Abstract
Purpose
18F-FDG PET has been used for vascular disease, but its role in deep vein thrombosis (DVT) remains prospectively unexplored.
Patients and Methods
Whole-body 18F-FDG PET/CT scans were performed in patients 1 to 10 weeks after onset of symptomatic DVT (n = 12) and in control subjects without DVT (n = 24). The metabolic activity (SUVmax) of thrombosed and contralateral nonthrombosed vein segments was determined. The sensitivity and specificity of 18F-FDG PET/CT for the diagnosis of DVT were determined by receiver operating characteristic curve analyses. In 2 patients with DVT, changes in the metabolic activity of thrombosed vein segments in serial 18F-FDG PET scans.
Results
The metabolic activity in thrombosed veins [SUVmax, 2.41 (0.75)] was visually appreciable and significantly higher than in nonthrombosed veins in either the contralateral extremity of patients with DVT [SUVmax, 1.09 (0.25), P = 0.007] or control subjects [1.21 (0.22), P < 001]. The area under the receiver operating characteristic curve for SUVmax was 0.9773 (P < 001), indicating excellent accuracy. An SUVmax threshold of greater than 1.645 was 87.5% sensitive and 100% specific for DVT. Metabolic activity in thrombosed veins correlated significantly with time from DVT symptom onset (decrease in SUVmax of 0.02/d, P < 0.05). Best-fit-line analyses suggested that approximately 84 to 91 days after acute DVT, the maximum metabolic activity of thrombosed veins would return to normal levels.
Conclusions
18F-FDG PET/CT is accurate for detecting acute symptomatic, proximal DVT. Metabolic activity in thrombosed veins decreases with time, suggesting that 18F-FDG PET may be helpful in assessing the age of the clot.
Keywords: duplex ultrasonography, deep vein thrombosis, 18F-FDG PET/CT, sensitivity, specificity, diagnosis, FDG, venothromboembolic disease
Since the initial reports 35 years ago, duplex ultrasonography (US) has virtually replaced contrast venography for the diagnosis of acute deep vein thrombosis (DVT).1,2 In addition to excellent overall performance, the examination does not involve radiation exposure or nephrotoxic contrast agents, can be performed portably, and is widely available. Despite the many advantages of duplex US for the identification of DVT, it is technically dependent on the experience of the individual performing and interpreting the procedure and can also be technically compromised by obesity, edema, or the presence of wounds or overlying bandages. For DVT within the body cavity, compression maneuvers cannot be performed and nonocclusive thrombus may limit the utility of color Doppler US.
18F-FDG is a glucose analog taken up avidly by cells that are metabolically active, including macrophages, endothelial cells, and lymphocytes. In addition to its important role in oncology, 18F-FDG combined with PET can also be used to image the vasculature (reviewed in Joshi et al3). 18F-FDG accumulates in macrophage-rich areas in atherosclerotic lesions and is also very sensitive for the diagnosis of large-vessel vasculitis, reflecting the acute inflammatory responses of the vessel wall.4
Inflammation also contributes to the development of DVT when endothelial cell injury or activation leads to increased expression of cell adhesion molecules promoting leukocyte adhesion and thrombus initiation. Subsequently, other cells, such as neutrophils and macrophages, accumulate within the developing venous thrombus (reviewed in Saha et al5). Because of this robust inflammatory response, increased uptake of 18F-FDG on PET scans may also occur in septic6,7 and aseptic venous thrombosis.8–11
In the current study, the role of 18F-FDG PET was examined in the evaluation of acute, proximal, symptomatic DVT. The primary hypothesis was that the metabolic activity of thrombosed vein segments would be significantly higher than nonthrombosed vein segments. The secondary hypothesis was that the metabolic activity of thrombosed vein segments would decrease with the time from DVT symptom onset.
PATIENTS AND METHODS
Study Cohort
This was a prospective, case-cohort study of patients referred to a university tertiary care referral center with symptomatic, unprovoked, unilateral, proximal DVT within 30 days of study enrollment. The institutional review board approved this study (protocol no. 18327). Symptoms consistent with acute DVT included acute swelling, pain, erythema, or cord tenderness of the lower extremity. Proximal DVT was diagnosed using venous duplex US to identify an area of noncompressibility and lack of color flow in 1 or more proximal veins of the lower extremity, including the femoral, iliac, and popliteal veins. Deep vein thrombosis was objectively confirmed by 2 investigators who reviewed ultrasound images and interpretations and adjudicated all thrombotic events.
Exclusion criteria included isolated distal DVT, upper extremity DVT, bilateral DVT, current pregnancy (all female patients of childbearing age underwent a serum β-human chorionic gonadotropin test before enrollment), prior history of DVT, and inability to provide informed consent or complete the 18F-FDG PET/CT because of distance or other reasons. Distal DVT was defined as noncompressibility of 1 or more vein segments below the popliteal vein. Provoked venous thromboembolism (VTE) was defined as VTE occurring in the setting of known thrombophilia (factor V Leiden mutation, hyperhomocysteinemia, protein C or S deficiency, prothrombin GA20210 mutation, antiphospholipid syndrome, or antithrombin deficiency), known active cancer, use of estrogen, thalidomide (because of its direct effects on the endothelium12 and the increased risk of DVT13) or vascular endothelial growth factor inhibitor, surgery or hospitalization within 30 days, recent immobility (defined as complete bed rest for ≥3 days), the presence of an indwelling vascular device such as a peripherally inserted central catheter, or severe varicose veins.
Once informed consent had been obtained, a comprehensive history and physical examination was performed, and the date of DVT symptom onset was recorded. Basic demographic and clinical information was prospectively captured, and patients were followed up through completion of 18F-FDG PET/CT scans, which were completed within 30 days of study enrollment.
Two investigators blindly selected male and female, control subjects (2:1 matching scheme on the prespecified variables of age and sex) undergoing 18F-FDG PET imaging as part of their routine oncologic care (lymphoma, n = 13/24; melanoma, n = 7/24; sarcoma, n = 2/24; thyroid cancer, n = 1/24; nerve sheath tumor, n = 1/24). Inclusion criteria for control subjects were aged 21 years and older undergoing 18F-FDG PET using the identical imaging protocol and scanner during the same period as our case subjects. Exclusion criteria for control subjects were history of VTE within the preceding 5 years, peripheral arterial or vascular disease, metastatic disease affecting the lower extremities, acute infection, hospitalization or surgery within the preceding 30 days, myeloma, or current use of thalidomide.
Acquisition and Interpretation of 18F-FDG PET Images
Before injection of FDG, subjects fasted for 6 hours or longer and fasting serum glucose was confirmed to be 200 mg/dL or less in all subjects. Subjects were injected intravenously at a site remote from the symptoms of VTE with 10 to 15 mCi (370–555 mBq) of 18F-FDG and then rested quietly for 90 minutes before scanning. Using a Siemens Biograph 4 PET/CT (Siemens Medical Solutions USA, Inc, Malvern, Pa), an 18F-FDG PET/CT scan was performed using diagnostic dose-modulation CT technique with oral barium-based contrast from the mid forehead through the knees and extending distally through the area of thrombosis. Intravenous iodinated contrast was not administered. Images were displayed and reviewed as axial, coronal, and sagittal attenuation-corrected and non–attenuation-corrected PET-only images, CT-only images, attenuation-corrected fused PET/CT images, and PET 3D maximum-intensity-projection (MIP) images.
After acquisition, images were first examined visually for asymmetric areas of 18F-FDG uptake by vein segments. In patients with DVT, MIP and fused axial coronal and sagittal PET/CT images were used to identify the deep veins of the lower extremities where duplex US had confirmed the presence of thrombosed veins. For each study subject, regions of interest were tightly delineated around thrombosed vein segments. For comparison, regions of interest were also closely drawn around nonthrombosed vein segments in the contralateral extremity. SUVmax values for each region of interest were recorded, and ratios of SUVmax in thrombosed vein segments to SUVmax in nonthrombosed contralateral vein segments were calculated for each patient with DVT. To prevent the introduction of inadvertent bias, the same vein segments were studied in both extremities. To reduce partial-volume-effect errors, an attempt was made to measure metabolic activity in thrombosed and contralateral nonthrombosed vein segments that were as similar as possible in diameter. Measurements were also made in vessels that were surrounded by fat, to reduce background metabolic activity.
Control subjects underwent 18F-FDG PET/CT imaging using the identical scanner and image acquisition protocol. In identical fashion to the case subjects, MIP and fused axial coronal and sagittal PET/CT images were used to delineate regions of interest in proximal lower-extremity vein segments bilaterally in each control subject. Vein segments were matched with DVT patients (popliteal, n = 22; or superficial femoral, n = 2). The SUVmax for each region of interest was recorded for each extremity, and the average SUVmax was calculated. The intraindividual coefficient of variance between the left and right vein segments in control subjects was 6.8%.
Statistical Analyses
The sample size for this pilot study was chosen to provide a cohort large enough to identify differences between groups and provide reasonable estimates of the sensitivity and specificity of 18F-FDG PET/CT in the evaluation of DVT. Descriptive statistics (mean, median, and interquartile range) were used to calculate summary data. Data are presented as mean (SD), unless otherwise indicated. For all analyses, continuous variables were assessed for normality visually and with skewness and kurtosis tests. Proportions and their 95% confidence intervals, when appropriate, were calculated (version 11.0; StataCorp, College Station, Tex). Parametric, 2-tailed t tests or analysis of variance were used for continuous variables, and the χ2 and Fisher exact tests were used for categorical variables with a predetermined α level of P < 0.05. Logistic linear regression analysis was used to identify correlations between SUVmax and time from onset of DVT symptoms to completion of 18F-FDG PET/CT. The sensitivity and specificity of measurements of metabolic activity (SUVmax) for determining acute DVT were using receiver operating characteristic curve analysis.14
RESULTS
From November 1, 2008, through January 31, 2010, 67 patients with DVT were prospectively assessed for study participation. Of these, 55 were excluded (Table 1), and thus, 12 patients with DVT met inclusion criteria, provided informed consent, and were prospectively enrolled in the study. Most patients had proximal DVT without clinical evidence of pulmonary embolism and were white (Table 2). All patients with DVT received optimal treatment with standard, parenteral antithrombotic therapy (heparinoids) followed by at least 6 months of oral anticoagulation with vitamin K antagonists (target international normalized ratio, 2–3). Case subjects and controls were well matched on the specified variables of age and sex (Table 2).
TABLE 1.
Reasons for Exclusion
| Reason for Exclusion (n = 55) | n (%) |
|---|---|
| Isolated distal DVT | 3 (5.4) |
| Bilateral, proximal DVT | 3 (5.4) |
| Family history of VTE | 2 (3.6) |
| Active pregnancy | 2 (3.6) |
| Known thrombophilia* | 1 (1.8) |
| Active malignancy | 7 (12.7) |
| Oral contraceptives, estrogen, hormone replacement therapy | 5 (9.1) |
| Surgery or hospitalization within 30 d | 13 (23.6) |
| Immobility† | 2 (3.3) |
| Duplex ultrasonography results not available for adjudication | 6 (10.9) |
| Inability to provide informed consent | 3 (5.4) |
| Inability to complete 18F-FDG PET because of distance | 4 (7.3) |
| Other | 2 (3.6) |
Includes factor V Leiden mutation, hyperhomocysteinemia, protein C/S deficiency, prothrombin gene mutation, antiphospholipid antibody syndrome, or antithrombin deficiency.
Defined as complete bed rest for 3 or more days.
TABLE 2.
Characteristics of the Study Cohort
| Demographic | DVT Subjects |
Control Subjects |
Statistical Difference |
|---|---|---|---|
| Age, mean (SD), y | 55.8 (15.7) | 52.2 (13.7) | NS |
| Male, n (%) | 7 (58.3) | 16 (66.6) | NS |
| White, n (%) | 12 (100) | 23 (95.8) | NS |
| Weight, mean (SD), kg | 93.3 (31.1) | 85.8 (14.4) | NS |
| BMI, mean (SD), kg/m2 | 30.8 (12.1) | 27.6 (4.1) | NS |
| Fasting glucose, mean (SD), mg/dL | 94.6 (9.6) | 95.8 (9.2) | NS |
| Type of index VTE, n (%) | |||
| DVT without PE* | 9 (75) | — | — |
| DVT with PE* | 3 (25) | — | — |
| Medical comorbidities, n (%) | |||
| Diabetes mellitus | 1 (8.3) | 1 (4.1) | NS |
| Cardiovascular disease | 0 (0) | 0 (0) | NS |
These were exclusion criteria for the control subjects.
BMI indicates body mass index; NS, not significant; PE, pulmonary embolism.
All patients underwent venous duplex US to confirm the diagnosis of DVT within 72 hours of symptom onset. The most common thrombosed vein segment was the popliteal vein (n = 9/12, 75%). The superficial femoral (n = 3/12, 25%), common femoral (n = 1/12, 8.3%), and greater saphenous vein segments (n = 1/12, 8.3%) were less commonly involved. Overall, 25% (n = 3/12) of patients had DVT involving 2 or more proximal vein segments.
The mean (SD) time between DVT symptom onset and completion of 18F-FDG PET/CT scan was 32.5 (22.5) days (median, 26 days; interquartile range, 7–70 days). All patients completed 18F-FDG PET scanning without adverse events or complications.
Metabolic Activity Was Increased in Thrombosed Vein Segments
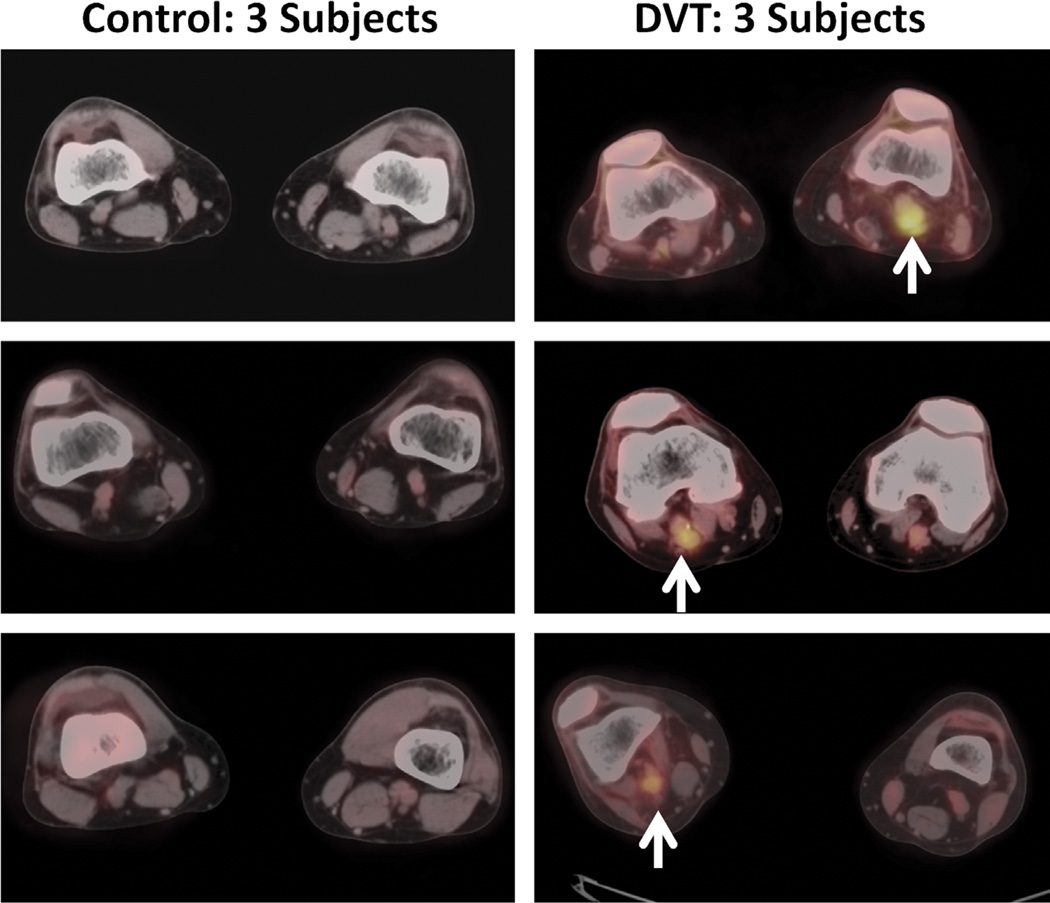
Increased metabolic activity was easily appreciated visually by 18F-FDG PET/CT in thrombosed vein segments compared with the matching, contralateral vein segments without thrombosis (Fig. 1). Asymmetry in thrombosed vein segments was noted in all patients (incidence, 100%). In addition, in patients who underwent 18F-FDG PET/CT imaging within 14 days of onset of DVT symptoms (n = 5), diffuse increased metabolic activity also extended into the perivascular soft tissues, likely the consequence of secondary adjacent inflammatory changes. These changes were not observed in patients whose 18F-FDG PET/CT imaging occurred after 14 days of DVT symptom onset (n = 7).
FIGURE 1.
Representative transaxial 18F-FDG PET/CT images from control subjects without DVT (left panels) and patients with DVT (right panels, white arrows).
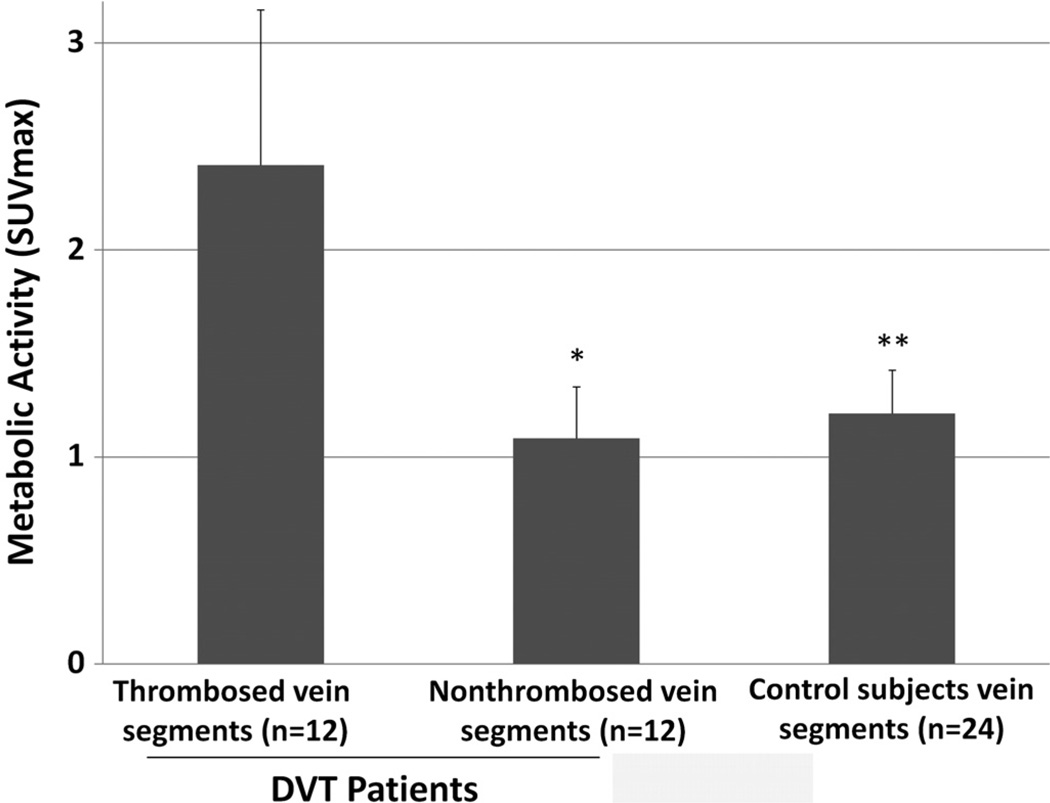
In patients with DVT, the SUVmax was significantly higher in thrombosed vein segments compared with matched, nonthrombosed vein segments in the contralateral leg [2.41 (0.75) vs 1.09 (0.25), P = 0.007; Fig. 2]. The SUVmax ratio between thrombosed and nonthrombosed contralateral vein segments was 2.20 (0.46). The SUVmax in thrombosed vein segments did not differ between left (n = 5) and right (n = 7) extremities [2.42 (0.67) vs 2.40 (0.85), P = NS] or between popliteal and superficial femoral thrombosed vein segments [2.76 (0.80) vs 2.65 (0.90), P = NS], the most common thrombosed vein segments in our study. By comparison, the SUVmax in control subjects without DVT was significantly lower than thrombosed vein segments [1.21 (0.21) vs 2.41 (0.75), P < 0.0001; Fig. 2]. The SUVmax in control subjects without DVT did not differ from nonthrombosed vein segments in patients with DVT [1.21 (0.22) vs 1.09 (0.25), P = NS; Fig. 2]. Among the 24 control subjects who were scanned as part of their routine oncologic care (staging, restaging, therapeutic assessment, or surveillance), 13 had metabolically active tumor identified at the time of the FDG PET/CT scan and 11 did not. In those with active cancer, metabolic activity in vein segments was not statistically different from that in the control patients without active cancer [1.20 (0.18) vs 1.95 (0.21), P = NS].
FIGURE 2.
Mean (SD) SUVmax in thrombosed and contralateral, nonthrombosed vein segments of patients with DVT (n = 12) or control subjects (n = 24). *P < 0.007 versus thrombosed vein segments. **P < 0.001 versus thrombosed vein segments.
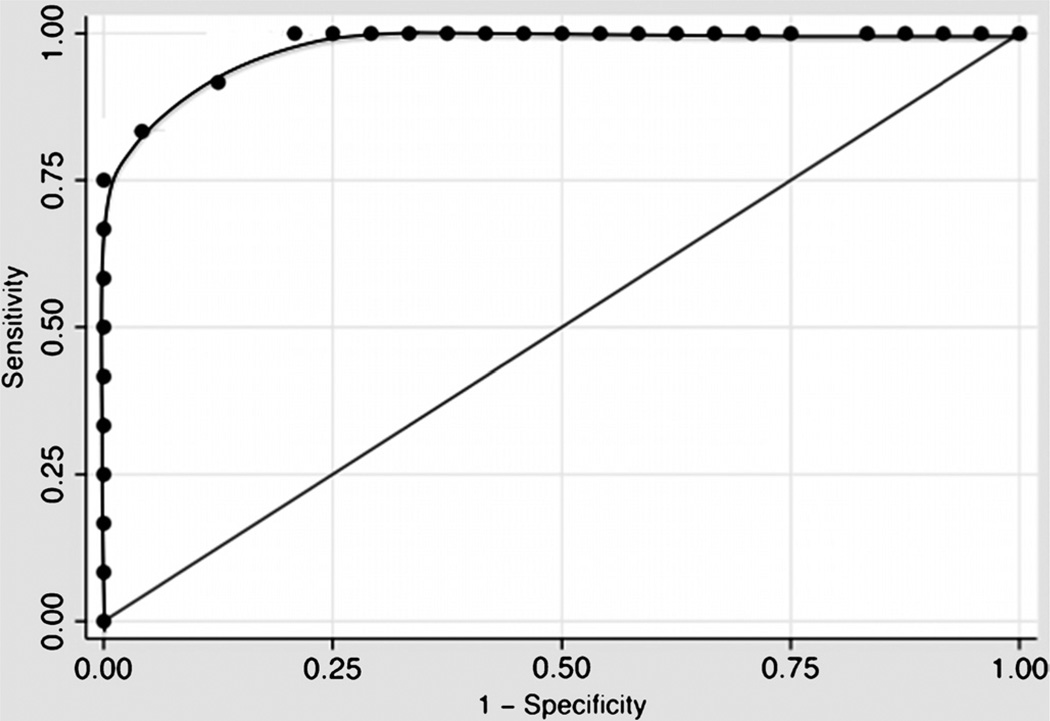
18F-FDG PET Was Accurate for the Diagnosis of DVT
Receiver operating characteristic curve analyses were used to identify the accuracy of 18F-FDG PET/CT for identifying acute DVT. The area under the receiver operating characteristic curve for SUVmax was 0.9844 (P < 0.0001; Fig. 3), indicating excellent accuracy. Using a SUVmax threshold of 1.645 or higher provided 87.5% sensitivity but 100% specificity (positive predictive value = 100% and negative predictive value = 92.3%) for the diagnosis of DVT. Decreasing the SUVmax threshold to 1.49 or higher increased the sensitivity to 100% but decreased specificity to 87.5% (positive predictive value = 84.2% and negative predictive value = 100%).
FIGURE 3.
Receiver operating characteristic curve of the SUVmax for the diagnosis of DVT by 18F-FDG PET/CT imaging.
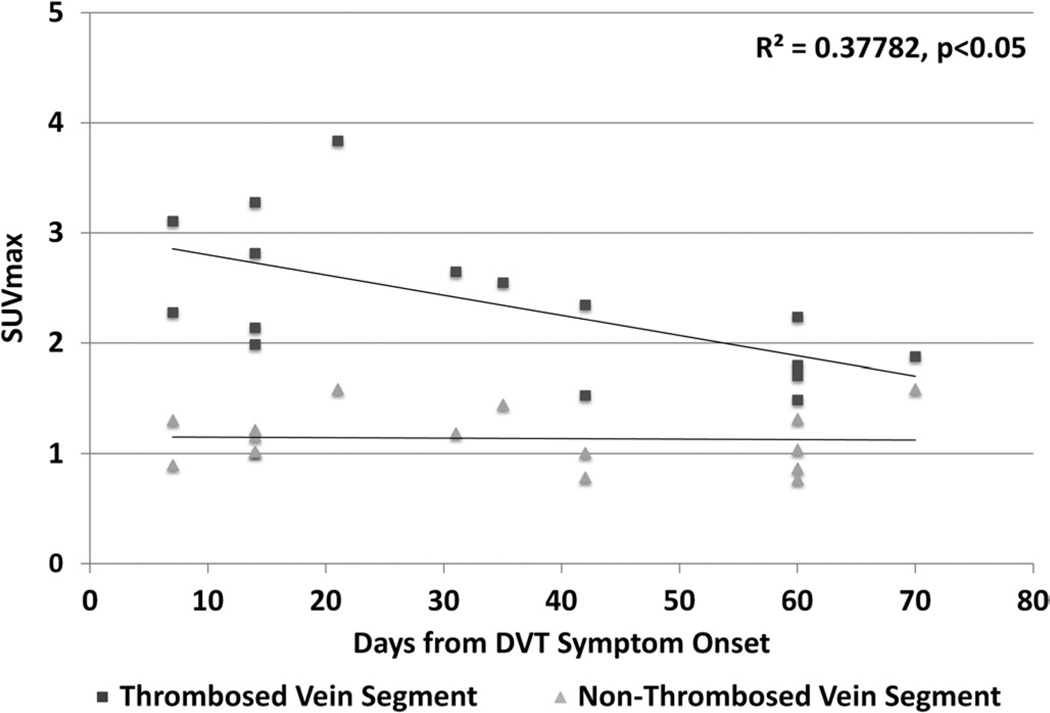
Metabolic Activity in Thrombosed Vein Segments Correlates With Time From DVT Symptom Onset
In prespecified linear regression analyses, we identified a negative correlation between metabolic activity in thrombosed vein segments and the time from onset of DVT symptoms (Fig. 4; decrease in SUVmax of 0.02/d, P < 0.05). There was no correlation between the SUVmax of nonthrombosed contralateral vein segments and the time from onset of DVT symptoms. Using the best-fit-line analyses for the scatterplot of SUVmax of thrombosed vein segments versus matching, nonthrombosed, contralateral vein segments, we estimated that, 84 to 91 days after DVT onset, the SUVmax of thrombosed vein segments would return to levels seen in nonthrombosed vein segments (Fig. 4).
FIGURE 4.
The ratio of metabolic activity (SUVmax) in thrombosed vein segments to matching, nonthrombosed vein segments decreases with time from DVT symptom onset.
We also performed serial 18F-FDG PET/CT imaging in 2 patients with DVT (n = 3 scans per patient) and, consistent with our regression analysis, the SUVmax of thrombosed vein segments declined over time during these serial imaging studies (Figs. 5 and 6). The SUVmax of matching nonthrombosed vein segments in the contralateral extremity of each patient remained remarkably consistent over time (interday coefficient of variance, 3.89%). Neither of these patients had evidence of recurrent thrombosis either clinically or radiographically.
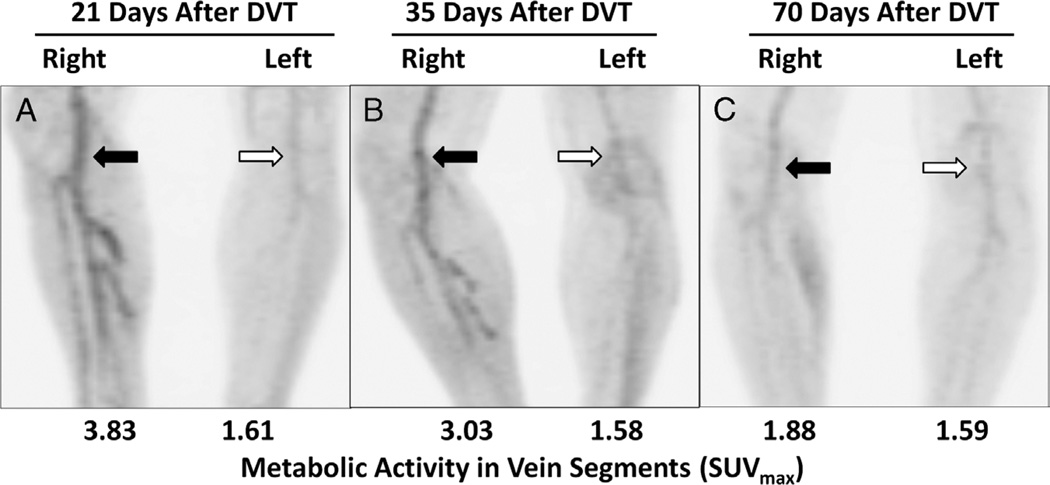
FIGURE 5.
The metabolic activity of thrombosed vein segments decreases with time from DVT symptom onset. Maximum-intensity-projection images of serial 18F-FDG PET scans in a 54-year-old man with acute right lower extremity DVT involving superficial femoral, popliteal, and calf veins. PET scans were obtained 21, 35, and 70 days after DVT symptom onset. A, The metabolic activity (eg, SUVmax) was initially higher in thrombosed vein segments (black arrow) when compared with the contralateral extremity (white arrow). B, At 35 days after DVT onset, the SUVmax in thrombosed vein segments (black arrow) had declined but was still higher than matching vein segments in the contralateral, nonthrombosed extremity (white arrow). C, At 70 days, metabolic activity in thrombosed vein segments (black arrow) was barely appreciable above normal vascular activity (white arrow).
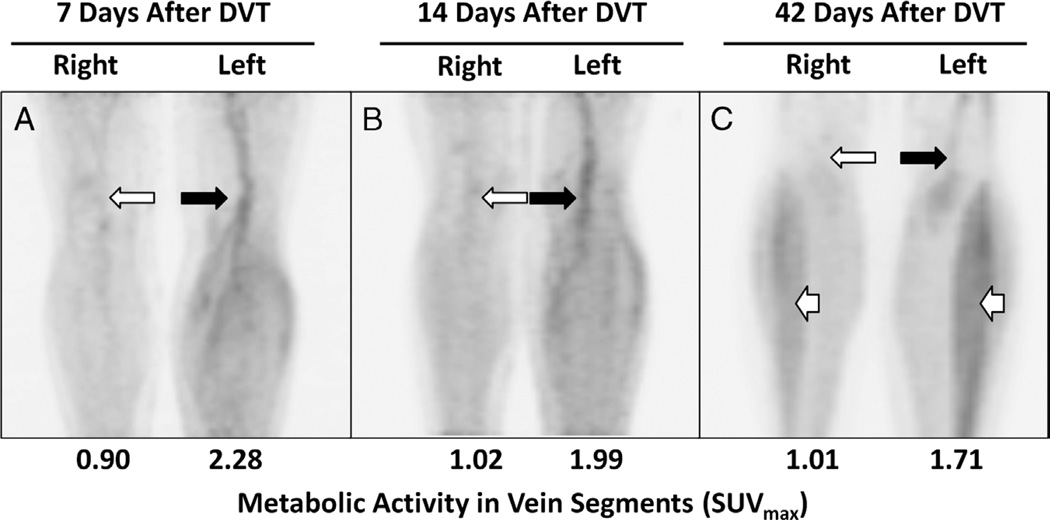
FIGURE 6.
The metabolic activity of thrombosed vein segments decreases with time from DVT symptom onset. Maximum-intensity-projection images of serial 18F-FDG PET scans in a 50-year-old woman with acute left lower extremity DVT involving superficial femoral, popliteal, and calf veins. PET scans were obtained 7, 14, and 42 days after DVT symptom onset. A, The metabolic activity (eg, SUVmax) was initially higher in thrombosed vein segments (black arrow) when compared with the contralateral extremity (white arrow). B, At 14 days after DVT onset, the SUVmax in thrombosed vein segments (black arrow) had declined slightly but was still higher than matching vein segments in the contralateral, nonthrombosed extremity (white arrow) and appeared visually unchanged. C, At 42 days, metabolic activity in thrombosed veins has continued to decrease (black arrow). Metabolic activity in nonthrombosed vein segments remains low (white arrow). Note also prominent muscle activity in the calves at 42 days, likely related to recent exercise (white arrowheads).
DISCUSSION
Duplex US is widely used for the diagnosis of acute DVT. Although noninvasive and readily available, methods to reliably distinguish acute from chronic thrombosis by duplex US have not been well validated. Newer techniques, including US elastography and photoacoustic imaging, may eventually prove to be useful but remains investigational.15,16 18F-FDG PET/CT is a very sensitive imaging modality used commonly in oncology, which also has increasing utility in vasculature imaging (reviewed in Joshi et al3). Acute thrombosis, often reflecting underlying systemic inflammation, is associated with endothelial cell activation and increased expression of cell adhesion molecules. These events subsequently promote leukocyte adhesion with accumulation of neutrophils and macrophages, which are metabolically active and avidly take up 18F-FDG, within the developing thrombus (reviewed in Saha et al5).
To our knowledge, this is the first prospective, comparative study to examine the role of 18F-FDG PET/CT in patients with symptomatic, proximal DVT and supports and extends smaller reports of increased 18F-FDG uptake in DVT.8,11,17 In the current study, we found that thrombosed vein segments universally appeared visually asymmetric. Furthermore, 18F-FDG uptake in thrombosed vein segments was significantly increased in thrombosed vein segments. The presence of visual asymmetry and a SUVmax threshold of 1.645 or higher were very sensitive for the diagnosis of acute, proximal DVT with 100% specificity. The uptake of 18F-FDG did not differ with regard to extremity or vein segments, and despite the small sample size, the absolute magnitude of difference in the SUVmax between thrombosed and nonthrombosed vein segments (Fig. 1) was sufficient to provide 99.9% power to avoid a type 2 error (using a 2-sided, α = 0.05).
In our patients with DVT, metabolic activity was not increased in the nonthrombosed contralateral vein segments. Thus, if bilateral DVT is absent, comparing metabolic activity between the 2 extremities may assist clinicians in the diagnosis of DVT as seen on 18F-FDG PET/CT. Furthermore, the SUVmax of nonthrombosed vein segments (whether in our n = 12 DVT patients or n = 24 control subjects) was remarkably consistent between subjects with little variation.
Finally, these data also suggest that 18F-FDG PET may allow an assessment of the age of DVT. In both regression analyses and 2 patients who underwent serial PET imaging, the metabolic uptake and visually asymmetric of thrombosed vein segments declined with increasing time from DVT symptom onset. On the basis of these preliminary data, the SUVmax in thrombosed vein segments may take up to 3 months to normalize after an episode of acute, proximal DVT.
Although 18F-FDG PET/CT would not replace duplex US for the routine diagnosis of DVT, 18F-FDG PET/CT have utility in a multitude of clinical settings for the evaluation of DVT. For example, acute thrombus may be suspected within the body cavity where duplex US cannot easily image (eg, iliofemoral DVT), particular when inability to compress veins limits the diagnostic utility (eg, obesity, plaster casts). Moreover, 18F-FDG PET/CT is used commonly in oncology,18 a population at high risk for VTE,19 and may identify incidental thrombosis prompting additional investigations. Finally, 18F-FDG PET/CT may be an important diagnostic tool for the assessment of the age of the thrombus—a distinction that is important given the marked differences in therapeutic approach. Acute DVT is treated with systemic anticoagulation and not uncommonly pharmacomechanical thrombolysis. Chronic DVT, however, may be treated with serial imaging only and thus distinguishing acute from chronic DVT is of key clinical importance. As duplex US-based techniques to difference acute from chronic DVT, including elastography and photoacoustic imaging,15,16 remain largely investigational and/or not widely available, the data of the current study provide novel evidence for the potential role of 18F-FDG PET/CT in making this distinction.
Clinically, the distinction between “acute” and “chronic” DVT has been somewhat arbitrarily established. The success of venous recanalization, preservation of valve function, and symptom relief may depend on the timing of therapy after thrombosis. These factors influence the effectiveness of the intervention and the subsequent incidence of postthrombotic syndrome (PTS). Fresh thrombi respond better to catheter-directed thrombolysis than do organized clots. It has been suggested that 10 days may be the optimal interval from onset of symptoms during which to instigate treatment. However, the ATTRACT trial in the United States has used 14 days and the CaVenT trial has used 21 days as the cutoff for recruitment of patients for catheter-directed therapy.20–22 In the literature, it is recognized that the age at which a clot is not longer amenable to thrombolysis requires further study to determine the optimal time frame for intervention that will prevent valvular destruction and venous hypertension, which may result in an increase of the likelihood of longer-term sequelae.23–26 After the development of proximal DVT, it is suggested that anticoagulation be maintained for 3 to 6 months, and the use of graduated compression stockings for 2 years significantly reduces the incidence of PTS.27 The results of the current study suggest that thrombus may remain metabolically active up to 10 weeks. It is tempting to postulate that demonstration of increased metabolic activity in clot may signify that the clot is still capable of active propagation during this time, but this remains to be studied. 18F-FDG PET may represent a novel mechanism by which a better refinement can be made between thrombus that is acute and metabolically active and that which is chronic, organized, and metabolically inert. These distinctions may translate to better refinement and more physiological assessment of the definition of “acute” versus “chronic” clot, which may have therapeutically significant implications.
The strengths of the study include the prospective study design, the objective adjudication of all DVT by duplex US, and the inclusion of carefully matched control subjects. We also carefully recorded the onset of DVT symptoms and excluded patients with isolated distal DVT, asymptomatic DVT, and provoked DVT due to surgery and hospitalization (which could introduce bias into the measured metabolic activity of vein segments due to generalized, systemic inflammation, or surgical manipulation). However, the disadvantage of such a study design is a lack of systematic information regarding the appearance of DVT on 18F-FDG PET in the face of intercurrent illnesses and inflammatory conditions. For example, patients with PTS have chronic extremity swelling, pain, skin ulcers, and inflammatory changes. These inflammatory changes could mimic the appearance of acute DVT. Conversely, the effect of antiinflammatory drugs on uptake of 18F-FDG by acute clot has not been assessed and was not a controlled factor in this specific study.
The primary limitation of this study was the small sample size, and this work should be considered preliminary. Nevertheless, this study still represents, to our knowledge, the only prospective evaluation of the role of 18F-FDG PET/CT in patients with DVT. Despite the small sample size, the magnitude of differences and correlation with time from DVT symptom onset remained robust. Furthermore, the inclusion of a matched control cohort, as well as using the contralateral vein segments from patients with DVT, allowed us to rigorously characterize and compare metabolic activity in nonthrombosed veins.
An additional limitation of this study was that serial or repeat 18F-FDG PET/CT imaging was not performed on any DVT patients more than 70 days after DVT symptom onset. Thus, the prediction that the metabolic activity of thrombosed vein segments would return to normal at 84 to 91 days after acute DVT would require prospective confirmation. “Optimized” SUVmax cutoff values in the identification of acute thrombosis would also require independent confirmation. Bilateral duplex US was not performed on every subject. Nevertheless, none of our patients who only had unilateral ultrasound had symptoms of DVT in the contralateral leg and none of these patients had evidence of DVT on 18F-FDG PET/CT. Because prospective studies have suggested that routine bilateral duplex US is not necessary in the absence of symptoms, active malignancy, or recent surgery or trauma,28 this is unlikely to represent a major limitation. Finally, it is recognized that other processes besides DVT could increase metabolic activity in the venous vasculature. Although 18F-FDG PET/CT may be very sensitive for the diagnosis of DVT, the specificity was less than 90% (and inferior to reported values obtained by duplex US), and confirmatory testing with duplex US would be necessary.
CONCLUSIONS
To our knowledge, this is the first prospective comparative study to demonstrate that 18F-FDG PET/CT is a sensitive imaging tool for detecting symptomatic, proximal DVT. Metabolic activity in thrombosed vein segments decreases with time DVT onset, suggesting that 18F-FDG PET/CT may have utility in assessing the age of the thrombus and in establishing the physiologically relevant revisions of the definitions of acute versus chronic thrombus, which could have therapeutic significance.
ACKNOWLEDGMENTS
The authors thank Dr Robby Campbell for his thoughtful reading of this article.
Conflicts of interest and source of funding: This work was directly or indirectly supported by the National Institutes of Health (NIH) and the University of Utah CCTS (grant numbers 1R01CA121003, 1K23HL092161, 5R01HL092746, 5R01HL091754, and UL1RR025764). This project was also facilitated by the Huntsman Cancer Institute through its Molecular Imaging Research Program and by grant R01CA135556 from the National Cancer Institute/NIH, by a Cancer Center Support Grant from the National Cancer Institute/NIH (3P30CA042014), and by the Huntsman Cancer Foundation.
REFERENCES
- 1.Meadway J, Nicolaides AN, Walker CJ, et al. Value of Doppler ultrasound in diagnosis of clinically suspected deep vein thrombosis. Br Med J. 1975;4:552–554. doi: 10.1136/bmj.4.5996.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flanigan DP, Goodreau JJ, Burnham SJ, et al. Vascular-laboratory diagnosis of clinically suspected acute deep-vein thrombosis. Lancet. 1978;2:331–334. doi: 10.1016/s0140-6736(78)92939-2. [DOI] [PubMed] [Google Scholar]
- 3.Joshi F, Rosenbaum D, Bordes S, et al. Vascular imaging with positron emission tomography. J of Int Med. 2011;270:99–109. doi: 10.1111/j.1365-2796.2011.02392.x. [DOI] [PubMed] [Google Scholar]
- 4.Neumann T, Oelzner P, Freesmeyer M, et al. Images in cardiovascular medicine. Diagnosis of large-vessel vasculitis by [18F] fluorodeoxyglucose–positron emission tomography. Circulation. 2009;119:338–339. doi: 10.1161/CIRCULATIONAHA.108.796391. [DOI] [PubMed] [Google Scholar]
- 5.Saha P, Humphries J, Modarai B, et al. Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arterioscler Thromb Vasc Biol. 2011;31:506–512. doi: 10.1161/ATVBAHA.110.213405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miceli M, Atoui R, Walker R, et al. Diagnosis of deep septic thrombophlebitis in cancer patients by fluorine-18 fluorodeoxyglucose positron emission tomography scanning: a preliminary report. J Clin Oncol. 2004;22:1949–1956. doi: 10.1200/JCO.2004.10.160. [DOI] [PubMed] [Google Scholar]
- 7.Bleeker-Rovers CP, Jager G, Tack CJ, et al. F-18-fluorodeoxyglucose positron emission tomography leading to a diagnosis of septic thrombophlebitis of the portal vein: description of a case history and review of the literature. J Int Med. 2004;255:419–423. doi: 10.1046/j.1365-2796.2003.01259.x. [DOI] [PubMed] [Google Scholar]
- 8.Do B, Mari C, Biswal S, et al. Diagnosis of aseptic deep venous thrombosis of the upper extremity in a cancer patient using fluorine-18 fluorodeoxyglucose positron emission tomography/computerized tomography (FDG PET/CT) Ann Nucl Med. 2006;20:151–155. doi: 10.1007/BF02985628. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal A, Agrawal R, Asopa R, et al. Detection of superior sagittal sinus thrombosis on FDG PET. Clin Nucl Med. 2009;34:161–163. doi: 10.1097/RLU.0b013e3181966fb3. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P, Kumar R, Jeph S, et al. 18F-FDG PET-CT in the diagnosis of tumor thrombus: can it be differentiated from benign thrombus? Nucl Med Comm. 2011;32:782–788. doi: 10.1097/MNM.0b013e32834774c8. [DOI] [PubMed] [Google Scholar]
- 11.Capete-Sanchez FM, Gandhi M, Evans TL, et al. Detection by 18F-FDG-PET/CT of upper extremity acute deep venous thrombosis. Hell J Nucl Med. 2011;14:81–82. [PubMed] [Google Scholar]
- 12.Kaushal V, Kaushal GP, Melkaveri SN, et al. Thalidomide protects endothelial cells from doxorubicin-induced apoptosis but alters cell morphology. J Thromb Haemost. 2004;2:327–334. doi: 10.1046/j.1538-7933.2003.00573.x. [DOI] [PubMed] [Google Scholar]
- 13.Zangari M, Anaissie E, Barlogie B, et al. Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood. 2001;98:1614–1615. doi: 10.1182/blood.v98.5.1614. [DOI] [PubMed] [Google Scholar]
- 14.Obuchowski NA. Receiver operating characteristic curves and their use in radiology. Radiology. 2003;229:3–8. doi: 10.1148/radiol.2291010898. [DOI] [PubMed] [Google Scholar]
- 15.Rubin JM, Xie H, Kim K, et al. Sonographic elasticity imaging of acute and chronic deep venous thrombosis in humans. J Ultrasound Med. 2006;25:1179–1186. doi: 10.7863/jum.2006.25.9.1179. [DOI] [PubMed] [Google Scholar]
- 16.Karpiouk AB, Aglyamov SR, Mallidi S, et al. Combined ultrasound and photoacoustic imaging to detect and stage deep vein thrombosis: phantomand ex vivo studies. J Biomed Opt. 2008;13 doi: 10.1117/1.2992175. 054061. [DOI] [PubMed] [Google Scholar]
- 17.Chang KJ, Zhuang H, Alavi A. Detection of chronic recurrent lower extremity deep venous thrombosis on fluorine-18 fluorodeoxyglucose positron emission tomography. Clin Nucl Med. 2000;25:838–839. doi: 10.1097/00003072-200010000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Wahl RL. Principles of cancer imaging with fluorodeoxyglucose. In: Wahl RL, Buchanan JW, editors. Principles and Practice of Positron Emission Tomography. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 100–110. [Google Scholar]
- 19.Garcia D, Quintana D. Thrombosis and malignancy: a case-based review. Semin Hematol. 2011;48:259–263. doi: 10.1053/j.seminhematol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Kearon C, Kahn S, Agnielli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 21.Comerota AJ. The ATTRACT trial: rationale for early intervention for iliofemoral DVT. Perspect Vasc Surg Endovasc Ther. 2009;21:221–224. doi: 10.1177/1531003509359311. quiz 224–225. [DOI] [PubMed] [Google Scholar]
- 22.Enden T, Sandvik L, Klow N, et al. Catheter-directed venous thrombolysis in acute iliofemoral vein thrombosis: the CaVenT study: rationale and design of a multicentre, randomized controlled, clinical trial ( NCT00251771) Am Heart J. 2007;154:808–814. doi: 10.1016/j.ahj.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Patterson BO, Hinchliffe R, Loftus IM, et al. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis [review] Arterioscler Thromb Vasc Biol. 2010;30:669–674. doi: 10.1161/ATVBAHA.109.200766. [DOI] [PubMed] [Google Scholar]
- 24.Segal J, Streiff M, Hofmann L, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med. 2007;146:211–222. doi: 10.7326/0003-4819-146-3-200702060-00150. [DOI] [PubMed] [Google Scholar]
- 25.Meissner M. Thrombolytic therapy for acute deep vein thrombosis and the venous registry. Rev Cardiovasc Med. 2002;3:S53–S60. [PubMed] [Google Scholar]
- 26.Mewissen M. Catheter-directed thrombolysis for lower extremity deep vein thrombosis. Tech Vasc Interv Radiol. 2001;4:111–114. doi: 10.1016/s1089-2516(01)90005-8. [DOI] [PubMed] [Google Scholar]
- 27.Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249–256. doi: 10.7326/0003-4819-141-4-200408170-00004. [DOI] [PubMed] [Google Scholar]
- 28.Pennell RC, Mantese VA, Westfall SG. Duplex scan for deep vein thrombosis—defining who needs an examination of the contralateral asymptomatic leg. J Vasc Surg. 2008;48:413–416. doi: 10.1016/j.jvs.2008.03.046. [DOI] [PubMed] [Google Scholar]