Abstract
BACKGROUND
Measurement of red blood cell (RBC) survival (RCS) is important for investigating pathophysiology and treatment of anemia. Our objective was to validate the multidensity biotin method for RCS determination in sheep, a commonly used model of RBC physiology. [14C]Cyanate served as the reference method for long-term RCS because the 51Cr method (the reference method for humans) is not reliable in sheep.
STUDY DESIGN AND METHODS
Aliquots of autologous RBCs from eight adult sheep were labeled with [14C]cyanate and four separate densities of biotin (BioRBCs) and reinfused. Short-term RCS was assessed by posttransfusion recovery at 24 hours (PTR24); long-term RCS was assessed by the time to 50% survival (T50) and mean potential life span (MPL).
RESULTS
Values for PTR24 of the four BioRBC densities were not different. Values for RCS as reflected by T50 and MPL were nearly identical for [14C]cyanate and the two intermediate-density BioRBC populations. In contrast, the lowest-density BioRBC population survived slightly longer (p < 0.01), but with a difference of no clinical significance. The highest-density BioRBC population importantly shortened RCS (p < 0.01 compared to the two intermediate densities).
CONCLUSION
This study provides evidence that BioRBCs labeled at four biotin densities can be used to independently and simultaneously measure short-term RCS and that BioRBCs labeled at the three lowest biotin densities can be used to accurately and simultaneously measure long-term RCS. Because the sheep RBC model is comparable to humans, this nonradioactive method has promise for use in RBC kinetic studies in neonates and pregnant women.
Characterization of the behavior of red blood cells (RBCs) in the bloodstream after transfusion (i.e., posttransfusion RBC kinetics) generally consists of measurement of RBC volume (RCV) and RBC survival (RCS) assessed by posttransfusion recovery at 24 hours (PTR24) and at later times, that is, to 50% survival (T50) and to the mean potential life span (MPL). Posttransfusion RBC kinetic studies provide information valuable in establishing the diagnosis and defining the pathogenesis of hematologic conditions and in determining optimal RBC transfusion and blood banking practices.1 Ideally, techniques for measuring posttransfusion RBC kinetics should be precise, safe, and easily applied. They should also be broadly applicable to diverse study populations and require minimal blood volumes for sampling, labeling, and analysis—particularly for infants.
Methods utilizing chromium-51 (51Cr) radionuclide RBC labeling have been adopted by the International Committee for Standardization in Hematology as the reference method for determining posttransfusion RCS in humans.2 Unfortunately, because some species experience excessive rates of loss of 51Cr from RBCs (3%–4% per day in sheep, horses, cattle, goats, cats, and rats, compared to approx. 1% in humans),3 the 51Cr labeling method is not reliable for all mammalian species. Moreover, the costs for radiation containment and disposal are substantial and increasing.
Recently, a method based on biotin-labeled RBCs (BioRBC) that does not require radiation exposure has been developed and validated for use in posttransfusion kinetic studies in a broad spectrum of mammalian species. In addition to overcoming the aforementioned limitations of the 51Cr method, the BioRBC method achieves similar sensitivity while requiring analytic blood volumes as small as 3 μL4,5 compared to the 1 to 2 mL recommended for the 51Cr method.3
The focus of this study was on validating the BioRBC labeling method in sheep. This is important because ovine fetal and newborn cardiovascular, pulmonary, and of relevance for this report, hematologic development and physiology resemble those of humans.6 In particular, ovine erythropoiesis in the fetus, neonate, and adult is similar to the human, and regulation of erythropoiesis and RBC kinetics in the ovine model are currently active areas of study.7–10 Accordingly, lambs and sheep are logical animal models for preclinical testing of this method for measuring RCV and RCS.
We recently demonstrated that autologous sheep RBCs labeled at four discrete, low biotin densities yielded RCV results that are equivalent to one another and are within 10% of values determined simultaneously using [14C]cyanate-labeled RBCs.11 Further, we have applied the multidensity method to measurement of RCV in human adults with similar success. We were able to extend these human studies to survival of the BioRBCs and determine short-term and long-term RCS in eight participants. However, use of a radioactive-labeled marker such as 51Cr was not included because of the technical and financial demands.
In this study, we extend these preclinical animal studies to assess the validity of RCS determined in the same animals using RBCs labeled with the same four biotin densities and with [14C]cyanate. Because of the additional complexities of dealing with an animal with a large spleen that sequesters up to 30% of RBCs, of the difficulties of measuring radiolabeled RBCs, and the need to fit a mathematical curve to accurately determine the proportion of each of the BioRBC peaks, we have only recently completed this analysis for the current report. To do so, we assessed short-term RCS by PTR24 and long-term RCS by T50 and by MPL. For long-term RCS, we hypothesized that these two variables would agree closely when determined using RBCs labeled with the lower BioRBC densities and that they would agree with RCS determined using [14C]cyanate.12
MATERIALS AND METHODS
Animal characteristics
The Institutional Animal Care and Use Committee at the University of Iowa approved all animal studies. This study reports on eight of the nine sheep previously included in a study of the validity of RCV determinations;13 one animal not studied serially was excluded. All animals were female and approximately 2 years of age. None had been previously exposed to BioRBCs or other biotinylated proteins.
Jugular venous catheter placement for infusion and blood sampling
Approximately 1 week before the start of the study, a catheter used for infusion and withdrawal was placed percutaneously in a jugular vein (Intracath 16 gauge × 8 in, Becton Dickinson Infusion Therapy Systems, Inc., Sandy, UT). Before the transfusion of labeled RBCs, daily blood samples were collected for determination of hemoglobin (Hb) concentration, RBC counts, RBC indices, and reticulocyte counts (Sysmex XT 2000i hematology analyzer, Sysmex Corp., Kobe, Japan). Subsequent hematologic testing was performed at weekly intervals throughout the 23-week study period to assess whether erythropoiesis was perturbed.
Labeling of RBCs
On the day that RBCs labeled with biotin and [14C]cyanate were to be transfused, 600 to 720 mL of venous blood (approx. 12% of total blood volume)14 was drawn via the jugular catheter into either syringes containing heparin or a collection bag containing citrate-phosphate-dextrose (Baxter Healthcare Corp., Fenwal Division, Deerfield, IL). These whole blood samples were centrifuged at room temperature; the plasma was removed and discarded. RBCs were labeled as described below.
Biotin labeling
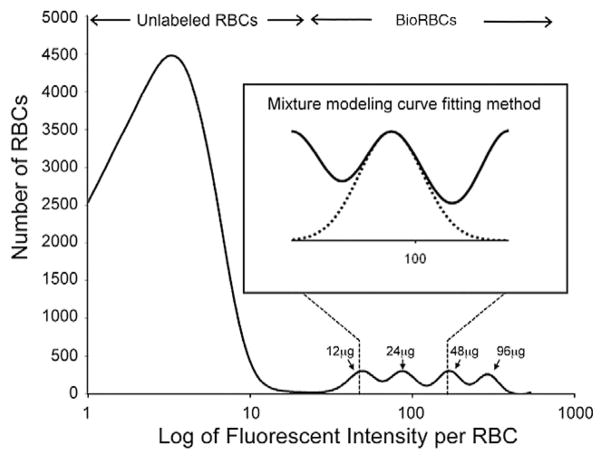
The method for biotin labeling of sheep RBCs at discrete densities has been previously described.15 Briefly, autologous RBCs were washed twice in a glucose containing pH 7.4 phosphate wash buffer to remove residual plasma. The washed RBCs were then labeled at twofold increasing concentrations of the biotinylation reagent, sulfo-succinimido-biotin (Pierce Chemical Co., Rockford, IL). To stabilize the biotinylation reagent, freshly prepared wash buffer was adjusted to pH 5, and dilutions of this biotinylation reaction mixture were chosen to yield reagent concentrations of 12, 24, 48, and 96 μg biotin/mL RBCs. The biotinylation reaction was conducted at pH 7.4 for 30 minutes, after which it was stopped by washing the RBCs twice with pH 7.4 wash buffer to remove the remaining reagent. This procedure yielded four separate, evenly spaced, overlapping BioRBC peaks.11–13,15,16 The lowest reagent concentration of 12 μg/mL RBCs (35 nmol/mL RBCs) was selected because this produced a BioRBC peak that was completely separated from the unlabeled RBC peak (Fig. 1).
Fig. 1.
Flow cytometry histogram of a representative post-RBC transfusion blood sample. The number of RBCs enumerated is plotted versus the log of fluorescent intensity for the four populations of sheep BioRBCs. Partial overlap among the four BioRBC peaks was observed as well as complete separation of the lowest-density BioRBC peak from the unlabeled RBC peak. The four populations of BioRBCs shown were produced by successive twofold incremental increases in the biotinylation reagent, sulfo-succinimido-biotin, starting with 12 μg/mL. The inset illustrates the curve produced by the mixed modeling method that was used to determine the percent RBC label enrichment.
[14C]Cyanate labeling
Aliquots of the washed RBCs from five of the eight sheep were labeled with [14C]cyanate using ex vivo incubation at 37°C for 60 minutes as previously described.17,18 These conditions drive the covalent reaction of [14C]cyanate with the terminal amino acid residue of Hb closer to completion thereby minimizing—but not completely eliminating—the confounding effects of the posttransfusion in vivo [14C]cyanate labeling. The continued labeling of Hb is an intracellular phenomena, which occurs primarily in the first 24 hours but may continue for the subsequent 1 to 3 days, but without evidence of any significant intercellular labeling or of breakdown and reacquisition of [14C]cyanate-labeled Hb by other RBCs.17,18
Study protocol
The four BioRBC populations were mixed in equal measured proportions and the one population of RBCs labeled with [14C]cyanate were alternately infused intravenously over a total of 3 to 4 minutes. The end of this infusion was designated as beginning of Study Day 0. The number of BioRBCs in each population density was calculated as the product of the RBC count per unit volume times the volume of transfused RBCs, determined gravimetrically. Using an estimated total blood volume of 70 mL/kg14 and the hematocrit (Hct) concentrations of individual sheep, the volume of each BioRBC population transfused was selected to result in an initial proportional enrichment of BioRBCs to total RBCs of approximately 2% to 3% of the total number of labeled and unlabeled RBCs as previously described.15 Approximately 80 mL of each BioRBC density and approximately 50 mL of [14C]cyanate-labeled RBCs were individually reinfused. After the reinfusion of the two labeled RBC aliquots, blood sampling for RCS was performed at 60 minutes, 24 hours, and weekly thereafter, until which time the individual BioRBC densities decreased below their lower limit of detection (<0.06% of circulating RBCs).16
Quantitation of individual BioRBC peaks used for determining RCS
Quantitation of the number of BioRBCs present in the four individual BioRBC peaks was performed on 4-μL aliquots of heparinized venous blood. Samples were washed in the same buffer used for biotin labeling and then incubated for 10 minutes at room temperature with Alexa 488-streptavidin (Invitrogen, Carlsbad, CA), washed again, and analyzed by flow cytometry as previously described.11 Sizable overlap was observed among the four adjacent peaks; that is, none of the tailing edges of adjacent peaks completely returned to baseline on the horizontal axis (Fig. 1). The choice of twofold increments was chosen to balance two opposing objectives: 1) to have the lowest density BioRBC sufficiently biotinylated to be separated from the unlabeled RBCs and 2) to have the highest density not so densely biotinylated that the BioRBCs are removed from circulation at an accelerated rate (unpublished observation).14
Overlap among the four BioRBC density peaks ranged from 1.2% to 4.0% of the total area under individual peaks for the initial day of the study (hence the suitability of using drop line for estimating RCV), and the degree of overlap among peaks increased with time as biotin was cleaved off the RBC surface and fluorescence intensity decreased. This mandated a more rigorous mathematical approach to quantitating the proportion of BioRBCs in each population when determining RCS. To best achieve this, a mathematical mixture modeling analysis was applied.19 In applying the mixture modeling, the number of BioRBCs included in each peak was expressed as a percentage of the total number of labeled and unlabeled RBCs counted in the blood sample. The mixture modeling curve fitting approach is more accurate than the drop line method used in our previous work because it specifically accounts for the overlap in the peaks, rather than assuming geometric symmetry in log of fluorescence BioRBC peaks.13 An alternative approach for avoiding this problem would have been to utilize nonoverlapping BioRBC densities, for example, threefold biotin density increments as we did in adult humans.14–20 However, this would require a greater degree of biotinylation that would cause a significant labeling artifact at the highest densities.
Quantitation of blood concentration of [14C]cyanate label bound to RBCs
Quantitation of the [14C]cyanate in posttransfusion samples was performed in triplicate using 0.5-mL samples of heparinized venous whole blood. Samples were processed for liquid scintillation quantitation of 14C covalently bound to Hb.16
Posttransfusion testing for antibodies to BioRBCs
Prior to exposure to transfused BioRBCs and every 4 weeks throughout the study period, plasma from 1 mL of venous blood was collected from study animals to test for the presence of existing antibodies and for the development of antibodies to BioRBCs. The specific BioRBC antibody testing procedure employed was similar to that reported by our group to detect antibodies to BioRBCs in humans.21 We purchased an anti-sheep immunoglobulin (Ig)G for use as a antiglobulin reagent (rabbit anti-sheep IgG [H+L], Cedarlane Labs, Burlington, Ontario, Canada). For the agglutination tests we used biotinylated sheep RBCs and nonbiotinylated sheep RBCs (negative control) prepared at a Hct of 4%. One drop of each RBC suspension was added individually to a series of paired test tubes. One drop of the test plasma was then added to each tube, and the mixtures were incubated for 30 minutes at 37°C. These were sedimented (300 × g for 6 min), washed, and resuspended, and the anti-IgG antiglobulin reagent was added at three concentrations. These three tubes were sedimented gently (300 × g for <1 min) and inspected macroscopically for agglutination. As a second negative control, normal saline was substituted for the test plasma. There is no available strictly comparable positive control (i.e., a sheep plasma with known BioRBC antibodies). However, we used plasma from lambs that developed an immune response after they were transfused with allogeneic RBCs. This plasma was shown to cause rouleaux formation of allogeneic sheep RBCs. We used this to produce a positive procedural control similar to commercially available human check cells used for this purpose in the human agglutination testing.
Determination of short- and long-term RCS variables
PTR24
Short-term survival of labeled RBCs after infusion was assessed using the PTR24 in the circulation. The percentage of labeled RBCs in the circulation 24 hours after infusion is referenced to the proportion of each population in circulation at 60 minutes posttransfusion because 60 minutes is required for the infused, labeled RBCs to reach equilibrium with a slowly mixing pool (probably the spleen) of endogenous RBCs.13
T50
Long-term RCS after infusion was measured using T50 and MPL. To determine the time after transfusion until disappearance of 50% of each population of labeled RBCs from the circulation, that is, “T50,” linear interpolation of the two nearest data points above and the two nearest below the 50% value were used. For the four BioRBC populations, the 100% reference value used in establishing the 50% value was the PTR24 BioRBC enrichment. For the [14C]cyanate population, the 100% reference value was established on the basis of the highest [14C]cyanate enrichment in the first week posttransfusion. This was done to allow for continuing in vivo binding of [14C]cyanate to Hb.
MPL
The MPL was estimated for each population of labeled RBCs by plotting RBC label enrichment against time after transfusion and then performing a least-squares linear curve fit of the RCS plot. MPL was defined by the number of days at the time axis intercept. For each density of BioRBCs the data used for the linear regression encompassed all data points between 24 hours and the time at which at least 20% of the population remained. The 20% value was selected because, at that point, several of the RCS curves became nonlinear. Because intracellular binding of [14C]cyanate to Hb continues for up to 3 days after ex vivo labeling,17,18 the earliest time point to be included in the linear regression data set was the time at which the highest concentration of [14C]cyanate was reached during the first week. The final time point included in the linear regression data set used to determine MPL was the time point at which [14C]cyanate had declined to or just slightly less than 20% of the highest concentration value.
Statistical analysis
Central tendency and variability of RCS variables (PTR24, T50, or MPL) for each population of labeled RBCs were expressed as the mean ± SEM. Differences in means for RCS variables among the populations of BioRBCs and [14C]cyanate-labeled RBCs were tested for significance by mixed model analysis for repeated measures or by t test, as indicated. If significant differences were detected, post hoc testing was performed using the Dunnett or Bonferroni correction for multiple comparisons, as indicated. These statistical analyses were performed with computer software (SAS, Version 9.2, 2002–2008, SAS Institute, Inc., Cary, NC). A p value of less than 0.05 was considered significant.
RESULTS
Assessment of sheep steady state
During the study, mean (±SEM) body weight increased from 75.5 ± 2.0 to 82.9 ± 1.4 kg (p = 0.0004). Initially, low whole blood Hb levels increased after the first 4 weeks and reticulocyte counts gradually declined until leveling off at 13 to 16 weeks (Table 1) providing evidence that the animals were approximately, but not perfectly, in steady state. We speculate that the increase in body weight was the result of the physiologic and nutritional changes accompanying the move from the supplier’s farm where the animals were maintained outdoors and free roaming to the sedentary indoor environment with more access to a more nutritious diet with more grain. We further speculate that the weight gain was primarily fat and as such did not significantly increase blood volume. The RBC indices, including mean RCV, mean concentration of RBC Hb, and RBC distribution width, did not change significantly during the study period indicating that nutritional iron deficiency was not responsible for the lower Hb levels in the pre- and early post-RBC transfusion period.
TABLE 1.
Sheep monthly mean (±SEM) hematologic variables measured
| Variable | Normal sheep adult values† | Pre-study | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 23 | p value |
|---|---|---|---|---|---|---|---|---|---|
| Hb (g/L) | 115 (90 to 150) | 101 ± 2.4 | 96 ± 3.0 | 118 ± 4.2* | 123 ± 5.3* | 120 ± 7.3* | 116 ± 2.1* | 118 ± 6.8* | <0.001 |
| Reticulocyte count (×109/L) | NA | 10.9 ± 1.3 | 10.5 ± 2.3 | 9.7 ± 0.81 | 8.6 ± 0.73 | 7.1 ± 0.74* | 7.0 ± 0.53* | 6.1 ± 0.80* | 0.014 |
| MCV (fL) | 34 (28 to 40) | 30.7 ± 0.27 | 31.0 ± 0.61 | 31.9 ± 0.86 | 31.6 ± 0.54 | 31.5 ± 0.57 | 31.7 ± 0.65 | 32.2 ± 0.69* | 0.01 |
| MCHC‡ (g/dL) | 32.5 (31 to 34) | 33.5 ± 0.59 | 33.4 ± 0.65 | 33.6 ± 0.70 | 33.9 ± 0.83 | 34.0 ± 0.89 | 33.8 ± 0.83 | 33.6 ± 0.72 | 0.14 |
| RDW§ (%) | NA | 29.2 ± 1.1 | 29.1 ± 1.2 | 30.3 ± 1.4 | 30.5 ± 3.4 | 30.1 ± 1.2 | 29.7 ± 1.2 | 29.9 ± 1.4 | NS |
p < 0.05 compared to the mean pre-study value by post hoc testing.
Mean (range) for normal sheep.32
MCHC = mean corpuscular Hb.
RDW = RBC distribution width.
Short-term RCS: PTR24
Group mean PTR24 for the four BioRBC densities averaged 93.5%; mean results for the four BioRBC densities did not differ (Fig. 2). The mean (±SEM) PTR24 for [14C]cyanate was 108 ± 2.4%, which is significantly greater than mean PTR24 for any of the four BioRBC densities (p < 0.0001) and significantly greater than 100% RCS (p = 0.0007). Thus, as observed in a previous study, labeling of RBCs exposed to [14C]cyanate ex vivo continued in vivo for up to 3 days, rendering [14C]cyanate unsuitable for assessing PTR24 in sheep.17,18
Fig. 2.
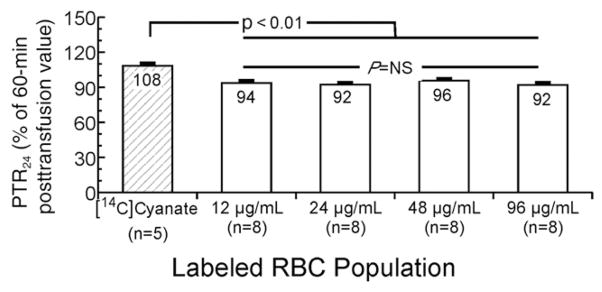
PTR24 for [14C]cyanate-labeled RBCs and the four BioRBC densities. Mean PTR24 agreed for the four populations of autologous BioRBCs, but all were different from that of the [14C]cyanate-labeled RBCs. Error bars depict ± 1 SEM.
Long-term RCS: T50 and MPL
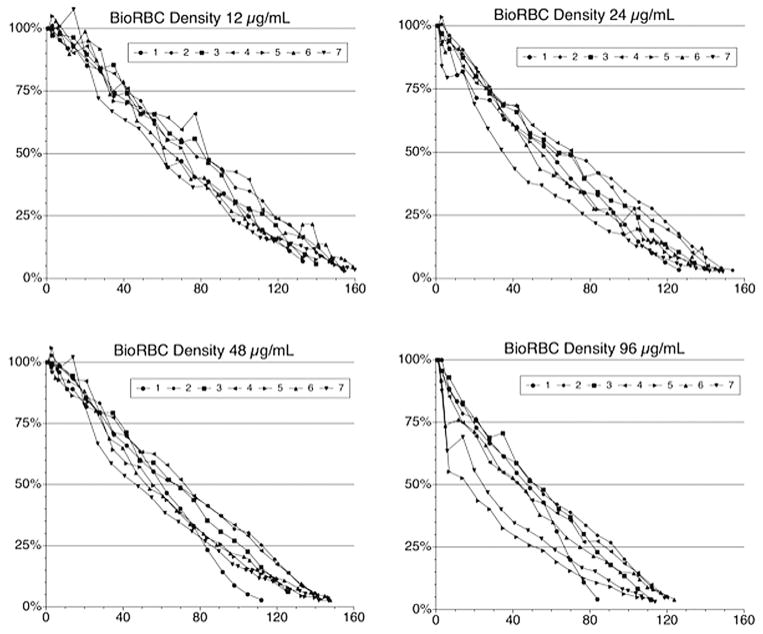
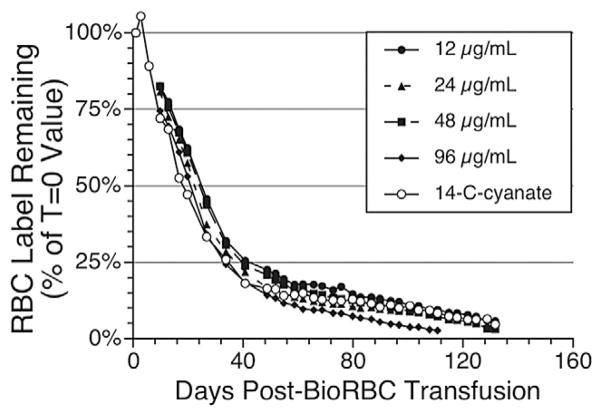
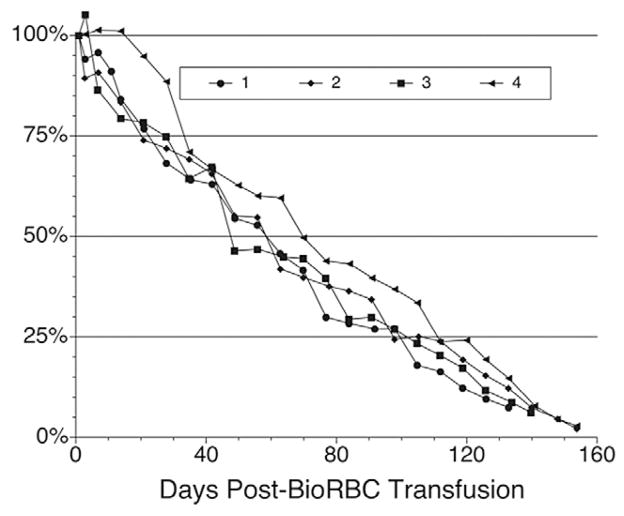
Examination of the survival curves for the eight study sheep (Figs. 3–5) revealed both similarities and differences among the five populations of labeled RBCs and among individual animals. When assessed using any of the three lowest BioRBC densities (12, 24, and 48 μg/mL) or [14C]cyanate-labeled RBCs, long-term RCS appears similar for any particular sheep. For all eight sheep, survival of all five populations (four BioRBC and one [14C]cyanate-labeled RBC) can be reasonably well fit by linear least-squares regression for disappearance of 80% of each RBC population; correlation coefficient was 0.976 ± 0.003 (n = 35 determinations; Figs. 4 and 5). The exceptions to this generalization, which were excluded in this analysis, were the survivals of 96 μg/mL density BioRBCs for Sheep 5 and Sheep 7 and the rapid, biphasic disappearance of all five populations of labeled RBC populations in Sheep 8 (Fig. 5). BioRBC density 96 μg/mL for Sheep 5 and 7 exhibited accelerated removal in the first week, after which they exhibited a slowing of removal. Sheep 8 exhibited striking (albeit linear) acceleration of RBC disappearance until only approximately 20% of the labeled RBCs remained in circulation; these data of Sheep 8 were included in the summary statistics for the correlation coefficient. In the early, rapidly disappearing phase observed in Sheep 8, mean MPL was 46.8 ± 1.9 days for the four BioRBC populations. After this rapid disappearance phase, a more gradual linear disappearance phase was observed that was quantitatively similar to that of the other seven animals. In this phase, MPLs for the three lowest-density BioRBC populations were 159, 163, and 152 days for 12, 24, and 48 μg/mL, respectively. The MPL for the highest density was 119 days.
Fig. 3.
Survival plots for the four populations of biotin-labeled RBCs for seven of the eight sheep studied. (Because of major differences in RCS, Sheep 8 is included separately in Fig. 5.) RCS was determined independently for each RBC population.
Fig. 5.
Sheep 8 RCS plots for the five populations of labeled RBCs (four BioRBC populations and one [14C]cyanate-labeled RBC population).
Fig. 4.
Survival plot for the raw data for the [14C]cyanate-labeled RBCs populations for seven of the eight sheep studied. (Because of major differences in RCS, Sheep 8 is included separately in Fig. 5.) As described under Materials and Methods, RCS was determined independently for each RBC population did not use all of the data points shown in the first few days after the retransfusion of [14C]cyanate-labeled RBCs. Also note that Sheep 5, 6, and 7 were not studied with this label.
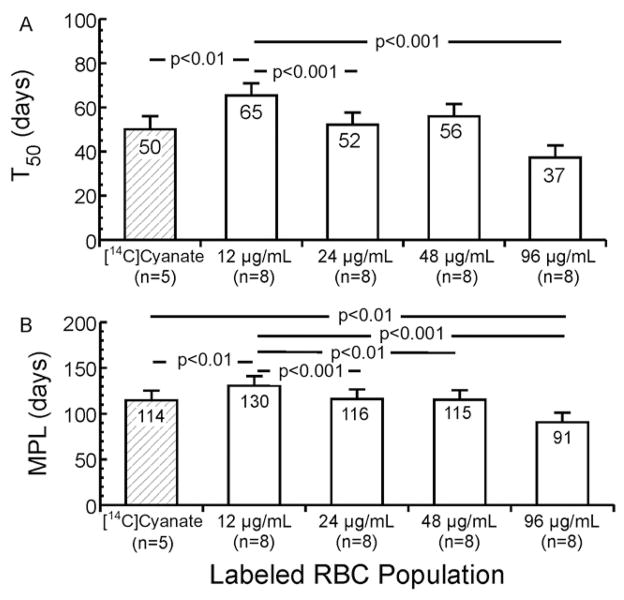
Statistical comparisons of group mean T50 for all eight sheep confirmed that that BioRBCs with the lowest biotin density (12 μg/mL) had the greatest T50 (i.e., the longest survival) and that RBCs labeled with the highest biotin density (96 μg/mL) had the shortest T50 (Fig. 6A). T50 of RBCs labeled at the intermediate densities of 24 and 48 μg/mL were not significantly different from each other. T50 of 24 μg/mL BioRBCs was significantly different from 12 μg/mL BioRBCs, but T50 of 48 μg/mL BioRBCs was not (p = 0.18). T50 of [14C]cyanate-labeled RBCs was shorter than T50 of BioRBCs labeled at 12 μg/mL.
Fig. 6.
T50 and MPL for [14C]cyanate-labeled RBCs and the four BioRBC densities. Mean T50 (A) for the lowest-density BioRBCs was longer than those of the 24 and 96 μg/mL BioRBCs densities and that of the [14C]cyanate-labeled RBCs. Mean MPL (B) for the lowest-density BioRBCs is longer than those of each of the higher-density BioRBCs and of [14C]cyanate-labeled RBCs. Error bars depict ± 1 SEM.
Group mean MPL offered a similar assessment of long-term survival (Fig. 6B). Mean MPL of the 12 μg/mL BioRBCs was significantly greater than those of the 24, 48, and 96 μg/mL BioRBCs, and [14C]cyanate-labeled RBCs exhibited shortened survival compared to BioRBC labeled at 12 μg/mL.
Antibody formation to sheep BioRBCs
Testing of plasma samples from study animals failed to demonstrate either the existing presence of antibodies or the development of antibodies that bound to any of the eight sheep’s BioRBCs.
DISCUSSION
A method to accurately and easily measure RCS is important to establish the diagnosis, to elucidate the patho-physiology, and to optimize therapies of anemia and other hematologic disorders and to guide research relevant to hematology, transfusion medicine, and blood banking.1 To this end, this study extends previous preclinical studies in the sheep, a commonly used model to study hematopoietic physiology of the fetus and low-birth-weight infant.7–10 This study validates the utility of BioRBCs to simultaneously measure both short- and long-term RCS in sheep using multiple populations of biotin-labeled RBCs.
All of the BioRBC populations yielded similar PTR24 results, providing evidence that all were useful for measuring short-term RCS and/or RBC recovery. The mean short-term PTR24 result of approximately 94% for each of the BioRBC populations is similar to the 98% PTR24 we reported for fresh, autologous, BioRBCs in adult humans.15 It is also similar to PTR24 51Cr RCS human adult data reported by Mollison and coworkers (96%)22 and by Bentley and coworkers (98.8%).23
Similarly, mean long-term MPL results in sheep obtained using the three lowest BioRBC densities (i.e., 130 ± 10, 115 ± 10, and 116 ± 10 days, respectively) were similar to those reported in adult humans by our group for the two lowest biotin densities (115 and 113 days)12 and by Mollison and coworkers22 (115 days) and Bentley and coworkers (110 days) using 51Cr.23 In this study, long-term RCS of RBCs labeled at the highest biotin density was significantly shortened as assessed by T50 and MPL. These findings are consistent with and support the findings of our previous RCS study in adult humans using multidensity BioRBCs.20 In that study, a limit for biotinylation density (96 μg biotin/mL RBCs) was identified above which long-term RCS decreases—because more densely biotinylated RBCs are altered and, consequently, prematurely removed from circulation. We conclude that the extent to which this takes place should be defined for each biotin labeling density in species in which BioRBCs are employed.
The observation from this study that long-term RCS of the lowest-density BioRBCs significantly exceeded that of simultaneously studied [14C]cyanate-labeled RBCs and the two intermediate-density BioRBCs merits consideration. Although the difference was small enough to have little clinical or practical importance, there are at least two possible biologic interpretations: 1) that [14C]cyanate labeling and this study’s intermediate biotin density labeling may alter sheep RBCs to minimally shorten long-term RCS such that low-density biotin labeling yields the best estimate of true RCS or 2) that very-low-density biotin labeling somehow extends the true long-term RCS. Unfortunately, data from this study do not resolve this question. This notwithstanding, this study provides evidence that labeling with any of the three lowest biotin densities yields estimates of long-term RCS similar to the [14C]cyanate method.
During the disappearance of the last 20% of the BioRBCs, the rate of disappearance slows. This slowing, which was more pronounced than in our recent report of RCS in adult humans,20 may reflect variability in the life span distribution of the cohort of RBCs present when labeling was performed.24 Alternatively, other factors such as subtle, minor degrees of biotinylation injury may have contributed to variability in the RBC life span.
Unlike the other seven animals, the RCS curve for Sheep 8 showed an initial rapid disappearance of both [14C]cyanate and BioRBCs until approximately 20% of labeled RBCs remained (Fig. 5). This was followed by a second phase exhibiting MPL similar to that observed in the other seven sheep. This biphasic RCS curve suggests the presence of two populations of RBCs: one with a shortened RCS and the other with a MPL survival equivalent to that observed in the other seven sheep. The absence of detectable antibodies to BioRBCs in Sheep 8 is consistent with a nonimmunologic mechanism for removal of BioRBCs. Although the mechanism(s) responsible is unknown, these results are similar to those described by Tucker for Finish Landrace sheep with a genetic deficiency of glutathione—a condition characterized by oxidant injury, consequent Heinz body formation, and shortened RCS.25
Given the importance of preclinical studies in investigating human disorders, it is worthwhile to briefly summarize the advantages and disadvantages of the multidensity biotin RBC labeling method relative to other labeling methods for RBC kinetic studies.26 First, as with 51Cr RBCs,2 the biotin labeling method permits accurate posttransfusion kinetic measurements of single populations of either allogeneic or autologous RBCs. Flow cytometric enumeration is accurate to a lower limit of 0.06% of total RBCs counted and allows tracking of disappearance of approximately 97% of BioRBCs. Such sensitivity and reproducibility are likely responsible for the superior reproducibility in the T50 and MPL estimates derived from the regression fits for the four BioRBC densities compared to the T50 and MPL estimates derived from [14C]cyanate regression. Unlike the 51Cr method, posttransfusion recovery and survival of multiple, distinct populations of BioRBCs can be determined simultaneously in the same individual.13–15,27 Second, elution of biotin from the RBC surface does not diminish the accuracy of kinetic measurements. This characteristic of the biotin method is because the flow cytometer counts individual BioRBCs as each passes through a beam of light; hence detection and enumeration do not depend on intensity of fluorescence per individual RBC—provided that the peaks are discrete and nonoverlapping.1 This contrasts with the 51Cr method in which elution of the label leads to significant uncertainty in estimates of long-term survival because elution rates for 51Cr method vary depending on the labeling technique used28 and among individual study subjects. Elution rates in normal human adults average approximately 1.4% loss per day22 but vary from 1% to 2% among individuals and differ at different time points after labeling.22 These variability factors introduce substantial uncertainty even in “elution corrected” determinations of PTR24, T50, and MPL.2,12,14,20,27,29
Third, tracking of RBCs with biotin requires smaller blood sample volumes (<20 μL) than most other methods for determining RCS. Fourth, the biotin method is technically practical because: 1) biotinylation reagents are inexpensive and readily available, 2) equipment for synthesis and measurement (a sterile hood and flow cytometer) are readily available, and 3) technician time for analysis is modest.12,14,20 Fourth, because measuring posttransfusion kinetics with BioRBCs avoids radiation exposure, it is ideal for studying vulnerable populations (i.e., infants, children, and pregnant women and their fetuses).
Finally, the BioRBC method has additional advantages over less commonly used methods to study RBC kinetics. For example, the “antigen difference method” uses flow cytometry to identify and enumerate donor and recipient RBCs based on the reaction of a specific antibody with its cognate allogeneic RBC antigen—a technique requiring antigenic differences between donor and recipient and availability of antibodies with specificity to these antigens for use in flow cytometric analysis.30 Although the antigen difference method is as sensitive as the biotin method, it cannot be utilized in kinetic studies of autologous RBCs (e.g., studies of new, experimental RBC preservative solutions). Additionally, the biotin method avoids costs of typing donor and recipient RBCs for antigenic differences and purchasing specific RBC antibodies. Thus, RCS studies of vulnerable population groups may now be resumed for the first time since the late 1960s, when clinical research studies involving the administration of radioactive compounds to such groups were curtailed.1
Relative to the 51Cr method and other RBC labeling methods employing radioactivity, RBC biotinylation has notable limitations. BioRBCs cannot be used to measure blood loss in the stool or to locate uptake of labeled RBCs in specific body organs, for example, spleen and liver.24 And as with any new method, concerns of possible toxicity must be addressed. Currently, there is no evidence of toxicity resulting from biotin labeling of RBCs when administered to human or animal subjects for RBC kinetic studies. When byproducts of the biotinylation process have been monitored for cellular toxicity by a sensitive cell culture system, none were found.15 Biotin per se (i.e., biotin not bound to RBCs or to other proteins) has been administered without toxicity intravenously and orally in amounts several orders of magnitude greater per unit body weight than the amounts given in this study.31 Finally, although antibodies to BioRBCs were not found in this study, antibodies to BioRBCs have been reported in 15% to 20% of exposed human adults. No adverse effects of apparent importance, including shortening of RCS, have been detected.21
Given the several advantages and relatively few disadvantages of multidensity BioRBC labeling method, we speculate that there are several important and clinically relevant areas of research and clinical practice in which this method can be usefully applied. Examples in transfusion medicine include simultaneous comparative evaluation of RBCs stored in multiple storage and preservative solutions, comparative evaluation of RBCs stored for varying periods of time after donation (i.e., fresh vs. stored), and comparative evaluation of RBCs selected for special characteristics that make them desirable in certain transfusion settings (e.g., neonatal and fetal autologous RBCs compared to adult donor allogeneic RBCs transfused into newborn infants). Additional examples include studies to define either pathophysiology or optimal therapy for RBC disorders such as hemolytic anemias and hemoglobinopathies. Even diseases that are not primarily hematologic such as diabetes have benefited from studies utilizing the multidensity BioRBC method to quantitate RCS of RBCs containing Hb A1c.1 We predict that the multidensity BioRBC labeling method will also continue to be important in preclinical studies, including pharmaco-dynamic studies assessing erythropoiesis-stimulating agents and other drugs influencing erythropoiesis and RCS. Such studies will benefit from the ability to label RBCs with different densities of biotin to produce populations that can be individually followed for determining RCS pre- and postdrug treatment.
In summary, this study provides evidence that RBCs labeled with biotin at discrete densities can be used to accurately estimate short- and long-term RCS of multiple RBC populations simultaneously or sequentially (or both) in the sheep. Because the sheep model is well established to be comparable to erythropoiesis in humans,6–10 and we have validated the biotin method to be safe, accurate, and offering advantages over the 51Cr method, we conclude that it will be useful in preclinical studies and in clinical studies of vulnerable populations such as neonates, infants, children, and pregnant women and their fetuses.
Acknowledgments
The authors acknowledge Robert S. Franco, PhD, for his careful review and insightful suggestions on the manuscript; James A. Roth, DVM, PhD, and Tom Raife, MD, for their advice on the BioRBC antibody assay; Earl Gingerich for analysis of laboratory samples; Jessica Goehring for data management; and Mark Hart for secretarial assistance. The Sysmex XT 2000i automatic hematology analyzer used in this study was provided on an on-loan basis from Sysmex Corp., Kobe, Japan.
This work was supported by the National Institutes of Health (NIH) Program Project Grant P01 HL046925. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This research was also supported by Award Number 1UL1RR029884 from the National Center for Research Resources in its use of the flow cytometer.
ABBREVIATIONS
- BioRBC(s)
biotinylated red blood cell(s)
- MPL
mean potential RBC life span in the circulation
- PTR24
posttransfusion recovery of RBCs after 24 hours in the circulation
- RCS
RBC survival
- RCV
circulating RBC volume
- T50
time to disappearance of 50% of the labeled RBCs from the circulation
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
References
- 1.Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109–14. doi: 10.1002/ajh.21298. [DOI] [PubMed] [Google Scholar]
- 2.International Committee for Standardization in Haematology. Recommended methods for radioisotope red-cell survival studies. Br J Haematol. 1980;45:659–66. doi: 10.1111/j.1365-2141.1980.tb07189.x. [DOI] [PubMed] [Google Scholar]
- 3.Gimlette T. Transfusion of autologous and allogeneic chromium-51 labelled red cells in ponies. J R Soc Med. 1978;71:576–81. [Google Scholar]
- 4.Lindsell CJ, Franco RS, Smith EP, Joiner CH, Cohen RM. A method for the continuous calculation of the age of labeled red blood cells. Am J Hematol. 2008;83:454–7. doi: 10.1002/ajh.21148. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann-Fezer G, Mysliwietz J, Mortlbauer W, Zeitler JJ, Eberle E, Honle U, Thierfelder S. Biotin labeling as an alternative nonradioactive approach to determination of red cell survival. Ann Hematol. 1993;67:81–7. doi: 10.1007/BF01788131. [DOI] [PubMed] [Google Scholar]
- 6.Lister G, Walter TK, Versmold PR, Dallman PR, Rudolph AM. Oxygen delivery in lambs: cardiovascular and hematologic development. Am J Physiol. 1979;237:H668–H675. doi: 10.1152/ajpheart.1979.237.6.H668. [DOI] [PubMed] [Google Scholar]
- 7.Blunt MH, Huisman TH, editors. The blood of sheep: composition and function. New York: Springer-Verlag; 1975. The hemoglobins of sheep; pp. 155–83. [Google Scholar]
- 8.Widness JA, Lowe LS, Bell EF, Mock DM, Kistard JA, Bard H. Adaptive responses during anemia and its correction in lambs. J Appl Physiol. 2000;88:1397–406. doi: 10.1152/jappl.2000.88.4.1397. [DOI] [PubMed] [Google Scholar]
- 9.Brace RA. Blood volume in the fetus and methods for its measurement. In: Nathanielsz PW, editor. Animal models in fetal medicine. Ithaca (NY): Perinatology Press; 1984. pp. 19–36. [Google Scholar]
- 10.Jones CT. The physiological development of the fetus and newborn. 1. New York: Academic Press; 1985. [Google Scholar]
- 11.Mock DM, Matthews NI, Strauss RG, Burmeister LF, Schmidt R, Widness JA. Red blood cell volume can be independently determined in vitro using sheep and human red cells labeled at different densities of biotin. Transfusion. 2009;49:1178–85. doi: 10.1111/j.1537-2995.2009.02095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of red cell survival using biotin labeled red cells: validation against 51Cr labeled red cells. Transfusion. 1999;39:156–62. doi: 10.1046/j.1537-2995.1999.39299154729.x. [DOI] [PubMed] [Google Scholar]
- 13.Mock D, Matthews N, Zhu S, Burmeister L, Zimmerman M, Strauss R, Schmidt R, Nalbant D, Freise K, Veng-Pedersen P, Widness J. Red blood cell (RBC) volume can be independently determined in vivo in the sheep using ovine RBCs labeled at different densities of biotin. Transfusion. 2010;50:2553–64. doi: 10.1111/j.1537-2995.2010.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. RBCs labeled at two biotin densities permit simultaneous and repeated measurements of circulating RBC volume. Transfusion. 2004;44:431–7. doi: 10.1111/j.1537-2995.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 15.Mock D, Matthews N, Zhu S, Burmeister L, Zimmerman M, Strauss R, Schmidt R, Nalbant D, Cress G, Widness J. Red blood cell (RBC) volume can be independently determined in vivo in humans using RBCs labeled at different densities of biotin. Transfusion. 2011;51:148–57. doi: 10.1111/j.1537-2995.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mock DM, Mock NI, Lankford GL, Burmeister LF, Strauss RG, Widness JA. Red cell volume can be accurately determined in sheep using a nonradioactive biotin label. Pediatr Res. 2008;64:528–32. doi: 10.1203/PDR.0b013e318183f119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mock DM, Lankford GL, Burmeister LF, Strauss RG. Circulating red cell volume and red cell survival can be accurately determined in sheep using the [14C]cyanate label. Pediatr Res. 1997;41:916–21. doi: 10.1203/00006450-199706000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Mock DM, Strauss RG, Lankford GL. 14C-cyanate labeling of sheep red cells: covalent binding to hemoglobin continues in vivo for a day. Pediatr Res. 1997;41:424–9. doi: 10.1203/00006450-199703000-00020. [DOI] [PubMed] [Google Scholar]
- 19.McLachlan GJ, Peel D. Finite mixture models. 1. New York: John Wiley and Sons, Inc; 2000. [Google Scholar]
- 20.Mock DM, Matthews NI, Zhu S, Strauss RG, Schmidt RL, Nalbant D, Cress GA, Widness JA. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion. 2011;51:1047–57. doi: 10.1111/j.1537-2995.2010.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordle DG, Strauss RG, Lankford GL, Mock DM. Antibodies provoked by the transfusion of biotin-labeled red cells. Transfusion. 1999;39:1065–9. doi: 10.1046/j.1537-2995.1999.39101065.x. [DOI] [PubMed] [Google Scholar]
- 22.Mollison PL, Engelfriet CP, Conteras M. Blood transfusion in clinical medicine. London: Blackwell Scientific Publications; 1993. [Google Scholar]
- 23.Bentley SA, Glass HI, Lewis SM, Szur L. Elution correction in 51Cr red cell survival studies. Br J Haematol. 1974;26:179–84. doi: 10.1111/j.1365-2141.1974.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 24.Klein H, Anstee D. Mollison’s blood transfusion in clinical medicine. 11. London: Blackwell Publishing; 2006. [Google Scholar]
- 25.Tucker EM. A shortened life span of sheep red cells with a glutathione deficiency. Res Vet Sci. 1974;16:19–22. [PubMed] [Google Scholar]
- 26.Suzuki T, Dale GL. Biotinylated erythrocytes: in vivo survival and in vitro recovery. Blood. 1987;70:791–5. [PubMed] [Google Scholar]
- 27.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of circulating red blood cell volume using biotin labeled red cells: validation against 51Cr labeled red cells. Transfusion. 1999;39:149–55. doi: 10.1046/j.1537-2995.1999.39299154728.x. [DOI] [PubMed] [Google Scholar]
- 28.Szymanski IO, Valeri CR. Factors influencing chromium elution from labelled red cells in vivo and the effect of elution on red-cell survival measurements. Br J Haematol. 1970;19:397–409. doi: 10.1111/j.1365-2141.1970.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 29.Ebaugh FG, Jr, Emerson CP, Ross JF. The use of radioactive chromium 51 as an erythrocyte tagging agent for the determination of red cell survival in vivo. J Clin Invest. 1953;32:1260–76. doi: 10.1172/JCI102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garratty G, Arndt PA. Applications of flow cytofluorometry to red blood cell immunology. Cytometry. 1999;38:259–67. doi: 10.1002/(sici)1097-0320(19991215)38:6<259::aid-cyto1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 31.Mock D. Biotin: physiology, dietary sources and requirements. In: Caballero B, Allen L, Prentice A, editors. Encyclopedia of human nutrition. Amsterdam; Boston (MA): Elsevier/Academic Press; 2005. pp. 201–10. [Google Scholar]
- 32.Pugh D. Appendix III: Table A normal values and conversions. In: Pugh D, editor. Sheep and goat medicine. Philadelphia (PA): Saunders; 2002. pp. 451–5. [Google Scholar]