Abstract
Therapeutic angiogenesis aims at treating ischemic diseases by generating new blood vessels from existing vasculature. It relies on delivery of exogenous factors to stimulate neovasculature formation. Current strategies using genes, proteins and cells have demonstrated efficacy in animal models. However, clinical translation of any of the three approaches has proved to be challenging for various reasons. Administration of angiogenic factors is generally considered safe, according to accumulated trials, and offers off-the-shelf availability. However, many hurdles must be overcome before therapeutic angiogenesis can become a true human therapy. This article will highlight protein-based therapeutic angiogenesis, concisely review recent progress and examine critical challenges. We will discuss growth factors that have been widely utilized in promoting angiogenesis and compare their targets and functions. Lastly, since bolus injection of free proteins usually result in poor outcomes, we will focus on controlled release of proteins.
Blood vessels that carry oxygen, nutrients, cells and signals are critical in both developmental and adult physiology. Without sufficient blood supply, tissues and organs cannot maintain regular activities. On the other hand, induction of neovasculature provides a potential strategy to treat many ischemic illnesses, especially cardiovascular diseases (CVDs) including coronary and peripheral arterial diseases. The morbidity, mortality and cost of CVDs are highlighted by the latest statistics from the American Heart Association [1]: an estimated 82,600,000 American adults (≥20 years old) have one or more types of CVDs; CVDs caused 813,804 of all 2,243,712 deaths (33.6%) or one of every 2.9 deaths in 2007, and coronary heart disease caused approximately one of every six deaths. The direct and indirect cost of CVD was estimated to be US$286 billion in 2007. The amount is higher than the $228 billion spent on cancer and benign neoplasms. As a potential therapy to revascularize ischemic tissues, including ischemic heart, therapeutic angiogenesis has drawn much attention in the last 20 years.
Neovasculature can be obtained by three approaches based on different mechanisms: promoting expression of angiogenic genes, supplying potent angiogenic factors and delivering progenitor or stem cells. No matter which approach is used, angiogenic activity has to be precisely controlled in order to achieve stable vascularization. For cell delivery, the potential and challenges of progenitor or stem cells to treat CVDs are discussed in detail [2,3]. For inducing neovasculature using angiogenic genes or growth factors, design of an effective delivery strategy is critical. Several reviews are available for gene therapy approaches [4,5]. Reviews on general strategies for growth factor delivery have been published in the last few years [6,7]. The goal of this perspective is to highlight recent developments in therapeutic angiogenesis using controlled delivery of growth factors. We will start with an overview of therapeutic angiogenesis and then focus on delivery vehicles of angiogenic proteins.
Vascularization
Blood vessel formation (vascularization) is highly coordinated and requires different types of cells and associated signaling molecules to be accomplished. Insufficient vascularization leads to ischemic conditions that inhibit tissue growth or survival, whereas excessive abnormal angiogenesis can promote tumor progression and other diseases such as macular degeneration. Three processes can lead to neovasculature: vasculogenesis, angiogenesis and arteriogenesis. Vasculogenesis, which often occurs in early development, is initiated by mesodermal stem cells that differentiate in situ to angioblasts and then to endothelial cells. Vasculogenesis gives rise to the primitive circulatory system, including the heart and capillary plexuses, and is followed by angiogenesis. Angiogenesis is a process that includes sprouting and intussusceptive growth of pre-existing blood vessels, and subsequent remodeling and maturation to form neovasculature [8]. Arteriogenesis usually takes place when main arteries, such as coronary and femoral arteries, are occluded. It involves generation of neovasculature from pre-existing collateral arteries. These pre-existing arteries may carry no significant function originally but are able to proliferate rapidly upon stimulation and bypass the occulted region to provide perfusion.
Unlike vasculogenesis, which happens primarily during the embryonic stage, angiogenesis and arteriogenesis frequently occur during adulthood. As long as there are active vessels, new vasculature can be generated following biophysical and physiological cues in the environment, of which the most highly studied are angiogenic factors. Notable angiogenic factors are VEGF, angiopoietin, TGF, FGF, HGF and PDGF, although dozens of other proteins are also known to participate in blood vessel formation. The importance of several angiogenic factors has been revealed by gene knockout, resulting in embryonic lethality [9,10].
Under normal physiological conditions, most secretory growth factors are associated with components of the extracellular matrix (ECM) including heparan sulfate proteoglycan and fibronectin [11]. Physiological fluctuations, such as insufficient nutrients and low oxygen concentration (hypoxia), induce the release of proangiogenic factors via cleavage of ECM by hydrolytic enzymes. A chemical gradient is created as released factors diffuse away in the surrounding tissue. Several downstream angiogenic processes are driven by this gradient, including activation of additional angiogenic genes, proteolytic degradation of the ECM, proliferation and migration of endothelial cells to form tubes, and recruitment of mural cells that stabilize the nascent vessels. The molecular and mechanical signals involved interact closely to generate mature vasculature. More detailed regulatory pathways during vessel maturation are discussed in an excellent review by Jain [12].
Therapeutic angiogenesis
Therapeutic angiogenesis was pioneered by JM Inser. His seminal work observing revascularization in response to VEGF165, an isoform of VEGF, injection in a rabbit hindlimb ischemia model demonstrated that single factor administration can generate functional blood vessels [13]. Subsequently, several other angiogenic factors have been utilized alone or combined to promote angiogenesis. Contrary to cancer treatment where drugs are selected to inhibit blood vessel growth, angiogenic therapy aims to develop neovasculature through various strategies. Therapeutic angiogenesis has been widely examined for treatment of many human diseases. Other processes, such as wound healing and organ repair and regeneration, also depend on a sufficient blood supply. Strategies for therapeutic angiogenesis include gene delivery, protein delivery and cell delivery. So far, no therapeutic angiogenesis treatment has been approved by the US FDA.
Gene therapy
Gene delivery can lead to high protein production efficiency providing a sustained source of protein. Furthermore, genes carried by viral vectors such as adeno-associated virus, lenti-virus and retrovirus are integrated into the host chromosome, resulting in expression of angiogenic genes even after cell division. However, this is not always desirable, as overproduction of a certain angiogenic factor could inhibit blood vessel formation, which requires a precise balance between different signals. One prior study revealed that unregulated overproduction of VEGF in the myocardium caused heart failure and vascular tumor formation [14]. Viral vectors, which are the most effective vehicle to transfer genes, can also trigger immune responses against viral components [15]. Other gene transfer methods using nonviral vectors, such as synthetic polymers, ultrasound and electric field, are less efficient and require further optimization [16,17]. Several clinical trials of gene therapy have been conducted, mostly applying adenovirus or plasmids to deliver vegf or fgf [18]. The outcomes of Phase I/II trials support the safety of angiogenic gene delivery, but the efficacy has not been concluded yet. Moreover, one Phase III trial investigating patients with critical limb ischemia that received the FGF1-expressing plasmid failed to demonstrate benefit. Another Phase III trial utilizing an adenoviral vector to express FGF4 in patients with angina also revealed no difference from the placebo group [19]. The discrepancy is possibly caused by variations in patient selection criteria and assessment methods.
Cell therapy
Cell-based therapy is another active field that is drawing much attention. Compared with single factor therapies, cell therapy is believed to have a more comprehensive and extensive effect. Cell selection is based on the capability of the candidate cells to differentiate into blood vessel-associated cells or secrete proangiogenic factors. Several stem/progenitor cells, such as bone marrow cells and endothelial progenitor cells, had been identified, isolated and applied in clinical trials with myocardial infarction (MI) patients [20]. The results indicate multiple benefits highlighted by improved blood perfusion and cardiac function. However, acquiring enough cells, on the order of millions per patient, is a significant challenge and their in vivo viability is usually very low. Most cells fail to integrate with the host tissue and die soon after delivery. In recent years, induced pluripotent stem cells generated by activating key transcriptional factors, provide an attractive avenue for cell therapy [21]. The beauty of this technique is that terminally differentiated cells can be reprogrammed back to an embryonic-like state and then differentiated to a desired cell type under specific culture conditions [22]. Autologous cells are used, which avoids many difficulties such as limited cell sources and immune rejection. However, the most effective way to achieve pluri-potency is based on viral vectors that carry similar safety concerns to gene therapy. As a very young technology, induced pluripotent stem-cell therapy still requires significant investigation followed by validation in clinical trials before its potential can be accurately evaluated [23].
Protein therapy
Protein delivery is the most straightforward strategy for therapeutic angiogenesis. Blood vessel formation is induced by simple injection of angiogenic proteins, with the extent of angiogenesis being controlled by dosage and duration of release. Compared with gene- and cell-based therapies, protein delivery is thought of as an ‘off-the-shelf’ treatment. Protein production and purification are fairly established; it can be stored in the lyophilized form and reconstituted easily in a buffer [24]. Most well-known angiogenic factors are now commercially available and widely used in research. All of these factors contribute to the advantages of angiogenic protein therapy. Nevertheless, maintaining angiogenic activity at a specific site of interest is a significant challenge. Angiogenic protein, such as viral vectors or cells, can be delivered either systemically or locally. Systemic administration, such as intravenous or intraperitoneal injection, is performed easily but is inefficient at targeting a desired tissue. Furthermore, most of the injected protein is rapidly cleared by the mononuclear phagocyte system with only a small fraction reaching the desired location [25]. As a result, high dosage and repeated injections are usually required [26]. Local administration, such as intracoronary, intramyo-cardial or intracerebral injection, can better confine the distribution of the protein but usually requires a special device (pumps) or highly invasive surgery [27]. Moreover, the delivered protein needs protection from proteolytic degradation [28].
Angiogenic factors
VEGF
The VEGF family is the most widely studied and one of the most crucial modulators involved in several steps of neovascularization [29]. There are five members discovered in the human VEGF family including VEGF-A (vascular permeability factor), VEGF-B, VEGF-C, VEGF-D and placenta growth factor. VEGF-A, often simply referred to as VEGF, was the first to be discovered and is the most studied. Generated by alternative splicing, VEGF has several isoforms among which VEGF121 and VEGF165 are predominant (the numbers refer to amino acid residues). All isoforms contain heparan sulfate binding except VEGF121 domains; their bioactivity is therefore localized and reliant on the presence of heparan sulfate [30,31]. All VEGF isoforms bind to VEGFR-1 (also known as Flt-1) and VEGFR-2 (also known as Flk-1 and KDR), which are receptor tyrosine kinases [32]. The activation of VEGFR results in phosphorylation and dimerization of the receptor. Downstream signaling is diverse and includes MAPK, PI3K and PLC-γ pathways. VEGF165 and other high-molecular-weight isoforms also bind to neuropilin-1, which serves as a co-receptor that enhances VEGF–VEGFR interaction and alters VEGF signaling [33,34]. Endothelium is the major tissue that expresses both VEGFR-1 and VEGFR-2 and, consequently, processes such as proliferation and survival of endothelium are highly regulated by VEGF. It is worth mentioning that splicing also generates a group of anti-angiogenic VEGFs, VEGFxxxb (xxx denotes the number of amino acid residues) [35]. For example, only in the six VEGF165b differs from VEGF165 amino acids at the carboxyl-terminus. It binds VEGFR-2 with a similar affinity but does not bind to neuropilin-1 because the essential residues for neuropilin-1 binding are altered. The resultant phosphorylation of VEGFR is insufficient and transient; therefore, it cannot activate the downstream signaling. In certain conditions, this switch from angiogenic to antiangiogenic variant may explain the cause of chronic ischemia [36].
For therapeutic purposes, local levels of angiogenic VEGF can be upregulated by using genes, proteins and cells. Thus far, vegf gene delivery has comprised the majority of clinical trials [37]. In some trials, significant neovasculature formation and increased blood perfusion were observed [38,39]. On the other hand, several studies concluded that VEGF expression has to be very tightly regulated in order to avoid side effects [40,41]. Animal studies have further confirmed that stable and functional vessel growth requires a high degree of control over VEGF production [42]. More investigations are needed in VEGF gene or protein delivery to fully realize the potential of this well-studied angiogenic factor alone or, more likely, with other factors.
FGF
Another potent and widely employed angiogenic factor is FGF. FGFs are expressed in diverse organisms from nematodes to vertebrates. In vertebrates, 22 FGFs with 30–70% amino acid sequence homology, bind selectively to four major receptors: FGFR1b/c, FGFR2b/c, FGFR3b/c and FGFR4 [43,44]. Direct comparison of amino acid sequences suggests that FGFs share conserved sequences in the primary FGFR binding site but differ in the secondary site, resulting in the selectivity between FGFRs [45]. FGFR is also a receptor tyrosine kinase and its activation induces phosphorylation and dimerization. Similar to VEGF, downstream signaling pathways include MAPK, PI3K and PLC-γ that control both genotype and phenotype. It is worth noting that heparan sulfate binds to all FGF–FGFR complexes and potentiates FGF signal transduction. The FGF pathway participates in many biological processes including embryonic development [46], wound healing [47], angiogenesis [48] and tumorigenesis. Thus, it is often regarded as one of the most pleiotrophic factors. Specifically in angiogenesis, it is well known that the effects of FGFs include migration [49], proliferation [50,51], differentiation and survival of blood vessel-associated cells. Recent studies also show that the integrity of the vasculature is highly regulated by FGF signaling. When FGF signaling is inhibited, endothelial junctions become compromised resulting in increased vessel permeability [52]. FGF signaling is also capable of controlling other angiogenic factor pathways, especially VEGF. This is evident because sustained stimulation by FGF is required to maintain VEGFR expression in the endothelium [53].
Among all FGF members, FGF2 is the most extensively studied and most often utilized in therapeutic angiogenesis. Multiple animal studies have demonstrated the potential of controlled delivery of FGF2 alone or combined with other factors in treating ischemic diseases [54,55]. So far, a few FGF2 Phase I human trials have been conducted in MI patients. No obvious toxicity was observed when high dosage of FGF2 was administered by intracoronary delivery, verifying that these levels of FGF2 are safe in humans [56,57]. However, long-term benefit was not observed, possibly due to quick inactivation of FGF2 in the human body [58,59].
Besides FGF2, other FGF members can also initiate angiogenesis and may be useful therapeutically. For example, fgf4 and fgf5 are two angiogenic genes that have been delivered via adenovirus at the embryonic stage for treating coronary heart diseases [19,60]. FGF9, in contrast to FGF2 that affects both endothelium and mesenchyme, is known to primarily target mesenchyme. Developmental studies suggest that FGF9 induces vegfa expression in lung mesenchyme and is necessary for capillary formation [61]. Another study reveals that FGF9 controls the survival of smooth muscle cells and the physiological response of vasculature; delivery of FGF2 and FGF9 together generates larger vessels and thicker smooth muscle layers than that of FGF2 alone [62].
Although FGF members share conserved amino acid sequences, the expression pattern and functions of each are still distinctive. Current knowledge of FGFs was mainly accumulated by gene knockout studies, which are not able to detect events at a molecular level. Consequently, the biological roles of many FGFs require further investigation to decipher. It is reasonable to expect that more FGFs or FGF combinations will be used in future therapies.
Other factors
Besides VEGF and FGF, other angiogenic factors have also been intensely investigated. The PDGF family consists of four members (-A to -D) that form homo- (-AA, -BB, -CC and -DD) or heterodimer (-AB), which binds to PDGF receptors (PDGFRα and β). PDGFs produced by diverse cell types are involved in the development of nervous and circulatory systems and other organs [63]. The importance of PDGF signaling in angiogenesis is highlighted in several aspects: PDGF-BB promotes FGF2 release and FGFR1 activation in vascular smooth muscle cells [64]. On the contrary, PDGF-BB can also inhibit FGF signaling by inducing formation of PDGFRα–FGFR1 heterodimers, which suggests its regulatory roles [65]. PDGF signaling is also important for maturation of vasculature as it controls the activity and behavior of mural cells [66,67]. Knockout of pdgfb and pdgfrβ in mouse embryos have been shown to reduce the number of vascular smooth muscle cells and pericytes associated with the endothelium. As a result the vessel becomes unstable and prone to hemorrhage [68]. In addition, a recent study finds that pdgfrβ is upregulated during cardiac regeneration in the zebrafish [69]. Its inhibition reduces the epicardial proliferation and epithelial-mesenchymal transition and impairs coronary vessel formation.
Angiopoietin-1, which binds to the Tie-2 receptor, is able to balance the effects of VEGF and enhance vasculature stability, and its efficacy has been demonstrated in animal studies [70,71]. HGF, which stimulates endothelial cell proliferation, can potentiate the mitogenic activity of VEGF and increase VEGF production [72]. It has also been shown to promote secretion of proteases that are involved in ECM degradation and cell migration [73]. Granulocyte colony-stimulating factor acts primarily as a cytokine to mobilize progenitor cells from the bone marrow and has been demonstrated to recruit CD34-positive cells to the infarct area and improve ventricular function in a clinical study [74]. However, its overall effect is still being debated [75]. Other angiogenic factors, such as IGF, erythropoietin and stem-cell factor, have also been investigated in clinical trials [76].
Another group of proteins drawing significant attention in therapeutic angiogenesis are morphogens such as Sonic hedgehog, Notch and Wnt [77–79]. The effects of morphogens are thought to be more comprehensive because they can regulate transcriptional upregulation of myriad pathways. However, their mechanisms in blood vessel development need to be investigated more thoroughly.
Blood vessels are critical to maintain normal physiology. Induction of neovasculature has the potential to treat many human diseases. No matter which approach is used, angiogenic activity has to be maintained and precisely controlled in order to achieve stable vascularization. In addition to a thorough understanding of angiogenic factors, improvements of delivery strategies will be critical for the success of therapeutic angiogenesis.
Controlled delivery of angiogenic factors
Regardless of the type of active agents that induce angiogenesis (genes, proteins or progenitor/stem-cells), the key bottleneck to the ultimate therapeutic efficacy is delivery. Controlled delivery is a multidisciplinary field focusing on effectively protecting therapeutic molecules, increasing their overall efficacy and targeting the desired tissues. In this multidisciplinary field, researchers bring insights from multiple fields, including physics, chemistry, biology and materials science, to create and optimize delivery strategies for maximum patient benefit. Compared with bolus delivery, which provides a burst and short period of biological effects, controlled delivery aims to sustain the bioactivity of therapeutic molecules in a steady and long-term fashion. In many cases, tissue regeneration takes weeks or months and requires persistent stimulation of growth factors [80]. Consequently, controlled delivery is expected to be more favorable in terms of therapeutic effects.
Delivery vehicles for angiogenic factor can be made from natural or synthetic materials. Many natural materials have good biocompatibility and degradability. However, controlling the quality of natural materials is challenging. Sources and batch variations often lead to different biological properties, which make mass production difficult. On the other hand, synthetic materials usually offer better quality control. The biomaterials field has matured enough that design of biocompatible and biodegradable materials with cell and tissue specificity becomes feasible. That said there are constant exchanges between the two approaches: natural component can enhance the biocompatibility of a synthetic material and the synthetic component can strengthen a natural material or offer new properties and utilities. Here, we categorize existing delivery vehicles broadly into hydrogels, particles, and others based on their physical properties (Table 1). Different applications will have different sets of requirements of the delivery vehicles and sometimes different types of vehicles are also used together.
Table 1.
Types of growth factor delivery vehicles and their representative materials.
| Forms | Materials | Natural or synthetic | Biodegradable |
|---|---|---|---|
| Hydrogels | Alginate | Natural | Yes |
| Hyaluronic acid | Natural | Yes | |
| Collagen and gelatin | Natural | Yes | |
| Fibrin | Natural | Yes | |
| Poly(ethyleneglycol) | Synthetic | No | |
| Peptide nanofiber | Synthetic | Yes | |
|
| |||
| Micro- and nano-particles | Poly(lactic-co-glycolic acid) | Synthetic | Yes |
| Chitosan | Natural | Yes | |
| Liposome | Synthetic | Yes | |
|
| |||
| Porous scaffolds | Poly(ε-carptolactone) | Natural/synthetic | Yes |
|
| |||
| Coacervate | Heparin and polycation | Natural/synthetic | Yes |
Hydrogel
A hydrogel is obtained when hydrophilic polymers are crosslinked to form a 3D network. Most polymers that yield hydrogels form viscous solution and their gelation is achieved by adding crosslinkers. There are three major properties that make hydrogels attractive in biomedicine [81]: the high water content of the hydrogel closely mimics the environment of most human tissues; having properties of a liquid and a solid, a hydrogel can be confined spatially and, in the meantime, allow diffusion of contents entrapped inside; many hydrogels have good biocompatibility that supports their clinical usage. With a long history of development, current hydrogels are made from a variety of sources including natural materials such as polysaccharides and proteins that are extracted from microorganisms or animals and synthetic materials. The following section will discuss a few representative examples of hydrogel delivery vehicles.
Alginate
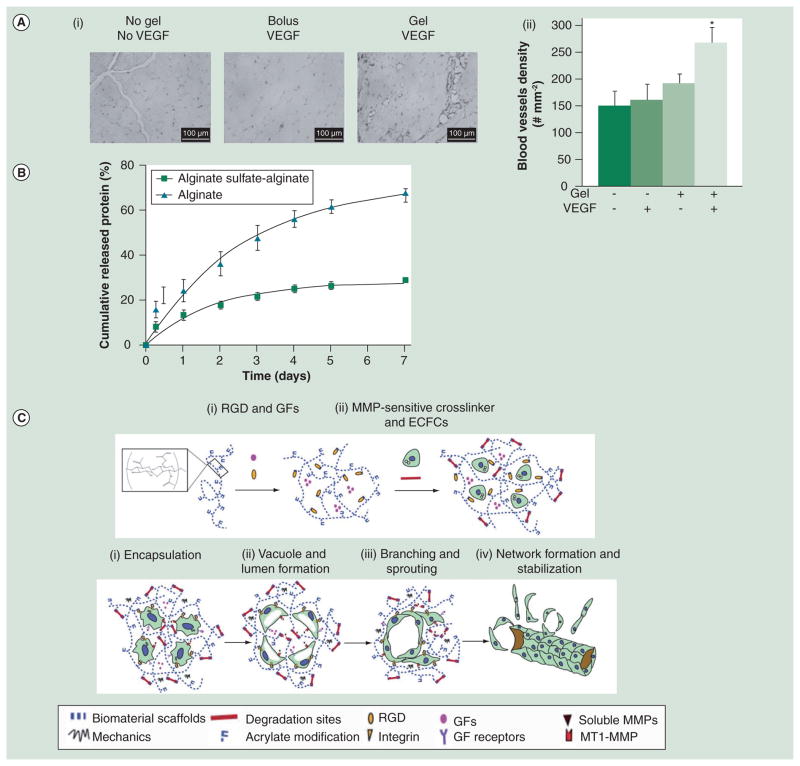
Alginate is a polysaccharide purified from algae and is an FDA-approved material used as a food additive. The repeating unit of alginate contains D-mannuronate and L-guluronate and both units form strong hydrogen bonds with water molecules. In the presence of crosslinkers such as Ca2+, alginate solution forms hydrogel spontaneously. However, loss of ion causes dissolution of an alginate gel; therefore, crosslinking is sometimes utilized to improve the mechanic property and control the degradation kinetics of an alginate gel [82]. A recent study reveals that VEGF entrapped in the alginate gel has a continuous and steady release over a month in vitro according to radioactivity measurement of the 125I-labeled VEGF [83]. VEGF injected within the alginate gel is still detectable 15 days post-injection compared with free VEGF that is degraded within 72 h. Taken together, this indicates that the alginate gel can offer spatio-temporal control of the release of VEGF. More importantly, in a hindlimb ischemic model, alginate-delivered VEGF stimulates the growth of perfused vessels and is more effective than bolus injection of free VEGF (Figure 1A). To enhance affinity to angiogenic factors, alginate can be modified without affecting its gelling property. For example, sulfation of alginate so that it mimics the structure of heparin was performed by conjugating urinate groups with sulfate groups via a N,N′-dicyclocarbodiimide coupling reaction (Figure 1B) [84]. The resultant gel has stronger binding to HGF and achieved a higher therapeutic effect than the unmodified gel.
Figure 1. Alginate and hyaluronic acid hydrogels.
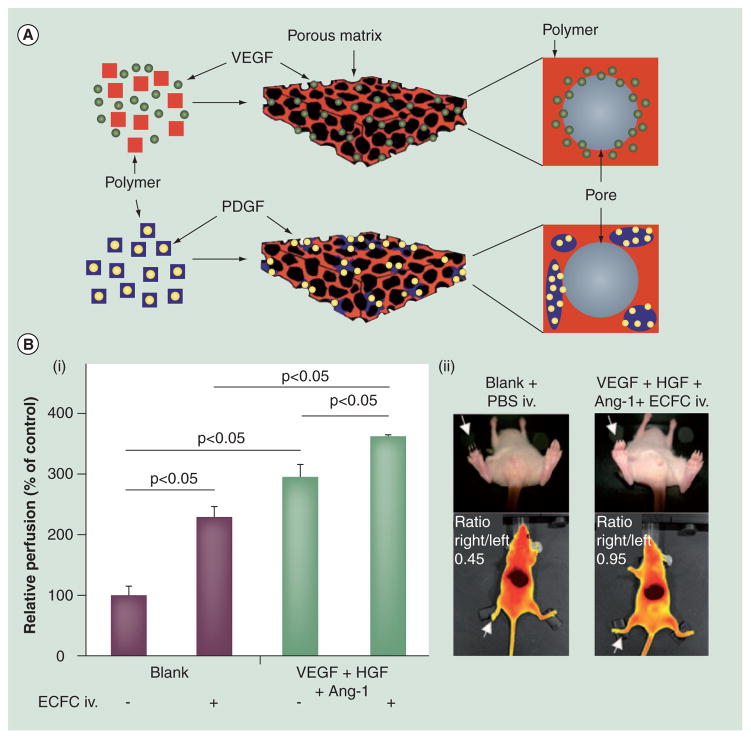
(A) (i) CD31 staining of tissue sections from ischemic hindlimbs 6 weeks post-injection of VEGF-alginate gels. (ii) Comparison of blood-vessel density: no injection (−−); bolus VEGF (−+); alginate gel without VEGF (+−); or alginate gel with VEGF (++). The statistical significance indicates enhanced vessel formation in the VEGF-alginate gel group. *p <0.02. (B) ELISA measures HGF release from alginate microbeads with or without sulfated alginate. Cumulative release percentage is calculated by dividing the amount of the released HGF at a given time point by the total amount of HGF. (C) Design of a hyaluronic acid (HA) hydrogel and its use in vascularization. Top: Acrylated HA (AHA) hydrogel. (i) AHA polymers are first modified with and stromal cell derived-a RGD-containing cell-adhesion peptide followed by mixing with a GF containing VEGF165, FGF2, TNF-α factor-1. (ii) An AHA hydrogel is obtained by crosslinking AHA polymers with MMP-sensitive linkers. Bottom: (i) ECFCs are encapsulated in the AHA hydrogel containing multiple biological signals. (ii) ECFCs in the AHA hydrogel form vacuoles 3–6 h postencapsulation. Continuing to grow in size, vacuolated ECFCs coalesce to form large lumens at day 1. (iii) From day 2, the ECFC tube structure forms via sprouting and branching, and it is accompanied by degradation of the AHA hydrogel. (iv) By day 3, a stable vascular network is observed in the AHA hydrogel.
ECFC: Endothelial colony-forming cells; GF: Growth factor mixture; MMP: Matrix metalloproteinase.
(A) Reprinted with permission from [83] © Wiley (2007).
(B) Reprinted with permission from [84] © Elsevier (2010).
(C) Reprinted with permission from [91] © American Society of Hematology (2011).
Hyaluronic acid
Another widely studied polysaccharide hydrogel is derived from hyaluronic acid (HA). HA, an anionic polymer composed of D-glucuronic acid and D-N-acetylglucosamine, is found widely in the human connective and skin tissues. Native HA has an extremely high molecular weight, up to millions of Daltons. To reduce hydrolysis by hyaluronidase, HA gels are usually formed by crosslinking, which often employs the carboxyl groups of HA that can react covalently with different crosslinkers [85,86]. Crosslinking can reduce the biocompatibility of HA. However, it can be mitigated by choosing appropriate cross-linkers [87,88]. HA carries abundant biochemical signals including its interaction with cell surface receptor CD44. Therefore, it provides mechanical support and specific biological signals [89]. As with alginate, modification of HA with synthetic molecules can provide it with new characteristics. For example, layers of polyelectrolytes were deposited on HA to change its surface property [90]. A recent study uses a HA gel conjugated with cell adhesion peptides and matrix metalloproteinase-cleavable sequence to entrap angiogenic factors and endothelial colony-forming cells [91]. When this gel is implanted in rats, it provides an excellent environment for cells to proliferate, differentiate and migrate. As a result, a functional vascular network forms in situ and integrates with the host vasculature (Figure 1C).
Collagen & gelatin
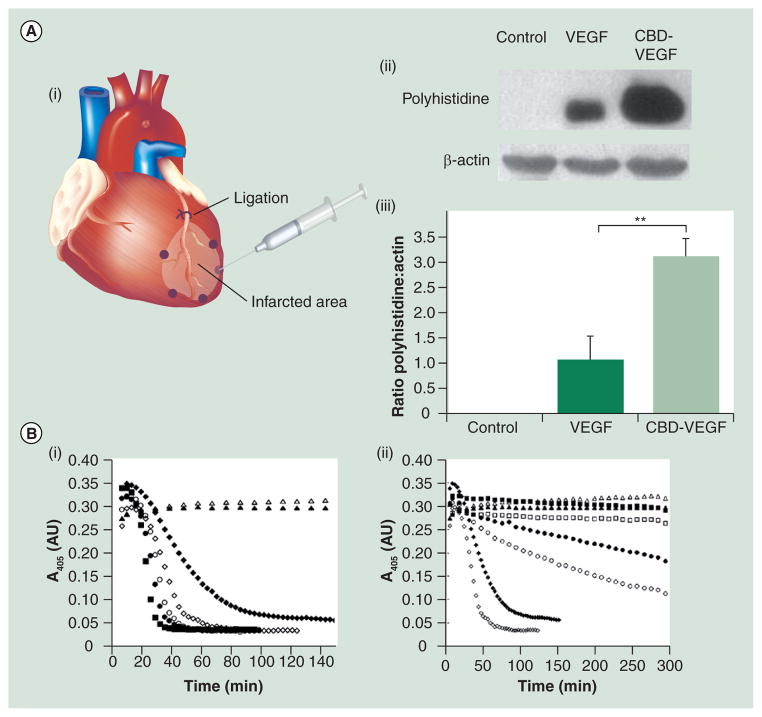
Collagen typifies protein-based vehicles. Collagen is one of the most well-known biomaterials [92]. Among 28 types of collagens identified, type I is the most abundant in the human body. There are many commercial products made of collagen widely used for therapeutic purposes. One study reports that type I collagen has an affinity to FGF2, but interactions between collagen and most growth factors are generally thought to be weak [93]. Consequently, different methods have been adopted to immobilize angiogenic factors on the collagen gel. For example, via a 1-ethyl-3 - [3 -dimethyla minopropyl] carbodiimide hydrodrochloride coupling reaction, VEGF was covalently anchored to the collagen gel [94]. The modified gel increased cell recruitment and vessel density in the ischemic myocardium compared with the unmodified one. In addition, a collagen-binding sequence, TKKTLRT, has been used for immobilization. In an MI model, TKKTLRT-modified VEGF reveals better retention in the border zone than the unmodified one (Figure 2A) [95]. Consequently, the modified VEGF delivered by a collagen patch promotes angiogenesis in the ischemic heart [96] and yields higher cardiac output and more blood perfusion than unmodified VEGF.
Figure 2. Collagen, fibrin and polyethylene glycol hydrogels.
(A) Injection of VEGF or the CBD-modified VEGF to the infarcted heart. (i) VEGF or CBD-VEGF was injected in the border zone indicated by dots. (ii) 3 h later, Western blot assessed the exogenous level of VEGF. (iii) Quantitative comparison revealed that CBD-VEGF was better retained in the border zones. **p <0.01.
(B) Plasmin catalyzes degradation of fibrin gels as detected by reduction of absorbance at 405 nm. (i) Aprotinin conjugation inhibits fibrin gel degradation. Degradation of fibrin gels (1 mg/ml) is performed at plasmin (100 nM) containing 5 nM (● soluble or ○ aprotinin-conjugated) or 10 nM (◆ soluble or ◇ aprotinin-conjugated) of fibrinogen. Control: fibrin (▲) and 30 nM of aprotinin-conjugated fibrin (△) not subjected to plasmin; plasmin contained no aprotinin (■). (ii) Concentration effect in plasmin inhibition. Fibrin gels are degraded by plasmin containing 10 nM (◆ soluble or ◇ aprotinin-conjugated), 30 nM (● soluble or ○ aprotinin-conjugated) or 50 nM (■ soluble or □ aprotinin-conjugated) of fibrinogen.
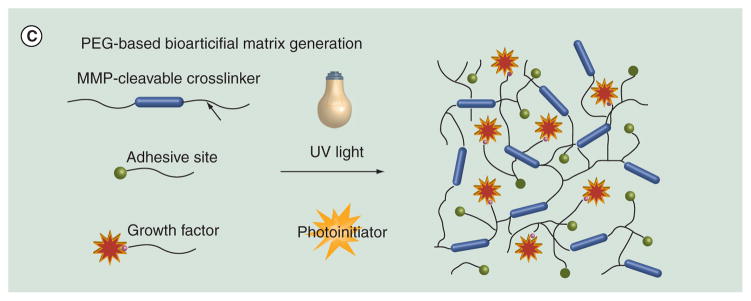
(C) Design of a PEG-based bioartificial matrix. The MMP-degradable sequence, adehesive ligands and growth factors are modified with PEG-acrylate groups. Photopolymerization crosslinks PEGylated precursors and generates in a hydrogel with multiple signals.
CBD: Collagen-binding domain; MMP: Matrix metalloproteinase; PEG: Poly(ethylene glycol).
(A) Reprinted with permission from [95] © American Heart Association (2009).
(B) Reprinted with permission from [110] © American Chemical Society (2007).
(C) Reprinted with permission from [119] © National Academy of Sciences, USA (2010).
Gelatin, being a denatured product of collagen, is made by acid or alkaline processing of collagen. The alkaline process hydrolyzes the amide moiety of asparagine or glutamine and generates carboxylic acid groups; consequently the isoelectric point increases to nine compared with the acidic process, which keeps the isoelectric point at five. Gelation of gelatin is highly dependent on temperature. When the temperature is below the sol-gel transition temperature, approximately 40°C, gelatin solution forms a gel by partially forming the triple-helix structure found in collagen. Crosslinking will strengthen the gelatin gels and reduce its degradation rate [97,98]. Gelatin gels have been examined widely in growth factor delivery with promising results [99,100]. Acidic gelatin is negatively charged at the physiological pH because its isoelectric point is five. Therefore, it binds growth factors with positively charged heparin-binding domains such as FGF2 [101]. The affinity makes basic gelation an excellent vehicle for controlled release of these factors. In a rabbit hindlimb ischemia model, intra-arterial administration of FGF2-loaded gelatin gel microspheres induces collateral vessel formation and generates more perfused vessels than the untreated group [102]. The same material increases cardiosphere-derived cell engraftment in the host cardiac tissue, induces neovasculature and synergistically improves functions of the ischemic heart [103].
Fibrin
Fibrin is another widely used protein gel. Fibrin is the major material in blood clots formed by activated fibrinogen. Fibrinogen (340 kD) is a water-soluble glycoprotein. Once cleaved by thrombin, intermolecular interaction increases and induces assembly of fibrin fibers. Clinical-grade fibrinogen is obtained by processing blood plasma, and the production procedures are relatively simple. There are many fibrin-based products on the market; most are used as surgical sealants in wound closure [104]. Fibrin gels provide a 3D environment for cell growth and are a valuable tool to observe cell migration, morphology change and other activities in vitro [105]. Specific to angiogenesis, endothelial cell tube formation is a well-adopted assay to test the angiogenic or anti-angiogenic ability of molecules [106]. Release of growth factors from a fibrin gel is fast because of the low affinity between fibrin and growth factors and fast degradation of fibrin. In many cases, burst release are over 50% and total release is complete within 2 weeks [107]. Consequently, there are several methods developed to improve the release kinetics:
Angiogenic factors are covalently linked to fibrin via a cleavable peptide linker [108]. The result indicates that the burst release is greatly reduced;
A heparin-binding peptide is conjugated to fibrin to sequester heparin and heparin-binding factors [109]. This approach retains more growth factors in the gel and slows the release substantially;
To prevent fibrinolysis, a protease inhibitor, aprotinin, is conjugated to the fibrin gel and the plasmin-mediated degradation decreases accordingly (Figure 2B) [110]. An ex vivo assay suggests that the slowly degraded gel leads to more vessel formation than the original one;
PEGylation that conjugates or crosslinks fibrinogen can also inhibit fibrinolysis and slows down protein release [111].
Poly(ethylene glycol)
Hydrogels formed by synthetic polymers such as poly(ethylene glycol) (PEG) are also used in angiogenic factor delivery. PEG, HO(C2H4O)n, is one of the most widely used materials in biomedicine because of its extremely low cytotoxicity and immunogenicity. PEG has a distinct ability to reduce clearance of materials by the reticuloendothelial system; therefore, it is a very useful modifier in prolonging drug circulation time in the human body [112,113]. PEG gels are formed by covalent crosslinking of PEG chains. Gel characteristics, such as stiffness and swelling properties, can be controlled easily. Modification of PEG monomers with reactive methacrylate groups and then exposure to UV light in the presence of radical initiator is a common method to crosslink PEG monomers. However, PEG is not biodegradable and only those with low molecular weights (MW < 5000) can be metabolized by the human body [114]. As a result, several functional groups are introduced into PEG molecules to provide degradability and also more utility [115,116]. Several PEG-based materials have been tested in therapeutic angiogenesis: VEGF and the integrin-binding domain RGD has been linked to a PEG gel. The resultant gel provides both sustained release of VEGF and guidance of vascular growth [117]. A heparin-functionalized PEG gel that slowed VEGF release attracted more endothelial cells surrounding the implantation site [118]. Last but not least, in a hindlimb ischemia model, a PEG gel carrying protease-degradable sites, cell-adhesion motifs and VEGF promoted functional recovery better than the control gels (Figure 2C) [119].
PEG is also a widely used building block to form composite hydrogels. For example, PEG/gelatin gels with tunable mechanic properties and degradation rates can be made by adjusting the ratio of PEG to gelatin utilized in the coupling reaction [120]. In addition, PEG is often utilized in synthesizing reversible thermogels. The polymer chain of a reversible thermogel has a distinct property in that its aggregation state varies with temperature. Those with the sol-gel points close to body temperature are especially attractive in biomedicine because they can offer injectability and spatial control of the delivery system [121]. Structure of the polymer that forms a thermogel has both hydrophobic and hydrophilic portions; PEG often serves as the hydrophilic portion and offers control of gelation property [122,123]. PEG-based thermogels have been examined for delivery of different drugs including small molecules, nanoparticles and proteins [124–126].
Peptide nanofibers
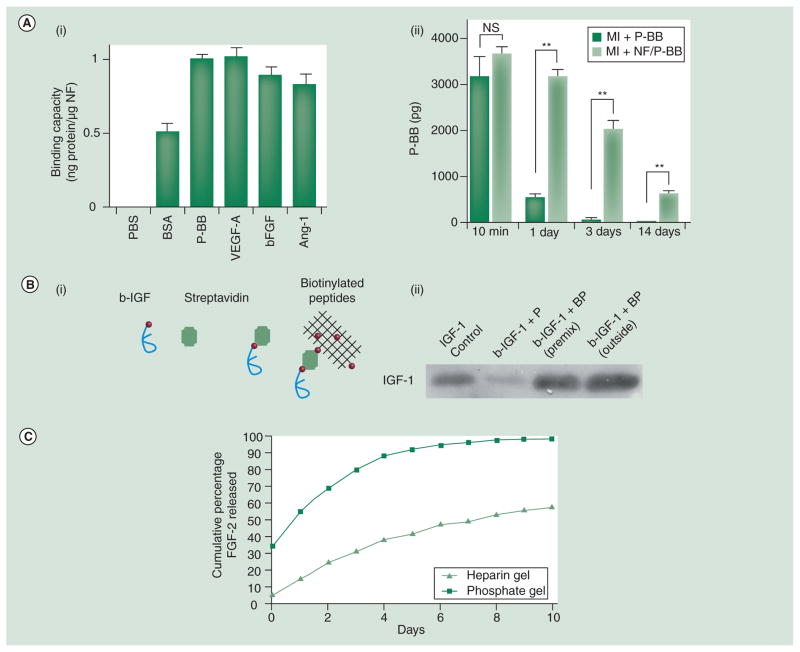
There are several types of peptide nanofibers. One is self-assembled from a 16-residue peptide that forms a β-sheet structure [127]. Gelation of nanofibers can occur immediately using specific triggers such as temperature, pH or ionic strength. Sharing similar properties to other hydrogels, nanofibers are capable of delivering angiogenic factors. Nanofibers injected with PDGF-BB into the infarct border zones of rat hearts indicates that PDGF-BB is confined locally [128]. In the MI and ischemia-reperfusion models, the nanofibers protect the PDGF-BB and results in better cardiac functions compared with free PDGF-BB (Figure 3A). Here, the binding between nanofibers and PDGF-BB is weak because there is no specific interaction between the vehicle and the growth factor. To increase the interactions, nanofibers and the growth factor can be biotinylated and complexed with streptavidin, respectively (Figure 3B) [129]. This method increased the binding fivefold and the dissociation constant was reduced to the micromolar range. Interestingly, a recent study found that nanofibers alone were able to slow deterioration after MI [130]. Examined by a porcine model, injection of nanofibers prevented ventricular remodeling and increased the efficiency of cell therapy. The exact mechanism of these nanofibers’ beneficial effects is currently unknown.
Figure 3. Peptide nanofibers.
(A) Injectable peptide NF delivers PDGF-BB in a controlled fashion. (i) The binding capacity of peptide NFs to PBS, BSA, PDGF-BB, VEGF, FGF2 or Ang-1 was determined by ELISA assays. (ii) PDGF-BB was injected alone or with peptide NFs in the border zones post-MI. The result suggests that PDGF-BB without peptide NFs was undetectable after 3 days, whereas PDGF-BB delivered by peptide NFs was still detectable after 14 days. **p <0.001. (B) Approach of immobilizing IGF-1 on peptide NFs. (i) IGF-1 and self-assembling peptides are biotinlyated first and then tethered together via tetravalent streptavidin. (ii) Western blot reveals that biotinylated IGF-1 only binds the BPs (b-IGF-1+BP) and not the non-biotinylated peptides (b-IGF-1+P). In addition, the affinity of b-IGF-1 to biotinylated peptides does not change before or after peptides assembly (b-IGF-1+BP premix vs b-IGF-1+BP outside). (C) The release of rhodamine-FGF2 (gray curve) from the PA-heparin gel is significantly slower than that from the PA-Na2HPO4 gel (black curve).
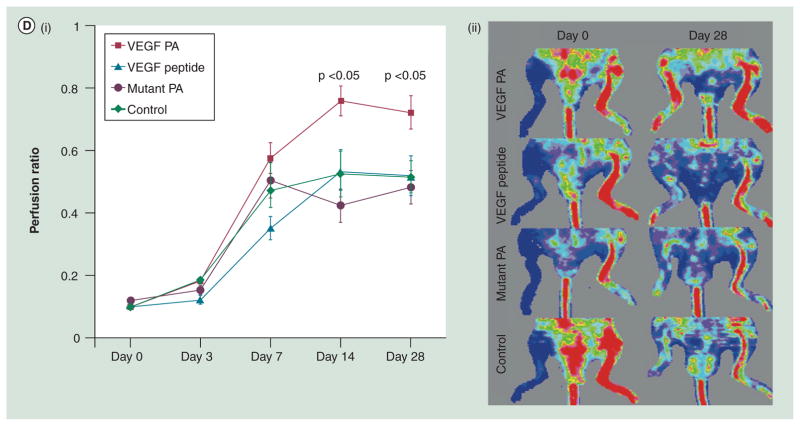
(D) Laser Doppler imaging compares blood perfusion at ischemic hindlimbs among all treatment groups, VEGF PA, VEGF peptide, mutated VEGF PA and saline control. (i) Perfusion ratios (ischemic hindlimb/non-ischemic hindlimb) reveal that the VEGF PA group has significantly higher perfusion than any other groups from day 14 to 28. (ii) Laser Doppler images show the perfusion of the same animals from day 0 to 28.
BP: Biotinylated self-assembling peptide; BSA: Bovine serum albumin; MI: Myocardial infarction; NF: Nanofiber; NS: No significance;
PA: Peptide amphiphile; PBS: Phosphate-buffered saline.
(A) Reprinted with permission from [128] © American Society for Clinical Investigation (2006).
(B) Reprinted with permission from [129] © National Academy of Sciences, USA (2006).
(C) Reprinted with permission from [132] © American Chemical Society.
.(D) Reprinted with permission from [134] © National Academy of Sciences, USA (2011).
Another type of peptide nanofiber is formed by the self-assembly of peptide amphiphiles that contain a hydrophobic tail, a middle peptide region that forms a β-sheet structure and a hydrophilic peptide epitope [131]. In an aqueous solution, peptide amphiphiles have the propensity to assemble into cylindrical micelles resembling those formed by lipid molecules. These peptides have no affinity to growth factors. One way to increase the affinity is to integrate heparin-binding peptide sequence into the PA, which will bind heparin and, consequently, heparin-binding growth factors (Figure 3C) [132]. This approach achieves similar binding affinity as biotinylation of growth factors in the above case. More recently, to create more functionality, the plain peptide sequences of these gels have been substituted to signaling sequences that provide functional surfaces. For example, a fibronectin-mimetic sequence was displayed on the surface to enhance cell adhesion [133]. In another report on the use of functionalized peptides, a VEGF-mimic peptide was displayed to make angiogenic nanofibers [134]. The in vitro experiment indicated that the displayed peptide exhibited functions similar to VEGF, and a hindlimb ischemia model demonstrated that its angiogenic activity was higher than unmodified peptide (Figure 3D). It is likely that more signaling peptides will be used to provide the nanofibers more utility in the future.
Others
Many other hydrogels have been examined in therapeutic angiogenesis including Matrigel™. Matrigel, comprising a mixture of proteins, including type IV collagen, laminin, heparan sulfate proteoglycan and entactin, is secreted by Engelbreth-Holm-Swarm mouse sarcoma cells. Matrigel is a useful material for tissue engineering and was reported to be an environment for growth and differentiation of embryonic stem cells and neonatal cardiomyocytes into cardiac tissues [135,136]. However, since this material is derived from a tumor tissue, it may raise safety concerns and, consequently, was not used as often as other natural hydrogels, especially in translational research.
Polyvinyl alcohol (PVA), (C2H4O)n, is an FDA-approved ingredient in drug formulation. It has a high capacity to absorb water and forms a solid gel by a freezing–thawing process in which PVA molecules form strong hydrogen bonds and crystallites [137]. Several freezing–thawing processes can strengthen the PVA gel but sometimes crosslinking is also applied to obtain a stronger gel [138]. PVA has an advantage over PEG in that its free hydroxyl groups enable simpler covalent modification. For example, a heparin/PVA gel can form by addition of PVA and heparin with methacrylate groups followed by photo-crosslinking [139]. A fibronectin/PVA gel has been made by converting hydroxyl groups to carboxylic acid groups and then conjugating fibronectin via a carbonyldiimidazole coupling reaction [140]. PVA-based hydrogels have been examined in controlled delivery of several drugs, including a composite material formed by PLGA microspheres/PVA hydrogels, which obtains a steady VEGF release and sustains the bioactivity up to 4 weeks [141–143].
One study uses a sucrose aluminum sulfate pellet coated with poly(hydroxyethyl methacrylate) hydrogel to co-deliver FGF2 and PDGF-BB and concludes that the synergistic effect between the two factors generates a more stable vasculature [144]. Lastly, a pH- and temperature-sensitive gel formed by poly(N-isopropylacrylamide-co-propylacrylic acid-co-butyl acrylate) with encapsulated FGF2 is injected in ischemic myocardium [145]. The result suggests a better retention of FGF2 at the injection site than free FGF2 and an improved cardiac function through enhanced angiogenesis.
Application of hydrogels in biomedicine has a long history. Currently, development of hydrogels has shifted from tuning their intrinsic properties, such as biocompatibility, degradation and stiffness, to making multifunctional and responsive hydrogels that can deliver diverse biomolecules or cells together and also respond to environmental changes. It is reasonable to expect that there will be more sophisticated and smarter gels in the near future.
Micro- & nano-particles
Micro- and nano-particles can be made from a variety of materials including metals, synthetic polymers and biomolecules. Because the sizes are close to mammalian cells, microparticles can diffuse in many tissues. Nanoparticles that have even smaller sizes can be uptaken into cells by different pathways. On the other hand, micro- or nano-particles that encapsulate therapeutic drugs could slow their release and protect their degradation, which may enhance the therapeutic outcome. Consequently, a significant amount of effort has been devoted to developing micro- and nano-particles. Now we have very good control on the size and porosity of these particles. Two materials often utilized, poly(lactic acid-co-glycolic acid) (PLGA) and chitosan, are discussed below.
Poly(lactic acid-co-glycolic acid)
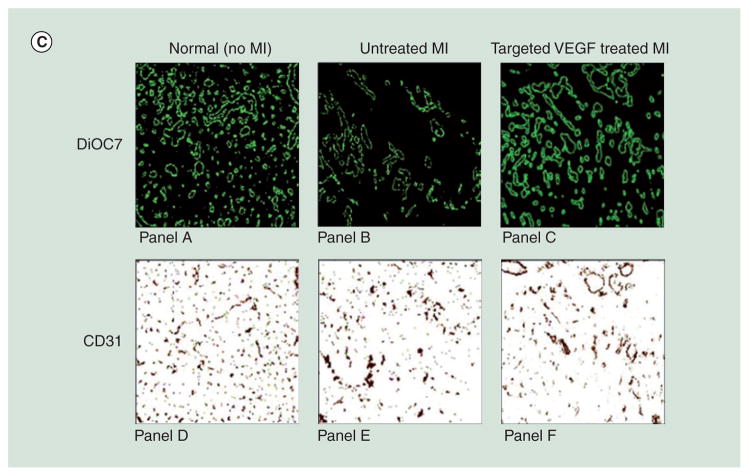
PLGA has been fabricated into micro- and nano-particles, foams and sponge. Owing to excellent biocompatibility and biodegradability, PLGA had been approved by the FDA for several indications and are widely applied in biomedical research as grafts and sutures. Different forms of PLGA are obtained by distinct synthetic procedures. PLGA particles are mostly obtained by emulsion-evaporation processes and their characteristics can easily be tailored by changing processing parameters [146]. On the other hand, PLGA foam/sponge is produced by particulate leaching or gas foaming methods [147]. Release of incorporated growth factors triggered by degradation of PLGA is highly dependent on their composition and characteristics. Several studies highlight the progress of PLGA in growth factor delivery: the sequential release of dual factors has been examined by embedding PDGF-incorporated particles in a PLGA foam that have VEGF dispersed within (Figure 4A) [148]. This strategy creates a significantly slower release of PDGF and faster release of VEGF. Similarly, partition of the two factors in the particle and foam enables control of the release pattern of each factor [149]. In addition to release profiles, VEGF-loaded microparticles have been tested to improve angiogenesis in an ischemia-reperfusion model [150]. Combining PLGA particles with cell therapy is also possible: VEGF, HGF and Ang-1-loaded microparticles created a proangiogenic environment for migration, homing and integration of vasculogenic progenitor cells [151]. The resultant vessels were more stable and functional compared with administration of progenitor cells only (Figure 4B).
Figure 4. Representative microparticles.
(A) Poly(lactic acid-co-glycolic acid) (PLGA) scaffolds enable different release kinetics of growth factors. VEGF is incorporated into a porous PLGA scaffold by mixing with PLGA followed by processing into a scaffold. This approach results in VEGF absorption on the scaffold. On the other hand, PDGF is incorporated by pre-encapsulation of PDGF into PLGA microspheres followed by processing into a scaffold. Compared with VEGF, PDGF has a significantly slower release kinetic. These two approaches can be combined to deliver dual factors with distinct kinetics. (B) Implantation of PLGA microparticles that release VEGF, HGF, and Ang-1 is combined with intravenous injection of ECFCs to enhance neovascularization in mice with hindlimb ischemia. (i) 14 days postadministration, relative blood flow (ischemic/nonischemic hindlimb) assesses vascular function of each group. (ii) Top: necrosis in toes has disappeared in the group receiving growth factor-carrying microparticles and ECFCs. Bottom: fluorescent imaging by indocyanine green injection compares blood perfusion in the toes. Red and yellow indicate high and low perfusion, respectively.
(C) Two methods determine effect of VEGF-containing liposomes in angiogenesis post-MI. Panels A–C: 3,3′-diheptyloxacarbocyanine iodide (green) stains perfused vessels. Panels D–F: CD31 staining (brown) labels endothelial cells. Both results reveal that MI decreases the quantity of blood vessels and VEGF-containing liposomes are able to induce neovasculature.
ECFC: Endothelial colony-forming cells; MI: Myocardial infarction; PBS: Phosphate-buffered saline.
(A) Reprinted with permission from [148] © Nature Publishing Group (2001).
(B) Reprinted with permission from [151] © American Heart Association (2010).
(C) Reprinted with permission from [167] © Federation of American Societies for Experimental Biology (2009).
Chitosan
Chitosan composed of D-glucosamine and N-acetyl-D-glucosamine is a polysaccharide made by deacetylation of chitin, which is mostly extracted from the exoskeleton of crustaceans. Chitosan is generally considered to be biocompatible and biodegradable, but it is also shown to have antibacterial and mucoadhesive properties; therefore, it has been applied extensively in the food industry and in drug development [152,153]. The amino groups of chitosan offer simple routes of functionalization including absorption, crosslinking and conjugation [154–156]. Chitosan forms nanoparticles with many biomolecules, especially those with negative charges such as DNA, heparin, alginate and HA [157–160]. The small size of chitosan nanoparticles increases cell uptake and, consequently, are effective at delivering genes and small RNA [161,162]. In addition, several studies use chitosan nanoparticles in growth factor delivery: the heparin-functionalized chitosan/poly(γ-glutamic acid) nanoparticles can respond to pH changes and adjust the release of the encapsulated FGF2 [163]. A decellularized scaffold carrying VEGF-encapsulated chito-san/heparin nanoparticles promoted more vasculature in vivo compared with the bare scaffolds [164].
Liposome
A liposome has a lipid bilayer structure similar to that of the cell membrane. Liposomes have played a significant role in drug delivery and their characteristics, such as size and surface property, can be controlled precisely by different preparation methods [165]. Because of the similar structure and composition, liposomes can fuse with the cell membrane and deliver the contents into a cell. As a result, they are suitable carriers of genes and certain drugs that function inside the cell. On the contrary, most angiogenic factors have receptors on the cell surface and, therefore, their encapsulation in a liposome may not be advantageous for overall efficacy. Application of liposomes in therapeutic angiogenesis mostly focuses on promoting expression of angiogenic genes [166]. Interestingly, a study uses antibody-conjugated liposomes to target P-selectin, which is upregulated post-MI [167]. The delivered VEGF promotes angiogenesis and improved cardiac functions (Figure 4C).
Other controlled-release vehicles
Other vehicles, such as porous scaffolds, have been adopted for the delivery of angiogenic factors. For example, a collagen-coated porous poly(ε-caprolactone) scaffold releases the entrapped VEGF and attracts endothelial cells to form neovasculature in situ [168]. A heparinized chitosan/poly(ε-caprolactone) nanofibrous scaffold has been utilized to create a VEGF gradient and guides cell growth [169]. Lastly, VEGF and PDGF-BB entrapped in a heparinized porous polyurethane scaffold had varying release at different rates according to their heparin-binding ability [170].
Recently, our group developed a new platform, coacervate, to deliver growth factors. The coacervate forms when two oppositely charged polyelectrolytes bind and neutralize each other to self-assemble into tiny oil droplets. The coacervation process happens immediately upon mixing of the components. Herein, we mimicked the structure of the (FGF–heparin–FGF receptor) complex and utilized a polycation to mimic the role of the FGF receptor (Figure 5A) [171,172]. Taking advantage of the broad range of growth factors that bind heparin, this platform can be used to deliver many growth factors useful in angiogenesis including VEGF, FGF, HGF and PDGF. Our studies demonstrate that this (polycation–heparin) coacervate was effective in loading the growth factors with a high efficiency, controlling their release in a sustained fashion and enhancing their bioactivity over free factors [173]. An in vivo examination shows that the injected FGF2 coacervate induced the formation of more functional and mature vasculature than that induced by free FGF2 (Figure 5B) [173]. The self-assembly process of the coacervate relying on polyvalent ionic interactions, thus, no crosslinking or other external trigger is required and the fluid nature of the coacervate allows for easy injection. We routinely use 31 G insulin needles for injection. Although coacervates formed by polycation and DNA or small RNA are used in nucleic acid delivery, this is the first application of coacervate in controlled delivery of proteins.
Figure 5. Coacervate: design and application.
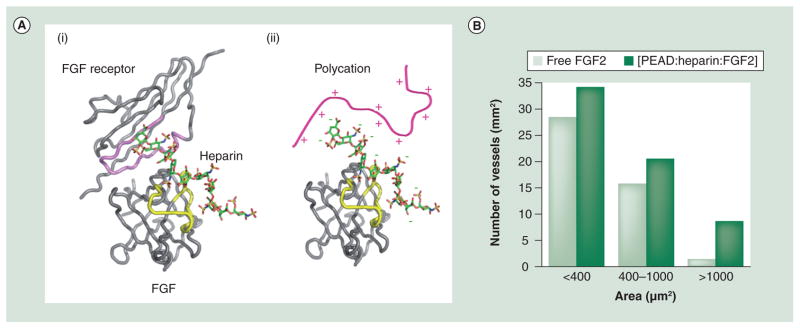
(A) (i) The crystal structure of the FGF–heparin–FGFR complex. The proteins are shown as coils and heparin as a stick model. The heparin-binding domains of FGFR and FGF are highlighted in pink and yellow, respectively. Both analyses showed that the heparin-binding regions contain a high density of positively charged amino acid residues such as arginine. (ii) A possible model of the matrix formed by ionic interactions between an arginine-based synthetic polycation and a heparin–growth factor complex. (B) Comparison of the number of blood vessels in a given size range between free and coacervate FGF2 groups as previously described; the value represents the cumulative number of all the slides examined. The coacervate induced more blood vessel formation than free FGF2. Furthermore, the coacervate group contained more large vessels (>1000 μm2, likely associated with arterioles and venules).
PEAD: Poly(ethylene argininylaspartate diglyceride).
Reprinted with permission from [172] © National Academy of Sciences, USA (2011)
Future perspective
In many cases, delivery of angiogenic factors targets ischemic tissues that are intrinsically different from normal tissues [174,175]. For example, ischemia causes hypoxic conditions that shift glucose metabolism from aerobic to anaerobic. Hence, the production of lactic acid is greatly increased and results in acidosis, which reduces pH down to approximately 6.5, significantly lower than the normal value of 7.4 [176]. In addition, ischemia is often accompanied by intense inflammatory responses including infiltration of neutrophils and macrophages, and secretion of reactive oxygen species and hydrolytic enzymes to the local region [177]. Lysosomal enzymes released from the necrotic tissue can degrade proteins. Furthermore, a new study found that the rapid development of microvasculature accompanying inflammation is not necessarily favorable and could trigger a refractory mechanism to clear angiogenic factors [178]. All of these elements constitute a harsh environment, which makes the bioactivity of delivered angiogenic growth factors difficult to maintain. Understanding and addressing this challenging environment will lead to new breakthroughs in controlled delivery for angiogenesis.
In addition, controlled delivery of angiogenic factors can benefit from materials innovation in multiple areas:
Development of materials that can respond to biological stimulants and control the size of the therapeutic zone;
Combination of materials with characteristics that can respond to biological cues in a coordinated sequence;
Distinct release kinetics and sequential release of multiple angiogenic factors that can be adjusted to achieve mature vasculature;
Novel engineering strategies that can create multiple gradients of growth factors and guide tissue growth in desired patterns;
Formulation of materials that are compatible with image-guided delivery.
Executive summary.
Therapeutic angiogenesis
Angiogenesis induced by gene, protein or cell delivery is a potential therapy for ischemic diseases. However, each approach faces its own challenges and none has been approved by the US FDA.
Delivery of angiogenic factors offers the off-the-shelf advantage but requires a suitable vehicle to control their release and maintain their bioactivity.
Vehicles for controlled release of angiogenic factors
-
Hydrogels (e.g., alginate hydrogel and peptide nanofibers):
Hydrogels have high water content that mimics the environment of most human tissues;
Hydrogels can offer spatiotemporal control of the release of growth factors;
Many hydrogels have good biocompatibility and their medical applications are well studied.
-
Micro- and nano-particles (e.g., poly(lactic acid-co-glycolic acid) microspheres and liposomes):
Micro- and nano-particles are injectable and can circulate in the bloodstream;
Microparticles can diffuse and accumulate in tissues. Nanoparticles can be uptaken by cells and target specific organelles;
Micro- and nano-particles protect encapsulated growth factors from proteolytic cleavage.
-
Porous scaffolds (e.g., poly(ε-caprolactone) scaffolds):
Porous scaffolds provide good mechanical support and guidance for tissue growth;
High surface area facilitates exchange of nutrients and biological signals.
-
Coacervates (e.g., [polycation–heparin] coacervates):
Native heparin and heparin-binding growth factors are utilized without modification;
Coacervates require no elaborate steps for preparation and offer good injectability.
Key Terms
- Angiogenesis
Physiological process that generates neovasculature from pre-existing blood vessels
- Ischemia
Insufficient blood supply caused by blockage or narrowing of blood vessels
- Controlled release
Drug-delivery strategy in which the release pattern, distribution and activity are controlled by certain means
- Peptide nano fibers
Nanofibrous materials produced by self-assembly of artificial peptides or peptide amphiphiles
- Coacervation
Spontaneous process in which two oppositely charged polyelectrolytes interact with each other to self-assemble into tiny oil droplets
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
This project is supported in part by the NIH grant HL089658. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪ ▪ of considerable interest
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics – 2011 update. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 3.Grisar JC, Haddad F, Gomari FA, Wu JC. Endothelial progenitor cells in cardiovascular disease and chronic inflammation: from biomarker to therapeutic agent. Biomark Med. 2011;5(6):731–744. doi: 10.2217/bmm.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪ ▪.Hedman M, Hartikainen J, Yla-Herttuala S. Progress and prospects: hurdles to cardiovascular gene therapy clinical trials. Gene Ther. 2011;18(8):743–749. doi: 10.1038/gt.2011.43. Summarizes recent progress and future direction of angiogenic gene therapy. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa K, Tilemann L, Fish K, Hajjar RJ. Gene delivery methods in cardiac gene therapy. J Gene Med. 2011;13(10):566–572. doi: 10.1002/jgm.1609. [DOI] [PubMed] [Google Scholar]
- 6.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12(6):295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 7.Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Adv Mater. 2009;21(32–33):3269–3285. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 8.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 10.Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Gene Dev. 1999;13(9):1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- 11.Wijelath ES, Rahman S, Namekata M, et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain. Circ Res. 2006;99(8):853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 13.Takeshita S, Zheng LP, Brogi E, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93(2):662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102(8):898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 15.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302(5644):415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 16.Taniyama Y, Tachibana K, Hiraoka K, et al. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105(10):1233–1239. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen DN, Green JJ, Chan JM, Langer R, Anderson DG. Polymeric materials for gene delivery and DNA vaccination. Adv Mater. 2009;21(8):847–867. doi: 10.1002/adma.200801478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105(8):724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry TD, Grines CL, Watkins MW, et al. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol. 2007;50(11):1038–1046. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Circulation. 2004;109(22):2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1(1):55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 23▪ ▪.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration. Circulation. 2010;122(5):517–526. doi: 10.1161/CIRCULATIONAHA.109.881441. Describes mechanisms and applications of stem-cell therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Structural Genomics Consortium, China Structural Genomics Consortium, Northeast Structural Genomics Consortium et al. Protein production and purification. Nat Meth. 2008;5(2):135–146. doi: 10.1038/nmeth.f.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarous DF, Shou M, Stiber JA, et al. Pharmacodynamics of basic fibroblast growth factor: route of administration determines myocardial and systemic distribution. Cardiovasc Res. 1997;36(1):78–85. doi: 10.1016/s0008-6363(97)00142-9. [DOI] [PubMed] [Google Scholar]
- 26.Bethel A, Kirsch JR, Koehler RC, Finklestein SP, Traystman RJ. Intravenous basic fibroblast growth factor decreases brain injury resulting from focal ischemia in cats. Stroke. 1997;28(3):609–616. doi: 10.1161/01.str.28.3.609. [DOI] [PubMed] [Google Scholar]
- 27.Laham RJ, Rezaee M, Post M, et al. Intracoronary and intravenous administration of basic fibroblast growth factor: myocardial and tissue distribution. Drug Metab Dispos. 1999;27(7):821–826. [PubMed] [Google Scholar]
- 28.Sommer A, Rif kin DB. Interaction of heparin with human basic fibroblast growth factor: protection of the angiogenic protein from proteolytic degradation by a glycosaminoglycan. J Cell Physiol. 1989;138(1):215–220. doi: 10.1002/jcp.1041380129. [DOI] [PubMed] [Google Scholar]
- 29.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 30.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4(12):1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruhrberg C, Gerhardt H, Golding M, et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Gene Dev. 2002;16(20):2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling? In control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 33.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 34.Kawamura H, Li X, Goishi K, et al. Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood. 2008;112(9):3638–3649. doi: 10.1182/blood-2007-12-125856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8(11):880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manetti M, Guiducci S, Romano E, et al. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis/novelty and significance. Circ Res. 2011;109(3):e14–e26. doi: 10.1161/CIRCRESAHA.111.242057. [DOI] [PubMed] [Google Scholar]
- 37.Tongers J, Roncalli JG, Losordo DW. Therapeutic angiogenesis for critical limb ischemia. Circulation. 2008;118(1):9–16. doi: 10.1161/CIRCULATIONAHA.108.784371. [DOI] [PubMed] [Google Scholar]
- 38.Hedman M, Hartikainen J, Syvänne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and instent restenosis and in the treatment of chronic myocardial ischemia. Circulation. 2003;107(21):2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 39.Ripa RS, Wang Y, Jørgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006;27(15):1785–1792. doi: 10.1093/eurheartj/ehl117. [DOI] [PubMed] [Google Scholar]
- 40.Alfranca A. VEGF therapy: a timely retreat. Cardiovasc Res. 2009;83(4):611–612. doi: 10.1093/cvr/cvp228. [DOI] [PubMed] [Google Scholar]
- 41.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49(10):1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 42.Tafuro S, Ayuso E, Zacchigna S, et al. Inducible adeno-associated virus vectors promote functional angiogenesis in adult organisms via regulated vascular endothelial growth factor expression. Cardiovasc Res. 2009;83(4):663–671. doi: 10.1093/cvr/cvp152. [DOI] [PubMed] [Google Scholar]
- 43.Ornitz DM, Xu J, Colvin JS, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271(25):15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 2006;281(23):15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkataraman G, Raman R, Sasisekharan V, Sasisekharan R. Molecular characteristics of fibroblast growth factor–fibroblast growth factor receptor–heparin-like glycosaminoglycan complex. Proc Natl Acad Sci USA. 1999;96(7):3658–3663. doi: 10.1073/pnas.96.7.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawada A, Shinya M, Jiang YJ, Kawakami A, Kuroiwa A, Takeda H. Fgf/MAPK signalling is a crucial positional cue in somite boundary formation. Development. 2001;128(23):4873–4880. doi: 10.1242/dev.128.23.4873. [DOI] [PubMed] [Google Scholar]
- 47.Werner S, Peters KG, Longaker MT, Fuller-Pace F, Banda MJ, Williams LT. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci USA. 1992;89(15):6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tigges U, Hyer EG, Scharf J, Stallcup WB. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135(3):523–532. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 49.Vitorino P, Meyer T. Modular control of endothelial sheet migration. Gene Dev. 2008;22(23):3268–3281. doi: 10.1101/gad.1725808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinsella MG, Irvin C, Reidy MA, Wight TN. Removal of heparan sulfate by heparinase treatment inhibits FGF2-dependent smooth muscle cell proliferation in injured rat carotid arteries. Atherosclerosis. 2004;175(1):51–57. doi: 10.1016/j.atherosclerosis.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 51.Sahni A, Francis CW. Stimulation of endothelial cell proliferation by FGF2 in the presence of fibrinogen requires αvβ3. Blood. 2004;104(12):3635–3641. doi: 10.1182/blood-2004-04-1358. [DOI] [PubMed] [Google Scholar]
- 52.Murakami M, Nguyen LT, Zhang ZW, et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118(10):3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murakami M, Nguyen LT, Hatanaka K, et al. FGF-dependent regulation of VEGF receptor 2 expression in mice. J Clin Invest. 2011;121(7):2668–2678. doi: 10.1172/JCI44762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hosaka A, Koyama H, Kushibiki T, et al. Gelatin hydrogel microspheres enable pinpoint delivery of basic fibroblast growth factor for the development of functional collateral vessels. Circulation. 2004;110(21):3322–3328. doi: 10.1161/01.CIR.0000147779.17602.18. [DOI] [PubMed] [Google Scholar]
- 55.Takehara N, Tsutsumi Y, Tateishi K, et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52(23):1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 56.Laham RJ, Chronos NA, Pike M, et al. Intracoronary basic fibroblast growth factor (FGF2) in patients with severe ischemic heart disease: results of a Phase I open-label dose escalation study. J Am Coll Cardiol. 2000;36(7):2132–2139. doi: 10.1016/s0735-1097(00)00988-8. [DOI] [PubMed] [Google Scholar]
- 57.Bush M, Samara E, Whitehouse M, et al. Pharmacokinetics and pharmacodynamics of recombinant FGF2 in a phase I trial in coronary artery disease. J Clin Pharmacol. 2001;41(4):378–385. doi: 10.1177/00912700122010230. [DOI] [PubMed] [Google Scholar]
- 58.Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2. Circulation. 2002;105(7):788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 59.Aviles RJ, Annex BH, Lederman RJ. Testing clinical therapeutic angiogenesis using basic fibroblast growth factor (FGF2) Brit J Pharmacol. 2003;140(4):637–646. doi: 10.1038/sj.bjp.0705493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lynch P, Lee TC, Fallavollita JA, Canty JM, Suzuki G. Intracoronary administration of AdvFGF-5 (fibroblast growth factor-5) ameliorates left ventricular dysfunction and prevents myocyte loss in swine with developing collaterals and ischemic cardiomyopathy. Circulation. 2007;116(Suppl 11):I71–I76. doi: 10.1161/CIRCULATIONAHA.106.681866. [DOI] [PubMed] [Google Scholar]
- 61.White AC, Lavine KJ, Ornitz DM. FGF9 and SHH regulate mesenchymal vegfa expression and development of the pulmonary capillary network. Development. 2007;134(20):3743–3752. doi: 10.1242/dev.004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62▪.Frontini MJ, Nong Z, Gros R, et al. Fibroblast growth factor 9 delivery during angiogenesis produces durable, vasoresponsive microvessels wrapped by smooth muscle cells. Nat Biotech. 2011;29(5):421–427. doi: 10.1038/nbt.1845. Demonstrates that co-delivery of FGF2 and FGF9 obtains mature vasculature. [DOI] [PubMed] [Google Scholar]
- 63.Hoch RV, Soriano P. Roles of PDGF in animal development. Development. 2003;130(20):4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 64.Millette E, Rauch BH, Defawe O, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB-induced human smooth muscle cell proliferation depends on basic FGF release and FGFR-1 activation. Circ Res. 2005;96(2):172–179. doi: 10.1161/01.RES.0000154595.87608.db. [DOI] [PubMed] [Google Scholar]
- 65.Faraone D, Aguzzi MS, Ragone G, Russo K, Capogrossi MC, Facchiano A. Heterodimerization of FGF-receptor 1 and PDGF-receptor-α: a novel mechanism underlying the inhibitory effect of PDGF-BB on FGF-2 in human cells. Blood. 2006;107(5):1896–1902. doi: 10.1182/blood-2005-04-1524. [DOI] [PubMed] [Google Scholar]
- 66.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007;100(5):607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 67.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116(22):4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hellstrom M, Kal NM, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126(14):3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 69.Kim J, Wu Q, Zhang Y, et al. PDGF signaling is required for epicardial function and blood vessel formation in regenerating zebrafish hearts. Proc Natl Acad Sci USA. 2010;107(40):17206–17210. doi: 10.1073/pnas.0915016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shyu K-G, Manor O, Magner M, Yancopoulos GD, Isner JM. Direct intramuscular injection of plasmid DNA encoding angiopoietin-1 but not angiopoietin-2 augments revascularization in the rabbit ischemic hindlimb. Circulation. 1998;98(19):2081–2087. doi: 10.1161/01.cir.98.19.2081. [DOI] [PubMed] [Google Scholar]
- 71.Tao Z, Chen B, Tan X, et al. Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc Natl Acad Sci USA. 2011;108(5):2064–2069. doi: 10.1073/pnas.1018925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Belle E, Witzenbichler B, Chen D, et al. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97(4):381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 73.Tomita N, Morishita R, Taniyama Y, et al. Angiogenic property of hepatocyte growth factor is dependent on upregulation of essential transcription factor for angiogenesis, ets-1. Circulation. 2003;107(10):1411–1417. doi: 10.1161/01.cir.0000055331.41937.aa. [DOI] [PubMed] [Google Scholar]
- 74.Ince H, Petzsch M, Kleine HD, et al. Prevention of left ventricular remodeling with granulocyte colony-stimulating factor after acute myocardial infarction. Circulation. 2005;112(Suppl 9):I73–I80. doi: 10.1161/CIRCULATIONAHA.104.524827. [DOI] [PubMed] [Google Scholar]
- 75.Hill JM, Bartunek J. The end of granulocyte colony-stimulating factor in acute myocardial infarction? Circulation. 2006;113(16):1926–1928. doi: 10.1161/CIRCULATIONAHA.106.623777. [DOI] [PubMed] [Google Scholar]
- 76.Beohar N, Rapp J, Pandya S, Losordo DW. Rebuilding the damaged heart: the potential of cytokines and growth factors in the treatment of ischemic heart disease. J Am Coll Cardiol. 2010;56(16):1287–1297. doi: 10.1016/j.jacc.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7(6):706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 78.Jakobsson L, Franco CA, Bentley K, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12(10):943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 79.Gore AV, Swift MR, Cha YR, et al. Rspo1/ Wnt signaling promotes angiogenesis via VEGFC/VEGFR3. Development. 2011;138:4875–4886. doi: 10.1242/dev.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Markkanen JE, Rissanen TT, Kivelä A, Ylä-Herttuala S. Growth factor-induced therapeutic angiogenesis and arteriogenesis in the heart – gene therapy. Cardiovas Res. 2005;65(3):656–664. doi: 10.1016/j.cardiores.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 81▪ ▪.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18(11):1345–1360. Summarizes development and application of hydrogels in biomedicine. [Google Scholar]
- 82.Chan AW, Whitney RA, Neufeld RJ. Kinetic controlled synthesis of pH-responsive network alginate. Biomacromolecules. 2008;9(9):2536–2545. doi: 10.1021/bm800594f. [DOI] [PubMed] [Google Scholar]
- 83.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5(3):590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 84.Ruvinov E, Leor J, Cohen S. The effects of controlled HGF delivery from an affinity-binding alginate biomaterial on angiogenesis and blood perfusion in a hindlimb ischemia model. Biomaterials. 2010;31(16):4573–4582. doi: 10.1016/j.biomaterials.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 85.Pitarresi G, Palumbo FS, Cavallaro G, Faré S, Giammona G. Scaffolds based on hyaluronan crosslinked with a polyaminoacid: novel candidates for tissue engineering application. J Biomed Mater Res A. 2008;87(3):770–779. doi: 10.1002/jbm.a.31825. [DOI] [PubMed] [Google Scholar]
- 86.Darr A, Calabro A. Synthesis and characterization of tyramine-based hyaluronan hydrogels. J Mater Sci Mater Med. 2009;20(1):33–44. doi: 10.1007/s10856-008-3540-0. [DOI] [PubMed] [Google Scholar]
- 87.Yeom J, Bhang SH, Kim BS, et al. Effect of cross-linking reagents for hyaluronic acid hydrogel dermal fillers on tissue augmentation and regeneration. Bioconjugate Chem. 2010;21(2):240–247. doi: 10.1021/bc9002647. [DOI] [PubMed] [Google Scholar]
- 88.Tan H, Li H, Rubin JP, Marra KG. Controlled gelation and degradation rates of injectable hyaluronic acid-based hydrogels through a double crosslinking strategy. J Tissue Eng Regen Med. 2011;5(10):790–797. doi: 10.1002/term.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 90.Yamanlar S, Sant S, Boudou T, Picart C, Khademhosseini A. Surface functionalization of hyaluronic acid hydrogels by polyelectrolyte multilayer films. Biomaterials. 2011;32(24):5590–5599. doi: 10.1016/j.biomaterials.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hanjaya-Putra D, Bose V, Shen Y-I, et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood. 2011;118(3):804–815. doi: 10.1182/blood-2010-12-327338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eyre DR. Collagen: molecular diversity in the body’s protein scaffold. Science. 1980;207(4437):1315–1322. doi: 10.1126/science.7355290. [DOI] [PubMed] [Google Scholar]
- 93.Kanematsu A, Marui A, Yamamoto S, et al. Type I collagen can function as a reservoir of basic fibroblast growth factor. J Control Release. 2004;99(2):281–292. doi: 10.1016/j.jconrel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 94.Miyagi Y, Chiu LLY, Cimini M, Weisel RD, Radisic M, Li RK. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials. 2011;32(5):1280–1290. doi: 10.1016/j.biomaterials.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J, Ding L, Zhao Y, et al. Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation. 2009;119(13):1776–1784. doi: 10.1161/CIRCULATIONAHA.108.800565. [DOI] [PubMed] [Google Scholar]
- 96.Gao J, Liu JZ, Gao Y, et al. A myocardial patch made of collagen membranes loaded with collagen-binding human vascular endothelial growth factor accelerates healing of the injured rabbit heart. Tissue Eng Part A. 2011;17(21–22):2739–2747. doi: 10.1089/ten.TEA.2011.0105. [DOI] [PubMed] [Google Scholar]
- 97.Giraudier S, Hellio D, Djabourov M, Larreta-Garde V. Influence of weak and covalent bonds on formation and hydrolysis of gelatin networks. Biomacromolecules. 2004;5(5):1662–1666. doi: 10.1021/bm049670d. [DOI] [PubMed] [Google Scholar]
- 98.Saito H, Murabayashi S, Mitamura Y, Taguchi T. Characterization of alkali-treated collagen gels prepared by different crosslinkers. J Mater Sci Mater Med. 2008;19(3):1297–1305. doi: 10.1007/s10856-007-3239-7. [DOI] [PubMed] [Google Scholar]
- 99.Li L, Okada H, Takemura G, et al. Sustained release of erythropoietin using biodegradable gelatin hydrogel microspheres persistently improves lower leg ischemia. J Am Coll Cardiol. 2009;53(25):2378–2388. doi: 10.1016/j.jacc.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 100.Kohara H, Tabata Y. Enhancement of ectopic osteoid formation following the dual release of bone morphogenetic protein 2 and Wnt1 inducible signaling pathway protein 1 from gelatin sponges. Biomaterials. 2011;32(24):5726–5732. doi: 10.1016/j.biomaterials.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 101.Tabata Y, Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials. 1999;20(22):2169–2175. doi: 10.1016/s0142-9612(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 102.Hosaka A, Koyama H, Kushibiki T, et al. Gelatin hydrogel microspheres enable pinpoint delivery of basic fibroblast growth factor for the development of functional collateral vessels. Circulation. 2004;110(21):3322–3328. doi: 10.1161/01.CIR.0000147779.17602.18. [DOI] [PubMed] [Google Scholar]
- 103.Takehara N, Tsutsumi Y, Tateishi K, et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52(23):1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 104.Mintz PD, Mayers L, Avery N, Flanagan HL, Burks SG, Spotnitz WD. Fibrin sealant: clinical use and the development of the University of Virginia tissue adhesive center. Ann Clin Lab Sci. 2001;31(1):108–118. [PubMed] [Google Scholar]
- 105.Brown LF, Lanir N, Mcdonagh J, Tognazzi K, Dvorak AM, Dvorak HF. Fibroblast migration in fibrin gel matrices. Am J Pathol. 1993;142(1):273–283. [PMC free article] [PubMed] [Google Scholar]
- 106.Lafleur MA, Handsley MM, Knäuper V, Murphy G, Edwards DR. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs) J Cell Sci. 2002;115(17):3427–3438. doi: 10.1242/jcs.115.17.3427. [DOI] [PubMed] [Google Scholar]
- 107.Oju J, Soo Hyun R, Ji Hyung C, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release. 2005;105(3):249–259. doi: 10.1016/j.jconrel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 108.Ehrbar M, Metters A, Zammaretti P, Hubbell JA, Zisch AH. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J Control Release. 2005;101(1–3):93–109. doi: 10.1016/j.jconrel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 109.Wood MD, Borschel GH, Sakiyama-Elbert SE. Controlled release of glial-derived neurotrophic factor from fibrin matrices containing an affinity-based delivery system. J Biomed Mater Res A. 2009;89(4):909–918. doi: 10.1002/jbm.a.32043. [DOI] [PubMed] [Google Scholar]
- 110.Smith JD, Chen A, Ernst LA, Waggoner AS, Campbell PG. Immobilization of aprotinin to fibrinogen as a novel method for controlling degradation of fibrin gels. Bioconjugate Chem. 2007;18(3):695–701. doi: 10.1021/bc060265o. [DOI] [PubMed] [Google Scholar]
- 111.Drinnan CT, Zhang G, Alexander MA, Pulido AS, Suggs LJ. Multimodal release of transforming growth factor-β1 and the BB isoform of platelet derived growth factor from PEGylated fibrin gels. J Control Release. 2010;147(2):180–186. doi: 10.1016/j.jconrel.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 112.Goren D, Horowitz AT, Zalipsky S, Woodle MC, Yarden Y, Gabizon A. Targeting of stealth liposomes to erbB-2 (Her/2) receptor: In vitro and in vivo studies. Brit J Cancer. 1996;74(11):1749–1756. doi: 10.1038/bjc.1996.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pirollo KF, Chang EH. Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends Biotechnol. 2008;26(10):552–558. doi: 10.1016/j.tibtech.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 114.Webster R, Didier E, Harris P, et al. PEGylated proteins: evaluation of their safety in the absence of definitive metabolism studies. Drug Metab Dispos. 2007;35(1):9–16. doi: 10.1124/dmd.106.012419. [DOI] [PubMed] [Google Scholar]
- 115.Rizzi SC, Ehrbar M, Halstenberg S, et al. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part II: biofunctional characteristics. Biomacromolecules. 2006;7(11):3019–3029. doi: 10.1021/bm060504a. [DOI] [PubMed] [Google Scholar]
- 116.Reid B, Tzeng S, Warren A, Kozielski K, Elisseeff J. Development of a PEG derivative containing hydrolytically degradable hemiacetals. Macromolecules. 2010;43(23):9588–9590. doi: 10.1021/ma1020648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zisch AH, Lutolf MP, Ehrbar M, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17(13):2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 118.Tae G, Scatena M, Stayton PS, Hoffman AS. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomater Sci Polym Ed. 2006;17(1–2):187–197. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 119.Phelps EA, Landázuri N, Thulé PM, Taylor WR, García AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci USA. 2010;107(8):3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hutson CB, Nichol JW, Aubin H, et al. Synthesis and characterization of tunable poly(ethylene glycol): gelatin methacrylate composite hydrogels. Tissue Eng Part A. 2011;17(13–14):1713–1723. doi: 10.1089/ten.tea.2010.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54(1):37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 122.Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drug-delivery systems. Nature. 1997;388(6645):860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 123▪.Badi N, Lutz JF. PEG-based thermogels: applicability in physiological media. J Control Release. 2009;140(3):224–229. doi: 10.1016/j.jconrel.2009.04.012. Introduces poly(ethylene glycol)-based thermogels and their physical characteristics. [DOI] [PubMed] [Google Scholar]
- 124.Censi R, Vermonden T, Deschout H, et al. Photopolymerized thermosensitive poly(HPMAlactate)-PEG-based hydrogels: effect of network design on mechanical properties, degradation, and release behavior. Biomacromolecules. 2010;11(8):2143–2151. doi: 10.1021/bm100514p. [DOI] [PubMed] [Google Scholar]
- 125.Gao Y, Ren FZ, Ding BY, et al. A thermo-sensitive PLGA-PEG-PLGA hydrogel for sustained release of docetaxel. J Drug Target. 2011;19(7):516–527. doi: 10.3109/1061186X.2010.519031. [DOI] [PubMed] [Google Scholar]
- 126.Wang CH, Hwang YS, Chiang PR, Shen CR, Hong WH, Hsiue GH. Extended release of Bevacizumab by thermosensitive biodegradable and biocompatible hydrogel. Biomacromolecules. 2011;13(1):40–48. doi: 10.1021/bm2009558. [DOI] [PubMed] [Google Scholar]
- 127.Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci. 1993;90(8):3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hsieh PCH, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116(1):237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129▪.Davis ME, Hsieh PCH, Takahashi T, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103(21):8155–8160. doi: 10.1073/pnas.0602877103. Strategy-enhancing affinity between peptide nanofibers and growth factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin YD, Yeh ML, Yang YJ, et al. Intramyocardial peptide nanofiber injection improves postinfarction ventricular remodeling and efficacy of bone marrow cell therapy in pigs. Circulation. 2010;122(11 Suppl 1):S132–S141. doi: 10.1161/CIRCULATIONAHA.110.939512. [DOI] [PubMed] [Google Scholar]
- 131.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 132.Rajangam K, Behanna HA, Hui MJ, et al. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006;6(9):2086–2090. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 133.Rexeisen EL, Fan W, Pangburn TO, et al. Self-assembly of fibronectin mimetic peptide-amphiphile nanofibers. Langmuir. 2009;26(3):1953–1959. doi: 10.1021/la902571q. [DOI] [PubMed] [Google Scholar]
- 134▪.Webber MJ, Tongers J, Newcomb CJ, et al. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proc Natl Acad Sci USA. 2011;108(33):13438–13443. doi: 10.1073/pnas.1016546108. First study examining VEGF-mimicking nanofibers in promoting angiogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guo XM, Zhao YS, Chang HX, et al. Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation. 2006;113(18):2229–2237. doi: 10.1161/CIRCULATIONAHA.105.583039. [DOI] [PubMed] [Google Scholar]
- 136.Naito H, Melnychenko I, Didié M, et al. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114(Suppl 1):I72–I78. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 137.Hassan CM, Peppas NA. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules. 2000;33(7):2472–2479. [Google Scholar]
- 138.Martens P, Anseth KS. Characterization of hydrogels formed from acrylate modified poly(vinyl alcohol) macromers. Polymer. 2000;41(21):7715–7722. [Google Scholar]
- 139.Nilasaroya A, Poole-Warren LA, Whitelock JM, Martens JP. Structural and functional characterization of poly(vinyl alcohol) and heparin hydrogels. Biomaterials. 2008;29(35):4658–4664. doi: 10.1016/j.biomaterials.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 140.Nuttelman CR, Mortisen DJ, Henry SM, Anseth KS. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J Biomed Mater Res. 2001;57(2):217–223. doi: 10.1002/1097-4636(200111)57:2<217::aid-jbm1161>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 141.Horiike S, Matsuzawa S. Application of syndiotacticity-rich PVA hydrogels to drug delivery system. J Appl Polym Sci. 1995;58(8):1335–1340. [Google Scholar]
- 142.Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117(1):68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 143.Cavalieri F, Chiessi E, Villa R, et al. Novel PVA-based hydrogel microparticles for doxorubicin delivery. Biomacromolecules. 2008;9(7):1967–1973. doi: 10.1021/bm800225v. [DOI] [PubMed] [Google Scholar]
- 144.Lu H, Xu X, Zhang M, et al. Combinatorial protein therapy of angiogenic and arteriogenic factors remarkably improves collaterogenesis and cardiac function in pigs. Proc Natl Acad Sci USA. 2007;104(29):12140–12145. doi: 10.1073/pnas.0704966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Garbern JC, Minami E, Stayton PS, Murry CE. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials. 2011;32(9):2407–2416. doi: 10.1016/j.biomaterials.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed. 2006;17(3):247–289. doi: 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- 147.Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(D, L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17(14):1417–1422. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- 148▪.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotech. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. Seminal work developing poly(lactic acid-co-glycolic acid)-based materials for dual delivery of growth factors. [DOI] [PubMed] [Google Scholar]
- 149.Ennett AB, Kaigler D, Mooney DJ. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A. 2006;79(1):176–184. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 150.Formiga FR, Pelacho B, Garbayo E, et al. Sustained release of VEGF through PLGA microparticles improves vasculogenesis and tissue remodeling in an acute myocardial ischemia-reperfusion model. J Control Release. 2010;147(1):30–37. doi: 10.1016/j.jconrel.2010.07.097. [DOI] [PubMed] [Google Scholar]
- 151.Saif J, Schwarz TM, Chau DYS, et al. Combination of injectable multiple growth factor-releasing scaffolds and cell therapy as an advanced modality to enhance tissue neovascularization. Arterioscler Thromb Vas Biol. 2010;30(10):1897–1904. doi: 10.1161/ATVBAHA.110.207928. [DOI] [PubMed] [Google Scholar]
- 152.No HK, Meyers SP, Prinyawiwatkul W, Xu Z. Applications of chitosan for improvement of quality and shelf life of foods: a review. J Food Sci. 2007;72(5):R87–R100. doi: 10.1111/j.1750-3841.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- 153.Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62(1):59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 154.Schiffman JD, Schauer CL. Cross-linking chitosan nanofibers. Biomacromolecules. 2006;8(2):594–601. doi: 10.1021/bm060804s. [DOI] [PubMed] [Google Scholar]
- 155.Fernandez-Megia E, Novoa-Carballal R, Quiñoá E, Riguera R. Conjugation of bioactive ligands to PEG-grafted chitosan at the distal end of PEG. Biomacromolecules. 2007;8(3):833–842. doi: 10.1021/bm060889x. [DOI] [PubMed] [Google Scholar]
- 156.Boddohi S, Killingsworth CE, Kipper MJ. Polyelectrolyte multilayer assembly as a function of pH and ionic strength using the polysaccharides chitosan and heparin. Biomacromolecules. 2008;9(7):2021–2028. doi: 10.1021/bm8002573. [DOI] [PubMed] [Google Scholar]
- 157.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5(4):387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 158.Liu ZG, Jiao YP, Liu FN, Zhang ZY. Heparin/chitosan nanoparticle carriers prepared by polyelectrolyte complexation. J Biomed Mater Res A. 2007;83A(3):806–812. doi: 10.1002/jbm.a.31407. [DOI] [PubMed] [Google Scholar]
- 159.Yang SJ, Chang SM, Tsai KC, Chen WS, Lin FH, Shieh MJ. Effect of chitosan–alginate nanoparticles and ultrasound on the efficiency of gene transfection of human cancer cells. J Gene Med. 2010;12(2):168–179. doi: 10.1002/jgm.1418. [DOI] [PubMed] [Google Scholar]
- 160.Verheul RJ, Slutter B, Bal SM, Bouwstra JA, Jiskoot W, Hennink WE. Covalently stabilized trimethyl chitosan–hyaluronic acid nanoparticles for nasal and intradermal vaccination. J Control Release. 2011;156(1):46–52. doi: 10.1016/j.jconrel.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 161.Mao HQ, Roy K, Troung-Le VL, et al. Chitosan–DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70(3):399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 162.Mao SR, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62(1):12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 163.Tang DW, Yu SH, Ho YC, Mi FL, Kuo PL, Sung HW. Heparinized chitosan/poly(γ-glutamic acid) nanoparticles for multi-functional delivery of fibroblast growth factor and heparin. Biomaterials. 2010;31(35):9320–9332. doi: 10.1016/j.biomaterials.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 164.Tan Q, Tang H, Hu JG, et al. Controlled release of chitosan/heparin nanoparticle-delivered VEGF enhances regeneration of decellularized tissue-engineered scaffolds. Int J Nanomed. 2011;6:929–942. doi: 10.2147/IJN.S18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maherani B, Arab-Tehrany E, Mozafari MR, Gaiani C, Linder M. Liposomes: a review of manufacturing techniques and targeting strategies. Curr Nanosci. 2011;7(3):436–452. [Google Scholar]
- 166.Ye L, Haider HK, Esa WB, et al. Liposome-based vascular endothelial growth factor-165 transfection with skeletal myoblast for treatment of ischaemic limb disease. J Cell Mol Med. 2010;14(1–2):323–336. doi: 10.1111/j.1582-4934.2008.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Scott RC, Rosano JM, Ivanov Z, et al. Targeting VEGF-encapsulated immunoliposomes to MI heart improves vascularity and cardiac function. FASEB J. 2009;23(10):3361–3367. doi: 10.1096/fj.08-127373. [DOI] [PubMed] [Google Scholar]
- 168.Singh S, Wu BM, Dunn JCY. Delivery of VEGF using collagen-coated polycaprolactone scaffolds stimulates angiogenesis. J Biomed Mater Res A. 2012;100 (3):720–727. doi: 10.1002/jbm.a.34010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Du FY, Wang H, Zhao W, et al. Gradient nanofibrous chitosan/poly epsilon-caprolactone scaffolds as extracellular microenvironments for vascular tissue engineering. Biomaterials. 2012;33(3):762–770. doi: 10.1016/j.biomaterials.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 170.Davies NH, Schmidt C, Bezuidenhout D, Zilla P. Sustaining neovascularization of a scaffold through staged release of vascular endothelial growth factor-A and platelet-derived growth factor-BB. Tissue Eng Part A. 2012;18(1–2):26–34. doi: 10.1089/ten.tea.2011.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zern BJ, Chu H, Wang Y. Control growth factor release using a self-assembled [polycation:heparin] complex. PLoS ONE. 2010;5(6):e11017. doi: 10.1371/journal.pone.0011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172▪.Chu H, Gao J, Chen CW, Huard J, Wang Y. Injectable fibroblast growth factor-2 coacervate for persistent angiogenesis. Proc Natl Acad Sci USA. 2011;108(33):13444–13449. doi: 10.1073/pnas.1110121108. First in vivo study investigating effectiveness of the coacervate in growth-factor delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chu H, Johnson NR, Mason NS, Wang Y. A [polycation:heparin] complex releases growth factors with enhanced bioactivity. J Control Release. 2011;150(2):157–163. doi: 10.1016/j.jconrel.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 174.Meller R. The role of the ubiquitin proteasome system in ischemia and ischemic tolerance. Neuroscientist. 2009;15(3):243–260. doi: 10.1177/1073858408327809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brevetti G, Giugliano G, Brevetti L, Hiatt WR. Inflammation in peripheral artery disease. Circulation. 2010;122(18):1862–1875. doi: 10.1161/CIRCULATIONAHA.109.918417. [DOI] [PubMed] [Google Scholar]
- 176.Marzouk SA, Buck RP, Dunlap LA, Johnson TA, Cascio WE. Measurement of extracellular pH, K+, and lactate in ischemic heart. Anal Biochem. 2002;308(1):52–60. doi: 10.1016/s0003-2697(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 177.Mehta JL, Li DY. Inflammation in ischemic heart disease: response to tissue injury or a pathogenetic villain? Cardiovasc Res. 1999;43(2):291–299. doi: 10.1016/s0008-6363(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 178.Le KN, Hwang CW, Tzafriri AR, Lovich MA, Hayward A, Edelman ER. Vascular regeneration by local growth factor release is self-limited by microvascular clearance. Circulation. 2009;119(22):2928–2935. doi: 10.1161/CIRCULATIONAHA.108.823609. [DOI] [PMC free article] [PubMed] [Google Scholar]