Abstract
Improving spectral resolution in cochlear implants is key to improving performance in difficult listening conditions (e.g. speech in noise, music, etc.). Current focusing might reduce channel interaction, thereby increasing spectral resolution. Previous studies have shown that combining current steering and current focusing reduces spread of excitation and improves virtual channel discrimination in a single-channel context. It is unclear whether the single-channel benefits from current focusing extend to a multi-channel context, in which the physical and perceptual interference of multiple stimulated channels might overwhelm the benefits of improved spectral resolution. In this study, signal discrimination was measured with and without current focusing, in the presence of competing stimuli on nearby electrodes. Results showed that signal discrimination was consistently better with current focusing than without, regardless of the amplitude of the competing stimuli. Therefore, combining current steering and current focusing may provide more effective spectral cues than are currently available.
Keywords: current steering, current focusing, psychophysics, cochlear implant, virtual channels
1. Introduction
Most cochlear implant (CI) users have excellent speech recognition in quiet conditions but have more difficulty understanding speech in noise or listening to music. Although 4 spectral channels are sufficient for understanding speech in quiet (Shannon et al., 1995), many more channels are required for understanding speech in noise or listening to music (Friesen et al., 2001; Smith et al., 2002; Shannon et al., 2004). While modern CIs typically have 12–22 intra-cochlear electrodes, most CI listeners perform as if they only have access to 4–8 independent spectral channels (Friesen et al., 2001). The reduced number of effective spectral channels is thought to be due to the broad current spread from stimulated electrodes and the resulting channel interaction from overlapping neural populations. CI simulation studies have shown that increasing the number of functional spectral channels (by reducing channel interaction) improves speech perception, particularly in noise (e.g. Fu et al., 1998; Fu and Nogaki, 2005).
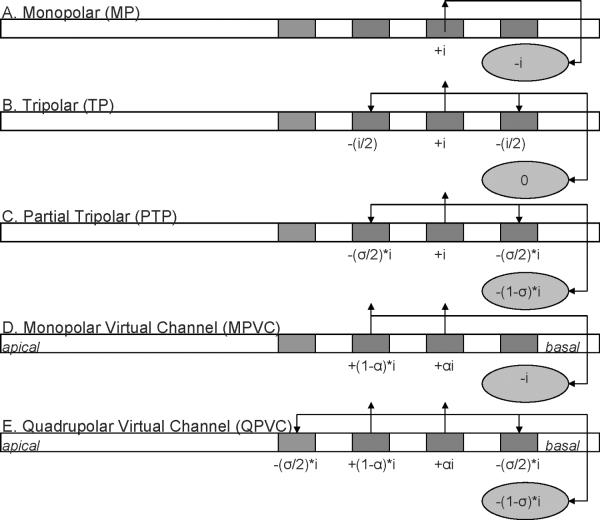
Virtual channels (VCs) have been proposed to increase the number of spectral channels beyond the number of physical electrodes. VCs are typically created by simultaneously stimulating two adjacent electrodes in-phase (see figure 1). The proportion of current on the more basal electrode is denoted by α, and the remainder of the current (1−α) is delivered to the more apical electrode. The current is “steered” between the physical electrodes by changing the value of α. Several studies have shown that CI users can reliably discriminate α steps as small as 0.2 mm between a pair of physical electrodes (e.g., Donaldson et al., 2005; Landsberger and Srinivasan, 2009). Unfortunately, single-channel VC discrimination has not been shown to consistently benefit multi-channel perception. VCs have been implemented in Advanced Bionics' Fidelity 120 speech processing strategy, with no clear advantage in speech perception over the standard 16-channel continuous interleaved sampling (CIS) strategy (e.g. Berenstein et al., 2008; Brendel et al., 2008). Saoji et al. (2009) and Busby et al. (2008) have shown that the current spread from a VC is similar to the current spread from the stimulation of an individual physical electrode. This suggests that channel interactions due to current spread may limit the spectral resolution with VCs to a similar degree as with physical electrodes, with CI users receiving only 4–8 independent spectral channels. Reducing current spread would most likely also reduce channel interaction, which would, in turn, increase the number of independent spectral channels.
Figure 1.
Illustration of different stimulation modes. The oval under each electrode array depicts the extra-cochlear electrode; “i” indicates the amount of stimulation current applied to each electrode. Note that only the first phase of an anodic-first biphasic pulse is shown. For PTP and QPVC stimulation modes, σ indicates the fraction of current returned by the intracochlear electrodes (i.e. the degree of current focusing). For MPVC and QPVC stimulation modes, α indicates the proportion of current delivered to the more basal electrode in the current steering pair.
With monopolar (MP) stimulation, the active intracochlear electrode is stimulated and an extra-cochlear electrode is used as a ground (see Figure 1 – A), producing relatively broad current spread. Tripolar (TP) stimulation (see Figure 1 – B) has been proposed to reduce current spread via “current focusing” (e.g., Litvak et al., 2007; Berenstein et al., 2008; Bierer and Faulkner, 2010; Landsberger et al., 2012). With TP stimulation, an active electrode is stimulated and the two flanking (ground) electrodes are stimulated in opposite-phase relative to the active electrode, with each receiving half the current of the active electrode. Because the current loop is entirely intra-cochlear, the current spread is reduced. Physiological (e.g. Bierer and Middlebrooks, 2002; Snyder et al., 2004), computational (e.g. Spelman et al., 1995; Briaire and Frijns, 2000, Litvak et al., 2007), and psychophysical (Bierer and Faulkner, 2010; Landsberger et al., 2012) studies have shown that TP stimulation reduces current spread compared to MP stimulation.
One aspect of TP stimulation is that the amount of current required to achieve a comfortable listening level is often above the compliance limit of the device. A variation on TP stimulation, called partial tripolar (PTP) stimulation (see Figure 1 – C) has been proposed to reduce current spread while staying within device compliance limits (e.g. Litvak et al., 2007; Bierer and Faulkner, 2010; Landsberger et al., 2012). With PTP stimulation, only a fraction of the current (denoted as σ) is returned to the adjacent, intra-cochlear electrodes and the remainder of the current (1− σ) is returned to the extra-cochlear electrodes (a fully TP configuration is denoted by σ = 1). Previous studies have shown that current spread can be significantly reduced for σ values >0.5 (Litvak et al., 2007; Bierer and Faulkner, 2010; Landsberger et al., 2012). However, even if current spread is reduced with PTP stimulation, the number of spectral channels transmitted is limited by the number of physical electrodes. With a 16-electrode array, PTP stimulation only allows for 14 spectral channels, as the most apical and basal electrodes can only be used as intra-cochlear grounds. While 14 independent spectral channels would be better than the 4–8 independent channels currently available to most CI users, 14 channels are still too few for difficult listening tasks (Shannon et al., 2004; Smith et al., 2002).
Typically, VCs are implemented by simultaneously stimulating two active intra-cochlear electrodes, and the extra-cochlear grounds are all in-phase, similar to MP stimulation (see Figure 1 -- D). For the remainder of this manuscript, these will be referred to as Monopolar Virtual Channels (MPVCs). Landsberger and Srinivasan (2009) proposed combining current steering and current focusing by using four electrode clusters, called Quadrupolar Virtual Channels (QPVCs), thereby increasing the number of spectral channels while reducing current spread. With QPVCs, four intra-cochlear electrodes are used (see Figure 1 – E). The middle two electrodes are stimulated in-phase and the proportion of current delivered to the basal electrode of the pair is denoted by α, similar to current steering with MPVCs. The outer two flanking electrodes are stimulated in opposite phase; the proportion of current delivered to each electrode is σ/2, and the remainder (1− σ) is returned to the extra-cochlear ground, similar to PTP stimulation (see Figure 1). Landsberger and Srinivasan (2009) found better VC discrimination with QPVCs than with MPVCs. Srinivasan et al. (2010) found that there was a sharper peak in the spread of excitation (measured with psychophysical forward masked excitation curves) with QPVCs than with MPVCs, which might explain better VC discrimination with QPVC stimulation.
Single-channel psychophysical metrics (e.g., electrode or rate discrimination) have not been shown to be strongly correlated with multi-channel speech perception measures (e.g., Hughes and Abbas, 2006; Zwolan et al., 1997). Thus, it not surprising that current steering via MPVCs has not been shown to significantly improve speech perception (Berenstein et al., 2008; Brendel et al., 2008; Donaldson et al., 2011), despite the increased number of single-channel pitch percepts (Firszt et al., 2007; Donaldson et al., 2005; Landsberger and Srinivasan, 2009; Donaldson et al., 2011). Multi-channel metrics, such as spectral ripple resolution (Henry and Turner, 2003; Henry et al., 2005; Won et al., 2007) and spectral modulation detection (Litvak et al., 2007) tend to have more robust correlations with speech perception, probably because multi-channel metrics more accurately account for channel interactions across electrodes than do single-channel measures. Therefore, while VC discrimination may be improved (Landsberger and Srinivasan, 2009) and the spread of excitation reduced with QPVC stimulation (Srinivasan et al., 2010), it remains to be seen if these benefits are maintained in a multi-channel context.
In this study, signal discrimination (VCs or physical electrodes) was measured with and without competing “distracter” stimuli on nearby electrodes, using MPVC or QPVC stimulation. Within a trial, signals and distracters were the same stimulation mode (either MPVC or QPVC), as would be presumably implemented in a signal processing strategy. The signal alone conditions effectively replicated the single channel results found in Landsberger and Srinivasan (2009). The addition of sequentially interleaved distracters extended the results to a parametrically controllable multi-channel context. As much of the channel interaction in a speech processing strategy stems from the sequential interleaving of the stimulated channels, the multi-channel stimulus described in this study is likely more relevant to performance through a speech processor as the patterns of stimulation are similar. We hypothesized that if QPVC stimulation significantly reduced current spread, VC discrimination would be better with QPVC stimulation, even with the distracters. If true, this might indicate that current-focusing effectively sharpens the “skirts” of the excitation pattern, increasing the “peakiness” of the spectral envelope. Such sharpening of the spectral envelope may benefit CI users in a signal processing strategy. If signal discrimination with MPVC discrimination is poorer with than without the distracters, this might explain why current steering (as in Fidelity 120) has not been shown to improve speech perception.
2. Methods
2.1 Subjects
Seven post-lingually deafened CI subjects implanted with the Advanced Bionics CII or HiRes90K device and the HiFocus 1J electrode array (none inserted with positioners) participated in the experiment. Relevant subject demographics are shown in Table 1. The experiment was conducted using the Bionic Ear Data Collection System (BEDCS version 1.17) with a custom-built MATLAB front-end. All subjects were paid for their participation and all provided informed consent in accordance with the local Institutional Review Board.
Table 1.
CI subject demographics.
| Subject | Gender | Age | Device Strategy Electrode Type | Duration of Deafness (yrs) Duration of CI use (yrs) |
|---|---|---|---|---|
| C1 | M | 77 | CII Fidelity 120 HiFocus |
1 / 8 |
| C3 | F | 53 | HR90K Fidelity 120 HiFocus |
11 / 5 |
| C4 | F | 62 | HR90K HiRes HiFocus |
6 / 5 |
| C7 | F | 60 | HR90K Fidelity 120 HiFocus |
45 / 5 |
| C8 | F | 62 | HR90K Fidelity 120 HiFocus |
1 / 3 |
| C9 | M | 67 | CII HiRes HiFocus |
58 / 9 |
| C14 | M | 44 | HR90K Fidelity 120 HiFocus |
1 / 6 |
2.2 Stimuli
Stimuli consisted of three interleaved biphasic pulse trains: one “signal” nd two “distracters.” The signal and distracter pulse trains were sequentially interleaved (see Figure 2). Each pulse train duration was 300 ms. The stimulation rate was 550 pulses per second per electrode (pps/e). The pulse phase duration was 226 μs. Within a stimulus, the stimulation mode was fixed for the signal and distracters (all MPVC or QPVC).
Figure 2.
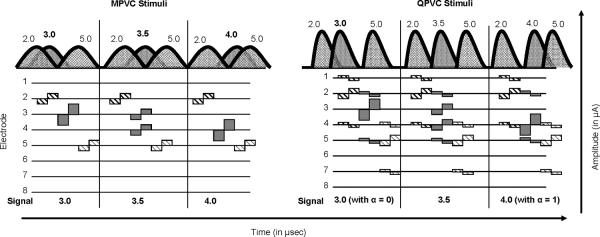
Illustration of current spread (top) and electrodograms (bottom) for MPVC (left panel) and QPVC stimuli (right panel). The current spread diagram illustrates the location of the distracter stimuli (hatched -- at locations 2.0 and 5.0) and the signal stimuli (gray shaded -- at location 3.0, 3.5 or 4.0). The electrodograms show the timing of the interleaved stimuli as well as stimulation patterns for current-steered and current- focused stimuli (QPVC only).
The signal was steered to 1 of 3 locations within the apical or middle portion of the array (see Figure 2). For the apical region, the signal was steered to locations 3.0, 3.5 or 4.0, where 3.0 and 4.0 refers to stimulation of electrode 3 or 4, respectively (α = 0.0 or 1.0), and 3.5 refers to a VC consisting of simultaneous stimulation of electrodes 3 and 4 (α = 0.5). For MPVC stimulation, 100% of current was returned to the extra-cochlear electrode (σ = 0.0). For QPVC stimulation, 75% of the current was returned to intra-cochlear electrodes 2 and 5 (37.5% to each), and 25% was returned to the extra-cochlear electrode (σ = 0.75). Similarly, signals were delivered to the middle cochlear region. The signal was steered to locations 7.0, 7.5, or 8.0, with fixed QPVC intra-cochlear return electrodes 6 and 9. Note that for subject C1, signal locations 9.0, 9.5 and 10.0 were used (using QPVC return electrodes 8 and 11) for the middle cochlear region because adequate loudness growth could not be obtained on electrode 7. The signals were presented at loudness-balanced, comfortably loud listening levels (see below for more details).
Distracters were presented adjacent to the signal locations. For the apical signal locations, distracters were presented on electrodes 2.0 and 5.0 (see Figure 2). For MPVC stimulation, 100% of the distracter current was returned to the extra-cochlear electrode (σ = 0.0). For QPVC stimulation, an α of 0 was used for each distracter. Thus, on electrode 2, 75% of the current was returned to intra-cochlear electrodes 1 and 4, and 25% was returned to the extra-cochlear electrode (σ = 0.75). Similarly, for the electrode 5 distracter, 75% of the current was returned to intra-cochlear electrodes 4 and 7, and 25% was returned to the extra-cochlear electrode (σ = 0.75). A similar distribution was used for the middle cochlear region. For the 7.0, 7.5 and 8.0 signal locations, the distracters were presented on electrodes 6 (with QPVC returns on electrodes 5 and 8) and 9 (with QPVC returns on electrodes 8 and 11). Although a PTP configuration could have been used for the distracters with return electrodes of 1 and 3, a QPVC configuration with α = 0 was used to maintain a consistent stimulation mode within each trial as a speech processing strategy would presumably also maintain a consistent stimulation mode. The distracters were presented at relatively soft and loud listening levels (see below).
2.3 Procedure
Dynamic range estimation and loudness balancing
Thresholds for the distracter stimuli were measured using a two-interval, forced-choice (2IFC) adaptive procedure (3-down/1-up), converging on the 79.4% correct level (Levitt, 1971). The procedure was terminated after 10 reversals; the first 2 reversals used a 1 dB step size, and the last 8 reversals used a 0.5 dB step size. The average of the last 6 reversals was considered the threshold for that run, and the average threshold across two runs was considered the stimulus threshold. Loudness growth for each signal and distracter was measured using the Advanced Bionics' 11-point loudness scale, where 0 corresponds to “No Sound”, 1 corresponds to “Barely Audible”, 6 corresponds to “Most Comfortable Level,” 8 corresponds to “Maximal Comfortable Level,” and 10 corresponds to “Very Uncomfortable.” Stimuli were initially played at 5 μA and raised in 5 μA steps (MPVC) or initially played at 30 μA and raised in 10 μA steps (QPVC) until the subject pointed to “Uncomfortable” (9 on the 11-point scale), then lowered in 5 μA (MPVC) or 10 μA (QPVC) steps until the subject pointed to “Maximal Comfort” (8 on the 11-point scale). The procedure was repeated at least 2 times to ensure that the “Maximal Comfort Level” (8 on the 11 point scale) was consistent, and the largest amplitude was taken to be the “Maximal Comfort Level.” The dynamic range (DR) was estimated to be the difference between the “Maximal Comfort Level” and threshold in μA.
Signal stimuli (i.e., locations 3.0, 3.5, and 4.0 in the apical region and 7.0, 7.5, and 8.0 in the middle region) were loudness balanced using a knob adjustment (Griffin PowerMate). Apical and middle signals were balanced in separate procedures. For the apical signals, channel 3.0 (using QPVC stimulation) was the reference; for the middle signals channel 7.0 (using QPVC stimulation) was the reference. MPVC signals were loudness balanced to QPVC reference signals. The reference was presented at the “Most Comfortable Level” (6 on the 11-point loudness scale). The reference and comparison stimuli were presented in an alternating manner, with a 300-ms inter-stimulus-interval (ISI). The subject used the knob to adjust the amplitude of the comparison stimulus until it was equally loud as the reference. This procedure was repeated a minimum of 6 times and the loudness-balanced level was taken as the average across runs. Thus, within each cochlear region (apical or middle), all QPVC and MPVC signal stimuli were equally loud.
Distracter stimuli were loudness balanced using the same procedure as above. For each cochlear region, the two distracters were loudness balanced separately. In the apical region, MPVC channel 2.0 was loudness balanced to QPVC channel 2.0 and MPVC channel 5.0 was loudness balanced to QPVC channel 5.0. Similarly, in the middle cochlear region, MPVC channel 6.0 was loudness balanced to QPVC channel 6.0 and MPVC channel 9.0 was loudness balanced to QPVC channel 9.0 (for subject C1, distracters in the middle region were channels 8.0 and 11.0, but the same procedure was used). Two distracter levels were also balanced in separate procedures. For the “soft” distracters, the reference level was 40% of the reference electrode DR with QPVC stimulation. For the “loud” distracters, the reference level was 70% of the reference electrode DR with QPVC stimulation. MPVC distracters were loudness balanced to QPVC references. Thus, within the apical and middle regions, and within the soft and loud distracter levels, all MPVC and QPVC distracter stimuli were equally loud. The reference levels for distracters were set by % DR in order to maintain roughly equivalent loudnesses of the distracters across subjects. Subjects subsequently confirmed that the soft distracters were at roughly 3–4 (“soft” to “medium soft”) and the loud distracters were at roughly 6–7 (“most comfortable” to “loud but comfortable”) on the AB loudness scale.
VC discrimination
VC discrimination was measured using a 3IFC procedure for three distracter conditions (no distracter, soft distracter, and loud distracter) at each cochlear region (apical or middle) and for both stimulation modes (MPVC or QPVC). Two intervals (randomly assigned) contained the reference stimulus (i.e., the same VC signal location) and the third interval contained the probe stimulus (i.e., a different VC signal location). The location of the distracters remained the same across intervals. The ISI was 300 ms. Subjects were asked to pick the interval that was different. VC discrimination was measured for each distracter and stimulation mode condition in separate procedures (i.e., VC discrimination was not directly compared across mode or distracter conditions). Within each condition, all signal locations were compared with each other 30 times to determine the proportion of correct responses for the three pairwise comparisons. Signal and distracters were amplitude-roved by ± 0.4 dB to further reduce any potentially confounding loudness cues.
3. Results
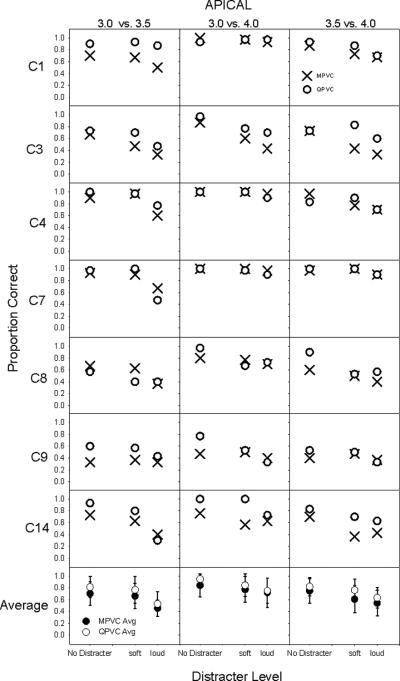
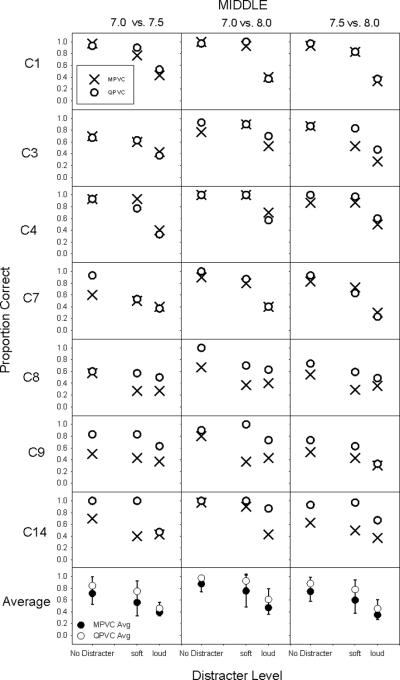
Figure 3 shows the proportion of correct responses for the apical region as a function of distracter level; each row shows individual subject data, each column shows the signal comparison (i.e. 3.0 vs 3.5, 3.0 vs 4.0 and 3.5 vs 4.0). The different symbols represent discrimination with MPVC and QPVC stimulation modes. Figure 4 shows similar data, but for the middle cochlear region. As expected, discrimination was poorer with the distracters than without. Distracter level seemed to have an inconsistent effect, strongly affecting discrimination for some subjects (C1 and C3 in the middle region) but not others (C7 and C8 in the apical region). While the loud distracter seemed to impact discrimination for most subjects, signal discrimination scores remained high (near 70% correct) for subjects C1, C4 and C7 in the apical region with QPVC stimulation. In general, signal discrimination with QPVC stimulation was as good as with MPVC stimulation, if not better.
Figure 3.
Proportion correct signal discrimination results for all subjects for the apical region. The left column shows the comparison between stimulation sites 3.0 and 3.5, the middle column shows the comparison between stimulation sites 3.0 and 4.0, and the right column shows the comparison between stimulation sites 3.5 and 4.0. The x-axis shows the three distracter levels and the y-axis shows the proportion of correct responses. The bottom row shows MPVC (filled symbols) and QPVC (open symbols) averages and standard deviations across all subjects for each of the comparisons.
Figure 4.
Similar plot as Figure 3, but for the middle region. Note that for subject C1, the signal was located between electrodes 9 and 10, and the distracters were located at locations 8.0 and 11.0.
Three-way repeated measures analyses of variance (RM ANOVAs) were performed for each cochlear region (apical and middle), with distracter level (no distracter, soft distracter or loud distracter), stimulation mode (MPVC or QPVC) and α-value (0 vs. 0.5, 0.5 vs. 1 or 0 vs. 1) as main effects. Apical and middle regions were analyzed separately as loudness was not balanced across cochlear regions. For both cochlear regions, there was a main effect of stimulation mode (F[1, 6] = 8.99, p<0.05 for the apical region and F[1, 6] = 8.49, p<0.05 for the middle region), indicating that QPVC stimulation yields significantly better discrimination than MPVC stimulation. There were also main effects of distracter level (F[2, 12] = 35.43, p<0.001 for the apical region and F[2, 12] = 36.34, p<0.001 for the middle region) and α-value/location (F[2, 12] = 22.72, p<0.001 for the apical region and F[2, 12] = 22.99, p<0.001 for the middle region) for both cochlear regions. There were no significant interactions. Multiple post-hoc t-tests with Bonferroni corrections showed significant differences between all distracter levels for both apical and middle cochlear regions, with the no distracter condition yielding the best performance, followed by the soft distracter condition and lastly the loud distracter condition. Likewise, multiple post-hoc t-tests with Bonferroni corrections showed that the α = 0 vs. α = 1 discriminations (i.e. 3.0 vs 4.0 or 7.0 vs 8.0) were significantly better than the α = 0 vs. α = 0.5 (i.e. 3.0 vs 3.5 or 7.0 vs 7.5) and the α = 0.5 vs. α = 1 (i.e. 3.5 vs 4.0 or 7.5 vs 8.0) discriminations. There was no significant difference between the α = 0 vs. α = 0.5 and the α = 0.5 vs. α = 1 discriminations. As adjacent electrodes in the Advanced Bionics HiFocus 1j electrode array are 1.1 mm apart, the results showed that discrimination with 1.1 mm spatial extent was better than discrimination with 0.55 mm extent.
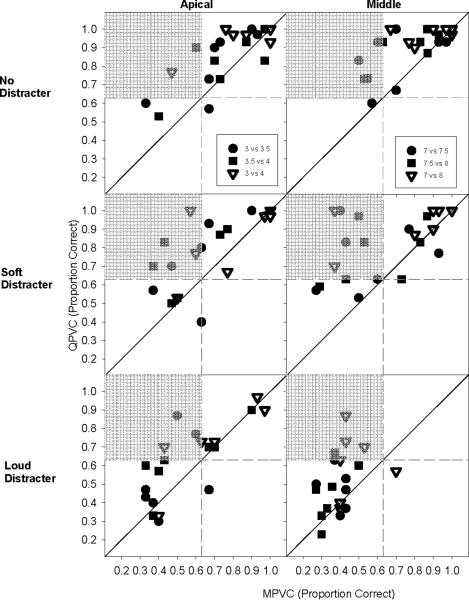
We compared stimulation modes across conditions (both distracter level and cochlear location), as shown in the scatter plots in Figure 5. Across plots, the majority of points are above the diagonal (where discrimination was equal for MPVC and QPVC stimulation), indicating that signal discrimination was generally better with QPVC than with MPVC stimulation. The vertical and horizontal dashed lines in each plot indicate discrimination at the d' = 1 level (63% correct) for MPVC and QPVC stimulation modes, respectively. The bottom left quadrant in each plot therefore represents stimuli that were not discriminable (d' < 1) with either stimulation mode. Similarly, points in the upper left quadrant (shaded) of each plot represent stimuli that were discriminable with QPVC stimulation (d' ≥ 1), but not with MPVC stimulation (d' < 1). The mean improvement for QPVC stimulation relative to MPVC stimulation across cochlear regions was 0.11 points with no distracter, 0.16 points with the soft distracters, and 0.88 points with the loud distracters (in units of proportion correct). With no distracter, many subjects performed near ceiling with both MPVC and QPVC stimulation, which may explain why there were smaller improvements observed with QPVC stimulation in the no distracter condition than in the soft distracter condition.
Figure 5.
QPVC discrimination as a function of MPVC discrimination, for the apical (left column) and middle cochlear regions (right column), and for the no distracter, soft distracter and loud distracter conditions (rows 1, 2 and 3, respectively). 1.1 mm comparisons (3 vs. 4; 7 vs. 8) are shown by the 7.5 vs. 8) are shown by the open symbols and 0.55 mm comparisons (3 vs. 3.5; 3.5 vs. 4; 7 vs. 7.5; 7.5 vs. 8) are shown by the filled symbols. The diagonal line indicates equivalent QPVC and MPVC discrimination performance. The vertical and horizontal dashed lines indicate discrimination where d' = 1 (63% correct) for MPVC and QPVC stimulation, respectively. The shaded regions indicate where d' was less than 1 with MPVC stimulation but better than 1 with QPVC stimulation.
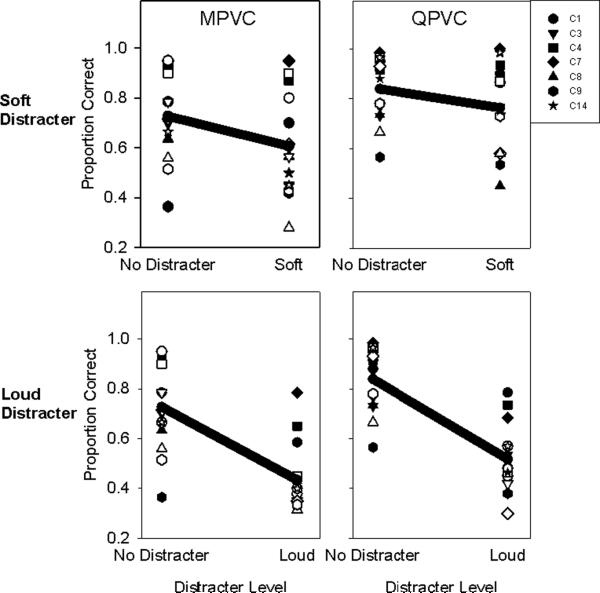
The data were re-plotted to show the effect of the distracters within each stimulation mode for α = 0.5 comparisons (i.e. 1 VC between the two adjacent electrodes). Figure 6 shows the decrease in proportion of correct responses from the no distracter condition with either the soft distracter (top row) or loud distracter (bottom row). Both individual data and average data are shown. In the no distracter condition, the mean proportion of correct MPVC responses is 0.72 and mean proportion of correct QPVC responses is 0.84. Proportion of correct responses falls to 0.61 and 0.76 (MPVC and QPVC respectively) in the soft distracter condition, and to 0.43 and 0.52 (MPVC and QPVC respectively) in the loud distracter condition. Two-tailed, paired t-tests showed that within a stimulation mode, drops in performance with the added distracters were all significant (t13 = 4.34, 2.52, 7.85, 7.99 respectively, with p<0.05).
Figure 6.
Change in performance from the no distracter to the soft (top row) and loud distracter conditions (bottom row), for all subjects and electrodes tested, for MPVC (left column) and QPVC (right column) stimulation modes. Symbols indicate individual subjects, with filled symbols representing apical electrode pairs and open symbols representing middle electrode pairs. The solid black line indicates the mean response.
4. Discussion
QPVC stimulation combines current steering (with VCs) and current focusing (similarly to TP/PTP stimulation). The intent is to provide a stimulation mode which improves spectral resolution by both stimulating in more locations spatially (through current steering) and to reduce channel interaction (through current focusing). Previous single-channel studies have shown that VC discrimination was better (Landsberger and Srinivasan, 2009) and that the local peaks of excitation within adjacent electrodes (1.1 mm spatial extent) was more sharply peaked with QPVC than with MPVC stimulation (Srinivasan et al., 2010). The results of the present study demonstrate that the benefits of QPVC stimulation (as observed by Landsberger and Srinivasan, 2009) persist in a multi-channel context. Specifically, the results showed that mean discrimination was better with current focusing (QPVC stimulation) than without (MPVC stimulation) in both single and multi-channel contexts, but that the benefit varied considerably across subjects.
4.1 Effect of current focusing
QPVC discrimination was better than MPVC discrimination at all distracter levels, demonstrating that current focusing improves target signal discrimination in both a single- and multi-channel context. QPVC stimulation may be most beneficial when spectral channels cannot be discriminated with MPVC stimulation (e.g., the shaded quadrants in Figure 5). Of particular note, there were 3 subjects who could not distinguish adjacent physical electrodes with MPVC stimulation but were able to discriminate the electrodes with QPVC stimulation (i.e., the loud distracter condition in the middle cochlear region, as shown in the bottom right panel of Figure 5 with the open symbols). Although 0.55 mm discrimination was not improved (i.e. the 3.0 vs 3.5 or 3.5 vs 4.0 comparisons), the improved discrimination of the physical electrodes (1.1 mm) might also improve the functional spectral resolution for these subjects. Conversely, subjects who exhibited “floor effects” and could not discriminate signals with either stimulation mode might not benefit from the small improvements in discrimination with QPVCs (lower left quadrants of Figure 5). In the same vein, it is possible that for subjects who exhibited strong ceiling effects with either stimulation mode and consequently showed little improvement with QPVC stimulation (upper right quadrants of Figure 5), stronger benefits might have been observed for smaller α value comparisons. Indeed, the present “no distracter” condition replicated the experiments in Landsberger and Srinivasan (2009), but with a smaller subset of α-values. In that study, no large ceiling effects were observed, as α-steps of 0.2 were tested (compared to 0.5 in the present study).
In this experiment, subjects were essentially asked to discriminate shifts in a spectral profile. In the no distracter condition, the spectral profile was very simple, and the stimulus allowed full access to the “skirts” of the signal. In the soft and loud distracter conditions, the competing distracter stimuli might have obstructed the shift in the skirt of the signal. It is unclear whether subjects discriminated the signal by attending to shifts in the peak of excitation or at the edges of excitation. Current focusing may have sharpened the skirts of excitation of the distracters, allowing a more complete glimpse of the signal within the spectral envelope. In essence, current focusing might enhance the peak to valley contrasts of the spectral envelope. This would be a step towards restoring the sharper functional tuning inherent in normal hearing listeners. Although the present experiment used QPVC stimulation (combining current steering and current focusing), the benefits of current focusing, particularly for subjects who were able to discriminate physical electrodes in the presence of competing stimuli with QPVC stimulation, might be achieved at least in part with TP/PTP stimulation.
4.2 Effect of distracters
As expected, target signal discrimination declined significantly in the presence of the distracters regardless of stimulation mode. However, the effect of distracters was more pronounced with MPVC stimulation than with QPVC stimulation. In many cases, MPVCs that could easily be discriminated when presented in isolation could not be discriminated (at the d' > 1 level) with concurrent stimulation on adjacent electrodes. Figure 6 shows that VC discrimination deteriorates from a single-channel context to a multi-channel context (soft and loud distracters, top and bottom rows, respectively), for all subjects, cochlear regions and stimulation modes. For the no distracter condition, mean MPVC discrimination was > 70% correct, indicating that in isolation, at least one VC was discriminable between adjacent electrodes. This result is in agreement with Firszt et al. (2007) who found that more than 60% of ears in the apical and middle regions could discriminate at least one VC. However, in the loud distracter condition, average MPVC discrimination fell to near chance level (see Figure 6).
The effect of the loud distracters on signal discrimination was quite strong for the middle cochlear region (bottom right column, Figure 5), as discrimination d' was < 1 in 20/21 cases with MPVC stimulation. The deterioration of MPVC signal discrimination ability in the presence of distracters (shown in the present data) may shed light on why MPVCs offer little advantage in speech perception over standard CIS processing using physical electrodes (e.g., Berenstein et al., 2008; Brendel et al., 2008; Donaldson et al., 2011), despite promising single-channel psychophysical data (e.g,, Donaldson et al., 2005, 2011; Firszt et al, 2007).
4.3 Inter-subject Variability
While mean MPVC and QPVC signal discrimination declined with increasing distracter level, individual subjects' susceptibility to the distracters varied considerably. For example, signal discrimination in the apical region was relatively unchanged across distracter levels for subjects C8 and C9, for both the MPVC and QPVC stimulation modes (see Figure 3). Signal discrimination in the middle region declined sharply for subjects C1 and C7 as the distracter level increased from soft to loud (see Figure 4). Also, while mean signal discrimination was better with QPVC than with MPVC stimulation for all distracter levels, the benefit for individual subjects and/or experimental conditions was highly variable. For example, signal discrimination in the middle region was considerably better with QPVC stimulation for subjects C8, C9 and C14, but not for subjects C1 and C4. The variability in the data may be due to several sources. For example, some subjects performed at ceiling with both stimulation modes and thus could not benefit from QPVC stimulation (e.g., C1 and C4 performance in the no distracter and soft distracter conditions in the middle region).
Another source of variability in the data may be the geometry of the electrodes relative to the neural population (i.e., distance of the electrode to the nerve) and the health of the electrode-neural interface (e.g., the degree of spiral ganglion cell survival, ossification, etc.). Litvak et al. (2007) modeled the reduction in current spread with TP/PTP stimulation for electrodes positioned close and far from the nerve. They found that while electrodes close to the nerve exhibited narrower current spread regardless of stimulation mode, electrodes farther from the nerve exhibited a greater reduction in current spread with TP/PTP stimulation relative to MP stimulation. This suggests that QPVC stimulation might not produce a large reduction in current spread when electrodes are very close to the nerve, which in turn might not improve signal discrimination with QPVC stimulation, relative to MPVC stimulation. Electrodes at a larger distance from the nerve might exhibit greater differences between stimulation modes, with better signal discrimination with QPVC than with MPVC stimulation, but with possibly lower performance for both stimulation modes compared to performance when the electrode is close to the nerve. Bierer and Faulkner (2010) found that psychophysical tuning curves can differ considerably in width for both MP and PTP stimulation, even within the same subject. Exceptionally broad tuning curves might indicate “dead regions,” with very sparse spiral ganglion cell survival and presumably poor spatial selectivity. If the electrodes tested in the present experiment were near dead regions, VC discrimination would be expected to be poor, regardless of stimulation mode. Conversely, if the electrodes tested in this experiment were near regions of high spiral ganglion cell survival, subjects might have achieved a ceiling signal discrimination performance with both stimulation modes. Thus, for both MPVC and QPVC stimulation, spectral resolution may be limited by the electrode geometry and pattern of neural survival.
4.4 Relationships to other multi-channel metrics and to speech perception
We are ultimately interested in whether QPVC stimulation provides a benefit in speech perception relative to MPVC stimulation. Although previous single-channel studies have shown that QPVC stimulation increases the number of discriminable steps between adjacent electrodes, (Landsberger and Srininvasan, 2009), single channel psychophysical measures of spectral discrimination often do not predict the multi-channel performance we are attempting to improve. Studies relating single channel psychophysical metrics (e.g. electrode discrimination or pitch ranking) to speech perception have had mixed results, with some studies (e.g. Hughes and Abbas, 2006; Zwolan et al., 1997) finding no relationship and other studies (e.g. Henry et al., 2000; Nelson et al., 1995; Throckmorton and Collins, 1999) finding significant correlations.
Multi-channel measures of spectral sensitivity such as spectral ripple resolution (e.g. Henry et al., 2005) and spectral modulation detection (Litvak et al., 2007) have been more consistently correlated to speech performance. Such multi-channel measures may more reliably predict speech performance because, in stimulating the entire array, they incorporate the effects of channel interaction. The present study, which incorporates channel interaction through the interleaving of competing distracter stimuli, shows that spectral envelope discrimination is improved with current focusing. Because the stimuli used in the present experiment incorporate channel interaction, it is likely that performance in the present experiment will predict speech performance similarly to spectral ripple discrimination or spectral modulation detection experiments.
Although other multi-channel stimuli have shown correlations with speech perception, the multi-channel stimuli in this study differ from previous stimuli in a few important ways. Typically (e.g., Henry et al., 2005; Litvak et al., 2007), speech perception and a corresponding psychophysical measure of spectral resolution are both measured using CI subjects' clinical processors and associated settings (e.g., frequency allocation, amplitude mapping, etc.). As a result, the performance on the two tests is not independent. For example, a poorly mapped parameter (e.g. dynamic range, automatic gain control, frequency allocation tables) will limit performance similarly on both tasks. The present study bypassed subjects' clinical processors and presented stimuli directly through the BEDCS system. As a result, the variability in performance across subjects is less likely to be influenced by clinical processor parameters. If the present results were to be related to speech perception, the correlation might suffer as consistent effects of a fixed clinical map will be eliminated. Another difference is that the present experiment provides localized measures of spectral resolution whereas spectral ripple discrimination and detection typically measure performance across large section of the array. Spectral ripple tasks can potentially be performed by only attending to the “best” region (where electrode position and neural survival are optimal). As such, spectral resolution may be over-estimated in those studies. By comparing the multi-channel VC discrimination scores in the present study (measured in localized cochlear regions) to speech perception results, we might be able to determine which spectral regions are most critical for speech perception.
4.5 Implications for CI signal processing
Due to the improved spectral envelope discrimination, QPVC stimulation within a CI signal processing strategy might benefit detection of formant shifts and transitions. QPVC stimulation may sharpen the spectral envelope and enhance spectral peaks and valleys. Loizou and Poroy (2001) showed that CI listeners typically require a 4–6 dB spectral contrast in vowels for accurate identification, while NH listeners only require 1 dB of contrast (as is typically observed across formants in the unprocessed acoustic signal). If QPVC stimulation sufficiently sharpens the spectral envelope, CI users may better perceive weak spectral cues at lower contrasts which might improve speech understanding. Current steering may also allow for better coding of formant transitions. Luo et al. (2010) recently showed that CI subjects could use MPVC stimulation to detect spectral transitions between physical electrodes, when presented in a single-channel context. By sharpening the spectral envelope, QPVC stimulation might provide even greater benefit, and the present multi-channel data suggests that the benefits may extend to more complex, speech-like stimuli.
The present study demonstrated that signal discrimination, whether between physical electrodes (α = 1) or virtual channels (α = 0.5), is sensitive to competing stimuli on nearby electrodes. This implies that MPVCs may not provide additional spectral cues beyond the physical electrodes when implemented in a speech processing strategy (e.g., Fidelity 120), due to channel interactions. The present data showed that QPVC stimulation was less susceptible to distracter stimuli, and therefore may be more robust to channel interactions. The crucial test for the viability of QPVC stimulation will be speech perception, particularly in noise. The present data suggests that QPVC stimulation may provide better speech performance than MPVC stimulation, at least for some CI patients.
Although the present study demonstrates improved spectral resolution with QPVC stimulation, there are complications with implementing a speech processing strategy utilizing QPVC stimulation. QPVC (and TP) stimulation requires ground electrodes on the apical and basal edges of the multi-electrode configuration. As a result, the most apical and basal electrodes (1 and 16 respectively for the Advanced Bionics device) can only be used as grounds with a strategy implementing only QPVC (or TP) stimulation. Effectively, this limits the range of place of stimulation to be only the cochlear extent which corresponds to electrodes 2 through 15. In order to prevent a reduction in the range of spectral information, a clinical implementation of a QPVC (or TP) strategy would require an alternate stimulation mode (such as MP or BP) for electrodes 1 and 16. The spatial extent could be extended even further by using “phantom” electrode stimulation (Saoji and Litvak, 2010) on electrodes 1 and 16.
Another limitation of QPVC stimulation (or TP) is that more current is required to maintain a fixed loudness than with MP (or MPVC) stimulation. In order to remain within device compliance, longer phase durations are required to reach adequate loudness. Ultimately, phase duration (and the number of channels presented in a sweep) limit the stimulation rate. Using the 226 μsec phase duration tested in this experiment, a 14-channel strategy would have a stimulation rate of only 158 pps/e. However, a more reasonable phase duration (128 μsec) combined with 8 maxima in an n-of-m strategy would allow a stimulation rate of 488 pps/e as discussed in Landsberger and Srinivasan (2009). Although a rate of 488 pps/e is lower than what is typically used clinically, studies (e.g. Vandali et al., 2000; Holden et al., 2002) have shown no consistent or significant or substantial effect of higher stimulation rates. Patients using SPEAK (at 250 pps/e; Whitford et al., 1995) in the Nucleus device or Crystalis (at 500 pps/e; Lazard et al., 2010) in the Neurelec device perform quite well with similarly low stimulation rates. Furthermore, the need for temporal resolution is greatly reduced when adequate spectral information is presented (e.g. Fu et al., 2005; Xu et al., 2005).
A further limitation with QPVC stimulation (as well as other current focusing stimulation modes) is that multiple current sources are required which can independently stimulate simultaneously in or out of phase. The only device presently on the market that meets this requirement (and therefore is capable of implementing current focusing) is the Advanced Bionics HR90K device. The Med-El i100 platform (e.g. Sonata, Pulsar and Concerto) has the ability to simultaneously stimulate on multiple electrodes, but cannot stimulate simultaneously out-of-phase. As a result, the i100 devices can create MPVC stimulation, but not QPVC stimulation. All commercial devices from Cochlear have only one current source and therefore are limited to stimulation in MP, BP, or common ground (CG) stimulation modes. However, recent research from Cochlear has indicated that they are investigating properties of current focusing with independent current sources (e.g. van den Honert and Kelsall, 2007) suggesting that future iterations of their device might have this capability.
Current focusing improves signal discrimination in a multi-channel context.
Competing stimuli on nearby electrodes reduce signal salience.
Ineffectiveness of virtual channels may be explained by these data.
Acknowledgements
This work was supported by NIDCD Grants and Fellowship Numbers:. R01-DC-001526, R03-DC-010064, and F31 DC011205-01. We gratefully acknowledge the CI subjects who participated in this study. We also thank John J. Galvin III for editorial help.
Abbreviations
- (CI)
Cochlear Implant
- (VC)
Virtual Channel
- (MPVC)
Monopolar Virtual Channel
- (QPVC)
Quadrupolar Virtual Channel
- (CIS)
Continuous Interleaved Sampling
- (MP)
Monopolar
- (TP)
Tripolar
- (PTP)
Partial Tripolar
- (BEDCS)
Bionic Ear Data Collection System
- (pps/e)
Pulses per second per electrode
- (2IFC)
Two-interval forced choice
- (DR)
Dynamic range
- (ISI)
Inter-stimulus interval
- (RM ANOVA)
Repeated measures analysis of variance
- (NH)
Normal hearing
- (AGC)
Automatic gain control
- (CG)
Common ground
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Berenstein CK, Mens LHM, Mulder JJS, Vanpouke FJ. Current Steering and Current Focusing in Cochlear Implants: Comparison of Monopolar, Tripolar, and Virtual Channel Electrode Configurations. Ear Hear. 2008;29:250–260. doi: 10.1097/aud.0b013e3181645336. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Faulkner KF. Identifying cochlear implant channels with poor electrode-neuron interface: partial tripolar, single-channel thresholds and psychophysical tuning curves. Ear Hear. 2010;31:247–58. doi: 10.1097/AUD.0b013e3181c7daf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer JA, Middlebrooks JC. Auditory cortical images of cochlear-implant stimuli: dependence on electrode configuration. J Neurophysiol. 2002;87:478–92. doi: 10.1152/jn.00212.2001. [DOI] [PubMed] [Google Scholar]
- Brendel M, Buechner A, Krueger B, Frohne-Buechner C, Lenarz T. Evaluation of the Harmony soundprocessor in combination with the speech coding strategy HiRes 120. Otol Neurotol. 2008;29:199–202. doi: 10.1097/mao.0b013e31816335c6. [DOI] [PubMed] [Google Scholar]
- Briaire JJ, Frijns JH. Field patterns in a 3D tapered spiral model of the electrically stimulated cochlea. Hear Res. 2000;148:18–30. doi: 10.1016/s0378-5955(00)00104-0. [DOI] [PubMed] [Google Scholar]
- Busby PA, Battmer RD, Pesch J. Electrophysiological spread of excitation and pitch perception for dual and single electrodes using the Nucleus Freedom cochlear implant. Ear Hear. 2008;29:853–64. doi: 10.1097/AUD.0b013e318181a878. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Kreft HA, Litvak L. Place-pitch discrimination of single-versus dual-electrode stimuli by cochlear implant users (L) J Acoust Soc Am. 2005;118:623–6. doi: 10.1121/1.1937362. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Dawson PK, Borden LZ. Within-subjects comparison of the HiRes and Fidelity120 speech processing strategies: speech perception and its relation to place-pitch sensitivity. Ear Hear. 2011;32(2):238–250. doi: 10.1097/AUD.0b013e3181fb8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firszt JB, Koch DB, Downing M, Litvak L. Current steering creates additional pitch percepts in adult cochlear implant recipients. Otol Neurotol. 2007;28:629–36. doi: 10.1097/01.mao.0000281803.36574.bc. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–63. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Chinchilla S, Nogaki G, Galvin JJ., 3rd Voice gender identification by cochlear implant users: the role of spectral and temporal resolution. J Acoust Soc Am. 2005;118:1711–8. doi: 10.1121/1.1985024. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Nogaki G. Noise susceptibility of cochlear implant users: the role of spectral resolution and smearing. J Assoc Res Otolaryngol. 2005;6:19–27. doi: 10.1007/s10162-004-5024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Shannon RV, Wang X. Effects of noise and spectral resolution on vowel and consonant recognition: acoustic and electric hearing. J Acoust Soc Am. 1998;104:3586–96. doi: 10.1121/1.423941. [DOI] [PubMed] [Google Scholar]
- Henry BA, McKay CM, McDermott HJ, Clark GM. The relationship between speech perception and electrode discrimination in cochlear implantees. J Acoust Soc Am. 2000;108:1269–80. doi: 10.1121/1.1287711. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW. The resolution of complex spectral patterns by cochlear implant and normal-hearing listeners. J Acoust Soc Am. 2003;113:2861–73. doi: 10.1121/1.1561900. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: normal hearing, hearing impaired, and cochlear implant listeners. J Acoust Soc Am. 2005;118:1111–21. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- Holden LK, Skinner MW, Holden TA, Demorest ME. Effects of stimulation rate with the Nucleus 24 ACE speech coding strategy. Ear Hear. 2002;23:463–76. doi: 10.1097/00003446-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Abbas PJ. The relation between electrophysiologic channel interaction and electrode pitch ranking in cochlear implant recipients. J Acoust Soc Am. 2006;119:1527–37. doi: 10.1121/1.2163273. [DOI] [PubMed] [Google Scholar]
- Landsberger DM, Srinivasan AG. Virtual channel discrimination is improved by current focusing in cochlear implant recipients. Hear Res. 2009;254:34–41. doi: 10.1016/j.heares.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger DM, Padilla M, Srinivasan AG. Reducing current spread using current focusing in cochlear implant users. Hear Res. 2012 doi: 10.1016/j.heares.2011.12.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazard DS, Bordure P, Lina-Granade G, Magnan J, Meller R, Meyer B, Radafy E, Roux PE, Gnansia D, Pean V, Truy E. Speech perception performance for 100 post-lingually deaf adults fitted with Neurelec cochlear implants: Comparison between Digisonic(R) Convex and Digisonic(R) SP devices after a 1-year follow-up. Acta Otolaryngol. 2010;130:1267–73. doi: 10.3109/00016481003769972. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(Suppl 2):467+. [PubMed] [Google Scholar]
- Litvak LM, Spahr AJ, Emadi G. Loudness growth observed under partially tripolar stimulation: model and data from cochlear implant listeners. J Acoust Soc Am. 2007;122:967–81. doi: 10.1121/1.2749414. [DOI] [PubMed] [Google Scholar]
- Litvak LM, Spahr AJ, Saoji AA, Fridman GY. Relationship between perception of spectral ripple and speech recognition in cochlear implant and vocoder listeners. J Acoust Soc Am. 2007;122:982–91. doi: 10.1121/1.2749413. [DOI] [PubMed] [Google Scholar]
- Loizou PC, Poroy O. Minimum spectral contrast needed for vowel identification by normal hearing and cochlear implant listeners. J Acoust Soc Am. 2001;110:1619–27. doi: 10.1121/1.1388004. [DOI] [PubMed] [Google Scholar]
- Luo X, Landsberger DM, Padilla M, Srinivasan AG. Encoding pitch contours using current steering. J Acoust Soc Am. 2010;128:1215–23. doi: 10.1121/1.3474237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Van Tasell DJ, Schroder AC, Soli S, Levine S. Electrode ranking of “place pitch” and speech recognition in electrical hearing. J Acoust Soc Am. 1995;98:1987–99. doi: 10.1121/1.413317. [DOI] [PubMed] [Google Scholar]
- Saoji AA, Litvak LM, Hughes ML. Excitation patterns of simultaneous and sequential dual-electrode stimulation in cochlear implant recipients. Ear Hear. 2009;30:559–67. doi: 10.1097/AUD.0b013e3181ab2b6f. [DOI] [PubMed] [Google Scholar]
- Saoji AA, Litvak LM. Use of “phantom electrode” technique to extend the range of pitches available through a cochlear implant. Ear Hear. 2010;31:693–701. doi: 10.1097/AUD.0b013e3181e1d15e. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–4. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Fu QJ, Galvin J., 3rd The number of spectral channels required for speech recognition depends on the difficulty of the listening situation. Acta Otolaryngol Suppl. 2004:50–4. doi: 10.1080/03655230410017562. [DOI] [PubMed] [Google Scholar]
- Smith ZM, Delgutte B, Oxenham AJ. Chimaeric sounds reveal dichotomies in auditory perception. Nature. 2002;416:87–90. doi: 10.1038/416087a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RL, Bierer JA, Middlebrooks JC. Topographic spread of inferior colliculus activation in response to acoustic and intra-cochlear electric stimulation. J Assoc Res Otolaryngol. 2004;5:305–22. doi: 10.1007/s10162-004-4026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelman FA, Pfingst BE, Clopton BM, Jolly CN, Rodenhiser KL. Effects of electrical current configuration on potential fields in the electrically stimulated cochlea: field models and measurements. Ann Otol Rhinol Laryngol Suppl. 1995;166:131–6. [PubMed] [Google Scholar]
- Srinivasan AG, Landsberger DM, Shannon RV. Current focusing sharpens local peaks of excitation in cochlear implant stimulation. Hear Res. 2010;270:89–100. doi: 10.1016/j.heares.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throckmorton CS, Collins LM. Investigation of the effects of temporal and spatial interactions on speech-recognition skills in cochlear-implant subjects. J Acoust Soc Am. 1999;105:861–73. doi: 10.1121/1.426275. [DOI] [PubMed] [Google Scholar]
- Vandali AE, Whitford LA, Plant KL, Clark GM. Speech perception as a function of electrical stimulation rate: using the Nucleus 24 cochlear implant system. Ear Hear. 2000;21:608–24. doi: 10.1097/00003446-200012000-00008. [DOI] [PubMed] [Google Scholar]
- van den Honert C, Kelsall DC. Focused intracochlear electric stimulation with phased array channels. J Acoust Soc Am. 2007;121:3703–16. doi: 10.1121/1.2722047. [DOI] [PubMed] [Google Scholar]
- Whitford LA, Seligman PM, Everingham CE, Antognelli T, Skok MC, Hollow RD, Plant KL, Gerin ES, Staller SJ, McDermott HJ, Gibson WR, Clark GM. Evaluation of the Nucleus Spectra 22 processor and new speech processing strategy (SPEAK) in postlinguistically deafened adults. Acta Otolaryngol. 1995;115:629–37. doi: 10.3109/00016489509139378. [DOI] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Rubinstein JT. Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. J Assoc Res Otolaryngol. 2007;8:384–92. doi: 10.1007/s10162-007-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Thompson CS, Pfingst BE. Relative contributions of spectral and temporal cues for phoneme recognition. J Acoust Soc Am. 2005;117:3255–67. doi: 10.1121/1.1886405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolan TA, Collins LM, Wakefield GH. Electrode discrimination and speech recognition in postlingually deafened adult cochlear implant subjects. J Acoust Soc Am. 1997;102:3673–85. doi: 10.1121/1.420401. [DOI] [PubMed] [Google Scholar]