Abstract
Acinar-to-ductal metaplasia (ADM) results from pancreatic injury or KRAS activation, and is an early step in pancreatic cancer progression. In this Cancer Cell issue, Ardito et al. and Navas et al. demonstrate that ADM and KRAS-driven pancreatic cancer require EGFR signaling, revealing a mechanism for developmental reprogramming that primes tumorigenesis.
Most tissues respond to injuries such as those induced by toxins, trauma, or infections through orchestrated cellular programs that either mitigate further damage or enable regeneration. One such response is the process of metaplasia, which involves the conversion or replacement of one differentiated cell type with another in a given tissue. Metaplasia helps protect tissues as they adjust to the insult and resulting changed environment, and is usually reverted once normal conditions are re-established. This defense mechanism comes with an unfortunate cost – it is becoming increasingly clear that sustained metaplasia can serve as an early precursor to malignant transformation in several organs, including pancreas, stomach, and lung.
In the pancreas, acute or chronic inflammation leads to replacement of damaged acini with duct-like cells, referred to as acinar-to-ductal metaplasia (ADM) (Reviewed in Reichert and Rustgi, 2011) (Figure 1). The relationship between ADM and pancreatic ductal adenocarcinoma (PDA) progression has been studied extensively in genetically engineered mouse models. In these models, oncogenic KRAS mutation — the earliest known genetic alteration in human PDA — promotes the focal development of ADM in the absence of exogenous inducers of inflammation. Rather than representing a reversible state, mutant KRAS-expressing metaplastic ducts progress into ductal precursor lesions known as pancreatic intraepithelial neoplasias (PanINs), which gradually acquire additional genetic changes including CDKN2A and/or p53 mutation and evolve into PDA. Treatment of mice with the cholecystokinin analogue cerulein, which induces pancreatic inflammation and widespread ADM, greatly accelerates PanIN formation and progression to PDA when KRAS mutations are present. Thus, it is thought that activated KRAS locks cells that have undergone ADM in the ductal state, preventing restoration of normal differentiation, and instead creating a reservoir of cells susceptible to additional oncogenic changes.
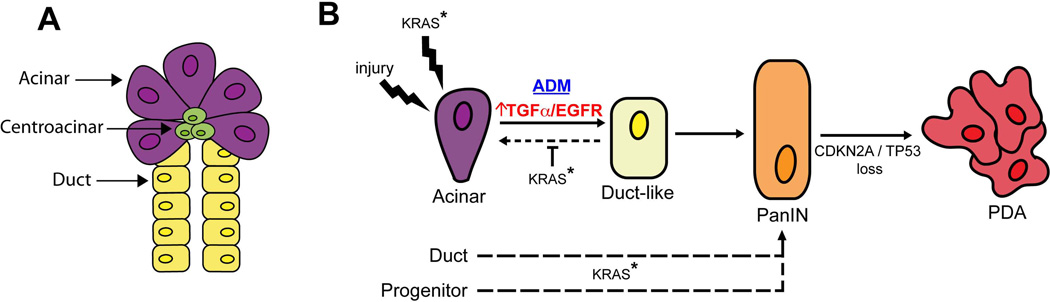
Figure 1. Role of EGFR signaling in PDA initiation and progression.
A. Pancreas anatomy: Acinar cells produce and secrete digestive enzymes, while ductal cells form channels that transport acinar secretions into the intestinal tract. Centroacinar cells lie at the interface between acinar and ductal cells and may have progenitor-like properties.
B. Models of PDA progression: Following injury, acinar cells undergo acinar-to-ductal metaplasia (ADM), a process that is normally reversible following resolution of the tissue damage. Activating KRAS mutation (KRAS*), the earliest oncogenic alteration in PDA pathogenesis, can also induce ADM while preventing reversion to acinar differentiation. ADM lesions can progress to Pancreatic Intraepithelial Neoplasia (PanIN), which in turn gives rise to PDA following loss of the CDKN2A and/or p53 tumor suppressors. EGFR signaling is required both for the initiation of ADM as well as for the survival of cells within established ADM and PanIN lesions. PDA may also originate from pancreatic duct cells and presumed pancreatic progenitors.
The first clues suggesting a role for the Epidermal Growth Factor Receptor (EGFR) pathway in ADM came from transgenic mouse models in which overexpression of the EGFR ligand, TGFα, in the pancreas caused spontaneous ADM, and progressive pancreatic tumorigenesis when crossed to KRAS, p53 or CDKN2A mutant strains (Reichert and Rustgi, 2011). In vitro experiments using pancreatic explants showed that EGFR activation could act cell-autonomously in converting acinar cells into metaplastic ducts (Means et al., 2005; Miyamoto et al., 2003). The physiological relevance of this pathway has now been established in two new studies by groups led by Crawford and Siveke (Ardito et al.) and Guerra and Barbacid (Navas et al.). They employed genetic and pharmacological inactivation of EGFR in KRAS-driven mouse models to directly determine the contribution of endogenous EGFR pathway signaling to the development of ADM and PDA. Consistent with prior studies, they found that EGFR expression is upregulated in ADM and PanIN lesions in these models and in human pancreatitis specimens. Knockout of EGFR in the pancreas or treatment of mice with pharmacological EGFR inhibitors suppressed ADM provoked by activated KRAS or by cerulein. Moreover, acute EGFR inhibition resulted in apoptosis in established ADM and PanIN lesions. Importantly, Navas et al. found that EGFR knockout completely prevented PDA development in their KRAS model even in the context of deletion of CDKN2A, whereas both groups found that EGFR deletion delayed but did not eliminate PDA formation in KRAS-p53 mutant models. Together, these results establish that EGFR is required for both the initiation and survival of ADM (and PanIN) lesions, and show that its ablation restricts the development of PDA. KRAS mutations are also early initiating lesions in lung cancer, and are present as later alterations in intestinal cancers, thus it is striking that Navas et al. found that EGFR deletion had no effect on tumorigenesis in KRAS-driven mouse models of these malignancies. Therefore, rather than playing a generic function in KRAS-mediated transformation, EGFR has specific roles in PDA initiation, acting to facilitate the developmental reprogramming of pancreatic acinar cells.
How does EGFR promote ADM? Although this remains incompletely understood, there are some intriguing leads. First, among the EGF family ligands (e.g. EGF, TGFα, AREG), TGFα is uniquely induced during ADM both in vitro and in vivo, and appears to be the main mediator of ADM. Accordingly, deletion of ADAM17, which cleaves and activates TGFα and AREG, blocks ADM to a similar extent as EGFR deletion, whereas prior studies have found that AREG fails to recapitulate the ADM phenotypes induced by TGFα (Wagner et al., 2002). TGFα is notable for its ability to cause recycling of the receptor back to the cell surface thereby allowing for sustained moderate levels of signaling (von Zastrow and Sorkin, 2007) while other EGFR ligands can be more potent EGFR activators but induce receptor down-regulation. Hence, the selective role for ADAM17-TGFα-EGFR axis in ADM and tumor initiation suggests that a sustained threshold of EGFR activity is required for these processes. How EGFR and TGFα expression are induced in this context remains to be defined.
The signaling pathways downstream of EGFR that mediate ADM are less clear. Ardito et al. show that acute EGFR inhibition reduced levels of phospho-ERK1/2 and GTP-bound active RAS in ADM lesions in KRAS mice and acinar explants, respectively. However, Navas et al. did not observe changes in ERK1/2 activity in their model. Differences between the mouse models used in these two studies may account for some of these discrepancies. Further studies will be required to fully define the proximal effectors of EGFR signaling and associated biochemical mechanisms that facilitate ADM. In terms of more downstream pathways, prior reports have provided evidence that induction of Notch transcriptional activity contributes to ADM in vitro in response to TGFα-EGFR signaling (Miyamoto et al., 2003), and thus Notch warrants additional analysis as a potential effector of endogenous EGFR in ADM in vivo.
Does EGFR also contribute to the malignant growth of established PDA? Activated EGFR is detected in a subset of human PDAs, and EGFR inhibition shows efficacy in some patient-derived PDA xenografts and PDA cell lines (Jimeno et al., 2008). However, Erlotinib provided only limited benefit when combined with gemcitabine in a clinical trial with unselected PDA patients (Moore et al., 2007) and when administered at advanced stages in the KRAS-p53 mouse model. This suggests that advanced PDA may have reduced dependence on EGFR signaling as compared to earlier stage lesions. Further studies will be required to ascertain how later stage cancers escape EGFR dependency and conversely to identify a molecular signature for those that remain EGFR-dependent. One such signature may involve loss of epithelial gene expression as Ardito et al. found that EGFR expression is extinguished in poorly differentiated PDA. Moreover, published studies demonstrate that PDA cell lines showing mesenchymal features are relatively resistant to Erlotinib as compared to well-differentiated PDA lines (Collisson et al., 2011).
PDA is the fourth most common cause of cancer deaths in the United States with a 5-year survival rate under 5%. Existing clinical trials with conventional and targeted therapies have only modest effects on patient outcomes, and hence new approaches to disease management are urgently needed. Since inherited predisposition to chronic pancreatitis greatly increases PDA risk, and since precursor lesions harboring KRAS mutations are common in otherwise normal pancreatic tissue of elderly individuals, there may be benefit in the development of preventative strategies that eradicate ADM and PanIN lesions. The present studies support the potential of targeting TGFα-ADAM17-EGFR axis as an approach to PDA prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardito, et al. [Google Scholar]
- Navas, et al. [Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, Tan AC, Coffa J, Rajeshkumar NV, Kulesza P, Rubio-Viqueira B, Wheelhouse J, Diosdado B, Messersmith WA, Iacobuzio-Donahue C, et al. Coordinated epidermal growth factor receptor pathway gene overexpression predicts epidermal growth factor receptor inhibitor sensitivity in pancreatic cancer. Cancer Res. 2008;68:2841–2849. doi: 10.1158/0008-5472.CAN-07-5200. [DOI] [PubMed] [Google Scholar]
- Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Jr, Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest. 2011;121:4572–4578. doi: 10.1172/JCI57131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Weber CK, Bressau F, Greten FR, Stagge V, Ebert M, Leach SD, Adler G, Schmid RM. Transgenic overexpression of amphiregulin induces a mitogenic response selectively in pancreatic duct cells. Gastroenterology. 2002;122:1898–1912. doi: 10.1053/gast.2002.33594. [DOI] [PubMed] [Google Scholar]