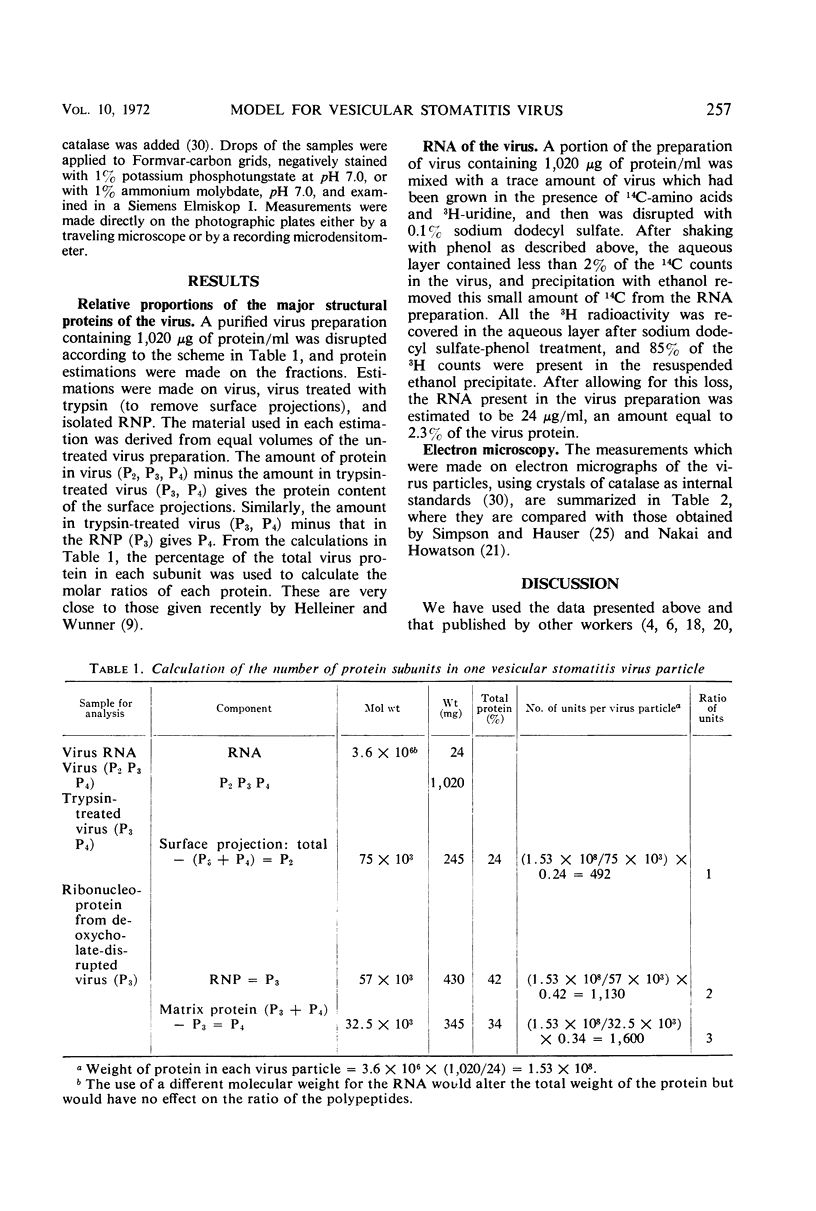
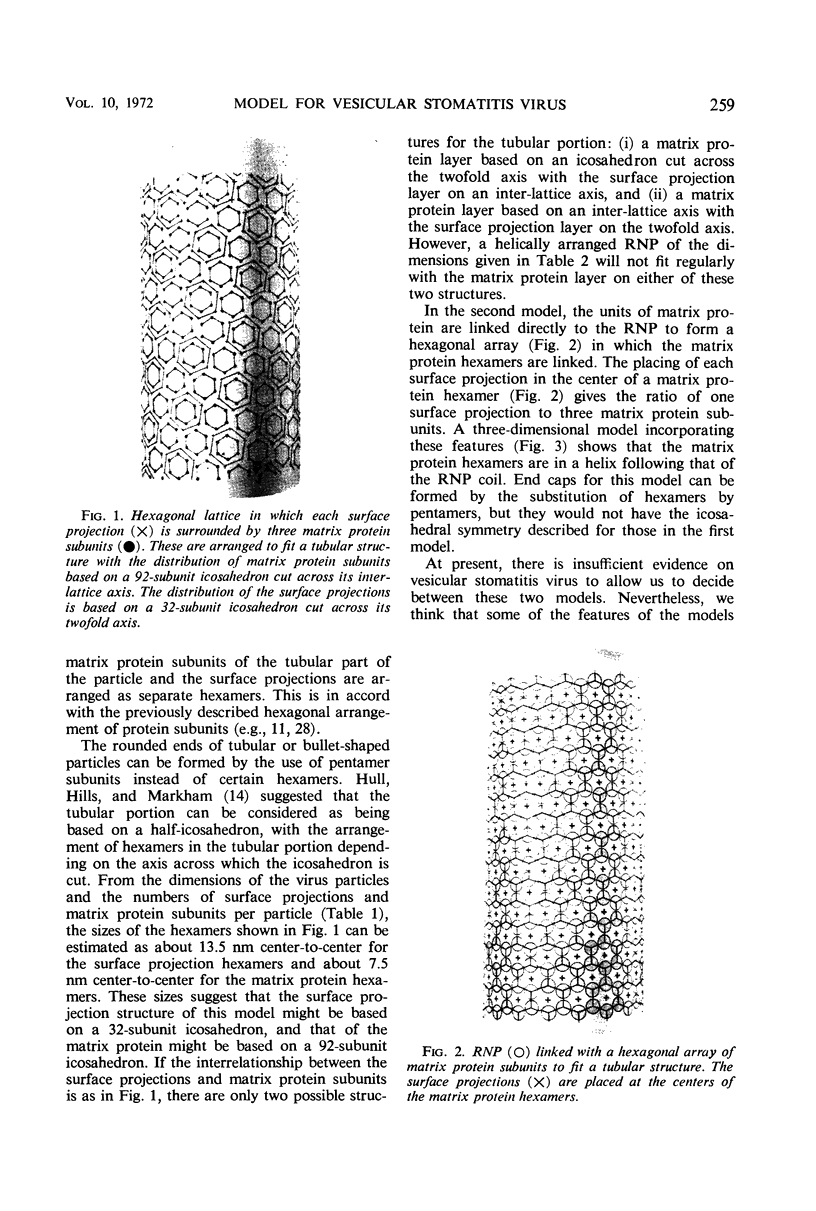
Abstract
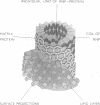
Vesicular stomatitis virus contains single-stranded ribonucleic acid of molecular weight 3.6 × 106 and three major proteins with molecular weights of 75 × 103, 57 × 103, and 32.5 × 103. The proteins have been shown to be subunits of the surface projections, ribonucleoprotein, and matrix protein, respectively. From these values and from estimates of the proportions of the individual proteins, it has been calculated that the virus has approximately 500 surface projections, 1,100 protein units on the ribonucleoprotein strand, and 1,600 matrix protein units. Possible models of the virus are proposed in which the proteins are interrelated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Waterson A. P. Some observations on the envelope of an influenza virus. J Gen Microbiol. 1967 Jan;46(1):107–110. doi: 10.1099/00221287-46-1-107. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Comparison of membrane protein glycopeptides of Sindbis virus and vesicular stomatitis virus. J Virol. 1970 Aug;6(2):176–182. doi: 10.1128/jvi.6.2.176-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- HOYLE L., HORNE R. W., WATERSON A. P. The structure and composition of the myxoviruses. III. The interaction of influenza virus particles with cytoplasmic particles derived from normal chorioallantoic membrane cells. Virology. 1962 Aug;17:533–542. doi: 10.1016/0042-6822(62)90152-6. [DOI] [PubMed] [Google Scholar]
- Helleiner C. W., Wunner W. H. A simple method for counting 14C- and 3H-proteins in polyacrylamide gels. Anal Biochem. 1971 Feb;39(2):333–338. doi: 10.1016/0003-2697(71)90423-4. [DOI] [PubMed] [Google Scholar]
- Hills G. J., Campbell R. N. Morphology of broccoli necrotic yellows virus. J Ultrastruct Res. 1968 Jul;24(1):134–144. doi: 10.1016/s0022-5320(68)80022-x. [DOI] [PubMed] [Google Scholar]
- Hitchborn J. H., Hills G. J. A study of tubes produced in plants infected with a strain of turnip yellow mosaic virus. Virology. 1968 May;35(1):50–70. doi: 10.1016/0042-6822(68)90304-8. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Comparative sedimentation coefficients of RNA extracted from plaque-forming and defective particles of vesicular stomatitis virus. J Mol Biol. 1966 Dec 28;22(2):381–384. doi: 10.1016/0022-2836(66)90143-4. [DOI] [PubMed] [Google Scholar]
- Hull R., Hills G. J., Markham R. Studies on alfalfa mosaic virus. II. The structure of the virus components. Virology. 1969 Mar;37(3):416–428. doi: 10.1016/0042-6822(69)90225-6. [DOI] [PubMed] [Google Scholar]
- Hummeler K., Koprowski H., Wiktor T. J. Structure and development of rabies virus in tissue culture. J Virol. 1967 Feb;1(1):152–170. doi: 10.1128/jvi.1.1.152-170.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN L. E., PLUMMER G., MAYOR H. D. THE FINE STRUCTURE OF FOAMY VIRUS. Virology. 1965 Jan;25:156–159. doi: 10.1016/0042-6822(65)90266-7. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Nakai T., Howatson A. F. The fine structure of vesicular stomatitis virus. Virology. 1968 Jun;35(2):268–281. doi: 10.1016/0042-6822(68)90267-5. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Frank H. Fine structure of influenza A2 (Singapore) as revealed by negative staining, freeze-drying and freeze-etching. J Gen Virol. 1971 Jan;10(1):37–51. doi: 10.1099/0022-1317-10-1-37. [DOI] [PubMed] [Google Scholar]
- Petric M., Prevec L. Vesicular stomatitis virus--a new interfering particle, intracellular structures, and virus-specific RNA. Virology. 1970 Aug;41(4):615–630. doi: 10.1016/0042-6822(70)90427-7. [DOI] [PubMed] [Google Scholar]
- Reginster M., Calberg-Bacq C. M. Further observations on the effects of caseinase C on the envelope of influenza and Newcastle disease viruses. J Ultrastruct Res. 1968 Apr;23(1):144–152. doi: 10.1016/s0022-5320(68)80038-3. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E. Structural components of vesicular stomatitis virus. Virology. 1966 Aug;29(4):654–667. doi: 10.1016/0042-6822(66)90289-3. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany J. M., Blough H. A. Estimation of the number of surface projections on myxo- and paramyxoviruses. Virology. 1970 Jun;41(2):392–394. doi: 10.1016/0042-6822(70)90096-6. [DOI] [PubMed] [Google Scholar]
- Vergara J., Longley W., Robertson J. D. A hexagonal arrangement of subunits in membrane of mouse urinary bladder. J Mol Biol. 1969 Dec 28;46(3):593–596. doi: 10.1016/0022-2836(69)90200-9. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]