and Protein Research. 1989a;34:129–133. doi: 10.1111/j.1399-3011.1989.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Otvös L, Jr., et al. Coupling strategies in solid-phase synthesis of glycopeptides. Peptide Res. 1989b;2:362–366. [PubMed] [Google Scholar]
- Perich JW. Synthesis of phosphopeptides using modern chemical approaches. In: Gregg BF, editor. Methods in Enzymology. Academic Press; 1997. pp. 245–266. [DOI] [PubMed] [Google Scholar]
- Perich JW, Ede NJ, Eagle S, Bray AM. Synthesis of phosphopeptides by the Multipinast method: Evaluation of coupling methods for the incorporation of Fmoc- Tyr(PO3Bzl,H)-OH, Fmoc- Ser(PO3Bzl,H)-OH and Fmoc- Thr(PO3Bzl,H)-OH. Letters in Peptide Science. 1999;6:91–97. [Google Scholar]
- Perich JW, Reynolds EC. Fmoc/solid-phase synthesis of Tyr(P)-containing peptides through t-butyl phosphate protection. International Journal of Peptide and Protein Research. 1991;37:572–575. doi: 10.1111/j.1399-3011.1991.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Plaue S. Synthesis of cyclic peptides on solid support Application to analogs of hemagglutinin of influenza virus. International Journal of Peptide and Protein Research. 1990;35:510–517. doi: 10.1111/j.1399-3011.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]
- Pontiroli AE. Peptide hormones: Review of current and emerging uses by nasal delivery. Advanced Drug Delivery Reviews. 1998;29:81–87. doi: 10.1016/s0169-409x(97)00062-8. [DOI] [PubMed] [Google Scholar]
- Quibell M, Owen D, Packman LC, Johnson T. Suppression of piperidine-mediated side product formation for Asp(OBut)-containing peptides by the use of N-(2-hydroxy-4-methoxybenzyl) (Hmb) backbone amide protection. J. Chem. Soc., Chem. Commun. 1994:2343–2344. [Google Scholar]
- Rich DH, Singh J. The carbodiimide method. In: Gross E, Meienhofer J, editors. The Peptides. Vol. 1. Academic Press; New York: 1979. pp. 241–314. [Google Scholar]
- Sakakibara S, Shimonishi Y, Kishida Y, Okada M, Sugihara H. Use of anhydrous hydrogen fluoride in peptide synthesis. I. Behavior of various protective groups in anhydrous hydrogen fluoride. Bull Chem Soc Jpn. 1967;40:2164–7. doi: 10.1246/bcsj.40.2164. [DOI] [PubMed] [Google Scholar]
- Saladin PM, Zhang BD, Reichert JM. Current trends in the clinical development of peptide therapeutics. IDrugs. 2009;12:779–84. [PubMed] [Google Scholar]
- Sarin VK, Kent SBH, Merrifield RB. Properties of swollen polymer networks: Solvation and swelling of peptide-containing resins in solid-phase peptide synthesis. J. Am. Chem. Soc. 1980;102:5463–5470. [Google Scholar]
- Shin Y, et al. Fmoc-based synthesis of peptide-thioesters: applications to the total chemical synthesis of a glycoprotein by native chemical ligation. J. Am. Chem. Soc. 1999;121:11684–11689. [Google Scholar]
- Sjölin P, Elofsson M, Kihlberg J. Removal of Acyl Protective Groups from Glycopeptides: Base Does Not Epimerize Peptide Stereocenters, and β-Elimination Is Slow. The Journal of Organic Chemistry. 1996;61:560–565. doi: 10.1021/jo951817r. [DOI] [PubMed] [Google Scholar]
- Solé NA, Barany G. Optimization of solid-phase synthesis of [Ala8]-dynorphin A. J. Org. Chem. 1992;57:5399–5403. [Google Scholar]
- Stawikowski M, Cudic P. A novel strategy for the solid-phase synthesis of cyclic lipodepsipeptides. Tetrahedron Letters. 2006;47:8587–8590. doi: 10.1016/j.tetlet.2006.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam JP, Lu Y-A. Coupling difficulty associated with interchain clustering and phase transition in solid phase peptide synthesis. J. Am. Chem. Soc. 1995;117:12058–12063. [Google Scholar]
- Tam JP, Wu CR, Liu W, Zhang JW. Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. Journal of the American Chemical Society. 1991;113:6657–6662. [Google Scholar]
- Vigneaud V.d., et al. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J Am Chem Soc. 1953;75:4879–4880. [Google Scholar]
- Wade JD, Bedford J, Sheppard RC, Tregear GW. DBU as an Na-deprotecting reagent for the fluorenylmethoxycarbonyl group in continuous flow solid- phase peptide synthesis. Peptide Res. 1991;4:194–199. [PubMed] [Google Scholar]
- Wakamiya T, Saruta K, Yasuoka J.-i., Kusumoto S. An Efficient Procedure for Solid-Phase Synthesis of Phosphopeptides by the Fmoc Strategy. Chemistry Letters. 1994;23:1099–1102. [Google Scholar]
- Zardeneta G, Chen D, Weintraub ST, Klebe RJ. Synthesis of phosphotyrosyl-containing phosphopeptides by solid-phase peptide synthesis. Analytical Biochemistry. 1990;190:340–347. doi: 10.1016/0003-2697(90)90205-n. [DOI] [PubMed] [Google Scholar]
Key References
- Atherton E, Sheppard RC. Solid Phase Peptide Synthesis: A Practical Approach. IRL Press; Oxford: 1989. [Google Scholar]; An extensive collection of Fmoc-based synthetic methods and techniques.
- Barany G, Merrifield RB. 1979. See above. [DOI] [PubMed]; The definitive, comprehensive overview of the solid-phase method.
- Fields GB, editor. Methods In Enzymology. Vol. 289. Academic Press; Orlando, FL: 1997. Solid-phase peptide synthesis. [Google Scholar]; A collection of SPPS techniques and applications.
- Houben-Weyl Methods of Organic Chemistry . Synthesis of Peptides and Peptidomimetics. Vol 22a–e. Thieme Chemistry; New York: 2004. [Google Scholar]; A comprehensive description of peptide synthesis methods.
- Chan WC, White PD. Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Oxford University Press; 2000. [Google Scholar]; A contemporary collection of Fmoc-based synthetic methods and techniques.