Abstract
Self assembly between cations and anions is ubiquitous throughout nature. Important biological structures such as chromatin often use polyvalent assembly between a polycation and a polyaninon. Biomedical importance of synthetic polycations arises from their affinity to polyanions such as nucleic acid and heparan sulfate. However, the limited biocompatibility of synthetic polycations hampers the realization of their immense potential. By examining biocompatible cationic peptides, we hypothesize that a biocompatible polycation should be biodegradable and made from endogenous cations. We designed an arginine-based biodegradable polycation and demonstrated that it was orders of magnitude more compatible than conventional polycations in vitro and in vivo. This biocompatibility diminishes when L-arginine is substituted with D-arginine or when the biodegradable ester linker changes to a biostable ether linker. We believe this design can lead to many biocompatible polycations that can significantly advance a wide range of applications including controlled release, tissue engineering, biosensing, and medical devices.
1. Introduction
The application of synthetic polycations in biomedine and biotechnology is limited by their unsatisfactory biocompatibility.[1] To design a biocompatible polycation, we seek inspiration from nature. Cationic peptides are important mediators in a variety of homeostatic processes including histone that regulates DNA replication and gene expression,[2] hepcidin that balances iron concentrations,[3] heparin-binding domains that control growth-factor stability and activity,[4] and defensins that act as anti-microbial agents in host defense.[5] These polypeptides are composed of hydrophobic and positively charged domains that control their physiological functions.[6] Structurally, cationic peptides are a series of amino acids linked through peptide bonds yielding a polymer hydrolysable at the presence of appropriate enzymes. We hypothesized that mimicry of the essential structure of cationic peptides would result in a biocompatible polycation. Firstly, natural cationic peptides undergo hydrolysis catalyzed by their respective proteases. Secondly, the positive charges of cationic peptides arise from endogenous amino acids such as L-arginine and L-lysine. A minimalistic chemical mimicry of these cationic peptides is a synthetic hydrolyzable polycation based on cationic amino acids.
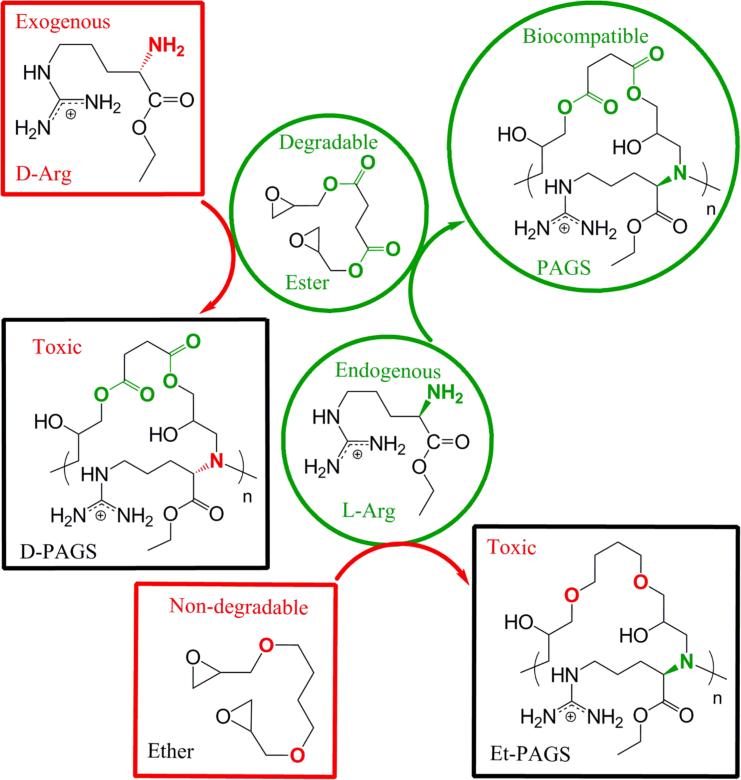
Specifically, we chose to use ester functional groups in the polymer to introduce hydrolyzability because of its well defined synthetic routes and familiarity to the biomaterials community.[7] We chose to use L-arginine because it is the most basic natural amino acid and its prevalence in endogenous polyanion-binding domains.[4, 8] The incorporation of L-arginine facilitates ionization of the resultant polycations at a neutral pH. We chose to integrate L-arginine into a polyester using a ring opening reaction between the α-amino group of arginine and the epoxy ring of diglycidyl succinate (Fig. 1). The resultant polycation is referred to as poly(L-argininate glyceryl succinate), or PAGS. Besides arginine, the other building blocks of PAGS are derived from succinic acid and glycerol. Succinic acid is readily found in the body, and plays a key role in the citric acid cycle in cellular respiration.[9] Glycerol derivatives are prevalent throughout the body in lipids and many signaling molecules. We anticipate that using derivatives of endogenous building blocks are necessary to design a polycation with good biocompatibility. The following data suggests that the biocompatibility of PAGS is orders of magnitude higher than PEI in vitro and in vivo. We chose PEI as a control because it is a polycation most scientists are familiar with. To probe the necessity of the key design features, we synthesized control polymers with D-arginine and a biostable backbone.[10] These perturbations on endogenous building block and degradability significantly reduced the biocompatibility of PAGS.
Figure 1.
The design of PAGS and the control polymers that probe the importance of endogenous cations and degradability. All polymers were synthesized by polycondensation between equimolar amounts of arginine ethyl ester and the diglycidyl starting material. Endogenous L-Arg and degradable diglycidyl succinate made PAGS; substituting L-Arg with the exogenous D-Arg yielded D-PAGS, and substitution of diglycidyl succinate with the biostable 1,4-butanediol diglycidyl ether produced Et-PAGS.
2. Results and Discussion
Polycondensation between L-arginine ethyl ester and diglycidyl succinate in N,N-dimethylforamide yields PAGS as a pale yellow powder, soluble in water. The polymer is purified by repetitive cycles of evaporation under vacuum and solvent washes using methanol and ethyl acetate. Nuclear magnetic resonance spectroscopy (NMR) reveals a change in chemical shift from approximately 3.2 ppm in the diglycidyl ester to approximately 4.0 ppm in PAGS (Fig. S1). This shift corresponds to the opening of the epoxy ring in the diglycidyl ester. The intense C=O stretch at 1735 cm-1 in the Fourier transform infrared spectroscopy (FTIR, Fig. S2) confirms the formation of ester bonds, and the intense band at 1673 cm-1 with a shoulder at 1635 cm-1 indicates the presence of the guanidinium side chain of arginine.[11] PAGS has a glass transition temperature of 42.8°C and a melting temperature of 88.1°C as revealed by differential scanning calorimetry. pH titration revealed that the pKa for PAGS was approximately 10.5 (Fig. S3), which is comparable to PEI with a pKa range of 9-10.[12]
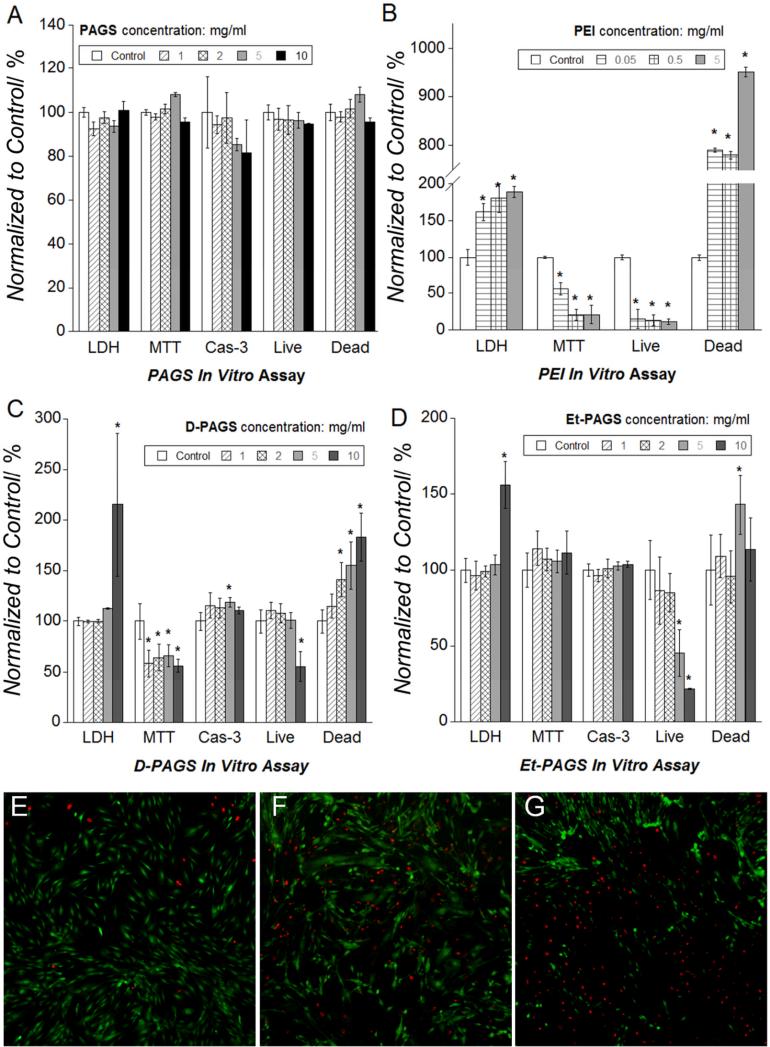
We investigated the cytocompatibility of PAGS in vitro using non-immortalized baboon smooth muscle cells (SMCs). In vitro biocompatibility assays of polycations often use cell lines, which tend to be more robust than primary cells. We chose non-immortalized cells because they are likely more accurate in predicting the in vivo biocompatibility of PAGS (MW = 10,500 Da). PEI of nearly identical molecular weight PEI (MW = 10,000 Da) served as the control because it is a well studied polycation and has been used in a wide variety of biomedical applications.[12-13] There is no standardized procedure to determine a polycation's biocompatibility in vitro, so we chose four assays that can evaluate toxicity from different perspectives and at progressively more severe levels. We examined the effects of PAGS on cell membrane integrity using lactate dehydrogenase (LDH) assay, metabolic activity using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, apoptosis using caspase-3 assay, and cell viability using calcein AM and ethiduim homodimer-1 staining followed by fluorescence measurements (live/dead assay). Many existing polycations disrupt cell membranes within minutes of contact, and consequently the extracellular concentration of LDH, an intracellular enzyme, increases significantly.[14] MTT metabolic activity assay provides a good indication to the level of stress cells experience.[15] If the stress crosses a threshold, cell apoptosis will occur and caspase-3 levels correlate with the severity of apoptosis.[16] Finally, differences in cell viability resulted from apoptosis and necrosis were evaluated by live/dead assay.[17]
We examined the in vitro biocompatibility of PAGS by subjecting the cells to media with increasing concentrations of either PAGS or PEI. The PAGS concentrations used were 1, 2, 5, and 10 mg/ml, and the PEI concentrations used were 0.05, 0.5, and 5 mg/ml. Cell incubation time with polycation supplemented media was either 4 h or 24 h. The 4 h incubation time was used to analyze earlier stresses and the 24 h incubation was used to assess cell death. A 4 h incubation time demonstrated that PAGS was non-toxic up to at least 10 mg/ml (Fig. 2A) as determined by LDH, MTT, and caspase-3 assays. Live/dead assay indicated that even after a 24 h exposure to a PAGS concentration of 10 mg/ml, cells were as viable as control populations subjected to normal culture medium. The majorities of cells were alive and displayed normal morphology (Fig. S4). In contrast, PEI induced significant cell toxicity as indicated by LDH and MTT assays at a concentration as low as 0.05 mg/ml (Fig. 2B). Furthermore, extensive cell death was observed by the expression of ethidium homodimer-1 with 0.05 mg/ml PEI (Fig. S5). The morphology of cells exposed to PEI was consistent with the results of the viability assay indicating massive cell death (Fig. S6). These assays revealed that PAGS has excellent in vitro biocompatibility and represent at least a 200-fold improvement over PEI.
Figure 2.
In vitro cytocompatibility of PAGS tested with non-immortalized baboon SMCs using lactate dehydrogenase (LDH), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), caspase-3 assay, and live/dead assays. (A) PAGS experimental groups are normalized to the control (SMCs exposed to normal cell culture medium). The assays showed that the cells had intact membrane, exhibited normal metabolic activities and were viable when exposed to up to at least 10 mg/ml of PAGS. Cells were incubated for 4 h except in live/dead assay, which was incubated for 24 h. (B) PEI exhibited toxicity at a concentration as low as 0.05 mg/ml under identical conditions using identical assays. (C) When the endogenous L-Arg is substituted by D-Arg, the resultant polymer displayed significantly higher toxicity as indicated by all 4 assays. (D) When the degradable ester linkage is replaced by a much more stable ether bond, the resultant polymer, Et-PAGS, exhibited significantly higher toxicity as indicated by more cell membrane leakage and increased cell death. Multicomparison ANOVA, Tukey method, p < 0.05 was considered statistically significant. Any statistically significant difference between the experimental and the control group is noted by an “*”. Fluorescent (100x) micrographs of live (calcein AM, green) and dead (ethiduim homodimer-1, red) cells incubated for 24 h. with 10 mg/ml of (E) PAGS, (F) D-PAGS, and (G) Et-PAGS indicate that endogenous building blocks and biodegradability are necessary for cytocompatibility.
When exogenous D-arginine was used to prepare the polymer, the resultant material caused significant metabolic reduction at all concentrations and decreased cell viability above 2 mg/ml (Fig. 2C). The substitution of the biodegradable ester linkage with a more stable ether linkage led to a significant loss of cell membrane integrity at 10 mg/ml and decreased cell viability above 5 mg/ml (Fig. 2D). It should be noted that polycations can coat the cells and affect cell division.[18] Representative live/dead images of cells cultured at the presence of the polycations showed that PAGS is more biocompatible than D-PAGS and Et-PAGS (Fig. 2E-G). These observations are consistent with the hypothesis on the importance of using endogenous cations and degradability on the biocompatibility of polycations.
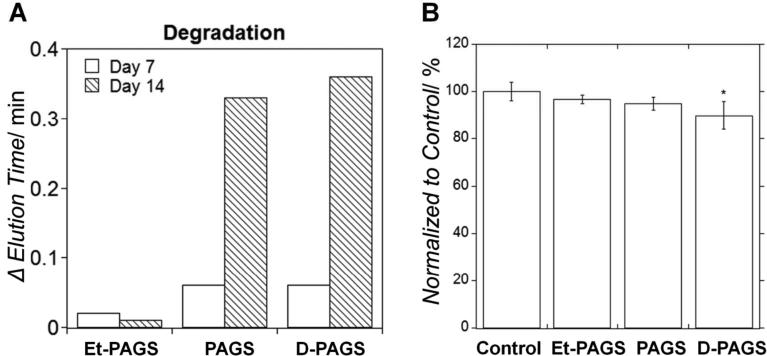
The difference in degradation of the 3 PAGS variations was observed by the change in elusion time on GPC after the polymers were exposed to cholesterol esterase in PBS.[19] The ether linkage in Et-PAGS rendered the polymer effectively non-degradable, whereas the change from L- to D-arginine didn't affect polymer degradation (Fig. 3A). The potential effect of degradation products on cell metabolic activity was investigated by conducting MTT assay using SMCs at the presence of conditioning medium containing polymer degradation products. D-PAGS degradation products is the only one that showed mild cytotoxicity (Fig. 3B).
Figure 3.
The effect of structural variations on degradation of PAGS. (A) Polyether Et-PAGS didn't degrade within 14 days. Polyesters with either L- or D-arginine showed significant degradation. (B) The degradation products of D-PAGS exhibited mild cytotoxicity, whereas the L-arginine-containing PAGS and the relatively biostable Et-PAGS showed no cytotoxicity. *Multicomparison ANOVA, Tukey method, p < 0.05.
Many state-of-the-art polycations have been tested in vitro at concentrations < 0.1 mg/ml. To the best of our knowledge, none displayed biocompatibility close to that of PAGS. It should be noted that cytotoxicity is cell dependent.[20] The literature values cited in this discussion were obtained with a wide variety of cell lines. Polycations are known to disrupt cell membranes, resulting in the leakage of LDH. Polycations such as polylysine have been shown to release significant amounts of LDH within 30 minutes of exposure.[21] For PAGS, no difference in LDH level was observed between the normal culture medium and PAGS media after a 4 h exposure. Several propositions suggested that polycation toxicity increases with increasing MW and charge density [expressed as (number of charge carriers)/(molecular weight of the polymer repeating unit)] and decreasing orders of amines respectively.[21b, 22] PAGS exhibited orders of magnitude higher biocompatibility than existing polycations regardless of which proposed criterion was used. Relative to PEI (10,000 Da), which was cytotoxic at a concentration as low as 0.05 mg/ml, PAGS (10,500 Da) exhibited no toxicity up to at least 10 mg/ml. Thus PAGS was at least 200 times more compatible than PEI of near identical MW. The charge density differs between PEI and PAGS. Each PEI repeating unit (43 g/mol) has one positive-charge carrier (amine). This leads to a charge density of 0.0233. Each PAGS repeating unit (418 g/mol) has two positive-charge carriers (guanidine and amine). This leads to a charge density of 0.00478. Thus the charge density of PEI is approximately 5 times that of the PAGS. A synthetic polycation with a similar charge density to PAGS is poly(vinyl pyridinium bromide) (PvPBr, charge density: 0.0054), which was shown to be cytotoxic to L929 murine fibroblast cell line at 0.1 mg/ml.[21] Thus PAGS was at least 100 times more compatible than a polycation with approximately the same charge density. For order of amines, the third proposed criterion, PAGS contained tertiary amines and guanidiniums. We could not find a synthetic polycation that contained both. Thus we compared PAGS with polyarginine and polyamidoamine (PAMAM). These two polycations contain guanidiniums and tertiary amines respectively. Polyarginine was cytotoxic to HeLa cells at 0.03 mg/ml.[23] Other arginine-rich polymers, such as an arginine-modified PAMAM exhibited cytotoxicity to HeLa cells at 0.04 mg/ml.[24] Poly(3-guanidinopropyl methacrylate), a guanidinium-containing polymer was toxic to COS-7 cells at 0.03 mg/ml.[25] Finally, PAMAM exhibited toxicity to L929 murine fibroblasts at 1 mg/ml.[21b] Again, this demonstrated that PAGS possessed unprecedented biocompatibility relative to existing polycations.
In vitro assays can provide valuable indications of biocompatibility and help determine the mechanism of toxicity. Complex responses at the organ and whole body level can only be obtained through in vivo analyses. We investigated the in vivo biocompatibility of PAGS by intraperitoneal injection of 150 μl of polycation solution in saline in six-week old BALB/c mice weighing approximately 20 g. Each mouse was weighed and injected with either a PAGS solution (8 mg/20 g body weight), PEI solution (0.2 mg/20 g body weight), or saline. Organs including heart, liver, lungs, spleen, kidneys, and bladder were collected on day 1, 5 and 30 after injection. Tissues were fixed and stained by hematoxylin and eosin (H&E) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. The organs were analyzed for tissue damage (H&E) and cell apoptosis (TUNEL).
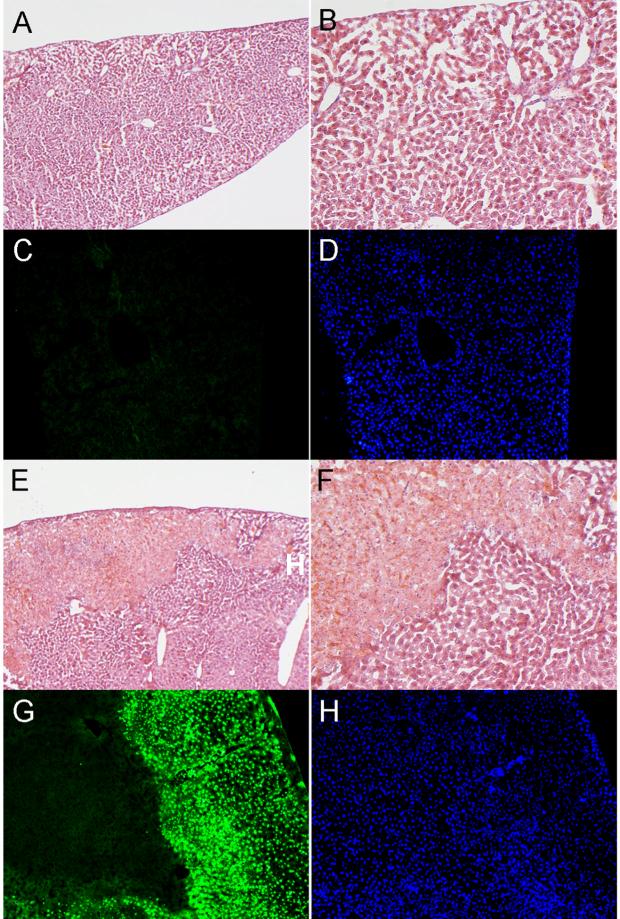
The major organs of animals injected with PAGS showed normal tissue architecture relative to saline control throughout all time points (liver: Fig. 4A and B; heart, lung, kidney, and spleen: Fig. S7). Mice injected with PAGS showed no observable change in apoptosis compared to control mice (Fig. 4C). Mice injected with PEI were the only animals that showed signs of toxicity. Of the organs harvested, only liver tissues harvested on day 1 suffered significant damage (Fig. 4E and F). On average, these liver tissues exhibited 15% cell death. Similarly, significant apoptosis was observed in livers of the day 1 PEI group only. All other PEI tissues at other time points did not exhibit apoptosis. All tissues in the PAGS group at all time points showed no signs of tissue damage (Fig. 4G). The tissues from all the organs except livers in the day 1 PEI group were indistinguishable histopathologically from healthy controls in terms of inflammation and necrosis. The day 1 PEI group liver exhibited 15% focal centrilobular necrosis on average. Hepatocytes surrounding the necrotic areas were characterized by grade 2 (out of 3 grades of severity) inflammation. The inflammation is predominantly acute in nature with infiltrate containing mostly neutrophils and expression of a small number of lymphocytes. Bile ducts were spared from the inflammatory responses. There was no significant bile stasis, bridging fibrosis or cirrhosis identified. No micro- or macro-vesicular steatosis was noted. Also, there was no associated atypical or malignant transformation of hepatocytes. Hepatocytes in the uninvolved areas were normal.
Figure 4.
In vivo biocompatibility of PAGS. Mice were either injected with 8 mg of PAGS or 0.2 mg of PEI intraperitoneally. Of all the organs isolated, liver was the only one that showed any significant histological change. A representative image of liver tissue in the PAGS group, H&E staining (A, 100x and B, 200x) revealed normal tissue architecture and TUNEL (C, green fluorescence) revealed no apoptosis. (D) DAPI staining revealed nuclei in the same tissue section as C. For PEI at 0.2 mg/animal, H&E staining (E, 100x and F, 200x) revealed that liver tissue suffered approximately 15% necrosis. (G) TUNEL staining showed extensive apoptosis in the livers of the PEI groups. (H) DAPI staining revealed nuclei in the same tissue section as G. All images were from samples isolated day 1 post injection. All image acquisition parameters are identical for PAGS and PEI.
The amount of PAGS used for the in vivo experiments was 40 times higher than that of PEI. Even so, animals exposed to PAGS displayed no toxicity and tissues maintained normal histological architecture. The charge density of PAGS is at least 1/5 that of PEI. Thus the total charge in the PAGS samples was at least 8 times that of PEI. Therefore PAGS is orders magnitude more compatible than PEI either by matching MW or total charge. Because of arginine's size (MW = 174), it is impossible to create an arginine-based polycation that equates the charge density of PEI (monomer MW = 43). In summary, the in vitro and in vivo evaluations demonstrated that PAGS possesses excellent biocompatibility unfound in existing polycations.
We set out to investigate the importance of biodegradability and use of endogenous building blocks on the biocompatibility of polycations. Many polycations satisfy one of the two parameters. To the best of our knowledge, none meets both. Polylysine (11.2 kDa) has been shown to be highly toxic (0.1 mg/ml) and induce apoptosis in a wide range of cells.[21a, 26] Polyarginine has shown toxicity to HeLa cells at a concentration of 0.03 mg/ml.[23] These polycations are built from endogenous amino acids, but are orders of magnitude less biocompatible than PAGS. The ester bonds in PAGS are more susceptible to hydrolysis than the amide bonds in polyarginine and polylysine. This difference likely contributed to the significant improvement of PAGS's biocompatibility. To improve biocompatibility, many synthetic polycations have been designed to be hydrolysable.[1, 27] These polycations have in general displayed higher biocompatibility than non-degradable ones. The highest reported concentration for their in vitro tests was 1 mg/ml. To the best of our knowledge, there has been no report on the in vivo biocompatibility of these existing hydrolyzable polycations. The essential difference between these hydrolyzable polycations and PAGS is the cations in PAGS derive from L-arginine, supporting the hypothesis that endogenous cations are necessary for a biocompatible polycation.
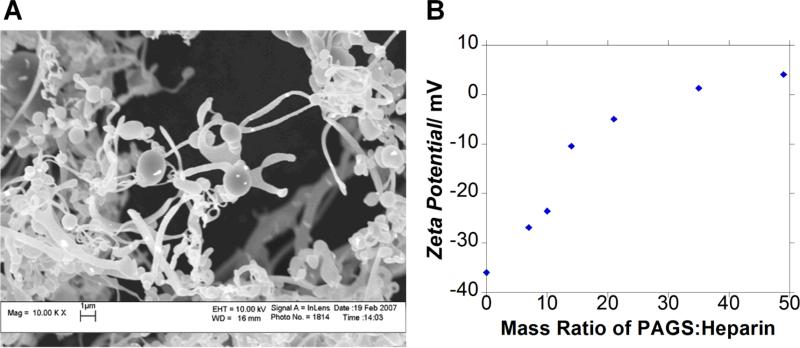
A synthetic polycation can be used for many biomedical applications. Control of the synthesis process should allow the control of its interaction with biological polyanions. One can take advantage of these interactions between oppositely charged macromolecules to precipitate biological polyanions such as nucleic acids and glycosaminoglycans. As a preliminary test, we explored the potential use of PAGS, we investigated the binding between PAGS and heparin, one of the most negatively charged glycosaminoglycans. The resultant [polycation:heparin] complex can be used to deliver heparin-binding growth factors. The interaction between heparin and heparin-binding growth factors is expected to stabilize and potentially activate the growth factors upon binding with the corresponding growth factor receptors.[28] Furthermore, it is possible to control the release of the growth factors by adjusting the polyvalency between the polycation and heparin. PAGS complexes heparin and precipitates it out of phosphate buffered saline as a fibrillar matrix (Fig. 5A). The diameter of the fibrils ranged from approximately 1 μm to sub-micron in diameter and sheets range from 5-20 μm in diameter. Globular structures were also observed dispersed among the fibrils. The addition of PAGS to the heparin solution increased the zeta potential following a sigmoidal curve (Fig. 5B).
Figure 5.
The interaction between PAGS and biological polyanions. (A) PAGS conjugates heparin to form a fibrillar matrix as revealed by SEM at a magnification of 10,000x. (B) Titration of heparin with PAGS as analyzed by zeta potential.
Synthetic polycations can be applied to many other applications including cell encapsulation, polyelectrolyte multilayers, biosensing, and coating of medical devices and implants. Polycation toxicity has been a great concern in these applications and a more biocompatible polycation would significantly advance their clinical translation.[29] A polycation can interact with alginate, a polymer widely used for cell encapsulation, to form a hydrogel with increased bioactivity and improved mechanical properties.[30] Polycations can also stabilize and control the molecular weight cut-off of the alginate microcapsule membrane.[31] Additionally, polycations can mediate cell encapsulation by forming multilayers through Coulomb interactions with polyanions. Polyelectrolyte multilayers are advantageous for cell encapsulation because the layer-by-layer assembly allows control of the local biochemical environment.[32]
3. Conclusions
We believe the design principle employed in this research is applicable to other building blocks and can lead to a variety of novel biomaterials. Other cationic amino acids such as lysine and diacids such as fumaric acid may yield polycations with additional functional groups and unique properties. The reported design allows structural flexibility and functional diversity including backbone rigidity, hydrophilicity, charge density, total charge, and side chain length. These inherent controls of polymer properties can be used to direct the interaction between the polycations and polyanions, an important determinant in many biomedical applications. The availability of biocompatible polycations may broaden the applications of biological polyanions and change their methods of administration.
4. Experimental
D-Arginyl ethyl ester Synthesis
D-Arginyl ethyl ester was synthesized by vigorously stirring D-arginine monohydrochloride and excess anhydrous ethanol in an ice bath. Acetyl chloride was added dropwise to the cold solution. The mixture was refluxed overnight. The reactants and side products were evaporated under reduced pressure to obtain the product.
Diglycidyl succinate
Diallyl succinate was synthesized by esterfication of succinic acid and allyl alcohol under the catalysis of sulfuric acid. The reaction mixture was refluxed for overnight and then sodium bicarbonate was added to neutralize the acid. The organic phase was extracted using ethyl acetate and brine. The product was then exposed to anhydrous sodium sulfate to remove residual water. Diglycidyl succinate was synthesized by epoxidation of diallyl succinate with meta-chloroperoxybenzoic acid (mCPBA) in dichloromethane. The reaction was refluxed at 40°C for overnight. Reaction mixture was then run through an ionic resin column containing tertiary amine beads to remove unreacted mCPBA. Diglycidyl suucinate was further purified using flash chromatography and stored at -20°C.
Polymer synthesis
The arginine-based polymer were synthesized via polycondensation reaction of a 1:1 molar ratio of L- or D-arginine ethyl ester and diglycidyl succinate or 1,4-butandiol diglycidyl ether in N,N-dimethylforamide (DMF) under N2. The reaction mixture was stirred and kept at 60°C for 7 days. The resultant polymers were purified by repeat precipitation in ethyl ether or ethyl acetate until NMR showed the products were pure enough. FTIR and gel permeation chromatography were used to further characterize the polymers.
pH titration
Acid titrations were performed by using 10 ml of 1 mg/ml PAGS solution. 0.1 M HCl was added sequentially while the solution was being stirred. The pH values were recorded by a SevenEasy pH meter (Metter-Toledo, Columbus, OH).
In Vitro Biocompatibility
Baboon smooth muscle cells (SMCs) were obtained from carotid arteries harvested from 17-20 kg juvenile male baboons and thoroughly characterized. Cells were cultured in MCDB 131 medium (Mediatech, Manassas, VA) supplemented with 10% FBS, 1.0% L-glutamine, 50 μg/mL ascorbic acid and 20 μg/mL gentamycin at 37°C with 5% CO2. BaSMCs (passages 6-14) were used for in vitro biocompatibility. To test lactate dehydrogenase (LDH) activity, metabolic activity and cell viability, 8×103 cells were seeded on 96-well plates one day before assay. For apoptotic assay, 5×104 cells were seed on 6-well plates. Different concentrations of polymer solution were prepared by dissolving polymers in MCDB 131 culture medium and incubated with cells for 4 h to test LDH activity, metabolic activity and apoptotic assay or 24 h to test cell viability. Extracellular LDH activity was measured using CytoTox 96® Non-Radioactive Cytotoxicity (Promega Madison, WI). Metabolic activity, caspase-3 activity and cell viability were measured by Vybrant® MTT Cell Proliferation Assay Kit, EnzCheck® Caspase-3 Assay Kit, and Live/Dead® Viability/Cytotoxicity Kit (Molecular Probes, Eugene, OR), respectively. All data were normalized to the control, which was culture medium without any polymer.
In Vivo Biocompatibility
Male Balb/C mice weighing 19-21 g were injected intraperitoneally with PAGS/saline solution (n=10), PEI/saline solution (n=9), or saline (n=3) as control. Animals were cared for in compliance with protocols approved by the Committee on Animal Care of the Georgia Institute of Technology following NIH guidelines for the care and use of laboratory animals (NIH publication No. 85-23 rev. 1985). Major organs including heart, liver, lung, kidney, spleen, and bladder with prostate gland were harvested at day 1, 5, and 30 post-injection. The organs were rinsed with PBS, fixed with 10% formalin and then submersed in 30% sucrose at 4°C. For cryosection, all samples were embedded in Tissue-Tek OCT Compound (Sakara Finetek USA, Torrance, CA). Cross-sections (10 μm thick, the longitudinal axis cut) were stained with the standard hematoxylin and eosin (H&E) staining method to examine inflammation and fibrosis. Sections were also stained for nuclear DNA fragmentation using DeadEnd™ Fluorometric TUNEL System (Promega, Madison, WI) to examine apoptosis. All slides were analyzed blindly by Dr. Adeboye Osunkoya at Emory University.
Heparin Conjugation
PAGS (8 mg/ml) and heparin (10 mg/ml) were dissolved in molecular grade water. Increasing amounts of PAGS solution was added dropwise to the heparin solution and incubated at room temperature for 15 minutes with mild agitation. From prepared complexes, 750 μl was diluted to a final volume of 1.5 ml and analyzed for zeta potential measurements. The [PAGS:heparin] complex was dropped directly on SEM sample stud, lyophilized, and sputtered with gold for SEM observation.
Polymer degradation
Degradation of PAGS, Et-PAGS, and D-PAGS were performed following established protocols.[19] Briefly, the polymers were dissolved in PBS. Bovine pancreatic cholesterol esterase (Sigma-Aldrich, St. Louis, MO) was added to reach the final concentration of 4 units/(mg of polymer). The reaction was carried out at 37 °C for 7 or 14 days. The retention time of the polymers before and after the enzymatic treatment was determined by a Viscotek GPCmax VE2001 equipped with a VE 3580 RI detector (Malvern, Westborough, MA). A Suprema Max 3,000Å column (PSS, Warwick, RI) and PBS were used as the stationary and mobile phases respectively.
MTT assay of degradation products
The degradation products were collected by passing through an Amicon Ultra centrifugal dialysis membrane with a cut-off molecular weight of 3 kDa (Millipore, Billerica, MA). The fraction that had a MW less 3 kDa was collected and lyophilized overnight. The degradation products were added to the cell culture medium (conditioning medium) in which the MTT assay was performed using the same protocol described above.
Statistical Analysis
For each condition tested, the results of four replicates were reported as a mean value with the standard deviation. Multicomparisons ANOVA, Tukey Method, was used as a statistic tool to compare the different experimental values; p < 0.05 was considered significantly different.
Supplementary Material
Acknowledgments
This research is supported by an NSF grant # DMR-1005766. We thank Anh Nguyen for experimental assistance. We greatly appreicate the Nils Kröger laboratory for assistance with dynamic light scattering experiments.
References
- 1.a Jon S, Anderson DG, Langer R. Biomacromolecules. 2003;4:1759–62. doi: 10.1021/bm034176f. [DOI] [PubMed] [Google Scholar]; b Lynn D, Langer R. J Am Chem Soc. 2000:10761–8. [Google Scholar]
- 2.Felsenfeld G. Science. 1978;271:115–22. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- 3.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 4.Faham S, Hileman RE, Fromm JR, Linhardt RJ, Rees DC. Science. 1996;271:1116–20. doi: 10.1126/science.271.5252.1116. [DOI] [PubMed] [Google Scholar]
- 5.Ganz T. Science. 2002;298:977–9. doi: 10.1126/science.1078708. [DOI] [PubMed] [Google Scholar]
- 6.a Hancock RE. Lancet Infect Dis. 2001;1:156–64. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]; b Ganz T. Nature. 2001;412:392–3. doi: 10.1038/35086680. [DOI] [PubMed] [Google Scholar]
- 7.Albertsson A, Varma I. Aliphatic polyesters: Synthesis, properties and applications. Springer-Verlag; Berlin: 2002. [Google Scholar]
- 8.Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL. Nature. 2000;407:1029–34. doi: 10.1038/35039551. [DOI] [PubMed] [Google Scholar]
- 9.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Garland Science. 4 ed. New York, NY: 2002. p. 1616. [Google Scholar]
- 10.Here biostable polymers are defined as those that degrade much slower than biodegradable polymers.
- 11.Braiman M. J Phys Chem B. 1999:4744–50. [Google Scholar]
- 12.Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Proc. Natl. Acad. Sci. 2005;102:5679–84. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansouri S, Winnik FM, Tabrizian M. Expert Opinion on Drug Delivery. 2009;6:585–97. doi: 10.1517/17425240902967599. [DOI] [PubMed] [Google Scholar]
- 14.Babich H, Zuckerbraun HL, Wurzburger BJ, Rubin YL, Borenfreund E, Blau L. Toxicology. 1996;106:187–96. doi: 10.1016/0300-483x(95)03189-m. [DOI] [PubMed] [Google Scholar]
- 15.Mossman T. J Immunol Methods. 1983:55–63. [Google Scholar]
- 16.Thornberry NA, Lazebnik Y. Science. 1998;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 17.Kaneshiro E, Wyder MA, Wu Y, Cushion M. J Microbiol Methods. 1993;17:1–16. [Google Scholar]
- 18.Diaspro A, Silvano D, Krol S, Cavalleri O, Gliozzi A. Langmuir. 2002;18:5047–50. [Google Scholar]
- 19.Shirahama H, Shiomi M, Sakane M, Yasuda H. Macromolecules. 1996;29:4821–8. [Google Scholar]
- 20.Chanana M, Gliozzi A, Diaspro A, Chodnevskaja I, Huewel S, Moskalenko V, Ulrichs K, Galla HJ, Krol S. Nano Letters. 2005;5:2605–12. doi: 10.1021/nl0521219. [DOI] [PubMed] [Google Scholar]
- 21.a Hong S, Leroueil PR, Janus EK, Peters JL, Kober MM, Islam MT, Orr BG, Baker JR, Jr., Banaszak Holl MM. Bioconjug Chem. 2006;17:728–34. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]; b Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. Biomaterials. 2003;24:1121–31. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 22.a Dekie L, Toncheva V, Dubruel P, Schacht EH, Barrett L, Seymour LW. J Control Release. 2000;65:187–202. doi: 10.1016/s0168-3659(99)00235-7. [DOI] [PubMed] [Google Scholar]; b Ryser HJ. Nature. 1967;215:934–6. doi: 10.1038/215934a0. [DOI] [PubMed] [Google Scholar]
- 23.Bendifallah N, Rasmussen FW, Zachar V, Ebbesen P, Nielsen PE, Koppelhus U. Bioconjug Chem. 2006;17:750–8. doi: 10.1021/bc050283q. [DOI] [PubMed] [Google Scholar]
- 24.Kim T-I, Bai CZ, Nam K, Park J-S. J Control Release. 2009;136:132–9. doi: 10.1016/j.jconrel.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Funhoff AM, van Nostrum CF, Lok MC, Fretz MM, Crommelin DJ, Hennink WE. Bioconjug Chem. 2004;15:1212–20. doi: 10.1021/bc049864q. [DOI] [PubMed] [Google Scholar]
- 26.Hunter AC. Adv Drug Deliv Rev. 2006;58:1523–31. doi: 10.1016/j.addr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 27.a Lim Y, Kim SM, Lee Y, Lee W, Yang T, Lee M, Suh H, Park J. J Am Chem Soc. 2001;123:2460–1. doi: 10.1021/ja005715g. [DOI] [PubMed] [Google Scholar]; b Lim YB, Kim CH, Kim K, Kim SW, Park JS. J Am Chem Soc. 2000;122:6524–5. [Google Scholar]; c Wang J, Mao HQ, Leong KW. J Am Chem Soc. 2001;123:9480. doi: 10.1021/ja016062m. [DOI] [PubMed] [Google Scholar]; d Putnam D, Langer R. Macromolecules. 1999;32:3658–62. [Google Scholar]
- 28.a Pellegrini L. Curr. Opin. Struct. Biol. 2001;11:629–34. doi: 10.1016/s0959-440x(00)00258-x. [DOI] [PubMed] [Google Scholar]; b Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL. Nature. 2000;407:1029–34. doi: 10.1038/35039551. [DOI] [PubMed] [Google Scholar]
- 29.a Chanana M, Gliozzi A, Diaspro A, Chodnevskaja I, Huewel S, Moskalenko V, Ulrichs K, Galla HJ, Krol S. Nano Lett. 2005;5:2605–12. doi: 10.1021/nl0521219. [DOI] [PubMed] [Google Scholar]; b Wilson JT, Cui W, Chaikof EL. Nano Lett. 2008;8:1940–8. doi: 10.1021/nl080694q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsich E, Borgogna M, Donati I, Mozetic P, Strand BL, Salvador SG, Vittur F, Paoletti S. J Biomed Mater Res A. 2008;84:364–76. doi: 10.1002/jbm.a.31307. [DOI] [PubMed] [Google Scholar]
- 31.Orive G, Tam SK, Pedraz JL, Halle JP. Biomaterials. 2006;27:3691–700. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 32.a Lynn DM. Advanced Materials. 2007;19:4118. [Google Scholar]; b Decher G. Science. 1997;277:1232–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.