Abstract
Plasmacytoid (p) dendritic cells (DC) are highly-specialized APC that, in addition to their well-recognized role in anti-viral immunity, also regulate immune responses. Liver-resident pDC are considerably less immunostimulatory than those from secondary lymphoid tissues and are equipped to promote immune tolerance/regulation through various mechanisms. IL-27 is an IL-12-family cytokine that regulates the function of both APC and T cells, although little is known about its role in pDC immunobiology. In this study, we show that mouse liver pDC express higher levels of IL-27p28 and EBV-induced protein (Ebi)3 compared to splenic pDC. Both populations of pDC express the IL-27Rα/WSX-1; however, only liver pDC significantly upregulate expression of the co-regulatory molecule B7 homolog-1 (B7-H1) in response to IL-27. Inhibition of STAT3 activation completely abrogates IL-27-induced upregulation of B7-H1 expression on liver pDC. Liver pDC treated with IL-27 increase the percentage of CD4+Foxp3+ T cells in MLR, which is dependent upon expression of B7-H1. pDC from Ebi3-deficient mice lacking functional IL-27, show increased capacity to stimulate allogeneic T cell proliferation and IFN-γ production in MLR. Liver but not spleen pDC suppress delayed-type hypersensitivity responses to OVA, an effect that is lost with Ebi3−/− and B7-H1−/− liver pDC compared to wild-type (WT) liver pDC. These data suggest that IL-27 signaling in pDC promotes their immunoregulatory function and that IL-27 produced by pDC contributes to their capacity to regulate immuneresponses in vitro and in vivo.
Keywords: dendritic cells, cytokines, cytokine receptors, tolerance
INTRODUCTION
The liver is a unique immunologic organ with inherent characteristics that promote immune tolerance [1, 2]. Constant exposure to gut-derived products via the portal venous blood conditions the liver microenvironment to suppress unwanted inflammatory responses to harmless or inert Ags, such as proteins from food or derived from commensal bacteria [2]. The liver contains a diversity of cells with Ag-presenting capacity, including liver sinusoidal endothelial cells, Kupffer cells or liver-resident macrophages, hepatic stellate cells, and dendritic cells (DC)3. The distribution of these APC within the liver sinusoids leads to specialized functional interplay with one another and with NK cells, T cells, and hepatocytes, and helps shape this unique microenvironment [2].
DC are bone marrow (BM)-derived, professional APC that are the most potent activators of T cell-mediated immune responses constituting a critical link between innate and adaptive immunity. The two main subsets in the mouse are conventional DC (cDC; CD11c+CD11b+NK1.1−) and plasmacytoid DC (pDC; CD11b−CD11clowB220+CD8α−). Steady-state pDC express mouse plasmacytoid DC Ag-1 (mPDCA-1) or BM stromal Ag (BST)-2/CD317 [3], and the endocytic receptor, Siglec-H [4]. They express high levels of endosomal TLR 7, 8 and 9 that sense single-stranded viral RNA (ssRNA; TLR7 and 8) and unmethylated, CpG-rich viral and bacterial ssDNA (TLR9) [5]. pDC are the primary type-I IFN producers in response to viral infection [5–7]. They are weaker T cell stimulators than cDC, and can promote T cell hyporesponsiveness and regulatory T cell (Treg) function [8–10]. Consequently, pDC have been linked to the development of tolerance in various experimental models and disease states [11].
Liver pDC express low levels of MHC class II and co-stimulatory molecules (CD40, CD80, and CD86) compared to their counterparts in secondary lymphoid tissues, such as the spleen [12, 13]. They also express comparatively high levels of the immunoregulatory cytokine IL-10 and the B7 family coregulatory molecule, B7 homolog-1 (B7-H1 or programmed death ligand-1, PD-L1) [14, 15], which are known to negatively regulate immune responses [16–19]. Despite the implications of these findings for the function of liver pDC, understanding of the molecular mechanisms that regulate liver pDC function and the expression of immunoregulatory molecules by these cells remains underexplored.
IL-27 is a heterodimeric, IL-12-family cytokine comprised of the p40-like molecule EBV-induced protein (Ebi)3 and the p35-like molecule p28 [20]. It is produced by APC, including macrophages and DC, and signals through the heterodimeric IL-27R that consists of T cell cytokine receptor (TCCR)/WSX-1 and the shared gp130 chains [21]. Signaling through the IL-27R activates STAT 1, 3, 4, and 5, where STAT1 and STAT3 appear to be the primary downstream targets, especially in T cells [22]. Activation of STAT1 by IL-27 has been shown to drive Th cell responses toward a Th1/IFN-γ-producing phenotype that favors immune activation and pathogen clearance [23]. By contrast, there is considerable evidence [24–26] that IL-27 plays an important role in the induction of IL-10-producing, Foxp3−, T-regulatory 1 (Tr1) cells [27]. In support of an immunoregulatory influence on the outcome of T cell responses, IL-27 has been found to suppress the development of inflammatory Th17 cells [28, 29].
The IL-27R is expressed widely on hematopoietic cells and plays distinct roles in their function, depending on the cell type on which it is expressed. Mouse splenic CD11c+ DC lacking the IL-27Rα/WSX-1 exhibit prolonged upregulation of activation markers and enhanced T cell stimulatory capacity following LPS stimulation [30], suggesting that IL-27 exerts immunoregulatory effects on DC. In addition, exposure of human monocyte-derived DC to IL-27 decreases their T cell stimulatory capacity in a B7-H1-dependent manner [31]. It was reported recently that mouse splenic pDC express Ebi3 and that it is upregulated following TLR ligation [32]. Moreover, pDC exposed to exogenous TGF-β increase IL-27 production [33]. Despite these recent important observations, studies linking IL-27 and pDC function have not been reported.
Oral tolerance is a phenomenon where feeding of Ag induces Ag-specific hyporesponsiveness upon subsequent exposure to the same Ag. It has been shown that induction of oral tolerance is dependent on liver and portal vein blood flow [34]. DC play an important role in oral tolerance through the capture and presentation of orally administered Ag, and various immunologic sites have been implicated in this process [35–37]. Liver pDC are reported as the key DC subset that mediates oral tolerance and that can also suppress Ag-specific delayed-type hypersensitivity (DTH) responses [38, 39]. Systemic depletion of PDCA-1+ pDC blunts the effects of Ag feeding in oral tolerance, and adoptive transfer of PDCA-1+ liver pDC from OVA-fed mice at the time and site of immunization with OVA-pulsed DC suppresses Ag-specific DTH responses upon rechallenge [39]. Additionally, IL-27 production is elevated in mesenteric lymph node DC (CD11c+CD11b+ and CD11c+CD11b−) from OVA-fed mice [40]. These data indicate a regulatory role for liver pDC in promoting Ag-specific tolerance and suggest that upregulation of IL-27 may contribute to this phenomenon. To date, however, there are no reports that link IL-27, liver pDC, and Ag-specific immune regulation.
Herein, we report for the first time that mouse liver pDC produce IL-27p28 and express the IL-27Rα/WSX-1. Exposure to exogenous IL-27 augments the expression of B7-H1 on liver pDC, an effect that is mediated by STAT3. IL-27-conditioned liver pDC induce similar levels of allogeneic CD4+ T cell proliferation compared to untreated control liver pDC, but increase the proportion of CD4+Foxp3+ T cells in these cultures, a phenomenon that is dependent upon B7-H1. pDC from Ebi3-deficient mice that do not produce IL-27 exhibit greater CD4+ T cell stimulatory capacity in vitro compared to wild type (WT) pDC. In vivo, liver but not spleen pDC from OVA-fed mice suppress DTH responses, an effect that is lost with Ebi3−/− or B7-H1−/− liver pDC. These data suggest that IL-27 acts on pDC to promote their regulatory function and that IL-27 produced by liver pDC contributes to their ability to suppress immune responses in vitro and in vivo.
MATERIALS AND METHODS
Mice
Six-week old C57BL/6 (B6; H2b) and BALB/cByJ (BALB/c; H2d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in the specific pathogen-free Central Animal Facility of the University of Pittsburgh School of Medicine. Ebi3−/− mice (Ebi3tm1Rsb/J) purchased from Jackson and B7-H1−/− mice (pairs kindly provided by Dr. Lieping Chen, Johns Hopkins University, Baltimore, MD), both on a B6 background, were bred and maintained at the University of Pittsburgh. Experiments were conducted under an Institutional Animal Care and Use Committee (IACUC)-approved protocol and in accordance with National Institutes of Health guidelines. The animals were fed a diet of Purina rodent chow (Ralston Purina, St. Louis, MO) and received tap water ad libitum.
Media and reagents
The endogenous DC poietin recombinant human Flt3L (CHO cell-derived) was obtained from Amgen (Seattle, WA). Complete medium was composed of RPMI-1640 (BioWhittaker, Walkersville, MD) supplemented with 10% v/v fetal calf serum (Nalgene, Miami, FL), non-essential amino acids, L-glutamine, sodium pyruvate, penicillin–streptomycin, and 2-mercaptoethanol (all from Life Technologies, Gaithersburg, MD). The TLR9 ligand CpG type B oligodinucleotide (ODN) 1826, certified endotoxin free, was obtained from Invivogen (San Diego, CA). Recombinant murine IL-27 was purchased from eBioscience (San Diego, CA) and STAT3 Inhibitor VII and the STAT3 inhibitor JSI-124/Cucurbitacin were purchased from Calbiochem/EMD Chemicals (Gibbstown, NJ).
Purification of liver and spleen pDC
pDC were purified as described [14]. Briefly, livers and spleens were harvested from mice administered Flt3L (10 µg/mouse/day; 10 days) and digested in collagenase (Sigma, St. Louis, MO). DC were enriched from total liver non-parenchymal cells or splenocytes by density gradient centrifugation using Histodenz (Sigma). Liver and spleen cells were depleted of NK1.1+ cells to remove NK cells and NKDC [41] by incubation with biotin-conjugated NK1.1 (BD Pharmingen, San Diego, CA) and antibiotin magnetic microbeads (Miltenyi Biotec, Auburn, CA), followed by depletion on an LS column (Miltenyi Biotec). pDC were positively selected using PDCA-1 magnetic microbeads (Miltenyi Biotec). Purity was consistently between 93 and 97%, where the majority of contaminating cells were B220− CD11c+ cDC, with negligible contamination by B220+CD11c− B cells, as reported previously [14]. In some experiments, cDC were isolated from NK1.1−PDCA-1− cells by CD11c positive selection and stimulated with the TLR4 ligand LPS at 1 µg/ml.
Flow cytometric analysis of cell phenotype
Liver and spleen pDC were incubated in 5% v/v normal goat serum and FcγR-blocking Ab (anti-CD16/32, BD Pharmingen) to block non-specific binding. For cell surface staining, cells were analyzed by 5-color analysis using FITC-, PE-, APC-/AlexaFluor647-, Pacific Blue-, and PE/Cy7-conjugated Abs. For analysis of PDCA-1+ pDC, surface expression of MHC class II (I-Ab), CD40, CD80, CD86, B7H1, and WSX-1/IL-27Rα was quantified on a population of B220+CD11clow gated cells. The gating strategy and expression of B220/CD45R, CD11c, and Siglec-H on purified PDCA-1+ pDC is included in the supplemental data (Suppl. Fig. 1).
Intracellular cytokine and Foxp3 staining
Purified PDCA-1+ pDC were cultured for 18 h with 1 µg/ml CpG B ODN or 10–25 ng/ml IL-27 and treated with Golgi Plug Protein Transport Inhibitor (1 µl/ml; BD Pharmingen) for the final 5 h. The cells were labeled for surface proteins as described above. For analysis of cytokine production, cells were fixed with 4% v/v paraformaldehyde and permeabilized using 0.1% saponin. pDC were stained for intracellular expression of IL-27p28 (BioLegend, San Diego, CA) and IL-12p35 (R&D Systems, Minneapolis, MN) and T cells stained for expression of Foxp3. For analysis of Foxp3 expression following cell surface staining, cells were incubated overnight using the Foxp3 Fix/Perm kit (eBioscience).
ELISA
Cytokine levels in culture supernatants were determined by ELISA using kits from BioLegend (IL-6, IL-10, IL-12p40, and IFN-γ), and R&D Systems (IL-27p28), respectively, following the manufacturers’ instructions.
RNA isolation and semi-quantitative RT-PCR
Total RNA was extracted using TRIzol (Invitrogen) and then reverse transcribed using the iScript cDNA Synthesis kit (Bio-Rad). IL-27p28, Ebi3, IL-12p35, WSX-1, and β-actin DNA products were amplified with Fast SYBR Green PCR Master Mix (Applied Biosystems; Carlsbad, CA) using an ABI PRISM 7000 Fast Sequence Detection System (Applied Biosystems). Relative gene expression was determined by comparing to a standard curve for each gene and then normalized to β-actin. Primers used for IL-27p28 were forward: 5′-ATCTCGATTGCCAGGAGTGA-3′ and reverse: 5′-GTGGTAGCGAGGAAGCAGAGT-3′; Ebi3, forward: 5′- ATTGCCACTTACAGGCTCGG-3′ and reverse: 5′- AAGCAGGGGGATGCCAGA-3′; IL-12p35, forward: 5′- CTGTGCCTTGGTAGCATCTATG-3′ and reverse: 5′- GCAGAGTCTCGCCATTATGATTC-3′; WSX-1, forward: 5′- CAAGAAGAGGTCCCGTGCTG-3′ and reverse: 5′- TTGAGCCCAGTCCACCACAT-3′; and β-actin, forward : 5′-AGAGGGAAATCGTGCGTGAC-3′ and reverse: 5′- CAATAGTGATGACCTGGCCGT-3′.
Western immunoblotting
Freshly-isolated liver or spleen PDCA-1+ pDC were washed in PBS and cell pellets lysed using Cell Lytic M (Sigma) supplemented with complete Mini EDTA-free protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Cellular debris was removed by centrifugation at 13,000 × g. Protein was quantified and 20 µg loaded onto 10% Tris-Glycine gels and separated using the XCell SureLock Blot Module (Invitrogen). Gels were transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blotted using primary Abs: Ebi3 (2.5 µg/ml; eBioscience) or IL-12/IL-35 p35 (1 µg/ml; R&D Systems) and GAPDH (Novus Biologicals, Littleton, CO). Primary Abs were followed by HRP-conjugated secondary Abs (Jackson ImmunoResearch Laboratories, West Grove, PA). Protein was visualized using Super Signal West Pico ECL kit (Pierce – Thermo Fisher Scientific, Rockford, IL).
T cell purification
Bulk T cells were purified from spleens and lymph nodes of normal BALB/c mice. Single cell suspensions were incubated with an Ab cocktail consisting of anti-CD45R/B220 (RA3-6B2), anti-CD16/CD32 (2.4G2), anti-TER-119, anti-I-A/I-E, anti-CD11b, and anti-Ly6G (RB6-8C5) obtained from BD Pharmingen. Non-T cells were eliminated from the cell suspension by negative selection using Dynabeads (Invitrogen) following the manufacturer’s instructions.
MLR
Unstimulated, CpG B- or IL-27-conditioned pDC (2×104/well) were used as stimulators of normal allogeneic BALB/c T cells (2×105/well) in a 3 d MLR using 96-well, round-bottom plates at a 1:10, DC:T cell ratio. T cells were labeled with the cell tracer CFSE (Invitrogen) for identification and proliferation analysis. At the end of the culture period, supernatants were harvested for cytokine quantitative analysis by ELISA, and cells were harvested for flow cytometric analysis of T cell proliferation and Foxp3 expression.
Ag feeding and delayed-type hypersensitivity (DTH)
Protocol for DTH was adapted from Goubier et al [39]. Briefly, DC were mobilized in B6 wild-type, Ebi3−/− and B7-H1−/− mice, as described above. On day 9 of Flt3L administration, the mice were fed 25 mg OVA (Grade II, Sigma) in 200 µl PBS by gavage. After 18 h, the animals were euthanized and liver pDC purified as described above. For immunization, pDC (105) were co-injected s.c at the base of the tail with day 7, GM-CSF/IL-4-propagated CD11c-purified bone marrow-derived DC (BMDC) [42] at a 1:1 ratio in 50 µl PBS. The BMDC were pulsed with 1 mg/ml OVA (Grade VII, Sigma) (BMDC-OVA) for 3–4 h prior to injection. Un-pulsed BMDC or BMDC-OVA were injected alone as controls. On day 7 post-immunization, mice were challenged s.c. in the hind footpad with 12.5 mg/ml heat-aggregated (HA) OVA (Grade VII, Sigma) in 20 µl PBS. HA-OVA was prepared by incubating OVA in PBS at 80°C for 60 min followed by centrifugation at 1900 g for 10 min [43]. The supernatant was removed and HA-OVA resuspended in PBS for injection. PBS alone was injected into the opposite hind footpad as a control. Footpad thickness was measured at time 0 prior to injection and daily thereafter through 96 h post-challenge using Quick Mini Series 700 digital calipers (Mitutoyo). Spleens were harvested after 96 h and total splenocytes (2.5×106/ml) stimulated with 1 mg/ml OVA (Sigma) for 48 h. Supernatants were collected for quantitative analysis of cytokines by ELISA.
Statistical analysis
Statistical significance was determined by unpaired Student’s ‘t’ test or two-way ANOVA where appropriate, using GraphPad Prism version 5.00 for Windows (GraphPad Software; San Diego, CA). A value of p ≤ 0.05 was considered significant. All experiments were carried out independently for a minimum of 3 times, unless indicated otherwise in the figure legends.
RESULTS
Liver pDC phenotype and cytokine profile correlates with weak T cell allostimulatory ability
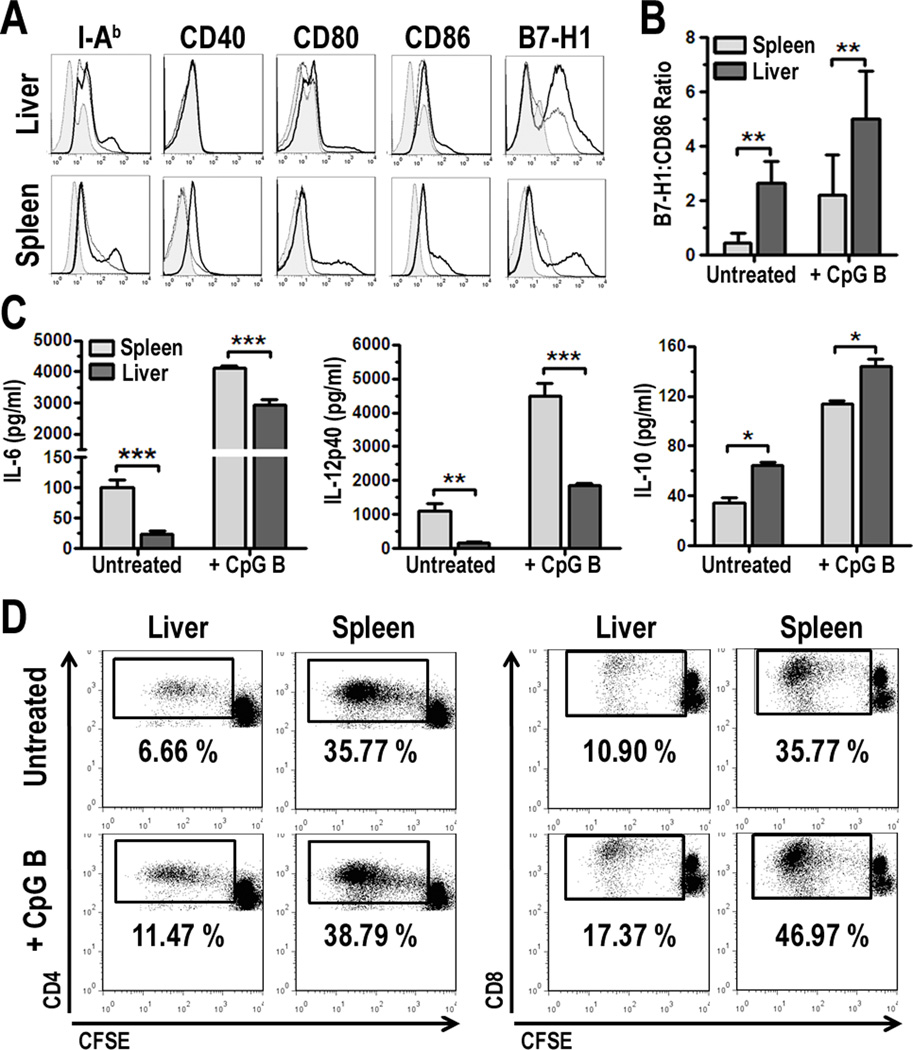
Due to their overall paucity in normal liver, we used Flt3L to expand liver DC [13, 44–48], in particular pDC. We found that freshly-isolated, PDCA-1-immunobead-purified liver pDC from B6 mice exhibited an immature phenotype, characterized by low cell surface expression of MHC II/I-Ab and the costimulatory molecules CD40, CD80, and CD86 (Fig. 1A). The level of expression of these molecules was generally lower than on pDC isolated similarly and concomitantly from the spleen, even following overnight stimulation with the TLR9 ligand CpG B ODN (Fig. 1A). By contrast, liver pDC exhibited higher levels of the co-regulatory molecule B7-H1 compared with similarly-isolated splenic pDC. The ratio of co-regulatory to co-stimulatory molecules expressed on the surface of APC can strongly influence the functional outcome of their interaction with T cells and subsequent immune reactivity [49]. We found that liver pDC expressed a significantly higher B7-H1:CD86 ratio relative to splenic pDC, in the steady-state and following TLR9 stimulation (Fig. 1B).
FIGURE 1.
Liver and spleen pDC differ in phenotype and function. A, B6 pDC were mobilized in vivo using Flt3L and enriched from total liver non-parenchymal cells or splenocytes as described in the Materials and Methods. PDCA-1-purified pDC were cultured in the absence or presence of 1 µg/ml CpG B ODN for 18 h, collected and analyzed by 5-color flow cytometry. Surface molecule expression was analyzed on B220+CD11clow cells. Isotypes are represented by the gray-filled histograms, unstimulated cells (thin line), and CpG B-stimulated (bold line). Data are representative of 6 independent experiments; B, The relative mean fluorescence intensities (MFI) [Sample MFI – Isotype MFI] for B7-H1 was divided by the MFI for CD86 to generate the B7-H1:CD86 ratio. Data were averaged from 4 independent experiments; C, Culture supernatants from cells analyzed in Fig. 1A were harvested and cytokines quantified by ELISA. Data represent 3 independent experiments; D, Liver or spleen pDC were cultured with CFSE-labeled, allogeneic BALB/c T cells for 3 d. Cultures were harvested and proliferation quantified by flow cytometry. Data represent 5 independent experiments, * p < 0.05, ** p< 0.01, ***p < 0.001.
The immature cell surface phenotype of liver pDC was associated with lower pro-inflammatory (IL-6 and IL-12p40) and conversely, higher anti-inflammatory cytokine (IL-10) production compared to splenic pDC, either in the absence of or following CpG stimulation (Fig. 1C). Moreover, liver pDC were inferior in their ability to stimulate allogeneic CD4+ or CD8+ T cell proliferation compared to pDC from the spleen in 3 d CFSE MLR (Fig. 1D). These data are consistent with observations we have reported previously regarding the phenotype and function of murine liver pDC before and after stimulation with LPS in vitro [15], or with CpG B or the NOD2 ligand muramyl dipeptide (MDP) in vivo [14].
IL-27p28 and the IL-27R/WSX-1 are expressed at comparatively high levels by liver pDC
IL-27 is an emerging IL-12-family member comprised of the p40-like molecule Ebi3 and the p35-like molecule p28 [50]. Early reports on the impact of IL-27 on T cells [23] suggested that IL-27 was important for driving Th1-mediated immune responses. It was reported subsequently that IL-27 signaling could drive the induction of IL-10-producing, Foxp3− Tr1 cells, as well as inhibit the induction of Foxp3+ Treg and IL-17-producing Th17 cells [24, 26, 51–53]. Interestingly, the p28 subunit alone possesses immune regulatory function. Thus, it was shown recently [54] that IL-27p28 can act as an antagonist of gp130-mediated signaling, indicating a unique role for this molecule independently of Ebi3. Although it is known that IL-27 is produced by APC, primarily activated macrophages but also DC [20, 55], less is understood about the functional biology of IL-27 in relation to DC function compared to that of T cells.
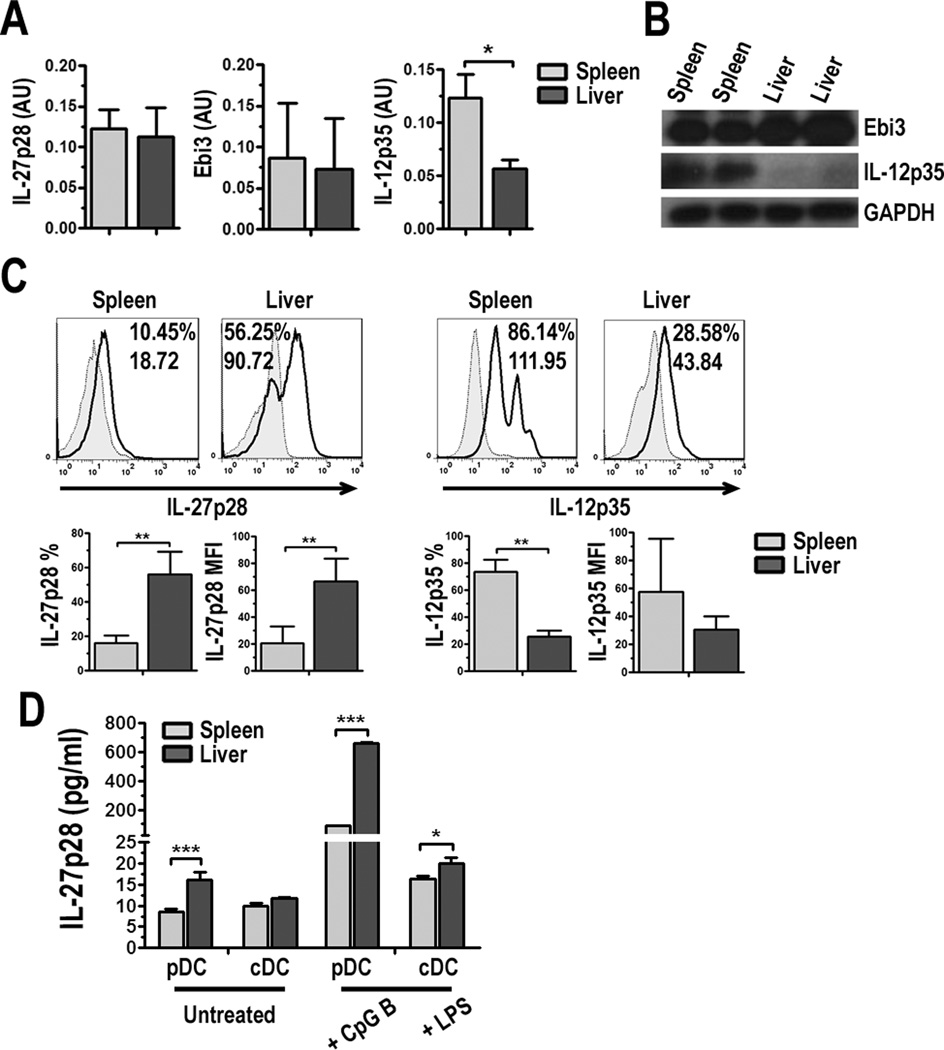
Semi-quantitative RT-PCR analysis of freshly-isolated PDCA-1+ pDC shows similar levels of IL-27p28 and Ebi3 mRNA transcripts in liver and spleen pDC (Fig. 2A). Interestingly, Western blot analysis revealed greater levels of Ebi3 protein in liver compared to spleen pDC (Fig. 2B), and intracellular flow cytometry (Fig. 2C) and quantification in culture supernatants by ELISA (Fig. 2D) revealed greater IL-27p28 levels in liver pDC, suggesting possible post-transcriptional differences between pDC in these organs. It was reported previously that cDC from the liver produce more IL-27 compared to spleen cDC [56]. We did observe this phenomenon; however, we detected greater levels of IL-27p28 production by pDC compared to cDC from the liver and spleen, especially following activation (Fig. 2D). Since Ebi3 is a shared component of both IL-27 and IL-35, an additional regulatory cytokine [57], we also tested IL-12p35 levels in these cells. We observed lower IL-12p35 mRNA transcripts (Fig. 2A) by RT-PCR and protein expression by Western blot (Fig. 2B) and flow analysis (Fig. 2C) in liver pDC compared to spleen, suggesting IL-35 is not contributing to the known immune regulatory function of liver pDC.
FIGURE 2.
Liver pDC produce comparatively high levels of IL-27p28 protein but low levels of IL-12p35. A, RNA was purified from freshly-isolated liver or spleen PDCA-1-purified pDC as described in Materials and Methods. RNA was reverse transcribed into cDNA and semi-quantitative PCR was performed. mRNA transcript levels of IL-27p28, Ebi3, and IL-12p35 were calculated relative to β-actin and data are presented as arbitrary units (AU). B, Total protein was extracted from freshly-isolated liver or spleen PDCA-1+ pDC as described in Materials and Methods and Western blot performed. The membranes were probed for Ebi3 or IL-12p35 and then stripped and reprobed for GAPDH. C, pDC were cultured for 18 h and Golgi Plug protein transport inhibitor was added for the final 5 h of culture. Cell surface proteins were stained and cells were fixed and permeabilized prior to intracellular staining for IL-27p28 or IL-12p35. Values indicate percent positive cells and relative MFI compared to isotype controls (gray-filled histograms). Percentage of IL-27p28 or IL-12p35 positive cells and MFI were averaged from 5 and 4 independent experiments, respectively; D, Liver and spleen pDC and cDC were cultured in the absence or presence of 1 µg/ml CpG B ODN or LPS for 18 h. Cell culture supernatants were collected and IL-27p28 quantified by ELISA. The change in scale from “Untreated” to “+ CpG B” eliminates visibility of error bars for liver pDC. Data represent 3 independent experiments, * p < 0.05, ** p < 0.01, *** p < 0.001.
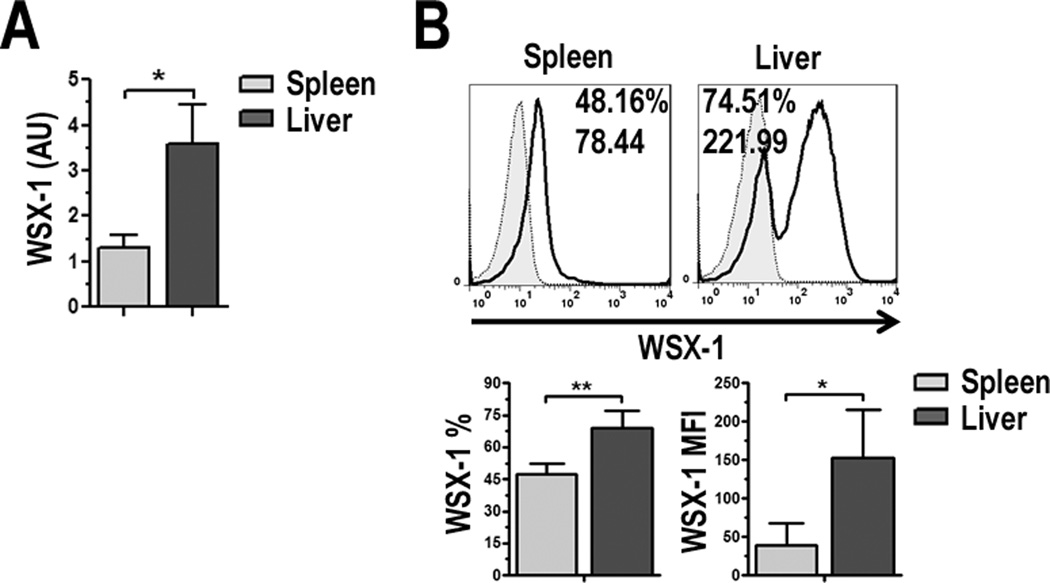
IL-27 signals through the heterodimeric receptor TCCR/WSX-1 and the gp130 subunit, which is a shared component in the IL-6R sub-family [21]. In addition to T cells, IL-27R/WSX-1 is expressed on other hematopoietic cells, including DC, at high levels in the resting state [21]. Semi-quantitative RT-PCR (Fig. 3A) and flow cytometric analysis (Fig. 3B) showed that WSX-1 was expressed at significantly higher levels on liver pDC compared to splenic pDC. Together, these data suggest pDC not only produce IL-27, but have the capacity to respond to it as well.
FIGURE 3.
pDC express the IL-27Rα/WSX-1. A, WSX-1 mRNA was determined by semi-quantitative RT-PCR for liver and spleen pDC and WSX-1 gene expression relative to β-actin was calculated. Data are expressed as AU; B, Liver and spleen pDC were stained for expression of IL-27Rα/WSX-1 and analyzed by flow cytometry. Values indicate percent positive cells and relative MFI compared to isotype controls (gray-filled histograms). Percentage of WSX-1 positive cells and MFI were averaged from 3 independent experiments, * p < 0.05, ** p < 0.01.
IL-27 augments B7-H1 expression on liver pDC
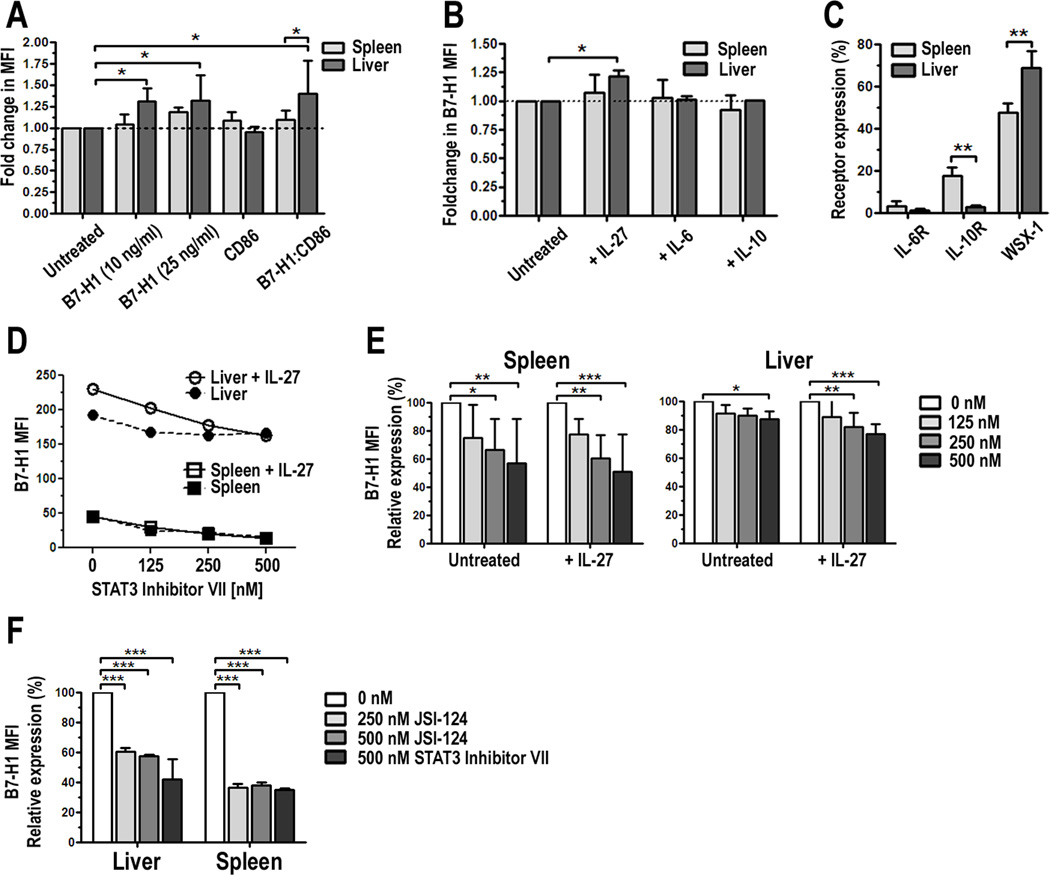
Previous studies on mouse [30] and human monocyte-derived DC [31] suggested that IL-27 can downregulate DC function. The high expression of WSX-1 we observed on pDC (Fig. 3) suggests they have the capacity to respond to IL-27 in the steady-state. Therefore, we cultured liver and spleen pDC overnight in the presence of IL-27. After 18 h, cells were harvested and their phenotype examined by flow cytometry. We found that liver pDC significantly upregulated cell surface expression of B7-H1 but concomitantly downregulated expression of CD86, whereas there was no significant change in surface molecule expression of corresponding splenic pDC (Fig. 4A and Suppl. Fig. 2). As a result of increased B7-H1 and decreased CD86 expression, there was a significant increase in the B7-H1:CD86 ratio in IL-27-conditioned liver pDC compared to untreated control liver pDC, which displayed high baseline expression of B7-H1 (Fig. 1A) and a high B7-H1:CD86 ratio (Fig. 1B). Interestingly, at a lower concentration of IL-27 (10 ng/ml), there was less of an increase in B7-H1 MFI by splenic pDC, but a similar response by liver pDC compared to the higher concentration of IL-27 (25 ng/ml). Despite upregulation of B7-H1, intracellular flow cytometric analysis and ELISA showed that conditioning in IL-27 did not alter production of IL-6, IL-10, IL-12p70 or IFN-α by liver or spleen pDC (data not shown).
FIGURE 4.
IL-27 augments B7-H1 expression in liver pDC. PDCA-1-purified liver or spleen pDC were cultured for 18 h in the presence of 10 or 25 ng/ml IL-27. Cells were harvested, stained, and the expression of B7-H1 and CD86 examined by flow cytometry. Results are presented as fold change in B7-H1 MFI on IL-27-conditioned cells compared with untreated cells (set to 1.0); B, Liver pDC were cultured with 25 ng/ml IL-27, IL-6, or IL-10 for 18 h and B7-H1 expression was analyzed. Results represent fold change in B7-H1 MFI; C, Surface expression of the IL-6Rα, IL-10Rα and WSX-1/IL-27Rα were analyzed on liver and spleen pDC. Data represent percent positive cells, D, Experiments in A were repeated in the absence or presence of increasing concentrations of a STAT3 inhibitor (STAT3 Inhibitor VII). Data represent 4 independent experiments; E, Percent change in B7-H1 expression in the presence of the STAT3 inhibitor compared to untreated cells was calculated and averaged from 4 independent experiments; F, Experiments in D and E were repeated with a second STAT3 inhibitor JSI-124/Cucurbitacin at the indicated concentrations and compared to STAT3 Inhibitor VII. Results represent percent change in B7-H1 MFI in the presence of the STAT3 inhibitors and are an average of two independent experiments, * p < 0.05, ** p < 0.01, *** p < 0.001.
pDC also produce IL-6 and IL-10, which are two cytokines abundant in the liver. Therefore, we tested whether exposure of pDC to these cytokines would also upregulate B7-H1 as was observed following exposure to IL-27. As shown in Fig. 4B, only IL-27 significantly upregulated the expression of B7-H1 on liver pDC. Subsequent analysis revealed that pDC expressed low levels of the IL-6Rα and IL-10Rα (Fig. 4C), especially relative to the high expression of WSX-1 (Figs. 3 and 4C). These data suggest a unique role for IL-27 and WSX-1 in the regulatory function of liver pDC.
STAT3 regulates IL-27-mediated upregulation of B7-H1 on liver pDC
It has been reported that STAT3 nuclear activity is greater in the liver than in other organs [58]. As a result, a higher basal level of STAT3 activation contributes to the inherent immaturity of liver DC. IL-27R/WSX-1 signaling in T cells has shown that STAT3 is a significant downstream signaling molecule that regulates the effects of IL-27 [24, 59]. Moreover, there is recent evidence that B7-H1 is upregulated on tumor cells through STAT3 activation [60]. Based on the observations that activated STAT3, B7-H1 and IL-27 are elevated in the liver in the steady state, we postulated that STAT3 might be involved in upregulation of B7-H1 by IL-27 on liver pDC. To test this, liver and spleen pDC were cultured overnight in the presence of IL-27 and a highly selective STAT3 inhibitor at increasing concentrations. We observed a concentration-dependent decrease in B7-H1 expression following STAT3 inhibition (Fig. 4D and 4E) in both liver and spleen pDC. Additionally, we found that inhibition of STAT3 reduced IL-27-induced upregulation of B7-H1 on liver pDC (Fig. 4D) back to or below baseline levels. To confirm this was indeed a STAT3-dependent effect, we tested a second STAT3-specific inhibitor, JSI-124/Cucurbitacin, and obtained similar results (Fig. 4F).
IL-27-conditioned liver pDC increase percentage of CD4+Foxp3+ T cells which is dependent on B7-H1
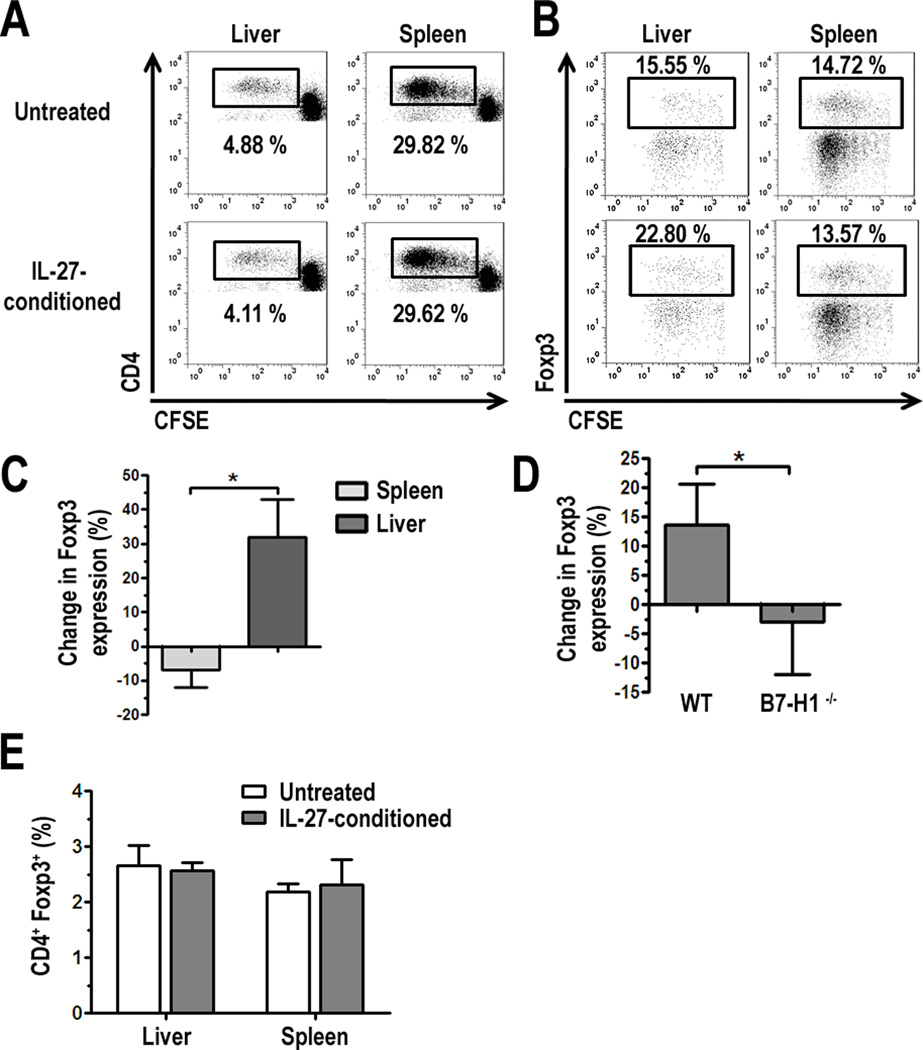
Next we tested whether there were any functional consequences, in addition to the phenotypic changes observed in pDC, following their exposure to exogenous IL-27. pDC conditioned overnight in IL-27 were washed then cultured in MLR with CFSE-labeled, allogeneic BALB/c splenic T cells for 3 d. Cultures were harvested and stained for flow cytometric analysis of T cell proliferation by CFSE dilution. IL-27-conditioned liver pDC showed minimal differences in CD4+ T cell allostimulatory capacity compared to untreated cells (Fig. 5A). However, further analysis of the proliferating CD4+ T cells (cells gated in 5A) revealed an increased percentage of Foxp3+ cells in cultures stimulated with IL-27-conditioned liver, but not spleen pDC (Fig. 5B). When the percent change in Foxp3 expression in CD4+ T cells cultured with IL-27-conditioned pDC relative to untreated pDC was calculated, we found a marked difference in the ability of IL-27-conditioned liver and spleen pDC to influence Foxp3 expression (Fig. 5C): thus, liver pDC favored a 30% increase and spleen pDC favored a modest decrease in Foxp3 expression. We next tested if this increase in CD4+Foxp3+ T cells was due to the upregulation of B7-H1 by IL-27. When WT or B7-H1−/− liver pDC were conditioned in IL-27 and then cultured in MLR, we observed that the frequency of CD4+Foxp3+ T cells was significantly reduced in cultures with B7-H1−/− liver pDC (Fig. 5D), suggesting a critical link between IL-27 and B7-H1 expression by liver pDC, and Foxp3+ Treg. The increase in the proportion of Foxp3+ cells was restricted to proliferating cells, as we did not observe any differences in MLR between untreated and IL-27-conditioned liver or spleen pDC in the non-dividing CD4+ (CFSEhi) population (Fig. 5E).
FIGURE 5.
IL-27-conditioned liver pDC increase the percentage of CD4+Foxp3+ T cells in MLR. A, Freshly-isolated liver or spleen PDCA-1+ pDC were cultured for 18 h with 25 ng/ml IL-27, washed, counted, and cultured in MLR with CFSE-labeled BALB/c splenic T cells. After 3 d, cells were harvested and stained for analysis of CD4+ T cell proliferation (A) and expression of Foxp3 (B) by flow cytometry. Data represents 3 independent experiments for both A and B; C, The percent change in Foxp3 expression in proliferating CD4+ T cells (cells gated in A) in cultures with IL-27-conditioned liver or spleen pDC compared to untreated pDC was calculated and average across 3 independent experiments; D, WT or B7-H1−/− liver pDC were cultured with IL-27 for 18 h and subsequently cultured with BALB/c T cells. Intracellular expression of Foxp3 was analyzed by flow cytometry after 3 d MLR and results were averaged across 3 independent experiments; E, Foxp3 expression was quantified in non-dividing CD4+ cells (CD4+ CFSEhi cells) from experiments shown in A, and averaged across 3 independent experiments, * p < 0.05.
IL-27 produced by pDC impairs their T cell allostimulatory capacity
To further evaluate the contribution of IL-27 to the function of pDC, we utilized Ebi3−/− mice that lack functional IL-27. Liver and spleen pDC were purified and stimulated with CpG B. Phenotypic analysis of WT and Ebi3−/− pDC revealed few differences in the expression of key cell surface molecules (Suppl. Fig. 3). Although not statistically significant, Ebi3−/− liver and spleen pDC showed a trend towards lower B7-H1 and WSX-1 expression following CpG B stimulation. Ebi3−/− spleen pDC showed slightly reduced CD86 MFI, but a significantly greater percentage of MHC class II/I-Ab+ cells following overnight exposure to CpG B. We did not observe a change in WSX-1 expression on WT pDC following exposure to exogenous IL-27 (data not shown), however, pDC from Ebi3−/− mice did express a slightly, but not statistically significant lower level of WSX-1 (Suppl. Fig 3), which suggested that IL-27 signaling through WSX-1 may partially upregulate the receptor in an autocrine manner.
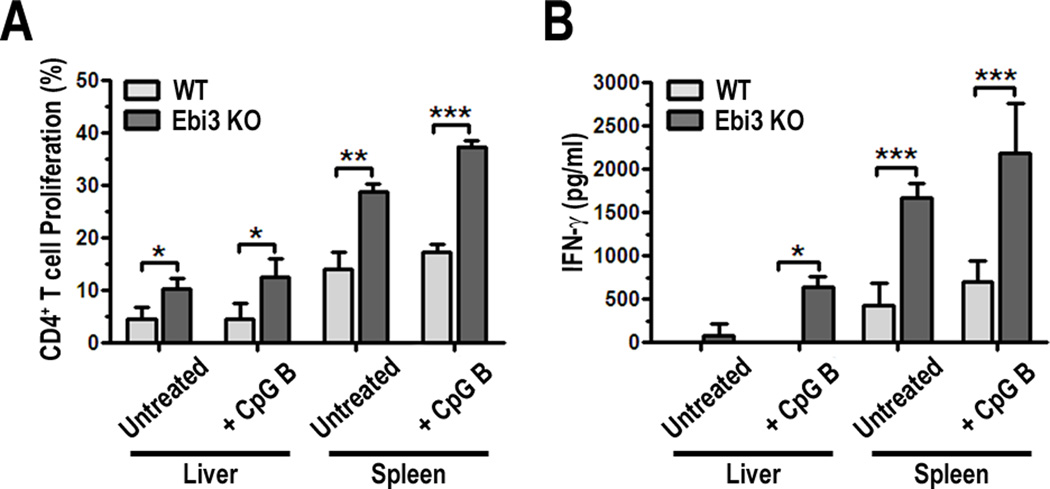
To determine if lack of functional IL-27 had an impact on pDC-stimulated allogeneic T cell responses, WT or Ebi3−/− pDC were cultured in MLR with CFSE-labeled BALB/c T cells for 3 days. T cell proliferation was quantified by flow cytometry and IFN-γ production in culture supernatants was analyzed by ELISA. Analyses of MLR cultures showed that Ebi3−/− liver and spleen pDC induced more CD4+ T cell proliferation compared to WT pDC, when either untreated or following overnight stimulation with CpG B (Fig. 6A). No significant differences in CD8+ T cell proliferation were observed (data not shown). Increased CD4+ T cell proliferation correlated with higher levels of IFN-γ in culture supernatants, (Fig. 6B), suggesting that IL-27 produced by pDC regulates their T cell stimulatory capacity. When T cells were stimulated in the absence of pDC (anti-CD3/anti-CD28 Ab stimulation), IL-27 did not suppress T cell proliferation or promote Foxp3 expression (Suppl. Fig. 4). These data suggest that the elevated T cell stimulatory capacity of Ebi3−/− pDC, and therefore, the regulatory effect of IL-27, is due to a pre-conditioning of the pDC by IL-27 in vivo and not a direct effect on the T cells.
FIGURE 6.
Ebi3−/− pDC exhibit greater allogeneic CD4+ T cell stimulatory capacity and induce more IFNγ production compared to WT pDC. A, Unstimulated or CpG B ODN-stimulated (18 h) PDCA-1+ WT and Ebi3−/− liver or spleen pDC were cultured with allogeneic, CFSE-labeled BALB/c T cells. After 3 d MLR, cells were harvested and stained for analysis of T cell proliferation by CFSE dilution; B, IFN-γ was quantified in supernatants from cultures in 6A by ELISA, * p < 0.05, ** p < 0.01, *** p < 0.001.
IL-27 and B7-H1 mediate liver pDC ability to suppress DTH responses
Despite their ability to promote anti-viral immunity, pDC also exert tolerogenic effects and modulate immune responses [11]. Specifically, systemic depletion of PDCA-1+ cells blunts the development of oral tolerance, and adoptive transfer of liver pDC from Ag-fed mice at the site and time of immunization suppresses Ag-specific DTH responses [39]. Thus, we hypothesized that IL-27 expressed by liver pDC might mediate their capacity to suppress DTH to fed Ag.
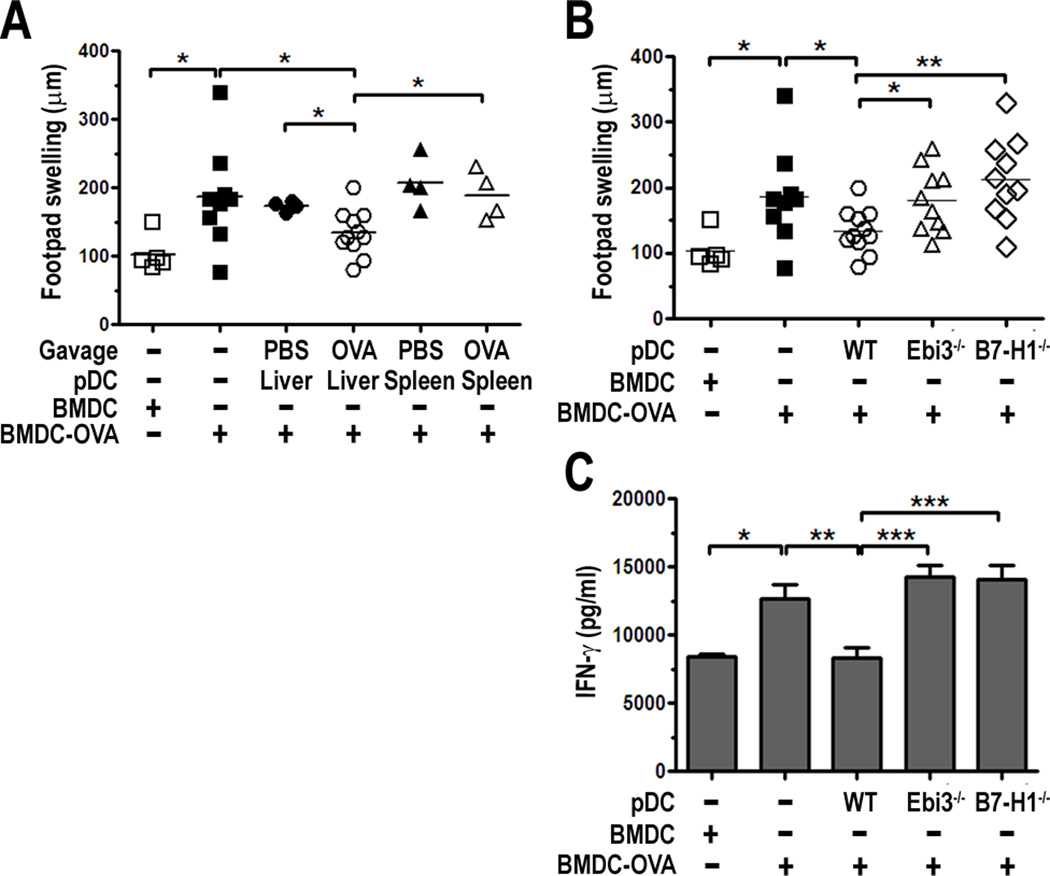
First, we tested whether suppression of DTH responses was limited to pDC from the liver, and confirmed their ability to do this in an Ag-dependent manner. Liver and spleen PDCA-1+ pDC were isolated from mice fed OVA by gavage, or PBS as a control. pDC were co-injected s.c. at the base of the tail at a 1:1 ratio with syngeneic BM-derived DC (BMDC) that were pulsed with OVA (BMDC-OVA). BMDC or BMDC-OVA were injected alone as controls. After 7 days, all mice were challenged with heat-aggregated OVA (HA-OVA) in the hind footpad and footpad swelling measured at 24 h. PBS was injected into the opposing footpad as a control. Compared to BMDC alone, BMDC-OVA induced a significant level of footpad swelling upon challenge with HA-OVA at 24 h (Fig. 7A). Liver pDC from OVA-fed mice, but not from PBS-fed controls significantly suppressed footpad swelling when co-injected at the same site and time of immunization with BMDC-OVA (Fig. 7A). Spleen pDC from OVA-fed mice or PBS-fed controls failed to suppress DTH responses (Fig. 7A). Together, these results confirm that suppression of DTH responses is specific to liver pDC and is dependent upon Ag acquired though feeding.
FIGURE 7.
WT, but not Ebi3−/− or B7-H1−/− liver pDC from OVA-fed mice suppress DTH responses. B6 mice were immunized with B6 BMDC-OVA ± liver or spleen pDC from PBS-fed control or OVA-fed mice (A), or WT, Ebi3−/− or B7-H1−/− liver pDC from OVA-fed mice (B) by s.c. injection at the base of the tail. 7 d later, mice were challenged in the footpad with HA-OVA and footpad swelling measured at 24 h; C, Total splenocytes from challenged mice were cultured for 48 h with 1 mg/ml OVA and supernatants harvested for quantification of IFNγ by ELISA, * p < 0.05, ** p < 0.01, *** p < 0.001.
To test whether IL-27 was required for the regulatory effect of liver pDC in the model of DTH to OVA, WT or Ebi3−/− liver pDC from mice fed OVA were co-injected with BMDC-OVA as described above. Mice were challenged in the footpad with HA-OVA and footpad swelling quantified at 24 h post-challenge. Liver pDC from Ebi3−/− mice failed to suppress DTH responses compared to WT liver pDC (Fig. 7B). When splenocytes from these mice were restimulated ex vivo with OVA, we detected greater levels of IFN-γ in cultures supernatants from mice that received Ebi3−/− liver pDC compared to WT liver pDC (Fig. 7C). To highlight the significance of these findings and the importance of IL-27 in the functional biology of liver pDC, we compared these results to liver pDC from mice lacking B7-H1, which is a known potent regulator of immune responses. B7-H1−/− liver pDC also failed to suppress DTH responses compared to WT pDC. Footpad swelling (Fig. 7B) and splenocyte production of IFN-γ (Fig. 7C) were at a similar level as observed for mice receiving Ebi3−/− liver pDC, suggesting IL-27 and B7-H1 play a significant role in the immunoregulatory capacity of liver pDC.
DISCUSSION
In the present study, we show that pDC express IL-27 as well as the IL-27R/WSX-1. Liver pDC express higher levels of both proteins compared to pDC from the spleen, and also exhibit a unique capacity to respond to IL-27 through upregulation of the coregulatory molecule B7-H1. We found STAT3 to be critical for this phenomenon, as its pharmacologic inhibition completely abrogated the effect of IL-27. A functional consequence of liver pDC conditioning by IL-27 was an ability to increase the incidence of CD4+Foxp3+ cells in allogeneic MLR. IL-27 contributes to the ability of pDC to regulate T cell responses in vitro, and our data also identify IL-27 and B7-H1 as critical molecules utilized by liver pDC to suppress DTH responses to OVA in vivo.
Although studied typically for their potent anti-viral function through production of type I IFNs [6, 7], pDC have garnered significant attention for their immunosuppressive function, and multiple mechanisms of immune regulation and tolerance by pDC have been studied by our lab and others [11]. Important features of pDC that shape their immunoregulatory function include their low level of Ag-presenting (MHC class II) and costimulatory molecules (CD80 and CD86) and unique regulation of MHC class II expression [61] which lead to poor T cell stimulatory function compared to cDC [8]. As we show here, pDC residing in the liver microenvironment show even lower expression of MHC class II and costimulatory molecules (CD40, CD80, and CD86) compared to pDC isolated concomitantly from the spleen. Both pDC populations exhibit high baseline expression of the coregulator B7-H1, however levels on liver pDC are greater than on spleen pDC. Liver-resident APC, including pDC, are continuously exposed to bacterial degradation products (e.g., endotoxin) via the portal blood [58] leading to constant stimulation of TLRs and other pattern recognition receptors (PRRs), which may contribute to their comparatively high baseline expression of B7-H1. We have demonstrated previously that an elevated B7-H1:CD86 ratio on pDC is positively correlated with successful weaning of immunosuppressive drug therapy or operational tolerance in liver transplant patients, suggesting that this may be an important characteristic associated with the tolerogenic capacity of pDC [62]. Analysis of the ratio of B7-H1 to CD86 MFI on liver and spleen pDC in this study clearly highlights the regulatory potential of liver pDC, based on their significantly elevated ratio of coregulatory to costimulatory molecule expression compared to pDC from the spleen.
In addition to their more immature phenotype compared with splenic pDC, liver pDC produce lower levels of the pro-inflammatory cytokines, IL-6, IL-12p40 and IL-12p70 [14], but greater levels of the immunoregulatory cytokine IL-10 in the steady state, or after stimulation with LPS [15] or CpG B. Similar to the fact that pDC are poor T cell allostimulators relative to cDC, liver pDC are comparatively weak stimulators compared with pDC from the spleen, of both CD4+ and CD8+ T cells.
Previous studies have identified IL-27 to play a role in the functional biology of DC, in addition to its known effects on T cell polarization and function. Liver cDC, mobilized through hydrodynamic injection of plasmids encoding GM-CSF, showed elevated levels of IL-27p28 compared to their counterparts in the spleen [56]. We observed a similar difference between liver and spleen cDC following LPS stimulation, but also found that pDC have a greater capacity for IL-27p28 production relative to cDC, and we detected higher levels of both p28 and Ebi3 in liver pDC compared to spleen pDC. Our detection of the p28 subunit in the culture supernatants of liver pDC (and intracellularly by flow cytometry) lends support to the tolerogenic potential of liver pDC, as p28 has been suggested to exert immunoregulatory function when secreted independently of Ebi3 [54, 63]. In support of our findings on IL-27, PDCA-1+ splenic pDC exposed to exogenous TGF-β (which is produced by multiple cell types in the liver, [2]) show elevated IL-27 and decreased IL-6 production compared to untreated controls [33], suggesting that the immunoregulatory molecules found within the normal liver microenvironment (i.e. TGF-β) may contribute to elevated IL-27 production by liver pDC. pDC also express p35, which can heterodimerize with Ebi3 to form the regulatory cytokine IL-35. However, low expression of p35 by liver pDC, which we have shown exhibit greater regulatory phenotype and function compared to pDC in the spleen, suggests that p35 and Ebi3 may not be forming functional IL-35 in these cells. The higher expression of p35 by spleen pDC, which have greater immunostimulatory capacity compared to liver pDC, supports this observation. The presence of two distinct populations of IL-12p35+ cells from the spleen is an interesting observation and may indicate phenotypically and functionally different subpopulations. The IL-12p35hi spleen pDC may be responsible for the greater levels of IL-12p70 we have reported previously in these cells [15].
IL-27 binds to the heterodimeric IL-27R comprised of IL-27Rα/WSX-1/TCCR and gp130 [21]. Although gp130 is a shared subunit for multiple cytokines, WSX-1 is specific to IL-27 and is expressed by pDC, suggesting they have the capacity to respond to IL-27. Indeed, in the presence of exogenous IL-27, liver but not spleen pDC upregulated B7-H1 but not CD86 expression. Consequently, IL-27-conditioned liver pDC have a significantly elevated B7-H1:CD86 ratio compared to untreated cells. Furthermore, liver pDC are more sensitive to lower concentrations of IL-27, highlighting the significance of higher WSX-1 expression on these cells. Studies on T cells have revealed important roles for STAT1 and STAT3 signaling in mediating downstream effects of IL-27 [23, 24]. Wölfle et al [64] recently showed that STAT3 directly binds the B7-H1 (PD-L1) promoter and drives its expression. When we used a soluble inhibitor to block STAT3 activation in vitro, upregulation of B7-H1 by IL-27 was abrogated. Additionally, B7-H1 levels on liver and spleen pDC in the absence of IL-27 also decreased, confirming that STAT3 promotes B7-H1 expression on pDC. IL-6 and IL-10 also signal through STAT3, however, the increase in B7-H1 expression we observed was unique to IL-27. Subsequent analysis showed low expression of both the IL-6R and IL-10R. These data not only suggest that these cytokine receptors may not critically influence pDC function, but also highlight the significance of high expression of the IL-27Rα/WSX-1 in the functional biology of these cells. Interestingly, we noted that exposure to IL-27 did not alter IFN-α, IL-6, IL-10, or IL-12p70 production by pDC, despite a report that IL-27 blunts TNF-α and IL-12p40 production in activated peritoneal macrophages via a STAT3-dependent mechanism [65].
In studies using WSX-1-deficient mice, Yoshida et al [30] showed that, in the absence of IL-27 signaling, spleen cDC exhibited enhanced T cell stimulatory capacity in MLR, suggesting that IL-27 negatively regulates DC function. In agreement with these data, human monocyte-derived DC exposed to exogenous IL-27 at high levels show a reduction in T cell stimulatory capacity, which is dependent on B7-H1 expression [31]. Although we did not observe any significant phenotypic differences in Ebi3−/− pDC compared to WT pDC, a trend towards lower B7-H1 and WSX-1 expression and a greater incidence of Ebi3−/− MHC class II (I-Ab)+ cells supports the existence of an autocrine feedback loop by IL-27 in pDC. When IL-27-conditioned pDC were cultured with allogeneic T cells, there was a greater proportion of proliferating CD4+ T cells expressing the Treg transcription factor Foxp3 in cultures with liver, but not spleen pDC. This effect was lost when IL-27-conditioned B7-H1−/− liver pDC were used as stimulators. Supporting a regulatory role for IL-27 in pDC immune function, Ebi3−/− pDC showed an enhanced ability to stimulate allogeneic T cell proliferation and subsequent IFN-γ production. The addition of IL-27 to these cultures did not suppress T cell proliferation to levels observed with WT pDC, suggesting that the regulatory effect of IL-27 in WT pDC, that is absent in Ebi3−/− pDC, is due to a pre-conditioning by IL-27 in vivo, and not a direct effect of IL-27 produced by pDC on T cells. Overall, IL-27 may influence liver pDC regulatory function by upregulating B7-H1 expression and enhancing their ability to promote Foxp3+ Treg while reducing their T cell allostimulatory capacity.
In a series of DTH and contact hypersensitivity (CHS) experiments, Goubier et al [39] showed recently that liver pDC (CD11c+CD11b-NK1.1−) accounted for the suppressive capacity of total CD11c+ hepatic DC. Liver pDC suppressed both Ag-specific DTH and CHS responses to model Ags, whereas they reported spleen pDC failed to inhibit immune reactivity in their CHS model. Furthermore, systemic depletion of pDC blunted the induction of oral tolerance to OVA. Our data provide strong evidence that liver but not spleen pDC suppress DTH responses to OVA in an Ag-dependent manner. Moreover, we have identified novel mechanisms contributing to this phenomenon. IL-27, as evidenced using Ebi3−/− liver pDC, and B7-H1, as evidenced using B7-H1−/− liver pDC, are critical for the ability of liver pDC to significantly suppress DTH responses. These data suggest that liver pDC can sufficiently acquire fed Ag and limit priming of the immune response during the immunization phase. Since pDC can inhibit cDC activation [66], it remains to be seen in this model whether pDC: suppress BMDC-OVA directly to inhibit the priming phase; migrate to the lymph nodes to inhibit T cell activation; or both. The capacity of liver pDC to partially but not significantly suppress DTH responses in an Ag-independent manner (PBS-Liver pDC, Fig. 7A) in addition to their significant suppression in an Ag-dependent fashion (Fig. 7B and 7C) suggests they are able to actively regulate immunity through multiple mechanisms.
In summary, our studies show that IL-27 plays a critical regulatory role in the functional biology of liver pDC. First, IL-27 promotes cell surface expression of B7-H1 in liver pDC which, in turn, increases the incidence of CD4+ T cells expressing Foxp3. The effects of IL-27 on liver compared to spleen pDC suggest the potential for unique signaling molecules downstream of the IL-27R in these cells, which may be critical in regulating their tolerogenic capacity. Second, IL-27 is critical for liver pDC ability to regulate immune responses in vitro and in vivo, with the potential for regulation of cDC, T cells, or both. Together, these results suggest that IL-27 and liver pDC may be an important target or tool for therapeutic intervention to limit immune reactivity or promote tolerance.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Hongmei Shen and the Starzl Transplantation Institute Flow Cytometry facility for their assistance and Dr. Hēth R. Turnquist for help with RT-PCR data analysis.
Footnotes
This work was supported by National Institutes of Health (NIH) grants R01 AI60994 and P01 AI81678 and by the Roche Organ Transplantation Research Foundation (874279717) (A.W.T.). B.M.M. was supported non-concurrently by NIH Training Grants T32 AI074490 and AI089443. G.R. was supported by an American Diabetes Association Junior Faculty Award (1-10-JF-43) and the Starzl Transplantation Institute Joseph Patrick Fellowship in Transplantation Research. B.R.R. was supported by an American Heart Association Predoctoral Fellowship (11PRE7070020). T.L.S. was supported by a Basic Science Fellowship from the American Society of Transplantation and a Roger Jenkins Fellowship from the American Liver Foundation.
Abbreviations used in this paper:
DC, dendritic cell; BM, bone marrow; cDC, conventional dendritic cell; pDC, plasmacytoid dendritic cell; PDCA-1, plasmacytoid dendritic cell antigen-1; Treg, regulatory T cell; B7-H1, B7 homolog-1; DTH, delayed-type hypersensitivity; Ebi3, Epstein-Barr Virus-induced protein 3; WT, wild type; Foxp3, forkhead box P3; B6, C57BL/6; ODN, oligodinucleotide; HA, heat-aggregated; CHS, contact hypersensitivity; MFI, mean fluorescence intensity.
REFERENCES
- 1.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 2.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 3.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski MJ, Cerundolo V, Crocker PR. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 5.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 6.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 7.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 8.Abe M, Wang Z, de Creus A, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5:1808–1819. doi: 10.1111/j.1600-6143.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010;115:5366–5375. doi: 10.1182/blood-2009-10-248260. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–2676. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 13.Kingham TP, Chaudhry UI, Plitas G, Katz SC, Raab J, DeMatteo RP. Murine liver plasmacytoid dendritic cells become potent immunostimulatory cells after Flt-3 ligand expansion. Hepatology. 2007;45:445–454. doi: 10.1002/hep.21457. [DOI] [PubMed] [Google Scholar]
- 14.Castellaneta A, Sumpter TL, Chen L, Tokita D, Thomson AW. NOD2 ligation subverts IFN-alpha production by liver plasmacytoid dendritic cells and inhibits their T cell allostimulatory activity via B7-H1 up-regulation. J Immunol. 2009;183:6922–6932. doi: 10.4049/jimmunol.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokita D, Sumpter TL, Raimondi G, Zahorchak AF, Wang Z, Nakao A, Mazariegos GV, Abe M, Thomson AW. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. J Hepatol. 2008;49:1008–1018. doi: 10.1016/j.jhep.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 18.Sher A, Fiorentino D, Caspar P, Pearce E, Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991;147:2713–2716. [PubMed] [Google Scholar]
- 19.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 20.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 21.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 22.Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 24.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 25.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 27.Levings MK, Roncarolo MG. T-regulatory 1 cells: a novel subset of CD4 T cells with immunoregulatory properties. J Allergy Clin Immunol. 2000;106:S109–S112. doi: 10.1067/mai.2000.106635. [DOI] [PubMed] [Google Scholar]
- 28.Sasaoka T, Ito M, Yamashita J, Nakajima K, Tanaka I, Narita M, Hara Y, Hada K, Takahashi M, Ohno Y, Matsuo T, Kaneshiro Y, Tanaka H, Kaneko K. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G568–G576. doi: 10.1152/ajpgi.00329.2010. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Rohowsky-Kochan C. Interleukin-27-Mediated Suppression of Human Th17 Cells Is Associated with Activation of STAT1 and Suppressor of Cytokine Signaling Protein 1. J Interferon Cytokine Res. 2011;31:459–469. doi: 10.1089/jir.2010.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 31.Karakhanova S, Bedke T, Enk AH, Mahnke K. IL-27 renders DC immunosuppressive by induction of B7-H1. J Leukoc Biol. 2011;89:837–845. doi: 10.1189/jlb.1209788. [DOI] [PubMed] [Google Scholar]
- 32.Wirtz S, Becker C, Fantini MC, Nieuwenhuis EE, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-kappa B activation. J Immunol. 2005;174:2814–2824. doi: 10.4049/jimmunol.174.5.2814. [DOI] [PubMed] [Google Scholar]
- 33.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, Mazza EM, Boon L, Grassi F, Fioretti MC, Fallarino F, Puccetti P, Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in longterm tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 34.Yang R, Liu Q, Grosfeld JL, Pescovitz MD. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. J Pediatr Surg. 1994;29:1145–1148. doi: 10.1016/0022-3468(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 35.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 36.Viney JL, Mowat AM, O'Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–5825. [PubMed] [Google Scholar]
- 37.Spahn TW, Weiner HL, Rennert PD, Lugering N, Fontana A, Domschke W, Kucharzik T. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur J Immunol. 2002;32:1109–1113. doi: 10.1002/1521-4141(200204)32:4<1109::AID-IMMU1109>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 38.Dubois B, Joubert G, Gomez de Aguero M, Gouanvic M, Goubier A, Kaiserlian D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology. 2009;137:1019–1028. doi: 10.1053/j.gastro.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 39.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiokawa A, Tanabe K, Tsuji NM, Sato R, Hachimura S. IL-10 and IL-27 producing dendritic cells capable of enhancing IL-10 production of T cells are induced in oral tolerance. Immunol Lett. 2009;125:7–14. doi: 10.1016/j.imlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Pillarisetty VG, Katz SC, Bleier JI, Shah AB, Dematteo RP. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-gamma via autocrine IL-12. J Immunol. 2005;174:2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- 42.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, Falo LD, Thomson AW. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–1523. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 43.de Siqueira AL, Russo M, Steil AA, Facincone S, Mariano M, Jancar S. A new murine model of pulmonary eosinophilic hypersensitivity: contribution to experimental asthma. J Allergy Clin Immunol. 1997;100:383–388. doi: 10.1016/s0091-6749(97)70253-7. [DOI] [PubMed] [Google Scholar]
- 44.O'Connell PJ, Morelli AE, Logar AJ, Thomson AW. Phenotypic and functional characterization of mouse hepatic CD8 alpha+ lymphoid-related dendritic cells. J Immunol. 2000;165:795–803. doi: 10.4049/jimmunol.165.2.795. [DOI] [PubMed] [Google Scholar]
- 45.De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005;174:2037–2045. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 46.Abe M, Tokita D, Raimondi G, Thomson AW. Endotoxin modulates the capacity of CpG-activated liver myeloid DC to direct Th1-type responses. Eur J Immunol. 2006;36:2483–2493. doi: 10.1002/eji.200535767. [DOI] [PubMed] [Google Scholar]
- 47.Morelli AE, O'Connell PJ, Khanna A, Logar AJ, Lu L, Thomson AW. Preferential induction of Th1 responses by functionally mature hepatic (CD8alpha− and CD8alpha+) dendritic cells: association with conversion from liver transplant tolerance to acute rejection. Transplantation. 2000;69:2647–2657. doi: 10.1097/00007890-200006270-00027. [DOI] [PubMed] [Google Scholar]
- 48.Sumpter TL, Packiam V, Turnquist HR, Castellaneta A, Yoshida O, Thomson AW. DAP12 promotes IRAK-M expression and IL-10 production by liver myeloid dendritic cells and restrains their T cell allostimulatory ability. J Immunol. 2011;186:1970–1980. doi: 10.4049/jimmunol.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 50.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 51.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 52.Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, Sellati R, Jiang H, Zhang S, Li H, Zhao J, Ting AT, Mayer L, Unkeless JC, Labadia ME, Hodge M, Li J, Xiong H. Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgamma t. Eur J Immunol. 2008;38:1204–1214. doi: 10.1002/eji.200838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, Decker T, Lohoff M. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 54.Stumhofer JS, Tait ED, Quinn WJ, 3rd, Hosken N, Spudy B, Goenka R, Fielding CA, O'Hara AC, Chen Y, Jones ML, Saris CJ, Rose-John S, Cua DJ, Jones SA, Elloso MM, Grotzinger J, Cancro MP, Levin SD, Hunter CA. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 2010;11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villarino AV, Hunter CA. Biology of recently discovered cytokines: discerning the pro- and anti-inflammatory properties of interleukin-27. Arthritis Res Ther. 2004;6:225–233. doi: 10.1186/ar1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y, Jiang G, Yang HR, Gu X, Wang L, Hsieh CC, Chou HS, Fung JJ, Qian S, Lu L. Distinct response of liver myeloid dendritic cells to endotoxin is mediated by IL-27. J Hepatol. 2009;51:510–519. doi: 10.1016/j.jhep.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 58.Lunz JG, 3rd, Specht SM, Murase N, Isse K, Demetris AJ. Gut-derived commensal bacterial products inhibit liver dendritic cell maturation by stimulating hepatic interleukin-6/signal transducer and activator of transcription 3 activity. Hepatology. 2007;46:1946–1959. doi: 10.1002/hep.21906. [DOI] [PubMed] [Google Scholar]
- 59.Owaki T, Asakawa M, Morishima N, Mizoguchi I, Fukai F, Takeda K, Mizuguchi J, Yoshimoto T. STAT3 is indispensable to IL-27-mediated cell proliferation but not to IL-27-induced Th1 differentiation and suppression of proinflammatory cytokine production. J Immunol. 2008;180:2903–2911. doi: 10.4049/jimmunol.180.5.2903. [DOI] [PubMed] [Google Scholar]
- 60.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, Wasik MA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, Mount AM, Belz GT, O'Keeffe M, Ohmura-Hoshino M, Ishido S, Stoorvogel W, Heath WR, Shortman K, Villadangos JA. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 62.Tokita D, Mazariegos GV, Zahorchak AF, Chien N, Abe M, Raimondi G, Thomson AW. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation. 2008;85:369–377. doi: 10.1097/TP.0b013e3181612ded. [DOI] [PubMed] [Google Scholar]
- 63.Shimozato O, Sato A, Kawamura K, Chiyo M, Ma G, Li Q, Tagawa M. The secreted form of p28 subunit of interleukin (IL)-27 inhibits biological functions of IL-27 and suppresses anti-allogeneic immune responses. Immunology. 2009;128:e816–e825. doi: 10.1111/j.1365-2567.2009.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolfle SJ, Strebovsky J, Bartz H, Sahr A, Arnold C, Kaiser C, Dalpke AH, Heeg K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41:413–424. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- 65.Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 66.Gigley JP, Khan IA. Plasmacytoid DC from Aged Mice Down-Regulate CD8 T Cell Responses by Inhibiting cDC Maturation after Encephalitozoon cuniculi Infection. PLoS One. 2011;6:e20838. doi: 10.1371/journal.pone.0020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.