Abstract
The majority of neurodegenerative diseases have an important age component, and thus, understanding the molecular changes that occur during normal aging of the brain is of utmost relevance. In search for the basis of the age-related cognitive decline found in humans, monkeys and rodents, we study the rhesus monkey. Surprisingly, there is no loss of neurons in aged monkey brains. However, we reported white matter and myelin abnormalities in aged monkeys, similar to those observed in Alzheimer’s disease and multiple sclerosis patients. In a microarray analysis comparing young and old monkey white matter, we discovered that Klotho is downregulated in the aged brain. We then asked whether there is a connection between the age-related cognitive decline, myelin abnormalities and Klotho downregulation. If such a connection is found, compounds that upregulate Klotho expression could become of therapeutic interest for the treatment of multiple sclerosis, and perhaps even Alzheimer’s disease.
One of the enormous challenges facing modern medicine is the treatment of neurodegenerative diseases. Neurons are post-mitotic cells and once they are born in utero they have to last a lifetime. A long list of genetic and environmental insults can weaken and kill neurons. Therefore, our goal is to identify reagents that are neuroprotective and would rescue these amazingly active cells from premature death. The most prevalent neurodegenerative diseases are Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington disease (HD) and amyotrophic lateral sclerosis (ALS). In the last 30 years, in rare cases of these disorders, mutations, gene duplications, expanded triple repeats or deletions have been identified and demonstrated to cause either autosomal dominant or recessive inheritability. However, except for HD, which is always dominantly inherited, the majority of AD, PD and ALS cases are called sporadic or idiopathic, meaning that one genetic cause has not been elucidated or that multiple genes with low individual effects interact to cause disease. Although not considered a typical neurodegenerative disease because neurons are not the direct target of destruction, multiple sclerosis (MS) results from progressive insults on myelin of the CNS resulting in neurological symptoms. MS is an autoimmune disease in which the white matter myelin is attacked by the immune system leading to demyelination and axonal injury.
In AD, PD, HD and ALS selective neuronal populations are the victims of an, as of yet, unknown attack or lack of a protecting factor, as seen by the plethora of manifestations that bring the patient to a neurologist. From memory loss and cognitive decline to tremor and muscle weakness, these diseases always incapacitate the patients and shorten their lifespan causing a heavy psychological and financial strain to family members. With an increasing aging population these diseases also exert tremendous burden on national health care systems.
In this article we will focus on a less traveled path in neurodegenerative diseases, the abnormalities in the form of demyelination vastly seen in the white matter in MS, to a lesser extent in AD and during normal aging. Next, we will examine the role of Klotho protein on brain white matter, strategies to augment expression of this protein as a means of potential therapy for the treatment of demyelinating neurodegenerative diseases and our recent efforts to identify small molecules that increase Klotho at the transcriptional level.
Features of neurodegenerative diseases relevant to the role of Klotho
AD is the most prevalent form of dementia among the elderly. The major neuropathological findings in AD are the presence of amyloid β (Aβ) plaques, neurofibrillary tangles, brain atrophy, and synaptic and neuronal loss. Currently, Aβ, a proteolytically derived fragment of the amyloid precursor protein, is recognized as a major initiating factor in the cascade of events that leads to progressive neurodegeneration in AD with evidence from multiple laboratories of its toxicity towards neurons and their synapses [1]. However, only the reduction of Aβ levels, currently being tried in multiple clinical studies, would prove that indeed Aβ is the culprit in AD. Meanwhile, other potential pathways responsible for neurodegeneration need to be considered in AD and other diseases. The greatest known risk factor for AD is increasing age. One out of eight people over 65 years of age have AD and nearly half of all people over 85 years of age have AD. Thus, studying factors that contribute to aging is of utmost importance. We focused our interest on the normal aging of the brain in the rhesus monkey, an animal model that develops cognitive decline with age similarly to humans.
In aged white matter, but not the gray matter of these nonhuman primates, we have detected neuroinflammation in the form of activated microglia [2] and reactive astrocytes [3]. In addition, we have observed attack on myelin by the complement system [4], the activation of the calcium-activated cysteine proteinase, calpain, an enzyme known to degrade cytoskeletal proteins [5,6] and abnormalities of the Node of Ranvier [7]. All these results indicating white matter degeneration complement the ultra-structural studies demonstrating age-related dysmyelination [8].
By MRI, the white matter in AD patients exhibits areas of hypomyelination seen as hyper-intensities. One hundred times less prevalent than AD, multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system that often progresses to significant chronic disability. Impaired remyelination is a common pathologic feature of MS and may largely account for disability and incomplete remissions in MS [9]. Several groups have established that remyelination is incomplete in MS lesions, despite numerous oligodendrocyte precursor cells (OPCs) found within and near the lesions [10–12]. Thus, a factor that would induce OPC maturation may be missing or an inhibitor of myelination is overactive [13].
Klotho, the anti-aging protein
Klotho is a recently discovered protein with profound effects on health and lifespan in mammals [14,15]. In mice, Klotho overexpression extends lifespan, while its disruption accelerates aging-like phenotypes, including atherosclerosis, osteoporosis, infertility and cognitive decline. Thus, a reduction in Klotho level is detrimental to the entire organism. Klotho is mainly expressed in the brain and in the kidney where it plays an antioxidative function and controls calcium and phosphate homeostasis. In addition, Klotho has been shown to have anti-inflammatory [16,17] and tumor suppressor properties [18]. Klotho is expressed as a type I transmembrane protein that is shed by ADAM10 and 17 [19] and is detectable in serum and cerebrospinal fluid [20]. The transmembrane form of Klotho has been shown to act as a coreceptor with FGF receptors to facilitate high affinity signaling of FGF23 in the kidney. The shed form of Klotho has been shown to possess many functions, including suppression of insulin/IGF1 and wnt signaling [21] and regulation of calcium and phosphate homeostasis (Table 1) [22,23]. Interestingly, shed Klotho can act as a ligand to an, as yet, unknown receptor to transduce signaling in the target cell, or as a glycosidase to modify a receptor or ion channel as shown for the TRPV5 channel [24,25]. Indeed, the extracellular domain of Klotho has homology to the family 1 glycosidases, which are enzymes that hydrolyze terminal glycosidic linkages in sugars, glycoproteins and glycolipids.
Table 1.
Pleiotropic functions of secreted Klotho protein.
| Glycoproteins regulated by secreted Klotho | Klotho effects | Ref. |
|---|---|---|
| Ion channels | ||
| Transient receptor potential vanilloid type isoform-5 | Increase in Ca2+ influx | [25] |
| Transporters | ||
| Renal outer medullary potassium channel-1 | Increase in K+ efflux | [33] |
| Na-dependent phosphate co-transporter, type-IIa | Decrease in Pi influx | [34] |
| Pit-1, 2 (Na-dependent phosphate co-transporter, type-III) | Decrease in Pi influx | [35] |
| Receptors | ||
| IGF-1 receptors | Inhibition | [15] |
| TGF-β1 receptor (type-II) | Inhibition | [36] |
Modified with permission from [23].
The anti-aging properties of Klotho are also associated with increased resistance to oxidative stress [15] and inflammation [17]. The possible link between Klotho and the inflammatory cytokines, such as TNF, was shown previously. Klotho’s downregulation is associated with increased inflammation in kidney [16], inflammatory bowel disease [26] and rheumatoid arthritis [27]. Klotho is known for the suppression of TNF-α-induced expression of adhesion molecules in a model of endothelial inflammation [28]. This effect of Klotho was accompanied by the attenuation of NF-κB activation, IκB phosphorylation and inhibition of eNOS phosphorylation induced by TNF-α. The protective effect of Klotho through the negative regulation of NF-κB signaling was demonstrated also in the model of inflammation in diabetic kidneys [16] and kidney injury [29]. The mechanism of protection involves the inhibition of RelA Ser-536 phosphorylation as well as promoter DNA binding of this phosphorylated form of RelA [16]. In future studies we will explore in our experimental system the possibility of the involvement of the inhibition of NF-κB signaling in the mechanism of Klotho protection from TNF cytotoxicity.
Recently, clinical phenotypes of loss-of-function mutations in human Klotho were reported. A 13-year-old girl with a homozygous missense mutation (H193R) of the Klotho gene exhibited decreased expression and secretion of Klotho protein resulting in osteopenia, but not neuropsychiatric symptoms [30]. The authors concluded that the loss-of-function mutations in human Klotho impair FGF23 bioactivity, underscoring the essential role of Klotho in FGF23-mediated phosphate and vitamin D homeostasis in humans. In the Klotho-hypomorphic mouse, the degeneration of mesencephalic dopaminergic neurons was observed that was related to increased vitamin D exposure [31]. Klotho has been shown to inhibit 1-α hydroxylase, the enzyme responsible for the production of 1,25 dihydroxy vitamin D3, the active vitamin D [32]. Thus, lower Klotho levels in these hypomorphic mice induced vitamin D production and toxicity, and this toxicity was rescued by vitamin D restriction. So far, this mechanism of Klotho function has only been shown in the kidney. Nevertheless, the finding that lower levels of Klotho are toxic to organs other than the brain supports the idea that increasing Klotho levels would be beneficial.
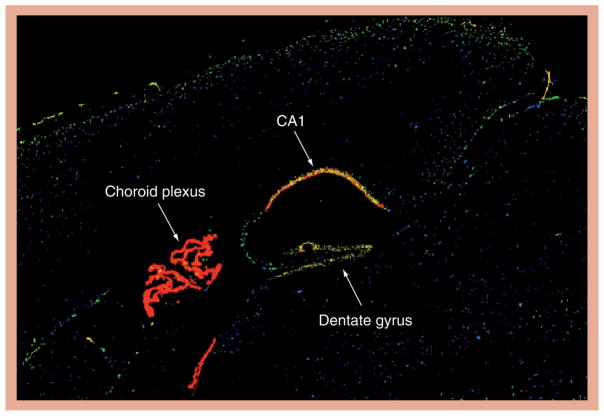
In the brain, Klotho is highly expressed in the choroid plexus and in neurons, especially in the hippocampus (Figure 1), as well as in the kidney and reproductive organs [14]. However, in contrast with the kidney, where the function of Klotho has been elucidated as shown in Table 1, in brain, the precise function and signaling pathways of Klotho are entirely unknown [15,25,33–36]. The neural Klotho may play a role in the aging phenotypes and protect against the development of age-related neurodegenerative diseases. In humans, the Klotho-VS variant is associated with reduced longevity and reasoning abilities, and mice lacking Klotho develop cognitive deficits [37–40]. We have previously reported that Klotho is downregulated in the brain white matter of normally aging rhesus monkeys [41]. This decrease in Klotho [41] correlated with age-related myelin loss and damage observed in the monkey brains [4–7,42] and was also seen in brains of aged rats and mice. Interestingly, brains from an AD mouse model exhibited Klotho expression that was even lower than that of their age-matched littermates. At the ultrastructural level, Klotho-knockout mice have significantly decreased myelination of the optic nerve and corpus callosum and, biochemically, brains of knockout mice express lower levels of myelin mRNAs and proteins [Chen et al. The anti-aging protein klotho enhances oligodendrocyte maturation and myelination of the central nervous system (2012), Submitted Manuscript]. We reported that one of the reasons for the age-dependent downregulation of Klotho is the increased methylation of its GC rich promoter [43]. Interestingly, the augmented methylation when compared with young animals was observed in the white matter of aged rhesus monkeys, but not in their gray matter.
Figure 1. Sagittal view of Klotho mRNA distribution in mouse brain demonstrated by in situ hybridization.
Note that the highest expression is in the choroid plexus, followed by hippocampal neurons, especially those in CA1. Reproduced from [102].
We have recently shown that in cell culture, Klotho enhances the maturation of OPCs into mature oligodendrocytes [Chen et al. The antiaging protein klotho enhances oligodendrocyte maturation and myelination of the central nervous system (2012), Submitted Manuscript]. Neuropathologically, Klotho-deficient mice display characteristics of neuronal degeneration in the cortex [14,44] and hippocampus [44]. Thus, it appears that the absence of only one gene has a major impact on the health and function of neurons and oligodendrocytes. Furthermore, increases in Klotho may rescue the phenotypes observed in the aging brain and in neurodegenerative diseases. Therefore, given the age-dependent decline in Klotho expression, combined with its multiple age-related deficiencies, has prompted us to explore therapeutic strategies that would augment Klotho expression in the brain for the treatment of demyelinating neurodegenerative diseases, such as MS and AD. When identified, Klotho-enhancing compounds could also alleviate other severe disorders where Klotho is downregulated, including all cancers tested to date by expression profiling (see [101] for details) and chronic kidney disease [23].
Strategies for modulating Klotho levels
Several potential strategies can be envisioned to increase the brain’s level of Klotho. First is direct delivery of exogenous Klotho. However, the introduction of this 130 kDa protein into the brain is prohibitive due to its molecular weight and its inability to cross the blood–brain barrier. Alternatively, identification of small orally bioavailable molecules that can cross the blood–brain barrier and that increase Klotho levels would be preferable. These molecules could affect Klotho levels or Klotho-dependent signaling pathways via several possible mechanisms. For example, molecules could act as Klotho mimetics by binding and activating, as yet unknown, receptors, or they could increase Klotho DNA transcription or protein translation or decrease Klotho degradation. With the lack of knowledge with regard to Klotho signaling pathways finding mimetics is not currently a viable strategy. However, the remaining strategies are all reasonable options that could provide useful probes for further understanding Klotho’s role in both normal physiology and in various neurodegenerative conditions.
Recently, we have initiated a program to identify small molecules that would increase Klotho at the transcriptional level. These efforts included devising a high-throughput screen (HTS) to identify compounds that increase Klotho protein expression. This assay was then utilized to screen over 150,000 compounds. The resulting compounds will be useful in future studies to determine whether Klotho-enhancing molecules could alleviate neurodegenerative changes that occur in aging and disease, specifically in MS and AD animal models. Although the molecular mechanisms by which Klotho enhancement may alleviate the MS or AD course of disease could be different, the resulting increase in Klotho is expected to be beneficial in both conditions.
Assay for HTS
In order to identify small molecules that increase Klotho protein expression, we cloned the 1.8 kb of DNA immediately upstream of the Klotho translation start site into the pGL3 luciferase reporter vector. Stable cell lines were then generated by co-transfection of the Klotho reporter with pcDNA3.1 into HEK-293 cells. The assay was miniaturized to a 384-well format and an initial compound, N-phenyl-1H-indole-2-carboxamide, was identified in a validation set of compounds as being able to elevate luciferase expression by ~30% and served as a positive control for screening. The remaining laboratory for drug discovery in neurodegeneration (LDDN) library of ~150,000 compounds (assembled from various commercial and noncommercial sources and enriched for compounds with predicted brain permeability based on low-molecular-weight, cLogP, and relatively low numbers of hydrogen bond donors and acceptors) were tested at a concentration of 1 μM for the ability to increase luciferase expression as a marker of Klotho promoter activation. After the screen, hits were defined as any compound causing at least a 30% increase in luciferase expression. A total of 414 compounds or 0.27% of all compounds met this threshold of activation. All 414 compounds were retested at five concentrations (2–0.02 μM) to validate the molecule as a hit and to obtain an initial indication of dose-dependent response. Two hundred compounds were selected for further study and prioritized based on potency, dose response and chemical structure (i.e., lack of reactive functional groups, molecular weight <500, cLogP <5 and synthetic feasibility).
Compounds that had been identified in other luciferase-based HTS campaigns conducted at the laboratory for drug discovery in neurodegeneration were removed from further consideration to concentrate on only those that affect Klotho expression as opposed to being nondiscriminant enhancers of gene transcription. Of the 50 compounds prioritized for further characterization, 38 were available for reordering from their original source. Fresh stocks were utilized to ensure the initial hit was not a side product of freeze–thaw degradation or some other contaminating byproduct specific to an individual lot. Fresh stocks were applied to the HTS platform at 12 concentrations (30–0.0001 μM). Luciferase expression and cell cytotoxicity assays were performed in parallel to examine both activation and toxicity. None of the compounds tested showed evidence of toxicity at any of the concentrations examined. Thirty of 38 compounds tested in 12-point dose–response assays revealed dose-dependent profiles. These were further prioritized to ten compounds based on dose–response and compound structure for additional validation with qRT-PCR. All of these compounds range in molecular weight from 288–410, have a cLogP value <5 (except one, which is 5.6) and lack overtly reactive functional groups. The majority of these compounds are quite tractable as lead compounds for optimization. However, based on the initial biological findings (i.e., qRT-PCR and western blot analyses) two structurally unrelated hits, compounds 21 and 25, have been selected for initial optimization studies and eight unselected compounds represent possible back-up molecules [45]. All of these compounds could eventually provide useful probes for elucidating mechanisms related to Klotho transcriptional control.
Validation of compounds in cell culture
Since the HTS was performed on a promoter luciferase construct, it is crucial to prove that Klotho protein expression and function are also increased. For protein expression opossum kidney cells or choroid plexus cells were used because these cells have detectable levels of Klotho and they represent the organs (i.e., kidney and brain, respectively) that express Klotho endogenously. In both these cell types, the two selected compounds enhanced Klotho protein expression as seen by western blot analysis. For the functional analysis of Klotho we have developed an assay in which we detect phosphorylated ERK in response to Klotho in cells transfected with the FGFR1c receptor and with the addition of FGF23. Klotho has been previously reported to be a co-receptor for FGFR1 and is needed for the high-affinity binding of FGF23 to the receptor [46]. Furthermore, to rule out the possibility that another protein was responsible for ERK phosphorylation, cells were treated with Klotho siRNA and the ERK phosphorylation was almost entirely abolished. Finally, to prove that these compounds work at the transcriptional level, Klotho mRNA was significantly increased, as assessed by qRT-PCR, following treatment with compounds [45]. In summary, our lead compounds identified in the HTS are inducing endogenous Klotho mRNA and protein expression, and this elevated Klotho is physiologically active.
Current efforts & future plans for compounds that enhance Klotho transcription
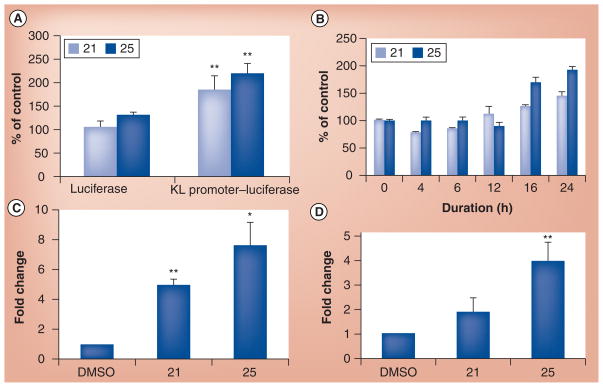
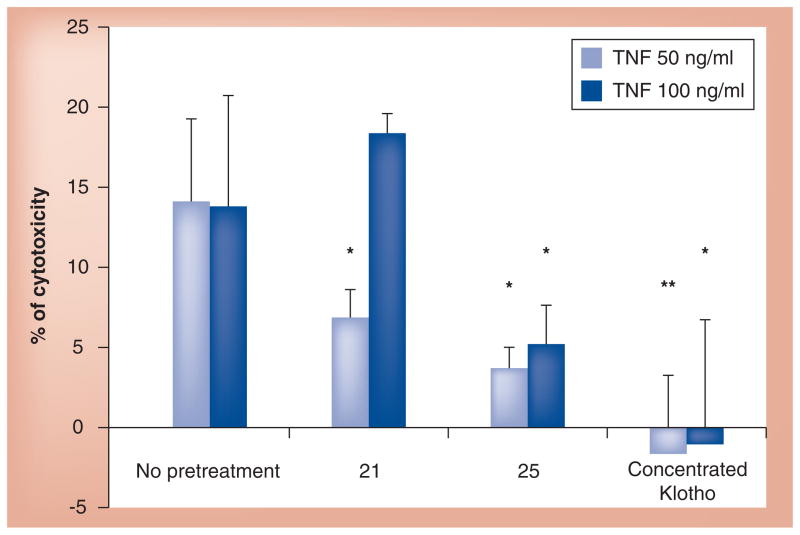
The Klotho promoter–luciferase assay was an artificial system used in the HTS to identify Klotho-enhancing compounds and, hence, a more physiologic assay to demonstrate the efficacy of the lead compounds was necessary, and since the last publication we have conducted some further analysis. These initial tests and data suggest promise, but the results are preliminary with full experimental data and methods to be verified and published at a later date. A short discussion has been provided below. We wished to determine whether we can take advantage of our recent finding that Klotho induces OPC maturation and test the compounds in an OPC maturation assay. However, we discovered that among the rat primary neural cell tested for Klotho expression by western blot, OPCs do not express detectable levels of Klotho, while astrocytes and neurons do. We then decided to use co-cultures of primary astrocytes and OPCs with the assumption that the compounds would increase Klotho expression in the astrocytes, which would then secrete Klotho. The shed astrocytic-derived Klotho would then bind to an, as of yet, unknown receptor on OPCs to induce their maturation. As a control for the experiment we used the recombinant shed form of Klotho that we previously showed is able to induce OPC maturation acting as a humoral factor. Compound 21 (identified and prioritized from the HTS assay) and recombinant Klotho induced the expression of myelin basic protein, a well-accepted marker of OPC maturation, while compound 25 only showed a trend towards this effect. To prove that these compounds acted via induction of Klotho mRNA expression, primary rat hippocampal neurons and astrocytes were individually treated with compounds 21 and 25 and Klotho mRNA expression was assessed by qRT-PCR. Figure 2 shows that both compounds enhanced Klotho promoter–luciferase activity. We used concentrated Klotho shed into the conditioned medium of HEK293 cells over-expressing the extracellular 980 amino acids of Klotho. This secreted form of Klotho was able to rescue from cell death co-cultures of oligodendrocytes and astrocytes treated with TNF-α at two different concentrations. Based on these results we examined whether compounds 21 and 25 could also rescue these glial cells from inflammatory stress induced by TNF-α. Indeed, both compounds significantly reduced cell death in this assay, as shown in Figure 3. With these results in hand, we are encouraged to investigate the anti-inflammatory potential of Klotho and Klotho-enhancing compounds on other neuroinflammatory paradigms.
Figure 2. Compounds 21 and 25 induce Klotho mRNA expression.
(A) The increase in luciferase signal is specific to KL promoter activation. (B) Time course of KL promoter activation in HEK293 cells stably transfected with a KL promoter-luciferase construct. q-PCR analysis of KL expression induced by the compounds in (C) primary hippocampal neurons and (D) in primary astrocytes.
KL: Klotho.
Figure 3. Concentrated endogenously secreted Klotho and compounds 21 and 25 rescue co-cultures of oligodendrocyte precursor cells and astrocytes from TNF-α-induced cytotoxicity.
Cells were pretreated with compounds for 24 h or with concentrated Klotho for 4 h and then exposed to 50 or 100 ng/ml of TNF-α for an additional 24 h. Cell cytotoxicity was assessed by CellTiter-Glo® (Promega).
Currently, SAR studies are ongoing for the two prioritized compounds. A large number of analogs for both series have been purchased and tested in the Klotho promoter-luciferase assay. Those with nanomolar EC50 are being tested for their efficacy to induce OPC maturation. Based on the preliminary SAR results and the results from the neural cell assays, novel Klotho-enhancing compounds are now being synthesized with the aims to increase potency, improve solubility and maximize brain permeability and pharmacokinetic properties. Future plans include testing analogs in animal models of AD and MS. In addition, these compounds will be useful for elucidating the mechanism of action for increasing Klotho levels via enhancing Klotho transcription.
Conclusion
The absence of a single gene in mice causes a plethora of age-related manifestations including osteoporosis, infertility, emphysema, arteriosclerosis, hair loss, cognitive impairment, synaptic loss and premature death at an average 60 days [14]. Interestingly, Klotho-deficient mice exhibit hallmarks of human aging, but develop normally until 40 days of age. Thus, it appears that Klotho is not needed during development but its deficiency in adulthood and late life leads to multiple organ failure and early death. Indeed, we reported that Klotho protein is downregulated in the aged brains of monkeys, rats and mice [41], and to an even higher extent in animal models of AD. In situ hybridization studies described in the Allen Atlas of the mouse brain [102] indicate that Klotho is highly expressed in the choroid plexus and in hippocampal neurons. Thus, the brain is bathed in cerebrospinal fluid that contains high concentrations of Klotho in addition to the local expression found in neurons. Mice lacking Klotho exhibit neurodegeneration of their hippocampal neurons [44] suggesting that in this region of the brain Klotho may act as a protective autocrine hormone, and its absence induces neuronal loss. Klotho shed by neurons or originating from the choroid plexus may also affect the differentiation of OPCs into mature, myelinating oligodendrocytes. Thus, based on our results, we posit that Klotho is a neuroprotective hormone/factor that is downregulated with age and compounds that could elevate its concentration in the brain would be beneficial in the treatment of demyelinating and neurodegenerative diseases. Although various strategies can be envisioned as means to increase Klotho levels in the brain, our efforts to date have been focused on the identification, optimization and biological characterization of small molecules that increase Klotho gene transcription. These molecules and potentially others that operate via alternate mechanisms will be valuable pharmacological probes of Klotho regulation in normal physiology and also for evaluation in animal models of demyelination such as MS, and in animal models of AD. Such compounds could also be studied in additional mouse models of neurodegenerative diseases, including PD, HD and ALS. We hope that Klotho, which was only discovered in 1997, will become a major player in the ongoing fight against neurodegenerative diseases.
Future perspective
Our ability to prevent or cure neurodegenerative diseases is very limited or inexistent at this time. However, these conditions share many common characteristics such as abnormally folded proteins, inflammation and selective neuronal death. Just like in the treatment of cardiovascular diseases, where antihypertensive and cholesterol-lowering drugs are prescribed, we may need a cocktail of drugs to treat age-related neurodegeneration. We hope that over the next 5–10 years Klotho-enhancing compounds will prove to be neuroprotective and will be one of the drugs used in concert with protein aggregation-lowering drugs and anti-inflammatory drugs to prevent and alleviate neurodegenerative diseases.
Executive summary.
There are a number of features of neurodegenerative diseases relevant to the role of Klotho, especially in Alzheimer’s disease and multiple sclerosis.
Strategies for modulating Klotho levels are discussed and assays for high-throughput screening commented on. Following this, the compounds were validated in the cell culture.
Future work includes further validation of compounds in physiologic cell-based assays and in animal models of neurodegenerative diseases.
Key Terms
- Alzheimer’s disease
Most common form of dementia in the elderly
- Multiple sclerosis
Inflammatory disease in which the fatty myelin sheaths around the axons of the brain and spinal cord are damaged, leading to demyelination and scarring as well as a broad spectrum of symptoms
- Myelin
Fatty sheath surrounding the axons, which is necessary for the rapid communication between nerve cells
- Inflammation
Protective attempt by the organism to remove injurious stimuli such as pathogens, damaged cells or irritants, and to initiate the healing process
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
This work was supported by a grant from the Alzheimer Drug Discovery Foundation and by NIH-NIA grant AG00001 to CR Abraham. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Selkoe D, Mandelkow E, Holtzman D. Deciphering Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(1):a011460. doi: 10.1101/cshperspect.a011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sloane JA, Hollander W, Moss MB, Rosene DL, Abraham CR. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol Aging. 1999;20(4):395–405. doi: 10.1016/s0197-4580(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 3.Sloane JA, Hollander W, Roseane DL, Moss MB, Kemper T, Abraham CR. Astrocytic hypertrophy and altered GFAP degradation with age in subcortical white matter of the rhesus monkey. Brain Res. 2000;862(1–2):1–10. doi: 10.1016/s0006-8993(00)02059-x. [DOI] [PubMed] [Google Scholar]
- 4.Hollander W, Jaffe R, Abraham CR. Activation of early components of complement targets myelin and oligodendrocytes in the aged rhesus monkey brain. Neurobiol Aging. 2006;27(4):633–644. doi: 10.1016/j.neurobiolaging.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Sloane JA, Hinman JD, Lubonia M, Hollander W, Abraham CR. Age-dependent myelin degeneration and proteolysis of oligodendrocyte proteins is associated with the activation of calpain-1 in the rhesus monkey. J Neurochem. 2003;84(1):157–168. doi: 10.1046/j.1471-4159.2003.01541.x. [DOI] [PubMed] [Google Scholar]
- 6.Hinman JD, Duce JA, Siman RA, Hollander W, Abraham CR. Activation of calpain-1 in myelin and microglia in the white matter of the aged rhesus monkey. J Neurochem. 2004;89(2):430–441. doi: 10.1046/j.1471-4159.2004.02348.x. [DOI] [PubMed] [Google Scholar]
- 7.Hinman JD, Peters A, Cabral H, et al. Age-related molecular reorganization at the node of Ranvier. J Comp Neurol. 2006;495(4):351–362. doi: 10.1002/cne.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442(3):277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- 9.Franklin R. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3(9):705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 10.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346(3):165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 11.Wolswijk G. Oligodendrocyte precursor cells in chronic multiple sclerosis lesions. Mult Scler. 1997;3(2):168–169. doi: 10.1177/135245859700300221. [DOI] [PubMed] [Google Scholar]
- 12.Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18(2):601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mi S, Miller RH, Tang W, et al. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65(3):304–315. doi: 10.1002/ana.21581. [DOI] [PubMed] [Google Scholar]
- 14▪▪.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. The discovery of Klotho. [DOI] [PubMed] [Google Scholar]
- 15.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Banerjee S, Dey N, et al. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60(7):1907–1916. doi: 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011;13(3):254–262. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 18.Wolf I, Levanon-Cohen S, Bose S, et al. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27(56):7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 19.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA. 2007;104(50):19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565(1–3):143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317(5839):803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 22.Kuro-o M. Klotho. Pflugers Arch Eur J Physiol. 2010;459(2):333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 23.Kuro-o M. Klotho and the aging process. Korean J Int Med. 2011;26(2):113–122. doi: 10.3904/kjim.2011.26.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang Q, Hoefs S, van der Kemp AW, et al. The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310(5747):490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 25.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro-O M, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA. 2008;105(28):9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurston RD, Larmonier CB, Majewski PM, et al. Tumor necrosis factor and interferon-γ down-regulate Klotho in mice with colitis. Gastroenterology. 2010;138(4):1384–1394. 1394 e1–e2. doi: 10.1053/j.gastro.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witkowski JM, Soroczyńska-Cybula M, Bryl E, Smoleńka Z, JóŸwik A. Klotho – a common link in physiological and rheumatoid arthritis-related aging of human CD4+ lymphocytes. J Immunol. 2007;178(2):771–777. doi: 10.4049/jimmunol.178.2.771. [DOI] [PubMed] [Google Scholar]
- 28.Maekawa Y, Ishikawa K, Yasuda O, et al. Klotho suppresses TNF-α-induced expression of adhesion molecules in the endothelium and attenuates NF-κB activation. Endocrine. 2009;35(3):341–346. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 29.Moreno JA, Izquierdo MC, Sanchez-Niño MD, et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol. 2011;22(7):1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117(9):2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosakai A, Ito D, Nihei Y, et al. Degeneration of mesencephalic dopaminergic neurons in klotho mouse related to vitamin D exposure. Brain Res. 2011;1382:109–117. doi: 10.1016/j.brainres.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida T, Fujimori T, Nabeshima Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1α-hydroxylase gene. Endocrinology. 2002;143(2):683–689. doi: 10.1210/endo.143.2.8657. [DOI] [PubMed] [Google Scholar]
- 33.Cha SK, Hu MC, Kurosu H, et al. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol. 2009;76(1):38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24(9):3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22(1):124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doi S, Zou Y, Togao O, et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286(10):8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arking DE, Becker DM, Yanek LR, et al. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72(5):1154–1161. doi: 10.1086/375035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deary IJ, Harris SE, Fox HC, et al. KLOTHO genotype and cognitive ability in childhood and old age in the same individuals. Neurosci Lett. 2005;378(1):22–27. doi: 10.1016/j.neulet.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Nagai T, Yamada K, Kim HC, et al. Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J. 2003;17(1):50–52. doi: 10.1096/fj.02-0448fje. [DOI] [PubMed] [Google Scholar]
- 40.Houlihan LM, Harris SE, Luciano M, et al. Replication study of candidate genes for cognitive abilities: the Lothian Birth Cohort 1936. Genes Brain Behav. 2009;8(2):238–247. doi: 10.1111/j.1601-183X.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- 41▪▪.Duce JA, Podvin S, Hollander W, Kipling D, Rosene DL, Abraham CR. Gene profile analysis implicates Klotho as an important contributor to aging changes in brain white matter of the rhesus monkey. Glia. 2008;56(1):106–117. doi: 10.1002/glia.20593. The discovery that Klotho is downregulated in the aged brain. [DOI] [PubMed] [Google Scholar]
- 42▪.Hinman JD, Abraham CR. What’s behind the decline? The role of white matter in brain aging. Neurochem Res. 2007;32(12):2023–2031. doi: 10.1007/s11064-007-9341-x. A comprehensive review of age-related changes that occur in the white matter. [DOI] [PubMed] [Google Scholar]
- 43.King GD, Rosene DL, Abraham CR. Promoter methylation and age-related downregulation of Klotho in rhesus monkey. Age. 2011 doi: 10.1007/s11357-011-9315-4. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiozaki M, Yoshimura K, Shibata M, et al. Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience. 2008;152(4):924–941. doi: 10.1016/j.neuroscience.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 45▪▪.King GD, Chen C, Huang MM, et al. Identification of novel small molecules that elevate Klotho expression. Biochem J. 2012;441(1):453–461. doi: 10.1042/BJ20101909. The identifcation of Klotho enhancing compounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
Websites
- 101.Oncomine.oncomine.org
- 102.Allen Institute for Brain Science. Allen brain atlas. www.brain-map.org.