Abstract
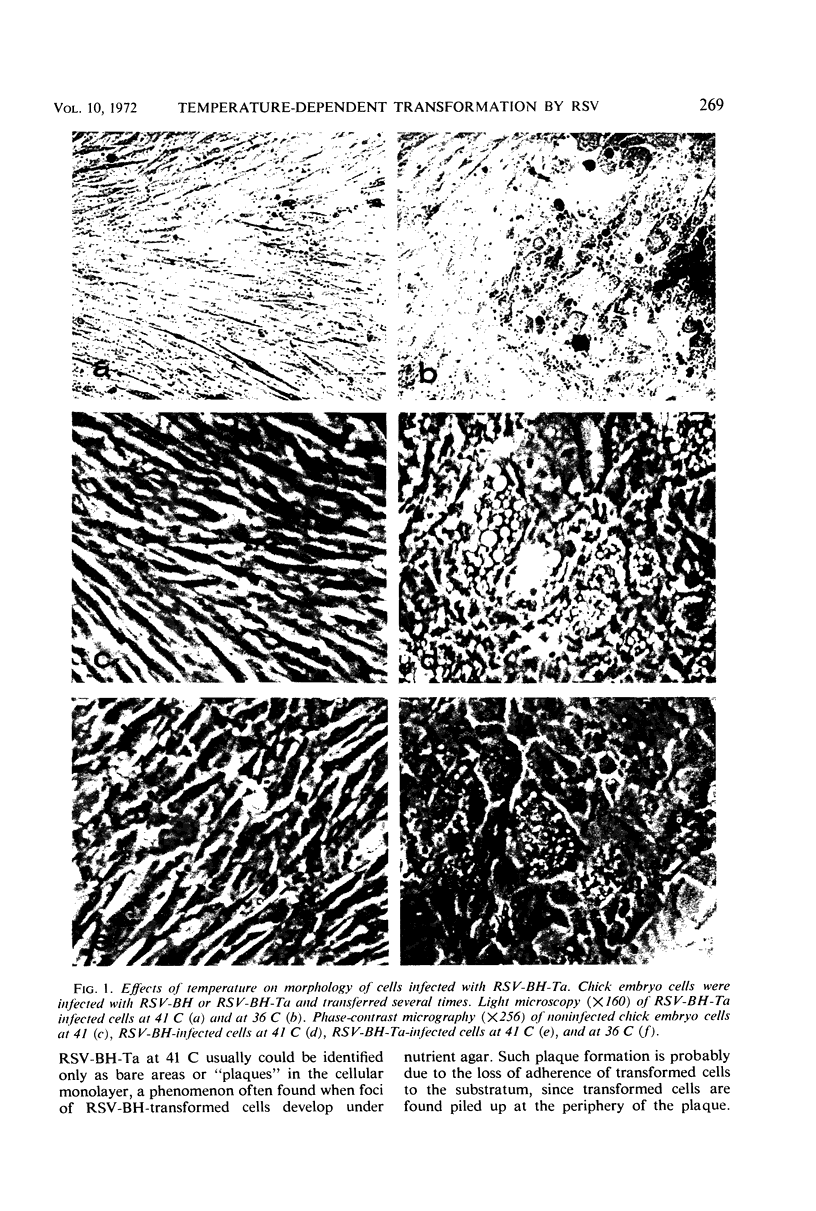
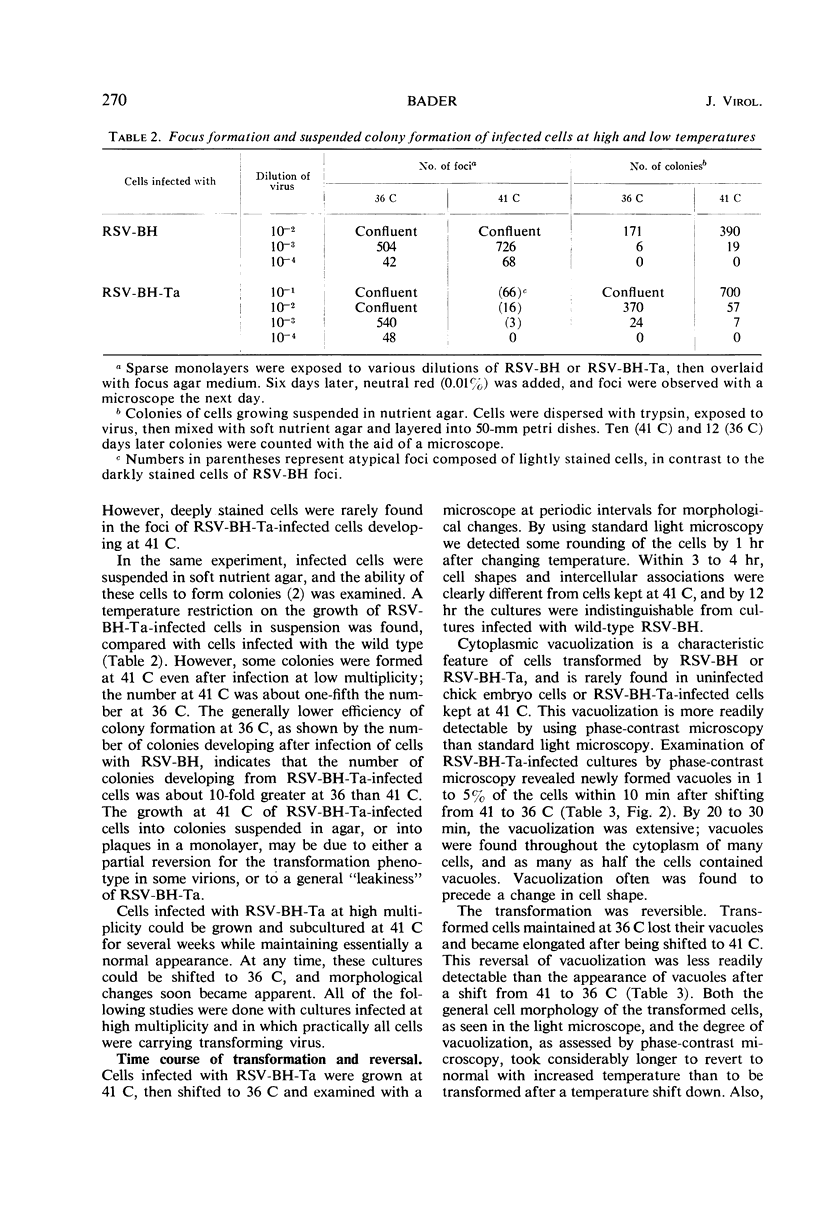
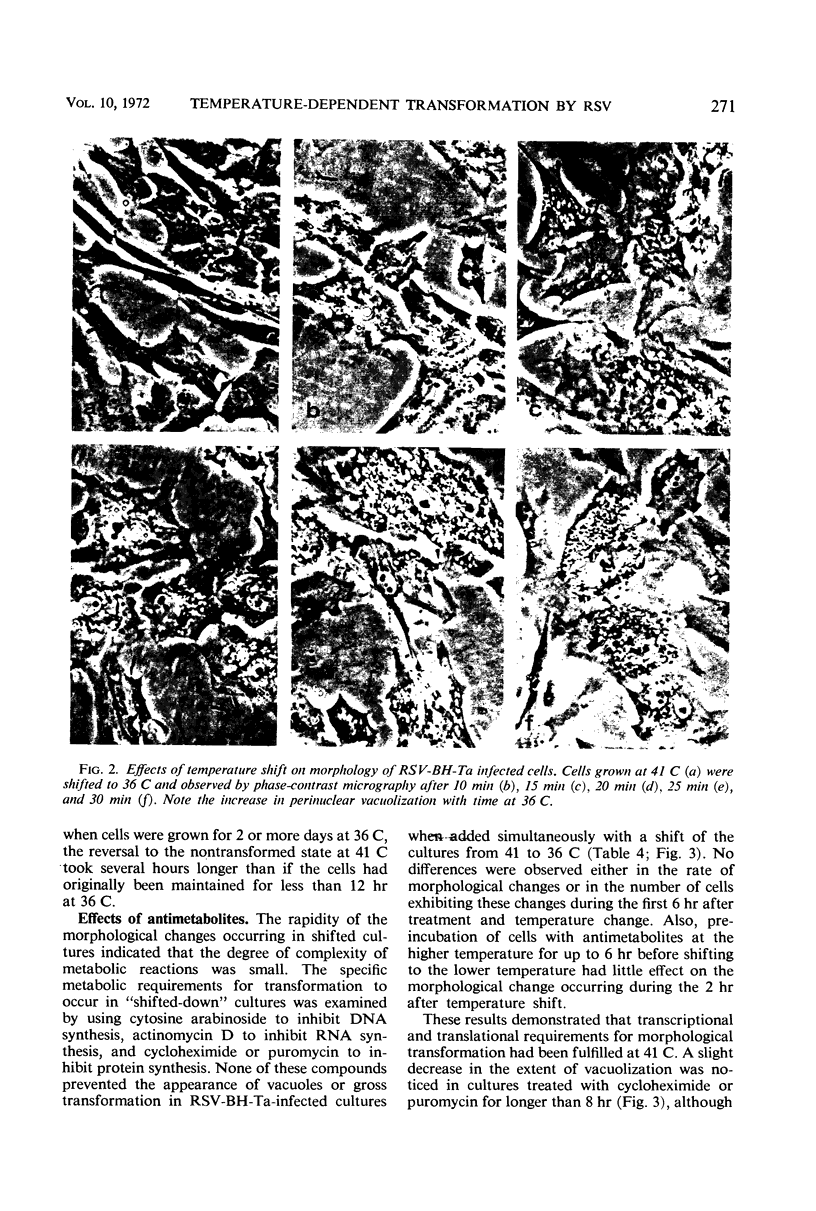
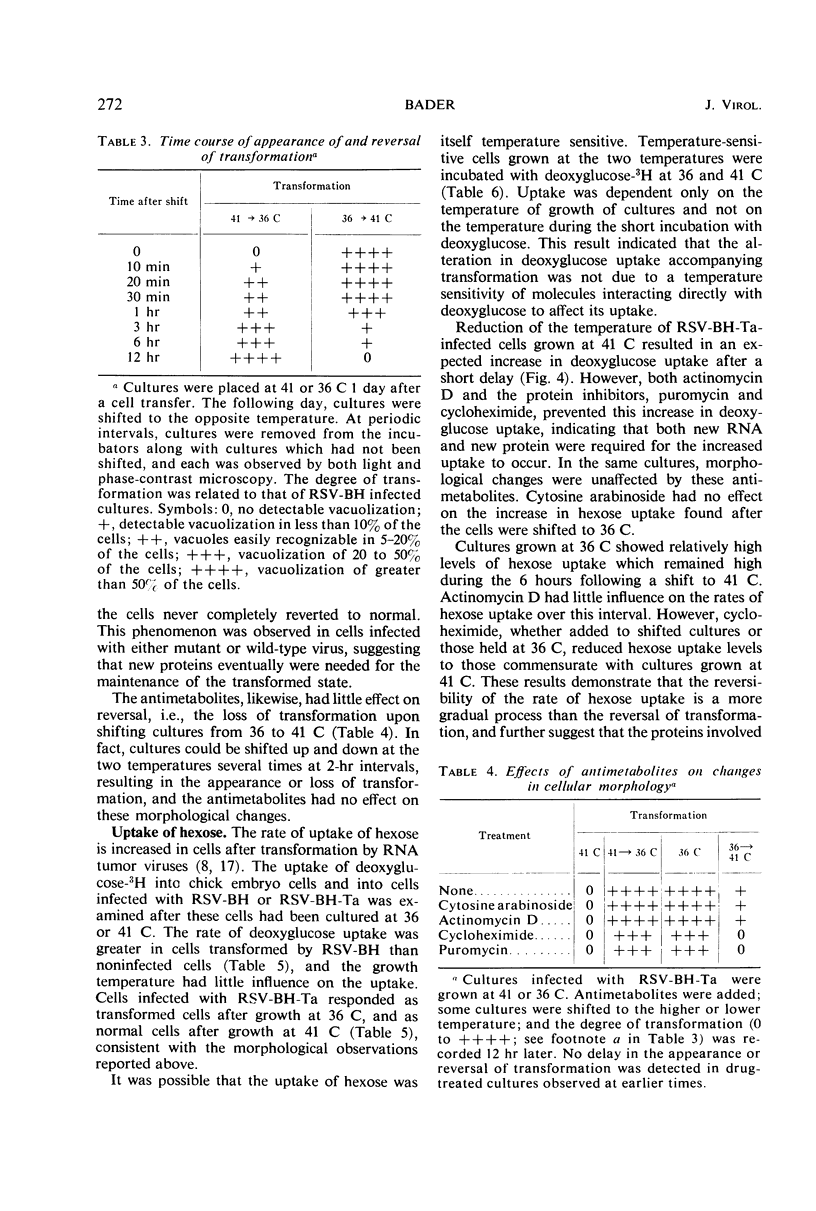
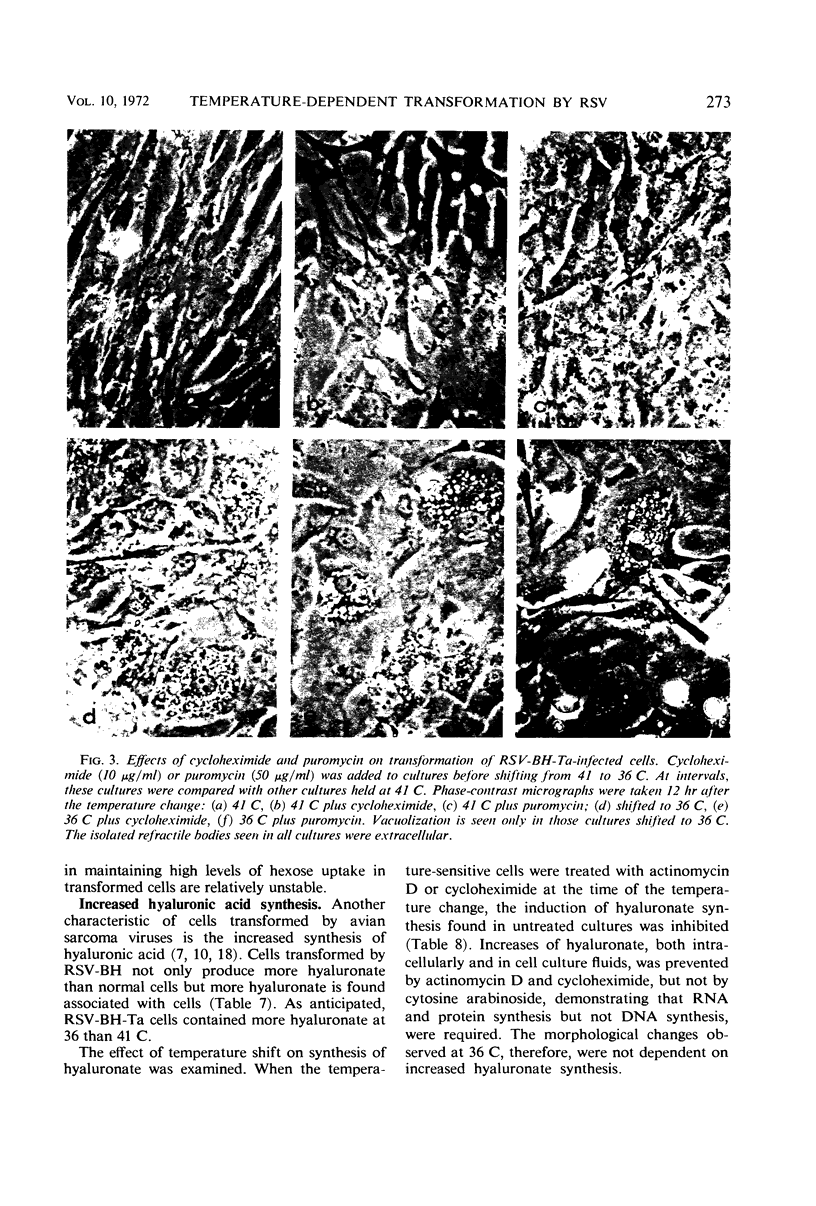
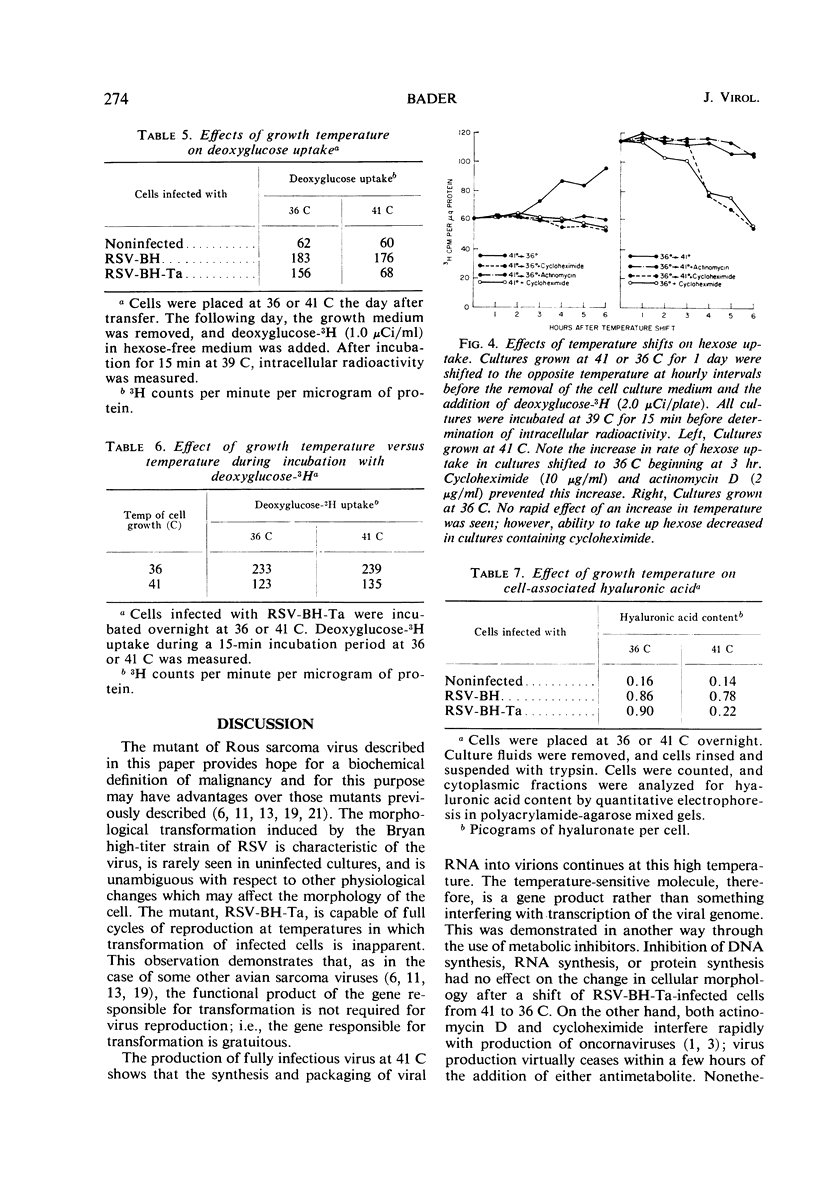
Chick embryo cells infected with a mutant (Ta) of the Bryan high-titer strain of Rous sarcoma virus (RSV-BH) are morphologically transformed at 36 C but appear similar to uninfected cells at 41 C. When cells infected with RSV-BH-Ta are switched from 41 to 36 C, morphological changes characteristic of transformation are observable within 10 min. The transformation is reversible; cells shifted from 36 to 41 C have been observed to lose their transformed morphology within 1 hr. The transformation after a shift in temperature is unaffected by inhibition of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), or protein synthesis, demonstrating that the proteins involved in the morphological change are already present. Transformed cells infected with RSV-BH or RSV-BH-Ta take up hexose and synthesize hyaluronic acid at higher rates than uninfected cells or RSV-BH-Ta-infected cells grown at 41 C. However, inhibition of either protein or RNA synthesis, but not DNA synthesis, prevented the induction of increased hexose uptake and hyaluronic acid synthesis after a shift of RSV-BH-Ta-infected cells from 41 to 36 C. Therefore, these biochemical changes are secondary to a more basic change responsible for morphological transformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADER J. P. THE ROLE OF DEOXYRIBONUCLEIC ACID IN THE SYNTHESIS OF ROUS SARCOMA VIRUS. Virology. 1964 Apr;22:462–468. doi: 10.1016/0042-6822(64)90067-4. [DOI] [PubMed] [Google Scholar]
- Bader J. P. A change in growth potential of cells after conversion by Rous sarcoma virus. J Cell Physiol. 1967 Dec;70(3):301–308. doi: 10.1002/jcp.1040700310. [DOI] [PubMed] [Google Scholar]
- Bader J. P., Bader A. V. Evidence for a DNA replicative genome for RNA-containing tumor viruses. Proc Natl Acad Sci U S A. 1970 Oct;67(2):843–850. doi: 10.1073/pnas.67.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J. P., Brown N. R. Induction of mutations in an RNA tumour virus by an analogue of a DNA precursor. Nat New Biol. 1971 Nov 3;234(44):11–12. doi: 10.1038/newbio234011a0. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Synthesis of the RNA of RNA-containing tumor viruses. I. The interval between synthesis and envelopment. Virology. 1970 Mar;40(3):494–504. doi: 10.1016/0042-6822(70)90192-3. [DOI] [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Characteristics of a conditional mutant of Rous sarcoma virus defective in ability to transform cells at high temperature. Virology. 1972 Feb;47(2):444–455. doi: 10.1016/0042-6822(72)90280-2. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. Alterations in the characteristics of sugar uptake by mouse cells transformed by murine sarcoma viruses. J Natl Cancer Inst. 1969 Nov;43(5):1091–1096. [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata Y., Bader J. P. Studies on the fixation and development of cellular transformation by Rous sarcoma virus. Virology. 1968 Nov;36(3):401–410. doi: 10.1016/0042-6822(68)90165-7. [DOI] [PubMed] [Google Scholar]
- Otten J., Bader J., Johnson G. S., Pastan I. A mutation in a rous sarcoma virus gene that controls adenosine 3',5'-monophosphate levels and transformation. J Biol Chem. 1972 Mar 10;247(5):1632–1633. [PubMed] [Google Scholar]
- Steck T. L., Kaufman S., Bader J. P. Glycolysis in chick embryo cell cultures transformed by Rous sarcoma virus. Cancer Res. 1968 Aug;28(8):1611–1619. [PubMed] [Google Scholar]
- TEMIN H. M., RUBIN H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958 Dec;6(3):669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- Temin H. M. The mechanism of carcinogenesis by avian sarcoma viruses. 1. Cell multiplication and differentiation. J Natl Cancer Inst. 1965 Oct;35(4):679–693. [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Temperature sensitive mutants of an avian sarcoma virus. Virology. 1969 Dec;39(4):930–931. doi: 10.1016/0042-6822(69)90030-0. [DOI] [PubMed] [Google Scholar]