Abstract
BACKGROUND
Recent reports have shown that the HNA-3a leukocyte antigen, a target for antibodies that cause severe transfusion-related acute lung injury, correlates with an arginine 154 (rather than glutamine) polymorphism in choline transporter–like protein 2 (CTL2) but did not show directly that R154 determines HNA-3a. CTL2 peptides containing R154 are recognized by only half of HNA-3a antibodies studied to date. Constructs that react with all HNA-3a antibodies are needed to fully define the HNA-3a epitope.
STUDY DESIGN AND METHODS
HEK293 cells were transfected with cDNA encoding full-length CTL2 linked to green fluorescent protein (GFP). Transfectants were selected for GFP expression and tested with antibodies specific for HNA-3a and -3b.
RESULTS
Each of 20 HNA-3a antibodies reacted preferentially with HEK293 cells expressing the R154 CTL2 construct. An HNA-3b antibody reacted only with CTL2 (Q154).
CONCLUSIONS
These findings provide direct evidence that R154 in the context of full-length CTL2 is both necessary and sufficient to create the HNA-3a epitope but suggest that posttranslational modifications of the protein, for example, S–S bonds or addition of glycans, are necessary for recognition of HNA-3a by many antibodies. This could complicate development of an assay for large-scale screening of blood donors to detect anti-HNA-3a.
Antibodies specific for the leukocyte antigen HNA-3a are prone to cause severe, often fatal, transfusion-related acute lung injury (TRALI)1,2 when transfused with blood products.3–5 Unfortunately, it has not been practical to test blood donors routinely for antibodies recognizing this antigen because it was thought to be specific for neutrophils, a cell difficult to use for donor screening, and the protein carrier for the antigen was unknown. Recently, two groups independently showed that the HNA-3a/b antigens are carried on choline-transporter–like protein 2 (CTL2), are expressed on lymphocytes and platelets in addition to neutrophils, and appear to be determined by a single nucleotide substitution in the CTL2 gene predicted to encode an arginine-glutamine polymorphism at CTL2 amino acid residue 154.6,7
Identification of the carrier protein for HNA-3a/b suggests new approaches toward developing assays to screen blood donors for the corresponding antibodies on a large scale. However, the 66-kDa CTL2 protein is predicted to span the cell membrane 10 times and to possess five extracellular loops.8 Owing to this complex structure, intact CTL2 is unlikely to lend itself to detergent solublization, purification, and immobilization to create a target suitable for antibody detection. As an alternative, we previously synthesized various CTL2 peptides containing R154 or Q154 and studied their reactions with anti-HNA-3a. The most satisfactory peptide proved to be a 36-mer (D131-K166) containing R154, but it was recognized in preference to the Q154 version by only 10 of 20 HNA-3a antibodies.9 Berthold and colleagues10 produced CTL2 fragments as GST fusion proteins in Escherichia coli and studied their reactions with anti-HNA-3a in Western blotting. None of the peptides possessing R154 was recognized in preference to its Q154 counterpart by more than 9 of 21 examples of anti-HNA-3a.10 To examine whether a larger recombinant version of CTL2 would mimic the native protein structure sufficiently well to react with all examples of anti-HNA-3a, we expressed the two alleles (R/Q154) of full-length CTL2 in HEK293 cells and studied their reactions with a panel of HNA-3a–specific antibodies.
MATERIALS AND METHODS
Expression of CTL2-green fluorescent protein in HEK293 cells
cDNA corresponding to full-length CTL2 isoform p211 was a gift from Dr T. Carey (University of Michigan, Ann Arbor, MI). This cDNA, coding for CTL2 (R154), was subcloned into expression vector pcDNA3.1/CT-GFP-TOPO (Invitrogen, Carslbad, CA). cDNA encoding Q154 was produced with a site-directed mutagenesis kit (Quickchange II XL, Agilent Technologies, Inc., Carlsbad, CA). cDNAs encoding the two versions of CTL2 were transfected into HEK293 cells using transfection reagent (Fugene HD, Roche, Atlanta, GA). Stable cell lines were selected using Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mg/mL G418, and 25 μg/mL gentamicin sulfate. Cells were selected for green fluorescent protein (GFP) expression using a cell sorter (BD FACS Aria IIu, BD Bioscience, San Jose, CA). Cells enriched for GFP were then cloned by limiting dilution to obtain monoclonal cell lines expressing high levels of HNA-3a or HNA-3b antigen.
Confocal microscopy
Cells were allowed to adhere to tissue culture grade plastic overnight. After being washed two times with phosphate-buffered saline, cells were examined on a multiphoton laser scanning microscope (Olympus Fluoview FV1000 MPE, Olympus America, Center Valley, PA) using a 40× immersion lense with a multi argon 488 laser. Images were generated with the accompanying software (FV10-ASW v3.1c, Olympus America).
Flow cytometry
Cell lines were harvested on the day of experiment by treatment with 0.05% trypsin/0.53 mmol/L ethylenedi-aminetetraacetate in Hanks’ balanced salt solution and washed twice in 3% bovine serum albumin phosphate-buffered saline solution. Forty microliters of a 5 × 103/μL suspension of HEK293 cells was added to microtiter plate wells. Ten microliters of normal or test plasma was added to each well and incubated for 1 hour at room temperature. The cells were then washed twice in buffer and resuspended in 50 μL of a 1:200 dilution of allophycocyanin-conjugated F(ab′)2 goat anti-human IgG solution (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 45 minutes at room temperature. After being washed, bound secondary antibody was detected using a flow cytometer (LSRII, BD Biosciences, San Jose, CA).
Antibodies
HNA-3a antibodies were from blood donors implicated in TRALI cases that were referred to the Platelet and Neutrophil Immunology Laboratory of the BloodCenter of Wisconsin for evaluation. Other samples were gifts from GTI Diagnostics, Inc. (Brookfield, WI); the American Red Cross (St Paul, MN); and Dr Patricia Kopko (Sacramento, CA). Studies to confirm specificity of these antibodies were described previously.9 The single example of anti-HNA-3b was a residual serum sample from the 2011 International Neutrophil Immunology workshop sponsored by the XIth European Platelet and Neutrophil Immunology Congress, 2010.
Statistical analysis
Differences of median values were analyzed in computer spreadsheet software (Excel, Microsoft Corp., Redmond, WA) using a paired t test with a two-tailed distribution.
RESULTS
HNA-3a and -3b antigens were strongly expressed by transfected HEK293 cells
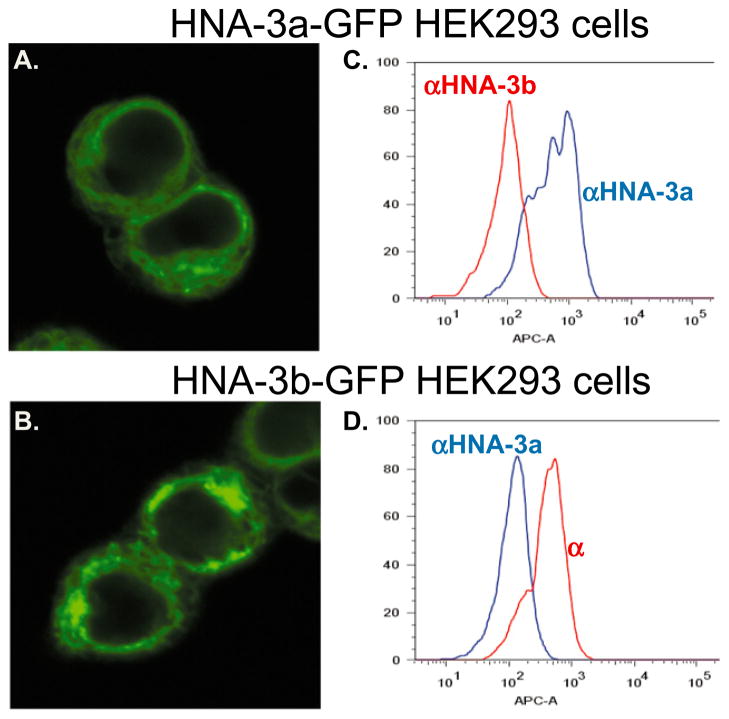
Figures 1A and 1B show that both versions of CTL2-GFP (R154, Q154) were strongly expressed in the selected cell lines. GFP was distributed throughout the plasma membrane, with aggregates apparent in some areas. As shown in Fig. 1C, a representative anti-HNA-3a reacted strongly with cells transfected with CTL2 (R154) but not with cells transfected with CTL2 (Q154). Only a single example of anti-HNA-3b was available for testing. As shown in Fig. 1D, this antibody recognized cells transfected with CTL2 (Q154) but not those transfected with CTL2 (R154). Sequencing studies showed that the CTL2 gene naturally present in HEK293 cells encoded HNA-3a/a. However, the signals produced by nontransfected cells incubated with anti-HNA-3a or with normal serum were indistinguishable, indicating that the cells used for transfection naturally express little or no membrane CTL2.
Fig. 1.
Stable expression of recombinant CTL2-GFP (HNA-3a/b-R/Q154) on HEK293 cells. (A and B) Laser-scanning confocal microscopic image of transfected HEK293 cells expressing CTL2 (R154) (A) and CTL2 (Q154) (B) selected for high expression of GFP-linked protein. (C) Cells expressing CTL2 (R154) were recognized by a patient antibody specific for HNA-3a, but not by one specific for HNA-3b. (D) Cells expressing CTL2 (Q154) were recognized by anti-HNA-3b, but not by anti-HNA-3a. Reactions against nontransfected HEK293 were identical to those lacking the antigens for which antibodies used in testing were specific (not shown).
From a comparison of median fluorescence intensity signals obtained when anti-HNA-3a at various dilutions was incubated with HEK293 cells transfected with CTL2 (R154) and with T cells from a donor homozygous for HNA-3a, membrane expression of CTL2 on the transfected cells was judged to be comparable to that found normally on T cells (data not shown).
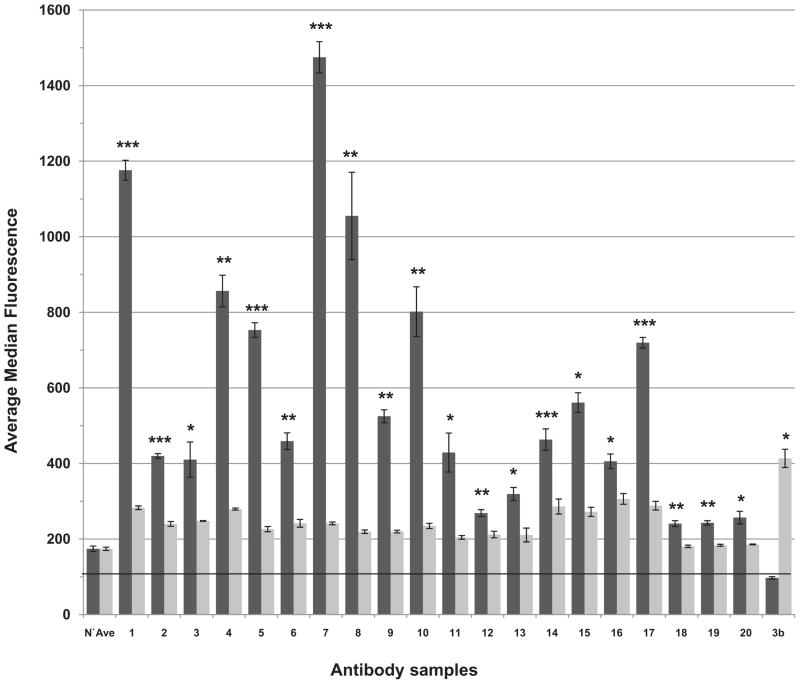
Cells transfected with CTL2 (R154) were preferentially recognized by each of 20 HNA-3a antibodies
Reactions of 20 different examples of anti-HNA-3a obtained from donors whose transfused blood was implicated as a cause of TRALI with the two cell lines are summarized in Fig. 2 where it can be seen that reactions of each antibody against cells expressing CTL2 (R154) were significantly stronger than those against cells expressing CTL2 (Q154). In contrast, an HNA-3b–specific antibody preferentially recognized CTL2 (Q154).
Fig. 2.
Reactions of HNA-3a and HNA-3b antibodies with HEK293 cells expressing recombinant CTL2. Studies were done using flow cytometry and 10 μL of serum containing HNA-3a antibodies from 20 blood donors implicated in TRALI reactions (designated 1–20) and one patient with an HNA-3b antibody (designated 3b). HNA-3a antibodies were tested in triplicate and the HNA-3b antibody was tested in duplicate. Each of the 20 HNA-3a antibodies reacted preferentially with cells expressing CTL2 (R154) (dark bars) and the single example of anti-HNA-3b reacted preferentially with CTL2 (Q154) (light bars). N’Ave = reactions of four unselected normal sera. Results shown are the median ± 1.0 SD. Significance of the differences observed (R154 vs. Q154) is indicated as *p < 0.05, **p < 0.01, and ***p < 0.001.
DISCUSSION
Genotyping studies have shown that R154 and Q154 of CLTL2 correlate perfectly with the HNA-3a–positive and -negative phenotypes, respectively,6,7,12 but did not rule out the possibility that antigen expression is influenced by other CTL2 polymorphisms or by posttranslational modifications of the protein. Our finding that each of 20 HNA-3a antibodies distinguished clearly between recombinant CTL2 constructs identical except for the R/Q154 polymorphism provides direct evidence that R154 in the context of CTL2 is both necessary and sufficient to create the HNA-3a epitope.
Mutations other than the R/Q mutation at Position 154 that correlates with HNA-3a/b antigen expression that predict amino acid substitutions at Positions 98 and 215 of the first extracellular loop of human CTL2, Position 413 of the third loop, and Positions 589 and 593 of the fifth loop are registered in the human genome SNP database and could conceivably influence binding of some HNA-3a or HNA-3b antibodies.13 However, none of these mutations was present in the CTL2 cDNA used for our transfections and none were identified in a recent study by Flesch and colleagues14 in which CTL2 cDNA was sequenced in 67 unrelated normal subjects. However, Flesch and colleagues did find undescribed mutations predicting an amino acid switch (153Leu>Phe) in the mature protein in two donors and one predicting a 301Thr>Met switch in a third.14 The former mutation appeared to affect binding of a minority of HNA-3a antibodies as judged by absent or decreased agglutination of neutrophils carrying CTL2 (Phe153).
The HEK293 transfectants appear to be highly specific and sensitive for antibody detection, considering that, to conserve material, only 10 μL of serum was used to obtain data shown in Fig. 2. We are currently examining whether transfected HEK293 cells can be stabilized with fixative for long-term storage and used to screen blood donors for anti-HNA-3a/b on a large scale. However, a recombinant CTL2 fragment sufficiently large to mimic the HNA-3a/b antigens as they exist naturally on blood cells could be adapted to a solid-phase assay and would be much more satisfactory for this purpose. The R/Q154 polymorphism that governs HNA-3a/b antigen expression is located in the first and largest extracellular loop of CTL2, comprising Amino Acid Residues 55 through 231.6–8 Failure of the CTL2 D131-K166 peptide to detect approximately 50% of HNA-3a antibodies9 suggests that some antibodies require additional CTL2 amino acid residues for high affinity binding. It is possible therefore that a larger recombinant fragment of the first loop, or perhaps the entire loop will be suitable for detection of all antibodies. However, this loop contains eight cysteines,8 at least some of which are probably disulfide-linked8; correct linkage of some of these disulfides may be necessary for certain examples of anti-HNA-3a to bind.9 Moreover, asparagine residues at Positions 187 and 200 are predicted to be glycosylated8 and could be required by some examples of anti-HNA-3a, as has been demonstrated for antibodies recognizing several other blood group antigens encoded by amino acid polymorphisms.15–19 We have not yet confirmed whether the full-length CTL2 isomers studied here (Fig. 2) are glycosylated but a recent report by Kommareddi and colleagues11 indicates that HEK293 cells do glycoslylate transfected CTL2. If correct disulfide linkages and/or glycosylation events in CTL2 are essential for detection of some HNA-3 antibodies, expression of a recombinant CTL2 fragment suitable for large-scale antibody screening is likely to be challenging.
Acknowledgments
This work was supported by Grant HL-13629 (RHA) and HL-106286 from the National Heart Lung and Blood Institute by a grant from GTI Diagnostics, Inc.
AJK, JAP, RHA, DWB, and BRC each contributed to research design, oversight of laboratory studies, interpretation of results, and manuscript preparation; AJK and MS performed laboratory studies, contributed to interpretation of findings made, and helped with manuscript preparation.
ABBREVIATIONS
- CTL2
choline transporter–like protein 2
- GFP
green fluorescent protein
Footnotes
CONFLICT OF INTEREST
A patent application covering typing for the HNA-3a/b antigens and detection of HNA-3a–specific antibodies has been filed by BloodCenter of Wisconsin. GTI Diagnostics (Brookfield, WI) has an agreement with BloodCenter of Wisconsin concerning technology for HNA-3 typing and detection of HNA-3 antibodies. These relationships involve only BRC and RHA. None of the other authors declare conflicts of interest.
References
- 1.Toy P, Popovsky MA, Abraham E, Ambruso DR, Holness LG, Kopko PM, McFarland JG, Nathens AB, Silliman CC, Stroncek D National Heart, Lung and Blood Institute Working Group on TRALI. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33:721–6. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 2.Flesch BK, Petershofen EK, Bux J. TRALI—new challenges for histocompatibility and immunogenetics in transfusion medicine. Tissue Antigens. 2011;78:1–7. doi: 10.1111/j.1399-0039.2011.01713.x. [DOI] [PubMed] [Google Scholar]
- 3.Davoren A, Curtis BR, Shulman IA, Mohrbacher AF, Bux J, Kwiatkowska BJ, McFarland JG, Aster RH. TRALI due to granulocyte-agglutinating human neutrophil antigen-3a (5b) alloantibodies in donor plasma: a report of 2 fatalities. Transfusion. 2003;43:641–5. doi: 10.1046/j.1537-2995.2003.00374.x. [DOI] [PubMed] [Google Scholar]
- 4.Reil A, Keller-Stanislawski B, Gunay S, Bux J. Specificities of leucocyte alloantibodies in transfusion-related acute lung injury and results of leucocyte antibody screening of blood donors. Vox Sang. 2008;95:313–7. doi: 10.1111/j.1423-0410.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 5.Silliman CC, Curtis BR, Kopko PM, Khan SY, Kelher MR, Schuller RM, Sannoh B, Ambruso DR. Donor antibodies to HNA-3a implicated in TRALI reactions prime neutrophils and cause PMN-mediated damage to human pulmonary microvascular endothelial cells in a two-event in vitro model. Blood. 2007;109:1752–5. doi: 10.1182/blood-2006-05-025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A, Wesche J, Hammer E, Fürll B, Völker U, Reil A, Bux J. Characterization of the human neutrophil alloantigen-3a. Nat Med. 2010;16:45–8. doi: 10.1038/nm.2070. [DOI] [PubMed] [Google Scholar]
- 7.Curtis BR, Cox NJ, Sullivan MJ, Konkashbaev A, Bowens K, Hansen K, Aster RH. The neutrophil alloantigen HNA-3a (5b) is located on choline transporter-like protein 2 and appears to be encoded by an R>Q154 amino acid substitution. Blood. 2010;115:2073–6. doi: 10.1182/blood-2009-11-248336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair TS, Kozma KE, Hoefling NL, Kommareddi PK, Ueda Y, Gong TW, Lomax MI, Lansford CD, Telian SA, Satar B, Arts HA, El-Kashlan HK, Berryhill WE, Raphael Y, Carey TE. Identification and characterization of choline transporter-like protein 2, an inner ear glycoprotein of 68 and 72 kDa that is the target of antibody-induced hearing loss. J Neurosci. 2004;24:1772–9. doi: 10.1523/JNEUROSCI.5063-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis BR, Sullivan MJ, Holyst MT, Szabo A, Bougie DW, Aster RH. HNA-3a-specific antibodies recognize CTL2 peptides containing arginine, but not glutamine at position 154. Transfusion. 2011;51:2168–74. doi: 10.1111/j.1537-2995.2011.03145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berthold T, Wesche J, Kuhnert K, Fürll B, Hippe H, Hoppen J, Reil A, Muschter S, Bux J, Greinacher A. Epitope mapping of antibodies directed against the human neutrophil alloantigen 3a. Transfusion. 2011;51:2160–7. doi: 10.1111/j.1537-2995.2011.03115.x. [DOI] [PubMed] [Google Scholar]
- 11.Kommareddi PK, Nair TS, Thang LV, Galano MM, Babu E, Ganapathy V, Kanazawa T, McHugh JB, Carey TE. Isoforms, expression, glycosylation, and tissue distribution of CTL2/SLC44A2. Protein J. 2010;29:417–26. doi: 10.1007/s10930-010-9268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reil A, Wesche J, Greinacher A, Bux J. Geno- and phenotyping and immunogenicity of HNA-3. Transfusion. 2011;51:18–24. doi: 10.1111/j.1537-2995.2010.02751.x. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Biotechnology Information. Database of single nucleotide polymorphisms (dbSNP) Bethesda (MD): National Center for Biotechnology Information, National Library of Medicine; (dbSNP Build 134). Available from: URL: http://www.ncbi.nlm.nih.gov/snp?term=Human%20CTL2. [Google Scholar]
- 14.Flesch BK, Reil A, Bux J. Genetic variation of the HNA-3a encoding gene. Transfusion. 2011;51:2391–7. doi: 10.1111/j.1537-2995.2011.03155.x. [DOI] [PubMed] [Google Scholar]
- 15.Take H, Tomiyama Y, Shibata Y, Furubayashi T, Honda S, Mizutani H, Nishiura T, Tsubakio T, Kurata Y, Yonezana T. Demonstration of the heterogeneity of epitopes of the platelet-specific alloantigen, Baka. Br J Haematol. 1990;76:395–400. doi: 10.1111/j.1365-2141.1990.tb06374.x. [DOI] [PubMed] [Google Scholar]
- 16.Djaffar I, Vilette D, Pidard D, Wautier JL, Rosa JP. Human platelet antigen 3 (HPA-3): localization of the determinant of the alloantibody Lek(a) (HPA-3a) to the C-terminus of platelet glycoprotein IIb heavy chain and contribution of O-linked carbohydrates. Thromb Haemost. 1993;69:485–9. [PubMed] [Google Scholar]
- 17.Socher I, Zwingel C, Santoso S, Kroll H. Heterogeneity of HPA-3 alloantibodies: consequences for the diagnosis of alloimmune thrombocytopenic syndromes. Transfusion. 2008;48:463–72. doi: 10.1111/j.1537-2995.2007.01550.x. [DOI] [PubMed] [Google Scholar]
- 18.Issitt PD, Wilkinson SL. Further studies on the dependence of some examples of anti-M and anti-N on the presence of red-cell-borne sialic acid. Transfusion. 1983;23:117–9. doi: 10.1046/j.1537-2995.1983.23283172846.x. [DOI] [PubMed] [Google Scholar]
- 19.Springer GF, Desai PR. Human blood-group MN and precursor specificities: structural and biological aspects. Carbohydr Res. 1975;40:183–92. doi: 10.1016/s0008-6215(00)82680-4. [DOI] [PubMed] [Google Scholar]