Abstract
Night eating syndrome (NES) is characterized by evening hyperphagia and frequent awakenings accompanied by food intake. Patients with NES display a delayed circadian pattern of food intake but retain a normal sleep-wake cycle. These characteristics initiated the current study, in which the phase and amplitude of behavioral and neuroendocrine circadian rhythms in patients with NES were evaluated. Fifteen women with NES (mean age ± SD, 40.8 ± 8.7 y) and 14 control subjects (38.6 ± 9.5 y) were studied in the laboratory for 3 nights, with food intake measured daily. Blood also was collected for 25 h (every 2 h from 0800 to 2000 h, and then hourly from 2100 to 0900 h) and assayed for glucose and 7 hormones (insulin, ghrelin, leptin, melatonin, cortisol, thyroid-stimulating hormone [TSH] and prolactin). Statistical analyses utilized linear mixed-effects cosinor analysis. Control subjects displayed normal phases and amplitudes for all circadian rhythms. In contrast, patients with NES showed a phase delay in the timing of meals, and delayed circadian rhythms for total caloric, fat, and carbohydrate intake. In addition, phase delays of 1.0 to 2.8 h were found in 2 food-regulatory rhythms—leptin and insulin—and in the circadian melatonin rhythm (with a trend for a delay in the circadian cortisol rhythm). In contrast, circulating levels of ghrelin, the primary hormone that stimulates food intake, were phase advanced by 5.2 h. The glucose rhythm showed an inverted circadian pattern. Patients with NES also showed reduced amplitudes in the circadian rhythms of food intake, cortisol, ghrelin, and insulin, but increased TSH amplitude. Thus, patients with NES demonstrated significant changes in the timing and amplitude of various behavioral and physiological circadian markers involved in appetite and neuroendocrine regulation. As such, NES may result from dissociations between central (suprachiasmatic nucleus) timing mechanisms and putative oscillators elsewhere in the central nervous system or periphery, such as the stomach or liver. Considering these results, chronobiologic treatments for NES such as bright light therapy may be useful. Indeed, bright light therapy has shown efficacy in reducing night eating in case studies and should be evaluated in controlled clinical trials.
Keywords: night eating syndrome, circadian rhythms, mixed-effects cosinor analysis, phase shifts, amplitude
Patients with night eating syndrome (NES)—first described in 1955—demonstrate a phase delay in the circadian pattern of food intake, manifested by evening hyperphagia, nocturnal awakenings with food intake, and morning anorexia (Birketvedt et al., 1999; Manni et al., 1997; O’Reardon et al., 2004; Spaggiari et al., 1994; Stunkard et al., 1955). Notably, the circadian timing of the sleep-wake cycle, including sleep onset and offset, as measured by actigraphy and polysomnography, remains undisturbed in NES (O’Reardon et al., 2004; Rogers et al., 2006), suggesting a dissociation between the circadian rhythm of food intake and the sleep-wake cycle.
We demonstrated in an outpatient study that NES shows a delayed calorie intake pattern (O’Reardon et al., 2004). Similarly, in an inpatient investigation, patients with NES demonstrated higher nocturnal food intake, although their total daily calorie intake was similar to that of controls (Allison et al., 2005). That study also evaluated absolute differences in 25-h physiological profiles of hormone levels involved in food intake, energy balance, sleep, and stress (Allison et al., 2005). In that study, which utilized linear mixed-effects models, ghrelin levels were significantly lower in patients with NES than controls from 0100 h to 0900 h. In addition, insulin was higher at night and lower in the morning, and glucose was nonstatistically higher at night in patients with NES than in controls. Levels of thyroid-stimulating hormone (TSH), cortisol, melatonin, leptin, and prolactin did not differ across the 25 h between groups. In that study, we did not assess the circadian rhythm patterns of these measures; it thus remained unknown whether patients with NES show alterations in the circadian timing system.
We conducted linear mixed-effects cosinor analyses of neuroendocrine and behavioral measures to determine whether patients with NES show circadian phase changes in physiological measures, other than sleep-wake, in addition to those reported for food intake. We investigated whether patients with NES showed temporal displacement of multiple circadian rhythms, controlled both peripherally and centrally, within a normally timed sleep-wake cycle or whether they showed only displacement of caloric intake. It was hypothesized that patients with NES would display predominantly phase-delayed circadian rhythms of various behavioral and neuroendocrine factors, and that the timing of key rhythms involved in food intake and metabolism would be misaligned, both of which would indicate circadian timing system abnormalities in NES.
MATERIALS AND METHODS
Subjects
Fifteen female patients with NES (mean ± SD, 40.8 ± 8.7 y; body mass index [BMI], 36.1 ± 7 kg/m2) and 14 female controls (38.6 ± 9.5 y; BMI, 38.7 ± 7 kg/m2) completed the protocol. Subjects were recruited from an outpatient study that characterized NES, in which we investigated at-home sleeping and eating patterns using actigraphy, questionnaires, and sleep and food diaries (see O’Reardon et al., 2004, for details). All patients were initially assessed via the Night Eating Questionnaire (Allison et al., 2008) and a clinical interview. NES was defined as consumption of at least 25% of daily intake after the evening meal and/or 3 or more nocturnal awakenings with ingestion of food, as noted during 7 days of food and sleep diary entries. On average, the patients with NES consumed 35.9 ± 7.9% of their intake after dinner at baseline and awakened to eat 1.5 ± 1.0 times per night, compared with control subjects who consumed only 8.5 ± 6.2% of their daily intake after dinner and reported no nocturnal ingestions. Histories and physical examinations, blood work, electrocardiogram, urinalysis, and a urine pregnancy test were performed prior to study entry. Demographic features, including race, marital status, education level, and employment status, were matched between patients with NES and control subjects (see Rogers et al., 2006, for details). Furthermore, age (p = 0.5) and BMI (p = 0.6) did not differ significantly between groups.
Inclusion and exclusion criteria were as described in our previous reports (Allison et al., 2005; O’Reardon et al., 2004; Rogers et al., 2006). Inclusion criteria included an age range of 18 to 65 y and a BMI of >27 kg/m2. (Notably, although there is a link between NES and obesity such that NES is more prevalent with increasing adiposity, this relationship is not uniform, since NES also occurs in normal-weight individuals [reviewed in O’Reardon et al., 2005, Rogers et al., 2006]). Exclusion criteria included concurrent psychiatric disorders, including bipolar disorder, substance abuse/dependence, presence of suicidality, or medical disorders that affect appetite and eating patterns, including diabetes mellitus and human immunodeficiency virus (HIV). Those patients with diagnosed sleep apnea and/or who had previously worked night shifts were also excluded. Further details are described in previous reports based on these same patients (O’Reardon et al., 2004; Allison et al., 2005; Rogers et al., 2006). The University of Pennsylvania Institutional Review Board approved the experimental protocol and consent form. Subjects gave written informed consent prior to study entry and received monetary compensation for participation.
Procedure
Subjects arrived at the General Clinical Research Center at 1500 h and remained in private rooms for a 3-night protocol that included 2 nights of polysomnographic sleep assessment (reported in Rogers et al., 2006) and 25 h of blood sampling for neuroendocrine analysis (initial noncircadian analyses reported in Allison et al., 2005). They remained in <20 lux from 1900 h the night before blood draws began until study completion 38 h later. Subjects slept according to their normal prestudy sleep schedules with nursing staff documenting times of lights-off at night and lights-on upon morning awakening. Subjects were served 3 meals per day, consisting of a varied macronutrient diet, and they were also allowed to eat snacks ad libitum, available at their bedside.
Measurements
Food intake was recorded by subjects in diaries, as well as by having metabolic kitchen staff weigh food before and after meals. Caloric and macronutrient content of ingested food was calculated with the ESHA Food Processor (version 8; Salem, OR). On day 3, a catheter was placed in the antecubital vein at 0730 h, and blood samples were collected every 2 h from 0800 h to 2000 h, then every 1 h from 2100 h to 0900 h. Samples were immediately centrifuged and stored at −80 °C until being assayed for cortisol, ghrelin, glucose, insulin, leptin, melatonin, prolactin, and TSH. The precision of assays was as follows: cortisol (Diagnostic Products Inc., Los Angeles, CA) had a coefficient of variation (CV) of 6.94; melatonin and prolactin (Alpco, Windham, NH) had CVs of 9.2 and 5.25, respectively; leptin and insulin (Linco Research, St. Charles, MO) had CVs of 4.62 and 6.74, respectively; and TSH (M.P. Biomedical, Irvine, CA) had a CV of 5.1. Ghrelin was measured with a modification of a commercial RIA (Phoenix Pharmaceuticals, Belmont, CA) with a CV of 7.67 (McLaughlin et al., 2004). Glucose was analyzed via a glucometer. All assays had CVs within the acceptable range and all samples for individual subjects were assayed in duplicate to minimize variability.
Statistical Analyses
Data were analyzed using linear mixed-effects cosinor analysis (Mikulich et al., 2003), which allows for direct estimation of circadian amplitude and phase while accounting for systematic inter-individual differences. We used a cosinor model with a fixed 24-h period, while amplitude (half the peak-to-trough difference) and acrophase (timing of maximum) were estimated for each group. A random effect was placed on the intercept to account for interindividual differences in overall concentrations. Group differences in circadian parameters were evaluated with 2-sided t tests. SAS (version 8.2, SAS Institute, Inc., Cary, NC) was used for statistical analyses.
RESULTS
Energy Intake
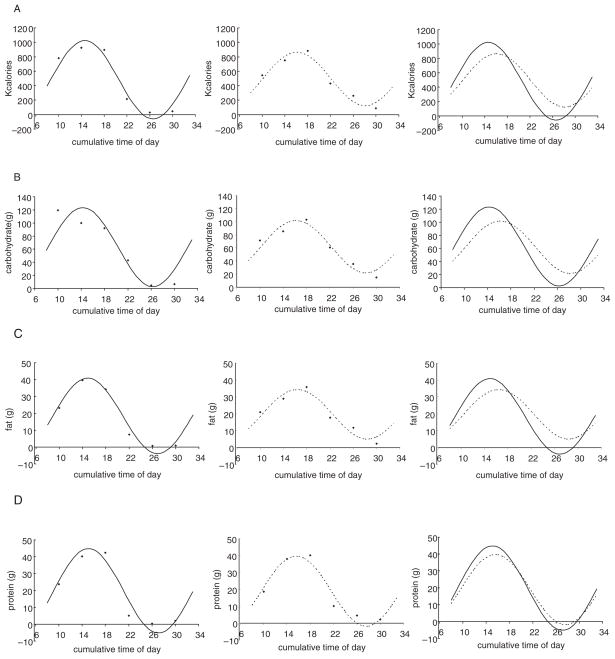
Dietary intake measures showed circadian rhythm alterations in patients with NES compared with control subjects that confirmed inclusion criteria were met. The circadian rhythm of total calorie intake was phase delayed by 1.5 h (t28 = 2.86, p = 0.008), and it displayed a 31.4% decrease in amplitude (t28 = −2.76, p = 0.01) in patients with NES (Fig. 1A, Table 1). The circadian rhythms of carbohydrate intake (phase: t28 = 2.49, p = 0.019; amplitude: t28 = −2.13, p = 0.042; Fig. 1B, Table 1) and fat intake (phase: t28 = 2.19, p = 0.037; amplitude: t28 = −2.47, p = 0.02; Fig. 1C, Table 1) also showed comparable and significant circadian phase delays and reductions in amplitude in NES patients. Although protein intake rhythms showed similar differences, they failed to reach significance (phase: t28 = 1.13, p = 0.27; amplitude: t28 = −1.44, p = 0.16; Fig. 1D, Table 1).
Figure 1.
Raw group-average data and fitted cosinor curves in patients with night eating syndrome (NES; - - -) and control subjects (—) for total calorie (A), carbohydrate (B), fat (C), and protein (D) intake. Circadian rhythms of total calorie, carbohydrate, and fat intake were phase delayed and decreased in amplitude in patients with NES compared with controls.
Table 1.
Absolute values for NES and control groups (mean ± SEM), and amplitude and phase differences from control subjects for energy intake, and 7 hormones and glucose in patients with NES.
| Measure | NES | Control | Amplitude Difference in NES (%) | Phase Difference in NES (h)a |
|---|---|---|---|---|
| Total calories | 2959 ± 154 | 2765 ± 206 | −31.4** | −1.5** |
| Carbohydrate (g) | 370.9 ± 26.9 | 378.8 ± 29.7 | −33.9* | −1.9* |
| Fat (g) | 118.0 ± 11.3 | 110.9 ± 14.0 | −34.4* | −1.5* |
| Protein (g) | 113.5 ± 8.3 | 128.5 ± 13.0 | −16.8 | −0.5 |
| Melatonin (pg/ml) | 24.3 ± 3.4 | 21.7 ± 3.8 | −15.3 | −1.1* |
| TSH (μU/L) | 2.3 ± 0.1 | 1.5 ± 0.1 | 30.9* | −0.7 |
| Prolactin (ng/ml) | 17.9 ± 0.7 | 16.3 ± 0.9 | −28.6 | −0.3 |
| Cortisol (mg/dl) | 8.6 ± 1.1 | 9.8 ± 1.3 | −25.7** | −0.7 |
| Ghrelin (pg/ml) | 248 ± 6.4 | 268 ± 5.5 | −49.6* | 5.2** |
| Leptin (ng/ml) | 40.1 ± 0.9 | 33.2 ± 0.9 | −3.9 | −1.0* |
| Glucose (mg/dl) | 109.3 ± 1.4 | 109.7 ± 2.4 | −56.5 | 11.6/−12.4c |
| Insulin (ng/ml) | 1.8 ± 0.1 | 1.7 ± 0.2 | −57.7** | −2.8** |
Means and SEM were calculated across the entire 25-h sampling period. The groups showed no significant differences in overall hormone or glucose values using the test of fixed effects from a linear mixed-effects model and no significant differences in overall energy intake using two-sided t tests (Allison et al., 2005). NES = night eating syndrome; TSH = thyroid-stimulating hormone.
Negative number indicates phase delay.
p < 0.05 using two-sided t tests.
p ≤ 0.01 using two-sided t tests.
Neuroendocrine Analyses
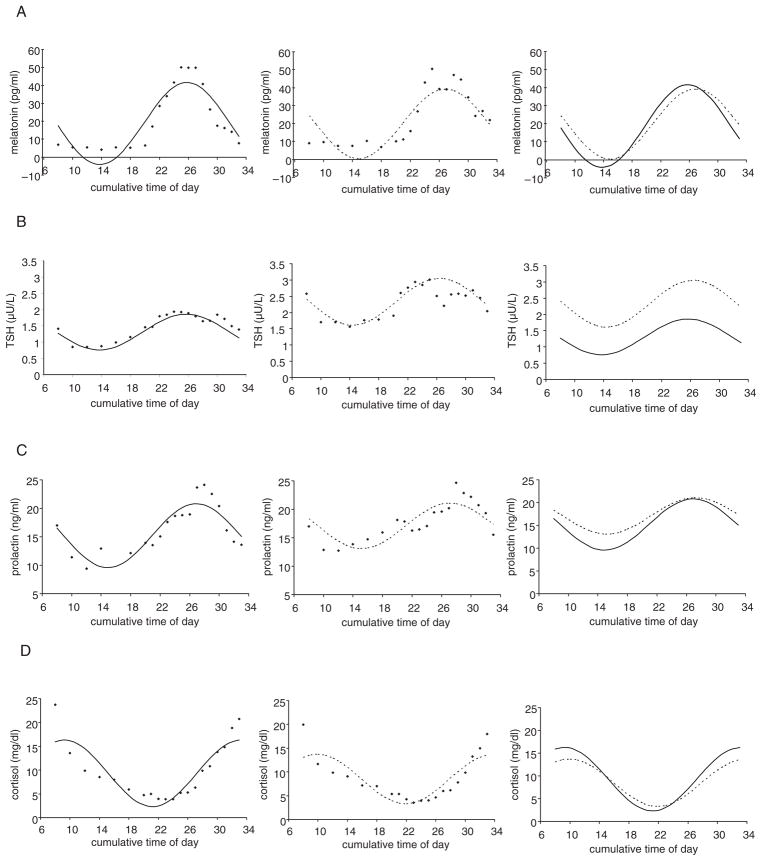
Neuroendocrine markers, like dietary intake measures, also showed significant phase and amplitude differences between patients with NES and control subjects. Melatonin rhythms were significantly phase delayed in patients with NES by 1.1 h (t25 = 2.17; p = 0.04) but showed no significant differences in amplitude (t25 = −1.10, p = 0.28; Fig. 2A, Table 1). TSH rhythms also were phase delayed by 0.7 h on average in patients with NES, but this difference did not reach significance (t26 = 1.46, p = 0.16; Fig. 2B, Table 1), although the rhythms displayed a significant increase in amplitude (30.9%; t26 = 2.07, p = 0.049). Prolactin rhythms in NES showed no significant phase differences (t28 = 0.48, p = 0.63) and showed a nonsignificant reduction in amplitude (t28 = −1.95, p = 0.06; Fig. 2C, Table 1). Cortisol rhythms were phase delayed by 0.7 h in patients with NES, but did not reach significance (t28 = 1.76, p = 0.089; Fig. 2D, Table 1), although the rhythms demonstrated a 25.7% reduction in amplitude (t28 = −3.34, p = 0.002).
Figure 2.
Raw group-average data and fitted cosinor curves in patients with night eating syndrome (NES; - - -) and control subjects (—) for melatonin (A), thyroid-stimulating hormone (TSH; B), prolactin (C), and cortisol (D). Melatonin circadian rhythms were phase delayed, while cortisol, TSH, and prolactin did not show significant phase differences from controls. Cortisol rhythms were diminished in amplitude in patients with NES compared with controls, while TSH showed increased amplitude.
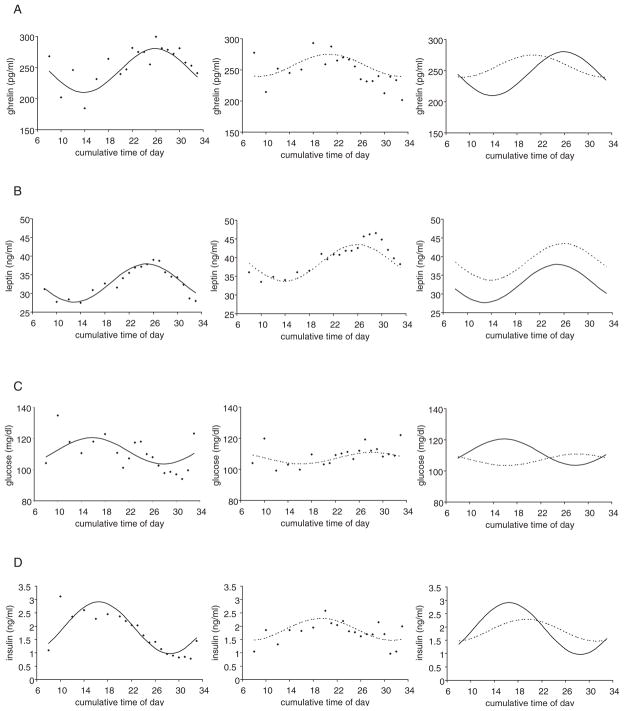
The hormones involved in appetite regulation showed marked differences between groups. The circadian rhythm of ghrelin—a predominantly stomach-derived appetite stimulant/orexigenic peptide—was phase advanced by 5.2 h in patients with NES (t26 = −4.15, p < 0.001), and was about half the amplitude (50.4% of the amplitude of controls; t26 = −2.45, p = 0.021; Fig. 3A, Table 1). By contrast, the circadian rhythm of leptin—an adipocyte-derived appetite suppressant/anorexigenic hormone that relays long-term current status of energy availability—was phase delayed by 1.0 h in patients with NES (t28 = 2.13, p = 0.042), but without a difference in amplitude (t28 = −0.38, p = 0.70; Fig. 3B, Table 1), although the levels across time points were higher in patients with NES. The glucose circadian rhythm was inverted in patients with NES relative to control subjects (11.6 h advanced or 12.4 h delayed; t28 = 2.04, p < 0.001), but showed no significant difference in amplitude (t28 = −1.64, p = 0.11; Fig. 3C, Table 1). The insulin circadian rhythm was phase delayed by 2.8 h in patients with NES (t28 = 3.15, p = 0.004), and was less than half the amplitude (42.3% of the amplitude of controls; t28 = −4.57, p < 0.001; Fig. 3D, Table 1).
Figure 3.
Raw group-average data and fitted cosinor curves in patients with night eating syndrome (NES; - - -) and control subjects (—) for hormones involved in food intake and metabolism: ghrelin (A), leptin (B), glucose (C), and insulin (D). Insulin was phase delayed and reduced in amplitude, while leptin showed delays without changes in amplitude in patients with NES, even though the overall levels across time points were higher in patients with NES. Glucose showed an inverted circadian rhythm. Ghrelin was out of phase with these rhythms showing a large phase advance and diminished amplitude in patients with NES.
DISCUSSION
This study was the 1st systematic evaluation of circadian rhythms of dietary intake and neuroendocrine measures in female patients with NES relative to healthy female controls of comparable age and BMI. Compared with control subjects—who showed circadian rhythm profiles similar to those reported in previous studies—patients with NES displayed significant abnormalities in both circadian phase and amplitude. Patients with NES showed a dysregulation between putative peripheral oscillators that provide signals for the central regulation of food intake, namely: 1) a phase advance in a putative peripheral oscillator in the stomach, regulating ghrelin, and 2) a phase delay in putative peripheral adipose tissue oscillators (Mendoza, 2007) regulating leptin release, and also in a putative peripheral liver oscillator, involved in food processing (Stokkan et al., 2001; Yamazaki et al., 2000). Furthermore, the central timing system regulating the melatonin rhythm also was phase delayed, similar to that observed for leptin. Thus, NES may have its etiological mechanisms in abnormalities of the peripheral (e.g., stomach, liver) and/or central (e.g., suprachiasmatic nuclei) circadian timing system. However, it must be noted that altered food intake could cause the observed hormonal patterns or vice versa; the patterns could be in a reinforcing cycle; and/or a 3rd factor (e.g., disturbed sleep) could cause both changes simultaneously. Thus, further studies, particularly those employing a constant routine or forced desynchrony protocol with controlled meal times and other factors are needed to establish cause and effect.
Circadian rhythm profiles in control subjects concurred with those reported in previous studies for prolactin (Czeisler and Klerman, 1999; Sassin et al., 1972), cortisol (Czeisler and Klerman, 1999; Dzaja et al., 2004; Schoeller et al., 1997), melatonin (Zhdanova et al., 1997), TSH (Allan and Czeisler, 1994; Peteranderl et al., 2002), leptin (Schoeller et al., 1997; Simon et al., 1998), insulin (Boden et al., 1996), glucose (Simon et al., 1994), and ghrelin (Cummings et al., 2001), thereby validating our approach for data collection and analysis. Thus, differences observed in NES indicate true rhythm abnormalities rather than protocol artifacts.
The observed changes in food intake may induce corresponding delays in metabolic regulators (e.g., insulin; Fogteloo et al., 2004) in patients with NES. The delays in the circadian rhythms of insulin and leptin are consistent with caloric intake delays, as noted previously (Allison et al., 2005; O’Reardon et al., 2004). By contrast, glucose and insulin are normally tightly coupled, but in NES, the glucose and insulin acrophases are markedly out of phase (see Fig. 3). The phase mismatch could indicate metabolic difficulties due to increased nocturnal carbohydrate intake (Allison et al., 2005) and altered timing of major eating bouts. Indeed, switching eating patterns from daytime to nighttime produces a timing mismatch between regulatory substances, and impairs the insulin response to glucose (Qin et al., 2003). Since both glucose and insulin rhythms show a distinct diminishing of amplitude, it is conceivable these 2 variables may show a marked dampening or possible absence of rhythm, despite use of the cosinor abstraction to identify amplitude and phase and fit curves for all measures.
The feeding-induced changes in metabolic regulators might phase-delay melatonin, cortisol, and TSH and entrain the central oscillator, because these substances can induce changes in circadian gene expression (reviewed in Mendoza, 2007). Alternatively, since patients with NES maintain a normal timing of sleep onset (Rogers et al., 2006), the delays in the melatonin circadian rhythm may be due to other direct effects on the central oscillator, such as late evening exposure to light (Khalsa et al., 2003; Minors et al., 1991; Van Cauter et al., 1994) that result from nighttime awakenings for food intake. Future studies, including those with experimental manipulation, are needed to discriminate between these hypotheses.
Patients with NES show maintenance of the normal positive phase relationship between leptin and TSH (Mantzoros et al., 2001) and normal negative phase relationships between leptin and cortisol (Licinio et al., 1998; Schoeller et al., 1997) and TSH and cortisol (Peteranderl et al., 2002). Thus, regardless of mechanisms, the putative peripheral oscillator that at least partially regulates leptin (Mendoza, 2007) appears phase delayed to the central oscillator, which may ultimately be important for biologic treatment of NES.
Patients with NES also had reduced amplitudes in the circadian rhythms of food intake as well as cortisol, ghrelin, and insulin, but showed increased TSH amplitude. Increased TSH levels in NES could be a result of their nighttime awakenings (Allan and Czeisler, 1994; Rogers et al., 2006). Similarly, these awakenings could have reduced the overall amplitude of ghrelin (Dzaja et al., 2004) and perhaps that of insulin (see Mullington et al., 2003).
Since ghrelin showed a notable lack of phase coherence with other circadian rhythms in NES, it may represent a major mechanism for this disorder. We hypothesize that some yet-to-be identified trigger, perhaps sleep deprivation (Schüssler et al., 2006), an altered food intake pattern (reviewed in Mendoza, 2007), or changes in insulin or glucose (Froy et al., 2007)—all shown to modify circadian rhythms—induces a phase advance and amplitude change in the putative peripheral oscillator in the stomach, the main site of ghrelin production (Ariyasu et al., 2001). This trigger may decouple ghrelin from other peripheral oscillators, from the central oscillator, and from the food-entrained oscillator (FEO; Mendoza, 2007). Such dissociations between peripheral and central oscillators have been reported in rodents (Mendoza, 2007; Stokkan et al., 2001; Yamazaki et al., 2000). The 5-hour phase advance in the acrophase of ghrelin initiates increased appetite and eating at an earlier phase (Cummings et al., 2001). The advance in timing of meals—and subsequent possible extended duration of eating produced by ghrelin—likely delays the leptin rhythm acrophase, as has been reported previously (Fogteloo et al., 2004; Schoeller et al., 1997), perhaps via a peripheral oscillator or FEO mechanism (Mendoza, 2007; Mühlbauer et al., 2004). Thus, in NES, the timing of the ghrelinleptin relationship, which is normally synchronized (Cummings et al., 2001), is out of sync by approximately 6 h (5 h advance and 1 h delay, respectively) compared with that in control subjects. This mismatch may represent a decoupling of the food intake system or a change in the phase of the FEO, and may represent a possible physiological marker for NES. This hypothesis awaits further testing, including use of an experimental approach. Since our study was not causal in nature, it is possible that cause and effect may differ for each measure.
NES shares features with seasonal affective disorder (SAD; Friedman et al., 2006). NES is an eating disorder, but with clear circadian (this study), sleep (Rogers et al., 2006), and clinical mood (Allison et al., 2005; Friedman et al., 2006) symptomology. NES patients are responsive to selective serotonin reuptake inhibitor treatment (O’Reardon et al., 2006; Stunkard et al., 2006) and show elevated midbrain serotonin transporter binding (Lundgren et al., 2008). This elevation is believed to increase the reuptake of serotonin, and thereby impair postsynaptic serotonin transmission. Thus, as is true for SAD, NES may be responsive to bright light therapy, because of its putative circadian phase shifting and serotonergic antidepressant mechanisms of action. Indeed, 2 case studies suggest morning bright light therapy is therapeutic in treatment of both disorders in patients with NES diagnosed with comorbid SAD or nonseasonal depression (Friedman et al., 2002, 2004). Future studies should determine the effects of bright light administration on circadian rhythms and behavior in NES, as well as examine whether circadian rhythm phase and amplitude become realigned and indistinguishable from controls following recovery.
In conclusion, previous studies have shown that NES involves a delay in the timing of food intake (Allison et al., 2005; O’Reardon et al., 2004). We extend these findings by showing that NES may be a disorder of circadian rhythm dysregulation (this study) with accompanying nighttime sleep disturbances (Rogers et al., 2006). Prolonged eating, possibly a result of an earlier ghrelin acrophase, may phase delay the putative peripheral oscillator for leptin and concurrently may phase delay the central oscillator controlling circadian signals for melatonin and cortisol. Thus, we theorize that patients with NES may show a dysregulation between peripheral oscillators that provide signals for the regulation of food intake and also may show a phase delay in the central oscillator. Our physiological findings may have clinical utility: they point to potential therapeutic chronobiologic options for treating NES, including bright light therapy. Such therapeutic options could be adjuvants or alternatives to previously proposed treatments including cognitive behavioral therapy, which focuses on stimulus control (e.g., restriction of access to food), regulation of circadian food intake, and sleep hygiene (Allison et al., 2004, 2005).
Acknowledgments
Research supported by NIH grants R01-DK56735 and M01-RR00040. KCA was supported by grant K12-HD043459 and RSA was supported by grant P01-DK49250. DFD and HVD were supported by NIH grants R01-NR04281 and R01-HL70154 and by the Institute for Experimental Psychiatry Research Foundation. NLR received support from the NHMRC Howard Florey Centenary Research Fellowship and NSW BioFirst awards. DEC was supported by NIH grants R01-DK61516 and P01-DK68384.
The authors gratefully acknowledge the subjects who participated in this protocol; the research nutritionist Lisa Basel-Brown, MS, RD; the nursing and metabolic kitchen staff of the CTRC of the Hospital of the University of Pennsylvania; Heather Collins, PhD, of the RIA/Biomarkers Core of the Diabetes Research Center of the University of Pennsylvania, supported by NIH grant DK19525; and Nicole S. Martino (Center for Weight and Eating Disorders, Department of Psychiatry) and Claire Fox (Division of Sleep and Chronobiology, Department of Psychiatry) for their work on this study. We also thank Richard Wurtman, MD, and his laboratory at the Massachusetts Institute of Technology for performing the melatonin assays and R. Scott Frayo of the University of Washington for performing the ghrelin assays.
Footnotes
Disclosure statement. This was not an industry supported study. Dr. Dinges has received research support from Cephalon, Inc., speaking honoraria from Cephalon, Inc., and Takeda, and has received both consulting fees and honoraria from Cephalon, Inc., Arena Pharmaceuticals, GSK, Mars Masterfoods, Inc., Merck, Neurogen, Novartis, Procter & Gamble, and Takeda. Dr. Rogers and Dr. Van Dongen have received research support from Cephalon, Inc. Dr. O’Reardon has received research support from Eli Lilly, BMS, Cyberonics, and Neuronetics Inc.; and has received consulting and/or speaking fees from Eli Lilly Pharmaceuticals, BMS, and Cyberonics Inc. Dr. Cummings has received consulting fees from Barosense, Tranzyme Pharma, and Elixer Pharmaceuticals. Drs. Ahima, Allison, Goel, Heo, and Stunkard have indicated no financial conflicts of interest.
References
- Allan JS, Czeisler CA. Persistence of the circadian thyrotropin rhythm under constant conditions and after light-induced shifts of circadian phase. J Clin Endocrinol Metab. 1994;79:508–512. doi: 10.1210/jcem.79.2.8045970. [DOI] [PubMed] [Google Scholar]
- Allison KC, Ahima RS, O’Reardon JP, Dinges DF, Sharma V, Cummings DE, Heo M, Martino NS, Stunkard AJ. Neuroendocrine profiles associated with energy intake, sleep, and stress in the night eating syndrome. J Clin Endocrinol Metab. 2005;90:6214–6217. doi: 10.1210/jc.2005-1018. [DOI] [PubMed] [Google Scholar]
- Allison KC, Lundgren JD, O’Reardon JP, Martino NS, Sarwer DB, Wadden TA, Crosby RD, Engel SG, Stunkard AJ. The Night Eating Questionnaire (NEQ): Psychometric properties of a measure of severity of the night eating syndrome. Eat Behav. 2008;9:62–72. doi: 10.1016/j.eatbeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Allison KC, Martino NS, O’Reardon JP, Stunkard AJ. CBT treatment for night eating syndrome: A pilot study. Obes Res. 2005;13:A83. [Google Scholar]
- Allison KC, Stunkard AJ, Thier SL. Overcoming Night Eating Syndrome: A Step-by-Step Guide to Breaking the Cycle. Oakland, CA: New Harbinger; 2004. [Google Scholar]
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- Bergendahl M, Iranmanesh A, Evans WS, Veldhuis JD. Short-term fasting selectively suppresses leptin pulse mass and 24-hour rhythmic leptin release in healthy midluteal phase women without disturbing leptin pulse frequency or its entropy control (pattern orderliness) J Clin Endocrinol Metab. 2000;85:207–213. doi: 10.1210/jcem.85.1.6325. [DOI] [PubMed] [Google Scholar]
- Birketvedt GS, Florholmen J, Sundsfjord J, Østerud G, Dinges D, Bilker W, Stunkard A. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA. 1999;282:657–663. doi: 10.1001/jama.282.7.657. [DOI] [PubMed] [Google Scholar]
- Boden G, Ruiz J, Urbain J, Chen X. Evidence for a circadian rhythm of insulin secretion. Am J Physiol. 1996;271:E246–E252. doi: 10.1152/ajpendo.1996.271.2.E246. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–132. [PubMed] [Google Scholar]
- Dzaja A, Dalal MA, Himmerich M, Uhr M, Pollmacher T, Schuld A. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. Am J Physiol Endocrinol Metab. 2004;286:E963–E967. doi: 10.1152/ajpendo.00527.2003. [DOI] [PubMed] [Google Scholar]
- Fogteloo AJ, Pijl H, Roelfsema F, Frölich M, Meinders AE. Impact of meal timing and frequency on the twenty-four-hour leptin rhythm. Horm Res. 2004;62:71–78. doi: 10.1159/000079326. [DOI] [PubMed] [Google Scholar]
- Friedman S, Even C, Dardennes R, Guelfi JD. Light therapy, obesity, and night-eating syndrome. Am J Psychiatry. 2002;159:875–876. doi: 10.1176/appi.ajp.159.5.875. [DOI] [PubMed] [Google Scholar]
- Friedman S, Even C, Dardennes R, Guelfi JD. Light therapy, nonseasonal depression, and night eating syndrome. Can J Psychiatry. 2004;49:790. [PubMed] [Google Scholar]
- Friedman S, Even C, Thuile J, Rouillon F, Guelfi JD. Night eating syndrome and winter seasonal affective disorder. Appetite. 2006;47:119–122. doi: 10.1016/j.appet.2006.03.159. [DOI] [PubMed] [Google Scholar]
- Froy O. The relationship between nutrition and circadian rhythms in mammals. Front Neuroendocrinol. 2007;28:61–71. doi: 10.1016/j.yfrne.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licinio J, Negrão A, Mantzoros C, Kaklamani V, Wong M, Bongiorno PB, Mulla A, Cearnal L, Veldhuis JD, Flier JS, et al. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc Natl Acad Sci U S A. 1998;95:2541–2546. doi: 10.1073/pnas.95.5.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren JD, Newberg AB, Allison KC, Wintering NA, Ploessl K, Stunkard AJ. 123I-ADAM SPECT imaging of serotonin transporter binding in patients with night eating syndrome: A preliminary report. Psychiatry Res. 2008;162:214–220. doi: 10.1016/j.pscychresns.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni R, Ratti MT, Tartara A. Nocturnal eating: Prevalence and features in 120 insomniac referrals. Sleep. 1997;20:734–738. doi: 10.1093/sleep/20.9.734. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Ozata M, Negrao AB, Suchard MA, Ziotopoulou M, Caglayan S, Elashoff RM, Cogswell RJ, Negro P, Liberty V, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: Evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab. 2001;86:3284–3291. doi: 10.1210/jcem.86.7.7644. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Abbasi F, Lamendola R, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab. 2004;89:630–635. doi: 10.1210/jc.2003-031572. [DOI] [PubMed] [Google Scholar]
- Mendoza J. Circadian clocks: Setting time by food. J Neuroendocrinol. 2007;19:127–137. doi: 10.1111/j.1365-2826.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- Mikulich SK, Zerbe GA, Jones RH, Crowley TJ. Comparing linear and nonlinear mixed model approaches to cosinor analysis. Stat Med. 2003;22:3195–3211. doi: 10.1002/sim.1560. [DOI] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A. A human phase response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- Mühlbauer E, Wolgast S, Finckh U, Peschke D, Peschke E. Indication of circadian oscillations in the rat pancreas. FEBS Lett. 2004;564:91–96. doi: 10.1016/S0014-5793(04)00322-9. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Chan JL, Van Dongen HPA, Szuba MP, Samaras J, Price NJ, Meier-Ewert HK, Dinges DF, Mantzoros CS. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- O’Reardon JP, Allison KC, Martino NS, Lundgren JD, Heo M, Stunkard AJ. A randomized, placebo-controlled trial of sertraline in the treatment of night eating syndrome. Am J Psychiatry. 2006;163:893–898. doi: 10.1176/ajp.2006.163.5.893. [DOI] [PubMed] [Google Scholar]
- O’Reardon JP, Peshek A, Allison KC. Night eating syndrome: Diagnosis, epidemiology and management. CNS Drugs. 2005;19:997–1008. doi: 10.2165/00023210-200519120-00003. [DOI] [PubMed] [Google Scholar]
- O’Reardon JP, Ringel BL, Dinges DF, Allison KC, Rogers NL, Martino NS, Stunkard AJ. Circadian eating and sleeping patterns in the night eating syndrome. Obes Res. 2004;12:1789–1796. doi: 10.1038/oby.2004.222. [DOI] [PubMed] [Google Scholar]
- Peteranderl C, Antonijevic IA, Steiger A, Murck H, Held K, Frieboes RM, Uhr M, Schaaf L. Nocturnal secretion of TSH and ACTH in male patients with depression and healthy controls. J Psychiatr Res. 2002;36:189–196. doi: 10.1016/s0022-3956(02)00004-3. [DOI] [PubMed] [Google Scholar]
- Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci. 2003;73:2467–2475. doi: 10.1016/s0024-3205(03)00628-3. [DOI] [PubMed] [Google Scholar]
- Rogers NL, Dinges DF, Allison KC, Maislin G, Martino N, O’Reardon JP, Stunkard AJ. Assessment of sleep in women with night eating syndrome. Sleep. 2006;29:814–819. doi: 10.1093/sleep/29.6.814. [DOI] [PubMed] [Google Scholar]
- Sassin JF, Frantz GF, Weitzman ED, Kapen S. Human prolactin: 24-hour pattern with increased release during sleep. Science. 1972;177:1205–1207. doi: 10.1126/science.177.4055.1205. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100:1882–1887. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüssler P, Uhr M, Ising M, Weikel JC, Schmid DA, Held K, Mathias S, Steiger A. Nocturnal ghrelin, ACTH, GH and cortisol secretion after sleep deprivation in humans. Psychoneuroendocrinology. 2006;31:915–923. doi: 10.1016/j.psyneuen.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Simon C, Brandenberger G, Saini J, Ehrhart J, Follenius M. Slow oscillations of plasma glucose and insulin secretion rate are amplified during sleep in humans under continuous enteral nutrition. Sleep. 1994;17:333–338. doi: 10.1093/sleep/17.4.333. [DOI] [PubMed] [Google Scholar]
- Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: Relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83:1893–1899. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- Spaggiari MC, Granella F, Parrino L, Marchesi C, Melli I, Terzano MG. Nocturnal eating syndrome in adults. Sleep. 1994;17:339–344. doi: 10.1093/sleep/17.4.339. [DOI] [PubMed] [Google Scholar]
- Stokkan K, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Allison KC, Lundgren JD, Martino NS, Heo M, Etemad B, O’Reardon JP. A paradigm for facilitating pharmacotherapy at a distance: Sertraline treatment of the night eating syndrome. J Clin Psychiatry. 2006;67:1568–1572. doi: 10.4088/jcp.v67n1011. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome: A pattern of food intake among certain obese patients. Am J Med. 1955;19:78–86. doi: 10.1016/0002-9343(55)90276-x. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, L’Hermite-Balériaux M, Refetoff S, Turek FW, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol Endocrinol Metab. 1994;266:E953–E963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Zhdanova I, Lynch HJ, Wurtman R. Melatonin: A sleep promoting hormone. Sleep. 1997;20:899–907. [PubMed] [Google Scholar]