Abstract
The objectives of this study were to ascertain the prevalence and potential sources of lead exposure among pregnant women residing in a socially-disadvantaged immigrant community in Albuquerque, New Mexico. Pregnant women (n = 140) receiving prenatal care through a community clinic participated in a structured interview and screening to measure their blood lead levels (BLLs). Potential sources of lead exposure were ascertained by the CDC and New Mexico Department of Health questionnaires. Self-reported risk factors were examined as predictors of BLLs using multiple linear regression and partial least squares discriminant analysis. Most patients were Spanish-speaking (88.6%), Latina (95%), foreign-born (87.1%), lacked health insurance (86.4%), and had a high school education or lower (84.3%). While risk factors were prevalent in this population, only three women (2.1%) had BLLs ≥3 μg/dL. Results of multivariate analyses demonstrated that pica symptoms in pregnancy, history of elevated BLLs before pregnancy, use of non-commercial pottery, and living in older houses were important predictors of elevated BLLs. Although the prevalence of other risk factors relevant to immigrant communities (i.e., use of traditional/folk remedies and cosmetics, seasonings and food products from Mexico) was high, they were not predictive of elevated BLLs. Clinics providing prenatal care to immigrant Hispanic communities should carefully assess patients’ pica symptoms, use of non-commercial pottery, and a history of elevated BLLs. Moreover, additional efforts need to focus on the development of screening questionnaires which better reflect exposures of concern in this population.
Keywords: Lead, Pregnancy, Hispanics, Immigrants, Environmental exposure, Screening, Questionnaires
Introduction
Exposure to lead, a well-established environmental pollutant, is associated with a wide range of multi-systemic adverse effects. Elevated blood lead levels (BLLs) during pregnancy are of particular concern because lead freely crosses the placenta and can cause adverse maternal health and birth outcomes, such as hypertension, miscarriage, preterm delivery, low birth weight, and developmental disabilities in affected children [1–6]. Neurobehavioral disabilities among lead-exposed children can result in long-term deficits in academic and cognitive skills [7]. In addition, the increased bone turnover that occurs during pregnancy can increase fetal exposure among women with high lead levels prior to pregnancy [8, 9]. Routine screening for lead exposure among pregnant women is not considered a standard of care, yet identifying and eliminating sources of lead exposure among pregnant women can substantially improve maternal and neonatal health outcomes [7].
Even BLLs below the 10 μg/dL, the Centers for Disease Control and Prevention (CDC) cutoff for ‘level of concern’, might result in adverse outcomes in these populations. A recent study of mothers aged 15–49 in New York reported that maternal BLLs <10 μg/dL resulted in statistically significant decreases in birth weight [10]. Updated CDC guidelines call for interventions and increased education for all pregnant women with BLLs greater or equal to 5 μg/dL in an effort to eliminate prenatal lead exposure [5, 11].
Targeted public health efforts for over three decades have greatly reduced average BLLs in the general U.S. population to less than 20% of levels measured in the 1970s [12]. This is partly due to eliminating lead-based paint, leaded gasoline, solder from canned food, and increasing regulations on imported food products and lead levels in food [13, 14]. However, the CDC identifies certain subgroups of U.S. women of childbearing age that remain at increased risk: (1) women working in manufactures which involve lead (e.g., smelting, auto repair, construction, firing ranges, painting, manufacturing of ceramics, stained glass, batteries, or plastics) or those who have indirect ‘take-home exposure’ from lead dust carried by a family member; (2) foreign-born recent immigrants; and (3) pregnant women with certain dietary and lifestyle risk factors such as pica symptoms (i.e., the intentional ingestion of nonfood items), iron and calcium deficiency [11]. Mexican–Americans may be particularly at increased risk of lead exposure due to the use of folk/traditional remedies, lead-glazed ceramic pottery, and Mexican-produced food products [15–18].
The primary objectives of this clinic-based study in the state of New Mexico were: (1) to estimate the prevalence of elevated BLLs among socially-disadvantaged pregnant women, predominantly recent immigrants, and (2) to identify behaviors that expose this population to potential sources of lead exposure.
Methods
Study Design and Population
A cross-sectional study design was utilized to measure BLLs and to ascertain risk factors of lead exposure among pregnant women attending a UNM community Maternal & Family Planning clinic in Albuquerque, New Mexico. The clinic serves predominately Spanish-speaking, immigrant women who lack health insurance. Eligible women included those who were ≥18 years of age, ≤20 weeks of gestation, spoke English or Spanish, and had an ultrasound-confirmed singleton pregnancy. Between November 2009 and September 2010, 144 consecutively chosen pregnant women, who were attending the clinic on the days when a study coordinator (SC) was on site, were offered participation and 140 enrolled (97.2% participation rate). The most common reasons for non-participation included not being interested, time constrains, or unwillingness to participate in phlebotomy.
The study was approved by the UNM Human Research Review Committee (HRRC); all study participants signed a written informed consent. After the interview, participants received a brochure in English or Spanish on prevention of lead exposure and a 2010 U.S. Environmental Protection Agency (EPA) desk calendar with month-to-month lead information.
Structured Interview
At enrollment, patients participated in a semi-structured interview in English or Spanish, depending on the patient’s preference. Socio-demographic, lifestyle, and reproductive health characteristics were ascertained. Self-reported data on medical and reproductive histories, including pregnancy dating, complications, and medical conditions were confirmed by review of electronic medical records for all participants.
Maternal behaviors with potential risk of lead exposure were ascertained by the 5-item CDC Blood Lead Screening Risk Questionnaire, the New Mexico Department of Health lead screening questionnaire, and additional questions about lead exposures identified in the literature. The CDC questionnaire collected information on housing features (e.g., regularly live or visit a house built prior to 1960 with peeling or chipped paint, renovations or remodeling that generates a lot of dust, proximity to an active lead smelter or facility that is likely to release lead), previous treatment or observations for lead poisoning among children or housemates, and jobs or hobbies that use lead or lead products among adults living in the household [2, 19]. Although the questionnaire was originally designed to be completed by parents about their children’s exposure, the reported sensitivity (75.7%) and negative predictive value (93.1%) for use in pregnant women were comparable to its reported accuracy in young children [1].
Additional questions about potential sources of lead exposure were included from the New Mexico Department of Health lead screening questionnaire [20]. These questions inquired about behavioral and lifestyle factors (e.g., pica behavior, use of traditional/folk remedies or cosmetics, non-commercially prepared pottery, ingestion of Mexican seasoning or candies, playing with jewelry in the mouth, living in another major city or another country, history of testing positive for lead in blood). Pica behavior, defined as an abnormal craving or appetite for nonfood substances, such as dirt, paint, or clay, is particularly an important risk factor among pregnant women, especially in urban environments with lead soil contamination. Additional questions, identified from previous studies, ascertained use of plastic or vinyl mini-blinds in the home [21, 22] and use of lighting candles at least a few times a month in the house [22].
Blood Lead Level Determination
At the same visit, a clinic nurse collected 10 mL of whole blood (EDTA vials) from each participant by venipuncture. Specimens were shipped on dry ice to the Quest Diagnostics clinical laboratory for blood lead analysis by the atomic absorption spectroscopy (AAS). The limit of detection (LOD) for blood lead using the AAS method is 3 μg/dL, sufficient to detect levels above both the CDC ‘intervention level’ and the CDC ‘level of concern’. Because AAS is more widely available, substantially cheaper than inductively coupled plasma mass spectrometry (ICP-MS), and sufficient to detect levels of concern, it is likely to be representative of methods used by clinical screening programs.
ICP-MS provides substantially increased sensitivity and can detect the presence of blood lead at levels well below the recommended action levels. However, this instrumentation is not always readily accessible to clinicians and public health programs, and analytical costs can be prohibitive for screening. Therefore, to assess whether BLLs below the recommended action levels were associated with self-reported potential sources of exposure and corresponded to results obtained by AAS, a subset of 39 samples was re-analyzed by ICP-MS (Elan DRC II, Perkin Elmer, Norwalk, CT) in a single batch at the trace metal laboratory at the Harvard University School of Public Health. These samples were from the last 39 subjects enrolled in the study for whom at least 1ml of whole blood was banked for confirmatory analysis. One gram of blood samples was digested with 2 mL of concentrated HNO3 acid for 24 h in a 15 mL plastic tube at the room temperature. Samples were left to digest for 24 h at room temperature after addition of 1 mL of hydrogen peroxide. Samples were then diluted to 10 mL with deionized water. Lead in blood was measured by ICP-MS with isotope dilution procedure. Data given were the average of five replicate measurements by the instrument. The calculated detection limit for blood lead analysis by this procedure was 0.05 μg/dL−1.
Data Analysis
Analyses were conducted in SAS® 9.2 (SAS Institute, Cary, NC) and PLS_Toolbox 5.0 in MATLAB (MathWorks, T. Matlab 2008Ra. Natick, MA). BLLs were log-transformed prior to analysis. Descriptive statistics and bivariate relationships were initially explored. The prevalence of each risk factor was compared among patients with detectable (≥3 μg/dL) and ‘non-detectable’ by AAS lead levels using Chi-square analysis. In addition, these risk factors were modeled as potential predictors of BLLs, measured on a continuous scale, by multiple linear regression for the 39 patients for whom BLLs were measured by ICP-MS.
To identify a combination of risk factors responsible for the best separation between subjects with ‘positive’ and ‘negative’ levels by AAS, a partial least squares discriminant analysis (PLSDA) was applied. PLSDA is a multivariate inverse least squares discrimination method which sharpens the separation between groups of observations by rotating mathematical components obtained by Principal Component Analysis (PCA) such that a maximum separation among classes (e.g., subjects with detectable and non-detectable levels) is obtained. PCA and PLSDA transforms original variables into new uncorrelated variables, called principal components. Each principal component is a linear combination of the original variables (risk factors). The coefficients of the principal components are calculated so that the first principal component (PC1) contains the maximum variance. The second principal component (PC2) is calculated to have the second most variance, and, importantly, is uncorrelated with the PC1 and so on. The first output from PCA is component coefficients or loadings, which are the coefficients of the linear combinations of the original variables that generate the principal components. The second output, principal components or scores, contains the coordinates of the original data in the new coordinate system. Biplots displaying both the principal component coefficients for each variable and the principal components for each subject in a single plot for the PC1 and PC2 were produced to visualize the clustering of samples with respect to risk factors that were the most or least important for separating patients based on their BLL results. Correlated variables and samples are located in the same quadrant on a biplot. Risk factors clustered in the same quadrant as the samples that tested ‘positive’ for lead were the most predictive of the BLL results.
Results
The mean age of study participants was 26.1 ± 5.5 years, and the average gestational age at recruitment was 14.5 ± 5.5 weeks (Table 1). The majority of the study participants were Spanish-speaking (88.6%), Latina (95%) and born outside of the U.S. (87.1%). Most women reported a high school education or lower (84.3%), lacked any form of health insurance (86.4%), and were married or living with a partner (79.3%). Half of the sample reported an unplanned pregnancy and 25.0% reported a history of adverse perinatal outcomes. There was no reported tobacco use at the time of the interview; however, 5.7% of women reported that they quit smoking after pregnancy recognition. In terms of alcohol use, 17.9% reported at least one binge drinking episode (≥4 standard drinks on one occasion) during the month around their last menstrual period (LMP) and 10% reported some alcohol use after LMP (mostly prior to pregnancy recognition).
Table 1.
Description of the study sample (N = 140)
| Patient characteristics | N (%) |
|---|---|
| Race | |
| White | 133 (95.0) |
| Black or African American | 2 (1.4) |
| American Indian | 0 |
| Asian Pacific Islander | 4 (2.9) |
| Other | 1 (0.7) |
| Ethnicity: Latina | 133 (95.0) |
| Foreign-born | 122 (87.1) |
| Marital status | |
| Single, never married | 28 (20.0) |
| Married or living with partner | 111 (79.3) |
| Separated from spouse | 1 (0.7) |
| Educational level | |
| High school education or less | 118 (84.3) |
| Some college or vocational school | 12 (8.6) |
| College degree or higher | 10 (7.1) |
| Health insurance | |
| No insurance | 121 (86.4) |
| Medicaid or other public insurance | 18 (12.9) |
| Employer-provided insurance | 1 (0.7) |
| Primary language: Spanish | 124 (88.6) |
| Primigravida | 35 (25.0) |
| Nulliparous | 40 (28.6) |
| Presence of chronic condition(s) | 9 (6.4) |
| Hypertension | 2 (1.4) |
| Diabetes | 1 (0.7) |
| Thyroid disorder | 1 (0.7) |
| Asthma or allergies | 3 (2.1) |
| History of adverse perinatal outcomes | 35 (25.0) |
| Miscarriage | 29 (20.7) |
| Stillbirth | 5 (3.6) |
| Ectopic pregnancy | 1 (0.7) |
| Unplanned pregnancy | 70 (50.0) |
| Smoking status | |
| Current smoker | 0 (0.0) |
| Never smoked | 122 (87.1) |
| Past smoker, quit prior to pregnancy | 10 (7.1) |
| Past smoker, quit during pregnancy | 8 (5.7) |
| Alcohol use | |
| One or more binge drinking episodes the month around LMPa | 25 (17.9) |
| Alcohol consumption anytime after LMP | 14 (10.0) |
|
| |
| Mean ± SD | |
|
| |
| Maternal age (years) | 26.1 ± 5.5 |
| Gestational age at enrollment (weeks) | 14.5 ± 5.5 |
Binge drinking among pregnant women is defined as ≥4 standard drinks on one occasion
LMP last menstrual period
For the entire sample, the most common self-reported sources of potential lead exposure included eating Mexican tamarind-chile candies or using Mexican seasonings (73.6%), living with an adult whose occupation might involve lead exposure (44.2%), having lived previously in a major city outside of New Mexico (43.6%), currently/ ever living in a house built before 1978 (47.1%) or built before 1960 (24.3%).
Among 140 participants, only three (2.1%) had a detectable BLL by AAS. Analysis by ICP-MS confirmed that two of these patients had BLLs above the CDC cutoff for intervention (8.5 and 5.6 μg/dL) and a third patient had BLL of 3.4 μg/dL. Of the 39 participants analyzed by ICPMS, the mean BLL was 1.06 ± 1.55 (median 0.61; range 0.26–8.5 μg/dL). Samples in the subset of 39 that had ‘undetectable’ BLLs by AAS were determined to have concentrations ≤1.1 μg/dL in ICP-MS confirmatory analysis.
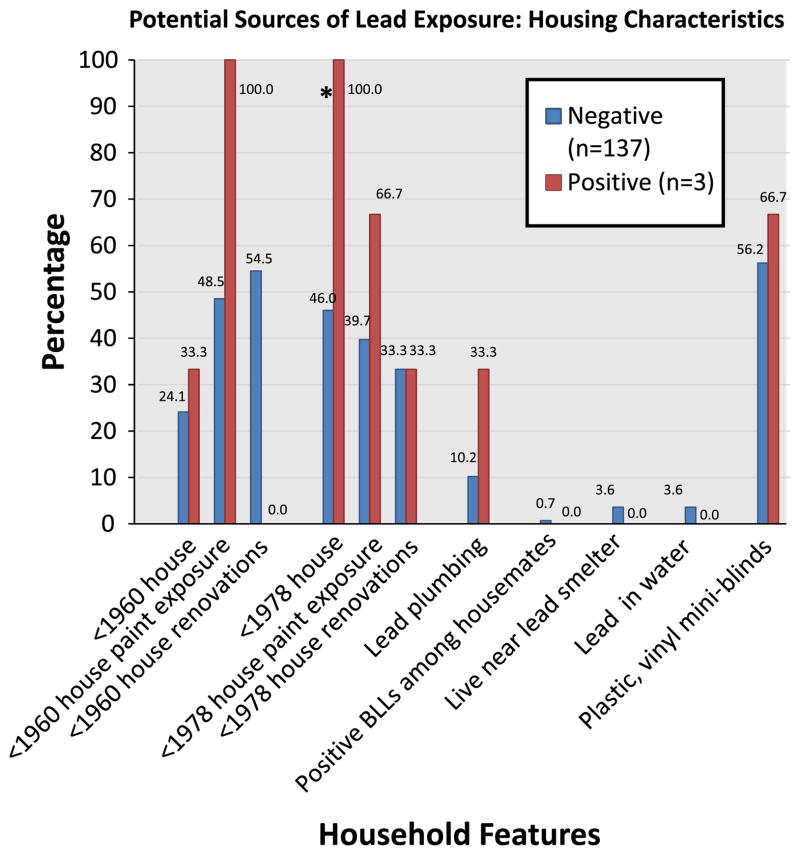
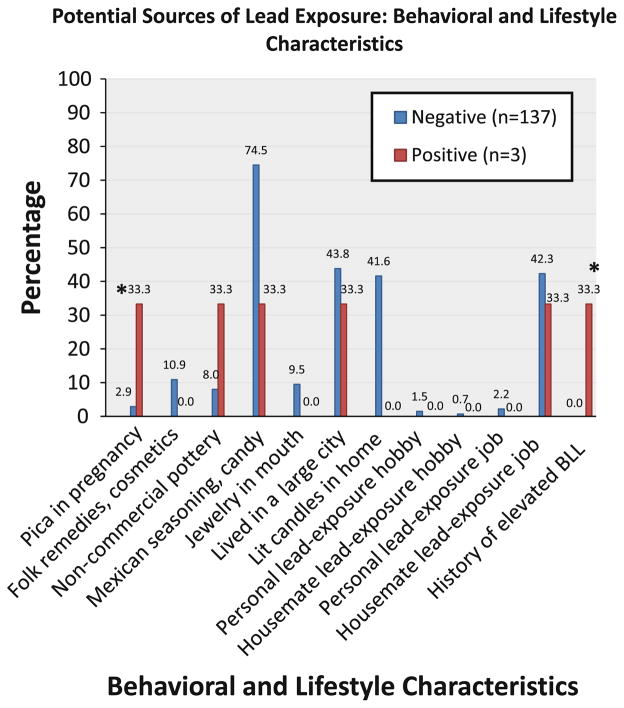
Individual risk factors of lead exposure among participants with BLLs ≥3 μg/dL and BLLs<3 μg/dL are summarized in Figs. 1 and 2. The only household feature that differed at a borderline significance level (p = 0.06) between the two groups was currently living or having ever lived in a house built before 1978. With respect to behavioral characteristics, subjects with positive BLLs had a higher prevalence of pica symptoms and a history of a positive blood test before pregnancy (both p-values<0.01). Other risk factors of concern in immigrant communities (e.g., use of traditional/folk remedies or cosmetics, Mexican chile seasoning or tamarind candies, having lived in a large city, and living with someone who has a hobby or job with lead-exposure) were not associated with increased BLLs in our sample.
Fig. 1.
Comparison of potential household sources of lead exposure between between women with negative and positive BLL tests. *Borderline significance (p = 0.06)
Fig. 2.
Comparison of potential sources of lead exposure from behavioral and lifestyle characteristics between women with negative and positive BLL tests. *Significant (p<0.01)
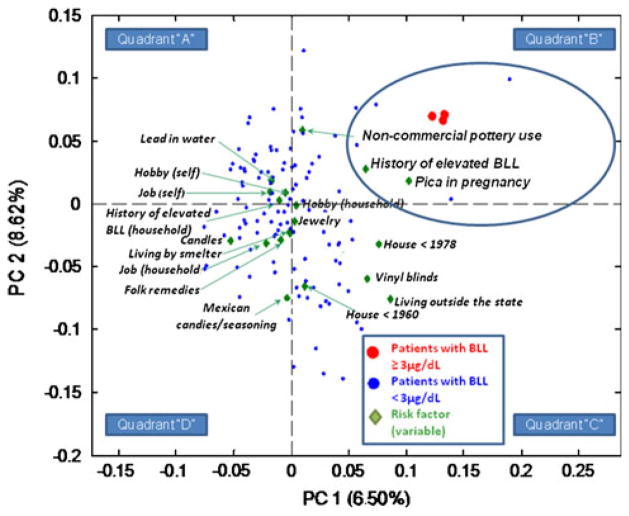
PLSDA analysis resulted in a model displayed as biplot in Fig. 3. The position of each subject and risk factor on the biplot is determined by respective principal components and component coefficients values calculated by PLSDA. The first principal component (quadrants ‘B’ and ‘C’ vs. ‘A’ and ‘D’, Fig. 3) captured 6.50% of the variance and succesfully separated patients with ‘positive’ and ‘negative’ BLLs. Risk factors which demonstrated the highest correlation with the three positive BLL tests (quadrant ‘B’) were: (a) pica symptoms; (b) use of non-commercially prepared pottery or dishes that may have lead-based glazes; and (c) a history of elevated BLLs before pregnancy. The second principal component explained an additional 8.62% of the variance and separated risk factors captured by the first principal component from more chronic exposures, such as living in an old house (quadrant ‘C’), use of vinyl blinds, and living in a major city outside the state (most participants listed Mexico city) in the past. Interestingly, the use of Mexican candies/seasonings, traditional/folk remedies or cosmetics, and a hobby of a study participant or her household member involving lead products were not important predictors identified by PLSDA in this sample.
Fig. 3.
Combination of potential sources of lead exposure, organized into principal components, which results in the best separation of subjects with positive and negative BLLs. Note: Correlated risk factors (green diamonds) which best separate patients with ‘positive’ (red dots) and ‘negative’ (blue dots) blood lead tests are located in the same quadrant on a plot. PC 1, first principal components (quadrants “B” and “C” vs. “A” and “D”). PC 2, second principal component (quadrants “A” and “B” vs. “C” and “D”). “Self” and “household” labels refer to risk factors applicable to a pregnant women and a household member, respectively
Multiple linear regression results, obtained on a subset of 39 women for whom BLLs analysed by ICP-MS were available, confirmed the results of PLSDA analysis. Namely, significant predictors included pica symptoms (p = 0.017), use of non-commercial pottery (p = 0.010), and testing positive for BLL before pregnancy (p<0.001).
Discussion
The decrease of elevated BLLs in the U.S. general population since the late 1970s is considered a major environmental health achievement. Data from the 2003–2008 National Health and Nutrition Examination Surveys (NHANES) indicated that BLLs among pregnant women were actually lower (0.64 μg/dL) than those in non-pregnant women (0.85 μg/dL), and less than 0.5% of the this nationally representative sample had elevated BLLs >5 μg/dL [23]. However, the report indicated that pregnant Mexican-American women had much higher lead levels (0.90 μg/dL) and women born in Mexico had twice as high BLLs (1.08 μg/dL) compared to their U.S.-born counterparts (0.62 μg/dL). Occupational and clinical surveillance data from 37 states collected by the CDC indicate that 0.6 per 100,000 women of childbearing age (16–44 years) had BLLs ≥40 μg/dL and 10.9 per 100,000 women had BLLs of ≥5 μg/dL [5].
Poor, urban, and immigrant populations remain at increased risk for lead exposure [15]. For example, the prevalence of elevated BLLs among pregnant women in the Mahoning County (Ohio), which was a center of steel production in the U.S. until the late 1970s, might be as high as 13% [1]. Prior studies suggest that sources of lead exposure and prevalence in asymptomatic women of childbearing age and pregnant women most commonly include gasoline, air, contaminated soil or water, certain vinyl products, some folk and alternative health remedies, residential lead-based paint, and occupations or hobbies that use lead [24, 25].
Immigrant pregnant women, especially recent immigrants, were reported to have higher BLLs compared to non-immigrants, and the BLLs among immigrants decreased with time spent in the U.S. [26]. The higher prevalence of lead exposure in immigrant populations might be due to the greater likelihood of living in older homes, closer proximity to lead smelters and other lead-producing factories, poorer nutrition, previous exposure to leaded gasoline, and occupational exposures [15, 26].
Although nearly all patients in this study had negative BLLs, there was an exceptionally high prevalence of consuming traditional Mexican candies and seasoning products (73.6%). Tamarind candy and folk remedies have previously been implicated in elevated BLLs in children according to some case reports [27]. Lead can be found in these candies and seasonings from ceramic candy containers, cellophane wrappers and ink on the wrappers, and from chile powder [27, 28]. Investigations of candy wrappers reported by the CDC found a Dulmex-brand Bolirindo lollipop with lead levels as high as 21,000 ppm, confirmed by the U.S. Food and Drug Administration (FDA), which lead to a public health warning [27]. Tamarind candies are often sold in small clay pots with leaded glazes, and the acidity of the tamarind fruit may further increase the leaching of the lead into the product [29]. Chile grown in Mexico have been found to contain high levels of lead from the soil in which they were grown and from processing plants [29]. Specific traditional Mexican products commonly cited by our study participants included chile suckers and candies, chile powder, tamarind, pulparindo, and Vero candies.
Prevalence of pica among pregnant women and its effects on the mother and fetus are not well described, although the prevalence in high-risk groups has declined during the 1950s and 1960s, and remained relatively stable since the 1970s [30]. The common risk factors for pica symptoms include low socioeconomic status, malnutrition, and living in underdeveloped areas [31]. Pica was previously associated with severe gestational lead poisoning, and some studies reported that the prevalence of pica might be as high as 39% among pregnant women with BLLs ≥20 μg/dL [14, 32].
In this study, the prevalence of positive BLLs was lower than expected, despite targeting recent immigrants with an average of four risk behaviors per participant. Even though our results cannot be generalized to all immigrant pregnant women, and the prevalence of elevated BLLs can be difficult to estimate due to a relatively small sample size, this study suggests that the prevalence of lead exposure among recent immigrants, similar to the general U.S. population, might have declined in recent years. Our results are in accordance with the recent surveillance data from the New Mexico Department of Health Lead Prevention Program which indicated that in 2006–2008 only 3 out of every 1,000 children tested for lead exposure had elevated BLLs [33]. However, these results cannot be generalized to other states in the Southwest.
An important health policy implication was highlighted by this study: in this population of predominantly immigrant Latinas, standard screening questionnaires would result in a very high false positive rate, if routinely used in the clinical settings. This could present an unnecessary burden to the healthcare system if all women who screened positive on the questionnaire underwent blood testing. There are two possible explanations for our findings of relatively low BLLs despite prevalent risk factors for lead exposure. First, successful public health efforts to decrease BLLs in the general U.S. population had an overall impact on the traditional risk factors (e.g., older housing, occupational exposure) which might be less contaminated with lead than in the past. While it is feasible that some immigrants have past exposures which might mobilize from endogenous bone stores during pregnancy, the most rapid initial drop in BLLs occurs during the first 2 months after the removal from environmental exposure [11]. In our study population, women resided on average for 8.2 ± 5.7 years in the United States. The second possible explanation is that the previously reported lead contamination of Mexican candies/seasonings and traditional folk remedies cannot be generalized to all such products. However, it is not clear whether screening questionnaires would have worked better in other parts of the United States. Our study raises the need to conduct more extensive evaluation of ‘non-traditional’ sources of lead exposures specific to immigrant populations in future studies. Our data also suggest that patients reporting pica symptoms and a history of elevated BLLs should be followed up more closely and screened for BLL in pregnancy.
Several limitations in the current study need to be mentioned. First, results of the study suggest a need for a more extensive analysis to determine the generalizability of the results to other immigrant communities. Second, while we assessed BLLs and asked participants to report risk factors of lead exposure, the specific products and environments were not directly tested for lead. Third, given the limit of detection of AAS, lead levels below 3 μg/dL could not be estimated for the entire sample; however, subset analysis by ICP-MS demonstrated that BLLs identified as undetectable by AAS had values ≤1.1 μg/dL. In addition, PLSDA for the entire sample and multiple linear regression for a subset of patients with BLLs measured by ICP-MS identified the same risk factors which further support our conclusions. Finally, given a low prevalence of elevated BLL tests, statistical power to detect association with some risk factors could have been limited. Given these limitations, it is important to emphasize that this was the first study in New Mexico to focus on socially-disadvantaged women from a low-income neighborhood, mostly Spanish-speaking immigrants, that otherwise might not be included in public health surveys. In addition, in an effort to minimize recall bias based on pregnancy outcome, patients in our study were recruited during the first part of pregnancy.
In summary, while our study confirmed well-established risk factors for chronic lead exposure (i.e., including living in older houses, history of elevated BLLs, use of non-commercial pottery) and emphasized the importance of evaluating sources of lead exposure specific to pregnant women (i.e., pica symptoms), lack of association with other risk factors relevant to immigrant communities (i.e., use of traditional/folk remedies and cosmetics, seasonings and food products from Mexico) warrant development of new screening questionnaires that more accurately assess sources of lead exposure among Latina pregnant women. However, until new screening questionnaires tailored to immigrant pregnant women are developed and validated, pregnant women reporting a regular use of non-commercial pottery, pica symptoms, and a prior history of elevated BLLs need to be screened for blood lead during pregnancy.
Acknowledgments
We are grateful to our study participants, the nurses and staff at the University of New Mexico (notably Elida Flores, Judith Vergara-Blake, Olivia Espinoza, and Ada Loera) and Sizhu Liu, M.S. for data management. The NMDOH provided educational materials for participants, and we thank Drs. Mary Shepherd and Janice Frustaglia for their assistance with the study and critical review of the manuscript. We are in debt to Dr. Chitra Amarasiriwardena at Harvard University for the ICP-MS analysis. This study was supported by the University of New Mexico Environmental Health Signature Program research grant.
Contributor Information
Ludmila N. Bakhireva, Email: lbakhireva@salud.unm.edu, Department of Pharmacy Practice and Administrative Sciences, College of Pharmacy, University of New Mexico, MSC09 5360, Albuquerque, NM 87131, USA. Department of Family and Community Medicine, School of Medicine, University of New Mexico, Albuquerque, NM, USA
Andrew S. Rowland, Department of Family and Community Medicine, School of Medicine, University of New Mexico, Albuquerque, NM, USA
Bonnie N. Young, Department of Pharmacy Practice and Administrative Sciences, College of Pharmacy, University of New Mexico, MSC09 5360, Albuquerque, NM 87131, USA
Sandra Cano, Department of Pharmacy Practice and Administrative Sciences, College of Pharmacy, University of New Mexico, MSC09 5360, Albuquerque, NM 87131, USA.
Sharon T. Phelan, Department of Obstetrics and Gynecology, School of Medicine, University of New Mexico, Albuquerque, NM, USA
Kateryna Artyushkova, Department of Chemical and Nuclear Engineering, University of New Mexico, Albuquerque, NM, USA J. Lewis.
William F. Rayburn, Department of Obstetrics and Gynecology, School of Medicine, University of New Mexico, Albuquerque, NM, USA
Johnnye Lewis, Department of Pharmaceutical Sciences, College of Pharmacy, University of New Mexico, Albuquerque, NM, USA.
References
- 1.Stefanak MA, Bourguet CC, Benzies-Styka T. Use of the centers for disease control and prevention childhood lead poisoning risk questionnaire to predict blood lead elevations in pregnant women. Obstetrics and Gynecology. 1996;87(2):209–212. doi: 10.1016/0029-7844(95)00397-5. [DOI] [PubMed] [Google Scholar]
- 2.Cleveland LM, Minter ML, Cobb KA, et al. Lead hazards for pregnant women and children: Part 2: More can still be done to reduce the chance of exposure to lead in at-risk populations. American Journal of Nursing. 2008;108(11):40–47. doi: 10.1097/01.NAJ.0000339156.09233.de. [DOI] [PubMed] [Google Scholar]
- 3.Borja-Aburto VH, Hertz-Picciotto I, Rojas Lopez M, et al. Blood lead levels measured prospectively and risk of spontaneous abortion. American Journal of Epidemiology. 1999;150(6):590–597. doi: 10.1093/oxfordjournals.aje.a010057. [DOI] [PubMed] [Google Scholar]
- 4.Torres-Sanchez LE, Berkowitz G, Lopez-Carrillo L, et al. Intrauterine lead exposure and preterm birth. Environmental Research. 1999;81(4):297–301. doi: 10.1006/enrs.1999.3984. [DOI] [PubMed] [Google Scholar]
- 5.Calvert GM, Roscoe RJ, et al. Centers for Disease Control and Prevention (CDC) Lead exposure among females of childbearing age—United States, 2004. Morbidity and Mortality Weekly Report. 2007;56(16):397–400. [PubMed] [Google Scholar]
- 6.Chen PC, Pan IJ, Wang JD. Parental exposure to lead and small for gestational age births. American Journal of Industrial Medicine. 2006;49(6):417–422. doi: 10.1002/ajim.20313. [DOI] [PubMed] [Google Scholar]
- 7.Gardella C. Lead exposure in pregnancy: A review of the literature and argument for routine prenatal screening. Obstetrical and Gynecological Survey. 2001;56(4):231–238. doi: 10.1097/00006254-200104000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Gulson BL, Jameson CW, Mahaffey KR, et al. Pregnancy increases mobilization of lead from maternal skeleton. Journal of Laboratory and Clinical Medicine. 1997;130(1):51–62. doi: 10.1016/s0022-2143(97)90058-5. [DOI] [PubMed] [Google Scholar]
- 9.Gulson BL, Pounds JG, Mushak P, et al. Estimation of cumulative lead releases (lead flux) from the maternal skeleton during pregnancy and lactation. Journal of Laboratory and Clinical Medicine. 1999;134(6):631–640. doi: 10.1016/s0022-2143(99)90104-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhu M, Fitzgerald EF, Gelberg KH, et al. Maternal low-level lead exposure and fetal growth. Environmental Health Perspectives. 2010;118(10):1471–1475. doi: 10.1289/ehp.0901561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ettinger AS, Wengrovitz AG, editors. Centers for Disease Control and Prevention (CDC) Guidelines for the identification and management of lead exposure in pregnant and lactating women. Atlanta, GA: U.S. Department of Health and Human Services; 2010. pp. 1–267. http://www.cdc.gov/nceh/lead/publications/leadandpregnancy2010.pdf. [Google Scholar]
- 12.Rothenberg SJ, Manalo M, Jiang J, et al. Maternal blood lead level during pregnancy in South Central Los Angeles. Archives of Environmental Health. 1999;54(3):151–157. doi: 10.1080/00039899909602253. [DOI] [PubMed] [Google Scholar]
- 13.Brown RW, Longoria T. Multiple risk factors for lead poisoning in Hispanic sub-populations: A review. Journal of Immigrant and Minority Health. 2010;12(5):715–725. doi: 10.1007/s10903-009-9245-8. [DOI] [PubMed] [Google Scholar]
- 14.Klitzman S, Sharma A, Nicaj L, et al. Lead poisoning among pregnant women in New York City: Risk factors and screening practices. Journal of Urban Health. 2002;79(2):225–237. doi: 10.1093/jurban/79.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleveland LM, Minter ML, Cobb KA, et al. Lead hazards for pregnant women and children: Part 1: Immigrants and the poor shoulder most of the burden of lead exposure in this country. Part 1 of a two-part article details how exposure happens, whom it affects, and the harm it can do. American Journal of Nursing. 2008;108(10):40–49. doi: 10.1097/01.NAJ.0000337736.76730.66. [DOI] [PubMed] [Google Scholar]
- 16.Erdem G, Hernandez X, Kyono M, et al. In utero lead exposure after maternal ingestion of Mexican pottery: Inadequacy of the lead exposure questionnaire. Clinical Pediatric (Phila) 2004;43(2):185–187. doi: 10.1177/000992280404300209. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Serrato MI, Mendoza-Alvarado LR, Rojas-Martinez R, et al. Factors associated with lead exposure in Oaxaca Mexico. Journal of Exposure Analysis and Environmental Epidemiology. 2003;13(5):341–347. doi: 10.1038/sj.jea.7500282. [DOI] [PubMed] [Google Scholar]
- 18.Sankury T, Cooper D, et al. Centers for Disease Control and Prevention (CDC) Lead poisoning from Mexican folk remedies–California. Morbidity and Mortality Weekly Report. 1983;32(42):554–555. [PubMed] [Google Scholar]
- 19.Roper WL, Houk VN, et al. Centers for Disease Control and Prevention (CDC) Preventing lead poisoning in young children. 4. CDC: A statement by the CDC; 1991. [Google Scholar]
- 20.New Mexico Department of Health (NMDOH) Lead Poisoning Prevention Program. Environmental Health Epidemiology Bureau; 2008. Prenatal lead risk questionnaire for those who are pregnant or are planning a pregnancy. http://nmhealth.org/eheb/lead.shtml. [Google Scholar]
- 21.Norman EH, Hertz-Picciotto I, Salmen DA, et al. Childhood lead poisoning and vinyl miniblind exposure. Archives of Pediatrics and Adolescent Medicine. 1997;151(10):1033–1037. doi: 10.1001/archpedi.1997.02170470067012. [DOI] [PubMed] [Google Scholar]
- 22.Sanborn MD, Abelsohn A, Campbell M, et al. Identifying and managing adverse environmental health effects: 3. Lead exposure. Canadian Medical Association Journal. 2002;166(10):1287–1292. [PMC free article] [PubMed] [Google Scholar]
- 23.Jones L, Parker JD, Mendola P. NCHS data brief no 52. Hyattsville, MD: National Center for Health Statistics; 2010. Blood lead and mercury levels in pregnant women in the United States, 2003–2008. http://www.cdc.gov/nchs/data/databriefs/db52.pdf. [PubMed] [Google Scholar]
- 24.Rastogi S, Nandlike K, Fenster W. Elevated blood lead levels in pregnant women: Identification of a high-risk population and interventions. Journal of Perinatal Medicine. 2007;35(6):492–496. doi: 10.1515/JPM.2007.131. [DOI] [PubMed] [Google Scholar]
- 25.Rischitelli G, Nygren P, Bougatsos C, et al. Screening for elevated lead levels in childhood and pregnancy: An updated summary of evidence for the US preventive services task force. Pediatrics. 2006;118(6):e1867–e1895. doi: 10.1542/peds.2006-2284. [DOI] [PubMed] [Google Scholar]
- 26.Agency for Toxic Substances and Disease Registry (ATSDR) Cooperative program with the Minority Health Profession Foundation. 2008 http://www.atsdr.cdc.gov/dtem/programs/cooperative_program_mhpf/index.html.
- 27.Courtney JG, Ash S, et al. Centers for Disease Control and Prevention (CDC) Childhood Lead Poisoning Associated with Tamarind Candy and Folk Remedies—California, 1999–2000. Morbidity and Mortality Weekly Report. 2002;51(31):684–686. [PubMed] [Google Scholar]
- 28.Fuortes L, Bauer E. Lead contamination of imported candy wrappers. Veterinary and Human Toxicology. 2000;42(1):41–42. [PubMed] [Google Scholar]
- 29.Virginia Deptartment of Health. Lead poisoning prevention and treatment updates. Lead Newsletter. 2006;2(2):1–4. [Google Scholar]
- 30.Horner RD, Lackey CJ, Kolasa K, et al. Pica practices of pregnant women. Journal of the American Dietetic Association. 1991;91(1):34–38. [PubMed] [Google Scholar]
- 31.Rose EA, Porcerelli JH, Neale AV. Pica: common but commonly missed. The Journal of the American Board of Family Practice. 2000;13(5):353–358. [PubMed] [Google Scholar]
- 32.Shannon M. Severe lead poisoning in pregnancy. Ambulatory Pediatrics. 2003;3(1):37–39. doi: 10.1367/1539-4409(2003)003<0037:slpip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.New Mexico Department of Health (NMDOH) NMDOH catalog of federal domestic assistance for the U.S. Environmental Protection Agency. 2009. Innovative approach which has a high likelihood of successfully identifying childhood lead poisoning. [Google Scholar]