Abstract
Lassa virus (LASV) is the most prevalent arenavirus in West Africa and is responsible for several hundred thousand infections and thousands of deaths annually. The sizeable disease burden, numerous imported cases of Lassa fever (LF) and the possibility that LASV can be used as an agent of biological warfare make a strong case for vaccine development. Currently there is no licensed LF vaccine and research and devlopment is hampered by the high cost of nonhuman primate animal models and by biocontainment requirements (BSL-4). In addition, a successful LF vaccine has to induce a strong cell-mediated cross-protective immunity against different LASV lineages. All of these challenges will be addressed in this review in the context of available and novel animal models recently described for evaluation of LF vaccine candidates.
Keywords: animal models, Lassa fever, vaccine research and development
Lassa virus infection in West Africa
Lassa virus (LASV), a human pathogen of the Arenaviridae, is transmitted to humans by a rodent reservoir, Mastomys natalensis, and is capable of causing lethal Lassa fever (LF) disease. LASV has the highest human impact of any of the hemorrhagic fever viruses (with the exception of Dengue fever) with an estimated 100,000–300,000 infections and 5000–10,000 deaths annually in western Africa [1–4]. Based on the prospective studies performed in four of the most affected countries (Guinea, Sierra Leone, Liberia and Nigeria) Richmond and Baglole estimated that 59 million people are at risk of primary LASV infections with an annual incidence of disease as high as 3 million and as many as 67,000 deaths per year [4]. The current LF predicted areas cover approximately 80% of Sierra Leone and Liberia, 50% of Guinea, 40% of Nigeria, 30% of each of Côte d’Ivoire, Togo and Benin and 10% of Ghana [3]. LASV was also detected in Mali [5] and LASV antibodies were detected in the Central African Republic, Democratic Republic of Congo and Senegal. Some experts believe that the population at risk includes most of the population of West Africa from Senegal to Nigeria and can be high as 200 million [4]. Recently performed genome-wide scans [6,7] suggest that LASV may have been a driver of natural selection in LARGE and IL-21 genes implicated in LASV infectivity (entry into susceptible cells and immune responses, respectively) in the West African population. It supports the hypothesis that LASV is a prevalent arenavirus in West Africa and selective pressures imposed by LASV may have led to the emergence of particular alleles conferring resistance to LASV infection.
LASV, as any member of the Arenaviridae, has a bisegmented, single-stranded RNA genome [8], and each segment contains two genes in ambisense orientation. The L RNA encodes a large protein (L or RdRp) and a small zinc-binding (Z) protein [9,10] with viral matrix functions [11]. The S RNA encodes the major structural proteins, nucleoprotein (NP) and glycoprotein precursor (GPC), cleaved into GP1 and GP2 [12,13]. The GP1 is expressed on the viral surface and is responsible for interaction with the major cellular receptor, α-dystroglycan (α-DG), a ubiquitous receptor for extracellular matrix proteins [14]. Interaction of LASV GP1 with α-DG perturbs cross-talk between DG and β1 integrins, contributing to cellular dysfunctions that are associated with LF clinical manifestations [15]. Recently two C-type lectin family members, DC-SIGN and LSECtin, and two TAM family members, Axl and Tyro3, have been identified as additional LASV and lymphocytic choriomeningitis virus (LCMV) receptors [16,17], which may be responsible for replication in hepatocytes. The GP2 is a typical transmembrane class I fusion glycoprotein mediating pH-dependent endocytosis [18,19]. However, LASV enters host cells through an unique cholesterol-dependent, clathrin-, caveolin- and actin-independent pathway which employs the multivesicular body and is dependent on the endosomal sorting complex required for transport [20,21].
Based on their antigenic properties and geographic distribution, arenaviruses are divided into two complexes: the Old World (OW) arenaviruses (or LCMV–LASV complex) and the New World (NW) arenaviruses. The prototypic LCMV has a global distribution. LASV and other members of the OW group circulate in Africa and the NW arenaviruses circulate in South and North America. Several new viruses recently isolated in Africa rapidly expanded the OW group, which until recently included nonpathogenic viruses Mopeia (MOPV), Morogo (MORV), Mobala (MOBV) and Ippy (IPPYV) [22]. In addition, Kodoko virus (KODV), which is related to but distinct from LCMV, was isolated in Guinea [23]. In 2008 Lujo virus (LUJV) was identified in Southern Africa during a nosocomial hemorrhagic fever outbreak with unprecedented high case fatality rate [24,25]. Screenings of M. natalensis trapped in Zimbabwe, where the first patient infected with LUJV was identified, resulted in the isolation of Luna virus (LUNV) genetically related to MOBV [26]. Novel tentative African arenaviruses also include Merino Walk virus (MWV) isolated from a rodent, Myotomys unisulcatus, collected at Eastern Cape, South Africa [27], and Menekre and Gbagroube viruses, the sequences of which detected in Hylomyscus sp. and Mus (Nannomys) setulosus, respectively [28]. Notably, the Gbagroube sequence was closely related to LASV, while the Menekre sequence clustered with IPPYV–MOBV–MOPV. Detection of LASV-like sequences in Mussetulosus suggests that co-evolution of African arenaviruses and their hosts can potentially include host-switching events, predicting isolation of novel arenavirus species in the future.
Genetic diversity among LASV strains is the highest among the Arenaviridae, and causes a great challenge for vaccine development. Based on the partial NP sequences of 54 strains of LASV, Bowen et al. showed that LASV isolates comprise four lineages, three of which are found in Nigeria, with the fourth found in Guinea, Liberia and Sierra Leone [29]. This diversity even raised concern about the status of LASV as a single species [29–31]. The prototype LP strain isolated by Buckley and Casals in 1969 from Eastern Nigeria occupied the most basal lineage I. Strains isolated from Southern Central and Northern Central Nigeria were placed in lineage II and III, respectively. The largest group of strains isolated from Guinea, Liberia and Sierra Leone occupied lineage IV. Phylogenetic analysis suggests that Nigerian strains from lineages I and II diverged prior to strains from the northern part of central Nigeria, Guinea, Liberia and Sierra Leone. A fifth lineage, which falls between III and IV, has been proposed for the AV strain isolated from a patient that was infected (presumably) in Ghana or Ivory Coast [32].
LASV vaccine candidates
Given the high annual incidence rate and morbidity/mortality, it is arguable that LF is one of the most neglected tropical diseases, to the point that some have pointed out that if LF was a developed world problem; there would be vociferous demands for control measures and vaccine [33]. An effective LASV vaccine is urgently needed not only for the general population, but also for healthcare and lab workers, as well as for military and other service personnel in West Africa. The vaccination strategies may differ for the various recipient populations. Whereas a multi-dose immunization regimen might be practical for medical providers and for military personnel, a single-dose vaccine would be ideal in endemic areas, where most of the target population is poor and live far from healthcare facilities [33].
Different vaccination strategies were applied to design LASV vaccine candidates. These strategies included nonreplicating vaccine approaches (inactivated LASV preps, virus-like particles (VLPs), peptide-based and DNA vaccines) and replication-competent vaccine strategies (recombinant and reassortant vaccines). A favorable safety profile is the most attractive feature of inactivated (‘killed’) vaccines or VLPs, but these approaches in general have low immunogenicity and efficacy. Indeed, immunization of rhesus monkeys with a preparation of purified LASV that had been inactivated by γ-irradiation induced high-titer antibody response against the three major viral proteins (NP, GP1 and GP2). However, this immunization failed to protect the animals from the fatal disease. All vaccinated animals died and viral load in plasma was equal to unvaccinated control rhesus monkeys [34].
LASV-like particles produced by a transient expression of LASV GP, NP and Z genes in mammalian cells were immunogenic in mice [35]. Although the LASV VLP has not been tested in vaccination challenge so far, the protective efficacy of the VLP is expected to be negligible, even less than in the case of inactivated LASV, because the VLPs do not contain viral RNA that act as ‘built-in’ adjuvants [36–40] to mount an effective adaptive immune responses. As any killed virus, LASV VLPs are poor inducers of MHC-I-dependent T-cell responses. Another weakness of the LASV VLP approach is a high contamination of VLP preps with host-derived glycoproteins [35]. This contamination raises questions regarding the feasibility of this technology in a manufacturing environment. Taken together, although ‘killed’ virus or VLPs can induce antibody responses in experimental subjects, the prospect of these approaches translating into an effective vaccine strategy is low.
Another potential approach is epitope-based vaccines. Using computer-assisted algorithms, five HLA-A2.1-binding LASV GP peptides and two LASV NP peptides have been identified [41,42]. Although immunization of HLA-A*0201 transgenic mice with LASV GPC-derived epitopes showed some cross-protection against viral challenge with LCMV [43], peptide vaccination is unlikely to be applicable for LASV because of significant safety concerns. While injection of a peptide vaccine into naive individuals might be safe, administration of an epitope-based vaccine to individuals recently infected with the virus or in immune individuals previously exposed (perhaps unknowingly/asymptomatically), the pathogen can strongly re-activate the pre-existing CD8+ T clone and induce TNF-dependent immunopathology with serious clinical consequences [44]. In LF endemic areas of West Africa, clinically healthy individuals with undetectable levels of anti-LASV antibodies still had robust cell-mediated immune (CMI) responses to LASV recombinant proteins [45,46]. Currently there is no licensed peptide vaccine. Nevertheless, if the concept is proven, the peptide-based approach can be potentially useful for a target population with a low probability of previous LASV exposure (e.g., foreign personnel working in local hospitals, travelers and military personnel).
A LASV DNA vaccination approach was tested in a LCMV murine challenge model based on the existence of common CD8+ T-cell epitopes inducing cross-protective immunity. A DNA vaccine expressing LASV NP showed partial (50%) cross-protection against LCMV and Pichinde virus (PICV) challenges in mice. DNA vaccination with a single epitope (LASV NP118–126) also partially protected mice from LCMV but not from PICV intracerebral (ic.) challenge [47] Protection induced by the LASV NP118–126 minigen vaccine in this challenge model provided some rationale for making multi-CD8+ CTL epitope constructs (‘string of beads’ vaccines) [48]. However, these approaches share the negative consequences of the application of an epitope-based strategy in a population with a high prevalence of individuals previously exposed to LASV (see above). Intradermal electroporation of guinea pigs and cynomolgus macaques with recombinant DNA expressing LASV GPC completely protected animals against fatal LF [49]. However, DNA electroporation and multiple immunizations required to induce protective immunity will be difficult to implement in rural endemic areas of West Africa.
Replication-competent, ‘live-attenuated’ vaccines are among the most cost-effective and widely used public health interventions. The vaccines for smallpox, polio and yellow fever dramatically reduced the incidence of these infectious diseases. Currently, advances in molecular virology and rational design of replication-competent vaccines provide new opportunities for development of the next generation of these vaccines that optimally balance safety and effectiveness [50].
There are several reasons to justify ‘live’ vaccines as an attractive approach to control LF: CMI plays the major role in LF patient recovery as well as protection; a live vaccine provides the most effective natural pathway to process and present protective antigens to MHC molecules; epidemiological observations in LF endemic areas of West Africa provide evidence that a single (survived) exposure will induce long-term protection against disease; a vaccine candidate formulated to contain both LASV NP and GP antigens will induce a broad cross-reactivity and a large pool of CD4+ memory T cells against all phylogenetic groups of LASV (T-helper cell epitopes of LASV-exposed individuals carry sequences that are highly conserved between OW and NW arenaviruses); a single-shot immunization approach is crucial for populations of remote rural areas of West Africa, which have a very limited medical infrastructure and where implementation of prime-boosting immunization is not practical.
The first vaccine for the prevention of viral hemorrhagic fevers caused by arenaviruses is Candid #1, a live-attenuated vaccine against Argentine hemorrhagic fever. This vaccine is safe, highly immunogenic and efficacious. In 2007, this vaccine was included in the Argentine National Immunization Plan and significantly reduced morbidity and mortality caused by Junin virus infection [51]. Currently there are four replication-competent LASV vaccine candidates based on vaccinia virus [52–54], vesicular stomatitis virus [55,56], MOPV [57–59] and yellow fever 17D [60,61] vectors. All of these vaccine candidates have been tested in different animal models including nonhuman primates (NHP; see below). While vaccinia-vectored LASV proteins tested as vaccine candidates in guinea pigs and NHP provided valuable information for vaccine design, a vaccinia-based platform is not applicable for African countries with high prevalence of HIV-1. At least two other vaccine candidates, rVSV/LASV and MOP/LAS reassortant (clone ML29), fully protected vaccinated NHP against fatal LF and demonstrated promising safety profiles. Recently established reverse genetics systems for LCMV, LASV and MOPV provide a powerful tool for further improvements of these vaccines and for rational design of new generation of replication-competent vaccines [62–65].
Based on outstanding safety and efficacy records and recent success in the molecular biology of flaviviruses, the genetic backbone of Yellow fever virus (YFV) 17D vaccine has been used for construction of chimeric YFV17D-based viruses expressing prM and E proteins of closely-related flaviviruses, Japanese encephalitis, Dengue and West Nile virus. ChimeriVax™ (Sanofi, Paris, France)-based vaccines against these flaviviruses are currently undergoing Phase II–III clinical testing (reviewed by [66]). The recombinant YFV17D/LAS vaccine candidate was designed in attempts to develop a bivalent vaccine to control both infections, YF and LF, in overlapping areas of West Africa. The YFV17D/LASVΔGPC recombinant virus was replication competent, deeply attenuated, induced immune responses against both pathogens, YFV and LASV, and protected 80% of guinea pigs against fatal LF in proof-of-concept homologous LASV-Josiah challenge experiments [60,67]. Unfortunately, this vaccine failed to protect common marmosets against fatal LF [Lukashevich IS, Unpublished Data]. Additional research and development efforts are required before this valuable option will be a LASV vaccine for humans [49].
To some extent, alphavirus replicon technology provides a reasonable compromise, in terms of safety and immunogenicity, between ‘killed’ vaccines and replication-competent platforms. Alphavirus replicon particles are single-cycle, replication-defective vehicles (vectors). They are not able to spread beyond the initially infected cells, but can deliver and transduce the gene(s) of interest in target cells. Direct comparison of the immune responses induced by alphavirus-vectored vaccines and inactivated vaccines showed a clear advantage of the alphavirus-vectored platform and currently numerous vaccine candidates are in preclinical and clinical development (reviewed in [68]).
RNA replicon vectors derived from an attenuated Venezuelan equine encephalititis virus (VEEV) were successfully used to express LASV GPC and NP proteins, and guinea pigs vaccinated with these replicon particles were fully protected against fatal LF [69]. The VEEV-based replicon technology was also used to make a bivalent replicon for simultaneous expression of LASV genes (NP and GP) and genes from unrelated viruses, LASV GP and Ebola virus (EBOV) GP. Vaccination of guinea pigs with dual-expression particles protected the animals against challenges with both viruses, LASV and EBOV [69]. This proof-of-concept study showed that bivalent replicon can efficiently express different genes from the same virus or genes from unrelated viruses (LASV and EBOV). Notably, co-expression of two major antigens of the same pathogen (e.g., equine arteritis virus) induced higher protection and had a more favorable safety profile than replicons expressing individual equine arteritis virus envelope proteins [70]. The multivalent feature of this system is certainly beneficial to optimize LASV vaccine formulation (e.g., simultaneous expression of GPC and NP genes); to address LASV genetic diversity (e.g., to express GPC from distantly related clades I and IV); and to enhance immunogenicity of experimental vaccines (e.g., to express ‘wild-type’ GPC for conventional antigen presentation and metabolically stable GPC for cross-priming CD8+ T-cell responses). Naturally, LASV GPC is not a cross-presented antigen. Effective vaccines for cross-priming CD8+ T-cell responses should express metabolically stable antigens [71,72]. A recent study showed that long-lived stable antigens improved vaccine immunogenicity as compared with antigens subjected to accelerated proteosomal degradation [73]. This technology is currently being used to manufacture a bivalent cross-protective LASV vaccine for preclinical studies [Lukashevich IS, Pushko P, Unpublished].
The US FDA ‘Animal Rule’
While health authorities of endemic and nonendemic countries have to apply the internationally recognized regulatory standards to new preventive and therapeutic biologics against emerging infectious threats, it seems that regulatory priorities differ. In the absence of market-driving forces, the approval process in nonendemic countries is very slow. In the USA, a successful LASV vaccine candidate will be probably evaluated by the FDA based on the agency’s Animal Rule (21 CRF 601.90, subpart H, 2007). The Animal Rule was proposed for regulation of ‘biodefense’ biologics at the end of the last century and was implemented in 2002. Up to today, there are no FDA-approved vaccines based on these regulations. Meanwhile, in 2007 a live-attenuated vaccine Candid #1 against Argentine hemorrhagic fever, with a clinically proven efficacy of 95%, was licensed in Argentina and incorporated in the National Immunization Plan. This vaccine was jointly developed by Argentine and US scientists. Application of Candid #1 in Argentina tremendously impacted the magnitude of epidemic outbreaks [51,74]. In the USA, this vaccine has an investigational new drug status. Notably, randomized, double-blind, placebo-controlled Phase I–III clinical trials were performed on adult males in endemic areas of Argentina. These trials showed that Candid #1 was safe and highly efficacious (95%) [51].
The Animal Rule states that under specific circumstances, when human trials would be unethical and unfeasible, the FDA may grant marketing approval to a new vaccine following efficacy trials in adequate and well-controlled animal studies [75]. Whereas preclinical toxicity and efficacy studies under the Animal Rule are the same as for clinical trial products, efficacy studies must be performed in more than one well-established and developed animal models. To qualify for approval under the FDA Animal Rule at least two animal models mimicking the pathophysiological mechanisms of human LF must be established in which correlates of protection must be clearly defined. It means that a strong relationship between the end point of the animal study and the prevention of the disease in humans must be demonstrated.
A solid body of evidence indicates that the clearance of LASV does not correlate with antibody induction and LASV is a poorly neutralized virus. Clinical and experimental studies in infected NHPs showed that viremia and circulated antibodies were detected simultaneously in LF patients and infected animals (reviewed in [1,2,33]). At the time of hospital admission, LASV antibodies were not associated with survival or positive prognosis. By contrast, the presence of LASV antibodies detectable in IFA early in the course of the disease correlated with fatal outcome, not survival [76]. Recent studies confirmed the previous observation and showed that patients simultaneously containing LASV antigen and specific IgM antibodies have significantly higher chances (>four-times) of death in comparison to patients with IgM alone [77]. Taken together, these results clearly indicate that during natural infection, protection and recovery are not associated with LASV antibody responses. Nevertheless, earlier studies in experimental animals and anecdotal evidence of passive antibody therapy indicate that neutralizing antibodies could be protective if they were available at sufficiently high titers [78–80].
To comply with the FDA regulations, valid animal models have to mimic major pathophysiological features of human LF disease. Although the pathogenesis of LF is still not clearly understood, a severe LASV infection in humans is a systemic disease and is characterized by unchecked viremia, lymphopenia, functional liver damage, vascular abnormalities and profound suppression of innate and adaptive immune responses (reviwed in [1,81]). LASV replicates in target tissues (liver, lung, spleen, lymph nodes, kidney and adrenal gland) without cytopathic effect, and the pathological damage to these tissues is usually not sufficient to implicate organ failure as the cause of death [82–84]. Death from LF is caused mostly by uncontrolled sepsis-like terminal cardiogenic shock and internal bleeding. Infection of target cells, macrophages and dendritic cells (DCs) fail to activate these cells and impair their antigen-presenting functions. The role of type I and II interferons (IFNs) are not clear. In humans LASV is a poor type I IFN inducer, LASV itself is relatively resistant to antiviral activities of IFN and the IFN sensitivity of LASV isolates does not correlate with disease progression [85]. In addition, recent experiments with genetically engineered live-attenuated arenaviral vaccine prototypes showed that IFNs are not required for the induction of potent adaptive immune responses [62]. In general, host factors such as a cell receptor polymorphism [7], innate immunity [86–89], proinflammatory cyto/chemokines [89–92], adaptive cell-mediated immune responses [45,46] as well as differences in the viral replicative capacity of LASV isolates [93] seem to play a role in the outcome of LASV infection in humans. A valid animal model has to reasonably mimic major pathophysiological features of human LF and replicate the human immune responses to LASV challenge. Intensive discussions during the recent Animal Models workshops sponsored by NIH resulted in understanding that different animal species can be used to mimic key patho-physiological features of human disease [94]. For example, guinea pigs are the most sensitive model to study lung pathology [95–97], while common marmosets (CM) [98] and a surrogate model of LASV hepatitis in LCMV-WE-infected rhesus macaques [99,100] are well positioned to study liver involvement. The immunogenic potency of LASV vaccine candidates can be also tested in mice [101]. Nevertheless, rhesus and cynomolgus monkeys are the most appropriate animals for challenge (efficacy) studies (see below).
LASV infection of mice & other experimental rodents
LASV in mice
The mouse model provides a great variety of immunological tools and the availability of knockout mouse strains can potentially be very helpful in elucidation of distinct mechanisms of LASV-induced pathology. The major problem is that LASV, a rodent-borne virus, is treated differently by the immune system of rodents and humans/NHPs [52,54]. A virus–host interaction in a natural rodent host is a complex interplay to keep balance between viral replication and host immune responses. On the other hand, in experimental settings, rodent models can be helpful to provide insights into mechanisms of LF pathogenesis and protective immune responses, providing an economical method of evaluation of vaccine candidates.
In general, the outcome of arenavirus infection in mice is complex, depends on host and viral factors, and can result in three major scenarios: a nonlethal acute infection with quick recovery mediated, at least in case of LCMV and LASV, by robust lifelong CMI; an acute lethal infection caused by immunopathological reactions; and a persistent infection that can be asymptomatic or can result in some nonfatal clinical manifestations [102]. Intracranial inoculation of LASV into adult immunocompetent mice resulted in clinical signs closely resembling those seen in adult mice infected with LCMV (Table 1) [103,104]. Brain histology of LASV-infected mice revealed inflammation of the meninges, the choroid plexus, the ependymal lining of the ventricles and the nearby neuroparenchyma similar to those described for LCMV pathology in adult mice [Lukashevich IS, Unpublished Data]. Outbred mice aged 3–6 weeks were the most sensitive to ic. inoculation of LASV and aging of mice slightly reduced the sensitivity to LASV challenge. In addition, in line with the LCMV mouse model, LASV ic. infection was not lethal for suckling mice. By contrast, MOPV, which is completely attenuated in guinea pigs and NHPs, killed 50–100% of suckling mice and this phenotype was mapped to the L RNA segment of MOPV [57,103,105,106].
Table 1.
Animal models of human Lassa fever.
| Virus/dose | Animals | Route of inoculation | Lethality/clinical features | Containment | Application | Ref. | |
|---|---|---|---|---|---|---|---|
| Rodent models of Lassa fever | |||||||
|
| |||||||
| LASV (Jos), 103 PFU | Outbred mice: | 2-day-old | ic. | 0% | BSL-4 | Antiviral research | [96,102] |
| 28-day-old | ic. | 87%, LCMV-like disease | [107,109] | ||||
| C3H/Sn: | 28-day-old | ic. | 100%, LCMV-like disease | BSL-4 | Antiviral research | [103,106] | |
| 28-day-old | sc. | 47%, LCMV-like disease | [107] | ||||
| 28-day-old | im. | 80%, LCMV-like disease | |||||
| C57BL/6: | 2-day-old | sc. | 44%, LCMV-like disease | BSL-4 | Antiviral research | [103,106,107] | |
| 50-day-old | sc. | 60%, LCMV-like disease | |||||
| CBA/J: | 28-day-old | ic. | 100%, LCMV-like disease | BSL-4 | Immunogenicity studies | [58,61,100] | |
| 28-day-old | ip. | 0%, full protection against ic. challenge | |||||
|
| |||||||
| LASV (Ba366), 106 PFU | HHD (tgHLA-A2.1/C57BL/6) | iv. | 22%, systemic disease, viremia, immunopathology | BSL-4 | Pathogenesis | [116] | |
|
| |||||||
| LASV (Jos) | STAT1 KO | ip. | 100%, ruffled fur, lethargy, paresis, convulsions, immunopathology | BSL-4 | Pathogenesis | [113,114] | |
|
| |||||||
| LASV, different strains, 103 PFU, LD50: 0.3 PFU | Strain 13 guinea pigs | sc., ip. | 100%, systemic infection mimicking human LF with predominant lung pathology | BSL-4 | Antiviral/pathogenesis Vaccine safety/efficacy |
[52,61,69, 95,96] | |
|
| |||||||
| Hartley guinea pigs | sc., ip. | ~30% | BSL-4 | Pathogenesis (host factors: fatal vs nonfatal infection) | [117] | ||
|
| |||||||
| LASV in nonhuman primates | |||||||
|
| |||||||
| LASV, different strains, 103 | Cynomolgus | sc., im. | 100%, systemic infection mimicking human LF disease | BSL-4 | Antiviral/pathogenesis | [34,54,56] | |
| Rhesus monkeys | sc., im. | Vaccine safety/efficacy | [88,96,118–124,128] | ||||
| PFU | Common marmosets | sc. | 100%, systemic infection mimicking human LF disease | BSL-4 | Antiviral/pathogenesis Vaccine safety/efficacy |
[59,97] | |
|
| |||||||
| Surrogate models of Lassa fever | |||||||
|
| |||||||
| PICV An 4763 | Golden Syrian hamsters | ip. | Severe infections with vascular dysfunctions | BSL-2 | Pathogenesis/antivirals | [138] | |
|
| |||||||
| PICV, host adopted, 2 × 103 PFU | Strain 13 guinea pigs | ip. | Systemic infection with some features resembing LF | BSL-2 | Pathogenesis/antivirals | [136,137] | |
| Hartley guinea pigs | ip. | Pathogenesis/antivirals | [132–135] | ||||
|
| |||||||
| PIRV VAV-488 | Golden Syrian hamsters | ip. | 100%, systemic disease with some features resembling human LF | BSL-3 | Pathogenesis/antivirals | [139] | |
|
| |||||||
| LCMV-WE, 103 PFU | Rhesus monkeys | iv. | fatal infection resembling human Lassa hepatitis | BSL-3 | Pathogenesis/vaccine | [98,99,140] | |
|
| |||||||
| SHEV, 5 × 101–5 × 105 PFU | Rhesus monkeys | im. | 64%, severe systemic disease with some features resembling human LF | BSL-2 | Pathogenesis/vaccine | [141] | |
ic.: Intracerebral ; im.: Intramuscular; ip.: Intraperitoneal; iv.: Intravenous; sc.: Subcutaneous.
The response to LASV varied markedly among different mouse strains and was also dependent on the age of the mice and the route of inoculation. Based on the sensitivity to ic. LASV infection, all tested mice were grouped into three categories: highly susceptible mice with fatality rate from 80 to 100%; relatively resistant mice with lethality approximately 30–60%, and resistant mice [104,107,108]. Among tested lineages, the first group included mice with H-2k haplotype, C3H/Sn (newborn and up to 4-week-old) and CBA/c mice (3–4-week-old). Aging of these mice was associated with the increased resistance to the infection and C3H mice of 9 weeks or older were almost unsusceptible to LASV infection. The second group included mice with the same H-2k haplotype, AKR and CBA/c (6–7-week-old), and C57Bl mice with H-2b haplotype. The resistant group was represented by Balb/c mice (H-2d) and C3H mice older than 9 weeks. Restricted cross-breeding experiments with (CBA/c × Balb/c)F1 or (CBA/c × C57Bl/6)F1 as well as testing of susceptibility or resistance to LASV infection of the backcross progeny between F1 hybrids and parental strains did not reveal any dominant patterns [Lukashevich IS, Godneva AT, Unpublished Data].
It is obvious that ic. inoculation leading to fatal meningitis in mice does not reflect pathogenesis in humans. Based on a LCMV-induced meningoencephalitis in mice, it is estimated that after disruptive ic. inoculation, more than 90% of the inoculum goes into the blood and less than 10% is retained to be replicated in the CNS. The virus released in blood induces robust expansion of virus-specific CD8+ CTLs, while the virus released in the CNS, a site of ‘immune privilege’, replicates virtually unchecked in leptomeningeal or choroid cells of the brain. Massive killing of the CNS cells with virus-specific CD8+ CTLs results in destruction of infected cells, increased intracellular pressure, brain edema and fatal meningitis [102,109]. We can speculate that pathogenesis of fatal disease in mice infected with LASV by the ic. route shares the immunopathological pathway with a LCMV-induced meningoencephalitis. At least, similar to LCMV infection, suppression of T-cell responses by cyclophosphamide treatment or by x-ray irradiation prevented fatal meningitis in LASV-infected mice [108].
Influence of other than ic. routes of infection on outcome of the LASV disease was studied in young adult outbred, CBA/c and C3H/Sn mice. Outbred mice and CBA/c mice fell into two opposite groups according to the outcome of the disease. Ic. inoculation killed almost all mice (80–100%), while intraperitoneal (ip.) or subcutaneous (sc.) infection did not produce any clinical signs of the disease. Notably, ip.-inoculated mice were fully protected against subsequent ic. challenge with LASV and this protection was associated with robust CD8+ CTL responses. Adoptive transfer of splenocytes from CBA donors ip.-inoculated with LASV prevented the development of lethal disease in recipient mice ic. challenged with LASV [57,110].
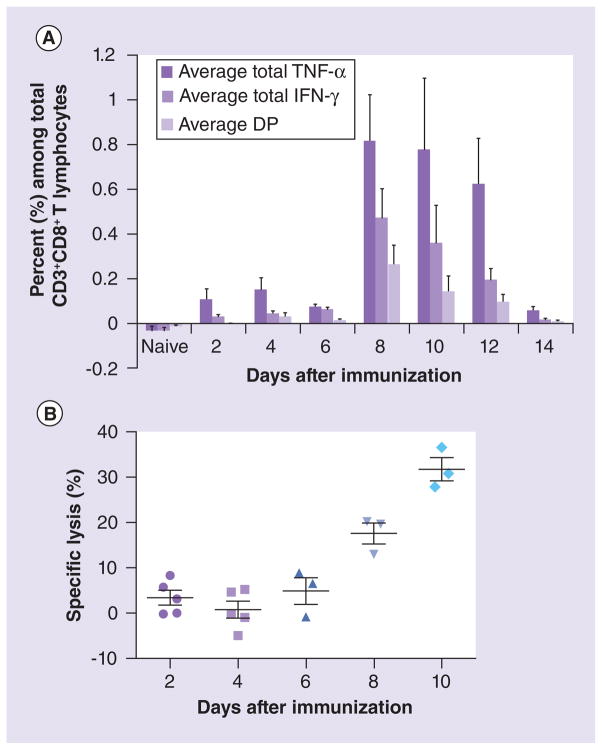
Although mice do not accurately model human LF disease, they can provide an economical assay to determine vaccine potency via the capacity of vaccine candidates to elicit protective CMI responses. This type of small animal model is especially needed when promising vaccine technology will be transferred from the laboratory to the manufacturing environment. In addition, this model must provide a lower level of biocontainment because pre-clinical vaccine development in the BSL-4 is not practical. In a recently described CBA/J-ML29 model [101], a T-cell cytotoxicity assay in vivo showed a correlation between LASV-specific cytotoxicity and protection induced by the LASV vaccine candidate (ML29). LASV-specific T-cell responses detected by IFN-γ ELISPOT and by intracellular cytokine staining peaked at day 8–10 when more than 30% target splenocytes pulsed with LASV GPC peptides were lysed in vaccinated mice (Figure 1). Notably, ML29-immune splenocytes protected mice in a dose-dependent manner and effectively cleared LASV from tissues. Recipient splenocytes depleted of CD8+ T cells did not protect mice against lethal challenge [58,101]. This model has been validated in common marmosets. Inoculation of 1000 PFU of ML29 induced in these animals sterilizing CMI responses and completely protected marmosets against fatal LF disease [59]. Based on these results, specific lysis of approximately 25–30% splenocytes pulsed with LASV GPC peptides and inoculated into CBA/J mice immunized with a LASV vaccine candidate can be established as a conservative end point, a surrogate of protection for NHPs. The reassortant ML29 can be also considered as a positive ‘vaccination control’ in this assay. The CBA/J-ML29 model can therefore be a useful immunological tool for evaluation of immunogenicity and efficacy of LASV vaccine candidates outside of BSL-4 containment facilities because this model can be used in BSL-3 containment [101].
Figure 1. Lassa virus-specific T-cell cytotoxic activity in vivo correlates with specific T-cell responses in vitro.
(A) Lassa virus (LASV)-specific T-cell responses detected by intracellular cytokine staining. The ML29-immune splenocytes were collected from mice (n = 5) at different time points after immunization, incubated with GPC peptides and then stained for either CD3 and CD4 surface markers or CD3 and CD8 surface markers to evaluate the LASV-specific responses in these two T-cell populations. Subsequently, cells were permeabilized and stained for IFN-J and TNF-D to evaluate the ability of these T lymphocytes to produce proinflammatory cytokines in response to LASV GPC stimulation. Frequencies shown are based on CD3+CD4+ gated T lymphocytes. DP for IFN-γ and TNF-α. (B) Detection of LASV-specific CTL. Mice (n = 5) were immunized with a single-dose of ML29 at the designated time points prior to CTL assay. At each time point, immunized mice received 5 × 106 target splenocytes from naive mice stained with CFSE and pulsed with LASV GPC peptides (Ag+) and 5 × 106 target splenocytes stained with Cell Tracker™ Far Red and left unpulsed (Ag−). Percent specific lysis was determined by the ratio of recovered Ag-labeled and nonlabeled target cells and adjusted for background from naive recipients. Only mice with >5000 recovered CFSE events were used for data analysis.
DP: Double positive; GPC: Glycoprotein precursor.
Adapted with permission from [101].
Recently some mouse strains with defects in innate and adaptive immunity were tested for susceptibility to NW arenaviruses using a nonintracranial route of challenge. 129AG mice lacking α/β and γ IFN receptors (IFN-α/βγR−/−) and mice lacking signal transducer and activator of transcription 1 factor (STAT-1 KO mice) were partially susceptible to Junin virus [111], TCRV [112] and MACV [113] and developed disseminated infection with some histological changes mimicking human pathology. By contrast, LASV did not induce fatal infection in IFN-α/βγR−/− mice [114]. The infected mice experienced only transient weight loss around day 11 post-inoculation and fully recovered their weight by day 17. Meanwhile, mice lacking only type I IFN receptors (IFN-α/βR−/−) developed some signs of the disease and did not fully recover their weight. Both groups of mice developed dissimilated infection, but IFN-α/βR−/− mice cleared infection more efficiently. However, the histological changes in brain and visceral tissues (lungs, liver and kidneys) were more prominent in IFN-α/βR−/− mice. This inconsistency was continued in STAT-1 and IFN-γ KO mice. Inoculation of 104 PFU of LASV induced clinical signs of the disease, including paresis and convulsions, in STAT-1 KO mice and resulted in lethal outcome for four out of six infected mice [115]. Meanwhile, all IFN-γ KO mice recovered and survived after inoculation with the same dose of LASV and only infection with very high doses (106 PFU) killed two out of six IFN-γ KO mice and all SAT-1 KO mice. Notably, LASV infection of genetically-engineered mice with different defects in innate and adaptive immune responses (Rag-2, NOD, NOD/SCID, KO CD4, KO CD8 and KO B cells mice) did not induce manifested disease.
These results provide additional evidence that LASV infection is treated fundamentally differently by the immune systems of rodents and humans/NHPs. As it has been mentioned, in humans LASV is a poor type I IFN inducer, LASV itself is relatively resistant to IFN-I, and sensitivity of LASV isolates does not correlate with disease progression. Recovery and protection in humans are dependent on T-cell responses and the role of antibody in natural infection is minimal if any. Experiments in CD4, CD8 and B-cell deficient mice infected with LASV indicate that adaptive immunity is not required to control LASV infection in mice. Involvement of STAT1-mediated IFN-responses in susceptibility of mice to LASV clearly indicates that the murine IFN system contributes to the delicate balance between virus replication and host innate immunity to establish and/or maintain persistent infection in natural hosts. In good confirmation with that, NP proteins of all tested arenaviruses, independently on their pathogenic potential for humans, block IRF3 and inhibit IFN responses. There is only one notable exception, NP of TCRV [116], which is not a rodent-borne virus.
An interesting observation was made in HHD mice genetically engineered to express a human/mouse-chimeric HLA-A2.1 instead of the murine MHC Class I gene products. In these mice, LASV induced disease with histological alterations resembling some features of human LF [117]. While MOPV-induced T-cell immunity protected HHD mice against LF, T-cell depletion of LASV-infected HHD mice prevented disease, implicating T-cell involvement in LASV pathogenesis. Notably, only one isolate, LASV Ba366/Guinea strain, efficiently replicated and induced disease in five out of 23 HHD mice iv. inoculated at very high dose, 106 PFU/mice. All other tested LASV strains, Josiah/SL, Lib90/Liberia, AV/IC/BF, CSF/Nigeria, failed to induce disease in HHD mice. Is not clear why only this strain induced pathology in 22% of HHD mice. It seems that T cells involvement described in this model is an unique feature of HDD mice inoculated with Ba366 strain of LASV. Notably, T cells-mediated pathology was never observed neither in LF patients nor in experimentally infected NHPs [1,2,33,97].
LASV in guinea pigs
Strain 13 (inbred) and Hartley (outbred) guinea pigs are widely used for studying arenaviral hemorrhagic fevers and for testing potential therapeutics and vaccine candidates (Table 1). In strain 13 guinea pigs, acute LASV infection (subcutaneous) resulted in fever, weight loss and death within 2 weeks after infection [96,97]. The inbred guinea pigs data were best fit by an exponential dose–response model but the outbred guinea pigs did not. The inbred animals are very sensitive to the virus with LD500.3 PFU. Hartley guinea pigs are more resistant to the infection and even a dose of 2 × 105 PFU killed approximately 30% of the infected animals. Passages of LASV in outbred guinea pigs resulted in selection of LASV strains with higher pathogenic potentials and fatality rates [118].
High viremia and viral loads in tissues (8–9 logs10 PFU) have been associated with LASV infection in strain 13 guinea pigs with observable titers usually occurring 4 days after infection and peak viremia occurring 10–12 days after infection. Viral titers have been lower in Hartley guinea pigs and viral replication appears to be limited in these animals when compared with strain 13 guinea pigs. High LASV titers were detected in lymph nodes, salivary gland, spleen, pancreas and lung very early after infection of strain 13 guinea pigs. Virus replication was also observed in the liver, kidney, adrenals and heart and low viral titers were recovered from the brain. LF in strain 13 guinea pigs was associated with liver damage, as evident by increased levels of liver enzymes in plasma. Clinical chemistry and hematology data also showed the reduced levels of serum albumin, lymphopenia and neutrophilia. Both the strain 13 and Hartley guinea pigs develop similar antibodies in response to LASV infection; however, neutralizing antibodies only develop in animals that survived 20–30 days after infection and increased over time. In most cases, interstitial pneumonia was the most prominent histological finding in LASV-infected guinea pigs. Mild myocarditis and necrotic lesions can occur in kidney and spleen, while lesions in the adrenals, salivary glands and brain are rare. In all, the pathology associated with LASV infection in strain 13 guinea pigs and the similarity to human disease makes these animals attractive for studying LF pathogenesis, especially for lung involvement. However, adaptive CMI responses induced by vaccination are different in guinea pigs and in NHPs [52,54].
Models of LF in NHPs
The NHPs are the only relevant challenge model for human LF. Closely related rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) monkeys have been most extensively used for evaluation of promising vaccine candidates or treatment (Table 1) [54,56,95,119–123]. LASV infection of NHPs resulted in fever, anorexia, weight loss and depression. In terminal stages, acute respiratory syndrome and hypothermia were observed in fatally infected monkeys. In most of these studies, necropsy was performed at the termination stage of the disease. Recent studies focusing on early stages of the LF in NHPs [124] and immune responses [125] confirmed previous observations and re-established markers of fatal LF: unchecked viremia, elevated liver enzymes, low or undetectable levels of proinflammatory cytokines (IL-1β, TNF-α, IL-8 and IP-10), and low and/or ineffective T-cell activation. In addition, high levels of IL-6 was found as an additional biological marker of fatal disease linked to hepatocyte regeneration, which had been previously described in fatally infected LF patients [84] and was recently confirmed in our surrogate model of LF hepatitis in rhesus macaques [99,100]. These studies also showed that early and strong T-cell responses were associated with effective control of virus replication and recovery. These observations are in good correlation with data obtained in human DC–T-cell cocultures. LASV and MOPV infections demonstrated very different responses in this system. While infection of MOPV resulted in generation of robust and functional CD4+ and CD8+ CTL responses, LASV infection induced only weak memory responses in human DC–T-cell cocultures [126].
NHPs can also serve as a valuable model for immunocompromized individuals in LF endemic areas in West Africa. Nigeria, the most LF-affected country in West Africa, is also ranking as the second worst HIV-1-affected country in the world. Our preliminary studies showed that there is intriguing overlap between the regions with high HIV-1 seroprevalence and LASV endemic areas in Nigeria. In IgG ELISA, a significantly higher number of LASV antibody positive samples were found among HIV-positive versus HIV-negative individuals (p < 0.005) [Abimiku A, Lukashevich IS, Unpublished Data]. These observations indicate that safety and immunogenicity of LASV vaccine candidates must be validated in immunocompromized animal models mimicking the HIV-1-affected population in West Africa. The successful application of simian-human immunodeficiency virus (SHIV)-infected rhesus macaques was already demonstrated for evaluation of the safety and efficacy of a rVSV-based vaccine against fatal EBOV infection [127]. In the recent study, an attenuated LASV vaccine candidate, ML29 [58], was tested in simian immunodeficiency virus (SIV)-infected rhesus macaques for its ability to elicit immune responses without instigating signs of LF disease. SIV-infected and uninfected rhesus macaques responded similarly to ML29 vaccination and none developed chronic arenavirus infection or signs of arenavirus disease. Notably, even ML29 was inoculated into a SIV-infected animal with high SIV loads and wasted appearance, a ML29 viremia was only barely-detectable. Seven out of the eight SIV-infected and ML29-vaccinated macaques had vigorous ML29-specific CMI responses which were also cross-protective against LCMV-WE in challenge experiments [Zapata JC, Poonia B, Bryant J et al. An attenuated Lassa vaccine in SIV-infected rhesus macaques does not persist or cause arenavirus disease but does elicit LASV-specific immunity (2012), Submitted]. Results of these two studies suggest that live-attenuated vaccines against LF (ML29) and pathogenic filo-virus (rVSV expressing EBOV GP) can be safe and immunogenic even in immunocompromied individuals.
Efficacy studies in NHP in BSL-4 containment are extremely costly. In addition, there is currently a shortage of rhesus macaques for biomedical research [128]. The development of less expensive and more reliable models of human LF for LASV vaccine research is therefore warranted. The common marmosets (CM, Callithrix jacchus) are small anthropoid primates that generally weighs between 320 and 450 g. The relatively small size of marmosets translates to lower cage and feeding costs and eases handling in a biosafety environment; these features confer substantial benefits when compared with the use of macaques. Completion of sequence analysis of the entire CM genome will clarify the genetic similarity between the CM and humans and will provide access to reliable immunological tools. Because of these advantages, CMs have been widely used in many studies involving gene therapy, bacterial infection, toxicology, immunology and vaccine development [128–131].
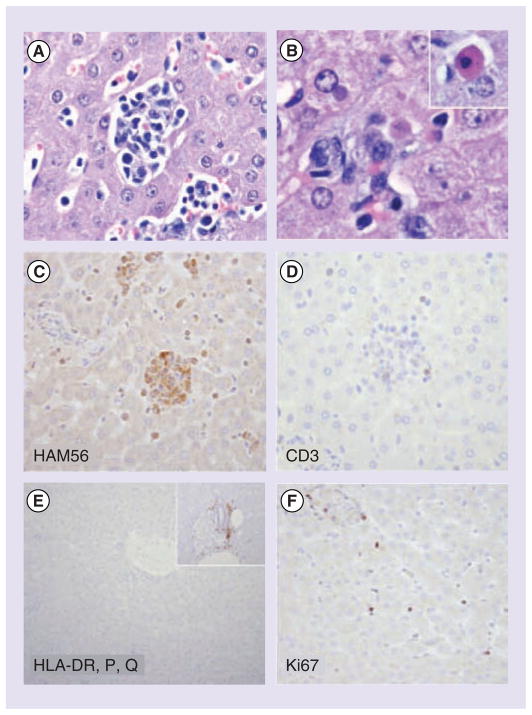
Experimental infection of CM with LASV resulted in a systemic disease with high viremia and viral RNA load in tested tissues, elevated liver enzymes, decreased plasma albumin, weight loss and severe morbidity, the latter that manifests 15–20 days after inoculation [98]. Morphological features mirror those described for human cases of fatal LF and include hepatic and adrenal necrosis, lymphoid depletion and interstitial nephritis. Immunochemistry studies of liver and lymphoid tissues revealed marked reduction in CD3+, CD20+ cells, the intensity of HLA-DP, DQ, DR staining and expression of MHC Class II molecules (Figure 2). These observations provided the first experimental evidence that replication of LASV in tissues is associated with immunological alterations that reflect an impaired adaptive immune response [98]. CM have been successfully used to evaluate safety, immunogenicity and efficacy of LASV vaccine candidate ML29 [59]. This study provides evidence that LASV infection of marmosets corresponds well with human LF, like the macaques. Challenge studies in CM will considerably reduce cost, especially if breeding colonies are established in-house. LASV infection of marmosets could therefore be the second ‘small’ NHP model to comply with the FDA Animal Rule. Recently, CM was proposed as an additional challenge model for EBOV and MARV vaccine studies [Hensley L, Pers. Comm.]
Figure 2. Hepatic pathology in Lassa-inoculated common marmosets.
(A) Multifocal random hepatic necrosis accompanied by a mixed inflammatory cell infiltrate consisting of macrophages and lymphocytes. (B) Degenerate hepatocytes contained well-circumscribed eosinophilic to amphophilic cytoplasmic inclusions, and individual hepatocyte necrosis was apparent (inset, Councilman body). Cellular aggregates in regions of hepatocellular necrosis were largely composed of HAM56 macrophages and devoid of (C) CD3-positive and (D) CD20-positive cells. (E) A marked reduction in the intensity of HLA-DP, DQ, DR staining was observed relative to normal control tissue (inset). (F) Increased numbers of cells positive for the proliferation marker Ki67/MIB1 were observed.
Surrogate models of LF
A limited access to BSL-4 facilities and the great expense of conducting studies under the highest level of containment resulted in development of surrogate models of LF in experimental animals (Table 1). Infection of guinea pigs with PICV, a risk group 2 pathogen, has been established in Hartley and strain 13 guinea pigs to study some mechanisms of LF-like pathogenesis. In Hartley guinea pigs a low passage 2 of PICV induced a mild disease, while a highly adopted strain (passage 18) caused a fatal disease with symptoms mimicking some features of LF [132]. In infection caused by PICV P18 strain, suppression rather than overexpression of proinflammatory cytokines was associated with terminal shock [133]. Attenuated and lethal variants of PICV induced differential patterns of NF-κB activation [134] in good confirmation with recent observations made on pathogenic OW arenaviruses [89]. Forty-eight nucleotide changes differentiate P2 and P18 PICV virus strains and most nonconservative amino substitutions were located in L and GP1 proteins. Reverse genetics analysis showed that mutations in both proteins are required to generate pathogenic strain P18 [135].
PICV was also adapted to induce fatal disease in strain 13 guinea pigs [136]. In this model, splenic macrophages and Kupffer cells were found as primary targets of the infection followed by hepatocytes, adrenal spongiocytes and alveolar macrophages. In infected animals, water and food intake decreased rapidly after day 8, contributing to weight loss. Severe involvement of the adrenal cortex suggested loss of electrolyte control and impairment of mineralocorticoid secretion. Fatty changes in liver additionally contributed to metabolic abnormalities. At gross necropsy, enlarged liver was the most prominent finding and signs of hemorrhage were not detected [137]. In this model, liver pathology was more prominent in comparison to LASV-infected guinea pigs (see above).
Infection of golden Syrian hamsters with 5 PFU (ip.) of nonadapted PICV strain An4763 was used to study vascular permeability by infusion of Evans blue into infected animals [138]. Two days after infection the virus was detected in the liver, kidney, spleen and lungs. On days 4–5, a significant drop in plasma albumin was observed followed by elevated levels of ALT and AST and pro-inflammatory mediators. On day 7, vascular leak, peaks of viral loads in tissues and high concentrations of plasma liver enzymes were observed with initial signs of weight loss. The vascular leakage was also observed in hamsters infected with YFV. Taken together, these results indicate that this model may be helpful to study vascular abnormalities during infections caused by hemorrhagic fever viruses and for evaluation of new interventions to control virus-induced vascular pathology.
Infection of hamsters with non-adopted Pirital virus, a risk group 3 NW arenavirus, resulted in weight loss, hemorrhage manifestations and signs of neurologic disorders [139]. The infected animals had high viremia, leukocytosis, coagulopathy, pulmonary hemorrhage, edema, hepatocellular and splenic necrosis, and high levels of serum transaminases. All of the Pirital virus-infected animals died within 9 days and pneumonitis and hepatocellular necrosis were most prominent necropsy findings. This model could serve as a low-cost and relatively safe alternative for studying the pathogenesis and testing antiviral compounds.
The systemic infection of rhesus macaques with the WE (vis-cerotropic) strain of LCMV induced a fatal LF-like disease characterized by disruption of liver functions and by strong hepatocyte proliferation [99,100]. LCMV-WE caused a rise in IL-6 and soluble receptors for IL-6 and TNF concomitant with a rise in viremia in good correlation with elevated levels of IL-6 in plasma observed in LASV-infected cynomolgus monkeys [125] and in LF patients [77]. The elevated IL-6 levels seem to be associated with hepatic proliferation and may serve as a marker of disease progression in addition to viremia and elevated liver enzymes in plasma.
Mucosal inoculation of rhesus macaques with LCMV-WE resulted in attenuated infection with a transient viremia and liver enzyme abnormalities [140]. The ARM strain of LCMV, a neurotropic variant highly adopted to mice, is sharing 88% amino acid homology with WE. The infection with ARM did not cause a manifested disease in monkeys but was able to induce robust CMI responses fully protecting animals against challenge with LCMV-WE [100]. The protected animals had no signs of hepatitis and hepatocyte proliferation.
Infection of NHPs with WE strain of LCMV still requires a BSL-3 containment. Infection of macaques with simian hemorrhagic fever virus offered a BSL-2 model of viral hemorrhagic fevers caused by pathogenic filoviruses and LASV [141]. SHEV is an arterivirus discovered in 1964 as a cause of hemorrhagic fevers in macaques at research facilities. Experimental infection of rhesus macaques with SHEV induced a hemorrhagic disease with a fatality rate of 64% and clinical and pathological similarities (coagulopathy, modulation of proinflammatory cytokines, involvement of lymphoid and hepatic systems) with other hemorrhagic fevers infections caused by EBOV, MARV (Marburg virus) and LASV. Immunosuppression and the high incidence of the secondary bacterial infections in numerous tissues was one of the major findings in this model. In many cases secondary bacterial infections aggravate prognosis of human viral hemorrhagic fevers and further study of SHEV infections in macaques will be helpful to better understand the contribution of secondary bacterial infections to viral hemorrhagic fever pathogenesis. However, as mentioned above, LF does not share some common features of ‘classical’ hemorrhagic fever (e.g., disseminated intravascular coagulation or the cytokine ‘storm’) and applicability of this model for LASV studies seems to be limited.
Expert commentary
Success of Candid #1 vaccine in reducing the incidence of Argentine hemorrhagic fever clearly demonstrates feasibility of development of a cost-effective vaccine to control LF in West Africa. Potential LASV vaccine candidate(s) will be evaluated based on appropriate animal models mimicking pathogenesis of human LF. In spite of significant efforts to replace NHPs with small animals in safety and efficacy assessment of vaccine candidates against LF, NHPs, especially rhesus and cynomolgus monkeys, are continuing to be the closest animal models to humans in terms of pathogenesis of the disease and development of protective immune responses. In addition, there is a clear trend, at least for filovirus vaccines and probably for vaccine candidates against LASV, to consider NHP as the only appropriate model under the Animal Rule [142]. In these circumstances, the relatively small size of marmosets, lower caging and feeding costs, and ease of handling in a BSL-4 biosafety environment represent substantial benefits compared with the use of macaques. Pathological features of LASV infection in marmosets mirror those described for human cases of LF and common marmosets are well positioned to serve as the second ‘small’ NHP model under the FDA Animal Rule.
Under current regulation policy, all rodent-based experimental models are compromised by the fact that arenaviruses are rodent-borne viruses and are treated differently by the immune systems of rodents (in natural hosts and/or experimentally infected rodents) and humans/NHPs. It is highly unlikely that experimental rodents, even the highly susceptible strain 13 guinea pigs, will be considered as an appropriate model for efficacy trials. That is also true for model systems based on infections with host-adapted LASV strains or with less pathogenic ‘surrogate’ arenaviruses (Table 1) (reviewed in [130]). Recently described animal models based on mice with artificially compromised immune systems (e.g., mice expressing humanized MHC Class I molecules [117], mice deficient for α/β and IFN-γ receptors [114]) can potentially be helpful for basic studies, but probably will not be applicable for efficacy trials. In addition, there is a consensus that some small animal models (e.g., guinea pigs infected with LASV) can be useful for studying the natural course of the disease and key pathophysiological features (e.g., lung involvement, pathogenesis of interstitial pneumonia).
Based on restricted human studies, an in vitro human model of the induction of primary T-cell responses, and advanced pathogenic studies in NHPs, there is a consensus that the control of LASV infection is associated with the induction of T-cell responses. Comparative studies of LASV versus MOPV in NHPs and in human macrophages and DCs provided valuable insights into immune responses in patients surviving acute LF and/or in individuals experiencing asymptomatic LASV infection. Based on these studies, MOPV infection in NHPs can be used as a model of nonfatal LF, and T-cell responses induced by MOPV-infected DCs may reflect the immune responses induced in survived LF patients. In this respect, MOP/LAS reassortant (ML29) recapitulating safety profile of MOPV and effective immune responses in nonfatal LF patients remains a promising vaccine candidate.
In spite of recent advances in our understanding of LASV interactions with the innate and adaptive immune systems resulting in T-cell-mediated control of viral replication, correlates of protection are still elusive and have yet to be established. While evaluation of LASV-specific CD8+ T-responses by ELISPOT and/or by intracellular cytokine staining in vitro after activation of T cells with LASV antigens provides valuable information, these tools are still poor correlates of protection. In a recently described CBA/J-ML29 model [101], a T-cell cytotoxicity assay in vivo showed a correlation between LASV-specific cytotoxicity and protection induced by the LASV vaccine candidate (ML29). Notably, CBA/J mice that received CD8+ T cell-depleted splenocytes from ML29-immunized donors all succumbed to a lethal challenge, demonstrating that CD8+ T cells are critical in protection. The CBA/J-ML29 model can therefore be a useful immunological tool for evaluation of immunogenicity and efficacy of LASV vaccine candidates outside of BSL-4 containment facilities. Immune correlate(s) may not cover all protective mechanisms, but they should be reproducibly consistent and quantitative to predict a positive effect of vaccination in humans/NHPs.
Surrogate virus models with less stringent biocontainment requirements (Table 1) will certainly be very helpful for basic studies and for evaluation of safety at early stages of preclinical development on a case-by-case basis. For example, infection of macaques with LCMV-WE may be used to evaluate the potential of vaccine candidates to prevent liver toxicity in experimentally vaccinated animals. In addition, infection of macaques with SHEV may provide helpful information regarding control of the secondary bacterial infections after experimental vaccination. However, these surrogate models cannot be considered as appropriate animals models according to the FDA Animal Rule.
Based on recent developments in the FDA Animal Rule policy, point-by-point comparison of characteristics of human disease to disease signs in the animal model will be required by FDA to validate the model. In addition, in efficacy trials individual animal records (case report forms) will also be requested for evaluation of vaccine efficacy and safety. All animal studies must be well documented and ideally performed under Good Laboratory Practice (GLP) standards. Introduction of GLP in BSL3/4 environmental is additional challenge for vaccine development.
While results of safety and efficacy studies in animal models must be submitted to the FDA, the agency will probably require efficacy trials in humans. The Animal Rule pertains to diseases that are so rare that trials are not feasible (e.g., outbreaks of hemorrhagic fevers caused by EBOV and MARV). By contrast, LF is a common disease in West Africa and this argument cannot be made. Indeed, efficacy evaluation of Candid #1 was performed in a Phase III trial in Argentina [51]. Notably, the prevalence of Junin virus infection in Argentina is lower than LASV infection in West Africa; 3500 cases/year (historical high before introduction of Candid #1) versus 3000–5000/year cases of LF in West Africa (conservative estimates). Due to strain variations of LASV, a multicenter trial in several endemic areas (countries) would probably have to be conducted to evaluate the cross-protective efficacy of vaccine candidates. Certainly, poor infrastructure in endemic areas will be a challenge for this trial. Currently ongoing capacity building in Nigeria and Sierra Leone and expanding international collaboration will be extremely helpful for future trials.
Five-year view
Despite impressive progress in the molecular biology of pathogenic arenaviruses and their interaction with susceptible cells and natural hosts, many questions of LF pathogenesis remain unanswered. Reverse genetic systems recently developed for LASV and MOPV will provide a powerful tool for pathogenesis studies and for rational design of a new generation of safe and efficacious vaccine candidates. Evaluation of these candidates will be crucially dependent on the availability of appropriate animal models complying with the FDA Animal Rule. Recently developed small animal models in mice and marmosets will be helpful for preclinical validation and for technology transfer from the laboratory to the manufacturing environment. Nevertheless, costly efficacy trials in NHPs in BSL-4 facilities will be the major licensure pathway for promising vaccine candidates. While transgenic and knockout mouse models will probably not be considered as appropriate models according to the Animal Rule, they will be very helpful in testing correlates of immunity and protection. Application of systems biology (vaccinology) to identify the signatures that predict protective immune responses to MOPV and MOP/LAS reassortant (ML29) in NHPs will be an important step in establishing validated correlates of protection.
In the absence of market-driving forces, the approval process for LF vaccine candidates in nonendemic countries will probably be very slow. Nevertheless, the Candid #1 story is a promising guide for LASV vaccine candidates. The major lesson learned from the past is that local needs and responsiveness of national authorities from endemic areas must be important driving forces supported by international development programs and charitable organizations. Recent international initiatives [2], signs of interest from biotechnology companies [35], and the successful story of development of a live-attenuated vaccine against Japanese encephalitis by a small biotech company, Acambis [143], provide some hope for the future.
Vaccine formulation will probably depend on the vaccination strategy and targeted groups. For the general population in LASV endemic areas a single-dose vaccination providing a lifelong protection against the disease caused by all genetic clades is the most desirable strategy. A live-attenuated vaccine expressing LASV GPC and NP would be an appropriate vaccine candidate to meet this goal. Reassortant ML29 is currently available preventive vaccine candidate inducing sterilizing and cross-protective immunity in NHPs. The ML29 can be also effective as a postexposure treatment [144]. Recently established LASV reverse genetics systems [64,145] provide powerful tools to secure safety profile and to further improve this promising vaccine. Based on Acambis’s successful experience [Monath T, Pers. Comm.], 5–7 years of clinical development with approximately $15–20 million/year investment are probably appropriate estimates for ML29.
The advanced VEEV TC-83 replicon technology also provides a safe and multivalent solution for making effective LASV vaccine candidates. This platform requires a prime–boost immunization strategy to achieve desirable levels of immune responses and protection. In practical terms, this strategy would be applicable for ‘organized’ target groups (e.g., first responders, personnel of local hospitals in endemic areas, international travelers visiting endemic areas, military personnel and staff of BSL-4 laboratories working with LASV).
Key issues.
Lassa virus (LASV) is the most prevalent arenavirus in West Africa and is responsible for several hundred thousand infections and thousands of deaths annually. LASV can also be potentially used as an agent of biological warfare.
There is no licensed Lassa fever (LF) vaccine and vaccine development is hampered by the high cost of nonhuman primate (NHP) animal models, by high biocontainment requirements (BSL-4), and by high heterogeneity of LASV species.
The FDA Animal Rule for regulation of ‘biodefense’ biologics was implemented in 2002. After 10 years, there are no vaccines approved under this regulation. During recent months, the NIH and FDA are finally taking joint efforts to aggressively apply the Animal Rule for development of ‘biodefense’ vaccines against the pathogenic filoviruses Ebola and Marburg. Any progress in clinical development of these vaccines, for example, pre-investigational new drug status, will be a positive impulse for LASV vaccine development as well.
There is an understanding that different animal species can be used to mimic key pathological features of a LF human disease and can be applicable for challenge (efficacy) trials. All of these animal species must be used for the evaluation of safety and efficacy of vaccine candidates to prove the benefits of a preventive strategy.
while the results of LASV vaccine candidate safety and efficacy studies in animal models must be submitted to the FDA, the agency will probably require efficacy trials in humans as well. In contrast to other ‘biodefense’ hemorrhagic fevers, LF is a common disease in West Africa and the regulatory pathway of Candid #1, a live-attenuated vaccine against Argentine hemorrhagic fever, will be a reasonable guideline for efficacy trials in West Africa.
Live-attenuated reassortant ML29 is currently the most advanced LASV vaccine candidate inducing sterilizing, cross-protective immunity and acting as a postexposure countermeasure. Based on preliminary estimates, US$15–20 million/year and 5–7 years will be required for clinical development of this vaccine.
Footnotes
Financial & competing interests disclosure
The author was supported by grants R01RR13980, R01AI52367, R01AI068961, and R01AI093450 (to IS Lukashevich) from the NIH. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- 2.Khan SH, Goba A, Chu M, et al. Mano River Union Lassa Fever Network. New opportunities for field research on the pathogenesis and treatment of Lassa fever. Antiviral Res. 2008;78(1):103–115. doi: 10.1016/j.antiviral.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Fichet-Calvet E, Rogers DJ. Risk maps of Lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3(3):e388. doi: 10.1371/journal.pntd.0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ. 2003;327(7426):1271–1275. doi: 10.1136/bmj.327.7426.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safronetz D, Lopez JE, Sogoba N, et al. Detection of Lassa virus, Mali. Emerging Infect Dis. 2010;16(7):1123–1126. doi: 10.3201/eid1607.100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen KG, Shylakhter I, Tabrizi S, Grossman SR, Happi CT, Sabeti PC. Genome-wide scans provide evidence for positive selection of genes implicated in Lassa fever. Philos Trans R Soc Lond, B, Biol Sci. 2012;367(1590):868–877. doi: 10.1098/rstb.2011.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabeti PC, Varilly P, Fry B, et al. International HapMap Consortium. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449(7164):913–918. doi: 10.1038/nature06250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukashevich IS, Stelmakh TA, Golubev VP, Stchesljenok EP, Lemeshko NN. Ribonucleic acids of Machupo and Lassa viruses. Arch Virol. 1984;79(3–4):189–203. doi: 10.1007/BF01310811. [DOI] [PubMed] [Google Scholar]
- 9.Djavani M, Lukashevich IS, Sanchez A, Nichol ST, Salvato MS. Completion of the Lassa fever virus sequence and identification of a RING finger open reading frame at the L RNA 5 End. Virology. 1997;235(2):414–418. doi: 10.1006/viro.1997.8722. [DOI] [PubMed] [Google Scholar]
- 10.Lukashevich IS, Djavani M, Shapiro K, et al. The Lassa fever virus L gene: nucleotide sequence, comparison, and precipitation of a predicted 250 kDa protein with monospecific antiserum. J Gen Virol. 1997;78(Pt 3):547–551. doi: 10.1099/0022-1317-78-3-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strecker T, Eichler R, Meulen J, et al. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles [corrected] J Virol. 2003;77(19):10700–10705. doi: 10.1128/JVI.77.19.10700-10705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auperin DD, Sasso DR, McCormick JB. Nucleotide sequence of the glycoprotein gene and intergenic region of the Lassa virus S genome RNA. Virology. 1986;154(1):155–167. doi: 10.1016/0042-6822(86)90438-1. [DOI] [PubMed] [Google Scholar]
- 13.Clegg JC, Wilson SM, Oram JD. Nucleotide sequence of the S RNA of Lassa virus (Nigerian strain) and comparative analysis of arenavirus gene products. Virus Res. 1991;18(2–3):151–164. doi: 10.1016/0168-1702(91)90015-n. [DOI] [PubMed] [Google Scholar]
- 14.Cao W, Henry MD, Borrow P, et al. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282(5396):2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 15.Rojek JM, Moraz ML, Pythoud C, et al. Binding of Lassa virus perturbs extracellular matrix-induced signal transduction via dystroglycan. Cell Microbiol. 2012;14(7):1122–1134. doi: 10.1111/j.1462-5822.2012.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimojima M, Ströher U, Ebihara H, Feldmann H, Kawaoka Y. Identification of cell surface molecules involved in dystroglycan-independent Lassa virus cell entry. J Virol. 2012;86(4):2067–2078. doi: 10.1128/JVI.06451-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimojima M, Kawaoka Y. Cell surface molecules involved in infection mediated by lymphocytic choriomeningitis virus glycoprotein. J Vet Med Sci. 2012;74(10):1363–1366. doi: 10.1292/jvms.12-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glushakova SE, Omelyanenko VG, Lukashevitch IS, et al. The fusion of artificial lipid membranes induced by the synthetic arenavirus ‘fusion peptide’. Biochim Biophys Acta. 1992;1110(2):202–208. doi: 10.1016/0005-2736(92)90360-x. [DOI] [PubMed] [Google Scholar]
- 19.Klewitz C, Klenk HD, ter Meulen J. Amino acids from both N-terminal hydrophobic regions of the Lassa virus envelope glycoprotein GP-2 are critical for pH-dependent membrane fusion and infectivity. J Gen Virol. 2007;88(Pt 8):2320–2328. doi: 10.1099/vir.0.82950-0. [DOI] [PubMed] [Google Scholar]
- 20.Kunz S. Receptor binding and cell entry of Old World arenaviruses reveal novel aspects of virus-host interaction. Virology. 2009;387(2):245–249. doi: 10.1016/j.virol.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Pasqual G, Rojek JM, Masin M, Chatton JY, Kunz S. Old world arenaviruses enter the host cell via the multivesicular body and depend on the endosomal sorting complex required for transport. PLoS Pathog. 2011;7(9):e1002232. doi: 10.1371/journal.ppat.1002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvato MS, Clegg JCS, Buchmeier MJ, et al. Family Arenaviridae, Virus Taxonomy, Classification and Nomenclature of Viruses. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier Inc; MA, USA: 2012. pp. 715–723. [Google Scholar]
- 23.Lecompte E, ter Meulen J, Emonet S, Daffis S, Charrel RN. Genetic identification of Kodoko virus, a novel arenavirus of the African pigmy mouse (Mus Nannomys minutoides) in West Africa. Virology. 2007;364(1):178–183. doi: 10.1016/j.virol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Paweska JT, Sewlall NH, Ksiazek TG, et al. Outbreak Control and Investigation Teams. Nosocomial outbreak of novel arenavirus infection, southern Africa. Emerging Infect Dis. 2009;15(10):1598–1602. doi: 10.3201/eid1510.090211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briese T, Paweska JT, McMullan LK, et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 2009;5(5):e1000455. doi: 10.1371/journal.ppat.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii A, Thomas Y, Moonga L, et al. Novel arenavirus, Zambia. Emerging Infect Dis. 2011;17(10):1921–1924. doi: 10.3201/eid1710.10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palacios G, Savji N, Hui J, et al. Genomic and phylogenetic characterization of Merino Walk virus, a novel arenavirus isolated in South Africa. J Gen Virol. 2010;91(Pt 5):1315–1324. doi: 10.1099/vir.0.017798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coulibaly-N’Golo D, Allali B, Kouassi SK, et al. Novel arenavirus sequences in Hylomyscus sp and Mus (Nannomys) setulosus from Côte d’Ivoire: implications for evolution of arenaviruses in Africa. PLoS ONE. 2011;6(6):e20893. doi: 10.1371/journal.pone.0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowen MD, Rollin PE, Ksiazek TG, et al. Genetic diversity among Lassa virus strains. J Virol. 2000;74(15):6992–7004. doi: 10.1128/jvi.74.15.6992-7004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozano ME, Posik DM, Albariño CG, et al. Characterization of arenaviruses using a family-specific primer set for RT-PCR amplification and RFLP analysis. Its potential use for detection of uncharacterized arenaviruses. Virus Res. 1997;49(1):79–89. doi: 10.1016/s0168-1702(97)01458-5. [DOI] [PubMed] [Google Scholar]
- 31.Bowen MD, Peters CJ, Nichol ST. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol Phylogenet Evol. 1997;8(3):301–316. doi: 10.1006/mpev.1997.0436. [DOI] [PubMed] [Google Scholar]
- 32.Günther S, Emmerich P, Laue T, et al. Imported Lassa fever in Germany: molecular characterization of a new Lassa virus strain. Emerging Infect Dis. 2000;6(5):466–476. doi: 10.3201/eid0605.000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher-Hoch SP, McCormick JB. Lassa fever vaccine. Expert Rev Vaccines. 2004;3(2):189–197. doi: 10.1586/14760584.3.2.189. [DOI] [PubMed] [Google Scholar]
- 34.McCormick JB, Mitchell SW, Kiley MP, Ruo S, Fisher-Hoch SP. Inactivated Lassa virus elicits a non protective immune response in rhesus monkeys. J Med Virol. 1992;37(1):1–7. doi: 10.1002/jmv.1890370102. [DOI] [PubMed] [Google Scholar]
- 35.Branco LM, Grove JN, Geske FJ, et al. Lassa virus-like particles displaying all major immunological determinants as a vaccine candidate for Lassa hemorrhagic fever. Virol J. 2010;7:279. doi: 10.1186/1743-422X-7-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20(1):17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Borrow P, Martínez-Sobrido L, de la Torre JC. Inhibition of the type I interferon antiviral response during arenavirus infection. Viruses. 2010;2(11):2443–2480. doi: 10.3390/v2112443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koyama S, Coban C, Aoshi T, Horii T, Akira S, Ishii KJ. Innate immune control of nucleic acid-based vaccine immunogenicity. Expert Rev Vaccines. 2009;8(8):1099–1107. doi: 10.1586/erv.09.57. [DOI] [PubMed] [Google Scholar]
- 40.Huang C, Kolokoltsova OA, Yun NE, et al. Junín virus infection activates the type I interferon pathway in a RIG-I-dependent manner. PLoS Negl Trop Dis. 2012;6(5):e1659. doi: 10.1371/journal.pntd.0001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boesen A, Sundar K, Coico R. Lassa fever virus peptides predicted by computational analysis induce epitope-specific cytotoxic-T-lymphocyte responses in HLA-A2.1 transgenic mice. Clin Diagn Lab Immunol. 2005;12(10):1223–1230. doi: 10.1128/CDLI.12.10.1223-1230.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Botten J, Alexander J, Pasquetto V, et al. Identification of protective Lassa virus epitopes that are restricted by HLA-A2. J Virol. 2006;80(17):8351–8361. doi: 10.1128/JVI.00896-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Botten J, Whitton JL, Barrowman P, et al. A multivalent vaccination strategy for the prevention of Old World arenavirus infection in humans. J Virol. 2010;84(19):9947–9956. doi: 10.1128/JVI.00672-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu F, Feuer R, Hassett DE, Whitton JL. Peptide vaccination of mice immune to LCMV or vaccinia virus causes serious CD8 T cell-mediated, TNF-dependent immunopathology. J Clin Invest. 2006;116(2):465–475. doi: 10.1172/JCI25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meulen J, Badusche M, Satoguina J, et al. Old and New World arenaviruses share a highly conserved epitope in the fusion domain of the glycoprotein 2, which is recognized by Lassa virus-specific human CD4+ T-cell clones. Virology. 2004;321(1):134–143. doi: 10.1016/j.virol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 46.ter Meulen J, Badusche M, Kuhnt K, et al. Characterization of human CD4(+) T-cell clones recognizing conserved and variable epitopes of the Lassa virus nucleoprotein. J Virol. 2000;74(5):2186–2192. doi: 10.1128/jvi.74.5.2186-2192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez-Carreno MP, Nelson MS, Botten J, Smith-Nixon K, Buchmeier MJ, Whitton JL. Evaluating the immunogenicity and protective efficacy of a DNA vaccine encoding Lassa virus nucleoprotein. Virology. 2005;335(1):87–98. doi: 10.1016/j.virol.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 48.Whitton JL, Sheng N, Oldstone MB, McKee TA. A ‘string-of-beads’ vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J Virol. 1993;67(1):348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grant-Klein RJ, Altamura LA, Schmaljohn CS. Progress in recombinant DNA-derived vaccines for Lassa virus and filoviruses. Virus Res. 2011;162(1–2):148–161. doi: 10.1016/j.virusres.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Lauring AS, Jones JO, Andino R. Rationalizing the development of live attenuated virus vaccines. Nat Biotechnol. 2010;28(6):573–579. doi: 10.1038/nbt.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ambrosio A, Saavedra M, Mariani M, Gamboa G, Maiza A. Argentine hemorrhagic fever vaccines. Hum Vaccin. 2011;7(6):694–700. doi: 10.4161/hv.7.6.15198. [DOI] [PubMed] [Google Scholar]
- 52.Clegg JC, Lloyd G. Vaccinia recombinant expressing Lassa-virus internal nucleocapsid protein protects guineapigs against Lassa fever. Lancet. 1987;2(8552):186–188. doi: 10.1016/s0140-6736(87)90767-7. [DOI] [PubMed] [Google Scholar]
- 53.Auperin DD, Esposito JJ, Lange JV, et al. Construction of a recombinant vaccinia virus expressing the Lassa virus glycoprotein gene and protection of guinea pigs from a lethal Lassa virus infection. Virus Res. 1988;9(2–3):233–248. doi: 10.1016/0168-1702(88)90033-0. [DOI] [PubMed] [Google Scholar]
- 54.Fisher-Hoch SP, Hutwagner L, Brown B, McCormick JB. Effective vaccine for Lassa fever. J Virol. 2000;74(15):6777–6783. doi: 10.1128/jvi.74.15.6777-6783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garbutt M, Liebscher R, Wahl-Jensen V, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78(10):5458–5465. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geisbert TW, Jones S, Fritz EA, et al. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2005;2(6):e183. doi: 10.1371/journal.pmed.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lukashevich IS. Generation of reassortants between African arenaviruses. Virology. 1992;188(2):600–605. doi: 10.1016/0042-6822(92)90514-p. [DOI] [PubMed] [Google Scholar]
- 58.Lukashevich IS, Patterson J, Carrion R, et al. A live attenuated vaccine for Lassa fever made by reassortment of Lassa and Mopeia viruses. J Virol. 2005;79(22):13934–13942. doi: 10.1128/JVI.79.22.13934-13942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lukashevich IS, Carrion R, Jr, Salvato MS, et al. Safety, immunogenicity, and efficacy of the ML29 reassortant vaccine for Lassa fever in small non-human primates. Vaccine. 2008;26(41):5246–5254. doi: 10.1016/j.vaccine.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bredenbeek PJ, Molenkamp R, Spaan WJ, et al. A recombinant Yellow Fever 17D vaccine expressing Lassa virus glycoproteins. Virology. 2006;345(2):299–304. doi: 10.1016/j.virol.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang X, Dalebout TJ, Bredenbeek PJ, et al. Yellow fever 17D-vectored vaccines expressing Lassa virus GP1 and GP2 glycoproteins provide protection against fatal disease in guinea pigs. Vaccine. 2011;29(6):1248–1257. doi: 10.1016/j.vaccine.2010.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pinschewer DD, Flatz L, Steinborn R, et al. Innate and adaptive immune control of genetically engineered live-attenuated arenavirus vaccine prototypes. Int Immunol. 2010;22(9):749–756. doi: 10.1093/intimm/dxq061. [DOI] [PubMed] [Google Scholar]
- 63.Emonet SE, Urata S, de la Torre JC. Arenavirus reverse genetics: new approaches for the investigation of arenavirus biology and development of antiviral strategies. Virology. 2011;411(2):416–425. doi: 10.1016/j.virol.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carnec X, Baize S, Reynard S, et al. Lassa virus nucleoprotein mutants generated by reverse genetics induce a robust type I interferon response in human dendritic cells and macrophages. J Virol. 2011;85(22):12093–12097. doi: 10.1128/JVI.00429-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kerber R, Rieger T, Busch C, et al. Cross-species analysis of the replication complex of Old World arenaviruses reveals two nucleoprotein sites involved in L protein function. J Virol. 2011;85(23):12518–12528. doi: 10.1128/JVI.05091-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28(3):632–649. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 67.Editorial. YF17D vector for Lassa fever vaccine. Int Innovation. 2010:77–79. [Google Scholar]
- 68.Vander Veen RL, Hank Harris DL, Kamrud KI. Alphavirus replicon vaccines. Animal Health Res Rev. 2012 doi: 10.1017/S1466252312000011. (Epub ahead of Print) [DOI] [PubMed] [Google Scholar]
- 69.Pushko P, Geisbert J, Parker M, Jahrling P, Smith J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J Virol. 2001;75(23):11677–11685. doi: 10.1128/JVI.75.23.11677-11685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balasuriya UB, Heidner HW, Davis NL, et al. Alphavirus replicon particles expressing the two major envelope proteins of equine arteritis virus induce high level protection against challenge with virulent virus in vaccinated horses. Vaccine. 2002;20(11–12):1609–1617. doi: 10.1016/s0264-410x(01)00485-6. [DOI] [PubMed] [Google Scholar]
- 71.Norbury CC, Basta S, Donohue KB, et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304(5675):1318–1321. doi: 10.1126/science.1096378. [DOI] [PubMed] [Google Scholar]
- 72.Huckriede A, Bungener L, Holtrop M, et al. Induction of cytotoxic T lymphocyte activity by immunization with recombinant Semliki Forest virus: indications for cross-priming. Vaccine. 2004;22(9–10):1104–1113. doi: 10.1016/j.vaccine.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Schliehe C, Bitzer A, van den Broek M, Groettrup M. Stable antigen is most effective for eliciting CD8+ T-cell responses after DNA vaccination and infection with recombinant vaccinia virus in vivo. J Virol. 2012;86(18):9782–9793. doi: 10.1128/JVI.00694-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKee KTJ, Enria DA, Barerra-Oro JG. Junin argentine hemorrhagic fever. In: Barett ADT, Stanberry LR, editors. Vaccines for Biodefense and Energing and Neglected Diseases. Elsevier; Amsterdam, The Netherlands: 2009. pp. 537–550. [Google Scholar]
- 75.Snoy PJ. Establishing efficacy of human products using animals: the US food and Drug Administration’s ‘Animal Rule’. Vet Pathol. 2010;47(5):774–778. doi: 10.1177/0300985810372506. [DOI] [PubMed] [Google Scholar]
- 76.Bausch DG, Rollin PE, Demby AH, et al. Diagnosis and clinical virology of Lassa fever as evaluated by enzyme-linked immunosorbent assay, indirect fluorescent-antibody test, and virus isolation. J Clin Microbiol. 2000;38(7):2670–2677. doi: 10.1128/jcm.38.7.2670-2677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Branco LM, Grove JN, Boisen ML, et al. Emerging trends in Lassa fever: redefining the role of immunoglobulin M and inflammation in diagnosing acute infection. Virol J. 2011;8:478. doi: 10.1186/1743-422X-8-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jahrling PB, Frame JD, Rhoderick JB, Monson MH. Endemic Lassa fever in Liberia. IV Selection of optimally effective plasma for treatment by passive immunization. Trans R Soc Trop Med Hyg. 1985;79(3):380–384. doi: 10.1016/0035-9203(85)90388-8. [DOI] [PubMed] [Google Scholar]
- 79.Jahrling PB. Protection of Lassa virus-infected guinea pigs with Lassa-immune plasma of guinea pig, primate, and human origin. J Med Virol. 1983;12(2):93–102. doi: 10.1002/jmv.1890120203. [DOI] [PubMed] [Google Scholar]
- 80.Jahrling PB, Peters CJ. Passive antibody therapy of Lassa fever in cynomolgus monkeys: importance of neutralizing antibody and Lassa virus strain. Infect Immun. 1984;44(2):528–533. doi: 10.1128/iai.44.2.528-533.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moraz ML, Kunz S. Pathogenesis of arenavirus hemorrhagic fevers. Expert Rev Anti Infect Ther. 2011;9(1):49–59. doi: 10.1586/eri.10.142. [DOI] [PubMed] [Google Scholar]
- 82.Walker DH, McCormick JB, Johnson KM, et al. Pathologic and virologic study of fatal Lassa fever in man. Am J Pathol. 1982;107(3):349–356. [PMC free article] [PubMed] [Google Scholar]
- 83.Knobloch J, McCormick JB, Webb PA, Dietrich M, Schumacher HH, Dennis E. Clinical observations in 42 patients with Lassa fever. Tropenmed Parasitol. 1980;31(4):389–398. [PubMed] [Google Scholar]
- 84.McCormick JB, Walker DH, King IJ, et al. Lassa virus hepatitis: a study of fatal Lassa fever in humans. Am J Trop Med Hyg. 1986;35(2):401–407. doi: 10.4269/ajtmh.1986.35.401. [DOI] [PubMed] [Google Scholar]
- 85.Asper M, Sternsdorf T, Hass M, et al. Inhibition of different Lassa virus strains by α and γ interferons and comparison with a less pathogenic arenavirus. J Virol. 2004;78(6):3162–3169. doi: 10.1128/JVI.78.6.3162-3169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baize S, Pannetier D, Faure C, et al. Role of interferons in the control of Lassa virus replication in human dendritic cells and macrophages. Microbes Infect. 2006;8(5):1194–1202. doi: 10.1016/j.micinf.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Baize S, Kaplon J, Faure C, Pannetier D, Georges-Courbot MC, Deubel V. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J Immunol. 2004;172(5):2861–2869. doi: 10.4049/jimmunol.172.5.2861. [DOI] [PubMed] [Google Scholar]
- 88.Baize S, Marianneau P, Loth P, et al. Early and strong immune responses are associated with control of viral replication and recovery in Lassa virus-infected cynomolgus monkeys. J Virol. 2009;83(11):5890–5903. doi: 10.1128/JVI.01948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayes MW, Carrion R, Jr, Nunneley J, Medvedev AE, Salvato MS, Lukashevich IS. Pathogenic Old World arenaviruses inhibit TLR2/Mal-dependent proinflammatory cytokines in vitro. J Virol. 2012;86(13):7216–7226. doi: 10.1128/JVI.06508-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lukashevich IS, Maryankova R, Vladyko AS, et al. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-α gene expression. J Med Virol. 1999;59(4):552–560. [PMC free article] [PubMed] [Google Scholar]
- 91.Mahanty S, Bausch DG, Thomas RL, et al. Low levels of interleukin-8 and interferon-inducible protein-10 in serum are associated with fatal infections in acute Lassa fever. J Infect Dis. 2001;183(12):1713–1721. doi: 10.1086/320722. [DOI] [PubMed] [Google Scholar]
- 92.Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol. 2003;170(6):2797–2801. doi: 10.4049/jimmunol.170.6.2797. [DOI] [PubMed] [Google Scholar]
- 93.Jahrling PB, Frame JD, Smith SB, Monson MH. Endemic Lassa fever in Liberia. III Characterization of Lassa virus isolates. Trans R Soc Trop Med Hyg. 1985;79(3):374–379. doi: 10.1016/0035-9203(85)90386-4. [DOI] [PubMed] [Google Scholar]
- 94.Korch J., Jr Animal models: what do we know and where do we go?. Animal Models Development Workshop; Bethesda, MD, USA. 17–18 September 2012. [Google Scholar]
- 95.Walker DH, Wulff H, Lange JV, Murphy FA. Comparative pathology of Lassa virus infection in monkeys, guinea-pigs, and Mastomys natalensis. Bull World Health Organ. 1975;52(4–6):523–534. [PMC free article] [PubMed] [Google Scholar]
- 96.Jahrling PB, Smith S, Hesse RA, Rhoderick JB. Pathogenesis of Lassa virus infection in guinea pigs. Infect Immun. 1982;37(2):771–778. doi: 10.1128/iai.37.2.771-778.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peters CJ, Jahrling PB, Liu CT, Kenyon RH, McKee KT, Jr, Barrera Oro JG. Experimental studies of arenaviral hemorrhagic fevers. Curr Top Microbiol Immunol. 1987;134:5–68. doi: 10.1007/978-3-642-71726-0_2. [DOI] [PubMed] [Google Scholar]
- 98.Carrion R, Jr, Brasky K, Mansfield K, et al. Lassa virus infection in experimentally infected marmosets: liver pathology and immunophenotypic alterations in target tissues. J Virol. 2007;81(12):6482–6490. doi: 10.1128/JVI.02876-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lukashevich IS, Tikhonov I, Rodas JD, et al. Arenavirus-mediated liver pathology: acute lymphocytic choriomeningitis virus infection of rhesus macaques is characterized by high-level interleukin-6 expression and hepatocyte proliferation. J Virol. 2003;77(3):1727–1737. doi: 10.1128/JVI.77.3.1727-1737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lukashevich IS, Rodas JD, Tikhonov II, et al. LCMV-mediated hepatitis in rhesus macaques: WE but not ARM strain activates hepatocytes and induces liver regeneration. Arch Virol. 2004;149(12):2319–2336. doi: 10.1007/s00705-004-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goicochea MA, Zapata JC, Bryant J, Davis H, Salvato MS, Lukashevich IS. Evaluation of Lassa virus vaccine immunogenicity in a CBA/J-ML29 mouse model. Vaccine. 2012;30(8):1445–1452. doi: 10.1016/j.vaccine.2011.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oldstone MB. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr Top Microbiol Immunol. 2002;263:83–117. doi: 10.1007/978-3-642-56055-2_6. [DOI] [PubMed] [Google Scholar]
- 103.Buckley SM, Casals J. Lassa fever, a new virus disease of man from West Africa. 3 Isolation and characterization of the virus. Am J Trop Med Hyg. 1970;19(4):680–691. doi: 10.4269/ajtmh.1970.19.680. [DOI] [PubMed] [Google Scholar]
- 104.Lukashevich IS. Lassa virus lethality for inbred mice. Ann Soc Belg Med Trop. 1985;65(2):207–209. [PubMed] [Google Scholar]
- 105.Wulff H, McIntosh BM, Hamner DB, Johnson KM. Isolation of an arenavirus closely related to Lassa virus from Mastomys natalensis in south-east Africa. Bull World Health Organ. 1977;55(4):441–444. [PMC free article] [PubMed] [Google Scholar]
- 106.Lukashevich IS, Vasiuchkov AD, Stel’makh TA, Scheslenok EP, Shabanov AG. The isolation and characteristics of reassortants between the Lassa and Mopeia arenaviruses. Vopr Virusol. 1991;36(2):146–150. [PubMed] [Google Scholar]
- 107.Lukashevich IS. Sov Med Rev. Harwood Academic Publishers GmbH; UK: 1987. Human Pathogens of the Arenaviridae family; pp. 133–186. [Google Scholar]
- 108.Lukashevich IS, Vela EM. Pathogenesis of Lassa virus infection in experimental animals. In: Vela EM, editor. Molecular Pathogenesis of Hemorrhagic Fever Viruses. Transw. Res. Net; Kerala, India: 2010. pp. 101–142. [Google Scholar]
- 109.McGavern DB, Homann D, Oldstone MB. T cells in the central nervous system: the delicate balance between viral clearance and disease. J Infect Dis. 2002;186(Suppl 2):S145–S151. doi: 10.1086/344264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barkar ND, Lukashevich IS. Lassa and Mozambique viruses: cross protection in experiments on mice and action of immunosuppressants on experimental infections. Vopr Virusol. 1989;34(5):598–603. [PubMed] [Google Scholar]
- 111.Kolokoltsova OA, Yun NE, Poussard AL, et al. Mice lacking α/β and γ interferon receptors are susceptible to junin virus infection. J Virol. 2010;84(24):13063–13067. doi: 10.1128/JVI.01389-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gowen BB, Wong MH, Larson D, et al. Development of a new tacaribe arenavirus infection model and its use to explore antiviral activity of a novel aristeromycin analog. PLoS ONE. 2010;5(9):e12760. doi: 10.1371/journal.pone.0012760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bradfute SB, Stuthman KS, Shurtleff AC, Bavari S. A STAT-1 knockout mouse model for Machupo virus pathogenesis. Virol J. 2011;8:300. doi: 10.1186/1743-422X-8-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yun NE, Poussard AL, Seregin AV, et al. Functional interferon system is required for clearance of Lassa virus. J Virol. 2012;86(6):3389–3392. doi: 10.1128/JVI.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Camus GS, Qui X, Bente DA, Strong UC, Jones SM. Resistance to a Lassa virus infection is interferon-dependent, but not B or T cell-mediated in mice. In. The 28th ASV Annual Meeting, Scientific Program and Abstract.; Vancouver, BC, Canada. 11–15 July 2009. [Google Scholar]
- 116.Martínez-Sobrido L, Giannakas P, Cubitt B, García-Sastre A, de la Torre JC. Differential inhibition of type I interferon induction by arenavirus nucleoproteins. J Virol. 2007;81(22):12696–12703. doi: 10.1128/JVI.00882-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Flatz L, Rieger T, Merkler D, et al. T cell-dependence of Lassa fever pathogenesis. PLoS Pathog. 2010;6(3):e1000836. doi: 10.1371/journal.ppat.1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Firsova IV, Shatokhina IV, Borisevich IV, et al. Use of guinea pigs for assessing the efficacy of vaccines against Lassa fever. Vopr Virusol. 2003;48(6):43–45. [PubMed] [Google Scholar]
- 119.Callis RT, Jahrling PB, DePaoli A. Pathology of Lassa virus infection in the rhesus monkey. Am J Trop Med Hyg. 1982;31(5):1038–1045. doi: 10.4269/ajtmh.1982.31.1038. [DOI] [PubMed] [Google Scholar]
- 120.Fisher-Hoch SP, Mitchell SW, Sasso DR, Lange JV, Ramsey R, McCormick JB. Physiological and immunologic disturbances associated with shock in a primate model of Lassa fever. J Infect Dis. 1987;155(3):465–474. doi: 10.1093/infdis/155.3.465. [DOI] [PubMed] [Google Scholar]
- 121.Jahrling PB, Hesse RA, Eddy GA, Johnson KM, Callis RT, Stephen EL. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J Infect Dis. 1980;141(5):580–589. doi: 10.1093/infdis/141.5.580. [DOI] [PubMed] [Google Scholar]
- 122.Peters CJ. Arenaviruses. In: Richman DD, Whitley RJ, Hayden FG, editors. Clinical Virology. Churchill Livingstone; New York, NY, USA: 1997. pp. 779–799. [Google Scholar]
- 123.Walker DH, Johnson KM, Lange JV, Gardner JJ, Kiley MP, McCormick JB. Experimental infection of rhesus monkeys with Lassa virus and a closely related arenavirus, Mozambique virus. J Infect Dis. 1982;146(3):360–368. doi: 10.1093/infdis/146.3.360. [DOI] [PubMed] [Google Scholar]
- 124.Hensley LE, Smith MA, Geisbert JB, et al. Pathogenesis of Lassa fever in cynomolgus macaques. Virol J. 2011;8:205. doi: 10.1186/1743-422X-8-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baize S, Marianneau P, Loth P, et al. Early and strong immune responses are associated with control of viral replication and recovery in Lassa virus-infected cynomolgus monkeys. J Virol. 2009;83(11):5890–5903. doi: 10.1128/JVI.01948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pannetier D, Reynard S, Russier M, et al. Human dendritic cells infected with the nonpathogenic Mopeia virus induce stronger T-cell responses than those infected with Lassa virus. J Virol. 2011;85(16):8293–8306. doi: 10.1128/JVI.02120-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, et al. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog. 2008;4(11):e1000225. doi: 10.1371/journal.ppat.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carrion R, Jr, Patterson JL. Vaccines against viral hemorrhagic fevers: non-human primate models. Hum Vaccin. 2011;7(6):667–673. doi: 10.4161/hv.7.6.14981. [DOI] [PubMed] [Google Scholar]
- 129.Mansfield K. Marmoset models commonly used in biomedical research. Comp Med. 2003;53(4):383–392. [PubMed] [Google Scholar]
- 130.Gowen BB, Holbrook MR. Animal models of highly pathogenic RNA viral infections: hemorrhagic fever viruses. Antiviral Res. 2008;78(1):79–90. doi: 10.1016/j.antiviral.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 131.Omatsu T, Moi ML, Hirayama T, et al. Common marmoset (Callithrix jacchus) as a primate model of dengue virus infection: development of high levels of viraemia and demonstration of protective immunity. J Gen Virol. 2011;92(Pt 10):2272–2280. doi: 10.1099/vir.0.031229-0. [DOI] [PubMed] [Google Scholar]
- 132.Aronson JF, Herzog NK, Jerrells TR. Pathological and virological features of arenavirus disease in guinea pigs.Comparison of two Pichinde virus strains. Am J Pathol. 1994;145(1):228–235. [PMC free article] [PubMed] [Google Scholar]
- 133.Scott EP, Aronson JF. Cytokine patterns in a comparative model of arenavirus haemorrhagic fever in guinea pigs. J Gen Virol. 2008;89(Pt 10):2569–2579. doi: 10.1099/vir.0.2008/002048-0. [DOI] [PubMed] [Google Scholar]
- 134.Bowick GC, Fennewald SM, Zhang L, et al. Attenuated and lethal variants of Pichindé virus induce differential patterns of NF-κB activation suggesting a potential target for novel therapeutics. Viral Immunol. 2009;22(6):457–462. doi: 10.1089/vim.2009.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liang Y, Lan S, Ly H. Molecular determinants of Pichinde virus infection of guinea pigs–a small animal model system for arenaviral hemorrhagic fevers. Ann N Y Acad Sci. 2009;1171 (Suppl 1):E65–E74. doi: 10.1111/j.1749-6632.2009.05051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jahrling PB, Hesse RA, Rhoderick JB, Elwell MA, Moe JB. Pathogenesis of a pichinde virus strain adapted to produce lethal infections in guinea pigs. Infect Immun. 1981;32(2):872–880. doi: 10.1128/iai.32.2.872-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Connolly BM, Jenson AB, Peters CJ, Geyer SJ, Barth JF, McPherson RA. Pathogenesis of Pichinde virus infection in strain 13 guinea pigs: an immunocytochemical, virologic, and clinical chemistry study. Am J Trop Med Hyg. 1993;49(1):10–24. doi: 10.4269/ajtmh.1993.49.10. [DOI] [PubMed] [Google Scholar]
- 138.Gowen BB, Julander JG, London NR, et al. Assessing changes in vascular permeability in a hamster model of viral hemorrhagic fever. Virol J. 2010;7:240. doi: 10.1186/1743-422X-7-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sbrana E, Mateo RI, Xiao SY, Popov VL, Newman PC, Tesh RB. Clinical laboratory, virologic, and pathologic changes in hamsters experimentally infected with Pirital virus (Arenaviridae): a rodent model of Lassa fever. Am J Trop Med Hyg. 2006;74(6):1096–1102. [PubMed] [Google Scholar]
- 140.Lukashevich IS, Djavani M, Rodas JD, et al. Hemorrhagic fever occurs after intravenous, but not after intragastric, inoculation of rhesus macaques with lymphocytic choriomeningitis virus. J Med Virol. 2002;67(2):171–186. doi: 10.1002/jmv.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Johnson RF, Dodd LE, Yellayi S, et al. Simian hemorrhagic fever virus infection of rhesus macaques as a model of viral hemorrhagic fever: clinical characterization and risk factors for severe disease. Virology. 2011;421(2):129–140. doi: 10.1016/j.virol.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Levis R. Regulatory considerations related for the development of filovirus vaccines. J Vaccines Vaccin. 2012;3(4):118. [Google Scholar]
- 143.Appaiahgari MB, Vrati S. Clinical development of IMOJEV® – a recombinant Japanese encephalitis chimeric vaccine (JE-CV) Expert Opin Biol Ther. 2012;12(9):1251–1263. doi: 10.1517/14712598.2012.704908. [DOI] [PubMed] [Google Scholar]
- 144.Carrion R, Jr, Bredenbeek P, Jiang X, et al. Vaccine platforms to control arenaviral hemorrhagic fevers. J Vaccines Vaccin. 2012 doi: 10.4172/2157-7560.1000160. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Albariño CG, Bird BH, Chakrabarti AK, Dodd KA, Erickson BR, Nichol ST. Efficient rescue of recombinant Lassa virus reveals the influence of S segment noncoding regions on virus replication and virulence. J Virol. 2011;85(8):4020–4024. doi: 10.1128/JVI.02556-10. [DOI] [PMC free article] [PubMed] [Google Scholar]