Abstract
Brain cancer tumors cause disruption of the selective properties of vascular endothelia, even causing disruptions in the very selective blood–brain barrier, which are collectively referred to as the blood–brain–tumor barrier. Nanoparticles (NPs) have previously shown great promise in taking advantage of this increased vascular permeability in other cancers, which results in increased accumulation in these cancers over time due to the accompanying loss of an effective lymph system. NPs have therefore attracted increased attention for treating brain cancer. While this research is just beginning, there have been many successes demonstrated thus far in both the laboratory and clinical setting. This review serves to present the reader with an overview of NPs for treating brain cancer and to provide an outlook on what may come in the future. For NPs, just like the blood–brain–tumor barrier, the future is wide open.
Keywords: brain tumors, clinical trials, drug delivery, imaging, nanoparticles, theranostics
Use of nanoparticles for brain cancer
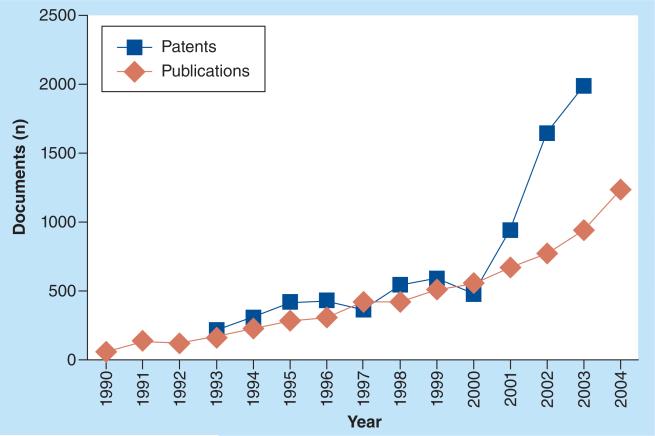
During one of the greatest periods of human achievement, the industrial revolution, humanity sought to expand ever outward and upward. A radical change has recently occurred, where in less than a century we have gone from developing macroscopic machines and structures to developing technology that is smaller than the width of a single human hair. We now see ourselves looking for smaller, more compact solutions to solve problems in the development of nanotechnology, seeking to become more efficient and less wasteful than a century ago. In addition, humans are now living longer due to an ever-improving understanding of our own physiology [1]. Nanomedicine has developed during this time, fueled by an ever-increasing pressure from society for more efficacious treatments as the cost of healthcare and populations continue to rise [2]. The general definition of nanotechnology is the creation and use of materials that have dimensions in the range of 1–100 nm [3–5]. However, many larger particles, sometimes as large as 1000 nm, have also been considered as nanoparticles (NPs), as long as they have dimensions comparable with biological functional units such as viruses [3–5]. There is a belief that NPs are the future of individualized medicine and the medical industry in general [4,6,7]. In 2006, a global survey was conducted by the European Science and Technology Observatory, which found that a total of 38 nanomedicine products have been approved for clinical use for a total market size of $6.8 billion [3]. Drug delivery is the most popular use for nanomaterials with a total of 23 clinically approved devices, a 75% share of total sales and a 76% share of scientific papers on nanomedicine [3]. NP systems have become a very popular research topic for drug delivery, with most applications aimed at diagnosing or treating cancer [5]. For years, medicines and treatments have been relatively ineffective at treating brain cancer [8–10]. Most of the current treatment options suffer from an inability of therapeutics to cross the blood–brain barrier (BBB) due to its restrictive transport properties. NPs provide a means to increase transport across the BBB and/or blood–brain–tumor barrier (BBTB) and for this reason have been exploited in the treatment of brain cancer [11–27]. In this review we will discuss the formation and use of NPs for the treatment of brain cancer. We will critically review the current state of the art and highlight opportunities for future developments. We have attempted to include seminal references for this discussion but admit that the fast-pace growth of this field, as seen in Figure 1, prevents inclusion of every reference, for which we apologize in advance.
Figure 1. The increasing rate of nanomedicine publications and patents.
Reproduced with permission from [3].
The synthesis of targeted NPs
In brain cancer research, several different types of NPs have been employed as imaging and delivery agents, including both solid–inorganic (magnetic Fe3O4 NPs [28–39], gadolinium NPs [26,40], gold NPs [AuNPs] [41] and semiconductor quantum dots [QDs] [42,43]) and organic-based (dendrimer [16,25,27,44], hydrogel [15] and polymer [45–68]) NPs. As expected, the synthetic routes for each system will be quite different. However, several unifying approaches based on whether the final product is predominately inorganic or organic allows for a quick review of common synthetic procedures. Here, we provide a cursory review of the general pathways for synthesizing both inorganic and organic NPs, and conclude by examining how both structures rely on active targeting for utilization in brain cancer therapy.
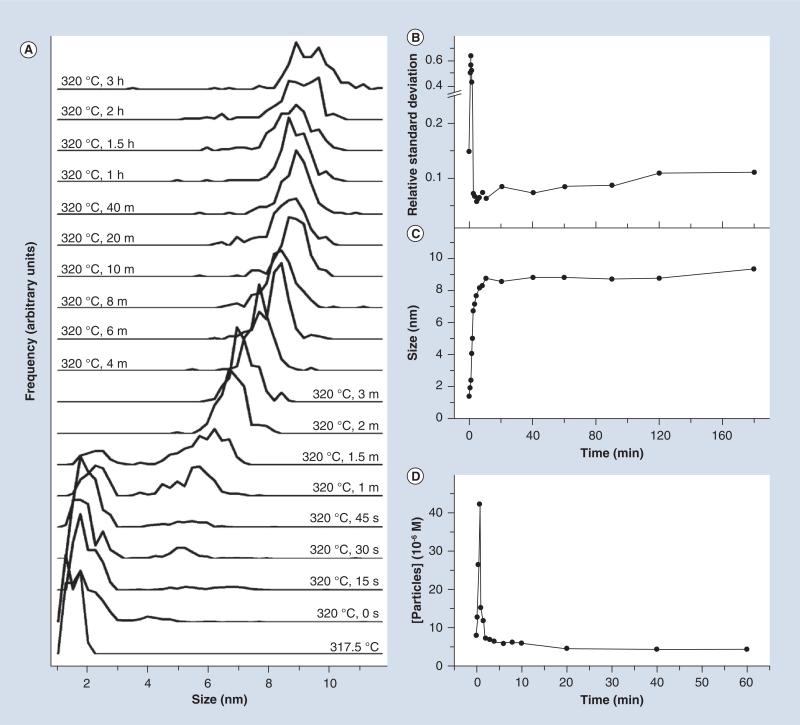
Inorganic NPs consist of a solid core material that can be either a magnetic metal oxide or a metallic or semiconductor framework. A sampling of the literature from the past decade finds that magnetic Fe3O4 NPs are the most popular inorganic motif for imaging brain tumors [28–39]. An excellent introductory review of synthetic details of magnetic metal oxide NPs has been presented by Hyeon [69]. Typically, synthesis of Fe3O4 NPs consists of reduction of an iron precursor under an inert atmosphere with a source of reactive oxygen at high temperatures [70]. The prolonged reflux of the reaction leads to a process known as Ostwald ripening, whereby smaller particles are incorporated into larger particles with time (Figure 2) [69]. The use of an exchangeable capping ligand (such as oleylamine) or a direct incorporation of a hydro-philic ligand completes the framework for subsequent modification. The synthesis of other metal oxide NPs, such as gadolinium oxide, also takes advantage of this approach [40], and semiconductor particles (termed QDs) are also produced in a similar fashion [71]. Alternatively, successful synthesis of these inorganic NPs has been obtained through coprecipiation methods involving the precipitation of metal ions with the corresponding counterions in solution, as well as microemulsion methods that utilize chemical interfaces to control size and morphology [72]. An excellent review of the magnetic properties, synthesis and functionalization of magnetic NPs has been provided by Lu et al. [72]. Similarly to these inorganic NPs, the generation of metallic NPs involves a metal precursor being reduced in the presence of a capping ligand to form metallic bonds. In the case of AuNPs, this process can be undertaken in both aqueous (Turkevich–Frens method [73,74]) and organic (Brust–Schiffrin method [75]) environments, with efficient tuning of the size based on the relative ratio of reactants and reaction times. Inorganic particles require further modification to improve water solubility and stability, with polyethylene glycol (PEG) being popular due to the ‘stealth’ character during blood circulation and low toxicity [76].
Figure 2. Controlling nanoparticle size.
(A) Frequency analysis of particle size during the synthesis of iron oxide nanoparticles as a function of time utilizing high temperature synthesis. Corresponding plots of (B) size dispersity, (C) overall size and (D) particle concentration are also shown for comparison.
Reproduced with permission from [139].
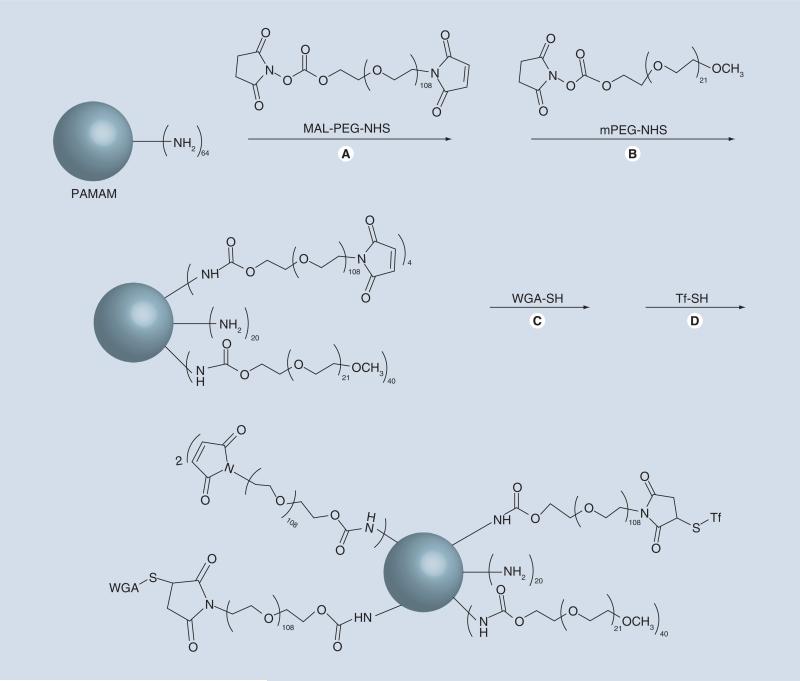
By contrast to inorganic NPs, formulations of organic NPs (including dendrimer, hydrogel and polymeric) rely on the direct physiochemical properties of the material in aqueous environments, which allow for dissolution but retain a soft, solid structure with nanoscale dimensions [77–79]. In many cases the core material is commercially available; however, these components are often further modified through conjugation to other polymers such as PEG [16]. For xample, He et al. utilized commercially available poly(amido amine) (PAMAM) as the core dendrimer with PEG functionalization through reaction of the dendrimer amine groups with activated N-hydroxysuccinimide groups on the PEG molecules (Figure 3) [16]. Organic NPs are often considered ‘soft’ particles, indicating easy deformability and flexibility in vivo.
Figure 3. Adding functionality to nanoparticles.
Synthetic scheme for the generation of monofunctional and bifunctional PEGylated PAMAM dendrimer nanoparticles (A & B), which allows for the incorporation of WGA and Tf-targeting moieties (C & D).
PAMAM: Poly(amido amine); PEG: Polyethylene glycol; Tf: Transferrin; WGA: Wheat germ agglutinin.
Reproduced with permission from [16].
It is important to note that while it seems relatively simple to distinguish inorganic from organic NPs, such distinction tends to oversimplify the complex differences between the two groups. For example, while the term ‘particle’ generally refers to a solid with definitive dimensions, this is most commonly associated with inorganic NPs. While this may be the case, organic structures often do not have such restrictions in terms of overall material density or shape, such as with water soluble polymers, hydrogels and dendrimers, which can lead to not only ‘soft’ properties but to unique biological processing. In addition, the relaxation of specific dimensions as well as standard chemical synthesis allows for modifications to drug incorporation and targeting. Readers interested in a deeper account of organic nanocarriers are directed to the review by Shi et al. for a more focused account of these important materials [80].
The ability to chemically modify polymer constructs is the foundation for generating targeted NPs, regardless of inorganic or organic designation. The use of condensation reactions have been heavily utilized in the literature for incorporating a myriad of targeting ligands to the NP scaffold, including EGF-related targets [32,40,41], transferrin [15,55,68], lactoferrin [34], trans-activating transcriptional activator [63], aptamers [66,67] and numerous other peptides [37,48,49]. The use of the peptide sequence known as Angiopep has recently become important for the targeting of brain cancer [27,58], as both the BBB and gliomas are known to overexpress the corresponding receptors [58]. Another popular targeting motif for brain cancer is chlorotoxin [28,30,31,38,54]. Veiseh et al. found that the incorporation of chlorotoxin onto functionalized Fe3O4 NPs (Figure 4) resulted in a significant increase in the total uptake within the brain tumors of mice after in vivo injection when compared with untargeted particles [31]. Similar to the schematic in Figure 4, the use of reactive groups (including carboxylic acids, amines, thiols and succinimide derivatives) allows for the direct chemical bonding of the targeting ligand to the particle, providing improved control and utility in preparing brain-specific NPs.
Figure 4. Synthesizing targeted nanoparticles.
(A) A multifunctional iron oxide nanoparticle for brain imaging. The incorporation of a molecular dye (Cy5.5) and targeting moiety (chlorotoxin) through chemical synthesis (B) was accomplished after incorporating PEG into the chitosan polymer (C) and functionalization of the chlorotoxin (D) through standard chemical coupling reactions.
PEG: Polyethylene glycol.
Reproduced with permission from [31].
In addition to single targeting additions to NPs for brain therapeutics, several groups have experimented with a dual targeting moiety system [25,44,56,66,67]. In work reported by Yan et al., PAMAM dendrimers were functionalized with Angiopep to translate through the BBB and RGDyk peptides were added to target brain tumor vasculature [44]. The authors concluded that the use of dual targeting allowed for better identification of the tumor boundaries, which could be useful for surgery. Gao et al. have also reported that the incorporation of both a small peptide for better BBB targeting and an additional aptamer to better target cancer cells onto a polymer NP [66]. The authors found that the dual targeting system had the highest tumor distribution. The success of these early studies indicates that the future of brain cancer imaging will inherently rely on multimodal NPs.
Noncovalent transport of therapeutics
With the advantages of highly controlled size, surface properties and chemical functionalization, NPs serve as excellent molecular transporters that can simultaneously improve solubility and protection while improving drug delivery and localization [41,81]. The issue of drug solubility is especially important; it has been estimated that nearly 40% of potential drug candidates are hydrophobic, limiting their application in vivo [81]. The BBB provides another level of complications, which hinders pharmaceutical transport into the brain tissue [82,83]. NPs provide a local environment that can stabilize these hydrophobic molecules while still retaining solubility in the outer solvation sphere. In the case of the solid inorganic particles mentioned (magnetic, metallic and semiconductor), the hydrophobic drugs are mainly incorporated at the NP surface within the polymer layer, while for organic NPs (and in the special case of mesoporous silica NPs) the same molecules are largely encapsulated within the interior of the NP [84]. Regardless of which methodology is used for brain tumor therapy, the NP must both facilitate transport through the vasculature system and the BBB, and effectively release the incorporated molecule upon reaching the target.
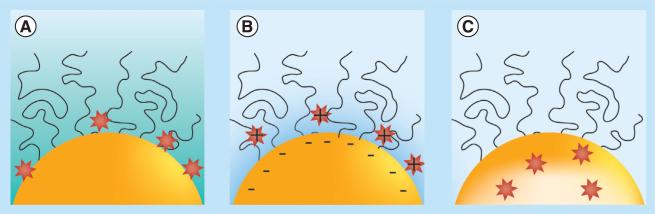
The noncovalent loading of target molecules can be broadly defined as any physical force that associates a molecule with the NP without forming a direct chemical bond. Figure 5 illustrates the typical physical forces that are utilized to incorporate drugs reported in the literature, including hydrophobic (Figure 5A) [41], electrostatic (Figure 5B) [36] and physical confinement (Figure 5C) [56], which are typical for loading imaging agents and drug payloads. The methodology chosen will naturally have its own strengths and weaknesses: for example, hydrophobic partitioning is useful for a broad range of macromolecules for improving solubility, but potential limitations include the possibility of encountering a more favorable hydrophobic environment such as a cell membrane (i.e., losing the drug before it makes it to the brain or brain tumor). Similar arguments can be made for electrostatic (i.e., high ionic strength media and competitive absorption) and physical barrier (i.e., site specific release and sufficient kinetic rates) methodologies. In the special case of brain imaging, the complications of the BBB can be particularly challenging [82,85].
Figure 5. Noncovalent incorporation of drug molecules.
Illustrations of three main noncovalent drug-loading schemes including (A) hydrophobic, (B) electrostatic and (C) physical confinement-based drug conjugation.
The use of NPs for encapsulation or surface-immobilized transport of dyes and imaging probes has a rich history in literature. The incorporation of therapeutics onto inorganic NPs has been demonstrated for both general in vivo applications and specifically for brain-targeted therapy. Cheng et al. reported that the hydro-phobic incorporation into PEGylated AuNPs of a silicon phthalocyanine dye (Pc 4) [86], which can be used as both a photodynamic therapeutic agent and a fluorescent marker, showed dramatic enhancement over covalent attachment of the drug to the NP [87]. The same team later found that the inclusion of an EGF peptide sequence could facilitate transport to the brain tumors [41]. Similarly, Kim and co-workers found that hydrophobic drugs could be incorporated into monolayers of polyelectrolyte coated AuNPs for cellular delivery [88]. Recently, Nie et al. have shown that PEGylated polyacrylamide hydrogel particles could be loaded with Coomassie Blue and provide specific tumor cell staining when targeting peptides were incorporated [15]. Liu et al. utilized free amino and carboxyl groups in their polymer-coated Fe3O4 magnetic NPs to immobilize the drug epirubicin for brain cancer therapy [36]. For organic-based NPs, the incorporation of drugs using encapsulation is by far the most popular technique (and can be done during the polymerization step), and the transport of therapeutics such as paclitaxel [47], doxorubicin [53,59], camptothecin [60] and ferrociphenol [65] have been reported. It should be noted that in addition to these methods, the covalent delivery of therapeutics (such as siRNA) has been reported for both inorganic [43] and organic [62] NPs utilizing cleavable disulfide bonds. Thus, the ability to functionalize NPs allows for both noncovalent and covalent therapeutic delivery, providing an important tool for rationally designing a NP transporter.
Labeled NPs as ‘theranostics’
It should be apparent that one of the most promising aspects of nanomedicine is the multi modality of a NP in any given application. The incorporation of both diagnostic and therapeutic modalities onto the same NP is a powerful approach to improving current drug delivery initiatives. This phenomenon has led to the term ‘theranostic’, which is attributed to Funkhouser, and is described by Kelkar and Reinke in their excellent theranostic review [89]. Moreover, Koo and coworkers have previously explored the importance of multimodality in brain cancer theranostics [90]. Therefore, we focus here only on the examples from the literature where dual modality NPs have been utilized for brain imaging.
The most important example of a NP theranostic agent is that of the magnetic NPs, which are inherently active for MRI applications. Liu et al. utilized polymer-coated magnetic NPs to both deliver the anticancer drug eprubicin and provide an MRI contrast agent in brain tissue [36]. Interestingly, the authors utilized ultra-sound to generate microbubbles that altered the permeability of the BBB to allow the magnetic NPs through [36]. Dilnawaz et al. have recently reported the delivery of pacilitaxel with glyceryl monoleate-coated magnetic NPs and demonstrated improved uptake of the drug in brain tumor tissue as well as significant accumulation of the NP itself to use as an MRI contrast agent in a rat model [39]. Other examples of magnetic NP-based theranostics are prevalent in the literature [33,35,38]. Many organic NP systems also utilize both MRI and drug delivery by incorporating a magnetic contrast agent covalently into the polymer framework. For example, Reddy et al. reported the use of a contrast agent with a polymer NP that also incorporated a fluorescent drug cargo [49].
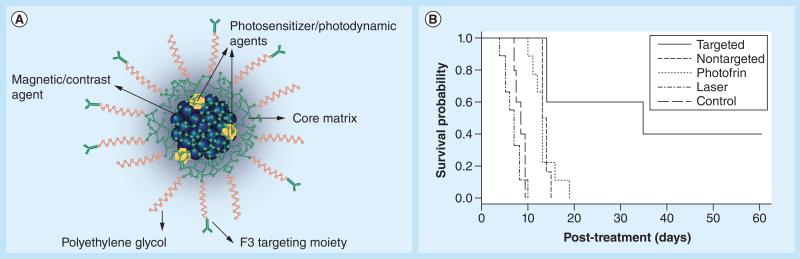
The work of Reddy et al. highlights an important progression of the theranostics, where both the NP and the therapeutic agent contribute to the theranostic aspect of the NP. Figure 6 shows an illustration of their system, which includes the photodynamic therapy (PDT) drug Photofrin® (Pinnacle Biologics, Inc., IL, USA). In most cases, a typical PDT agent can both generate singlet oxygen as well as emit fluorescence, allowing for simultaneous therapy and imaging in the same system. By utilizing drugs that can themselves be imaged, the distribution of a drug relative to that of its NP carrier can also be explored. Cheng et al. utilized metallic NPs to deliver the PDT drug, phthalocyanine, to brain tumors, and the use of the drug's fluorescence provided a powerful method for monitoring drug uptake [41]. Subsequent work by the same authors followed the biodistriubtion of the NPs and the drug, noting significant differences that were probably a result of drug release from the NPs due to reversible hydrophobic adsorption of the drug to the NPs [91]. The ability to create a ‘theranostic NP’ by using a ‘multimodal drug’ provides an exciting opportunity to explore pharmacokinetics in real time, and is a promising avenue for future research in brain cancer imaging and therapy.
Figure 6. Theranostic nanoparticles.
(A) Illustration of the theranostic organic nanoparticle designed by Reddy et al. [49], which included a magnetic contrast agent, fluorescent drug and targeting ligand (F3). (B) Survival probability as a function of time for mice bearing brain tumors under different conditions. The use of the theranostic nanoparticle drastically improved the survival rate relative to the other treatments tested.
Reproduced with permission from [49].
Strategies of overcoming the BBB
A major hurdle in successfully treating brain cancer is the presence of the selective barrier between brain tissue and the blood, appropriately named the BBB. The BBB is highly permeable to water, CO2, oxygen and lipid-soluble substances like alcohol [92]. The BBB is slightly permeable to electrolytes, but almost completely impermeable to plasma proteins and many nonlipid-soluble large organic molecules [92]. This selective permeability of the BBB arises from the tight junctions formed within the capillary endothelium of the brain, which eliminate the normal passage of molecules as found in most other capillaries of the body [11,12,92,93]. This selectivity limits the passage of small, nonlipid-soluble molecules to a molecular mass under a 400–600-Da threshold [11,12,92,93]. The BBB exists at tissue capillary membranes in essentially all areas of the brain, with the exception of some areas of the hypothalamus, pineal gland and area postrema [92]. These are areas of the brain that need to have easier (and thus faster) diffusion of molecules because they contain specialized sensory receptors that need to respond to very small and specific changes in body fluids or are regulated by large peptide hormones [92]. Some examples would include glucose concentration sensing and the passage of the hormone angiotensin II, which regulates thirst [92]. The BBB also has specific carrier molecules that are able to facilitate the transport of hormones that would otherwise be too large to pass through the BBB [92]. In addition to the obstacle of the specific permeability of the BBB is the role of efflux pumps, multidrug resistance proteins and the exposure of degrading enzymes in further decreasing the bioavail-ability of many popular chemotherapeutic drugs in the brain [23,24].
However, it is important to note that the presence of a brain tumor disrupts this very selective BBB, and creates an opportunity for the improved delivery of therapeutic agents [25]. Just as most therapeutics and NPs take advantage of the enhanced permeability and retention effect to accumulate particles and/or drugs over time due to leaky vasculature and an ineffective lymph system for cancer elsewhere in the body, a similar disruption still exists for tumors in the brain. Studies of brain microvasculature have shown that gaps do exists in the BBTB and that these gaps have an upper limit of somewhere between 20 and 100 nm [25,94–97].
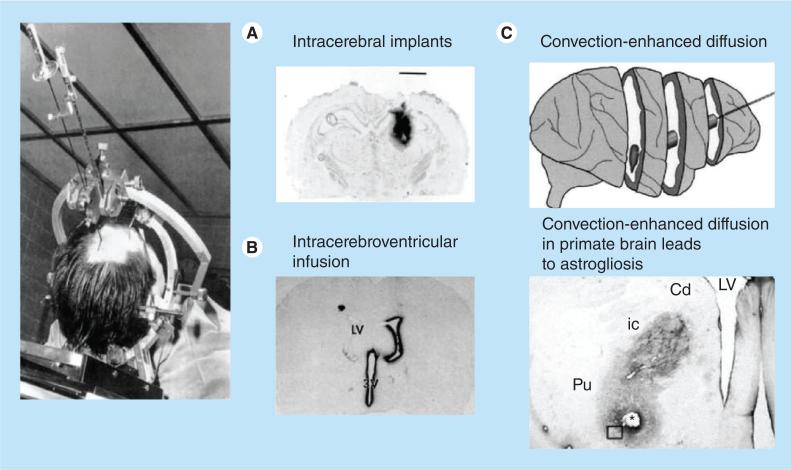
There are three major approaches to delivering therapeutics into the brain, which include invasive, pharmacological and physiological methods [23]. Invasive approaches rely on the direct administration of drugs into the brain through varying means such as stereotactically guided drug insertion through a catheter, placement of intracerebral drug implants and osmotic disruption of the BBB, among others [11,23]. These approaches (some of which are pictured in Figure 7) suffer from invasiveness, effective treatment volume of the drug, are costly, and require both anesthesia and hospitalization.
Figure 7. Invasive drug delivery methods for brain cancer therapy.
Examples of invasive approaches to deliver drugs to the brain. (A) Intracerebral drug implants. (B) Intracerebroventricular infusion. (C) Convection-enhanced diffusion. The asterisk demarcates the hole left in the brain by the catheter, the insertion of which is shown in the top image of (C).
Cd: Caudate; ic: Internal capsule; LV: Left ventricle; Pu: Putamen.
Reproduced with permission from [10].
Besides physical methods to bypass or disrupt the BBB/BBTB, the pharmacological approach relies on modification of drugs to enable penetration through the BBB. For example, reducing the relative number of polar groups on the compound of interest has also been attempted [23]. These approaches often lead to a loss of the activity of the drug. For example, increasing the lipophilicity of a molecule can improve penetration through the BBB, but may also make it susceptible to drug efflux pumps [23].
Physiological approaches take advantage of the requirement of the brain for essential nutrients needed for survival. Substances such as glucose, insulin, growth hormones and low-density lipoproteins, for example, require recognition by specific receptors or transport mechanisms in order to penetrate the BBB [23]. These approaches take advantage of the brain's high perfusion rate and the short distances that separate its capillaries, due to the fact that nearly every neuron in the brain is perfused by its own capillary [22,23]. Therefore, this method is most effective for drugs that interact with neurons for neural diseases, but can also be applicable to other diseases, especially cancer. This is due to the fact that tumors tend to grow quickly and must stimulate angiogenesis by VEGF and other growth factors, which tend to further increase the blood perfusion rate in and around tumors [98–100].
The physiological approach is advantageous as it uses naturally occurring internalizing receptors to assist in penetrating the BBB. One such method includes using transporter-mediated delivery, the use of peptides, antibodies or small molecules attached directly to the drug of interest or to its delivery vehicle, to initiate penetration of the BBB through specific interaction with transporter proteins that are expressed on the luminal (the side of the endothelial cells interacting with blood) and the basolateral (the opposite side of the endothelial cells that interacts with tissues) sides of the endothelial cells [11,12,23]. Another method is to use receptor-mediated transcytosis of molecules by interacting with cellular receptors that are highly expressed on the surface of endothelial cells, which form the BBB to allow drug molecules to pass through the endothelial cells [23]. A third physiological method is to take advantage of low-density lipoprotein receptor proteins (LRPs), which are receptors that mediate the internalization and degradation of multiple ligands [23,101]. LRPs are expressed on many tissues and in the CNS. LRPs are expressed on neurons and astrocytes, but are also overexpressed in malignant astrocytomas such as glioblastomas, which are the most common and aggressive forms of brain cancer [23,101]. Another method is to use adsorptive-mediated transcytosis, which involves the use of charged substances such as cationic proteins to bind to the luminal membrane of the endothelial cells to trigger a nonspecific uptake of the substance [23]. Some limitations of physiological approaches include the limited kinetics available to transport molecules, the requirements needed to bind to transporters and receptors, creating a drug that can remain active during the process (e.g., to avoid degradation by lysosomes) and the actual transport of the drug into the brain as opposed to it just remaining at the transporter or receptor [23].
The final approach to bypass the BBB is through the use of NPs. While this review will focus on the use of NPs to deliver therapeutics to brain cancer by bypassing the BBTB, NPs can be used in similar ways to deliver therapeutics through the undisturbed BBB [23,100]. In NP research, investigators have taken advantage of invasive [32,50,60,102–104], pharmacological [43,46,52,53,59,62,64] and physiological [16,27,32,38,41,58,63,68,81,105–107] methods to enhance brain cancer therapy. Importantly, however, research has shown that while each method can be used individually or simultaneously, the detrimental effects associated with each (long recovery times, decreased drug uptake and low receptor mediated transport) can be overcome through careful NP design [4,7,11,22,25]. In particular, investigators have also sought to exploit the physiological method to enhance NP delivery into the brain and into brain cancers. NPs offer many advantages as drug delivery vehicles, including increased circulation of drug, increased payloads, protection of the drugs from degradation and the ability to ‘solubilize’ drugs that have inherent pharmacological properties that make systemic delivery difficult [24,25,108–111]. This is accomplished by adding moieties to the NPs (as discussed earlier), which protect the drugs [108,109]. Additionally the particles can be decorated with moieties that promote uptake and transcytosis (i.e., exploiting physiological methods to enhance NP uptake) [24,25,100,108–110]. Here, we discuss how the unique properties of NPs described earlier have been used to significantly enhance the imaging and treatment of brain cancer, and how future efforts can take advantage of rationally designed NPs for improved therapy.
Imaging & treatment of brain cancer using NPs
While NPs can function as delivery vehicles with modifiable sizes, shapes, surfaces and targeting moieties that serve to increase the blood retention, bioavailability and specificity of cancer therapeutics, they can also allow loading of additional drugs for simultaneous multi drug delivery or even the addition of imaging probes for in vivo monitoring or diagnosis [24,25,84,108–111]. Furthermore, NPs sometimes offer inherent advantages arising from their composition. One such example is the use of metallic NPs that can be heated with light or radiofrequencies, or by a magnetic field for thermal ablation of tumors [112–114]. Another example is contrast enhancement by magnetic NPs used for MRI and contrast enhancement by AuNPs for computed tomography imaging [5,13,26,32,115,116].
While inorganic NPs have seen some use in clinical applications for cancer, many still require a greater understanding of their clearance and safety [24,117,201]. The inorganic NPs that have seen clinical use are mainly NPs used for MRI contrast agents, such as Fe3O4 and gadolinium NPs. Fe3O4 NPs with diameters around 50 nm have been relatively well-tolerated, while some cases of toxicity do exist for gadolinium NPs most notably for nephrogenic systemic fibrosis [118–120]. Many industrial studies exist for ultra-fine particles of diameters less than 100 nm, but to this day an organized and complete study of all inorganic NPs does not exist [24,117,201]. Therefore, the choice of material; its size, and the way in which it is coated or protected becomes of great importance in moving a NP into clinical use. It makes sense that choosing an inorganic material that is generally inert, such as Au, is better than choosing a material that has inherent side-effects such as unchelated gadolinium or QDs [120,121]. Furthermore, it is generally accepted that for a nonbiodegradable polymer NP to be able to be excreted it must have a diameter less than the renal filtration cutoff of approximately 5–6 nm [122]. Most polymer NPs benefit from being biodegradable or biocompatible and therefore have also been well-tolerated in clinical trials [4,24]. However, there is still very little data available on the long-term fate of polymers and possible toxicity they may generate in neuronal cells [123]. Most inorganic NPs, therefore, also employ an organic polymer as a protective layer that increases circulation half-life and protects both the particle from the body and the body from the particle [4,5,7,24,29,32,41,50,86,124]. There remain many questions regarding the long-term distribution and safety of unprotected inorganic NPs and there is much work still to be done. However, the inherent risks of brain cancer and its low median survival may outweigh the potential side-effects caused by the NPs [8–10]. Indeed some applications may see a use before a complete understanding of the toxicology of all NPs is presented, which has already been observed with gadolinium and Fe3O4 NPs.
Gadolinium and Fe3O4 NPs have received a lot of attention, owing to the fact that they are inherently magnetic and can be used in MRI as contrast enhancement for improved detection and monitoring of brain tumors. Fe3O4 NPs cause MRI contrast enhancement for mostly T2-weighted images, which provides contrast enhancement for tissues with high water content or, for instance, blood vessels [32,125]. Gadolinium NPs cause MRI contrast enhancement for mostly T1-weighted images, which provide contrast enhancement for tissues with high fat content and are better at creating appreciable contrast between white and gray matter, or between tissue types [26,40]. Gadolinium NPs increase the retention time or half-life of gadolinium in tumors, which is the major problem with current clinically used non-nanoparticulate gadolinium. Non-nanoparticulate gadolinium also exhibits toxicity in some patients due to leakage of the gadolinium from the chelating agent [24,31,40]. On the other hand, Fe3O4 NPs have been shown to be relatively nontoxic with no evidence of tissue damage or pathologic changes in the brain. These particles are also biodegradable [24,125,126].
QDs are highly advantageous as they can be tailored for fluorescence emission spectra from 400 to 2000 nm, although theoretical modeling studies indicate that spectral windows of 700–900 and 1200–1600 nm are the best for in vivo imaging [121]. However, because of their heavy metal content, QDs can potentially be toxic if accumulated in normal tissues without organic polymer protection [121]. Therefore, there is still work to be carried out in order to determine the long-term toxicity and degradation of QDs in vivo.
AuNPs have recently been investigated for use in delivering therapeutics to the brain as well as to brain cancer and have been receiving increasing attention [18,41,124,127–130]. AuNPs have the advantages of relatively straightforward synthesis, easy surface functionalization, small sizes, and the corresponding ability to be excreted by the body and remain relatively nontoxic [14,18,41,91,128,130]. In the past few years, there has been a marked increase in the amount of data on the biodistribution and toxicity of AuNPs. AuNPs have been examined by the USA National Institute of Standards and Technology as a potential standard for research based on nanosized particles [18,41,124,127–130]. Previous studies have found that AuNPs are able to actively cross the BBB with diameters of up to 50 nm, making them suitable for increased delivery through the disrupted BBTB [18,127,128,130].
In addition to solid inorganic NPs, organic-based delivery vehicles have also been heavily explored. Polymers encompass a vast amount of research in the NP field and account for 80% of the total amount of therapeutic products that have been approved for clinical use, as can be seen in Table 1 [3,4]. It is also true that most inorganic NPs also employ an organic polymer as a protective layer that increases circulation half-life and allows for the addition of targeting agents or imaging probes [4,5,7,24,29,32,41,50,86,124]. Like most other NPs, their use and investigation for treatment of brain cancer has just begun. A brief survey of the vast amount of research being done shows varying polymer compositions, with the most popular core compositions being made of poly(lactic-co-glycolic acid), polysorbate 80-coated poly(butyl cyano acrylate) and poly(ε-caprolactone) [45,52,53,56,57,59,66]. Dendrimers and hydrogels are also being investigated as organic NPs capable of crossing the BBB to deliver therapeutics to tumors. Dendrimers are repetitively and symmetrically branched organic molecules with uniform sizes, which allow encapsulation of therapeutic agents in their interior while also allowing attachment of drugs, imaging agents, functional groups, or even targeting moieties on their surface by covalent bonding or physical adsorption [16,27,44]. Dendrimers, like most other organic NPs, exhibit a tunable circulation lifetime and tolerable toxicity [27,44]. It has also been determined by one study that dendrimers that are approximately less than 11.7–11.9 nm in diameter are able to cross BBTB [25]. Hydrogels are NPs made of polymer chain networks that are hydrophilic, highly absorbent and possess a large degree of flexibility due to their significant water content [15,105].
Table 1.
The development status of carrier-based drug delivery systems for intravenous administration.
| Carrier† | Main component | Product (platform) | Targeting moiety (target) | Company (location) | Brain tumors: clinical phase (drug) | Market situation or clinical phase (indications) [drug] |
|---|---|---|---|---|---|---|
| Polymer based (several polymer-based drugs are on the market‡) | ||||||
| Polymer–drug conjugate | Polyaminoacids | CT2103, Opaxio™ (formerly Xyotax) | N/A | Cell Therapeutics (WA, USA) | Phase II (temozolomide) | Phase III (ovarian cancer) [paclitaxel] |
| Peptide–drug conjugate | N/A | GRN1005 | Angiopep-2 peptide (LRP-1) | AngioChem (Montreal, Canada) | Phase II (paclitaxel) | Phase II (malignant glioma) [paclitaxel] |
| N/A | N/A | N/A | VH0455 peptide (LDLR) | Vect-Horus (Marseille, France) | Preclinical | N/A |
| N/A | N/A | Trascend™ | Melanotransferrin (LRP) | biOasis Technologies (Vancouver, Canada) | Preclinical | N/A |
| N/A | N/A | NeuroTrans™ | Peptide RAP (LRP-2) | Raptor Pharmaceutical (CA, USA) | Preclinical | N/A |
| mAb-fusion proteins (Trojan horses) | ||||||
| N/A | N/A | AGT181 | mAb against HIR | ArmaGen Technologies (CA, USA) | Planned to enter Phase I soon (iduronidase enzyme) | N/A |
| Nanosized drug carriers | ||||||
| Polymeric NP | Cyclodextrin | CALAA01 | Tf | Arrowhead Research Corp., formerly Calando (CA, USA) | N/A | Phase I (melanoma) [siRNA] |
| Polymeric NP | Cyclodextrin | IT101 | N/A | Arrowhead Research Corp., formerly Insert Therapeutics (CA, USA) | N/A | Phase Ib-IIa (metastatic solid tumors) [camptothecin] |
| Polymeric NP | Cyanoacrylate | Livitag® | N/A | BioAlliance Pharma (Paris, France) | N/A | Phase II (hepatic tumors) [doxorubicin] |
| Polymeric NP | Polybutylcyanoacrylate | N/A | N/A | Sichuan University (Chengdu, China) | N/A | Phase II (hepatic tumors) [mitoxantrone] |
| Polymeric NP | Polyester§ | Bind014 | Prostate-specific membrane antigen | Bind Biosciences (MA, USA) | N/A | Phase I (prostatic tumor) [docetaxel] |
| Liposomes (several drug-loaded liposomes are on the market, but not for brain diseases)¶# | ||||||
| Liposomes | Lipids | 2B3-101 | Glutathione (glutathione receptors) | To-BBB (Leiden, The Netherlands) | Phase I, II (doxorubicin) | Phase I, II (brain cancer) [doxorubicin] |
| Liposomes | Lipids | NLCPT11 | N/A | University of California (CA, USA) | Phase I (irinotecan) | Phase I (brain cancer) [irinotecan] |
| Liposomes | Lipids | Marqibo® | N/A | Talon Therapeutics (CA, USA) | Phase I–II (vincristine) | On the market (leukemia) |
| Albumin | ||||||
| Albumin | Albumin | Abraxane® | N/A | Celgene Corp., formerly Abraxis Bioscience (NJ, USA) | N/A | On the market (metastatic breast cancer) [paclitaxel] |
Other carriers (polymers, dendrimers [among polymer-based carriers], nanogels, crosslinked bovine serum albumin 4-hydroxy-3-nitrophenylacetyl hapten, solid lipid nanoparticles [among nanosized drug carriers]) are in preclinical research for CNS delivery; with the exception of polymeric drugs, no products based on these platforms are on the market (even for other indications).
Currently more than 14 polymer–drug conjugates are in clinical trials for other targets [140]; several compounds are clinically approved [4,141]. Overall, only one (PK2) is based on active targeting (the carrier contains a moiety that can establish specific interactions with the target cells).
Micelles based on the polyester polylactic acid are commercially available for the treatment of breast cancer (Genexol-PM, Samyang Co. Seoul, Korea).
Tekmira (Burnaby Canada), Alnylam (MA, USA), Marina Biotech (WA, USA), Silence Therapeutics (London, UK) and Sirnaomics (MD, USA) are involved in Phase I clinical trials with lipid-based systems for the delivery of siRNA [142,143].
Ten untargeted liposomal preparations are clinically approved and others are in clinical trials [4].
BBB: Blood–brain barrier; HIR: Human insulin receptor; LDLR: Low-density lipoprotein receptor; LRP: Lipoprotein receptor protein; mAB: Monoclonal antibody; N/A: Not available; NP: Nanoparticle; Tf: Transferrin. Reproduced with permission from [123].
A further example of an organic-based delivery vehicle is liposomes, which are spherical in shape and consist of a phospholipid shell that can be used to encapsulate and deliver both hydrophobic and hydrophilic drugs. They are on average 100 nm in diameter [24,50,102,131,132]. Doxorubicin was the first drug to be delivered by liposomes to brain tumors [24,131,132]. High-density lipoprotein (HDL) NPs are closely related to liposomal nanocarriers, having the stability and monodispersity of inorganic NPs combined with the shielding ability of liposomes that improves circulation half-lives of therapeutics [106,107,133]. These HDL NPs can take advantage of a similar physiological approach as LRPs (as discussed earlier) to help mediate internalization of NPs [23,133]. These HDL NPs possess favorable surface properties for the addition of targeting or imaging agents and very small core sizes between 7 and 12 nm, which may make them an excellent future candidate for brain tumor therapy [106,107,133].
Many of these types of NPs have been researched for brain cancer for these reasons and can be broken down into three main functions: NPs for diagnostic imaging, NPs for therapy and NPs that are for both diagnostic and therapeutic purposes (also known as theranostics).
Diagnostic NPs
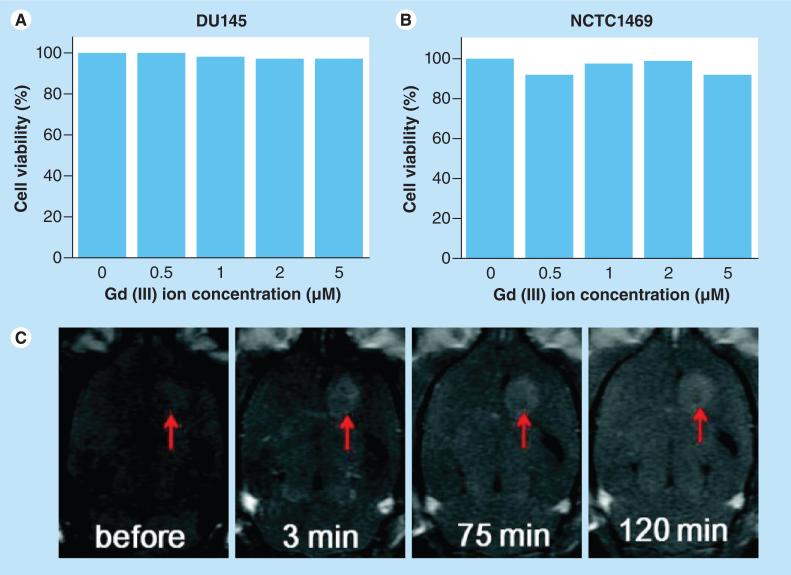
Gadolinium chelates are currently the standard of MRI contrast agents because the gadolinium (III) ion is the best known T1 contrast agent due to its large magnetic moment [26,40]. Gadolinium NPs with sizes ranging from 1 to 3 nm have been shown to be the best at increasing both the relaxation and contrast enhancement properties of gadolinium [26,40]. Gadolinium chelates may also leak into normal tissues during surgical breakdown of the BBB enhancing normal brain tissue, making tumors indistinguishable and rendering complete MRI-guided surgical resection more difficult [24,31,40]. An example of the use of a gadolinium NP, showing contrast enhancement of rat brain tumors in 2 h, and their corresponding lack of toxicity in vitro, is shown in Figure 8 [40].
Figure 8. A cytotoxicity test and representative magnetic resonance images of a rat brain tumor using ultrasmall gadolinium oxide nanoparticles for T1-weighted MRI contrast enhancement.
In vitro cytotoxicity tests for (A) DU145 and (B) NCTC1469 cell lines. (C) In vivo T1-weighted images of a rat brain with its tumor marked with arrows before and after injection of the ultrasmall gadolinium oxide nanoparticles.
Reproduced with permission from [40].
Fe3O4 NPs, more specifically dextran-coated superparamagnetic Fe3O4 NPs, are MRI contrast agents that have been approved by the US FDA for use in hepatic reticuloendothelial cell imaging, while ultrasmall superparamagnetic Fe3O4 NPs are in Phase III clinical trials for use as blood pool agents or for use with lymphography [134]. The first NPs created for brain tumor imaging were a form of Fe3O4 NPs known as monocrystal-line Fe3O4 NPs, which were conjugated to tumor-specific monoclonal antibodies called L6 [24,135]. These targeted particles cause MRI enhancement of brain tumors, and especially neovasculature of tumors, after being phagocytosed by cells within a brain tumor over a much longer time period of approximately 1–2 days [24,29]. This means that Fe3O4 NPs, unlike current contrast agents, are able to be given 24 h prior to surgery with little enhancement of normal tissues, giving a surgeon the ability to remove all contrast-enhanced tissue during intraoperative MRI [24].
Although not yet applied to brain tumors, QDs can also be conjugated with other contrast agents such as gadolinium for further diagnostic abilities along with any inherent fluorescent properties [42,121,136]. One study found that QDs can be used to deliver NPs to tumors by the enhanced permeability and retention effect, allow phagocytosis by tumor-infiltrating tissue macrophages and give an optical outline of the infiltrative margin of brain tumors [42].
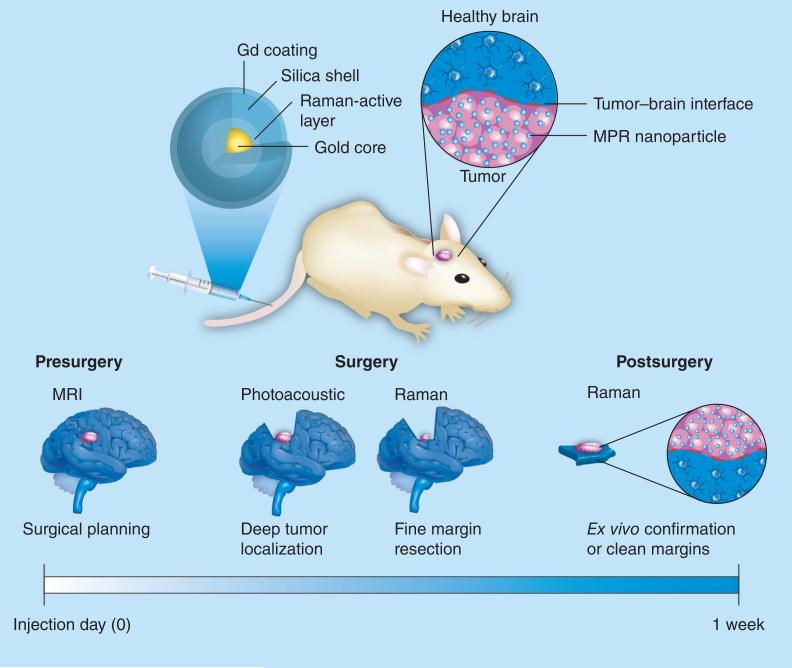
AuNPs have also seen success as diagnostic NPs for brain cancer. Figure 9 depicts the use of AuNPs to deliver gadolinium for MRI preoperative detection and surgical planning, as well as to simultaneously deliver photoacoustic and Raman imaging agents for delineation of tumor margins with at least picomolar sensitivity [14]. Although this system is purely diagnostic, one could see how additional therapeutics could be loaded in order to provide either systemic treatment with chemotherapeutics or localized treatment with the use of PDT drugs. AuNPs can also be loaded with fluorescent imaging agents for diagnostic purposes [41].
Figure 9. A gold nanoparticle-based delivery system for identification of brain tumor margins and improved surgical resection.
MPR: MRI-photoacoustic-Raman.
Reproduced with permission from [14].
Organic NPs have also seen great success as diagnostic NPs for brain cancer. Liposomes have been investigated for noninvasive real-time monitoring for detection and diagnosis of brain tumors by delivering gadolinium using convection-enhanced delivery as shown in Figure 10 [50,102–104]. Dendrimers have similarly been used to deliver gadolinium for MRI contrast enhancement as well as fluorescent imaging probes for optical detection of tumors [25,27,44]. One example, shown in Figure 11, is the use of a dendrimer to carry both gadolinium and a fluorescence imaging probe to brain tumors by using a novel two-step targeting approach by first targeting tumor vasculature with the αvβ3 integrin then by targeting LRP receptors to further accelerate BBTB transversal [27,44].
Figure 10. Convection-enhanced delivery of MRI contrast enhancement using gadolinium-loaded liposomes.
Reproduced with permission from [104].
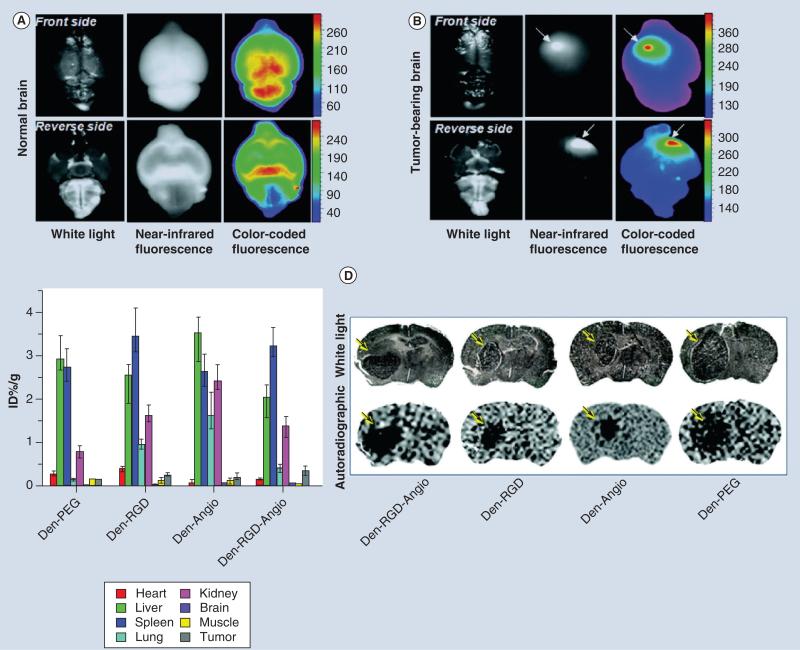
Figure 11. In vivo brain tumor imaging using an optical and paramagnetic dendrimeric nanoparticle (Den-RGD-Angio) targeted to both tumor vasculature and lipoprotein receptor protein receptors in a mouse brain.
Representative images of ex vivo mouse brains showing white-light, near-infrared fluorescence and color-coded fluorescence images for (A) a normal mouse brain and (B) a tumor-bearing mouse brain at 24-h post-injection of the Den-RGD-Angio. (C) Biodistribution of the nanoparticles by radioactive isotope labeling with 125I in tumor-bearing mice (n = 3) at 24-h post-injection. Columns represent mean values and bars present the data range. (D) Representative white-light microscopic images and autoradiographic images of tumor-bearing brain sections at 24-h post-injection of the radioactive nanoprobe, with arrows pointing to tumors.
PEG: Polyethylene glycol.
Reproduced with permission from [27].
For color images see online at www.futuremedicine.com/doi/pdf/10.2217/nnm.12.185.
Hydrogels have also been investigated as organic, diagnostic NPs for brain cancer, and one study developed a peptide-targeted hydrogel NP containing a Cy5.5 fluorescent imaging agent, and a blue dye called Coomassie Brilliant Blue [15]. These covalently loaded NPs showed a highly selective blue staining of brain tumors with negligible normal tissue staining in glioma-bearing rats [15]. The delivery of Coomassie Brilliant Blue allowed direct visualization of the brain tumor by the naked eye and can potentially be used as a successful means to guide tumor resection during intraoperative surgery [15].
Therapeutic NPs
For inorganic NPs, Fe3O4 and Au have been studied for the treatment of brain cancer. Studies on Fe3O4 NPs have shown success with antibody treatments as well as with thermotherapy induced by an alternating magnetic field [113,114,126]. AuNPs also offer the ability to achieve noncovalent drug delivery, which allows drugs to be delivered in vivo without needing the AuNPs to be taken up into tumor cells [41,86,87,91]. AuNPs can also utilize thermotherapy by heating gold with visible, infrared, or radiofrequency pulses to cause localized tumor damage [112,137]. However, to the best of our knowledge, this type of system for AuNPs has not yet been applied to brain tumors.
Organic NPs, polymers, liposomes, dendrimers and hydrogels have all been studied for brain cancer treatment. Untargeted formulations of organic NPs have been the only therapeutic NPs to make it to clinical trials, although none have been used for delivery of drugs to the brain [123]. Targeted delivery with organic NPs has been in clinical trials, although the percentage of the injected dose that is delivered to the brain is still only approximately 1% or less [123]. The bulk of the drug mainly localizes in the liver, which causes some concern regarding the long-term fate of the polymers as described before [123].
One recent success with polymer NPs in research has been the use of polymer NP-delivered gene therapy for brain tumors [51,54,62,63]. In one such study, Han et al. showed success in using an external magnetic field to guide a polymer NP gene delivery system, which is naturally magnetic due to a process called biomineralization, across the BBB [63]. Other polymer-based NPs have seen success in accessing brain tumors to deliver the chemotherapeutic agents: doxorubicin, camptothecin and paclitaxel [45,46,52,56,59,60,64]. Several studies have shown that liposomes improve the therapeutic activity of chemotherapeutics by increasing drug retention times in the brain tumor [102,131,132]. Research on dendrimer NPs has found that a dual-targeted, 14–20 nm PEGylated PAMAM dendrimer-based delivery vehicle loaded with doxorubicin was able to increase the inhibition of growth of glioma cells, and increase the transport ratio of doxorubicin in vitro [16]. Hydrogels have also been used in the treatment of brain cancer, in the form of a hydrogel matrix wafer to locally deliver cisplatin, another popular chemotherapeutic agent, to brain tumors [105].
Theranostic NPs
QDs, Fe3O4 NPs, AuNPs and polymers have all demonstrated levels of success as theranostic NPs. They have all been used as delivery vehicles to deliver both drugs and imaging agents [43,48,49,138]. However, Fe3O4 and AuNPs do have the ability to function as theranostic NPs completely on their own. This is because Fe3O4 NPs are magnetic resonance contrast agents, while AuNPs are able to function as computed tomography imaging contrast agents due to their density [116], and both can be used for thermotherapy [112,137,138].
QDs have been shown to be able to be used as theranostic delivery vehicles for targeted, multimodal imaging of brain cancer as well as to successfully deliver siRNA-based chemotherapeutics [43]. Fe3O4 NPs have even found success in using an externally applied magnetic field to actively target cancer and increase delivery of Fe3O4 NPs in tumors [138]. Although this system has not yet been applied to brain cancer, its ability to improve delivery of an MRI contrast agent along with optical fluorescent imaging agents and cancer therapeutics by using an applied external magnetic field targeted on cancer, make it a promising future candidate for the delicate nature of brain tumors [138].
AuNPs have shown the ability to deliver PDT drugs, which are fluorescent and allow the drug's distribution to be tracked, while also having the ability to be activated to cause tumor cell damage induced by laser light [41]. Polymer NPs have also seen success by using Fe3O4 encapsulation for MRI contrast enhancement with simultaneously encapsulating PDT drugs for treatment of brain tumors [48,49]. Therefore, a promising application for AuNPs and polymers is the delivery of drugs to brain tumors to allow improved intraoperative tumor margin identification for improved surgical resection of tumors as well as possible intraoperative PDT treatment for inoperable tumor margins.
Conclusion
The use of nanotechnology for therapy has grown exponentially over the last two decades. By comparison, the growth of nanotechnology in the imaging and treatment of brain cancer has only begun but has already shown great promise. In this review, we have briefly provided the reader with an overview of targeted NP design (inorganic or organic synthesis, functionalization and loading of drug pay-loads), as well as reviewed the exciting frontier of theranostics. In the second half of this article, we have explored current limitations in brain cancer therapy specifically associated with the BBB, and examined how NPs of all types (metallic, semiconductor, magnetic, dendrimer, liposomal, polymeric and hydrogel) are providing solutions. In reviewing the literature, it is clear that the use of nanometer-sized materials is ideally situated for treating this very difficult disease.
Future directions of NP-based brain cancer therapy
The future of NPs for brain tumor treatment will likely see simultaneous delivery of drugs as well as the increased use of targeting agents and imaging agents. The use of simultaneous delivery of different drugs allows treatment to be tailored for the patient, making NPs essentially delivery vehicles in which cocktails of drugs can be applied. The increased use of targeting agents will serve to increase accumulation in brain tumors and decrease accumulation in normal brain tissue, which will improve therapeutic efficacy. The increased use of imaging agents will help to diagnose cancer at an earlier stage, in order to have the best survival probability, as well as to monitor brain tumors over the duration of a treatment regime.
This future still remains a challenging one, as the creation of complex systems entails an understanding of individual components that make up the delivery vehicle as a whole. Future NP delivery systems that are able to employ simultaneous delivery of drugs, targeting to specific brain tumor surface markers and/or simultaneous imaging of their delivery will take a great deal of knowledge of how each component works to achieve such goals. Furthermore success with one core material may not mean success with another, and the study of the long-term health effects of these core materials and their biodistribution properties is still ongoing [24,201]. The most popular strategy remains to use either biodegradable materials or nondegradable materials with small enough core sizes to avoid this problem [4,24,122]. Another obstacle that will continue to be problematic is the role of drug efflux pumps, which have the possibility of rendering the delivery of a drug by a NP useless if the drug cannot remain in the tumor cells long enough to have an effect [23]. Many researchers have already begun finding innovative solutions to these problems and it is likely that it will only be a matter of time before NPs become a mainstay in the imaging and treatment of brain cancer. However, there is still much work to be done to convince the general public of the safety of NPs and to prove that NPs are indeed the future of brain tumor imaging and treatment.
Executive summary.
■ The application of nanoparticles in medicine is on the rise both in research literature and in the number of patents filed, with drug delivery being the most popular use.
■ The blood–brain barrier (BBB), drug efflux pumps, multidrug-resistant proteins and degrading enzymes limit the bioavailability of drugs used for brain cancer, and current treatments have so far been relatively ineffective.
■ Nanoparticles are a new way to overcome the BBB and can take advantage of invasive, pharmacological and physiological approaches to overcome the BBB to further increase the bioavailability of drugs in brain tumors.
■ Nanoparticles can be divided into two main categories: inorganic and organic nanoparticles, which encompass a wide range of materials with different physiochemical properties.
■ The synthesis of inorganic and organic nanoparticles allows for facile modifications that can be used to improve targeting and delivery to brain cancers as well as the ability to be loaded with therapeutics or theranostic multimodal vectors via covalent or noncovalent methods.
■ Nanoparticles offer many advantages, including: modifiable surface properties, modifiable shapes, modifiable sizes, the ability to deliver multiple types of drugs, the ability to allow attachment of contrast agents for in vivo diagnosis capabilities, and sometimes the ability to function as both an imaging agent and thermal therapy by their composition alone.
■ Prior to the widespread use of nanoparticles, further research into their long-term toxicity, and improvements in the bioavailability of the drugs they deliver to brain tumors will be needed.
■ Nanoparticles will continue to be a popular research topic and have the possibility of one day becoming a popular treatment option for those afflicted with brain cancer.
Acknowledgments
This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.From the Centers for Disease Control and Prevention Public health and aging: trends in aging – United States and worldwide. J. Am. Med. Assoc. 2003;289(11):1371–1373. [PubMed] [Google Scholar]
- 2.Dickson M, Gagnon JP. Key factors in the rising cost of new drug discovery and development. Nat. Rev. Drug Discov. 2004;3(5):417–429. doi: 10.1038/nrd1382. [DOI] [PubMed] [Google Scholar]
- 3.Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat. Biotech. 2006;24(10):1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 2007;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 5.Nune SK, Gunda P, Thallapally PK, Lin YY, Laird Forrest M, Berkland CJ. Nanoparticles for biomedical imaging. Expert Opin. Drug Deliv. 2009;6(11):1175–1194. doi: 10.1517/17425240903229031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitesides GM. The “right” size in nanobiotechnology. Nat. Biotechnol. 2003;21(10):1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat. Rev. Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 8.Wen PY, Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 9.Ullrich NJ, Pomeroy SL. Pediatric brain tumors. Neurol. Clin. 2003;21(4):897–913. doi: 10.1016/s0733-8619(03)00014-8. [DOI] [PubMed] [Google Scholar]
- 10■.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [Provides further information on the blood–brain barrier and its role in restricting the use of drugs for brain applications.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardridge WM. Vector-mediated drug delivery to the brain. Adv. Drug Deliv. Rev. 1999;36(2–3):299–321. doi: 10.1016/s0169-409x(98)00087-8. [DOI] [PubMed] [Google Scholar]
- 12.Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J. Neurochem. 1998;70(5):1781–1792. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 13.Kumar M, Medarova Z, Pantazopoulos P, Dai G, Moore A. Novel membrane-permeable contrast agent for brain tumor detection by MRI. Magn. Reson. Med. 2010;63(3):617–624. doi: 10.1002/mrm.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kircher MF, de la Zerda A, Jokerst JV, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012;18(5):829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nie G, Hah HJ, Kim G, et al. Hydrogel nanoparticles with covalently linked coomassie blue for brain tumor delineation visible to the surgeon. Small. 2012;8(6):884–891. doi: 10.1002/smll.201101607. [DOI] [PubMed] [Google Scholar]
- 16.He H, Li Y, Jia XR, et al. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials. 2011;32(2):478–487. doi: 10.1016/j.biomaterials.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Yan H, Wang J, Yi P, et al. Imaging brain tumor by dendrimer-based optical/paramagnetic nanoprobe across the blood-brain barrier. Chem. Commun. 2011;47(28):8130–8132. doi: 10.1039/c1cc12007g. [DOI] [PubMed] [Google Scholar]
- 18.Sousa F, Mandal S, Garrovo C, et al. Functionalized gold nanoparticles: a detailed in vivo multimodal microscopic brain distribution study. Nanoscale. 2010;2(12):2826–2834. doi: 10.1039/c0nr00345j. [DOI] [PubMed] [Google Scholar]
- 19.Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 1995;674(1):171–174. doi: 10.1016/0006-8993(95)00023-j. [DOI] [PubMed] [Google Scholar]
- 20.Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release. 2008;127(2):97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv. Drug Deliv. Rev. 2001;46(1–3):247–279. doi: 10.1016/s0169-409x(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 22.Pardridge WM. Re-engineering biopharmaceuticals for delivery to brain with molecular trojan horses. Bioconjug. Chem. 2008;19(7):1327–1338. doi: 10.1021/bc800148t. [DOI] [PubMed] [Google Scholar]
- 23■■.Gabathuler R. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol. Dis. 2010;37(1):48–57. doi: 10.1016/j.nbd.2009.07.028. [Excellent resource for understanding how to transport drugs across the blood–brain barrier.] [DOI] [PubMed] [Google Scholar]
- 24■■.Orringer D, Koo Y, Chen T, Kopelman R, Sagher O, Philbert M. Small solutions for big problems: the application of nanoparticles to brain tumor diagnosis and therapy. Clin. Pharmacol. Ther. 2009;85(5):531–534. doi: 10.1038/clpt.2008.296. [Provides an alternative review on nanoparticles for brain cancer therapy and diagnosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarin H, Kanevsky AS, Wu H, et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J. Transl. Med. 2008;6:80. doi: 10.1186/1479-5876-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faucher L, Guay-Begin AA, Lagueux J, Cote MF, Petitclerc E, Fortin MA. Ultra-small gadolinium oxide nanoparticles to image brain cancer cells in vivo with MRI. Contrast Media Mol. I. 2011;6:209–218. doi: 10.1002/cmmi.420. [DOI] [PubMed] [Google Scholar]
- 27.Yan H, Wang L, Wang J, et al. Two-order targeted brain tumor imaging by using an optical/paramagnetic nanoprobe across the blood brain barrier. ACS Nano. 2012;6:410–420. doi: 10.1021/nn203749v. [DOI] [PubMed] [Google Scholar]
- 28.Veiseh O, Sun C, Gunn J, et al. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 29.Anderson SA, Glod J, Arbab AS, et al. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood. 2005;105:420–425. doi: 10.1182/blood-2004-06-2222. [DOI] [PubMed] [Google Scholar]
- 30.Sun C, Veiseh O, Gunn J, et al. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4:372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veiseh O, Sun C, Fang C, et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009;69(15):6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadjipanayis CG, Machaidze R, Kaluzova M, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70:6303–6312. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hua MY, Liu HL, Yang HW, et al. The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas. Biomaterials. 2010;32:516–527. doi: 10.1016/j.biomaterials.2010.09.065. [DOI] [PubMed] [Google Scholar]
- 34.Xie H, Zhu Y, Jiang W, et al. Lactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials. 2010;32:495–502. doi: 10.1016/j.biomaterials.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 35.Mejias R, Perez-Yaguee S, Roca AG, et al. Liver and brain imaging through dimercaptosuccinic acid-coated iron oxide nanoparticles. Nanomedicine (Lond.) 2010;5:397–408. doi: 10.2217/nnm.10.15. [DOI] [PubMed] [Google Scholar]
- 36.Liu HL, Hua MY, Yang HW, et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc. Natl Acad. Sci. USA. 2010;107:15205–15210. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar M, Medarova Z, Pantazopoulos P, Dai G, Moore A. Novel membrane-permeable contrast agent for brain tumor detection by MRI. Magn. Reson. Med. 2010;63:617–624. doi: 10.1002/mrm.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kievit FM, Veiseh O, Fang C, et al. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano. 2010;4:4587–4594. doi: 10.1021/nn1008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dilnawaz F, Singh A, Mewar S, Sharma U, Jagannathan NR, Sahoo SK. The transport of non-surfactant based paclitaxel loaded magnetic nanoparticles across the blood brain barrier in a rat model. Biomaterials. 2012;33:2936–2951. doi: 10.1016/j.biomaterials.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 40.Park JY, Baek MJ, Choi ES, et al. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: account for large longitudinal relaxivity, optimal particle diameter, and in vivo T1 MR images. ACS Nano. 2009;3(11):3663–3669. doi: 10.1021/nn900761s. [DOI] [PubMed] [Google Scholar]
- 41.Cheng Y, Meyers JD, Agnes RS, et al. Addressing brain tumors with targeted gold nanoparticles: a new gold standard for hydrophobic drug delivery? Small. 2011;7:2301–2306. doi: 10.1002/smll.201100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson H, Muhammad O, Daneshvar H, et al. Quantum dots are phagocytized by macrophages and colocalize with experimental gliomas. Neurosurgery. 2007;60(3):524–529. doi: 10.1227/01.NEU.0000255334.95532.DD. discussion 529–530. [DOI] [PubMed] [Google Scholar]
- 43.Jung J, Solanki A, Memoli KA, et al. Selective inhibition of human brain tumor cells through multifunctional quantum-dot-based siRNA delivery. Angew. Chem. Int. Ed. 2010;49:103–107. doi: 10.1002/anie.200905126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan H, Wang J, Yi P, et al. Imaging brain tumor by dendrimer-based optical/paramagnetic nanoprobe across the blood-brain barrier. Chem. Commun. (Camb.) 2011;47:8130–8132. doi: 10.1039/c1cc12007g. [DOI] [PubMed] [Google Scholar]
- 45.Gelperina SE, Khalansky AS, Skidan IN, et al. Toxicological studies of doxorubicin bound to polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles in healthy rats and rats with intracranial glioblastoma. Toxicol. Lett. 2002;126:131–141. doi: 10.1016/s0378-4274(01)00456-8. [DOI] [PubMed] [Google Scholar]
- 46.Steiniger SC, Kreuter J, Khalansky AS, et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int. J. Cancer. 2004;109:759–767. doi: 10.1002/ijc.20048. [DOI] [PubMed] [Google Scholar]
- 47.Koziara JM, Oh JJ, Akers WS, Ferraris SP, Mumper RJ. Blood compatibility of cetyl alcohol/polysorbate-based nanoparticles. Pharm. Res. 2005;22(11):1821–1828. doi: 10.1007/s11095-005-7547-7. [DOI] [PubMed] [Google Scholar]
- 48.Kopelman R, Koo YEL, Philbert M, et al. Multifunctional nanoparticle platforms for in vivo MRI enhancement and photodynamic therapy of a rat brain cancer. J. Magn. Magn. Mater. 2005;293:404–410. [Google Scholar]
- 49.Reddy GR, Bhojani MS, McConville P, et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin. Cancer Res. 2006;12:6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 50.Noble CO, Krauze MT, Drummond DC, et al. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66:2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- 51.Lu W, Sun Q, Wan J, She Z, Jiang XG. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 2006;66:11878–11887. doi: 10.1158/0008-5472.CAN-06-2354. [DOI] [PubMed] [Google Scholar]
- 52.Ambruosi A, Khalansky AS, Yamamoto H, Gelperina SE, Begley DJ, Kreuter J. Biodistribution of polysorbate 80-coated doxorubicin-loaded [14C]-poly(butyl cyanoacrylate) nanoparticles after intravenous administration to glioblastoma-bearing rats. J. Drug Targeting. 2006;14:97–105. doi: 10.1080/10611860600636135. [DOI] [PubMed] [Google Scholar]
- 53.Petri B, Bootz A, Khalansky A, et al. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nanoparticles: revisiting the role of surfactants. J. Control. Release. 2007;117:51–58. doi: 10.1016/j.jconrel.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Veiseh O, Kievit FM, Gunn JW, Ratner BD, Zhang M. A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells. Biomaterials. 2008;30:649–657. doi: 10.1016/j.biomaterials.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren W, Chang J, Yan C, et al. Development of transferrin functionalized poly(ethylene glycol)/poly(lactic acid) amphiphilic block copolymeric micelles as a potential delivery system targeting brain glioma. J. Mater. Sci. Mater. Med. 2010;21:2673–2681. doi: 10.1007/s10856-010-4106-5. [DOI] [PubMed] [Google Scholar]
- 56.Xin H, Chen L, Gu J, et al. Enhanced anti-glioblastoma efficacy by PTX-loaded PEGylated poly(ε-caprolactone) nanoparticles: In vitro and in vivo evaluation. Int. J. Pharm. 2010;402:238–247. doi: 10.1016/j.ijpharm.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Gelperina S, Maksimenko O, Khalansky A, et al. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: influence of the formulation parameters. Eur. J. Pharm. Biopharm. 2010;74:157–163. doi: 10.1016/j.ejpb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Xin H, Jiang X, Gu J, et al. Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials. 2011;32:4293–4305. doi: 10.1016/j.biomaterials.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 59.Wohlfart S, Khalansky AS, Gelperina S, Begley D, Kreuter J. Kinetics of transport of doxorubicin bound to nanoparticles across the blood-brain barrier. J. Control. Release. 2011;154:103–107. doi: 10.1016/j.jconrel.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Sawyer AJ, Saucier-Sawyer JK, Booth CJ, et al. Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors. Drug Deliv. Transl. Res. 2011;1:34–42. doi: 10.1007/s13346-010-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parveen S, Sahoo SK. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur. J. Pharmacol. 2011;670:372–383. doi: 10.1016/j.ejphar.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 62.Jin J, Bae KH, Yang H, et al. In vivo specific delivery of c-met sirna to glioblastoma using cationic solid lipid nanoparticles. Bioconjug. Chem. 2011;22:2568–2572. doi: 10.1021/bc200406n. [DOI] [PubMed] [Google Scholar]
- 63.Han L, Zhang A, Wang H, Pu P, Kang C, Chang J. Construction of novel brain-targeting gene delivery system by natural magnetic nanoparticles. J. Appl. Polym. Sci. 2011;121:3446–3454. [Google Scholar]
- 64.Geldenhuys W, Mbimba T, Bui T, Harrison K, Sutariya V. Brain-targeted delivery of paclitaxel using glutathione-coated nanoparticles for brain cancers. J. Drug Target. 2011;19:837–845. doi: 10.3109/1061186X.2011.589435. [DOI] [PubMed] [Google Scholar]
- 65.Roger M, Clavreul A, Huynh NT, et al. Ferrociphenol lipid nanocapsule delivery by mesenchymal stromal cells in brain tumor therapy. Int. J. Pharm. 2012;423:63–68. doi: 10.1016/j.ijpharm.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 66.Gao H, Qian J, Cao S, et al. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials. 2012;33:5115–5123. doi: 10.1016/j.biomaterials.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 67.Gao H, Qian J, Yang Z, et al. Whole-cell SELEX aptamer-functionalised poly(ethyleneglycol)-poly(ε-caprolactone) nanoparticles for enhanced targeted glioblastoma therapy. Biomaterials. 2012;33:6264–6272. doi: 10.1016/j.biomaterials.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 68.Chang J, Paillard A, Passirani C, et al. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm. Res. 2012;29:1495–1505. doi: 10.1007/s11095-011-0624-1. [DOI] [PubMed] [Google Scholar]
- 69.Hyeon T. Chemical synthesis of magnetic nanoparticles. Chem. Commun. 2003;8:927–934. doi: 10.1039/b207789b. [DOI] [PubMed] [Google Scholar]
- 70.Laurent S, Forge D, Port M, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 71.Chuang CH, Lo SS, Scholes GD, Burda C. Charge separation and recombination in CdTe/CdSe core/shell nanocrystals as a function of shell coverage: probing the onset of the quasi type-II regime. J. Phys. Chem. Lett. 2010;1:2530–2535. [Google Scholar]
- 72.Lu AH, Salabas EL, Schüth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007;46(8):1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 73.Turkevich J, Stevenson PC, Hillier J. The nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951;11:55–75. [Google Scholar]
- 74.Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature Phys. Sci. 1973;241:20–22. [Google Scholar]
- 75.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiolderivatized gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994;241:801–802. [Google Scholar]
- 76.Gref R, Luck M, Quellec P, et al. “Stealth” corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloid Surface B. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 77.Tekade RK, Kumar PV, Jain NK. Dendrimers in oncology: an expanding horizon. Chem. Rev. 2009;109:49–87. doi: 10.1021/cr068212n. [DOI] [PubMed] [Google Scholar]
- 78.Vermonden T, Censi R, Hennink WE. Hydrogels for protein delivery. Chem. Rev. 2012;112:2853–2888. doi: 10.1021/cr200157d. [DOI] [PubMed] [Google Scholar]
- 79.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 80.Shi M, Lu J, Shoichet MS. Organic nanoscale drug carriers coupled with ligands for targeted drug delivery in cancer. J. Mater. Chem. 2009;19(31):5485. [Google Scholar]
- 81.Ferris DP, Lu J, Gothard C, et al. Synthesis of biomolecule-modified mesoporous silica nanoparticles for targeted hydrophobic drug delivery to cancer cells. Small. 2011;7:1816–1826. doi: 10.1002/smll.201002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patel MM, Goyal BR, Bhadada SV, Bhatt JS, Amin AF. Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs. 2009;23:35–58. doi: 10.2165/0023210-200923010-00003. [DOI] [PubMed] [Google Scholar]
- 83.Biddlestone-Thorpe L, Marchi N, Guo K, et al. Nanomaterial-mediated CNS delivery of diagnostic and therapeutic agents. Adv. Drug. Deliv. Rev. 2012;64:605–613. doi: 10.1016/j.addr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doane T, Burda C. Nanoparticle mediated non-covalent drug delivery. Adv. Drug Delivery Rev. 2012 doi: 10.1016/j.addr.2012.05.012. doi:10.1016/j.addr.2012.05.012 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thakur A, Gahane A, Bhadoriya SS, Jain SK, Jain R, Mishra H. Major hurdles for brain tumour therapy and the ways to overcome them: a review. J. Pharm. Res. 2011;4:1315–1318. [Google Scholar]
- 86.Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei B, Burda C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008;130:10643–10647. doi: 10.1021/ja801631c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng Y, Samia AC, Li J, Kenney ME, Resnick A, Burda C. Delivery and efficacy of a cancer drug as a function of the bond to the gold nanoparticle surface. Langmuir. 2010;26:2248–2255. doi: 10.1021/la902390d. [DOI] [PubMed] [Google Scholar]
- 88.Kim CK, Ghosh P, Pagliuca C, Zhu ZJ, Menichetti S, Rotello VM. Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells. J. Am. Chem. Soc. 2009;131:1360–1361. doi: 10.1021/ja808137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89■.Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug. Chem. 2011;22:1879–1903. doi: 10.1021/bc200151q. [Provides an excellent overview of theranostics.] [DOI] [PubMed] [Google Scholar]
- 90.Koo YE, Reddy GR, Bhojani M, et al. Brain cancer diagnosis and therapy with nanoplatforms. Adv. Drug. Deliv. Rev. 2006;58:1556–1577. doi: 10.1016/j.addr.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 91.Cheng Y, Meyers JD, Broome AM, Kenney ME, Basilion JP, Burda C. Deep penetration of a PDT drug into tumors by noncovalent drug-gold nanoparticle conjugates. J. Am. Chem. Soc. 2011;133:2583–2591. doi: 10.1021/ja108846h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guyton AC, Hall JE. Textbook of Medical Physiology. Elsevier Saunders; PA, USA: 2005. [Google Scholar]
- 93.Levin VA. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem. 1980;23(6):682–684. doi: 10.1021/jm00180a022. [DOI] [PubMed] [Google Scholar]
- 94.Vick NA, Bigner DD. Microvascular abnormalities in virally-induced canine brain tumors. J. Neurol. Sci. 1972;17(1):29–39. doi: 10.1016/0022-510x(72)90019-6. [DOI] [PubMed] [Google Scholar]
- 95.Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc. Res. 1999;58(3):312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 96.Shen T, Weissleder R, Papisov M, Bogdanov A, Jr, Brady TJ. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn. Reson. Med. 1993;29(5):599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- 97.Jung CW, Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging. 1995;13(5):661–674. doi: 10.1016/0730-725x(95)00024-b. [DOI] [PubMed] [Google Scholar]
- 98.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 99.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today. 2006;11(17–18):812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug. Chem. 2010;21(5):797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 101.Yamamoto M, Ikeda K, Ohshima K, Tsugu H, Kimura H, Tomonaga M. Increased expression of low density lipoprotein receptor-related protein/α2-macroglobulin receptor in human malignant astrocytomas. Cancer Res. 1997;57(13):2799–2805. [PubMed] [Google Scholar]
- 102.Saito R. Convection-enhanced delivery of Ls-TPT enables an effective, continuous, low-dose chemotherapy against malignant glioma xenograft model. Neurooncology. 2006;8(3):205–214. doi: 10.1215/15228517-2006-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krauze MT, McKnight TR, Yamashita Y, et al. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain Res. Brain Res. Protoc. 2005;16(1–3):20–26. doi: 10.1016/j.brainresprot.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 104.Mamot C, Nguyen JB, Pourdehnad M, et al. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J. Neurooncol. 2004;68(1):1–9. doi: 10.1023/b:neon.0000024743.56415.4b. [DOI] [PubMed] [Google Scholar]
- 105.Tauro JR, Gemeinhart RA. Matrix metalloprotease triggered delivery of cancer chemotherapeutics from hydrogel matrixes. Bioconjug. Chem. 2005;16(5):1133–1139. doi: 10.1021/bc0501303. [DOI] [PubMed] [Google Scholar]
- 106.Corbin IR, Chen J, Cao W, Li H, Lund-Katz S, Zheng G. Enhanced cancer-targeted delivery using engineered high-density lipoprotein-based nanocarriers. J. Biomed. Nanotechnol. 2007;3(4):367–376. [Google Scholar]
- 107.Chen W, Jarzyna PA, van Tilborg GAF, et al. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. FASEB J. 2010;24(6):1689–1699. doi: 10.1096/fj.09-139865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Langer R. Drug delivery and targeting. Nature. 1998;392(Suppl. 6679):S5–S10. [PubMed] [Google Scholar]
- 109.Torchilin VP. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006;58(14):1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 110.Sampson JH, Archer GE, Mitchell DA, Heimberger AB, Bigner DD. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin. Immunol. 2008;20(5):267–275. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. Multivalent effects of RGD peptides obtained by nanoparticle display. J. Med. Chem. 2006;49(20):6087–6093. doi: 10.1021/jm060515m. [DOI] [PubMed] [Google Scholar]
- 112.Terentyuk G, Akchurin G, Maksimova I, Maslyakova G, Khlebtsov N, Tuchin V. Cancer laser thermotherapy mediated by plasmonic nanoparticles. In: Tuchin V, editor. Handbook of Photonics for Biomedical Science. CRC Press; FL, USA: 2010. pp. 763–797. [Google Scholar]
- 113.Maier-Hauff K, Rothe R, Scholz R, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol. 2006;81(1):53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 114.Jordan A, Scholz R, Maier-Hauff K, et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neurooncol. 2005;78(1):7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 115.Anderson SA. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood. 2005;105(1):420–425. doi: 10.1182/blood-2004-06-2222. [DOI] [PubMed] [Google Scholar]
- 116.Popovtzer R, Agrawal A, Kotov NA, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8(12):4593–4596. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117■.Gwinn MR, Vallyathan V. Nanoparticles: health effects-pros and cons. Environ. Health Perspect. 2006;114(12):1818–1825. doi: 10.1289/ehp.8871. [Excellent review on the status of the currently researched health effects of nanoparticles.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sharma R, Saini S, Ros PR, et al. Safety profile of ultrasmall superparamagnetic iron oxide ferumoxtran-10: Phase II clinical trial data. J. Magn. Reson. Imaging. 1999;9(2):291–294. doi: 10.1002/(sici)1522-2586(199902)9:2<291::aid-jmri21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 119.Bernd H, De Kerviler E, Gaillard S, Bonnemain B. Safety and tolerability of ultrasmall superparamagnetic iron oxide contrast agent: comprehensive analysis of a clinical development program. Invest. Radiol. 2009;44(6):336–342. doi: 10.1097/RLI.0b013e3181a0068b. [DOI] [PubMed] [Google Scholar]
- 120.Khurana A, Runge VM, Narayanan M, Greene JF, Jr, Nickel AE. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (omniscan). Invest. Radiol. 2007;42(2):139–145. doi: 10.1097/01.rli.0000253505.88945.d5. [DOI] [PubMed] [Google Scholar]
- 121.Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 2005;16(1):63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 122.Soo Choi H, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat. Biotech. 2007;25(10):1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123■■.Costantino L, Boraschi D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov. Today. 2012;17(7–8):367–378. doi: 10.1016/j.drudis.2011.10.028. [Alternative review on polymers used for brain cancer diagnosis and treatment. Contains the newest, most relevant information.] [DOI] [PubMed] [Google Scholar]
- 124.Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem. Soc. Rev. 2011;40(3):1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 125.Muldoon LL, Sàndor M, Pinkston KE, Neuwelt EA. Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor. Neurosurgery. 2005;57(4):785–796. doi: 10.1093/neurosurgery/57.4.785. discussion 785–796. [DOI] [PubMed] [Google Scholar]
- 126.Hadjipanayis CG, Machaidze R, Kaluzova M, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70(15):6303–6312. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 128.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloids Surf. B Biointerfaces. 2008;66(2):274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 129.Jong WHD, Burger MC, Verheijen MA, Geertsma RE. Detection of the presence of gold nanoparticles in organs by transmission electron microscopy. Materials. 2010;3(9):4681–4694. doi: 10.3390/ma3094681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010;393(4):649–655. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 131.Siegal T, Horowitz A, Gabizon A. Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of a brain tumor model: biodistribution and therapeutic efficacy. J. Neurosurg. 1995;83(6):1029–1037. doi: 10.3171/jns.1995.83.6.1029. [DOI] [PubMed] [Google Scholar]
- 132.Fabel K, Dietrich J, Hau P, et al. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer. 2001;92(7):1936–1942. doi: 10.1002/1097-0142(20011001)92:7<1936::aid-cncr1712>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 133.Zhang Z, Chen J, Ding L, et al. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small. 2010;6(3):430–437. doi: 10.1002/smll.200901515. [DOI] [PubMed] [Google Scholar]
- 134.Frank JA, Miller BR, Arbab AS, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228(2):480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 135.Remsen LG, McCormick CI, Roman-Goldstein S, et al. MR of carcinoma-specific monoclonal antibody conjugated to monocrystalline iron oxide nanoparticles: the potential for noninvasive diagnosis. Am. J. Neuroradiol. 1996;17(3):411–418. [PMC free article] [PubMed] [Google Scholar]
- 136.Oostendorp M, Douma K, Hackeng TM, Post MJ, van Zandvoort MA, Backes WH. Gadolinium-labeled quantum dots for molecular magnetic resonance imaging: R1 versus R2 mapping. Magn. Reson. Med. 2010;64(1):291–298. doi: 10.1002/mrm.22342. [DOI] [PubMed] [Google Scholar]
- 137.Letfullin RR, Iversen CB, George TF. Modeling nanophotothermal therapy: kinetics of thermal ablation of healthy and cancerous cell organelles and gold nanoparticles. Nanomedicine. 2011;7(2):137–145. doi: 10.1016/j.nano.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 138.Foy SP, Manthe RL, Foy ST, Dimitrijevic S, Krishnamurthy N, Labhasetwar V. Optical imaging and magnetic field targeting of magnetic nanoparticles in tumors. ACS Nano. 2010;4(9):5217–5224. doi: 10.1021/nn101427t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kwon SG, Piao Y, Park J, et al. Kinetics of monodisperse iron oxide nanocrystal formation by “Heating-Up” process. J. Am. Chem. Soc. 2007;129(41):12571–12584. doi: 10.1021/ja074633q. [DOI] [PubMed] [Google Scholar]
- 140.Sanchis J, Canal F, Lucas R, Vicent MJ. Polymer-drug conjugates for novel molecular targets. Nanomedicine (Lond.) 2010;5(6):915–935. doi: 10.2217/nnm.10.71. [DOI] [PubMed] [Google Scholar]
- 141.Gaspar R, Duncan R. Polymeric carriers: Preclinical safety and the regulatory implications for design and development of polymer therapeutics. Adv. Drug Deliv. Rev. 2009;61(13):1220–1231. doi: 10.1016/j.addr.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 142.Stanton MG, Colletti SL. Medicinal chemistry of siRNA delivery. J. Med. Chem. 2010;53(22):7887–7901. doi: 10.1021/jm1003914. [DOI] [PubMed] [Google Scholar]
- 143.Yuan X, Naguib S, Wu Z. Recent advances of siRNA delivery by nanoparticles. Expert Opin. Drug Deliv. 2011;8(4):521–536. doi: 10.1517/17425247.2011.559223. [DOI] [PubMed] [Google Scholar]
- 201.Institut de recherche Robert-Sauvé en santé et en sécurité du travail (IRSST) du Québec Montréal. Health Effects of Nanoparticles. www.irsst.qc.ca/media/documents/pubirsst/r-469.pdf.