Abstract
Sleep deprivation is associated with considerable social, financial, and health-related costs, in large measure because it produces impaired cognitive performance due to increasing sleep propensity and instability of waking neurobehavioral functions. Cognitive functions particularly affected by sleep loss include psychomotor and cognitive speed, vigilant and executive attention, working memory, and higher cognitive abilities. Chronic sleep-restriction experiments—which model the kind of sleep loss experienced by many individuals with sleep fragmentation and premature sleep curtailment due to disorders and lifestyle—demonstrate that cognitive deficits accumulate to severe levels over time without full awareness by the affected individual. Functional neuroimaging has revealed that frequent and progressively longer cognitive lapses, which are a hallmark of sleep deprivation, involve distributed changes in brain regions including frontal and parietal control areas, secondary sensory processing areas, and thalamic areas. There are robust differences among individuals in the degree of their cognitive vulnerability to sleep loss that may involve differences in prefrontal and parietal cortices, and that may have a basis in genes regulating sleep homeostasis and circadian rhythms. Thus, cognitive deficits believed to be a function of the severity of clinical sleep disturbance may be a product of genetic alleles associated with differential cognitive vulnerability to sleep loss.
Keywords: Neurobehavioral performance, sleep restriction, attention and executive function, functional neuroimaging, prefrontal cortex, thalamus, sensory processing areas, genetics
SLEEP DEPRIVATION AND ACCIDENT RISK
The overall prevalence of insufficient sleep in adults has been estimated at 20%.1 The effects of insufficient sleep on cognitive processing are described below; of these, daytime sleepiness has been the most common measure assessed in population-based studies. One study determined the prevalence of daytime sleepiness using interviews conducted over 5.5 years which followed 1,007 randomly selected young adults ages 21 to 30 years in southeast Michigan.2 That study found the average nocturnal sleep time during weekdays was 6.7 hours and on weekends was 7.4 hours. Sleepiness was inversely proportional to hours slept, and difficulty falling asleep was more prevalent in single adults with a full-time job.2 Studies in young adults indicate that 8 to 9 hours of extended nocturnal sleep are needed to resolve sleepiness caused by decreased sleep time.3,4 The apparent chronic partial sleep deprivation experienced by the young adults surveyed in 1997 complements statistics showing young drivers, especially males, are at much higher risk for drowsy driving and sleep-related crashes.5–7
Sleep deprivation increases the risk of human-error-related accidents,8 with such accidents estimated to have an annual economic impact of $43 to $56 billion.9 Motor vehicle accidents related to fatigue, drowsy driving, and falling asleep at the wheel are particularly common, but often underestimated.10,11 Increased time awake, nocturnal circadian phase, reduced sleep duration, prolonged driving duration, and use of soporific medications all contribute to the occurrence of drowsy-driving-related and fatigue-related motor vehicle crashes.6,12,13 Moreover, studies of shift-workers,14–16 truck drivers,17–19 medical residents,20–22 and airline pilots23–26 all show an increased risk of crashes or near misses due to sleep deprivation in these populations.
Sleepiness-related motor vehicle crashes have fatality rates and injury severity levels similar to alcohol-related crashes.6 In addition, sleep deprivation produces psychomotor impairments equivalent to those induced by alcohol consumption at or above the legal limit.27 For example, in a study of simulated driving performance, impairments in lane-keeping ability after a night without sleep were equivalent to those observed at a blood alcohol content (BAC) of 0.07%.28 Similarly, a study of professional truck drivers found that deficits in performance accuracy and reaction time after 28 hours of sleep deprivation were equivalent to those found after alcohol intoxication (BAC at 0.1%).29 Thus, it appears that as continuous daytime waking exceeds 16 hours, psychomotor performance deficits increase to levels equivalent to BAC levels between 0.05% and 0.1%.27,29
Sleep deprivation poses risks to safe operation in all modes of transportation and to performance in other safety-sensitive activities.30 Improved understanding of the neural basis of such risks in operational environments has been achieved through the experimental study of how precisely sleep deprivation affects discrete cognitive abilities.
SLEEP DEPRIVATION AND SLEEP–WAKE REGULATION
The identification of the neural systems controlling circadian and sleep homeostatic mechanisms has improved our understanding of the effects of sleep deprivation on human neurobehavioral functions.23,31–33 Although much is known about the neurobiology of hypothalamic mechanisms involving sleep–wake regulation, less is known about how these systems interact and alter waking neurocognitive functions.34,35 Both wakefulness and sleep are modulated by an endogenous biological clock located in the suprachiasmatic nuclei (SCN) of the hypothalamus. Beyond driving the body to fall asleep and to wake up, the biological clock also modulates waking behavior, as reflected in sleepiness and cognitive performance, generating circadian rhythmicity in almost all neurobehavioral variables investigated.34,35 Theoretical conceptualizations of the daily temporal modulation of sleep and wakefulness (and to a lesser extent the modulation of waking cognitive functions) have been instantiated in the two-process mathematical model of sleep regulation36,37 and in mathematical variants of this model.38 The two-process model of sleep regulation has been used to describe the temporal profiles of sleep and wakefulness.34,35 The model consists of a sleep homeostatic process (S) and a circadian process (C), which interact to determine the timing of sleep onset and offset, as well as the stability of waking neurocognitive functions.34,35,39 The homeostatic process represents the drive for sleep that increases during wakefulness and decreases during sleep. When this drive increases above a certain threshold, sleep is triggered; when it decreases below another threshold, wakefulness is invoked. The circadian process represents daily oscillatory modulation of these threshold levels. The circadian drive for wakefulness may be experienced as spontaneously-enhanced alertness in the early evening even after a sleepless night. Deprivation of sleep, however, can elevate homeostatic pressure to the point that waking cognitive functions will be degraded even at the time of the peak circadian drive for wakefulness.40 The degradation of goal-directed cognitive behavior that can occur unpredictably in sleep-deprived individuals appears to reflect the transient intrusion of sleep neurobiology into waking neurobiology.41
Sleep Propensity
Sleep deprivation increases sleep propensity, measured using polysomnography, as a reduction in the latency to sleep onset,42 as well as by shortening of the latencies from lighter stages of non-rapid eye movement (NREM) sleep to deeper slow wave thalamocortical oscillations.43 For example, after a night without sleep, the daytime sleep latency of a healthy adult decreases, by an order of magnitude, to less than a minute or two on average, and the subsequent latency from sleep onset to slow wave sleep is halved.43 The Multiple Sleep Latency Test (MSLT) standardized sleep latency as a physiologic measure of sleepiness.42,44 MSLT results may vary for many reasons, including prior sleep efficiency, prior sleep time, drug effects, physical activity, and posture.45,46 The Maintenance of Wakefulness Test (MWT), a variant of the MSLT, also uses sleep latency to measure sleep propensity, but requires subjects to remain awake (resist sleep) rather than fall asleep.47 Like the MSLT, the MWT shows reduced sleep latency in response to sleep deprivation. Thus, whether attempting to fall asleep or resist sleep, the latency from waking to sleeping is significantly reduced by sleep deprivation.
Microsleeps and Wake State Instability
The increased propensity for sleep to occur quickly, even when being resisted by a sleep-deprived subject, is consistent with evidence suggesting that “microsleeps” intrude into wakefulness when sleep-deprived subjects fail to respond (i.e., lapse) during cognitive performance demands.48–51 Cognitive performance variability involving both errors of omission (i.e., behavioral lapses evident as failure to respond in a timely manner to a stimulus) and errors of commission (i.e., responses when no stimulus is present or to the wrong stimulus) is a primary consequence of sleep deprivation.40,52 Such variability during performance in sleep-deprived subjects has been hypothesized to reflect wake state instability.40,41 According to this theory, two competing neurobiologic systems exert an influence on the behavior of a sleep-deprived individual. More rostral areas of the brain exert a top-down drive to sustain alertness (i.e., motivated behavior), while more central and caudal areas increase the involuntary homeostatic drive to fall asleep. The interaction of these drives results in unreliable behavior, including heightened moment-to-moment variability in cognitive functions. A hallmark feature of cognitive variability is lapsing (i.e., brief periods of half a second to many seconds of no response). Lapses are often so brief, they cannot be detected in behavior without a special test, such as the Psychomotor Vigilance Test.41,53 As lapses increase in frequency, they increase in duration, and ultimately they can result in a full-blown sleep attack (i.e., no spontaneous recovery by the subject). Both acute total sleep deprivation and chronic partial sleep deprivation can produce a high rate of lapsing that ultimately progresses to full and sustained sleep onset during goal-directed behavior (e.g., motor vehicle operation).8,41
The difference between the lapse hypothesis and the state instability hypothesis is in the explanation for the variability in cognitive performance during sleep deprivation. The lapse hypothesis posits that cognitive performance during sleep deprivation is essentially “normal” until it becomes disrupted by lapses or brief periods of low arousal.51 By contrast, the state instability hypothesis40 posits that responses between lapses can also slow and get worse with time on task, that errors of commission (wrong responses) can comingle with errors of omission (lapses), and that variability in neurocognitive performance (more so than changes in average performance) increases as homeostatic sleep-initiating mechanisms become progressively more upregulated with sleep loss.8,41 Thus, the brain’s capacity to maintain alertness is hindered by the activation of sleep processes.
Wake state instability occurs when sleep-initiating mechanisms repeatedly interfere with wakefulness, depending on the severity of sleep deprivation, making cognitive performance increasingly variable and dependent on compensatory mechanisms.53,54 The ability of the sleep-deprived subject to engage in motivated behavior (e.g., walking) to compensate for or mask the cognitive effects of sleep loss is well recognized.55,56 However, such a compensatory effort to resist sleep ultimately cannot prevent intrusions of sleep initiation into wakefulness. In addition to reports of sleep-deprived subjects “semi-dreaming” (likely hypnagogic reverie) while engaged in verbal cognitive tasks,57,58 first-person reports exist of healthy sleep-deprived people falling asleep while ambulating in dangerous environments.59 Thus, state instability evident in the cognitive performance and biobehavioral signs (e.g., slow eyelid closures60–64) of sleep-deprived subjects, as reflected by the occurrence of microsleeps or sleep attacks, is directly related to increased variability in cognitive performance. The concomitant increase in errors of commission can also reflect an increased compensatory effort to resist sleep (i.e., trying to stop lapses by overresponding). Both cognitive errors of omission and of commission during sleep loss increase with time on task.
The effects of sleep deprivation on wake state instability during cognitive performance means that at any given moment in time the cognitive ability of the sleep-deprived individual is unpredictable, and a product of interactive, reciprocally inhibiting neurobiologic systems mediating sleep initiation and wake maintenance. Theoretically, wake state instability suggests there are multiple, parallel mechanisms by which waking and sleep states can interact. This theory is consistent with reports of the growing number of candidate molecules that may be involved in the co-occurrence of sleep and waking.32
Cognitive Performance during Sleep Deprivation
It has long been established that sleep deprivation degrades aspects of cognitive performance.52,57,65 The first published experimental study of the cognitive performance effects of sleep deprivation in humans was reported in 1896 and involved three adults experiencing 90 hours of continuous wakefulness.56 Since 1896, many studies measuring behavioral changes associated with sleep deprivation have been performed. A review of the literature reveals three general types of studies: long-term total sleep deprivation studies (>45 hours), short-term total sleep deprivation studies (≤45 hours), and partial sleep deprivation studies (sleep restriction to <7 hours/24 hours). There are literally hundreds of published studies on the effects of total sleep deprivation, but far fewer on the effects of partial sleep deprivation, and only a handful on the effects of chronic partial sleep restriction. Moreover, the cognitive performance measures used vary widely among studies. Three categories of measurement commonly used in sleep deprivation studies include cognitive performance, motor performance, and mood.66 Virtually all forms of sleep deprivation result in increased negative mood states, especially feelings of fatigue, loss of vigor, sleepiness, and confusion. Although feelings of irritability, anxiety, and depression are believed to result from inadequate sleep, experimental evidence of the existence of these mood states following sleep deprivation in a comfortable and predictable environment is thus far lacking. These alterations in mood, however, have been observed repeatedly when sleep deprivation occurs without regard for conditions.67 On the other hand, self-reports of fatigue and sleepiness are often blunted in chronic sleep restriction relative to the more linear effects of chronic partial sleep loss on cognitive performance.68
An early meta-analysis suggested that the effects of sleep deprivation on feelings of fatigue and related mood states are greater than the effects on cognitive performance and motor functions.66 This conclusion, however, appears to be the result of inadequate experimental controls and cognitive assessments in partial sleep deprivation studies conducted prior to 1997.69 Experiments in the past 10 years have found that chronic sleep restriction results in more rapid cumulative increases in cognitive performance errors than subjective measures of fatigue and mood, and the effects are in proportion to the dose of sleep and chronicity of restriction,68,70–73 although rapid versus gradual restriction of sleep can influence the rate of accumulation of cognitive deficits.68,74
Sleep deprivation induces a wide range of effects on cognitive functions (Table 1), although cognitive tasks vary considerably in their sensitivity to sleep loss. In general, regardless of the task, cognitive performance becomes progressively worse when time on task is extended; this is the classic “fatigue” effect that is exacerbated by sleep loss.48,75 However, performance on even very brief cognitive tasks that measure speed of cognitive “throughput,” working memory, and other aspects of attention have been found to be sensitive to sleep deprivation.76 Two confounding factors that can obscure the effects of sleep loss on many cognitive tasks are intersubject variability and intrasubject variability.53 For example, one individual’s poorest performance during sleep deprivation may be superior to that of the best performance of a non-sleep-deprived individual (this aptitude effect is the intersubject confound). Similarly, a person may be cognitively diminished by sleep loss, but continue to improve on a repeated task due to the effects of learning (this learning effect is the intrasubject confound). A second problem with many research reports on the cognitive effects of sleep deprivation concerns the nature of the dependent variables selected for analyses. A failure to understand that sleep deprivation increases variability within subjects (i.e., state instability) and between subjects (i.e., differential vulnerability to the effects of sleep deprivation) can mean that the effects of sleep loss on cognitive measures are missed because less sensitive metrics or data analyses are used.77,78
Table 1.
Summary of Cognitive Performance Effects of Sleep Deprivation
| Involuntary microsleeps occur. |
| Attention-intensive performance is unstable with increased errors of omission (lapses) and commission (wrong responses). |
| Cognitive slowing occurs in subject-paced tasks, whereas time pressure increases cognitive errors. |
| Psychomotor response time slows. |
| Both short-term recall and working memory performances decline. |
| Reduced learning (acquisition) of cognitive tasks occurs. |
| Performance requiring divergent thinking deteriorates. |
| Response suppression errors increase in tasks primarily subserved by the prefrontal cortex. |
| Response perseveration on ineffective solutions is more likely to occur. |
| Increased compensatory effort is required to remain behaviorally effective. |
| Tasks may begin well, but performance deteriorates as task duration increases. |
| Growing neglect of activities judged to be nonessential (loss of situational awareness) occurs. |
To provide an accurate and useful measure of performance during sleep loss and the dynamic expression of waking neurobehavioral integrity as it changes over time, cognitive assessments must be valid and reliable reflections of fundamental waking functions altered by sleep deprivation. As such, measures of attention, vigilance, and declarative memory are often used, with reaction time as the dependent variable. The Psychomotor Vigilance Test (PVT),79 a measure of behavioral alertness via sustained attention demands, is free of aptitude and learning effects and sensitive to sleep loss,41 sleep pathology, and functioning at an adverse circadian phase.53,80 The PVT requires continuous attention to detect randomly occurring stimuli. Such simple but attention-demanding tasks have proven to be reliable, valid, and sensitive measures of sleep deprivation, suggesting that the neural mechanisms of attention are among the most susceptible to sleep deprivation. The ubiquitous effects of sleep deprivation on attention-rich tasks should be understood relative to evidence that the dorsolateral prefrontal cortex is one of the critical structures in a network of anterior and posterior “attention control” areas. The prefrontal cortex has a unique executive attention role in actively maintaining access to stimulus representations and goals in interference-rich contexts.81
More complex cognitive tasks involving higher cognitive functions have often been regarded as insensitive to sleep deprivation (see reference 65 for a review), perhaps due to the types of complex neurocognitive tasks utilized in some studies. In particular, the use of novel logic-based tasks results in little change following sleep loss. When tasks are made more divergent, such as occurs with multitasking and flexible thinking, sleep deprivation has been reported to produce adverse effects on performance. Divergent skills involved in decision making that are affected by sleep loss include assimilation of changing information, updating strategies based on new information, lateral thinking, innovation, risk assessment, maintaining interest in outcomes, mood-appropriate behavior, insight, and communication and temporal memory skills.65 In one study utilizing divergent, complex skills, including visual temporal memory, confidence judgment, verb generation to noun presentation, and response inhibition, assessments were made in normal subjects from different age groups.65 Performance on these cognitive skill areas was poorer in older subjects, but when young subjects were evaluated after 36 hours of sleep deprivation, their performance declined to those levels of the older subjects. The authors suggest that decrements in cognitive performance due to aging may be similar to the effects of sleep deprivation. Neurocognitive deficits in healthy aging have been attributed to deficits in the prefrontal cortex.82 Both aging and sleep deprivation appear to reliably slow cognitive “throughput.”
Implicit to divergent thinking abilities is a heavy reliance on executive functions that require the prefrontal cortex. Executive function can be defined as “the ability to plan and coordinate a willful action in the face of alternatives, to monitor and update action as necessary and suppress distracting material by focusing attention on the task at hand.”83 Many tasks believed to engage different aspects of executive function have been used in sleep deprivation studies. Examples include the Wisconsin Card-Sorting Task, the Tower of London Test, Torrance Tests of Creative Thinking, the Hayling sentence completion task, and Thurstone’s verbal learning task (see references 65 and 83 for reviews). Commonalities among these tasks include reliance upon working memory and attention systems. Working memory involves the ability to hold and manipulate information and can involve multiple sensory–motor modalities. Tests include presentation of visual, auditory, or tactile sensory information, utilized in verbal, mathematical, or spatial memory functions. Deficits in cognitive performance requiring working memory result in difficulty determining the scope of a problem due to changing or distracting information,84–87 remembering the temporal order of information,88,89 maintaining focus on relevant cues,86,90–93 maintaining flexible thinking,91,94 taking inappropriate risks,95,96 having poor insight into performance deficits,65,97,98 perseverating on thoughts and actions,84,85,99–101 and having problems making behavioral modifications based on new information.87,91,101
Although there is evidence that sleep deprivation adversely affects prefrontal cortex-related executive attention and working memory abilities, these cognitive effects are often not nearly as conspicuous or easy to measure as those involving basic processes such as cognitive and psychomotor speed, and lapses. Moreover, although executive functions clearly rely upon cortical activity, the role of subcortical systems (hypothalamus, thalamus, and brainstem) in purported prefrontal cortex-mediated deficits remains to be determined. Functional neuroimaging studies confirm that sleep loss affects prefrontal cortex activity, but there is also evidence (reviewed below) that the thalamus and possibly other midbrain and brainstem nuclei have important roles in the mechanisms underlying cognitive performance deficits in sleep-deprived subjects.
Functional Neuroimaging after Acute Total Sleep Deprivation
Functional neuroimaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have been increasingly used to examine the influence of sleep deprivation on brain metabolism and to relate changes in neural activity to behavioral performance decline and compensation (see references 102–105 for reviews). Among various domains of cognitive function, attention and working memory tasks predominate. These paradigms—mediated by reasonably well-understood brain networks involving the frontal and parietal cortices and subcortical structures including the thalamus and basal ganglia (see references 106–112 for reviews)—have been used to characterize the effects of sleep deprivation. Other tasks employed in neuroimaging studies on the effects of sleep deprivation include arithmetic calculation,113–115 verbal learning,116–118 logical reasoning,119 spatial navigation,120 inhibition control,121 risky decision making,122 and emotional processing.123
PET STUDIES OF ACUTE TOTAL SLEEP DEPRIVATION
Wu and colleagues124 conducted one of the earliest PET neuroimaging investigations exploring the effects of sleep deprivation on metabolism in the human brain. Their study used a continuous attention-demanding performance test and reported a significant reduction of absolute metabolic rates in the frontal and temporal lobes, thalamus, basal ganglia, and cerebellum without an overall decrease of whole brain metabolism following sleep deprivation. Greater reductions in absolute metabolic rates in the prefrontal cortex, thalamus, basal ganglia, and limbic regions after sleep deprivation were associated with greater deficits in vigilant attention as measured by reaction times. A more recent study from the same group, with an expanded number of subjects, replicated the findings that metabolism in the thalamus, basal ganglia, and frontal lobe decreases after 24 hours of sleep deprivation, and further reported that one night of recovery sleep only partially reversed metabolic reductions in these regions.125 Using PET during the performance of serial addition/subtraction tasks that required both attention and arithmetic working memory, Thomas and colleagues114,115 also found significant decreases in metabolic rates in the prefrontal cortex, anterior cingulate cortex, thalamus, and inferior parietal and temporal cortices across an 85-hour sleep deprivation period. The authors also reported that decreases in the thalamus, prefrontal cortex, and parietal cortices correlated with decreased cognitive performance and alertness over time.
Molecular tracers have also been used in PET studies to measure cerebral receptor changes following sleep deprivation. One study, using PET with F-18 CPFPX (8-cyclopentyl-3-(3-fluoropropyl)-1-propylxanthine) to quantify cerebral A1 adenosine receptor binding (A1AR) before and after sleep deprivation, found that deprivation increased A1AR binding in the human brain, particularly in the orbitofrontal cortex.126 Another study used PET with C-11 raclopride and C-11 cocaine radiotracers to measure dopamine D2/D3 receptors and transporters, respectively, and examined the effects of sleep deprivation on dopamine neurotransmission in the human brain.127 Although sleep deprivation significantly decreased the specific binding of C-11 raclopride in the thalamus and striatum, which may reflect increases in dopamine cell firing and/or release after sleep deprivation, it did not change the binding of dopamine transporters in the striatum. A greater reduction in C-11 raclopride binding was associated with greater fatigue and sleepiness, and greater deficits in cognitive performance on visual attention and working memory tasks. The authors speculated that dopamine increases after sleep deprivation may underlie arousal maintenance in the presence of a greater homeostatic drive, but provide insufficient compensation for behavioral and cognitive impairment.127
fMRI STUDIES OF ACUTE TOTAL SLEEP DEPRIVATION
In contrast to PET, which requires the injection of invasive and rapidly decaying radioactive tracers, fMRI using blood oxygenation level dependent (BOLD) contrast is noninvasive, more cost effective, and easier to use. In addition to measuring tonic neural activation by comparing task and control conditions with a block design, BOLD fMRI can detect phasic hemodynamic responses to brief stimuli at a rate of up to once every 2 seconds by utilizing an event-related experimental design.128 With event-related BOLD fMRI, researchers can pseudo randomly intermix stimuli of different types and categorize events post hoc on the basis of the subject’s responses; this is necessary for studies to dissociate different performances during the same task conditions. Thus, BOLD fMRI has become the most widely used imaging method for localizing regional brain function and, as such, is more applicable to sleep deprivation studies than PET.
Attention
Attention tasks appear to be particularly sensitive to sleep loss. For example, Portas and colleagues129 used fMRI with a block design and measured brain activity during similar performance of a short attention task under different levels of arousal, including a normal level of arousal, a higher level of arousal induced by caffeine administration, and a lower level of arousal induced by sleep deprivation. They found that arousal level modulated activation in the thalamus, with greater activation after sleep deprivation, but did not modulate activation in the prefrontal cortex, anterior cingulate cortex, and parietal regions. Another study used event-related fMRI and measured brain activity using the Psychomotor Vigilance Test after a normal night of sleep and after 36 hours of total sleep deprivation.130 Faster reaction times were related to increased activation within a sustained attention cortical network and subcortical arousal and motor systems. By contrast, slower reaction times (which can include lapses), particularly after sleep deprivation, were associated with greater activation in the frontal and posterior midline regions, which may reflect a failure to disengage the “default-mode network.”130
A series of fMRI experiments using various tasks involving attention and memory have investigated the effects of sleep deprivation on functioning.113,131–145 Converging evidence from some fMRI studies have largely replicated the effects of sleep deprivation on decreased neural activity observed in PET studies. For example, using a serial subtraction task, Drummond and colleagues113 found decreased activation in the prefrontal cortex, parietal lobe, and premotor cortex after sleep deprivation. Choo et al136 found reduced activation in the parietal lobe and left thalamus after 24 and 35 hours of sleep deprivation during the performance of verbal working memory tasks. Similarly, other studies have also found decreased activation in the prefrontal cortex and parietal regions during the performance of a verbal working memory task.137,138 A replication study with two pairs of rested wakefulness and sleep deprivation scans, showed that brain activation patterns were highly correlated across sessions and the magnitude of decreased activation in parietal regions was preserved and correlated with behavioral decline after sleep deprivation.142 Tomasi and colleagues144 used a visual ball-tracking task and found decreased activation in the frontoparietal regions, but increased activation in the thalamus.
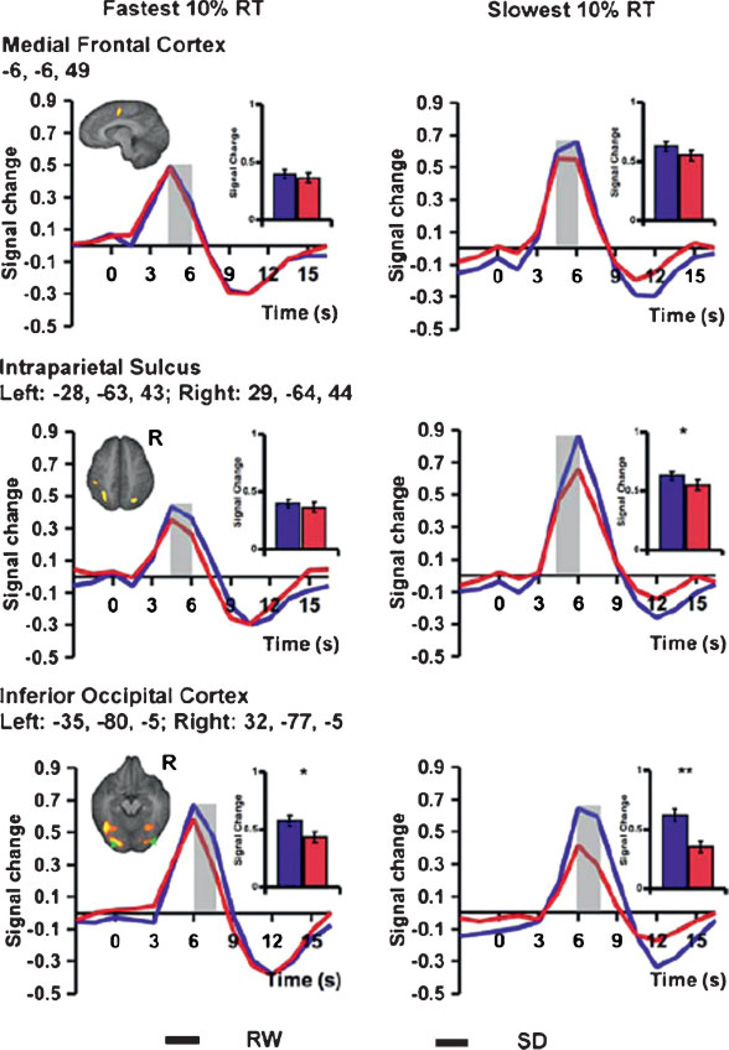
In a recent experiment on the effects of sleep deprivation on lapses during performance on a visual selective-attention task, Chee and colleagues141 also found reduced activation in the frontoparietal regions during lapses in addition to decreased mean activation in these regions after sleep deprivation (Fig. 1).141 Relative to lapses after a normal night’s sleep, lapses during sleep deprivation were associated with the expected reduction in activity in frontal and parietal control, but also a marked reduction in visual sensory cortex activation, as well as reduced thalamic activation. The latter contrasted with elevated thalamic activation during nonlapse periods. Despite these differences, the fastest responses after normal sleep and after sleep deprivation “elicited comparable frontoparietal activation, suggesting that performing a task while sleep deprived involves periods of apparently normal neural activation interleaved with periods of depressed cognitive control, visual perceptual functions, and arousal.”141 These findings support the state instability hypothesis by providing evidence that neural changes are occurring rapidly and frequently in the brain when sleep-deprived subjects are attempting to maintain goal-directed behavior in the presence of elevated homeostatic sleep drive.
Figure 1.
Functional magnetic resonance imaging (fMRI) responses from three cortical areas during a visual, global/local selective attention task performed by N = 24 healthy young adults when not sleep deprived (RW, in blue) and when sleep deprived (SD, in red) for one night. The graphs display differential neural responses in the medial frontal cortex (top), bilateral intraparietal sulcus (middle), and bilateral inferior occipital cortices (bottom), in association with the fastest 10% reaction times (RT) (left column) and the slowest 10% RTs (right column). A threshold of p < 0.001 was used to detect task-related activation. For both RW and SD states, slower responses were associated with higher peak fMRI signals in the medial frontal cortex and bilateral intraparietal sulcus (all p < 0.005). When comparing SD with RW, peak signal for the slowest 10% of trials was significantly lower in the parietal and occipital regions (right middle and bottom), but not in the medial frontal cortex (right top). SD also attenuated task-related thalamic activation (not shown). Peak signal in the occipital region after SD was significantly lower than RW even for the fastest 10% of trials (left bottom). However, there was no difference between RW and SD states in the frontal or parietal peak fMRI signals for the fastest responses across states (left top and middle). The shaded time points indicate those contrasted to assess significant state effects. The inset shows the mean peak signal associated with the time points under consideration. Error bars represent SEM. Significant differences between peak signals associated with a lapse and the average response for each state are marked with an asterisk. *p < 0.01, **p < 0.001. (Reprinted from Chee et al,141 with permission from The Journal of Neuroscience.)
Verbal Learning
Utilizing novel multivariate techniques as opposed to traditional group- and voxel-wise analyses, two independent studies132,134 identified decreased activation as a function of sleep deprivation, which correlated with memory performance decline in nonverbal and verbal recognition tasks. By contrast, other fMRI studies have found increased regional neural activity in the frontoparietal network following sleep deprivation, suggesting that a neurobiologic compensatory mechanism for behavioral and cognitive impairment results from sleep loss. For example, Drummond et al116 reported increased activation in the prefrontal cortex and parietal lobe during the performance of a verbal learning task after 35 hours of total sleep deprivation. Greater parietal activation was associated with better memory performance on this task. When arithmetic subtraction was combined with verbal learning in a divided attention task,131 fMRI results replicated increased activation in the prefrontal cortex, anterior cingulate cortex, and parietal regions, and showed a positive association between parietal activation and memory performance after sleep deprivation. Chee and colleagues133 reported increased activation in the prefrontal cortex during the performance of a more complex verbal working memory task, but not during the performance of a simple working memory task after sleep loss. Sleep deprivation also increased prefrontal cortex and temporal activation during the performance of a complex spatial navigation task.120 Similarly, Mander and colleagues143 found increased parietal activation during covert attention orientation following sleep deprivation. In addition, by manipulating the verbal learning task with two levels of word difficulty, increased activation was observed in the prefrontal cortex and parietal lobe in response to difficult, but not easy words after 36 hours of sleep deprivation.117 Moreover, using a logic reasoning task with parametrically manipulated levels of task difficulty,119 stronger linear neural responses were observed in the prefrontal cortex and parietal lobe associated with increasing task demands after sleep deprivation.
Taken together, the neuroimaging findings suggest the effect of sleep deprivation on brain regions subserving different cognitive functions may not be universal, but rather dependent on cognitive demands and on the difficulty level of the specific tasks used in a study. In addition, more complex or difficult tasks appear to permit recruitment of additional neural resources as part of compensatory neurocognitive mechanisms, which may make these tasks less vulnerable to sleep deprivation. By contrast, tasks that rely heavily on vigilant attention, even when simple and easy to do—such as PVT performance or driving—appear to involve no recruitable compensatory neurobiology to maintain performance in most (but perhaps not all) individuals.
Memory Acquisition and Retention
Sleep deprivation impairs visual short-term memory and limits its capacity. A recent fMRI study140 used parametrically manipulated perceptual or memory load in two visual tasks, and found that both tasks showed declines in behavioral performance and reductions in parietal and extrastriatal activation after sleep loss. Critically, sleep deprivation reduced the linear relationship between memory load and parietal activation at rested wakefulness. Moreover, cholinergic augmentation with donepezil reduced the negative effects of sleep loss on behavioral performance across both tasks and increased parietooccipital activation in a manner that correlated with performance in individuals who were vulnerable to sleep deprivation.146 Attention lapses following sleep loss compared with those after normal sleep were associated with reduced activation in the visual sensory cortex and thalamus.141
Acute total sleep deprivation also produced a significant deficit in hippocampal activity during episodic memory encoding, resulting in worse subsequent retention.118 Such findings suggest that a lack of sleep compromises the neural and behavioral capacity for committing new experiences to memory, which is essential for learning. Functional connectivity analysis showed that sleep deprivation increased connectivity between the hippocampus and basic alertness networks of the brainstem and thalamus on a memory consolidation task.118 Like other neuroimaging studies, these findings support the notion that lower brain areas involved in arousal, sensory/perceptual gating, and attentional functions contribute to impairments in memory following sleep deprivation. This conclusion was recently confirmed by a transcranial magnetic stimulation study showing that stimulation in the left lateral occipital cortex improved memory function after sleep loss.147
Emotional Memories and Emotional Processing
The role of sleep loss in consolidation of emotional (negative and positive) memories and in emotional processing has also been studied with fMRI.123,145 In one study, sleep deprivation deteriorated recollection of both neutral and positive stimuli, but not negative stimuli.145 Successful recollection of emotional stimuli elicited larger responses in the hippocampus and various cortical areas, including the medial prefrontal cortex, in the sleep group than in the sleep-deprived group.145 When sleep deprived, recollection of negative items (but not positive items) elicited larger responses in the amygdala and an occipital area than when sleep was obtained.145 Another study123 used an emotional stimulus viewing task and demonstrated enhanced neural responses to negative stimuli in the amygdala and reduced functional connectivity between the amygdala and medial prefrontal cortex.
Expectations of Reward
The effects of a night of sleep deprivation on expectations of reward found that sleep loss elevated expectations of higher reward on a gambling task, as nucleus accumbens activation increased following risky choices, and it attenuated neural responses to gambling losses in the insular and orbito-frontal cortices.122 However, sleep loss did not change behavioral performance on the gambling task, suggesting that neuroimaging may be more sensitive than behavior for detecting possible deficits in brain function following sleep deprivation.
INDIVIDUAL DIFFERENCES IN COGNITIVE VULNERABILITY TO ACUTE TOTAL SLEEP LOSS
Beyond task difficulty, interindividual differences may contribute to the inconsistent findings derived from functional neuroimaging studies. Behavioral studies have consistently reported large interindividual differences in cognitive responses to sleep deprivation, suggesting trait-like differential vulnerability148 (see next section for further discussion). Most fMRI studies have used small samples (usually 10 to 30 subjects) and employed group-level analyses, both of which mask individual variation in a heterogeneous population. Several recent fMRI studies have examined brain activation patterns in subjects who appeared to be either cognitively more vulnerable versus less vulnerable to sleep loss. In one study, Mu and colleagues138 compared brain activation between 10 more vulnerable and 10 less vulnerable young male subjects during performance of a working memory task at rested baseline (after normal sleep) and after sleep deprivation (when the vulnerability of subjects was defined based on their cognitive responses). Although both groups showed reduced frontoparietal activation after sleep deprivation, less vulnerable subjects showed significantly more activation both at rested wakefulness and after sleep deprivation. This finding is consistent with another working memory study that showed greater activation in left frontoparietal regions at rested wakefulness was associated with better memory performance after 24 hours of sleep deprivation.139 The results from these two studies support the “cognitive reserve hypothesis,” which suggests that subjects who are less cognitively vulnerable to sleep loss may have more preexisting cognitive resources or a greater capacity to engage alternative neural resources as demand increases during sleep deprivation.149
Unfortunately, results opposing this hypothesis have also been obtained. That is, better inhibition efficiency after sleep deprivation was associated with less activation in the right ventral prefrontal cortex at rested wakefulness using the go/no-go task.121 Although several factors may account for the inconsistency of findings, it is noteworthy that all of these studies failed to prospectively phenotype subjects’ cognitive vulnerability to sleep deprivation before imaging them, which would have helped separate state and trait variance in these experiments. Given these inconsistent findings, additional studies are needed to understand the neural mechanisms mediating trait-like differential cognitive vulnerability to sleep deprivation in normal individuals that has been carefully documented.148
Arterial Spin Labeled (ASL) Perfusion fMRI
Although findings from fMRI studies have significantly enriched our understanding of how sleep deprivation affects brain activation and how neural responses relate to cognitive and behavioral performance changes, several essential technical and methodological shortcomings of the BOLD contrast may limit its future application to sleep deprivation studies. BOLD contrast lacks absolute quantification and can only measure relative signal changes, which affects the accurate interpretation of observed sleep loss effects. Second, the presence of low frequency noise in the signal150,151 decreases the sensitivity of BOLD fMRI for tracking slow neural activity changes for longer than a few minutes.152,153 For this reason, BOLD imaging may be unsuitable for directly assaying sleep deprivation-induced neural activity changes occurring over a few days.
A recently developed neuroimaging technique—arterial spin labeled (ASL) perfusion fMRI—utilizes magnetically labeled arterial blood water as a diffusible tracer for blood flow measurements in a manner analogous to that used for 15O-PET scanning.154,155 This technique provides a noninvasive imaging method to quantify cerebral blood flow (in mL/100 g per minute) during the performance of cognitive tasks156–159 as well as during task-free resting states.160–162 ASL-perfusion fMRI also offers reliable measures of cerebral blood flow with excellent reproducibility over long time periods.153,163 These features suggest that ASL-perfusion fMRI may be a compelling method for functional imaging studies of sleep deprivation. Indeed, although no sleep deprivation study using ASL perfusion imaging has yet been published, preliminary data reported at scientific meetings164,165 support the feasibility of this technique.
Cognitive Deficits from Chronic Partial Sleep Restriction
Although total sleep deprivation is a useful experimental paradigm for studying the neurocognitive effects of sleep deprivation, in reality it is a much less representative form of sleep loss than chronic partial sleep restriction. Chronic sleep restriction is common in modern society, due to a wide range of factors, including medical conditions, sleep disorders, work demands, and social and domestic responsibilities.70,71 Many early studies of chronic partial sleep restriction reported conflicting effects on cognitive performance (see references 70 and 71 for reviews). Recent studies—using neurocognitive tasks sensitive to sleep deprivation and controlling those factors that in prior studies obscured accurate measurement of sleep restriction effects—found that 4 or more days of partial sleep restriction involving less than 7 hours sleep per night resulted in cumulative adverse effects on neurobehavioral functions.68,72,73 Repeated days of sleep restriction to between 3 and 6 hours time in bed increased daytime sleep propensity,73,166 decreased cognitive speed/accuracy as reflected in working memory tasks,68,74 and increased lapses of attention on the Psychomotor Vigilance Test.68,72,73
In the most extensive, controlled dose–response experiment on chronic sleep restriction to date, the neurocognitive effects of 14 days of sleep limitation to no more than 4, 6, or 8 hours in bed were compared with the effects of total sleep deprivation after 1, 2, and 3 nights without sleep.68 Cognitive tasks, which were performed every 2 hours from 7:30 am to 11:30 pm each day, included the Psychomotor Vigilance Test, a working memory task, and cognitive “throughput” tasks. Subjective sleepiness was assessed and electroencephalogram (EEG) recordings were continuously obtained for power spectral analyses. Three days of total sleep deprivation resulted in significantly larger deficits than any of the three chronic sleep restriction conditions. No cognitive deficits occurred following 8 hours time in bed for sleep each night. After 2 weeks of sleep restriction to 4 hours time in bed per night, deficits in attention, working memory, and cognitive “throughput” were equivalent to those seen after two nights of total sleep deprivation. Similarly, 2 weeks of restriction to 6 hours time in bed per night resulted in cognitive deficits equivalent to those found after one night of total sleep deprivation. The cumulative cognitive deficits increased in a nearly linear manner over days of 4 and 6 hours time in bed. Subjective sleepiness and fatigue ratings showed much smaller increases, suggesting an escalating dissociation between subjective perceptions of sleepiness and actual cognitive performance capability. Slow wave activity (delta power) in the sleep EEG also showed little response to chronic sleep restriction, in marked contrast to slow wave activity after total sleep deprivation. This is a particularly provocative finding because it has long been assumed that slow wave sleep/EEG activity are associated with restorative sleep functions.68,70 Apparently, as long as at least 4 hours of sleep time is permitted each night, slow wave activity does not reflect the homeostatic need for sleep during wakefulness. Thus, other aspects of physiologic functions occurring in the second half of a typical 7-to-8-hour sleep period appear essential for maintaining normal waking cognitive functions.
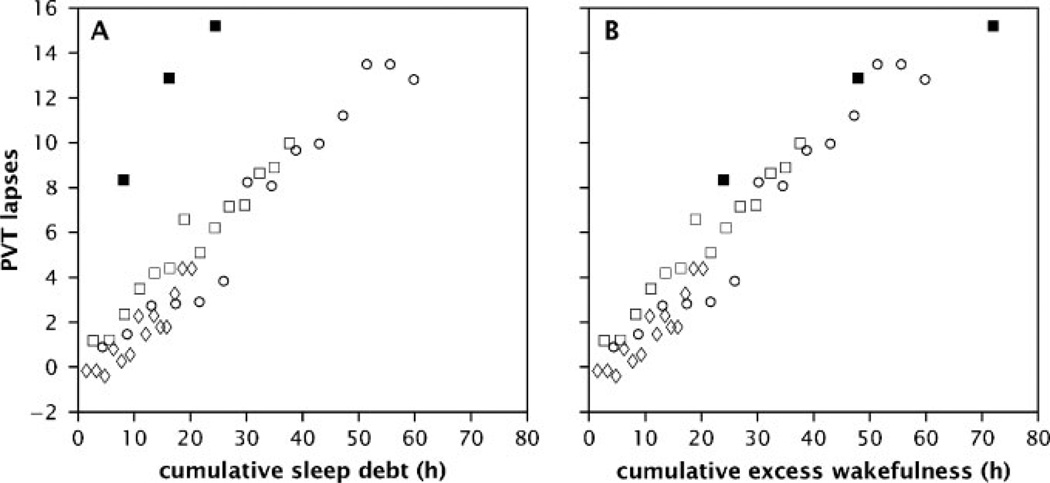
Surprisingly, the two aforementioned sleep restriction studies failed to find a linear relationship between the amount of sleep that subjects lost over days (i.e., sleep debt) and the magnitude of the cognitive performance deficits. Cognitive deficits accumulated much more rapidly when no sleep was allowed than when the same amount of sleep was lost more gradually over days of sleep restriction.68,74 Drake and colleagues74 interpreted this finding as evidence of adaptation to chronic sleep loss, although Van Dongen and colleagues68 suggested that the crucial factor in producing daytime cognitive performance deficits was the cumulative amount of time subjects spent awake in excess of their usual wakefulness period (Fig. 2). This finding suggests that there is a critical period of stable wake time within each circadian cycle after which neurocognitive deficits occur: this statistically estimated critical period was equal to 15.84 hours, and its associated sleep period was equal to 8.16 hours.68 There are as yet no published studies to establish whether the same neural responses occur when cumulative cognitive deficits from chronic sleep restriction reach levels comparable to the deficits induced by acute total sleep deprivation.
Figure 2.
A graphic comparison of performance on a behavioral alertness test following 14 days of partial sleep restriction or 3 days of total sleep deprivation, as a function of cumulative sleep debt (panel A) or cumulative excess wakefulness (panel B). Alertness was measured by lapses on the Psychomotor Vigilance Test (PVT). Cumulative sleep debt was determined by summating the difference between a statistically derived average sleep time of 8.16 hour/night and the actual hours of sleep each night (panel A). Cumulative excess wakefulness was determined by summating the difference between a statistically derived average daily wake time of 15.84 hour/day and the actual hours of wake each day (panel B). Each point represents the average time/day for each subject across 14 days of partial sleep restriction or 3 days of total sleep deprivation. Data from three partial sleep restriction groups (8 hours = diamond, 6 hours = square, and 4 hours = open circle) and one total sleep deprivation group (at days 1, 2, and 3 of total sleep deprivation = solid square) are shown. Panel A illustrates a difference (nonlinear relationship) between behavioral performance in the partial sleep restriction and total sleep deprivation groups as a function of cumulative sleep debt. Panel B demonstrates a similarity (linear relationship) between behavioral performance in the partial sleep restriction and total sleep deprivation groups as a function of cumulative excess wakefulness. The difference in analysis between panel A and panel B affects only the total sleep deprivation condition because subjects who receive 0 hours of sleep per day build up a statistically estimated average sleep debt of 8.16 hours per day, but extend their wakefulness by 24 hours per day. Thus, panel B shows a monotonic, near-proportional relationship between cumulative excess wakefulness and neurobehavioral performance deficits. (Reprinted with permission from Van Dongen et al.68)
Collectively, sleep restriction studies suggest that cumulative deficits in cognitive functions are more likely to occur and to accumulate to significant levels when sleep in healthy adults is reduced below 7 hours per night.70 However, as in total sleep deprivation experiments, this conclusion must be tempered by the fact that there are substantial interindividual differences not only in basal sleep need, but also in resistance and vulnerability to the cognitive effects of sleep loss.148 Moreover, the latter may have little relationship to the former. There is now compelling evidence that interindividual differences in cognitive deficits during sleep deprivation are systematic and trait-like, and the magnitude of these differences is substantial relative to the magnitude of the effect of prior sleep restriction.148 Consequently, individual differences in neurocognitive responses to sleep deprivation are not merely a consequence of variations in sleep history. Rather, they involve trait-like differential vulnerability to impairment from sleep loss, for which neurobiologic or genetic correlates have yet to be uncovered.
Genetics of Sleep Deprivation
The stable, trait-like interindividual differences observed in response to total sleep deprivation78,148,167,168—with intraclass correlations ranging from 58 to 92% for neurobehavioral measures78,148—strongly suggest an underlying genetic component. Until recently, however, the genetic basis of such differential vulnerability to sleep loss in normal healthy subjects has received little attention169–171 despite active ongoing genetics research in other related areas. For example, several studies have investigated frequency differences of sleep and circadian genetic polymorphisms in clinical (e.g., major depression and bipolar disorder172–175) and nonclinical subjects.176,177 Other studies have investigated specific genes involved in sleep and circadian rhythm sleep disorders, including insomnia, restless legs syndrome, narcolepsy, sleep apnea, and advanced and delayed sleep phase disorders (see references 178–180 for reviews).
By contrast, only a handful of studies have examined the role of human genetic polymorphisms on functioning in healthy subjects undergoing total sleep deprivation. One study found that the Val158Met polymorphism of catechol-O-methyltransferase (COMT) modulates the efficacy of modafinil on waking function, but neither this COMT genotype nor modafinil affected sleep-deprivation-induced changes in recovery sleep.181 Another study from the same group reported an association between an A2A receptor gene polymorphism and objective and subjective differences in caffeine’s effects on NREM sleep after total sleep deprivation.182
Three related studies investigated the role of the variable number tandem repeat (VNTR) polymorphism of the circadian gene PERIOD3 (PER3)—which shows similar allelic frequencies in African Americans and Caucasians/European Americans183,184 and is characterized by a 54-nucleotide coding region motif repeating in 4 or 5 units—in response to total sleep deprivation by using a small group of the same subjects specifically recruited for the homozygotic versions of this polymorphism. Compared with the 4-repeat allele, the longer, 5-repeat allele (PER35/5; n = 10) was associated with higher sleep propensity both at baseline and after total sleep deprivation, and worse cognitive performance, as assessed by a composite score of 12 tests, following total sleep deprivation.185 A subsequent report—using the same 24 subjects—clarified that the PER35/5 overall performance deficits were selective: they only occurred on certain executive function tests, and only at 2 to 4 hours following the melatonin rhythm peak, from ~ 06:00 to 08:00.186 Such performance differences are hypothesized to be mediated by sleep homeostasis.185,186 Another publication on the same subjects showed that PER35/5 subjects had more slow wave sleep and elevated sympathetic predominance and a reduction of parasympathetic activity during baseline sleep.187 These studies found no significant differences in the melatonin and cortisol circadian rhythms, PER3 mRNA levels, or in a self-report morningness–eveningness measure,185,186 although another study using these same subjects found PER3 expression and sleep timing were more strongly correlated in PER35/5 subjects.188 Other studies have shown that this polymorphism is associated with diurnal preference and delayed-sleep-phase syndrome.189–192 Though compelling, these studies have yet to be replicated using independent, larger samples and thus should be considered preliminary. Indeed, we recently examined this genetic polymorphism and its relationship with cumulative neurobehavioral and homeostatic functions in chronic sleep restriction,193 a condition more applicable to real world situations. We found that the PER3 VNTR polymorphism was not associated with individual differences in neurobehavioral responses to chronic sleep restiction, although it was slightly but significantly related to one marker of sleep homoeostatic response during chronic sleep restriction. The PER3 genotypes were comparable at baseline and showed equivalent inter-individual vulnerability to sleep restriction indicating that PER3 does not contribute to the neurobehavioral effects of chronic sleep loss.193
Sleep Fragmentation: Experiments and Reality
Arousals during sleep are defined by polysomnography as abrupt increases in the EEG frequency for a minimum of 3 seconds during NREM sleep and in association with increases in the electromyographic frequency during REM sleep.194 By definition, arousals do not result in awakenings; however, they have been associated with excessive daytime somnolence,195–201 cognitive performance deficits,195,198,202–206 and mood alterations.198,203,206,207 These studies show that sleep fragmentation has the same effect as sleep deprivation on waking behavior. Experimental sleep fragmentation models that use aural stimulation to produce transient changes in heart rate and blood pressure, but not EEG (or cortical) arousal, have demonstrated significant increases in daytime somnolence using the MSLT and MWT.208 In theory, one might expect waking neurocognitive deficits following the cumulative effect of multiple nights of sleep fragmentation. It has been suggested that arousals occurring at a rate of one per minute lead to daytime cognitive impairments associated with one night of sleep deprivation.195,209 This scenario may be all too frequent, especially when specific populations with intrinsic sleep disorders are considered.
Obstructive sleep apnea (OSA) is a common disorder affecting between 2 and 4% of the adult population.210–213 Episodic obstructive apneas are associated with hypoxemia and cortical arousals. OSA-related arousals are associated with increases in autonomic activity (heart rate and blood pressure) and may occur without cortical arousals.208,214–216 Some investigators suggest that the severity of OSA due to hypoxemic neural damage may be related to deficits in executive function.213,217,218 Reports indicate that neurophysiologic measures, such as the P300 latency of the event-related potentials in OSA patients show slowing and amplitude changes consistent with sleep-deprived subjects.219–222 These changes persist months after apnea reversal with continuous positive airway pressure.221,222 Sleep fragmentation has also been linked to deficits in sustained attention tasks as well as excessive daytime somnolence.197,198 More recently, investigations of OSA in adults223 and children224,225 demonstrate that sleep apnea creates a pro-inflammatory state, which may precipitate other important chronic medical conditions, such as hypertension, type 2 diabetes, obesity, metabolic syndrome, stroke, and heart failure. A relationship may also exist between such inflammation and neurocognitive dysfunction in apneic patients.226,227 At present, therapies targeting the inflammatory response in OSA have not been assessed with respect to their effects on cognitive function.
Neurocognitive deficits associated with OSA appear quite similar to those demonstrated in sleep deprivation and sleep fragmentation studies. A meta-analysis of cognitive dysfunction in sleep-disordered breathing patients using twenty-eight studies revealed several neurocognitive deficits associated with this spectrum of disease.228 Moderate to large effect sizes were noted for performance on sustained attention tasks (i.e., Four Choice Reaction Time Test, Psychomotor Vigilance Test, and Continuous Performance Test), driving simulation, delayed visual memory retrieval, and working memory tasks requiring mental flexibility (i.e., Wisconsin Cart Sorting Task and Stroop Interference Trial). Verbal fluency tests showed small to moderate effect sizes, and short attention tasks (i.e., trail-making tests and cancellation tests), vigilance tests (i.e., Mack-worth Clock Performance and Parasumaran Vigilance Task), delayed verbal retrieval tasks and general intellectual function (i.e., full-scale intelligence quotient [IQ], Wechsler Adult Intelligence Scale-Revised [WAIS-R] estimated IQ, psychomotor efficiency factor, Processing Speed Index, and Mini-Mental Status Exam) all showed small effect sizes. No differences were found for reasoning tasks (i.e., WAIS-R Subtests Comprehension, Picture Arrangement, and Picture Completion), concept formation (i.e., WAIS-R Subtest Similarities and Wisconsin Cart Sorting Task) and immediate visual or verbal memory tasks. Notably, there was not enough data to identify a quantifiable change in overall executive functions in sleep-disordered breathing patients. Thus, a comprehensive array of deficits appears over a broad range of neurocognitive domains in OSA and sleep-related disordered breathing. This meta-analysis highlights several cognitive arenas that need further investigation in this population including working memory and executive function.
Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) represent two overlapping disorders that often lead to sleep fragmentation and excessive daytime sleepiness. Recent reports indicate that children with attention-deficit hyperactivity disorder (ADHD), with symptoms suggestive of ADHD, or with conduct problems have a higher incidence of RLS and PLMD.229–234 Although attention deficits are easily demonstrable in these populations, it is unclear whether a cause-and-effect relationship between sleep fragmentation and cognitive deficits can be established. Recent studies comparing untreated RLS patients with control populations demonstrate statistically and clinically significant deficits in standard measures of executive function (similar to deficits reported after one night of total sleep deprivation in controls).235 In additional experiments, prefrontal cortex function was assessed in RLS patients and compared with controls who underwent two weeks of partial sleep restriction. The results demonstrated that RLS patients perform significantly better than sleep-deprived controls on executive function tasks, suggesting a possible adaptation to sleep deprivation in RLS patients.236 Taken together, these data suggest that sleep deprivation alone (as assessed in control subjects) may not account for the observed deficits in executive function noted in RLS. Indeed, other factors such as periodic limb movements, arousals, and/or dopaminergic dysfunction also may play a role. Additional neurocognitive assessments in RLS and PLMD are needed because these disorders offer a unique opportunity to study the effect of sleep fragmentation without hypoxemia on executive function. Any detectable cognitive deficits may be reversible with effective treatments such as dopaminergic therapy.
CONCLUSION
Sleep deprivation, whether a result of a clinical disorder or lifestyle choices, and whether acute or chronic, poses significant cognitive risks in the performance of many ordinary tasks such as driving and operating machinery. Theories of how sleep deprivation affects neurocognitive abilities are evolving rapidly as both the range of cognitive effects from sleep loss and the neurobiology of sleep–wake regulation are better understood. For example, recent experiments reveal that following chronic sleep restriction, significant daytime cognitive dysfunction accumulates to levels comparable to that found after severe acute total sleep deprivation. Executive performance functions subserved by the prefrontal cortex in concert with the anterior cingulate and posterior parietal systems seem particularly vulnerable to sleep loss. Following wakefulness in excess of 16 hours, deficits in attention and executive function tasks are demonstrable through well-validated testing protocols. Functional neuroimaging techniques indicate that wake state instability underlies many of the cognitive effects of sleep loss and involves sleep-promoting systems initiated during motivated behavior in which wake-promoting systems are active. Although neurophysiologic processes show similar changes across human brains following sleep deprivation, individual performance on cognitive measures vary greatly in response to sleep deprivation, suggesting trait-like (i.e., genetic) differential vulnerability or compensatory changes in the neurologic systems involved in cognition. New research has begun to investigate such possible genetic influences. Sleep-disordered breathing and nocturnal movement disorders show similar waking neurocognitive deficits to those seen in experimental sleep fragmentation protocols. Further studies of neurocognitive deficits in human disorders and following cumulative sleep restriction in healthy adults are needed. The results of such studies will have significant health implications for large segments of the population.
ACKNOWLEDGMENTS
This review was supported by grants NIH NR004281, AFOSR F49620-00-1-0266, and the National Space Biomedical Research Institute through NASA NCC 9–58 grant awarded to David F. Dinges. The review was also supported by a grant from the Institute for Translational Medicine and Therapeutics’ (ITMAT) Transdisciplinary Program in Translational Medicine and Therapeutics awarded to Namni Goel. The project described was supported in part by Grant Number UL1RR024134 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional support was provided by Children’s Research Center grant 2–80225 and a Restless Legs Syndrome Foundation Research Grant awarded to Jeffrey S. Durmer and a Chinese NSF Grant awarded to Hengyi Rao.
REFERENCES
- 1.Hublin C, Kaprio J, Partinen M, Koskenvuo M. Insufficient sleep—a population-based study in adults. Sleep. 2001;24(4):392–400. doi: 10.1093/sleep/24.4.392. [DOI] [PubMed] [Google Scholar]
- 2.Breslau N, Roth T, Rosenthal L, Andreski P. Daytime sleepiness: an epidemiological study of young adults. Am J Public Health. 1997;87(10):1649–1653. doi: 10.2105/ajph.87.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roehrs T, Merlotti L, Petrucelli N, Stepanski E, Roth T. Experimental sleep fragmentation. Sleep. 1994;17(5):438–443. doi: 10.1093/sleep/17.5.438. [DOI] [PubMed] [Google Scholar]
- 4.Roehrs TA, Timms V, Zwyghuizen-Doorenbos A, Roth T. Sleep extension in sleepy and alert normals. Sleep. 1989;12(5):449–457. doi: 10.1093/sleep/12.5.449. [DOI] [PubMed] [Google Scholar]
- 5.Pack AI, Pack AM, Rodgman E, Cucchiara A, Dinges DF, Schwab CW. Characteristics of crashes attributed to the driver having fallen asleep. Accid Anal Prev. 1995;27(6):769–775. doi: 10.1016/0001-4575(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 6.Knipling RR, Wang JS. Research note. Washington, DC: National Highway Traffic Safety Administration; 1994. Nov, Crashes and fatalities related to driver drowsiness/fatigue. [Google Scholar]
- 7.Carskadon MA. Adolescent sleepiness: increased risk in a high-risk population. Alcohol Drugs Driving. 1989–1990;5–6:317–328. [Google Scholar]
- 8.Dinges DF. An overview of sleepiness and accidents. J Sleep Res. 1995;4(S2):4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 9.Leger D. The cost of sleep-related accidents: a report for the National Commission on Sleep Disorders Research. Sleep. 1994;17(1):84–93. doi: 10.1093/sleep/17.1.84. [DOI] [PubMed] [Google Scholar]
- 10.McCartt AT, Ribner SA, Pack AI, Hammer MC. The scope and nature of the drowsy driving problem in New York State. Accid Anal Prev. 1996;28(4):511–517. doi: 10.1016/0001-4575(96)00021-8. [DOI] [PubMed] [Google Scholar]
- 11.Horne JA, Reyner L. Vehicle accidents related to sleep: a review. Occup Environ Med. 1999;56(5):289–294. doi: 10.1136/oem.56.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitler MM, Carskadon MA, Czeisler CA, Dement WC, Dinges DF, Graeber RC. Catastrophes, sleep, and public policy: consensus report. Sleep. 1988;11(1):100–109. doi: 10.1093/sleep/11.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stutts JC, Wilkins JW, Scott Osberg J, Vaughn BV. Driver risk factors for sleep-related crashes. Accid Anal Prev. 2003;35(3):321–331. doi: 10.1016/s0001-4575(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 14.Colquhoun WP. Accidents, injuries and shift work. In: Department of Health and Human Services, editor. Shift Work and Health. Washington, DC: Government Printing Office; 1976. [Google Scholar]
- 15.Folkard S, Monk TH. Shiftwork and performance. Hum Factors. 1979;21:483–492. [Google Scholar]
- 16.Richardson GS, Miner JD, Czeisler CA. Impaired driving performance in shiftworkers: the role of the circadian system in a multifactorial model. Alcohol Drugs Driving. 1989–1990;5–6:265–273. [PubMed] [Google Scholar]
- 17.Stoohs RA, Guilleminault C, Itoi A, Dement WC. Traffic accidents in commercial long-haul truck drivers: the influence of sleep-disordered breathing and obesity. Sleep. 1994;17(7):619–623. [PubMed] [Google Scholar]
- 18.McCartt AT, Rohrbaugh JW, Hammer MC, Fuller SZ. Factors associated with falling asleep at the wheel among long-distance truck drivers. Accid Anal Prev. 2000;32(4):493–504. doi: 10.1016/s0001-4575(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 19.Lyznicki JM, Doege TC, Davis RM, Williams MA. Sleepiness, driving, and motor vehicle crashes. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(23):1908–1913. doi: 10.1001/jama.279.23.1908. [DOI] [PubMed] [Google Scholar]
- 20.Marcus CL, Loughlin GM. Effect of sleep deprivation on driving safety in housestaff. Sleep. 1996;19(10):763–766. doi: 10.1093/sleep/19.10.763. [DOI] [PubMed] [Google Scholar]
- 21.Steele MT, Ma OJ, Watson WA, Thomas HA, Jr, Muelleman RL. The occupational risk of motor vehicle collisions for emergency medicine residents. Acad Emerg Med. 1999;6(10):1050–1053. doi: 10.1111/j.1553-2712.1999.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 22.Geer RT, Jobes DR, Tew JD, Stepsis LH. Incidence of automobile accidents involving anesthesia residents after on-call duty cycles. Anesthesiology. 1997;87:A938. [Google Scholar]
- 23.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4(8):649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 24.Neri DF, Oyung RL, Colletti LM, Mallis MM, Tam PY, Dinges DF. Controlled breaks as a fatigue countermeasure on the flight deck. Aviat Space Environ Med. 2002;73(7):654–664. [PubMed] [Google Scholar]
- 25.Price WJ, Holley DC. Shiftwork and safety in aviation. Occup Med. 1990;5(2):343–377. [PubMed] [Google Scholar]
- 26.Bourgeois-Bougrine S, Carbon P, Gounelle C, Mollard R, Coblentz A. Perceived fatigue for short- and long-haul flights: a survey of 739 airline pilots. Aviat Space Environ Med. 2003;74(10):1072–1077. [PubMed] [Google Scholar]
- 27.Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388(6639):235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- 28.Fairclough SH, Graham R. Impairment of driving performance caused by sleep deprivation or alcohol: a comparative study. Hum Factors. 1999;41(1):118–128. doi: 10.1518/001872099779577336. [DOI] [PubMed] [Google Scholar]
- 29.Williamson AM, Feyer AM. Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occup Environ Med. 2000;57(10):649–655. doi: 10.1136/oem.57.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulmer C, Wolman DM, Johns MME, editors. Committee on Optimizing Graduate Medical Trainee (Resident) Hours and Work Schedules to Improve Patient Safety. Institute of Medicine of the National Academies. Washington, DC: The National Academies Press; 2008. Resident duty hours: enhancing sleep, supervision, and safety. [PubMed] [Google Scholar]
- 31.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 32.Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5(Suppl):1071–1075. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- 33.Hastings MH. Circadian rhythms: a gut feeling for time. Nature. 2002;417(6887):391–392. doi: 10.1038/417391a. [DOI] [PubMed] [Google Scholar]
- 34.Van Dongen HPA, Dinges DF. Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. J Sleep Res. 2003;12(3):181–187. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 35.Goel N, Van Dongen HPA, Dinges DF. Circadian rhythms in sleepiness, alertness, and performance. In: Kryger MH, Dement WC, Roth T, editors. Principles and Practice of Sleep Medicine. 5th ed. Elsevier; In press. [Google Scholar]
- 36.Achermann P, Dijk DJ, Brunner DP, Borbély AA. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull. 1993;31(1–2):97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 37.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 38.Mallis MM, Mejdal S, Nguyen TT, Dinges DF. Summary of the key features of seven biomathematical models of human fatigue and performance. Aviat Space Environ Med. 2004;75(3) Suppl:A4–A14. [PubMed] [Google Scholar]
- 39.Jewett ME, Duffy JF, Czeisler CA, Khalsa SBS. The timing of the human circadian clock is accurately represented by the core body temperature rhythm following phase shifts to a three-cycle light stimulus near the critical zone. J Biol Rhythms. 2000;15(6):524–530. doi: 10.1177/074873040001500609. [DOI] [PubMed] [Google Scholar]
- 40.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139(3):253–267. [PubMed] [Google Scholar]
- 41.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 42.Carskadon MA, Dement WC. Daytime sleepiness: quantification of a behavioral state. Neurosci Biobehav Rev. 1987;11(3):307–317. doi: 10.1016/s0149-7634(87)80016-7. [DOI] [PubMed] [Google Scholar]
- 43.Dinges DF. Differential effects of prior wakefulness and circadian phase on nap sleep. Electroencephalogr Clin Neurophysiol. 1986;64(3):224–227. doi: 10.1016/0013-4694(86)90170-7. [DOI] [PubMed] [Google Scholar]
- 44.Richardson GS, Carskadon MA, Flagg W, Van den Hoed J, Dement WC, Mitler MM. Excessive daytime sleepiness in man: multiple sleep latency measurement in narcoleptic and control subjects. Electroencephalogr Clin Neurophysiol. 1978;45(5):621–627. doi: 10.1016/0013-4694(78)90162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roehrs TA, Carskadon MA. Standardization of method: essential to sleep science. Sleep. 1998;21(5):445. doi: 10.1093/sleep/21.5.445. [DOI] [PubMed] [Google Scholar]
- 46.Bonnet MH, Arand DL. Sleepiness as measured by modified multiple sleep latency testing varies as a function of preceding activity. Sleep. 1998;21(5):477–483. [PubMed] [Google Scholar]
- 47.Mitler MM, Gujavarty KS, Browman CP. Maintenance of wakefulness test: a polysomnographic technique for evaluation treatment efficacy in patients with excessive somnolence. Electroencephalogr Clin Neurophysiol. 1982;53(6):658–661. doi: 10.1016/0013-4694(82)90142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bjerner B. Alpha depression and lowered pulse rate during delayed actions in a serial reaction test: a study of sleep deprivation. Acta Physiol Scand. 1949;19(suppl 65):1–93. [Google Scholar]
- 49.Akerstedt T. Sleep/wake disturbances in working life. Electroencephalogr Clin Neurophysiol Suppl. 1987;39:360–363. [PubMed] [Google Scholar]
- 50.Torsvall L, Akerstedt T. Sleepiness on the job: continuously measured EEG changes in train drivers. Electroencephalogr Clin Neurophysiol. 1987;66(6):502–511. doi: 10.1016/0013-4694(87)90096-4. [DOI] [PubMed] [Google Scholar]
- 51.Williams HL, Lubin A. Impaired performance with acute sleep loss. Psychol Monogr. 1959;73:1–26. [Google Scholar]
- 52.Kribbs NB, Dinges DF. Vigilance decrement and sleepiness. In: Harsh JR, Ogilvie RD, editors. Sleep Onset Mechanisms. Washington, DC: American Psychological Association; 1994. pp. 113–125. [Google Scholar]
- 53.Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: a neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep Deprivation: Clinical Issues, Pharmacology and Sleep Loss Effects. New York, NY: Marcel Dekker Inc; 2005. pp. 39–70. [Google Scholar]
- 54.Rogers NL, Dorrian J, Dinges DF. Sleep, waking and neurobehavioural performance. Front Biosci. 2003;8:s1056–s1067. doi: 10.2741/1174. [DOI] [PubMed] [Google Scholar]
- 55.Horne JA, Pettitt AN. High incentive effects on vigilance performance during 72 hours of total sleep deprivation. Acta Psychol (Amst) 1985;58(2):123–139. doi: 10.1016/0001-6918(85)90003-4. [DOI] [PubMed] [Google Scholar]
- 56.Patrick GT, Gilbert JA. On the effects of loss of sleep. Psychol Rev. 1896;3:469–483. [Google Scholar]
- 57.Kleitman N. Sleep and Wakefulness. Chicago, IL: The University of Chicago Press; 1963. [Google Scholar]
- 58.Dinges DF. Are you awake? Cognitive performance and reverie during the hypnopompic state. In: Bootzin RR, Kihlstrom JF, Schacter DL, editors. Sleep and Cognition. Washington, DC: American Psychological Association; 1990. pp. 159–175. [Google Scholar]
- 59.Nansen F. Farthest North: The Incredible Three-Year Voyage to the Frozen Latitudes for the North. New York, NY: Random House Inc; 1999. [Google Scholar]
- 60.Mallis MM, Maislin G, Powell JW, Staszewski JJ, Grace R, Dinges DF. New drowsiness detection technologies testing their validity to track hypovigilance. Sleep. 1998;21(suppl 1):172. [Google Scholar]
- 61.Price NJ, Maislin G, Powell JW, et al. Unobtrusive detection of drowsiness-induced PVT lapses using infrared retinal reflectance of slow eyelid closures. Sleep. 2003;26(suppl):A177. [Google Scholar]
- 62.Morris TL, Miller JC. Electrooculographic and performance indices of fatigue during simulated flight. Biol Psychol. 1996;42(3):343–360. doi: 10.1016/0301-0511(95)05166-x. [DOI] [PubMed] [Google Scholar]
- 63.Caffier PP, Erdmann U, Ullsperger P. Experimental evaluation of eye-blink parameters as a drowsiness measure. Eur J Appl Physiol. 2003;89(3–4):319–325. doi: 10.1007/s00421-003-0807-5. [DOI] [PubMed] [Google Scholar]
- 64.Russo M, Thomas M, Thorne D, et al. Oculomotor impairment during chronic partial sleep deprivation. Clin Neurophysiol. 2003;114(4):723–736. doi: 10.1016/s1388-2457(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 65.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6(3):236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 66.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19(4):318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 67.Murray EJ. Sleep, Dreams, and Arousal. New York, NY: Appleton-Century-Crofts; 1965. [Google Scholar]
- 68.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 69.Van Dongen HPA, Rogers NL, Dinges DF. Understanding sleep debt: theoretical and empirical issues. Sleep Biol Rhythms. 2003;1:4–12. [Google Scholar]
- 70.Dinges DF, Rogers NL, Baynard MD. Chronic sleep deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, PA: WB Saunders Company; 2005. pp. 67–76. [Google Scholar]
- 71.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 72.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- 73.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12(1):1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 74.Drake CL, Roehrs TA, Burduvali E, Bonahoom A, Rosekind M, Roth T. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology. 2001;38(6):979–987. doi: 10.1111/1469-8986.3860979. [DOI] [PubMed] [Google Scholar]
- 75.Wilkinson RT. Sleep deprivation: performance tests for partial and selective sleep deprivation. In: Abt L, editor. Progress in Clinical Psychology. New York, NY: Grune and Stratton; 1969. pp. 28–43. [Google Scholar]
- 76.Dinges DF. Probing the limits of functional capability: the effects of sleep loss on short-duration tasks. In: Broughton RJ, Ogilvie RD, editors. Sleep, Arousal, and Performance. Boston, MA: Birkhäuser; 1992. pp. 177–188. [Google Scholar]
- 77.Olofsen E, Dinges DF, Van Dongen HP. Nonlinear mixed-effects modeling: individualization and prediction. Aviat Space Environ Med. 2004;75(3) Suppl:A134–A140. [PubMed] [Google Scholar]
- 78.Van Dongen HP, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat Space Environ Med. 2004;75(3) Suppl:A147–A154. [PubMed] [Google Scholar]
- 79.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;16:652–655. [Google Scholar]
- 80.Balkin TJ, Bliese PD, Belenky G, et al. Comparative utility of instruments for monitoring sleepiness-related performance decrements in the operational environment. J Sleep Res. 2004;13(3):219–227. doi: 10.1111/j.1365-2869.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 81.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 82.Corey-Bloom J, Wiederholt WC, Edelstein S, Salmon DP, Cahn D, Barrett-Connor E. Cognitive and functional status of the oldest old. J Am Geriatr Soc. 1996;44(6):671–674. doi: 10.1111/j.1532-5415.1996.tb01830.x. [DOI] [PubMed] [Google Scholar]
- 83.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5(6):463–475. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 84.Wimmer F, Hoffmann RF, Bonato RA, Moffitt AR. The effects of sleep deprivation on divergent thinking and attention processes. J Sleep Res. 1992;1(4):223–230. doi: 10.1111/j.1365-2869.1992.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 85.Horne JA. Sleep loss and “divergent” thinking ability. Sleep. 1988;11(6):528–536. doi: 10.1093/sleep/11.6.528. [DOI] [PubMed] [Google Scholar]
- 86.Blagrove M, Alexander C, Horne JA. The effects of chronic sleep reduction on the performance of cognitive tasks sensitive to sleep deprivation. Appl Cogn Psychol. 1995;9:21–40. [Google Scholar]
- 87.Banderet LE, Stokes JW, Francesconi R, Kowal DM, Naitoh P. Artillery teams in simulated sustained combat: performance and other measures. In: Johnson LC, Tepas DJ, Colquhoun WP, Colligan MJ, editors. Biological Rhythms, Sleep and Shiftwork. New York, NY: Spectrum; 1981. pp. 459–477. [Google Scholar]
- 88.Harrison Y, Horne JA. Sleep loss and temporal memory. Q J Exp Psychol A. 2000;53(1):271–279. doi: 10.1080/713755870. [DOI] [PubMed] [Google Scholar]
- 89.Morris GO, Williams HL, Lubin A. Misperceptions and disorientation during sleep. Arch Gen Psychiatry. 1960;2:247–254. [Google Scholar]
- 90.Norton R. The effects of acute sleep deprivation on selective attention. Br J Psychol. 1970;61(2):157–161. doi: 10.1111/j.2044-8295.1970.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 91.Harrison Y, Horne JA. One night of sleep loss impairs innovative thinking and flexible decision making. Organ Behav Hum Decis Process. 1999;78(2):128–145. doi: 10.1006/obhd.1999.2827. [DOI] [PubMed] [Google Scholar]
- 92.Blagrove M, Alexander C, Horne JA. The effects of chronic sleep reduction on the performance of cognitive tasks sensitive to sleep deprivation. Appl Cogn Psychol. 1994;9:21–40. [Google Scholar]
- 93.Blagrove M. The effects of length of sleep deprivation on interrogative suggestibility. J Exp Psychol Appl. 1996;2:48–59. [Google Scholar]
- 94.May J, Kline P. Measuring the effects upon cognitive abilities of sleep loss during continuous operations. Br J Psychol. 1987;78(Pt 4):443–455. doi: 10.1111/j.2044-8295.1987.tb02261.x. [DOI] [PubMed] [Google Scholar]
- 95.Harrison Y, Horne JA. Sleep loss affects risk-taking. J Sleep Res. 1998;7(suppl 2):113. doi: 10.1046/j.1365-2869.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- 96.Brown ID, Tickner AH, Simmonds DC. Effect of prolonged driving on overtaking criteria. Ergonomics. 1970;13(2):239–242. doi: 10.1080/00140137008931137. [DOI] [PubMed] [Google Scholar]
- 97.Dinges DF, Kribbs NB. Performing while sleepy: effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, Sleepiness and Performance. Chichester, UK: John Wiley and Sons; 1991. pp. 97–128. [Google Scholar]
- 98.Kjellberg A. Effects of sleep deprivation on performance of a problem-solving task. Psychol Rep. 1975;37(2):479–485. doi: 10.2466/pr0.1975.37.2.479. [DOI] [PubMed] [Google Scholar]
- 99.Harrison Y, Horne JA. Sleep deprivation affects speech. Sleep. 1997;20(10):871–877. doi: 10.1093/sleep/20.10.871. [DOI] [PubMed] [Google Scholar]
- 100.Harrison Y, Horne JA. Sleep loss impairs short and novel language tasks having a prefrontal focus. J Sleep Res. 1998;7(2):95–100. doi: 10.1046/j.1365-2869.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- 101.Herscovitch J, Stuss D, Broughton R. Changes in cognitive processing following short-term cumulative sleep deprivation and recovery oversleeping. J Clin Neuropsychol. 1980;2:301–319. [Google Scholar]
- 102.Drummond SPA, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25(5) Suppl:S68–S73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]
- 103.Dang-Vu TT, Desseilles M, Petit D, Mazza S, Montplaisir J, Maquet P. Neuroimaging in sleep medicine. Sleep Med. 2007;8(4):349–372. doi: 10.1016/j.sleep.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 104.Chee MW, Chuah LY. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr Opin Neurol. 2008;21(4):417–423. doi: 10.1097/WCO.0b013e3283052cf7. [DOI] [PubMed] [Google Scholar]
- 105.Chuah LY, Chee MW. Functional neuroimaging of sleep deprived healthy volunteers and persons with sleep disorders: a brief review. Ann Acad Med Singapore. 2008;37(8):689–694. [PubMed] [Google Scholar]
- 106.Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55(4):343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 107.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 108.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 109.Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8(5):223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 110.Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14(2):390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 111.Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- 112.McDowd JM. An overview of attention: behavior and brain. J Neurol Phys Ther. 2007;31(3):98–103. doi: 10.1097/NPT.0b013e31814d7874. [DOI] [PubMed] [Google Scholar]
- 113.Drummond SPA, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10(18):3745–3748. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- 114.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9(4):335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 115.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness II. Effects of 48 and 72 h of sleep deprivation on waking human regional brain activity. Thalamus Relat Syst. 2003;2:199–229. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 116.Drummond SPA, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403(6770):655–657. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 117.Drummond SP, Meloy MJ, Yanagi MA, Orff HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. 2005;140:211–223. doi: 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 118.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10(3):385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 119.Drummond SP, Brown GG, Salamat JS, Gillin JC. Increasing task difficulty facilitates the cerebral compensatory response to total sleep deprivation. Sleep. 2004;27(3):445–451. [PubMed] [Google Scholar]
- 120.Strangman G, Thompson JH, Strauss MM, Marshburn TH, Sutton JP. Functional brain imaging of a complex navigation task following one night of total sleep deprivation: a preliminary study. J Sleep Res. 2005;14(4):369–375. doi: 10.1111/j.1365-2869.2005.00488.x. [DOI] [PubMed] [Google Scholar]
- 121.Chuah YM, Venkatraman V, Dinges DF, Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26(27):7156–7162. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30(5):603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- 123.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep—a prefrontal amygdala disconnect. Curr Biol. 2007;17(20):R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 124.Wu JC, Gillin JC, Buchsbaum MS, et al. The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991;14(2):155–162. [PubMed] [Google Scholar]
- 125.Wu JC, Gillin JC, Buchsbaum MS, et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31(12):2783–2792. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- 126.Elmenhorst D, Meyer PT, Winz OH, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27(9):2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Volkow ND, Wang GJ, Telang F, et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28(34):8454–8461. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9(16):3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- 129.Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci. 1998;18(21):8979–8989. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28(9):1059–1068. [PubMed] [Google Scholar]
- 131.Drummond SPA, Gillin JC, Brown GG. Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res. 2001;10(2):85–92. doi: 10.1046/j.1365-2869.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 132.Bell-McGinty S, Habeck C, Hilton HJ, et al. Identification and differential vulnerability of a neural network in sleep deprivation. Cereb Cortex. 2004;14(5):496–502. doi: 10.1093/cercor/bhh011. [DOI] [PubMed] [Google Scholar]
- 133.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24(19):4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Habeck C, Rakitin BC, Moeller J, et al. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Brain Res Cogn Brain Res. 2004;18(3):306–321. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 135.Caldwell JA, Mu Q, Smith JK, et al. Are individual differences in fatigue vulnerability related to baseline differences in cortical activation? Behav Neurosci. 2005;119(3):694–707. doi: 10.1037/0735-7044.119.3.694. [DOI] [PubMed] [Google Scholar]
- 136.Choo WC, Lee WW, Venkatraman V, Sheu FS, Chee MW. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage. 2005;25(2):579–587. doi: 10.1016/j.neuroimage.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 137.Mu Q, Nahas Z, Johnson KA, et al. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005;28(1):55–67. doi: 10.1093/sleep/28.1.55. [DOI] [PubMed] [Google Scholar]
- 138.Mu Q, Mishory A, Johnson KA, et al. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep. 2005;28(4):433–446. doi: 10.1093/sleep/28.4.433. [DOI] [PubMed] [Google Scholar]
- 139.Chee MW, Chuah LY, Venkatraman V, Chan WY, Philip P, Dinges DF. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: correlations of fronto-parietal activation with performance. Neuroimage. 2006;31(1):419–428. doi: 10.1016/j.neuroimage.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 140.Chee MW, Chuah YM. Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc Natl Acad Sci U S A. 2007;104(22):9487–9492. doi: 10.1073/pnas.0610712104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chee MW, Tan JC, Zheng H, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28(21):5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lim J, Choo WC, Chee MW. Reproducibility of changes in behaviour and fMRI activation associated with sleep deprivation in a working memory task. Sleep. 2007;30(1):61–70. doi: 10.1093/sleep/30.1.61. [DOI] [PubMed] [Google Scholar]
- 143.Mander BA, Reid KJ, Davuluri VK, et al. Sleep deprivation alters functioning within the neural network underlying the covert orienting of attention. Brain Res. 2008;1217:148–156. doi: 10.1016/j.brainres.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tomasi D, Wang RL, Telang F, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex. 2009;19(1):233–240. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sterpenich V, Albouy G, Boly M, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol. 2007;5(11):e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chuah LY, Chee MW. Cholinergic augmentation modulates visual task performance in sleep-deprived young adults. J Neurosci. 2008;28(44):11369–11377. doi: 10.1523/JNEUROSCI.4045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luber B, Stanford AD, Bulow P, et al. Remediation of sleep-deprivation-induced working memory impairment with fMRI-guided transcranial magnetic stimulation. Cereb Cortex. 2008;18(9):2077–2085. doi: 10.1093/cercor/bhm231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27(3):423–433. [PubMed] [Google Scholar]
- 149.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 150.Zarahn E, Aguirre GK, D’Esposito M. Empirical analyses of BOLD fMRI statistics. I. Spatially unsmoothed data collected under null-hypothesis conditions. Neuroimage. 1997;5(3):179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]
- 151.Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline J. To smooth or not to smooth? Bias and efficiency in fMRI time-series analysis. Neuroimage. 2000;12(2):196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- 152.Aguirre GK, Zarahn E, D’Esposito M. Empirical analyses of BOLD fMRI statistics. II. Spatially smoothed data collected under null-hypothesis and experimental conditions. Neuroimage. 1997;5(3):199–212. doi: 10.1006/nimg.1997.0264. [DOI] [PubMed] [Google Scholar]
- 153.Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage. 2002;15(3):488–500. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- 154.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 155.Detre JA, Alsop DC. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur J Radiol. 1999;30(2):115–124. doi: 10.1016/s0720-048x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 156.Wang J, Rao H, Wetmore GS, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A. 2005;102(49):17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim J, Whyte J, Wang J, Rao H, Tang KZ, Detre JA. Continuous ASL perfusion fMRI investigation of higher cognition: quantification of tonic CBF changes during sustained attention and working memory tasks. Neuroimage. 2006;31(1):376–385. doi: 10.1016/j.neuroimage.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Olson IR, Rao H, Moore KS, Wang J, Detre JA, Aguirre GK. Using perfusion fMRI to measure continuous changes in neural activity with learning. Brain Cogn. 2006;60(3):262–271. doi: 10.1016/j.bandc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 159.Rao H, Wang J, Tang K, Pan W, Detre JA. Imaging brain activity during natural vision using CASL perfusion fMRI. Hum Brain Mapp. 2007;28(7):593–601. doi: 10.1002/hbm.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rao H, Gillihan SJ, Wang J, et al. Genetic variation in serotonin transporter alters resting brain function in healthy individuals. Biol Psychiatry. 2007;62(6):600–606. doi: 10.1016/j.biopsych.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 161.Rao H, Wang J, Giannetta J, et al. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120(5):e1245–e1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- 162.Wang Z, Faith M, Patterson F, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27(51):14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang J, Aguirre GK, Kimberg DY, Roc AC, Li L, Detre JA. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003;49(5):796–802. doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- 164.Asllani I, Habeck C, Borogovac A, et al. Effects of 48hr sleep deprivation on cerebral blood flow measured with arterial spin labeling MRI. Proceedings of the 15th Annual Meeting of the International Society for Magnetic Resonance in Medicine; International Society for Magnetic Resonance in Medicine; Berkeley, CA. 2007. 0507. [Google Scholar]
- 165.Rao H, Lim J, Wu WC, Detre JA, Dinges DF. Neural correlates of inter-individual differences in sleep deprivation susceptibility: a pilot perfusion imaging study on the Psychomotor Vigilance Test. Paper presented at: National Sleep Foundation Young Investigators Conference, Basic Research Session; March 2, 2008; Washington, DC. [Google Scholar]
- 166.Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18(2):107–113. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 167.Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R280–R290. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 168.Van Dongen HPA, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance. Clin Sports Med. 2005;24(2):237–249. doi: 10.1016/j.csm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 169.Van Dongen HPA, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: leitmotif for a research agenda. Sleep. 2005;28(4):479–496. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 170.von Schantz M. Phenotypic effects of genetic variability in human clock genes on circadian and sleep parameters. J Genet. 2008;87(5):513–519. doi: 10.1007/s12041-008-0074-7. [DOI] [PubMed] [Google Scholar]
- 171.Landolt HP. Genotype-dependent differences in sleep, vigilance, and response to stimulants. Curr Pharm Des. 2008;14(32):3396–3407. doi: 10.2174/138161208786549344. [DOI] [PubMed] [Google Scholar]
- 172.Partonen T, Treutlein J, Alpman A, et al. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann Med. 2007;39(3):229–238. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- 173.Johansson C, Willeit M, Smedh C, et al. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28(4):734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- 174.Desan PH, Oren DA, Malison R, et al. Genetic polymorphism at the CLOCK gene locus and major depression. Am J Med Genet. 2000;96(3):418–421. doi: 10.1002/1096-8628(20000612)96:3<418::aid-ajmg34>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 175.Benedetti F, Dallaspezia S, Fulgosi MC, et al. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- 176.Ebisawa T. Circadian rhythms in the CNS and peripheral clock disorders: human sleep disorders and clock genes. J Pharmacol Sci. 2007;103(2):150–154. doi: 10.1254/jphs.fmj06003x5. [DOI] [PubMed] [Google Scholar]
- 177.Lamont EW, James FO, Boivin DB, Cermakian N. From circadian clock gene expression to pathologies. Sleep Med. 2007;8(6):547–556. doi: 10.1016/j.sleep.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 178.Kimura M, Winkelmann J. Genetics of sleep and sleep disorders. Cell Mol Life Sci. 2007;64(10):1216–1226. doi: 10.1007/s00018-007-6532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tafti M, Maret S, Dauvilliers Y. Genes for normal sleep and sleep disorders. Ann Med. 2005;37(8):580–589. doi: 10.1080/07853890500372047. [DOI] [PubMed] [Google Scholar]
- 180.Dauvilliers Y, Maret S, Tafti M. Genetics of normal and pathological sleep in humans. Sleep Med Rev. 2005;9(2):91–100. doi: 10.1016/j.smrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 181.Bodenmann S, Xu S, Luhmann UF, et al. Pharmacogenetics of modafinil after sleep loss: catechol-O-methyltransferase genotype modulates waking functions but not recovery sleep. Clin Pharmacol Ther. 2009;85(3):296–304. doi: 10.1038/clpt.2008.222. [DOI] [PubMed] [Google Scholar]
- 182.Rétey JV, Adam M, Khatami R, et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81(5):692–698. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 183.Nadkarni NA, Weale ME, von Schantz M, Thomas MG. Evolution of a length polymorphism in the human PER3 gene, a component of the circadian system. J Biol Rhythms. 2005;20(6):490–499. doi: 10.1177/0748730405281332. [DOI] [PubMed] [Google Scholar]
- 184.Ciarleglio CM, Ryckman KK, Servick SV, et al. Genetic differences in human circadian clock genes among world-wide populations. J Biol Rhythms. 2008;23(4):330–340. doi: 10.1177/0748730408320284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Viola AU, Archer SN, James LM, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17(7):613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 186.Groeger JA, Viola AU, Lo JC, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31(8):1159–1167. [PMC free article] [PubMed] [Google Scholar]
- 187.Viola AU, James LM, Archer SN, Dijk DJ. PER3 polymorphism and cardiac autonomic control: effects of sleep debt and circadian phase. Am J Physiol Heart Circ Physiol. 2008;295(5):H2156–H2163. doi: 10.1152/ajpheart.00662.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Archer SN, Viola AU, Kyriakopoulou V, von Schantz M, Dijk DJ. Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep. 2008;31(5):608–617. doi: 10.1093/sleep/31.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26(4):413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 190.Pereira DS, Tufik S, Louzada FM, et al. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep. 2005;28(1):29–32. [PubMed] [Google Scholar]
- 191.Jones KH, Ellis J, von Schantz M, Skene DJ, Dijk DJ, Archer SN. Age-related change in the association between a polymorphism in the PER3 gene and preferred timing of sleep and waking activities. J Sleep Res. 2007;16(1):12–16. doi: 10.1111/j.1365-2869.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ebisawa T, Uchiyama M, Kajimura N, et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2(4):342–346. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One. 2009;4(6):e5874. doi: 10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 195.Bonnet MH. Performance and sleepiness as a function of frequency and placement of sleep disruption. Psychophysiology. 1986;23(3):263–271. doi: 10.1111/j.1469-8986.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 196.Levine B, Roehrs T, Stepanski E, Zorick F, Roth T. Fragmenting sleep diminishes its recuperative value. Sleep. 1987;10(6):590–599. [PubMed] [Google Scholar]
- 197.Martin SE, Brander PE, Deary IJ, Douglas NJ. The effect of clustered versus regular sleep fragmentation on daytime function. J Sleep Res. 1999;8(4):305–311. doi: 10.1046/j.1365-2869.1999.00169.x. [DOI] [PubMed] [Google Scholar]
- 198.Martin SE, Engleman HM, Deary IJ, Douglas NJ. The effect of sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1996;153(4 Pt 1):1328–1332. doi: 10.1164/ajrccm.153.4.8616562. [DOI] [PubMed] [Google Scholar]
- 199.Roehrs T, Shore E, Papineau K, Rosenthal L, Roth T. A two-week sleep extension in sleepy normals. Sleep. 1996;19:576–582. [PubMed] [Google Scholar]
- 200.Stepanski E, Lamphere J, Badia P, Zorick F, Roth T. Sleep fragmentation and daytime sleepiness. Sleep. 1984;7(1):18–26. doi: 10.1093/sleep/7.1.18. [DOI] [PubMed] [Google Scholar]
- 201.Stepanski E, Lamphere J, Roehrs T, Zorick F, Roth T. Experimental sleep fragmentation in normal subjects. Int J Neurosci. 1987;33(3–4):207–214. doi: 10.3109/00207458708987405. [DOI] [PubMed] [Google Scholar]
- 202.Bonnet MH. Performance and sleepiness following moderate sleep disruption and slow wave sleep deprivation. Physiol Behav. 1986;37(6):915–918. [PubMed] [Google Scholar]
- 203.Bonnet MH. Sleep restoration as a function of periodic awakening, movement, or electroencephalographic change. Sleep. 1987;10(4):364–373. doi: 10.1093/sleep/10.4.364. [DOI] [PubMed] [Google Scholar]
- 204.Bonnet MH. Infrequent periodic sleep disruption: effects on sleep, performance and mood. Physiol Behav. 1989;45(5):1049–1055. doi: 10.1016/0031-9384(89)90236-9. [DOI] [PubMed] [Google Scholar]
- 205.Bonnet MH. The effect of sleep fragmentation on sleep and performance in younger and older subjects. Neurobiol Aging. 1989;10(1):21–25. doi: 10.1016/s0197-4580(89)80006-5. [DOI] [PubMed] [Google Scholar]
- 206.Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol. 1991;71(3):1112–1118. doi: 10.1152/jappl.1991.71.3.1112. [DOI] [PubMed] [Google Scholar]
- 207.Kingshott RN, Cosway RJ, Deary IJ, Douglas NJ. The effect of sleep fragmentation on cognitive processing using computerized topographic brain mapping. J Sleep Res. 2000;9(4):353–357. doi: 10.1046/j.1365-2869.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- 208.Martin SE, Wraith PK, Deary IJ, Douglas NJ. The effect of nonvisible sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1997;155(5):1596–1601. doi: 10.1164/ajrccm.155.5.9154863. [DOI] [PubMed] [Google Scholar]
- 209.Downey R, Bonnet MH. Performance during frequent sleep disruption. Sleep. 1987;10(4):354–363. doi: 10.1093/sleep/10.4.354. [DOI] [PubMed] [Google Scholar]
- 210.Bresnitz EA, Goldberg R, Kosinski RM. Epidemiology of obstructive sleep apnea. Epidemiol Rev. 1994;16(2):210–227. doi: 10.1093/oxfordjournals.epirev.a036151. [DOI] [PubMed] [Google Scholar]
- 211.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40–64 years: a population-based survey. Sleep. 1997;20(1):65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Olson LG, King MT, Hensley MJ, Saunders NA. A community study of snoring and sleep-disordered breathing. Health outcomes. Am J Respir Crit Care Med. 1995;152(2):717–720. doi: 10.1164/ajrccm.152.2.7633732. [DOI] [PubMed] [Google Scholar]
- 213.Redline S, Young T. Epidemiology and natural history of obstructive sleep apnea. Ear Nose Throat J. 1993;7220–21(1):24–26. [PubMed] [Google Scholar]
- 214.Davies RJ, Vardi-Visy K, Clarke M, Stradling JR. Identification of sleep disruption and sleep disordered breathing from the systolic blood pressure profile. Thorax. 1993;48(12):1242–1247. doi: 10.1136/thx.48.12.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ringler J, Basner RC, Shannon R, et al. Hypoxemia alone does not explain blood pressure elevations after obstructive apneas. J Appl Physiol. 1990;69(6):2143–2148. doi: 10.1152/jappl.1990.69.6.2143. [DOI] [PubMed] [Google Scholar]
- 216.Martin SE, Engleman HM, Kingshott RN, Douglas NJ. Microarousals in patients with sleep apnoea/hypopnoea syndrome. J Sleep Res. 1997;6(4):276–280. doi: 10.1111/j.1365-2869.1997.00276.x. [DOI] [PubMed] [Google Scholar]
- 217.Naëgelé B, Thouvard V, Pépin JL, et al. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18(1):43–52. [PubMed] [Google Scholar]
- 218.Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS) Sleep. 2000;23(Suppl 4):S102–S108. [PubMed] [Google Scholar]
- 219.Miyamoto T, Miyamoto M, Takekawa H, Kubo J, Hirata K, Katayama S. A comparison of middle latency auditory-evoked response in obstructive sleep apnea syndrome before and after treatment. Psychiatry Clin Neurosci. 2001;55(3):251–252. doi: 10.1046/j.1440-1819.2001.00846.x. [DOI] [PubMed] [Google Scholar]
- 220.Paquereau J, Meurice JC, Neau JP, Ingrand P, Patte F. Auditory brain-stem responses (ABRs) in sleep respiratory disorders. Eur J Clin Invest. 1994;24(3):156–160. doi: 10.1111/j.1365-2362.1994.tb00981.x. [DOI] [PubMed] [Google Scholar]
- 221.Sangal RB, Sangal JM. Obstructive sleep apnea and abnormal P300 latency topography. Clin Electroencephalogr. 1997;28(1):16–25. doi: 10.1177/155005949702800104. [DOI] [PubMed] [Google Scholar]
- 222.Rumbach L, Krieger J, Kurtz D. Auditory event-related potentials in obstructive sleep apnea: effects of treatment with nasal continuous positive airway pressure. Electroencephalogr Clin Neurophysiol. 1991;80(5):454–457. doi: 10.1016/0168-5597(91)90094-e. [DOI] [PubMed] [Google Scholar]
- 223.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9(3):211–224. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 224.Waters KA, Mast BT, Vella S, et al. Structural equation modeling of sleep apnea, inflammation, and metabolic dysfunction in children. J Sleep Res. 2007;16(4):388–395. doi: 10.1111/j.1365-2869.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 225.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med. 2008;9(3):254–259. doi: 10.1016/j.sleep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Haensel A, Bardwell WA, Mills PJ, et al. Relationship between inflammation and cognitive function in obstructive sleep apnea. Sleep Breath. 2009;13(1):35–41. doi: 10.1007/s11325-008-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gozal D, Sans Capdevila O, McLaughlin Crabtree V, Serpero LD, Witcher LA, Kheirandish-Gozal L. Plasma IGF-1 levels and cognitive dysfunction in children with obstructive sleep apnea. Sleep Med. 2009;10(2):167–173. doi: 10.1016/j.sleep.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 228.Fulda S, Schulz H. Cognitive dysfunction in sleep-related breathing disorders: a meta-analysis. Sleep Res Online. 2003;5:19–51. [Google Scholar]
- 229.Chervin RD, Archbold KH, Dillon JE, et al. Associations between symptoms of inattention, hyperactivity, restless legs, and periodic leg movements. Sleep. 2002;25(2):213–218. [PubMed] [Google Scholar]
- 230.Chervin RD, Archbold KH, Dillon JE, et al. Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics. 2002;109(3):449–456. doi: 10.1542/peds.109.3.449. [DOI] [PubMed] [Google Scholar]
- 231.Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep. 2001;24(3):313–320. doi: 10.1093/sleep/24.3.313. [DOI] [PubMed] [Google Scholar]
- 232.Picchietti DL, England SJ, Walters AS, Willis K, Verrico T. Periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. J Child Neurol. 1998;13(12):588–594. doi: 10.1177/088307389801301202. [DOI] [PubMed] [Google Scholar]
- 233.Picchietti DL, Underwood DJ, Farris WA, et al. Further studies on periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. Mov Disord. 1999;14(6):1000–1007. doi: 10.1002/1531-8257(199911)14:6<1000::aid-mds1014>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 234.Walters AS, Picchietti DL, Ehrenberg BL, Wagner ML. Restless legs syndrome in childhood and adolescence. Pediatr Neurol. 1994;11(3):241–245. doi: 10.1016/0887-8994(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 235.Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2006;7(1):25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 236.Gamaldo CE, Benbrook AR, Allen RP, Oguntimein O, Earley CJ. A further evaluation of the cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2008;9(5):500–505. doi: 10.1016/j.sleep.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]