Abstract
Here we develop nanoparticles composed of lipid-like materials (lipidoids) to facilitate non-viral delivery of small interfering RNA (siRNA) to endothelial cells (ECs). Nanoparticles composed of siRNA and lipidoids with small size (~200 nm) and positive charge (~34 mV) were formed by self assembly of lipidoids and siRNA. Ten lipidoids were synthesized and screened for their ability to facilitate the delivery of siRNA into ECs. Particles composed of leading lipidoids show significantly better delivery to ECs than a leading commercially-available transfection reagent, Lipofectamine 2000. As a model of potential therapeutic application, nanoparticles composed of the top performing lipidoid, NA114, were studied for their ability to deliver siRNA targeting anti-angiogenic factor (SHP-1) to human ECs. Silencing of SHP-1 expression significantly enhanced EC proliferation and decreased EC apoptosis under a simulated ischemic condition.
Keywords: siRNA Delivery, Lipidoid, Endothelial Cell, Apoptosis, Ischemia
1. Introduction
The specific silencing of gene expression through RNA interference (RNAi) has tremendous potential to address previously untreatable diseases.[1–3] The safe and effective delivery of small interfering RNA (siRNA), however, remains challenging. Viral vector systems[4,5] have demonstrated in vivo RNAi delivery efficacy but face several of safety-related challenges including potential immunogenicity.[6] A variety of approaches has been reported to facilitate non-viral delivery of siRNA, including direct conjugation of delivery reagent,[7–10] formulation into liposomes,[11,12] and encapsulation by polymers.[13,14] Modification of siRNA with cholesterol,[7] lipoprotein,[8] peptide,[9] or antibody[10] has been reported to improve systematic siRNA delivery. Recently, systemic delivery of siRNA to the liver of non-human primates was demonstrated using a lipid formulation.[11] Despite these advances in non-viral siRNA delivery, the diversity of published delivery materials still remains limited, in part due to their slow, multi-step syntheses.[15] The customization of each synthetic reaction and multiple steps limits the ability to generate a significant library size with diversity.
Recently, chemical approaches were developed that allow the simple, rapid, and parallel generation of lipid-like delivery materials termed lipidoids, as delivery agents for RNAi therapeutics.[16] Lipidoid synthesis is based upon the conjugate addition of alkyl-acrylamides to primary or secondary amines. Synthesis can be performed in the absence of solvents, catalysts, or purification.[16] A library of over 1,200 structurally-diverse lipidoids was synthesized, and members were shown efficacious in three species, including non-human primates.[16–19]
During ischemic events, endothelial cells (ECs) can suffer from hypoxia-induced apoptosis. siRNA delivery to endothelial cells (ECs) may have utility in the treatment of ischemic disease by promoting angiogenesis or inhibiting apoptosis. To this end, we sought to develop a nanoparticulate, lipidoidal delivery system for the treatment of ECs under simulated ischemic conditions. Src homology 2 domain-containing protein tyrosine phosphatase-1 (SHP-1) has been known to inhibit EC proliferation and angiogenesis via the inactivation of vascular endothelial growth factor (VEGF) receptor-2 (KDR/Flk-1) in ECs.[20–22] In addition to its anti-angiogenic activity, SHP-1 can directly affect apoptosis in many cell types[23,24] by binding to death receptors such as TNFR-1 and FAS-R and blocking anti-apoptotic signals. Previous studies have demonstrated that SHP-1 expression is upregulated following myocardial or cerebral ischemia, which may contribute to the enhanced magnitude of myocardial[25] or cerebral[26] infarction. We hypothesized that siRNA-mediated silencing of SHP-1 expression could inhibit EC apoptosis and enhance EC proliferation, and thereby stimulate angiogenesis in ischemic tissue.
2. Results and Discussion
2.1. Screening of Lipidoids for siRNA Delivery to Endothelial Cells
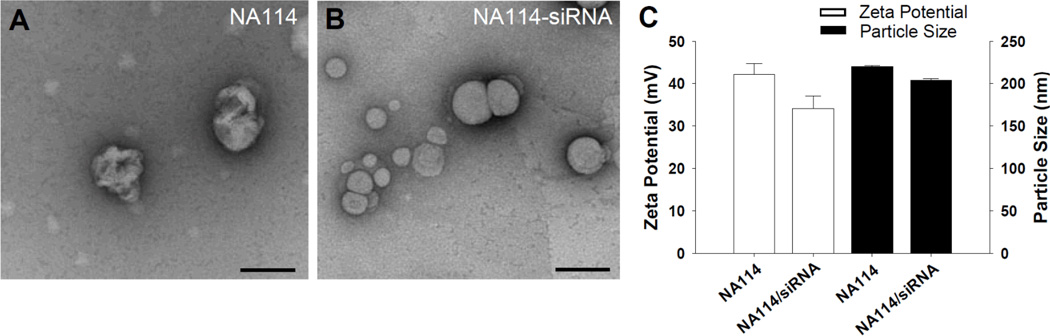
In vitro screening was performed to identify lipidoids that can deliver siRNA efficiently to ECs. Previously, 56 lipidoids were identified that were capable of delivering siRNA to HeLa cells with comparable knockdown efficiency to the commercially-available in vitro transfection agent Lipofectamine 2000.[16] Ten lipidoids were selected among those lipidoids and formulated at four different lipidoid/siRNA weight ratios (2.5w/w, 5.0w/w, 7.5w/w, and 10.0w/w), resulting in total 40 lipidoid/siRNA compositions. Lipidoids were synthesized through the conjugate addition of amine to acrylamide (Fig. 1A and 1B). Structural details of lipidoid (NA114) complexed with and without siRNA were visualized by negative-staining transmission electron microscopy (TEM), as shown in Figure 2A and 2B, respectively. These data showed that the lipidoid forms spherical particle with a size of 200 nm regardless of complexation with siRNA, which is consistent with the size distribution obtained from the dynamic light scattering (Fig. 2C). The tertiary amine of the lipidoids could be protonized in an acidic buffer (25 mM sodium acetate) used in this study, and hence result a positive charged particle. The lipidoids (NA114) loaded with and without siRNA have a zeta potential of 34 mV and 40 mV, respectively (Fig. 2C).
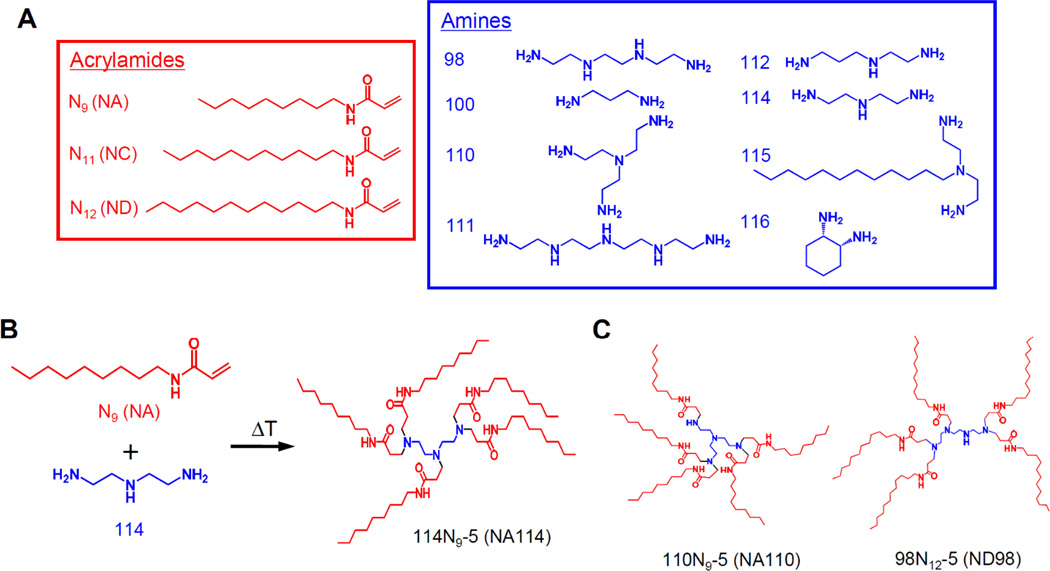
Figure 1.
Synthesis of lipidoids for siRNA delivery. (A) Alkyl-acrylamide and amine molecules were used to synthesize a combinatorial library of lipidoids. (B) Lipidoid synthesis (114N9-5; NA114) was performed through the conjugate addition of amine (114) to acrylamide (N9; NA). Depending on the number of addition sites in the amino monomer, lipidoids can be formed with anywhere from 1 to 7 tails. Lipidoids are named as follows; (amine number)(acrylamide name)-(the number of tails). For example, 100N12-3 (ND100) indicates “the reaction of 100 with N12 where 3 of 4 tails are present”. (C) The molecular structures of 110N9-5 (NA110) and 98N12-5 (ND98).
Figure 2.
Characterization of lipidoid nanoparticles. TEM images of (A) lipidoid (NA114) nanoparticles in the absence of siRNA and (B) lipidoid (NA114) nanoparticles complexed with siRNA (5:1 weight ratio of lipidoid to siRNA). Scale bars indicate 200 nm. (C) Zeta potential and particle size of lipidoid (NA114) and siRNA-lipidoid (NA114) nanoparticles (5:1 weight ratio of lipidoid to siRNA).
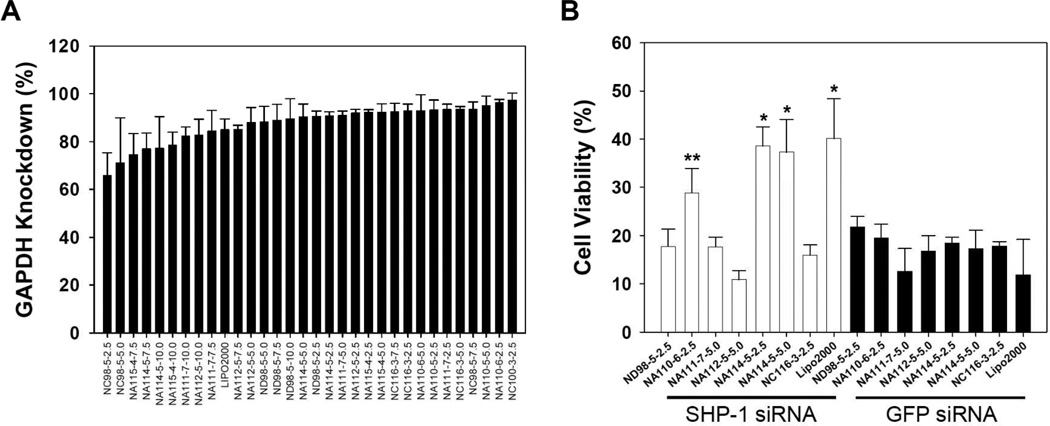
Lipidoidal-siRNA formulations were tested for their ability to deliver siRNA targeting the housekeeping molecule (glyceraldehydes-3-phosphate dehydrogenase; GAPDH) to human umbilical vein endothelial cells (HUVECs). We identified 22 lipidoid formulations with delivery efficacy comparable to Lipofectamine 2000, two days after GAPDH siRNA transfection (Fig. 3A). The seven top-performing formulations with minimal cellular toxicity were further tested to deliver therapeutic siRNA to HUVECs. Two days after transfection of SHP-1 siRNA (siSHP-1) and green fluorescent protein siRNA (siGFP, as control siRNA) (Fig. 3B), transfected HUVECs were transferred to hypoxic (1% oxygen) conditions with serum deprivation to simulate the low oxygen content and deficiency of survival growth factors in ischemic tissue.[27] Two lipidoids, 110N9-5 (“NA110”) and 114N9-5 (“NA114”), were found to increase HUVEC viability significantly (NA110; p<0.05 and NA114; p<0.01), compared to respective controls (siGFP delivery, Fig. 3B). These data suggest these two lipidoidal formulations may provide vehicles for the treatment of ECs in ischemic tissues.
Figure 3.
Screening of lipidoids for siRNA delivery to HUVECs. (A) GAPDH activity of HUVECs (n=4) at 2 days after siGAPDH transfection. The % reduction in GAPDH activity following transfection with siRNA-lipidoid complexes at various lipidoid/siRNA weight ratios is shown. Only lipidoids showing no significant cytotoxicity are included in this graph. (B) The viability of siRNA-transfected HUVECs (n=4) cultured for 2 days under hypoxic (1% oxygen) and serum-deprived condition (*; p<0.01, **; p<0.05, compared to siGFP group with respective lipidoids or Lipofectamine 2000).
Previous studies have shown that top-performing lipidoids contain several structural similarities; 1) amide linkages, 2) more than two alkyl tails, 3) tail length in the range of 8–12 carbons, and 4) one tail short of total substitution of the amine reactants, therefore containing one secondary amine.[16] The lipidoids NA110 and NA114 identified as optimal for siRNA delivery to ECs showed structural similarities to 98N12-5 (ND98), a lipidoid previously demonstrated to have activity in several in vivo models.[16] NA110 is a branched version of ND98, while NA114 has one less ethyleneimine unit relative to ND98 (Fig. 1B and 1C).
2.2. Delivery of Therapeutic siRNA Using Lipidoids to Endothelial Cells
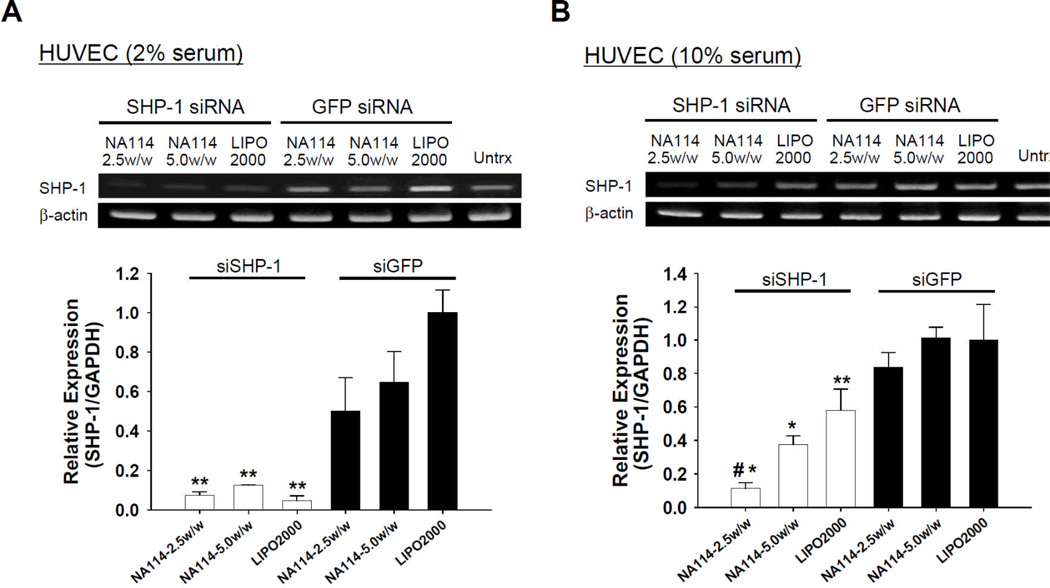
NA114, the optimal lipidoid identified in the present study, was used to deliver therapeutic siRNA (siSHP-1) into ECs and downregulate the target (SHP-1). Two days after siSHP-1 transfection with NA114, significant silencing of SHP-1 mRNA expression was achieved (p<0.05) in siSHP-1-transfected HUVECs compared with siGFP-transfected HUVECs (Fig. 4A). In HUVEC culture using endothelial growth medium-2 (EGM-2) with 2% fetal bovine serum (FBS), SHP-1 knockdown using NA114-siRNA nanoparticles was similar to that by Lipofectamine 2000 (Fig. 4A). Importantly, under culture condition with higher serum content (10% FBS), NA114 showed significantly greater SHP-1 silencing (p<0.01) than Lipofectamine 2000 (Fig. 4B). Generally, the presence of serum can hinder intracellular delivery of nucleic acids.[28] Thus, this lipidoid (NA114) may deliver more efficiently siRNA in the in vivo environment.
Figure 4.
Silencing of SHP-1 by siSHP-1 delivery in HUVECs. RT-PCR and quantitative real-time PCR (n=3) to examine SHP-1 expression in HUVECs at 2 days after siRNA transfection using NA114 and Lipofectamine 2000 under culture condition with (A) 2% FBS and (B) 10% FBS (*; p<0.01, **; p<0.05, compared to all siGFP-transfected groups and #; p<0.01, compared to siSHP-1-transfected group with Lipofectamine 2000). The relative expression of SHP-1 in each group was normalized to that of siGFP group using Lipofectamine 2000.
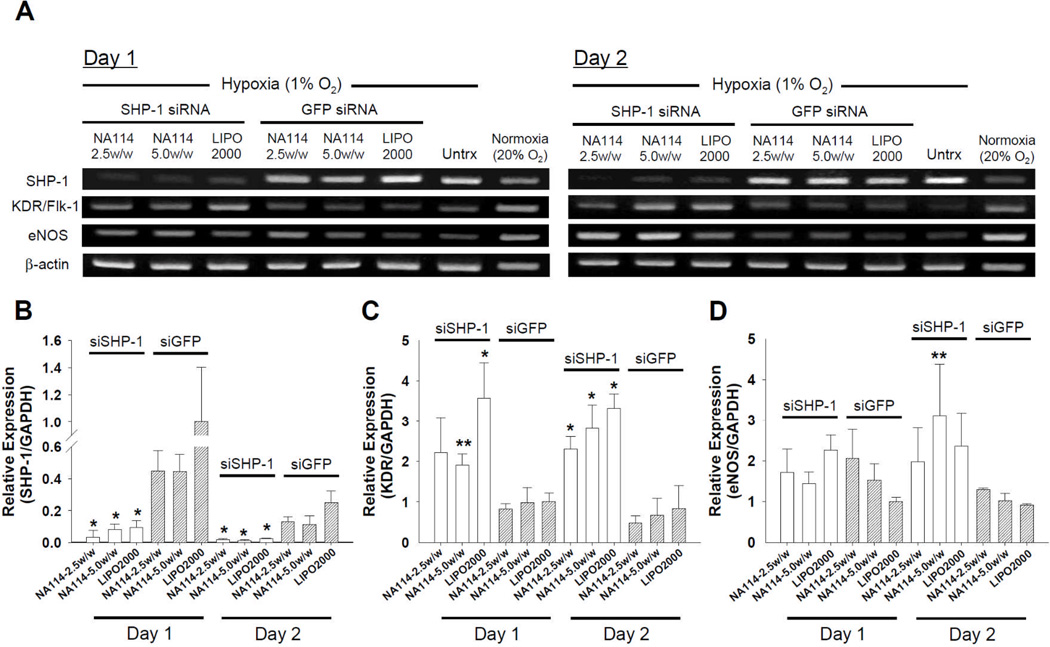
The silencing of SHP-1 by siRNA increased the expression of angiogenesis-inducing factors (e.g., KDR/Flk-1 and eNOS) in ECs (Fig. 5). Two days after siRNA delivery (transfection condition; EGM-2 with 2% FBS), siRNA-transfected HUVECs were moved to hypoxic (1% oxygen) condition with serum deprivation and cultured further for one and two days. While these conditions are known to increase SHP-1 expression,[29] siSHP-1 transfection using NA114 resulted in significant knockdown of SHP-1 expression (p<0.01), compared with siGFP transfection (Fig. 5A and 5B). The knockdown was maintained up to two days after culture (Fig. 5A and 5B), after which siRNA becomes diluted owing to cell division. Silencing of SHP-1 expression can induce activation of KDR/Flk-1, an important angiogenesis regulator in the VEGF signal pathway.[20,21] The expression of eNOS could be enhanced via activation of KDR/Flk-1.[20,21,29,30] siSHP-1 delivery mediated by NA114 increased KDR/Flk-1 expression and consequently eNOS expression in HUVECs (Fig. 5A, 5C, and 5D). The stimulation of KDR/Flk-1- and eNOS-mediated signal transduction may enhance angiogenesis in ischemic tissue and consequently reduce apoptosis of ischemic tissue.
Figure 5.
Gene expression profiles in siRNA-transfected HUVECs under hypoxic (1% oxygen) and serum-deprived (endothelial basal medium-2 (EBM-2) with no serum and growth factors) condition. (A) RT-PCR for SHP-1, KDR/Flk-1, and eNOS of siRNA-transfected HUVECs at day 1 and 2 after culture. Quantitative real-time PCR for (B) SHP-1, (C) KDR/Flk-1, and (D) eNOS. The gene expression in siRNA-transfected HUVECs (n=3) at day 1 and 2 was normalized to that in siGFP-transfected HUVECs using Lipofectamine 2000 at day 1 (*; p<0.01, **; p<0.05, versus all siGFP groups at comparable time point).
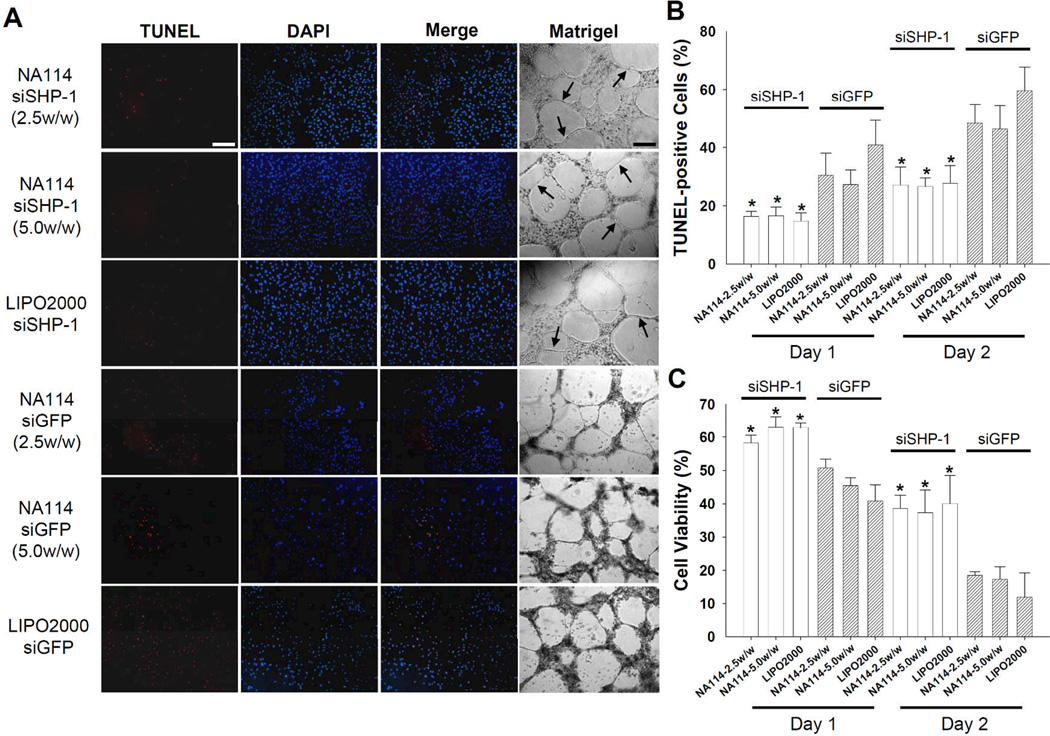
Intracellular delivery of siSHP-1 using NA114 inhibited apoptosis of ECs by hypoxia and serum deprivation. The favorable changes in gene expression profiles following siSHP-1 delivery (Fig. 5) substantially inhibited EC apoptosis and enhanced EC proliferation under hypoxic (1% oxygen) and serum-deprived condition (Fig. 6), which indicates that siSHP-1 therapy allows ECs to endure ischemic condition. On days one and two after culture under simulated ischemic condition, TUNEL staining revealed that the portion of TUNEL-positive cells (apoptotic cells) in siSHP-1-transfected cell populations was significantly lower (p<0.01) than that in siGFP-transfected cell populations (Fig. 6A and 6B). Additionally, siSHP-1-transfected HUVECs showed greater cell viability (p<0.01) compared to siGFP-transfected HUVECs (Fig. 6C).
Figure 6.
Inhibition of apoptotic activity in siSHP-1-transfected HUVECs cultured under hypoxic (1% oxygen) and serum-deprived (EBM-2 with no serum and growth factors) condition. (A) TUNEL staining of siRNA-transfected HUVECs and capillary formation (arrows) by siRNA-transfected HUVECs at 2 days after culture (×100). Scale bars indicate 200 µm. (B) The percentage ratio of TUNEL-positive cells (red; apoptotic cells) in DAPI-positive cells (blue; total cells) at day 1 and 2 after culture (n=4, *; p<0.01 versus all siGFP groups at comparable time point). (C) The viability of siRNA-transfected HUVECs at day 1 and 2 after culture (n=4, *; p<0.01 versus all siGFP groups at comparable time point).
The capacity of ECs to generate microvessels was also enhanced by siSHP-1 delivery. When cultured on growth factor reduced (GFR)-Matrigel, siSHP-1-transfected HUVECs showed the formation of robust capillaries compared with siGFP-transfected HUVECs (Fig. 6A). The functional delivery of siSHP-1 to ECs may provide an avenue for the treatment of ischemic disease via the promotion of angiogenesis and/or inhibition of apoptosis. Of note, previous studies reported that injection of plasmid vector-based shRNA targeting SHP-1 enhanced angiogenesis and reduced the necrotic area in ischemic myocardium[25] or hindlimb.[31]
3. Conclusions
The study reported herein suggests that lipidoid-siRNA nanoparticles may provide a vehicle for the delivery of RNAi therapeutics to ECs. In particular, the formulations described here may provide a vehicle for the treatment of ischemic diseases such as myocardial, hindlimb, or cerebral ischemia. The exploration of potential targets that are involved in more critical signaling in ischemic pathology (e.g., PHD1, an oxygen sensor that regulates the stability of the hypoxia inducible factors under ischemia[32]) should be performed together with the development of next generation of lipidoids with enhanced siRNA delivery efficiency.
4. Experimental
Lipidoid Synthesis
Lipidoid library synthesis was performed and characterized as previously described [16]. Amines and 1-aminoalkanes were purchased from Sigma-Aldrich (St. Louis, MO) and TCI America (Portland, OR). Acrylamides were synthesized by the drop-wise addition of acryloyl chloride to the appropriate 1-aminoalkane. Lipidoids were synthesized by conjugate addition of acrylamides to amines. All library reactions were carried out in 5-ml Teflon-lined glass screw-top vials. The amide portion of the lipidoid library was synthesized at the maximal ratio of acylamide/amine for each amine. 200 mg of amine was added to the corresponding amount of acrylamide. The mixture was stirred at 90°C for 7 days. After cooling, the lipid mixtures were used without purification unless otherwise specified. Representative library members were characterized by TLC, IR, NMR, and mass spectrometer [16].
Small Interfering RNAs (siRNAs)
All siRNAs were synthesized by Dharmacon (Lafayette, CO). SHP-1 siRNA (siSHP-1) for in vitro transfection was designed for targeting human SHP-1 (GenBank accession number: NM_080548). Green fluorescent protein (GFP) siRNA (siGFP) was used as a negative control siRNA. The sequences for the sense and anti-sense strands of siRNAs are as follows; 1) human SHP-1, (sense) 5′-GGA ACA AAU GCG UCC CAU AUU-3′, (anti-sense) 5′-UAU GGG ACG CAU UUG UUC CUU-3′, 2) GFP, (sense) 5′-GGC UAC GUC CAG GAG CGC ACC-3′, (anti-sense) 5′-UGC GCU CCU GGA CGU AGC CUU-3′.
In Vitro siRNA Transfection
To facilitate screening throughput, siRNA-lipidoid complexes were formed by simple mixing of siRNA-lipidoid solutions in microtiter plates. HUVECs (Lonza, Walkersville, MD) were cultured in EGM-2 (Lonza) supplemented with 2% (v/v) FBS (Lonza) and EGM-2 SingleQuot Kits (Lonza) at 37°C and 5% CO2. For transfection in 96-well plates (cell viability and apoptosis assay), HUVECs were seeded (10,000 cells per well) into each well of 96-well polystyrene plates and allowed to attach overnight. Cells were transfected with 50 ng of siRNA (per well) complexed with lipidoids at four lipidoid/siRNA weight ratios of 2.5, 5.0, 7.5, and 10.0. Working dilutions of each lipidoid were prepared at concentrations necessary to yield the different lipidoid/siRNA weight ratios in 25 mM sodium acetate buffer (pH 5.0). The diluted lipidoids were added to the siRNA solutions, and then the mixtures were incubated for 20 min at room temperature to allow for complex formation. The lipidoid/siRNA solution was immediately added to cells in each well. After transfection, cells were incubated for 2 days at 37°C and 5% CO2. Control transfection was performed with Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA), as described by the vendor. Transfection was performed in quadruplicate. For transfection in 6-well plates (gene expression analysis and Matrigel assay), HUVECs were seeded (2.0 × 105 cells per well) into each well of 6-well polystyrene plates and transfected with 1 µg of siRNA per well.
Particle Size and Zeta Potential
Particle size and zeta potential measurements were measured by using a ZetaPALS dynamic light scattering detector (Brookhaven Instruments Corp., Holtsville, NY). Correlation functions were collected at a scattering angle of 90°, and particle sizes were calculated using the MAS option of BICs particle sizing software using the viscosity and refractive index of water at 25°C. Particle sizes are expressed as effective diameters assuming a lognormal distribution. Average electrophoretic mobilities were measured at 25°C using BIC Phase Analysis Light Scattering (PALS) zeta potential software, and zeta potentials were calculated using the Smoluchowsky model for aqueous suspensions. Samples were prepared for biophysical characterization in the same manner as for transfection (5:1 weight ratio of lipidoid to siRNA) and then were diluted 1:10 in 25 mM sodium acetate buffer prior to measurement. Each formulation was run in triplicate. Particle size and zeta potential are expressed as the average values of each run.
Transmission Electron Microscopy (TEM)
Lipidoid/siRNA nanoparticles were formed as they were for transfection experiments (5:1 weight ratio of lipidoid to siRNA) and then droplets of the sample (5 µl) were applied to hydrophilized carbon-covered copper grids (300 meshes) for 30 min. The sample was subsequently rinsed with contrasting material (1% uranyl acetate at pH 4.5). The remaining stain solution was removed with a filter paper and air-dried. TEM microstructure was determined using a Tecnai™ FEG TEM (FEI Tecnai™ 12 Spirit Bio-twin, FEI Company, Hillsboro, OR) operating at 80 kV.
GAPDH Activity Measurement
Transfection of GAPDH siRNA (siGAPDH) (Ambion, Austin, TX) was performed to screen optimal lipidoids for siRNA delivery to HUVECs. After 2 days of transfection, GAPDH activity in siRNA-transfected HUVECs was measured using KDalert™ GAPDH assay kit (Ambion). GAPDH activity of siGAPDH-transfected HUVECs was expressed as a percent activity to that of siGFP-transfected HUVECs. In this assay, reduction of GAPDH activity by cytotoxic or other non-specific effects is observed in both groups (siGAPDH group and siGFP group), while non-cytotoxic and specific silencing results in reduction of GAPDH activity only in siGAPDH group.
Cell Culture under Hypoxic and Serum-Deprived Condition
Two days after transfection with siSHP-1 and siGFP, HUVECs were cultured under hypoxic and serum-deprived condition, a simulated ischemic condition. EGM-2 was changed to Endothelial Basal Medium-2 (EBM-2) without FBS and growth factors, and then siRNA-transfected HUVECs were further cultured for 1 or 2 days in a hypoxic incubator (MCO-18M, Sanyo, Japan) with air condition of 1% oxygen and 5% CO2 at 37°C. Low oxygen content was maintained through the controlled supply of nitrogen gas to the incubator.
Cell Viability Measurement
Cell viability was measured using the CellTiter 96® Aqueous One Solution assay kit (Promega, Madison, WI). Cellular metabolic activity was determined by measuring optical absorbance at 490 nm using a Victor3™ Multilabel plate counter (Perkin-Elmer Life Sciences, Wellesley, MA). The viability of siRNA-transfected HUVECs cultured under hypoxic (1% oxygen) and serum-free (EBM-2) condition for 1 or 2 days was converted to percent viability by comparison to that of HUVECs cultured under normal condition with normoxia (20% oxygen) and complete growth medium (EGM-2) for the comparable time period.
Capillary Formation Assay
48-well polystyrene plates were coated with Growth Factor Reduced (GFR)-Matrigel (BD Biosciences, San Jose, CA). Two days after transfection, siRNA-transfected HUVECs were detached with 0.05% (w/v) trypsin, suspended in EBM-2, and seeded at 4.0 × 104/cm2 onto 48-well plates pre-coated with GFR-Matrigel. The cell plates were incubated under hypoxic (1% oxygen) condition at 37°C and 5% CO2. After culture of 2 days, the capillary structures generated by seeded HUVECs were examined microscopically.
Determination of Apoptotic Activity
On 1 and 2 days after culture under hypoxic and serum-free condition, apoptosis of siRNA-transfected HUVECs was investigated by the terminal deoxynucleotide transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) method using a commercially available apoptosis detection kit (ApopTaq®, Chemicon, Temecula, CA). Cells stained with rhodamine by TUNEL staining method were counterstained with 4,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA). The stained images were examined with a fluorescence microscope (Carl Zeiss, Oberkochen, Germany). The portion of TUNEL-positive cells in total cell populations was calculated as the percentage ratio of TUNEL-stained cells to DAPI-stained cells.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Gene expression in HUVECs after siRNA transfection was examined with RT-PCR. Total RNA was isolated with RNeasy Mini kit (Qiagen, Chatsworth, CA) from each sample of the cells. A reverse transcription reaction was performed with 1 µg of pure total RNA using SuperScript™ III reverse transcriptase (Invitrogen). The synthesized cDNA was amplified by PCR with human specific primers (SHP-1, KDR/Flk-1, eNOS, and β-actin) using Platinum PCR master mix (Invitrogen). The amplification conditions followed several steps; 5 min at 95°C, followed by 25–35 cycles of denaturing (94°C, 30 sec), annealing (55–62°C, 30 sec), and extension (72°C, 45 sec) with a final extension at 72°C for 7 min. The PCR products were visualized by electrophoresis on a 2% agarose gel with ethidium bromide (E-Gel, Invitrogen). β-actin was served as an internal control.
Quantitative Real-Time PCR (TaqMan Method)
Quantitative real-time PCR was performed using a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Universe Fast PCR Master Mix (Applied Biosystems) was used for the reaction. Quantification of gene expression in HUVECs was analyzed with TaqMan® Gene Expression Assays (Applied Biosystems) for each target (human SHP-1: Hs00169359_m1, human KDR/Flk-1: Hs00176676_m1, human eNOS: Hs00167166_m1, human GAPDH: Hs02758991_g1). The expression level of target genes was determined by the comparative Ct method, whereby the target was normalized to the endogenous reference (GAPDH).
Statistical Analysis
Quantitative data are expressed as mean ± standard deviation. Statistical analysis was performed by the analysis of variance (ANOVA) using a Bonferroni test. A value of p<0.05 was considered statistically significant.
Acknowledgements
This work was supported by a grant (EB000244) from the National Institutes of Health and a grant from Alnylam Pharmaceuticals, Inc. S.-W.C. would like to thank the Korea Medical Institute for his postdoctoral fellowship.
Contributor Information
Seung-Woo Cho, Department of Anesthesiology, Children’s Hospital Boston, Harvard Medical School, 300, Longwood Avenue, Boston, MA 02115 (USA); Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA).
Michael Goldberg, Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA).
Sun Mi Son, Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA).
Qiaobing Xu, Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA).
Fan Yang, Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA).
Ying Mei, Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA).
Said Bogatyrev, Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA).
Robert Langer, Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA).
Daniel G. Anderson, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, 45 Carleton Street, E25-342, Cambridge, MA 02142 (USA), dgander@mit.edu
References
- 1.Whitehead KA, Langer R, Anderson DG. Nat. Rev. Drug Discov. 2009;8:129. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novina CD, Sharp PA. Nature. 2004;430:161. doi: 10.1038/430161a. [DOI] [PubMed] [Google Scholar]
- 3.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Nat. Rev. Drug Discov. 2007;6:443. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Rossi JJ. Methods Mol. Biol. 2005;309:261. doi: 10.1385/1-59259-935-4:261. [DOI] [PubMed] [Google Scholar]
- 5.Kumar P, Lee SK, Shankar P, Manjunath N. PLoS Med. 2006;3:e96. doi: 10.1371/journal.pmed.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomanin R, Scarpa M. Curr. Gene Ther. 2004;4:357. doi: 10.2174/1566523043346011. [DOI] [PubMed] [Google Scholar]
- 7.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Nature. 2004;432:173. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 8.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, Zimmermann T, Koteliansky V, Manoharan M, Stoffel M. Nat. Biotechnol. 2007;25:1149. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 9.McNamara 2nd JO, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Nat. Biotechnol. 2006;24:1005. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 10.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J. Nat. Biotechnol. 2005;23:709. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. Nature. 2006;441:111. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 12.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Nat. Biotechnol. 2005;23:1002. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 13.Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. Gene Ther. 2005;12:461. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 14.Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, Hovgaard MB, Schmitz A, Nyengaard JR, Besenbacher F, Kjems J. Mol. Ther. 2006;14:476. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Miller A. Angew. Chem. Int. Ed. Engl. 1998;37:1769. [Google Scholar]
- 16.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Narayanannair Jayaprakash K, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DW, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG. Nat. Biotechnol. 2008;26:561. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, Butler D, Charisse K, Dorkin R, Fan Y, Gamba-Vitalo C, Hadwiger P, Jayaraman M, John M, Jayaprakash KN, Maier M, Nechev L, Rajeev KG, Read T, Rohl I, Soutschek J, Tan P, Wong J, Wang G, Zimmermann T, de Fougerolles A, Vornlocher HP, Langer R, Anderson DG, Manoharan M, Koteliansky V, Horton JD, Fitzgerald K. Proc. Natl. Acad. Sci. U S A. 2008;105:11915. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang YH, Bao Y, Peng W, Goldberg M, Love K, Bumcrot DA, Cole G, Langer R, Anderson DG, Sawicki JA. Proc. Natl. Acad. Sci. U S A. 2009;106:3426. doi: 10.1073/pnas.0813348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akinc A, Goldberg M, Qin J, Dorkin JR, Gamba-Vitalo C, Maier M, Jayaprakash KN, Jayaraman M, Rajeev KG, Manoharan M, Koteliansky V, Rohl I, Leshchiner ES, Langer R, Anderson DG. Mol. Ther. 2009 doi: 10.1038/mt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagami H, Cui TX, Iwai M, Shiuchi T, Takeda-Matsubara Y, Wu L, Horiuchi M. Arterioscler. Thromb. Vasc. Biol. 2002;22:238. doi: 10.1161/hq0202.104001. [DOI] [PubMed] [Google Scholar]
- 21.Guo DQ, Wu LW, Dunbar JD, Ozes ON, Mayo LD, Kessler KM, Gustin JA, Baerwald MR, Jaffe EA, Warren RS, Donner DB. J. Biol. Chem. 2000;275:11216. doi: 10.1074/jbc.275.15.11216. [DOI] [PubMed] [Google Scholar]
- 22.Seo DW, Li H, Qu CK, Oh J, Kim YS, Diaz T, Wei B, Han JW, Stetler-Stevenson WG. J. Biol. Chem. 2006;281:3711. doi: 10.1074/jbc.M509932200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daigle I, Yousefi S, Colonna M, Green DR, Simon HU. Nat. Med. 2002;8:61. doi: 10.1038/nm0102-61. [DOI] [PubMed] [Google Scholar]
- 24.Cui T, Nakagami H, Iwai M, Takeda Y, Shiuchi T, Daviet L, Nahmias C, Horiuchi M. Cardiovasc. Res. 2001;49:863. doi: 10.1016/s0008-6363(00)00299-6. [DOI] [PubMed] [Google Scholar]
- 25.Sugano M, Tsuchida K, Hata T, Makino N. FASEB J. 2005;19:2054. doi: 10.1096/fj.05-4020fje. [DOI] [PubMed] [Google Scholar]
- 26.Wishcamper CA, Brooks DM, Douglas Coffin J, Lurie DI. Brain Res. 2003;974:88. doi: 10.1016/s0006-8993(03)02564-2. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W, Chen J, Cong X, Hu S, Chen X. Stem Cells. 2006;24:416. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 28.Green JJ, Shi J, Chiu E, Leshchiner ES, Langer R, Anderson DG. Bioconjug. Chem. 2006;17:1162. doi: 10.1021/bc0600968. [DOI] [PubMed] [Google Scholar]
- 29.Cho SW, Hartle L, Son SM, Yang F, Goldberg M, Xu Q, Langer R, Anderson DG. Biochem. Biophys. Res. Commun. 2008;376:158. doi: 10.1016/j.bbrc.2008.08.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen BQ, Lee DY, Zioncheck TF. J. Biol. Chem. 1999;274:33057. doi: 10.1074/jbc.274.46.33057. [DOI] [PubMed] [Google Scholar]
- 31.Sugano M, Tsuchida K, Maeda T, Makino N. Atherosclerosis. 2007;191:33. doi: 10.1016/j.atherosclerosis.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, Harten SK, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P. Nat. Genet. 2008;40:170. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]