1. Introduction
Macrophages are important cells of the immune system that are involved in both innate and cell-mediated immunity. Macrophages can eliminate foreign organisms or dead cellular debris by phagocytosis, and can present antigens derived from these entities to T or B lymphocytes. They also produce a broad palette of intercellular mediators such as cytokines, growth factors, and reactive oxygen species that alter the function of surrounding cells. Many of the macrophages found in lymphoid and non-lymphoid tissues are derived from peripheral blood monocytes that were recruited from the blood into the tissue in response to chemical signals from damaged tissue or chemotactic signals released from other cell types (Hunter et al, 2009). Once in the tissue, monocytes differentiate into macrophages or dendritic cells in response to the local immunoregulatory environment (Tacke and Randolph, 2006).
Tissue macrophages are often difficult to obtain in high numbers, so the ability to differentiate macrophages from peripheral blood precursors is an important research tool. A number of protocols are available to differentiate human monocytes into macrophages (Plesner, 2003, Brugger et al., 1991), however, human models are not always feasible for invasive experimental studies. For example, the immune response to human embryo implantation and early pregnancy is difficult to study due to the ethical limitations of using human embryos and obtaining healthy placental tissue. Nonhuman primates closely parallel human physiology and pathophysiology and the rhesus monkey is an important model for many human diseases. However, the conditions for differentiating macrophages from rhesus monkey peripheral blood monocytes has proved to be different than that for humans, and in our lab, provided inconsistent results.
Zheng et al (2008) obtained monocyte derived macrophages from the rhesus monkey by culturing monocytes in RPMI supplemented with 15% fetal bovine serum (FBS), 10% human serum, and 500 U/mL M-CSF for 5 days. Sopper et al (1996) cultured rhesus monkey peripheral blood monocytes in RPMI supplemented with 10% human serum and GM-CSF for 7 days. Although we obtained macrophages when monocytes were cultured in 10% human serum with M-CSF or GM-CSF, cell survival and differentiation was greatly increased with low serum conditions and the addition of M-CSF and IL-1β. We will describe this alternative method to differentiate macrophages in vitro in the rhesus monkey.
2. Materials and Methods
2.1 Cell culture
Peripheral blood was obtained in heparinized vacutainer collection tubes from healthy female rhesus monkeys and peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll gradient centrifugation. Blood was diluted 1:3 in RPMI-1640, 30 mLs of diluted blood was slowly layered over 15 mLs of Ficoll-Paque (GE Healthcare, Piscataway, NJ), and centrifuged at 800 × g for 30 minutes at room temperature. PBMCs were collected, washed in RPMI-1640 (Gibco, Grand Island, NY), and plated in tissue culture dishes (BD Bioscience, San Diego CA) at a density of 3×106 cells/mL and monocytes were obtained by adhesion for 24 hours in RPMI-1640 supplemented with 2 mM L-glutamine (Gibco), 100 U/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco), 0.1 mM sodium pyruvate (Gibco), 1% non-essential amino acids (Gibco), 50 μM 2-mercaptoethanol (Gibco), and 10% fetal bovine serum (Atlanta Biologicals) or 1% heat-inactivated human AB serum (Sigma, St. Louis, MO). After 24 hours cells were washed twice with RPMI-1640 to remove a majority of the non-adherent cells and cultured for an additional five days in the culture media described above supplemented with 18 ng/mL GM-CSF (Peprotech, Rocky Hill, NJ). 20 ng/mL M-CSF (Peprotech), and/or 10 ng/mL IL-1β (Peprotech). After 5 days, cells were washed with RPMI-1640 to remove non-adherent or dead cells, and the remaining cells were scraped from the plate and used for subsequent experiments.
2.2 Flow analysis of cell surface marker expression
Macrophages were incubated with saturating concentrations of monoclonal antibodies for 25 minutes at 4°C in PBS supplemented with 2% fetal calf serum (Atlanta Biological, Lawrenceville, CA). Antibodies included FITC conjugated anti-CD64 or anti-CD206, PE conjugated anti-CD16, anti-CD86, or anti-CD83, PerCP-Cy5.5 conjugated anti-HLA-DR, or APC conjugated anti-CD14 (BD Bioscience). Isotype-specific antibodies at the same concentration were used as the controls. Cells were washed with PBS supplemented with 2% FCS and resuspended in 2% paraformaldehyde. Flow cytometry data collection was done on a FACSCalibur flow cytometer (BD Biosciences) with Cell Quest software (BD Biosciences). The data were analyzed using Flow Jo software (Tree Star).
2.3 Cytokine analysis
Culture media were collected from control macrophages and macrophages stimulated for 24 hours with 1 ug/mL bacterial lipopolysaccharide (Sigma). The levels of TNF, IL-6, IL-10 and IL-12(p40) in the culture media were determined using a Non-Human Primate Cytokine Milliplex Map Kit (Millipore, Billerica, MA) analyzed with a Luminex 100 instrument (HLA/Molecular Diagnostics Lab, UW-Madison Hospital and Clinics).
2.4 Antigen uptake and processing
To determine the phagocytic and antigen processing ability of peripheral blood monocyte-derived macrophages, macrophages were incubated in culture medium with 100 ug/mL FITC labeled DQ-ovalbumin (Invitrogen, Carlsbad, CA) for 1 hour at 4 or 37°C. Cells were washed with PBS supplemented with 2% FCS and mean fluorescence intensity was determined on a flow cytometer and analyzed as described above.
3. Results
3.1 Macrophage phenotype
Cells grown for 5 days in culture media supplemented with 1% human serum, M-CSF, and IL-1β, showed an adherent morphology indicative of macrophages (Fig. 1) and had the highest viable cell yields (Fig. 2). Cells cultured in media supplemented with 10% FBS and GM-CSF, M-CSF, or M-CSF + IL-1β, but in the absence of human serum, did not exhibit macrophage differentiation and had low cell yields after the 5-day incubation. Low levels of cells were also obtained when cells were cultured in media supplemented with 1% human serum plus GM-CSF or M-CSF, but in the absence of IL-1β.
Fig. 1.
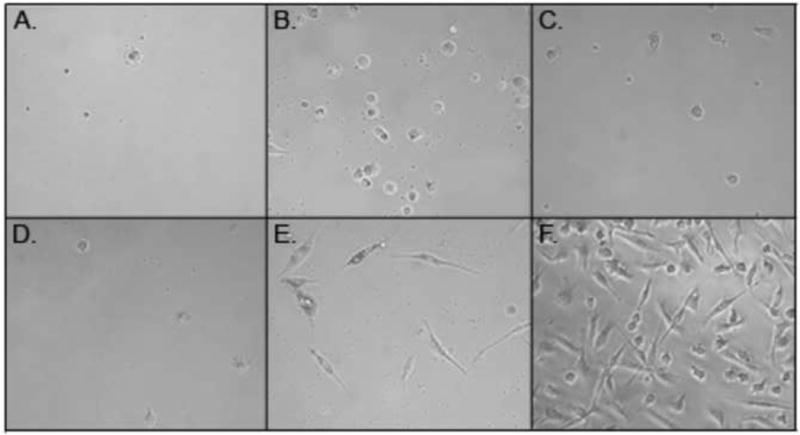
Peripheral blood monocyte-derived macrophages. Cells were cultured for 5 days in media supplemented with 10% FBS, GMCSF (A), MCSF (B), MCSF + IL-1β (C), or 1% HS, GMCSF (D), MCSF (E), MCSF + IL-1β (F).
Fig. 2.
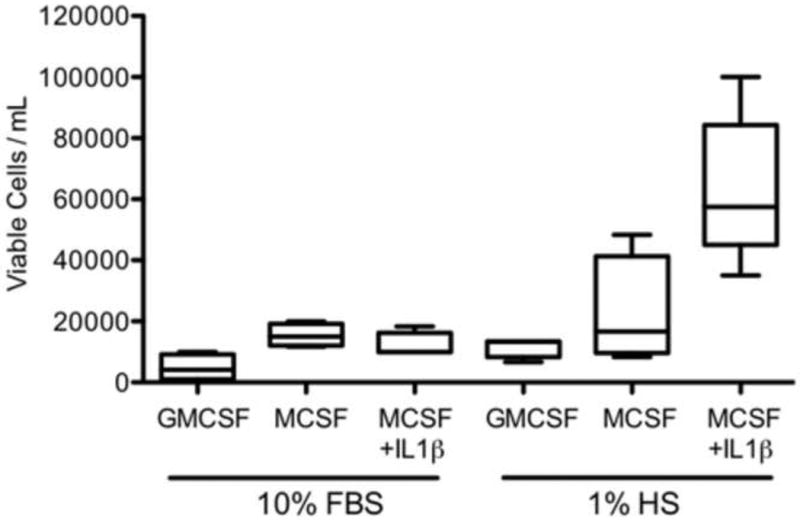
Number of viable cells obtained per milliliter of cells plated for each treatment.
The overall cell surface marker expression varied slightly from monkey to monkey, but in general CD14, CD64, CD16, HLA-DR, CD206, and CD86 were expressed on peripheral monocyte-derived macrophages, with CD14 and CD86 exhibiting the highest mean fluorescence (Fig. 3). CD83, a typical dendritic cell marker, was not expressed under the culture conditions described. Do to the low cell yield in other treatments, only cells cultured in 1% human serum, M-CSF, and IL-1β were evaluated.
Fig. 3.
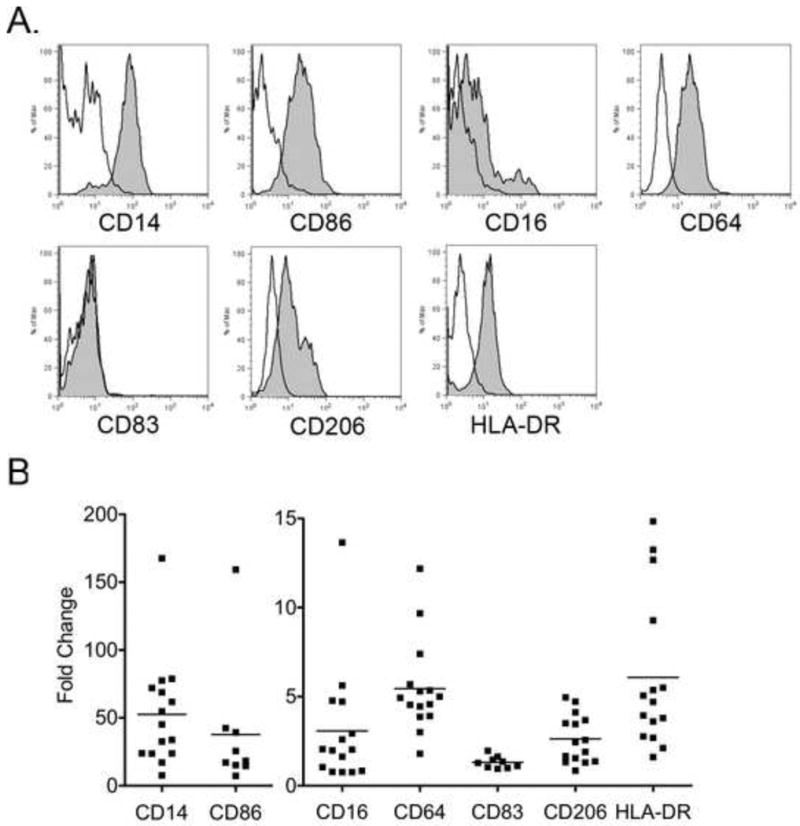
Flow cytometric analysis of peripheral blood monocyte-derived macrophages. A. Representative histograms of surface marker expression (gray) from one animal. Isotype control shown in white. B. Data are presented as fold increase over the background mean fluorescent intensity (MFI) of the isotype control; each data point represents results from an individual animal.
3.2 Cytokine production
Cytokine secretion in response to bacterial stimuli is an important function of macrophages. Macrophages stimulated with bacterial lipopolysaccharide (LPS) for 24 hours demonstrated high levels of the cytokines TNF, IL-6, IL-10, and IL-12 (p40) in the media compared to that of unstimulated controls (Fig. 4).
Fig. 4.
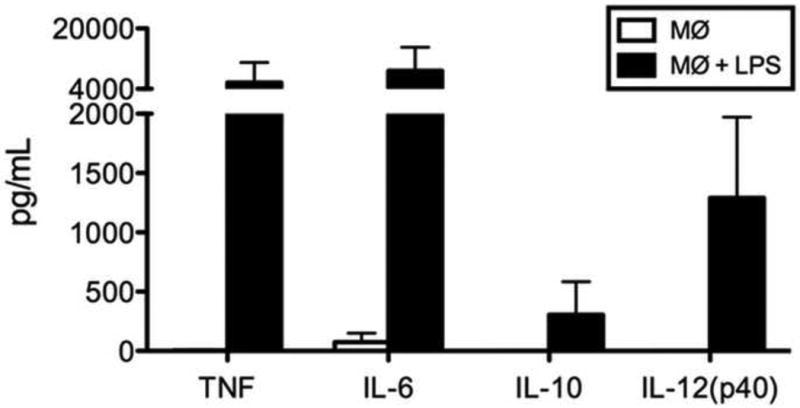
Cytokine secretion by resting macrophages and macrophages stimulated with LPS for 24 hours.
3.3 Antigen uptake and processing
Cells were tested for their functional ability to internalize and process DQ-ovalbumin. DQ-ovalbumin FITC becomes highly fluorescent upon proteolytic processing (denaturation) and is detectable by flow cytometry. Essentially all peripheral blood derived macrophages processed the ovalbumin at 37°C, whereas, cells cultured at 4°C had very little fluorescence, indicating that cellular uptake and metabolism are essential for antigen processing (Fig. 5).
Fig. 5.
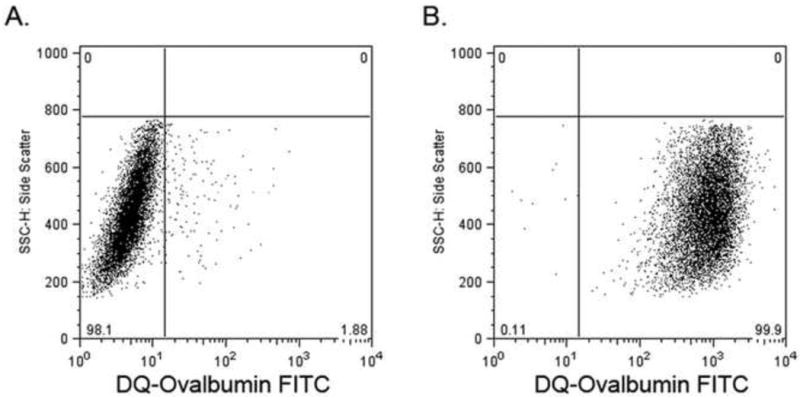
Antigen uptake and processing by macrophages. Cells were incubated with DQ-ovalbumin FITC for 1 hour at 4°C (A) or 37°C (B).
4. Discussion
This study demonstrates that culturing rhesus monkey peripheral blood monocytes with RPMI supplemented with 1% human serum, 20 ng/mL M-CSF, and 10 ng/mL IL-1β for 5 days is an effective way to generate macrophages from the rhesus monkey. The resulting macrophages are functional in that they respond to LPS by producing inflammatory cytokines and can take up and process antigen, characteristic and important functions of macrophages. The presence of human serum was necessary for monocyte survival and differentiation in the rhesus monkey. However, media can be supplemented with a combination of FBS and human serum and similar levels of survival are noted, suggesting FBS is not detrimental to monocyte survival, but also that it is not necessary.
In this study we found that supplementation with IL-1β, in addition to M-CSF and human serum, increased monocyte survival and differentiation. This observation is somewhat surprising given the inflammatory nature of this cytokine (Martinez et al., 2008). Since the unstimulated macrophages do not produce high levels of inflammatory cytokines expected from an activated macrophage, it can be concluded that the IL-1β present in the culture media does not elicit an inflammatory response from the differentiated macrophages. A possible role of IL-1β in this culture system may be to promote rhesus monocyte survival as it has been shown that IL-1β prolongs cell survival of human peripheral blood monoctyes (Hunter et al., 2009).
Using this approach to generate macrophages from peripheral blood allows further opportunities to design and establish rhesus monkey models for the study of disease processes. Further refinement of these methods can include using additional factors, conditioned medium, or co-culture cells to promote the differentiation of tissue-specific cell types.
Acknowledgments
We extend our appreciation to the Animal Care Staff at the Wisconsin National Primate Research Center for doing the blood draws, Kim Weisgrau for assistance with the FACSCalibur, and David Lorentzen for the use of the Luminex at the HLA/Molecular Diagnostics Lab, UW-Madison Hospital and Clinics. This research was supported by NIH grants HD053925, AI076734, and RR021876 to T.G.G. and RR000167 to the Wisconsin National Primate Research Center, University of Wisconsin-Madison. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This publication's contents are solely the responsibility of the authors and does not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brugger W, Kreutz M, Andreesen R. Macrophage colony-stimulating factor is required for human monocyte survival and acts as a cofactor for their terminal differentiation to macrophages in vitro. J of Leukocyte Bio. 1991;49:483–488. doi: 10.1002/jlb.49.5.483. [DOI] [PubMed] [Google Scholar]
- Hunter M, Wang Y, Eubank T, Baran C, Nana-Sinkam P, Marsh C. Survival of monocytes and macrophages and their role in health and disease. Frontiers in Bioscience. 2009;14:4079–4102. doi: 10.2741/3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Frontiers in Bioscience. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Plesner A. Increasing the yield of human mononuclear cells and low serum conditions for in vitro generation of macrophages with M-CSF. J. of Immuno. Methods. 2003;279:287–295. doi: 10.1016/s0022-1759(03)00234-5. [DOI] [PubMed] [Google Scholar]
- Sopper S, Demuth M, Stahl-Hennig C, Hunsmann G, Plesker R, Coulibaly C, Czub S, Ceska M, Koutsilieri E, Riederer P, Brinkmann R, Katz M, Ter Meulen V. The effect of simian immunodeficiency virus infection in vitro and in vivo on the cytokine production of isolated microglia and peripheral macrophages from rhesus monkey. Virology. 1996;220:320–329. doi: 10.1006/viro.1996.0320. [DOI] [PubMed] [Google Scholar]
- Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Ourmanov I, Hirsch VM. Persistent transcription of a nonintegrating mutant of simian immunodeficiency virus in rhesus macrophages. Virology. 2008;372:291–299. doi: 10.1016/j.virol.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


