Abstract
N-Methyl-D-aspartate receptors (NMDARs) are important for synaptic refinement during development. In CxNR1KO mice, cortical excitatory neurons lack NR1, the essential subunit of the NMDAR, and in their primary somatosensory (S1) cortex whisker-specific cellular patterns, “barrels,” are absent. Despite this cytoarchitectural defect, thalamocortical axons (TCAs) representing the mystacial vibrissae form topographically organized patterns and undergo critical period plasticity. This region-specific knockout mouse model allows for dissection of the mechanisms underlying patterning of the pre- and postsynaptic neural elements in the S1 cortex. In the absence of functional NMDARs, layer IV cell numbers are unaltered, but these cells fail to segregate into barrels. Furthermore, the dendritic fields of spiny stellate cells do not orient toward TCA terminal patches as in normal mice. Instead, they radiate in all directions covering larger territories, exhibiting profuse branching with increased spine density. Comparison of TCA patches with serotonin transporter (5-HTT) immunohistochemistry or Dil labeling also indicates that in the CxNR1KO cortex TCAs form smaller patches and individual axon terminal branching is not as well developed as in control cortex. Our results suggest that postsynaptic NMDAR activation is critical in communicating periphery-related sensory patterns from TCAs to barrel cells. When postsynaptic NMDAR function is disrupted, layer IV spiny stellate cells remain imperceptive to patterning of their presynaptic inputs and elaborate exuberant dendritic specializations.
INTRODUCTION
Neurons in different regions of the nervous system are often characterized, and classified according to the morphological features of their dendrites. These arboreal extensions from the soma and their particular patterning, branching, and presence or absence of dendritic spines reflect the functional characteristics and developmental history of each class of neuron. For many years it has been documented that elaboration of dendritic trees of many classes of neurons depends on their afferent inputs. A classical example of this is the “weeping willow” appearance of Purkinje cell dendrites in the cerebellar cortex, deprived of parallel fiber inputs (Altman and Anderson, 1972, 1973). Since then, numerous other examples of the influence of afferent inputs on dendritic modeling have been reported in a variety of species and neuronal regions (Globus et al., 1973; Rakic and Sidman, 1973; Caviness and Rakic, 1978; Harris and Woolsey, 1979; Steffen and Van der Loos, 1980; Purves, and Hume, 1981; Berry, 1982, Katz and Constantine-Paton, 1988; Nedivi et al., 1998; McAllister, 2000, 2002). More recent studies on dendritic differentiation have focused on a variety of molecules, rather than the afferent inputs, that might play a role in sculpting dendritic arbors as they are elaborated. These molecules range from neurotrophins such as brain-derived neurotrophic factor (BDNF) to Semaphorins, Netrins, Ena, Notch, BMPs, and Slits (McAllister et al., 1995, 1996, 1997, 2000; Gao et al., 1999; Polleux et al., 2000; Redmond and Ghosh, 2001; Whitford et al., 2002; see Scott and Luo, 2001, for a recent review). Intracellular signaling molecules such as Rho GTPases also play major roles in dendritic differentiation and stabilization of select sets of neurons (Luo et al., 1996; Threadgill et al., 1997; Gao et al., 1999; Lee et al., 2000; Li et al., 2000, 2002; Nakayama et al., 2000; Wong et al., 2000). While new and important information is emerging on the role of a growing number of molecular cues that direct dendritic differentiation of neurons, the classical question remains: How and to what extent does neural activity shape dendritic development, and in particular how do presynaptic inputs regulate and transfer specific patterns to postsynaptic cell dendrites?
A marvelous example of morphological pattern communication between the pre- and postsynaptic elements and regulation of dendritic orientation by afferent inputs is the rodent whisker-barrel pathway. The patterned array of mystacial vibrissae (whiskers) and sinus hairs on the snout is replicated by the patchy distribution of afferents and modular organization of their postsynaptic partners along the trigeminal pathway leading to the S1 cortex. These patterns are first established in the brainstem somatosensory nuclei (where they are called “barrelettes”), then in the ventroposteromedial (VPM) nucleus of the thalamus (where they are called “barreloids”), and finally in the S1 cortex (where they are called “barrels”) (Woolsey and Van der Loos, 1970; Van der Loos, 1976; Ma and Woolsey, 1984) The instructive role of the sensory periphery and the critical period for these central neural patterns have been demonstrated by peripheral lesion studies performed in perinatal rodents, or in mice selectively bred for aberrant numbers of whiskers (Van der Loos and Woolsey, 1973; Welker and Van der Loos, 1986; Woolsey, 1990; O’Leary et al., 1994; Killackey et al., 1995; Erzurumlu and Kind, 2001).
In the barrel cortex, TCAs are the first elements to develop whisker-specific, patchy terminal arbors (Erzurumlu and Jhaveri, 1990; Jhaveri et al., 1991; Blue et al., 1991). Once this pattern is conveyed to layer IV, spiny stellate (granule) cells orient their dendrites toward TCA patches and embrace them, forming cell-dense barrel walls, and dendrite- and TCA terminal-dense barrel centers or “hollows” (Woolsey and Van der Loos, 1970; Pasternak and Woolsey, 1975; Harris and Woolsey, 1979; Steffen and Van der Loos, 1980). How do layer IV cells detect the segregation of their presynaptic inputs from the VPM, and preferentially orient their dendritic arbors? Mechanisms underlying this seemingly simple communication between the pre-and postsynaptic elements that lead to an exquisitely patterned and physiologically precise cortical map (Petersen and Sakmann, 2001) have been an enduring mystery. Neural activity-mediated processes have been the prime candidates.
To elucidate the role of neural activity in patterning of the mouse barrel cortex, in particular, that of activity mediated via the NMDARs, we used the Cre/loxP system and generated mice (CxNR1KO mice) in which the gene for NR1, the essential NMDAR subunit, is selectively deleted in cortical excitatory neurons (Iwasato et al., 2000). In these mice, all subcortical neurons have normal NMDAR function, and whisker-specific patterns in the brainstem trigeminal nuclei and in the VPM develop normally. In the barrel cortex, TCAs do not show any layer- or region-specific targeting defects, but form smaller, less-distinct terminal patches only in the regions corresponding to the representation of larger whiskers. In contrast, stellate cells never develop barrels even in portions of the layer IV where rudimentary patterning of the TCAs is observed. These findings clearly indicate that postsynaptic NMDARs are one of the key players in transfer of the pattern information from the presynaptic inputs to the postsynaptic elements.
We further examined the cellular and dendritic differentiation of the barrel cortex in CxNR1KO mice. We show that layer IV spiny stellate cells fail to move about and develop cell-dense barrel walls around TCA patches. On the other hand, they develop extensive dendritic trees remaining impervious to patterning of TCAs, and are studded with higher numbers of spines. Thus, CxNR1KO mouse barrel cortex provides a unique model to examine how presynaptic inputs can influence dendritic differentiation of postsynaptic neurons and paves the way to unveiling of intracellular signaling mechanisms downstream from NMDARs in sculpting dendritic arbors and their patterns.
RESULTS
TCA Patterning in Control and CxNR1KO Mice
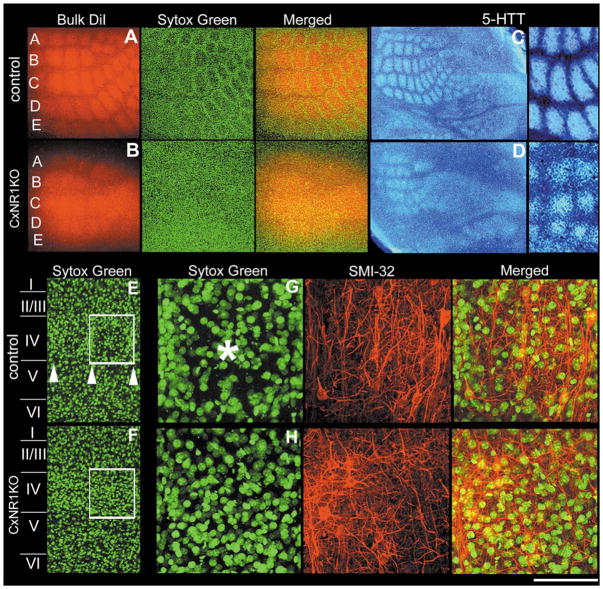
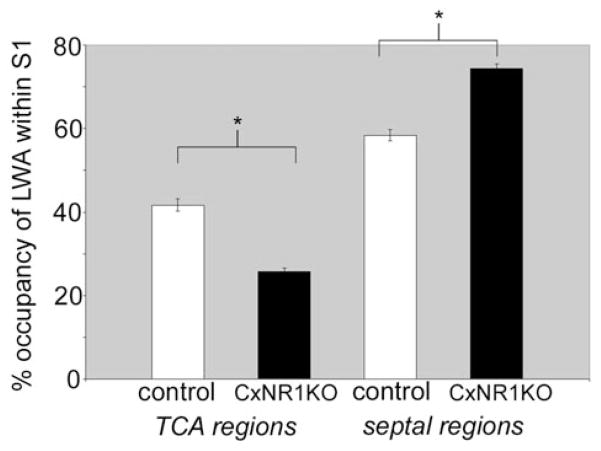
In layer IV of the normal mouse barrel cortex, TCA terminals form discrete patches that replicate the patterned organization of the whiskers on the contralateral snout (Figs. 1A, C), and each patch corresponds to and transmits information from a single whisker (Petersen and Sakmann, 2001). Stellate cells of layer IV aggregate around the TCA terminal patches and orient their dendritic fields toward the foci of TCA terminals. In the CxNR1KO barrel cortex, smaller and less-defined TCA patches can be detected (Figs. 1B, D), but no cellular patterning of the stellate cells is visible with routine stains for cyto- or nucleoarchitecture (Fig. 1B, Sytox green). Comparison of a normal barrel field area between the control (Figs. 1E, G) and CxNR1KO (Figs. 1F, H) cortex revealed a neuropil rich with dendritic and axonal processes but a nonuniform distribution of cells in control cases, and uniform distribution of cells in CxNR1KO cases. Similar observations were also made when other markers for TCAs such as serotonin transporter (5-HTT) antibody (Figs. 1C, D) or Nissl stains for cells were used (Fig. 4). 5-HTT is transiently expressed by developing thalamic sensory nuclei and their axonal terminals in the neocortex of rodents; thus, immunohistochemistry for 5-HTT specifically reveals the distribution and patterning of the TCAs in the barrel cortex (Lebrand et al., 1996, 1998; Cases et al., 1998; Hansson et al., 1998a,b; Zhou et al., 2000; Persico et al., 2001). Areal measurements from 5-HTT-immunostained control and CxNR1KO cortices revealed that TCA patches were significantly reduced in the CxNR1KO mice, and septal areas between TCA patches enlarged (Fig. 2).
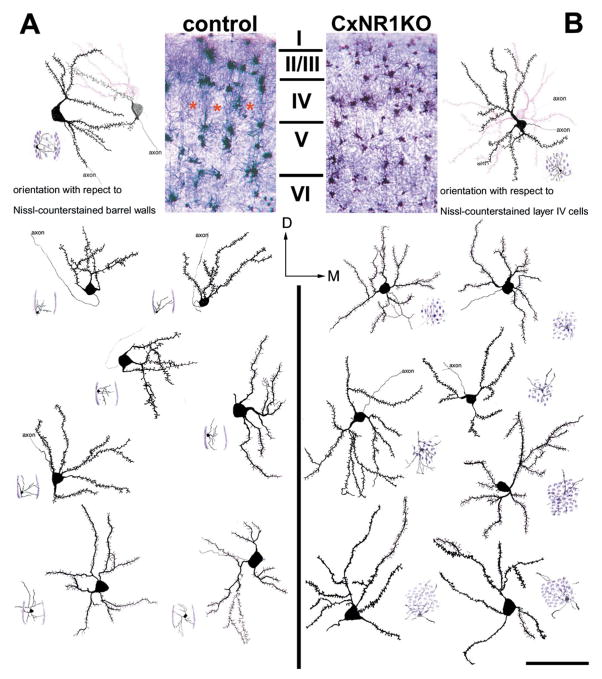
FIG. 1.
Layer IV cells in CxNR1KO S1 fail to organize into barrels while TCAs form rudimentary patterns. Series of micrographs of the same section and field in A illustrate the patterning of TCAs (red) and barrel cell nuclei (green) in the tangential plane in control mice. Similar series of photomicrographs in B illustrate the barrel cortex in the CxNR1KO mice in the same plane. C and D show inverted photomicrographs of TCA patterns visualized by anti-5-HTT immunohistochemistry in the tangential plane of the barrel cortex of control and CxNR1KO mice, respectively. Higher power images to the right show 5-HTT-immunopositive TCA regions versus the septal regions (quantified in Fig. 2). E and F show low-power views of layer IV and a single barrel in control (E) and uniform nucleoarchitecture in CxNR1KO (F) cortices. Series of micrographs in G and H are high-power views of a single barrel region depicted in the boxed regions in E and F, respectively. These series show the same region with different filters and merged images after staining with Sytox green for nuclei and SMI-32 for neuropil staining. Arrowheads in E mark the lower boundary of layer IV barrels and asterisk in G marks the center of a single barrel hollow which is absent in the CxNR1KO S1 cortex (H). Scale bar A, B = 400 μm; C, D = 750 μm; high power of C, D, and E, F = 150 μm; G, H = 50 μm.
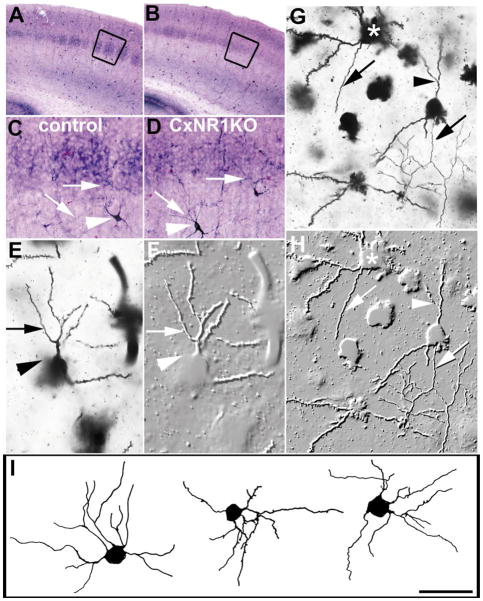
FIG. 4.
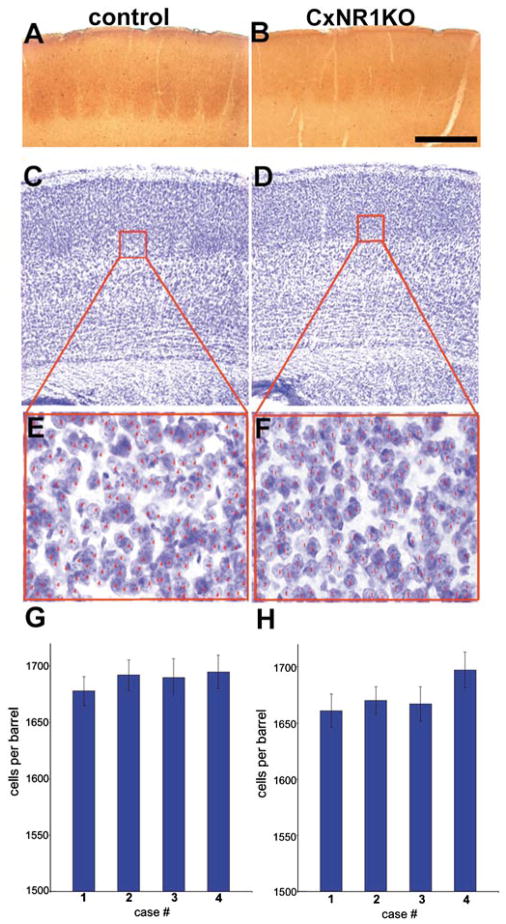
Normal cortical lamination and cell numbers in the CxNR1KO S1 cortex despite absence of barrel organization. A and B illustrate comparable barrel cortex regions in the coronal plane, stained for cytochrome oxidase histochemistry. Adjacent sections stained for Nissl (cresyl violet) illustrate distinct barrels in control and no barrels in the CxNR1KO cortex (C, D). The boxed regions were then viewed under higher magnification for cell counts (E, F). Histograms G and H illustrate the cell numbers per barrel for 4 control P21 and 4 CxNR1KO P21 mice. From each mouse, 7–8 consecutive sections were used to count cells in a barrel size area. Small red numbers on individual cells were placed using computer-aided microscopy to ensure that cells are not counted more than once, as described under Experimental Methods. Scale bar A, B = 300 μm; C, D = 350 μm; E, F = 45 μm; G, H error bars = SEM.
FIG. 2.
Reduced size of 5-HTT-immunopositive TCA patches and increased septal areas in the CxNR1KO S1 cortex. Graph illustrates the relative percentage of the large whisker representation area (LWA) in the S1 cortex occupied by 5-HTT-immunopositive TCAs and by septal regions for both control and CxNR1KO cases. n = 10 for each genotype. Error bars = SEM. Significance by t test for percentage area of TCA regions, P < 0.00002; and for percentage area of septal regions, P < 0.004.
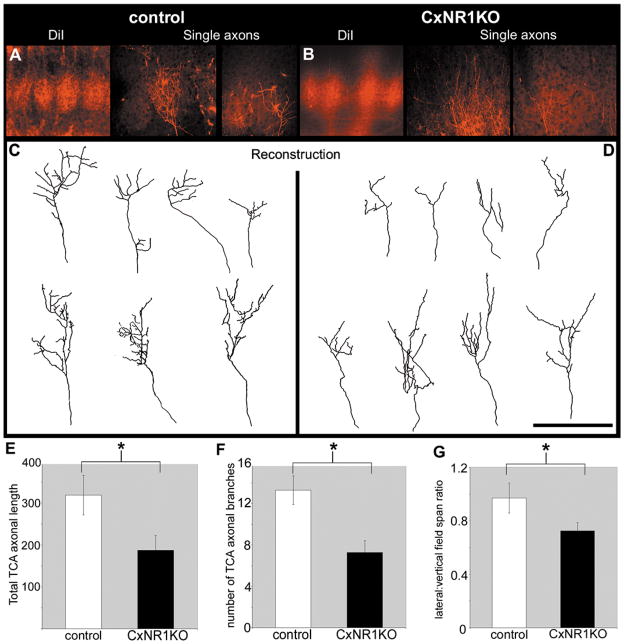
In a separate series of experiments, we recovered and reconstructed a few Dil-labeled single axons (Figs. 3A–D). While our n in these experiments is low, presently available data indicate that in the CxNR1KO cortex, individual TCAs do not develop well-defined terminal patches as in normal barrel cortex (Figs. 3A–D). In the mutant S1 cortex, total axonal length, number of branches, and lateral:vertical field span ratio of TCAs are noticeably decreased (Figs. 3E–G). Ongoing studies are aimed at collecting a much larger sample of single TCAs from different loci within the face map area in control and mutant cortices and analyzing them in depth.
FIG. 3.
Poorly defined single TCAs with reduced branching and decreased total axonal arbor length in the CxNR1KO S1 cortex. Focal Dil injections into the ventroposteromedial (VPM) nucleus reveal details of single axonal arbors in the S1 cortex of control (A) and CxNR1KO (B) P5 cases. Reconstructions of these single axons for control (C) and CxNR1KO (D) cases reveal morphological differences in the axonal arbor length and branching and lateral field spans. Quantification of total axonal arbor length of TCAs shows a significant reduction in the CxNR1KO compared to controls, P < 0.02 (E). The number of TCA branches are significantly reduced by almost 50% in the CxNR1KO compared to controls (F), P < 0.003. The ratios of lateral:vertical extent of individual TCA arbors are also significantly reduced in the CxNR1KO (G), P < 0.04, n = 7 for control, and n = 8 for CxNR1KO. Scale bar A, B = 300 μm; C, D = 100 μm. Error bars = SEM.
Defects in Cellular Organization of the Barrel Cortex in CxNR1KO Mice
The absence of a patterned organization of layer IV cells and extracellular matrix molecules that normally delineate barrel boundaries was noted consistently in the CxNR1KO mice from early developmental stages to several weeks postnatal (Iwasato et al., 2000). An earlier study, using mice with complete knockout of the NR1, and cell birthdating techniques showed that this mutation does not affect migration of neocortical neurons (Messersmith et al., 1997). To verify that the postsynaptic, cortical pattern defects in the CxNR1KO mice are not due to altered cell numbers, we performed nonbiased cell counts in the layer IV barrel fields and found no statistically significant difference between cell counts in control vs CxNR1KO cortex (Fig. 4) as determined by Student’s t test. We selected barrel size regions (150μm2) in serial Nissl stained sections or a 400 × 125 μm window within the layer IV of S1 cortex of P21 mice, and compared the cell numbers between control and CxNR1KO cases. The mean number of cells per barrel-size area was 1688.75 ± sem 6.67 for the control and 1673.75 ± sem 6.90 for the CxNR1KO cortex, and the density was 2.68 × 10−4/μm3, and 2.66 × 10−4/μm3 respectively. These numbers are close to those reported for the C1 barrel of adult mice (Pasternak and Woolsey, 1975). In a 400 × 125μm area within the S1 layer IV of two control and two CxNR1KO P21 cortices (5 sections from each cortex), again there were no significant differences in the mean number of cells or their density. Gross organization and lamination in the cortex of CxNR1KO mice were largely normal even though the layer IV cells failed to organize into barrel-wall barrel-hollow cytochrome oxidase activity or cyto-architectural patterns (Figs. 4A–F). Although we could not distinguish between neuronal and glial cell numbers, the relative numbers of these cells may be altered as well as their distribution; there is evidence of altered glycoconjugate boundaries and extracellular matrix (ECM) proteins such as tenascin-C in these CxNR1KO mice (Iwasato et al., 2000). Furthermore, ECM proteins as well as a number of cell-surface proteins have been known to interact with adhesion molecules (N-CAM in particular; see Edelman and Jones, 1995) to influence dendritic growth and neurite extension in vitro (Lafont et al., 1994).
Altered Dendritic Differentiation of Granule Cells in the Barrel Cortex of CxNR1KO Mice
The dendritic specialization and orientation of mouse barrel cortex layer IV cells have been previously documented using Golgi stains (Pasternak and Woolsey, 1975; Harris and Woolsey, 1979; Steffen and Van der Loos, 1980; Greenough and Chang, 1988). In both the tangential and coronal planes, spiny stellate and aspiny stellate classes of granule cells orient their dendrites toward barrel hollows, as their cell bodies accumulate along the barrel walls. However, the vast majority of these cells are spiny stellate cells (Class I cells described by Harris and Woolsey, 1979, 1983). We employed the same method to examine dendritic differentiation of stellate cells in the barrel cortex where all excitatory (including spiny stellate) cells lack functional NMDARs. Since CxNR1KO mouse barrel cortex lacks, cellular patterning, it was difficult to determine with certainty the level and the boundaries of the barrelfield region in tangential sections. Thus, we chose to examine these cells in sections cut in the coronal plane (Figs. 5A, B). In the coronal plane, it is easy to identify different cortical layers and match the levels of sections between control and knockout conditions. While this approach allowed us to examine dendritic fields and specialization of stellate cells localized to layer IV, our analysis was limited to the mediolateral extent of the dendritic fields and only 120-μm-thick rostrocaudal extent of their dendritic fields (Fig. 6). We were able to identify both spiny and aspiny stellate cells, but aspiny cells were very few in layer IV. Aspiny stellate cells are GABAergic interneurons, and a subset of them also stains for NADPH-diaphorase (Mitrovic and Schachner, 1996; Yan et al., 1996). In both control and CxNR1KO mouse cortex NADPH-positive neurons were mostly located in the infra and supra-granular layers while their processes entered the barrel fields (see Figs. 7A–D). Relatively few Golgi-impregnated aspiny stellate cells were encountered in the barrel field region of both control and mutant mice (Figs. 7E–I). With the low numbers of these cells we could not distinguish or characterize any differences between the two conditions.
FIG. 5.
Layer IV spiny stellate cells in CxNR1KO fail to exhibit orientation bias. Low-power photomicrographs of Golgi and Nissi-stained coronal sections illustrate the cortical layers and the barrel field in control (A) and CxNR1KO (B) mice. In each panel multiple examples of camera lucida drawings of spiny stellate cells are shown. Small cartoon diagrams located in the bottom corner of each cell depict the location of the cell with respect to a single barrel in the control cases (A) and within a uniform distribution of Layer IV cells in the CxNR1KO cortex. Note that all of the cells located in the barrel walls of the control cases have biased dendritic tree orientations, while none of the cells charted in the CxNR1KO cortex show this bias. Scale bar A, B = 250 μm; inset = 80 μm.
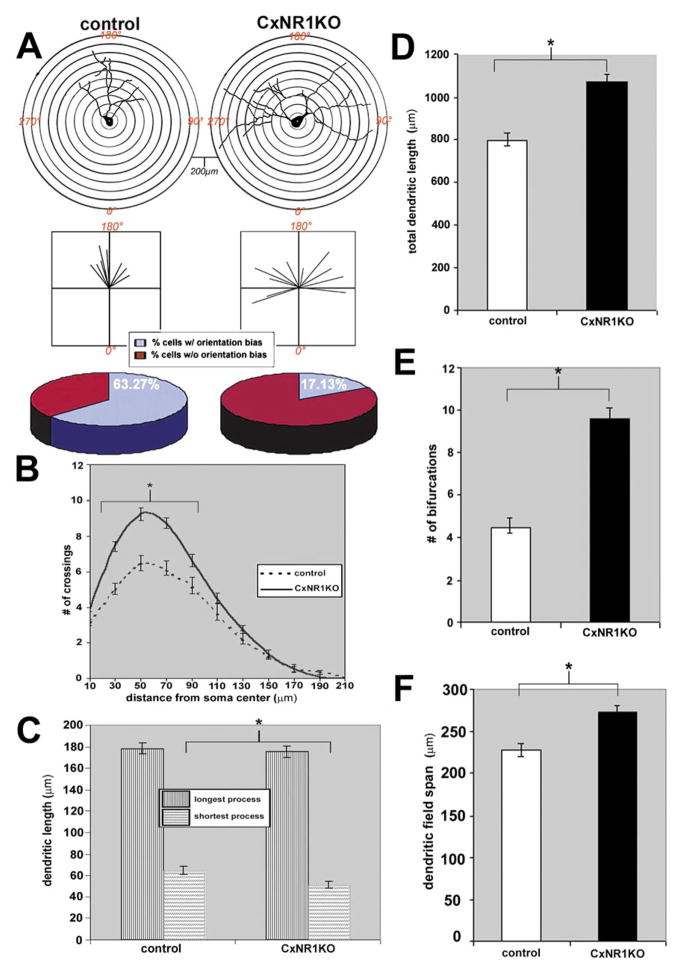
FIG. 6.
Quantification of dendritic parameters of spiny stellate cells in the CxNR1KO and control S1 cortex. (A) Top panel illustrates the Scholl ring analysis used to determine orientation bias of charted layer IV cells and their dendritic arbors; below are vector plots of the same cells and the pie charts indicate the relative percentage of cells scored as having an orientation bias in both control and CxNR1KO mice. B–F are graphs comparing control and CxNR1KO dendritic parameters; B illustrates the number of crossings as a function of distance from the soma P < 0.0019 for soma distances between 30 and 100 μm. (C) The mean lengths for the longest and shortest dendritic process P < 0.007 significant for the shortest process only; D compares the total dendritic length P < 0.001; E the number of bifurcations P < 0.00001; and F, the dendritic field span P < 0.0009. Error bars = SEM.
FIG. 7.
Aspiny layer IV cells in the CxNR1KO and control S1 cortex. Low-power photomicrographs of NADPH-stained coronal sections show distinct patterns in layer IV of the control barrel cortex (A) and less distinct patterns in the CxNR1KO cortex (B). Higher magnification of the boxed areas in micrographs A and B show a few aspiny stellate cells positive for NADPH (C, D). These cells (arrowheads) are often seen below or above layer IV with their axonal processes (arrows) extending into layer IV. (E, G) show two Golgi-stained aspiny stellate cells and photomontage-embossed images (F, H) from the layer IV of P33 control and CxNR1KO cortex, respectively. Arrowheads in E and F point to the cell body and the arrows to the same aspiny dendritic branch. In G and H, arrows point out to the same axonal process while arrowheads point to the aspiny dendritic branch. Note the spiny stellate cell (asterisk) just above and to the left of the aspiny cell. I illustrates camera lucida drawings of aspiny stellate cells from a P33 CxNR1KO barrel cortex. A, B = 600 μm; C, D = 150 μm; E, F = 30 μm; G, H = 40 μm; and I = 60 μm.
We chose to focus our study largely on the spiny stellate (Class I) cells (Harris and Woolsey, 1979, 1983) of layer IV since these are known to have specific orientation preferences toward TCA terminal patches with their somata forming cell-dense barrel walls. These cells were visualized under high magnification using a Leica DMRXA with Nomarski optics (see Figs. 8A–F) and several cases were reconstructed using camera lucida with 100x oil immersion (Fig. 5). Care was taken to identify cells that are located at mid levels of each section with their dendritic trees mostly present within the depth of the section. A total of 58 cells from 5 different cases of each genotype at age P16 were examined. Scholl ring analysis revealed striking differences in the orientation of layer IV spiny stellate cells between each genotype. In control cases 63% of the cells examined had asymmetric dendritic trees with pronounced orientation bias particularly in the 0° medial or 180° lateral axes toward TCA foci, while only 17% of the cells exhibited orientation bias in the CxNR1KO cases (Fig. 6A bottom panel). To examine the complexity of the dendritic processes as a function of distance from the soma the number of crossings that occur at each concentric 20 μm ring were counted for all cells (see Fig. 6A, top panel). The number of crossings follows a normal distribution in both control and CxNR1KO cases; however, the number of crossings that occurred at the 30- to 100-μm distance from soma, was almost 2-fold higher in the CxNR1KO compared to controls (Fig. 6B); the P value by Student’s t test was P < 0.0019. The number of dendritic bifurcations for spiny stellate cells was also significantly different; on average more than 2-fold increase was observed in CxNR1KO over controls (9.58 ± SEM, 0.469 for CxNR1KO compared to 4.47 ± SEM 0.232 bifurcations for control, P < 0.00001, Fig. 6E). The total dendritic length was measured for each spiny stellate cell and was significantly higher in CxNR1KO by 30% over control, P < 0.001, (Fig. 6D). The dendritic field span was defined as the greatest distance between the most distal dendrite tips of a given stellate cell. CxNR1KO cases had broader field spans with an increase of 20% over control field spans, P < 0.0009 (Fig. 6F). Interestingly, when the dendritic lengths were subdivided into the longest and shortest processes, there were no significant differences between CxNR1KO and control for the longest dendritic process, approximately 180 ± SEM 18 μm; however, there was a statistically significant difference observed for the shortest dendritic process, with CxNR1KO having significantly shorter length, 50.78 ± SEM 2.57 μm, compared to controls 64.12 ± SEM 4.07 μm, P < 0.007 (Fig. 6C).
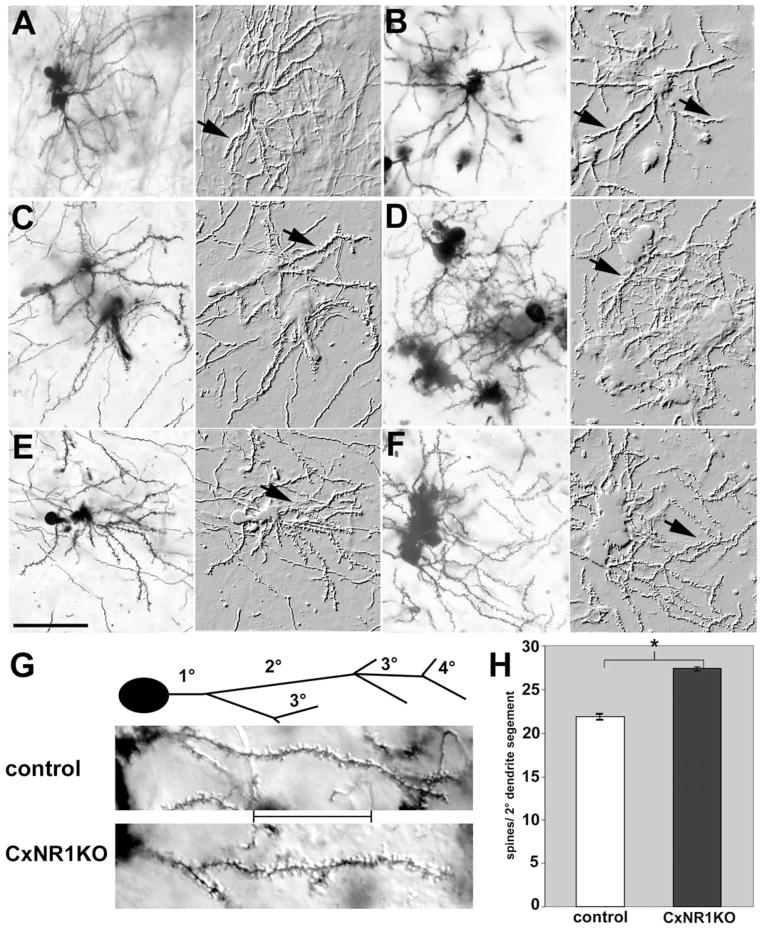
FIG. 8.
High-power images of layer IV spiny stellate cells and their spines in the CxNR1KO and control S1 cortex. Pairs of micrographs illustrate Golgi-Cox-impregnated spiny stellate cells in the barrel cortex of control (A, C, E) and CxNR1KO (B, D, F) mice at P16. Each pair is a photomicrograph of the same cell(s) first with light microscopy and on the right as photomontage-embossed images. Arrows indicate spiny dendritic processes. Note the biased dendritic orientation of control cells. (G) Higher power views of spines on secondary dendritic branches with the 20-μm segment inset between control and CxNR1KO panels. Spine counts from 232 such segments for each genotype were quantified and shown in H. Scale: A–F = 45 μm. Error bars = SEM, P < 0.00001.
Dendritic Spines of Stellate Cells in the Barrel Cortex of CxNR1KO Mice
A number of previous studies have underscored activity-dependent alterations in the morphology and density of dendritic spines (Rocha and Sur, 1995; McAllister et al., 1995, 1996, 1997). In our study randomly selected secondary dendritic segments of spiny stellate cells in layer IV barrel field were examined from 5 control and 5 CxNR1KO cases of age P16 (Fig. 8). The average number of spine counts per 20-μm stretch of dendrite was significantly different between control and CxNR1KO cases when examined by Student’s t test. On average CxNR1KO had a 20% increase in spine density on their secondary dendrites (see Figs. 8G–H). Typically control segments had 21.86 ± SEM 0.28 spines per segment, while CxNR1KO segments were studded with more spines, 27.35 ± SEM 0.29 per segment, P < 0.00001.
DISCUSSION
Patterned organization of neural connections is an essential structural substrate for processing sensory information. Pre- and postsynaptic elements act in concert to develop neural patterns representing the sensory periphery. The role of NMDAR-mediated neural activity in this process has attracted much attention in a variety of vertebrate sensory systems (Hahm et al., 1991; Cline et al., 1987; Bear et al., 1990; Simon et al., 1992; see Goodman and Shatz, 1993; Cramer and Sur, 1995; Katz and Shatz, 1996, for reviews). For several decades, it has been an intriguing question how one set of neurons confers pattern information to another set at a distant site, to the extent that the second-order neurons undergo structural and functional modeling that is in accord with their presynaptic partners. Here we show that, in the mouse barrel cortex, NMDARs play a crucial role in this process.
Region-specific disruption of the NMDAR function allows a unique way to address how pre- and postsynaptic neural elements develop patterns, and to what extent postsynaptic NMDARs play a role in this process. In CxNR1KO mice, whiskers and subcortical whisker representations all develop normally (Iwasato et al., 2000). In the barrel cortex TCAs representing the large whiskers are able to develop patterns, yet their postsynaptic partners, barrel cells, are imperceptive when they lack the NMDAR function. They fail to orient their dendrites, and furthermore, their dendritic fields and spine densities develop differently from their counterparts with normal NMDAR function.
One possible mechanism that could account for the absence of patterning of postsynaptic cells in regions where TCAs develop patterns might be related to differential cell survival. There is precedence for this possibility in subcortical trigeminal centers in mice with complete deletion of the NR1 or following pharmacological blockade of NMDARs (Ikonomidou et al., 1999; R. Corriveau, personal communication). Our cell counts, in comparable “barrel” regions in layer IV of control and CxNR1KO mice, on the other hand, indicate that cell densities are similar even at P21. Thus, differential cell death as one of the consequences of NR1 deletion could not account for the absence of barrels in the CxNR1KO mice. It is a major technical hurdle to visualize and determine cell movements during pattern formation in the barrel cortex. Our cell counts suggest that, if similar numbers of cells are present in the TCA projection zone of the barrel cortex, cells must move around to compose the barrel walls in normal mice, whereas they do not in the CxNR1KO mice. Along these same lines, if the stellate cells begin elaborating their dendritic trees toward barrel centers, i.e., TCA terminal foci, their somata are displaced, thus forming the barrel walls.
Dendritic tree analyses of normal and CxNR1KO mice clearly indicate that, in the absence of NMDAR function in layer IV spiny cells, there is no biased dendritic field orientation. While in control cortices spiny stellate cells located along the barrel walls orient their dendrites toward barrel hollows, thus showing a conspicuous dendritic field orientation bias. In contrast, the majority of CxNR1KO cortex spiny stellate cell dendrites we have charted radiated in all directions, with no specific orientation bias. To date, the role of the NMDARs in dendritic orientation of barrel cells has not been investigated. Interestingly, while the visual cortical development and patterning of TCAs (i.e., ocular dominance stripes), and the role of peripherally or intrinsically conveyed neural activity has been the subject of numerous studies in the feline and primate visual cortex (Katz and Shatz, 1996) relatively little attention has been devoted to how stellate cells in layer IV respond to these alterations in terms of their dendritic differentiation. In an earlier study, Katz and Constantine-Paton (1988) examined this issue in the optic tectum of experimentally induced three-eyed frogs where retinotectal afferents form stripes that are activity dependent, much like that seen in the feline or primate visual cortex. In this experimental model, these authors noted that afferent input can influence dendritic orientation of tectal cells, such that individual cells will preferentially elaborate their dendritic trees in one stripe of afferent terminals or the other. In later studies, the role of NMDARs in retinotectal targeting and topography has been investigated (Simon et al., 1992), but dendritic field orientation of individual tectal cells has not. Other recent studies in the frog tectum, however, indicate the role of neural activity and NMDARs in elaboration of dendritic fields of tectal neurons (Nedivi et al., 1998; Rajan and Cline, 1998; Rajan et al., 1999). In a similar vein, pharmacological applications of NMDAR blocker APV have been noted to increase dendritic spines in the lateral geniculate neurons of the ferret (Rocha and Sur, 1995). However, no specific analyses were carried out in this study to determine whether this manipulation also interferes with the dendritic field orientation of X or Y thalamic cells. In the CxNR1KO barrel cortex, soma size and cell numbers were unchanged while the dendritic orientation, number of branches, total dendritic length, field spans, and spine densities were increased. Such a significantly altered spatial distribution of dendritic processes in a manner that they extend beyond their normal territories suggests a restricted effect of the NMDARs in negatively regulating dendritic growth by removing inappropriately situated branches. The observation of more dendritic length per cell overall in CxNR1KO layer IV cells and many shorter processes provides further support that NMDARs may mediate competition within local regions of the postsynaptic cell membrane. Such effects may be due to an absence of selective pruning of inappropriate branches or due to an increased inhibition since inputs cannot be well correlated in the layer IV cells lacking NMDARs. Thus in the absence of NMDARs in excitatory layer IV cells their processes are more exuberant. It remains to be directly tested whether these processes fail to undergo selective pruning.
Several lines of in vitro evidence indicate that a number of extracellular molecules such as Semaphorins, and Slits, might play a major role in dendritic differentiation of cortical pyramidal cells. For example Sema3A can influence directional growth of apical dendrites of layer V pyramidal cells, whereas Slit1 can influence dendritic growth and branching (Polleux et al., 2000; Whitford et al., 2002). However, the role of these molecules in differentiation of stellate cell dendrites in layer IV has not been examined. An interesting observation is that, in mice with targeted deletion of the ephrin-A5, cortical barrels shift their position (Vanderhaeghen et al., 2000). However, effects on the dendritic field orientation and differentiation of the spiny cells in affected barrels have not been examined. Recent studies on the EphB receptor tyrosine kinases provide evidence that such receptors typically classified as a topographic guidance cue in fact are localized at excitatory synapses. Here they cluster and associate with NMDARs and are activated via phosphorylation by another tyrosine kinase family member Src (Dalva et al., 2000; Gerlai, 2002). Furthermore, EphB receptor activation potentiates NMDAR-mediated calcium influx, and NMDAR-dependent gene expression (Takasu et al., 2002). If NMDARs play a major role in dendritic differentiation and orientation of cortical layer IV cells, what are the downstream signaling events that ultimately lead to dendritic development? A number of studies have noted that neurotrophins play a major role in dendritic differentiation of cortical cells (McAllister et al., 1995, 1996, 1997; Horch et al., 1999; McAllister, 2000, 2002). Future studies using region-specific mutants will certainly lend insights into how neurotrophin-dependent differentiation of layer IV stellate cells might be linked to NMDARs and enable a modulation of local trophic factors by activity levels within the postsynaptic cell.
EXPERIMENTAL METHODS
Breeding of CxNR1KO Mice
CxNR1KO mice were generated using the Cre/loxP gene deletion approach with the cortex-specific Emx1 promoter, as previously described (Iwasato et al., 2000). We crossed Emx1Cre/Cre NR1+/− or Emx1Cre/+ NR1+/− mice with NR1flox/flox mice to obtain four types of mice: Emx1Cre/+ NR1flox/−, Emx1+/+ NR1flox/−, Emx1Cre/+ NR1flox/+, and Emx1+/+ NR1flox/+ mice. The Emx1Cre/+ R1flox/− mice are CxNR1KO mice and other littermates were used as control mice. The tissue was processed immediately to examine cellular, axonal, and dendritic parameters in genotype-blind fashion and genotypes were later determined by PCR. Experiments were performed in accordance with guidelines of the Institutional Animal Care and Use Committees.
Thalamocortical Afferents
We used the lipophilic tracer Dil (Molecular Probes) labeling from the VPM to reveal TCA patterns. Four control and 4 CxNR1KO P5-7 mice were sacrificed by overdose of sodium pentobarbital and perfused with phosphate-buffered physiological saline (PBS) followed by 4% paraformaldehyde in PBS. The brains were then removed and kept in fixative overnight. We implanted small crystals of the tracer in the VPM under a dissecting microscope. After 2–3 weeks of diffusion time at 37°C, we examined and charted out labeled TCA distribution in both flattened cortices and thalamocortical slices using a confocal microscope to acquire a z-series of multiple focal planes, optimized for visualization of the entire barrel field or single barrel under high-power oil objective (Nikon TE-300 with Radians 2000 laser scanning system, Bio-Rad). Several sections were counterstained with Sytox green, a fluorescent nuclear stain (1:5000; Molecular Probes, Eugene, OR) to visualize the nucleoarchitecture in relation to TCA patterns (see Figs. 1A, B, E–H). For single TCA analysis, we were able to recover 7 axons from control and 8 axons from CxNR1KO cortices at P5. The lateral and vertical axonal arbor field spans (as defined by the broadest distance between afferent arbor tips originating from a single TCA terminal in the lateral and vertical plane, respectively), the number of axonal branches, and total axonal arbor length were measured using the Metaview Image Analysis Program (Universal Imaging Corp.).
5-HTT Immunohistochemistry
Tangential sections (60 μm) of 9 control and 9 CxNR1KO P5-7 cortices were incubated in anti-5-HTT rabbit polyclonal antibody (Diasorin, 1:10,000; Stillwater, MN) overnight in PBS with 5% normal goat serum (NGS), 0.3% Triton X-100, sections were rinsed in PBS, and then incubated in a secondary, biotinylated goat anti-rabbit antibody (Sigma, 1:200; St. Louis, MO), followed by processing with ABC (Vector Labs; Burlingame, CA) and DAB (Sigma; St. Louis) for light microscopy visualization. Sections were mounted on gelatin-coated slides, dehydrated, cleared in xylene, and coverslipped in DePeX mounting media (BDH Lab Supplies; Poole, England). For quantitative analysis 5-HTT-immunostained tangential cortex sections were visualized under the light microscope and images of the barrel field were acquired by a Cool-Snap digital camera (Media Cybernetics; Carlsbad, CA) and inverted in Adobe Photoshop 6.0 (ADOBE Systems Inc.) for optimum resolution of the barrel field. Measurements of the entire large whisker representation area (LWA) and areas devoted to each 5-HTT-immunopositive TCA region within the LWA were made using the Meta-view Image Analysis Program (Universal Imaging Corp, Downingtown, PA). Statistical significance was determined by Student’s t test.
Histochemistry and Immunofluorescence
Sections (60 μm) from control and CxNR1KO cases at age P7 were incubated in a solution of 5 mg nitroblue tetrazolium, 20 mg NADPH (β-nicotinamide adenine dinucleotide phosphate) in 20 ml of Tris buffer (pH 7.1) at 37°C in a shaker incubator for 2–3 h. Sections were then rinsed in Tris buffer, mounted onto slides, and coverslipped with an aqueous medium. Cresyl violet Nissl staining and cytochrome oxidase histochemistry were performed as described previously (Iwasato et al., 2000). For qualitative examination of a subset of thick axonal elements coursing through barrel hollows and layer IV dendritic trees immunohistochemistry was performed on 60-μm sections using the SMI-32 antibody that recognizes the nonphosphorylated neurofilament-H proteins (1:10,000; Sternberger Monoclonals Inc.). Immunopositive signal was visualized using the Alexa Fluor 555-conjugated secondary antibody (1:500, Molecular Probes) and several sections were counter-stained with Sytox green (as described above).
Layer IV Cell Counts
Forebrains of 4 control and 4 CxNR1KO mice at P21 were sectioned in the coronal plane and kept in serial order. One series of sections was stained for cytochrome oxidase (CO) histochemistry (a routine marker for cortical barrels), and the other series with cresyl violet for examination of cytoarchitecture. We then identified layer IV of the barrel field in the normal and the CxNR1KO cortex by the CO patterns (Figs. 4A, B). Adjacent, cresyl violet-stained sections (Figs. 4C, D) were then matched to the areas of CO patches. A barrel size area was determined and fixed in the control S1 layer IV (outlined in red; Figs. 4C, E). The same exact area was fixed in CxNR1KO S1 layer IV (outlined in red, Figs. 4D, F) though there were no barrels, and the number of cells was determined. Images of the barrel field were acquired by a CoolSnap digital camera (Media Cybernetics). Cell counts were made using the Metaview Image Analysis Program (Universal Imaging Corp.) Cells per barrel were counted from 7–8 consecutive sections per mouse and are illustrated in Figs. 4E–H. In addition a 400 × 125 μm window was placed within the center of Layer IV (outlined in green Fig. 4C, D) in 10 Nissl-stained control and 10 CxNR1KO P21 sections and the numbers of cells within this area were counted using the Metaview Image Analysis Program. Average number of cells per control and knockout conditions were then compared using students t-test.
Layer IV Dendritic Analyses
To visualize the morphological details of the layer IV stellate cells in the barrel cortex, we employed the Golgi-Cox method as described by Glaser and Van der Loos (1981). In brief 8 control and 9 CxNR1KO P16 brains were fixed in 4% paraformaldehyde and fixed P16 brains were immersed in 20 ml of Golgi-Cox solution for 14 days impregnation, followed by 7 days in 30% sucrose. On a freezing microtome 120-μm-thick coronal and flattened cortex sections were collected in PBS in series. Sections were then incubated in 15% ammonium hydroxide (EM Science, Gibbstown, NJ) for 30 min under the fume hood in the dark, followed by 15 min in Kodak fixative solution (Eastman Kodak Co., Rochester, NY) and then rinsed thoroughly in distilled water. Sections were counterstained with cresyl violet to reveal layer IV barrel field. Cellular and dendritic details were examined under light microscope under 100x oil immersion and were reconstructed using a drawing tube. These drawings were scanned, resolutions were standardized in the ADOBE Photoshop 6.0, and careful note of the mediolateral axis with respect to the cell was made. To evaluate various aspects of dendritic growth, morphology, dendritic geometry, and branching parameters for all 58 CxNR1KO cases and 58 control cases, we adopted the method used by Scholl (1953). In brief a series of 20-μm-spaced concentric rings are brought over the soma center and the cell is oriented such that the medio-lateral axis of the forebrain is aligned along the 0–180° axis. Vector plots for each dendritic process were performed and cells were scored by the number of processes that aligned within each quadrant of these axes (see Fig. 6A); symmetric cells without any orientation preference will tend to have their processes spanning more than one quadrant and often along the 0–180 or 90–270° axes. Cells with greater than 75% of total dendritic length directed toward a single quadrant or axis were scored as having an orientation bias (see Fig. 6A) while other cells were scored as having no orientation bias.
All images were digitally acquired, calibrated to scale and using the MetaView imaging software stereological measurements were performed for the following dendritic parameters: number of bifurcations per cell, number of branches with increasing distance from soma, total dendritic length, dendritic field span, shortest and longest dendritic process, and soma area. Statistical analyses were performed on all quantified data by using the Student’s t test to validate significance between control and CxNR1KO values for each of the parameters.
Dendritic Spine Analysis
Using a high-resolution light microscope with Nomarski optics (Leica DMRXA) stretches of 20 μm secondary dendrite segments were randomly selected from the Golgi-Nissl-stained samples of layer IV stellate cells. For each segment a z-series was created to visualize the entire extent of the dendritic process and also capture the spine details in all focal planes. These were then compressed onto a single plane and spine counts were performed on these compressed images that capture all the spine details across the entire z-series (see Figs. 8G–H). For dendritic spine analyses 4 segments per cell from 58 cells for a total of 232, 20-μm segments, were analyzed for each genotype.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (T.I.), and by NIH/NINDS (NS39050, R.S.E.). We thank R. Ando for technical assistance and L. Marrero for help with the confocal imaging.
References
- Altman J, Anderson WJ. Experimental reorganization of the cerebellar cortex. I Morphological effects of elimination of all microneurons with prolonged X-irradiation at birth. J Comp Neurol. 1972;146:355–406. doi: 10.1002/cne.901460305. [DOI] [PubMed] [Google Scholar]
- Altman J, Anderson WJ. Experimental reorganization of the cerebellar cortex. II Effects of elimination of most microneurons with prolonged X-irradiation started at five days. J Comp Neurol. 1973;149:123–152. doi: 10.1002/cne.901490202. [DOI] [PubMed] [Google Scholar]
- Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. Cellular differentiation: Development of dendritic arborizations under normal and experimentally altered conditions. Neurosci Res Program Bull. 1982;20:451–461. [PubMed] [Google Scholar]
- Blue ME, Erzurumlu RS, Jhaveri S. A comparison of pattern formation by thalamocortical and serotonergic afferents in the rat barrel field cortex. Cereb Cortex. 1991;1:380–389. doi: 10.1093/cercor/1.5.380. [DOI] [PubMed] [Google Scholar]
- Cases O, Lebrand C, Giros B, Vitalis T, De Maeyer E, Caron MG, Price DJ, Gaspar P, Seif I. Plasma membrane transporters of serotonin, dopamine and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knock-outs. J Neurosci. 1998;18:6914–6927. doi: 10.1523/JNEUROSCI.18-17-06914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Jr, Rakic P. Mechanisms of cortical development: A view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- Cramer KS, Sur M. Activity-dependent remodeling of connections in the mammalian visual system. Curr Opin Neurobiol. 1995;5:106–111. doi: 10.1016/0959-4388(95)80094-8. [DOI] [PubMed] [Google Scholar]
- Cline HT, Debski EA, Constantine-Paton M. N-Methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci USA. 1987;84:4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Jones FS. Developmental control of N-CAM expression by Hox and Pax gene products. Philos Trans R Soc Lond B Biol Sci. 1995;349:305–312. doi: 10.1098/rstb.1995.0118. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somato-sensory cortex. Dev Brain Res. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: Sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao FB, Brenman JE, Jan LY, Jan YN. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1999;13:2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. EphB and NMDA receptors: Components of synaptic plasticity coming together. Trends Neurosci. 2002;25:180–181. doi: 10.1016/s0166-2236(02)02165-3. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: Application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psychol. 1973;82:175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Chang FL. Dendritic pattern formation involves both oriented regression and oriented growth in the barrels of mouse somatosensory cortex. Brain Res. 1988;471:148–152. doi: 10.1016/0165-3806(88)90160-5. [DOI] [PubMed] [Google Scholar]
- Hahm JO, Langdon RB, Sur M. Disruption of retinogeniculate afferent segregation by antagonists to NMDA receptors. Nature. 1991;351:568–570. doi: 10.1038/351568a0. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA in the developing rat brain: Early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience. 1998a;83:1185–1201. doi: 10.1016/s0306-4522(97)00444-2. [DOI] [PubMed] [Google Scholar]
- Hansson SR, Cabrera-Vera TM, Hoffman BJ. Infraorbital nerve transection alters serotonin transporter expression in sensory pathways in early postnatal rat development. Dev Brain Res. 1998b;111:305–314. doi: 10.1016/s0165-3806(98)00148-5. [DOI] [PubMed] [Google Scholar]
- Harris RM, Woolsey TA. Morphology of golgi-impregnated neurons in mouse cortical barrels following vibrissae damage at different post-natal ages. Brain Res. 1979;161:143–149. doi: 10.1016/0006-8993(79)90201-4. [DOI] [PubMed] [Google Scholar]
- Harris RM, Woolsey TA. Computer-assisted analyses of barrel neuron axons and their putative synaptic contacts. J Comp Neurol. 1983;220:63–79. doi: 10.1002/cne.902200107. [DOI] [PubMed] [Google Scholar]
- Horch HW, Kruttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri S, Erzurumlu RS, Crossin K. Barrel construction in rodent neocortex: Role of thalamic afferents versus extracellular matrix molecules. Proc Natl Acad Sci USA. 1991;88:4489–4493. doi: 10.1073/pnas.88.10.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Constantine-Paton M. Relationships between segregated afferents and postsynaptic neurones in the optic tectum of three-eyed frogs. J Neurosci. 1988;8:3160–3180. doi: 10.1523/JNEUROSCI.08-09-03160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Rhoades RW, Bennett-Clarke CA. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402–407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- Lafont F, Prochiantz A, Valenza C, Petitou M, Pascal M, Rouget M, Rousselet A. Defined glycosaminoglycan motifs have opposite effects on neuronal polarity in vitro. Dev Biol. 1994;165:453–468. doi: 10.1006/dbio.1994.1267. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1966;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Li Z, Aizenman CD, Cline HT. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- Li Z, Van Aelst L, Cline HT. Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nature Neurosci. 2000;3:217–225. doi: 10.1038/72920. [DOI] [PubMed] [Google Scholar]
- Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- Ma PM, Woolsey TA. Cytoarchitectonic correlates of the vibrissae in the medullary trigeminal complex of the mouse. Brain Res. 1984;306:374–379. doi: 10.1016/0006-8993(84)90390-1. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Cellular and molecular mechanisms of dendrite growth. Cereb Cortex. 2000;10:963–973. doi: 10.1093/cercor/10.10.963. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Conserved cues for axon and dendrite growth in the developing cortex. Neuron. 2002;33:2–4. doi: 10.1016/s0896-6273(01)00577-3. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- Messersmith EK, Feller MB, Zhang H, Shatz CJ. Migration of neocortical neurons in the absence of functional NMDA receptor. Mol Cell Neurosci. 1997;9:347–357. doi: 10.1006/mcne.1997.0646. [DOI] [PubMed] [Google Scholar]
- Mitrovic N, Schachner M. Transient expression of NADPH diaphorase activity in the mouse whisker to barrel field pathway. J Neurocytol. 1996;25:429–437. doi: 10.1007/BF02284813. [DOI] [PubMed] [Google Scholar]
- Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedivi E, Wu GY, Cline HT. Promotion of dendritic growth by CPG15, an activity-induced signaling molecule. Science. 1998;281:1863–1866. doi: 10.1126/science.281.5384.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Schlaggar BL, Tuttle R. Specification of neocortical areas and thalamocortical connections. Annu Rev Neurosci. 1994;17:419–439. doi: 10.1146/annurev.ne.17.030194.002223. [DOI] [PubMed] [Google Scholar]
- Pasternak JR, Woolsey TA. The number, size and spatial distribution of neurons in lamina IV of the mouse Sml neocortex. J Comp Neurol. 1975;160:291–306. doi: 10.1002/cne.901600303. [DOI] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F, Hall SF. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Sakmann B. Functionally independent columns of rat somatosensory barrel cortex revealed with voltage-sensitive dye imaging. J Neurosci. 2001;21:8435–8446. doi: 10.1523/JNEUROSCI.21-21-08435.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- Purves D, Hume RI. The relation of postsynaptic geometry to the number of presynaptic axons that innervate autonomic ganglion cells. J Neurosci. 1981;1:441–452. doi: 10.1523/JNEUROSCI.01-05-00441.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan I, Witte S, Cline HT. NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J Neurobiol. 1999;38:357–368. doi: 10.1002/(sici)1097-4695(19990215)38:3<357::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci. 1998;18:7836–7846. doi: 10.1523/JNEUROSCI.18-19-07836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Sidman RL. Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J Comp Neurol. 1973;152:133–161. doi: 10.1002/cne.901520203. [DOI] [PubMed] [Google Scholar]
- Redmond L, Ghosh A. The role of Notch and Rho GTPase signaling in the control of dendritic development. Curr Opin Neurobiol. 2001;11:111–117. doi: 10.1016/s0959-4388(00)00181-1. [DOI] [PubMed] [Google Scholar]
- Rocha M, Sur M. Rapid acquisition of dendritic spines by visual thalamic neurons after blockade of N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA. 1995;92:8026–8030. doi: 10.1073/pnas.92.17.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EK, Luo L. How do dendrites take their shape? Nature Neurosci. 2001;4:359–365. doi: 10.1038/86006. [DOI] [PubMed] [Google Scholar]
- Scholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–407. [PMC free article] [PubMed] [Google Scholar]
- Simon DK, Prusky GT, O’Leary DD, Constantine-Paton M. N-Methyl-D-aspartate receptor antagonists disrupt the formation of a mammalian neural map. Proc Natl Acad Sci USA. 1992;89:10593–10597. doi: 10.1073/pnas.89.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen H, Van der Loos H. Early lesions of mouse vibrissal follicles and their influence on dendrite orientation in the cortical barrelfield. Exp Brain Res. 1980;40:419–431. doi: 10.1007/BF00236150. [DOI] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P, Lu Q, Prakash N, Frisen J, Walsh CA, Frostig RD, Flanagan JG. A mapping label required for normal scale of body representation in the cortex. Nature Neurosci. 2000;3:358–365. doi: 10.1038/73929. [DOI] [PubMed] [Google Scholar]
- Van der Loos H. Barreloids in mouse somatosensory thalamus. Neurosci Lett. 1976;2:1–6. doi: 10.1016/0304-3940(76)90036-7. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: Structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Welker E, Van der Loos H. Quantitative correlation between barrel-field size and the sensory innervation of the whiskerpad: A comparative study in six strains of mice bred for different patterns of mystacial vibrissae. J Neurosci. 1986;11:3355–3373. doi: 10.1523/JNEUROSCI.06-11-03355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford KL, Marillat V, Stein E, Goodman CS, Tessier-Lavigne M, Chedotal A, Ghosh A. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33:47–61. doi: 10.1016/s0896-6273(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Wong WT, Faulkner-Jones BE, Sanes JR, Wong RO. Rapid dendritic remodeling in the developing retina: Dependence on neurotransmission and reciprocal regulation by Rac and Rho. J Neurosci. 2000;20:5024–5036. doi: 10.1523/JNEUROSCI.20-13-05024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA. Peripheral alterations and somatosensory development. In: Coleman EJ, editor. Development of Sensory Systems in Mammals. Wiley; New York: 1990. p. 461. [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (S1) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Yan XX, Jen LS, Garey LJ. NADPH-diaphorase-positive neurons in primate cerebral cortex colocalize with GABA and calcium-binding proteins. Cereb Cortex. 1996;6:524–529. doi: 10.1093/cercor/6.3.524. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK. Expression of serotonin transporter protein in developing rat brain. Dev Brain Res. 2000;119:33–45. doi: 10.1016/s0165-3806(99)00152-2. [DOI] [PubMed] [Google Scholar]