Abstract
Abstract
Background
The Hawaiian endemic genus Clermontia (Campanulaceae) includes 22 species, 15 of which, the double-corolla species, are characterized by an extra whorl of organs that appear to be true petals occupying what is normally the sepal whorl. Previous research has shown that the presence of homeotic petaloid organs in some other plant groups correlates with ectopic expression of B-function MADS box genes, but similar core eudicot examples of apparent groundplan divergence remain unstudied. B-function genes, which are not normally expressed in the sepal whorl, are required for determination and maintenance of petal identity. Here, we investigate the potential role of altered B-function gene expression contributing to the morphological diversity of this island genus.
Results
We examined the morphology and developmental genetics of two different species of Clermontia, one of which, C. arborescens, has normal sepals while the other, C. parviflora, has two whorls of petal-like organs. Scanning electron microscopy of cell surface morphologies of first and second whorl organs in the double-corolla species C. parviflora revealed conical epidermal cells on the adaxial surfaces of both first and second whorl petaloid organs, strongly suggesting a homeotic conversion in the former. Phylogenetic analysis of Clermontia species based on 5S ribosomal DNA non-transcribed spacer sequences indicated a probable single and geologically recent origin of the double-corolla trait within the genus, with numerous potential reversals to the standard sepal-petal format. Quantitative polymerase chain reaction analysis of homologs of the B-function genes PISTILLATA (PI), APETALA3 and TOMATO MADS 6 indicated ectopic expression of two PI paralogs in the first whorl of C. parviflora; no such homeotic expression was observed for the other two genes, nor for several other MADS box genes involved in various floral and non-floral functions. In the standard sepal-petal species C. arborescens, ectopic expression of PI homologs was not observed. In C. parviflora, the upregulation of PI homologs was precisely restricted to the perianth and stamen whorls, excluding a simple overexpression phenotype. In situ hybridization analysis of C. parviflora material similarly showed first and second whorl PI homolog expression in developing flower buds.
Conclusions
Our morphological and gene expression data strongly suggest that a drastic and heritable phenotypic change, at the level of floral groundplan, can originate from a homeotic mutation that is likely regulatory, being under precise spatiotemporal control as opposed to having pleiotropic characteristics. The uniqueness of this trait among core eudicots could be linked to increased ecological viability in an unstable island environment, a chance event which need not have posed any immediate adaptive benefit. We argue that the evolutionarily young morphological radiation of Clermontia may form a model system for general understanding of mechanisms of larger-scale angiosperm diversification in past, similarly unstable environments, in which small regulatory changes may have been responsible for modern-day groundplan differences.
Trial registration
Clinical Trials.gov- NCT01710735
Significance and Innovations
The present investigation is one of the first to examine the hypothesis of gross muscle contractile inhibition due to the presence of diagnostically relevant MFTrPs. Individuals suffering from clinically relevant levels of self-reported pain are able to tolerate maximum voluntary contraction testing, but delayed onset muscle soreness (DOMS) is a likely side-effect irrespective of symptom status. As a consequence, its confounding effect during subsequent testing must be taken into account.
Keywords: Myofascial pain, Trigger points, Neck/shoulder pain, Health status
Background
The ABC model of floral development, which describes the role of three classes of organ identity genes in the spatial arrangement of floral organs, was proposed in accordance with studies in two model core eudicots, Arabidopsis thaliana and Antirrhinum majus[1,2]. In the determination of reproductive organs, expression of C-function genes alone specifies carpels and expression of both C-function and B-function genes gives rise to stamens. In the outer perianth whorl, sepal identity is determined by the lack of B-function and C-function genes. In the inner perianth whorl, the lack of C-function genes and the activity of B-function genes are required for determination and maintenance of petal identity [3]. All previously described B-function genes belong to the family of MADS-box containing transcription factors.
While the ABC model is well known, little is known about the evolutionary events that have led to the striking structural diversity among petaloid organs, including sepal or petal loss and transfer of petaloid characters between floral whorls. The presence of homeotic petaloid organs in other plant groups, such as the Ranunculales, monocots, magnolids and basal angiosperms, have been shown to be correlated with ectopic expression of B-function MADS box genes [4-7]. It has been suggested that similar core eudicot examples of apparent divergence from the standard condition, in which petaloid organs are found only in the second floral whorl, may be associated with heterotopic expression of B-function genes. However, these systems have remained inadequately studied.
Within the core eudicots, perianth organization rarely deviates from the standard sepal-petal format. In cases where ectopic petaloid organs do occur in core eudicots, the organs remain morphologically differentiated from petals, as compared with the identical perianth whorls that can often be observed in the monocots. Furthermore, petaloid organs which occur outside of the inner perianth whorl, such as petaloid bracts in dogwood (Cornus), the petaloid sepal in Impatiens, and sepal-derived and stamen-derived petaloid organs in the Caryophyllales, have revealed employment of alternate petal identity programs in the formation of petaloid organs, discounting simple extension of B-class gene expression in these systems [8-11]. Similarly, some monocots have been shown to lack expression of B-function genes in the outer tepals [12].
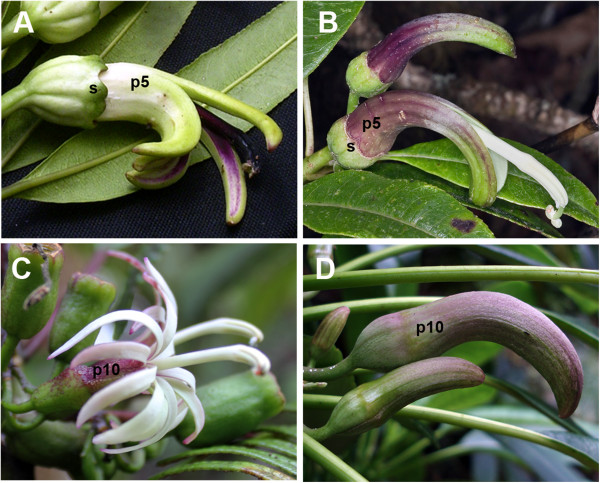
The Hawaiian endemic lobelioids, Campanulaceae, comprise 6 genera and 126 species and represents the largest plant radiation on any ocean island archipelago. Clermontia, a genus within the lobelioids, includes 22 species of bird-pollinated trees and shrubs possessing morphologically diverse flowers [13]. Clermontia flowers exhibit inferior ovaries and produce fleshly fruits. A drastic homeotic phenotype, in which sepals are replaced by an extra whorl of organs that appear to be true petals, characterizes 15 of the 22 species: the double-corolla species. This petal-petal format is a unique trait within the standard groundplan, consisting of a sepal-petal perianth, of the core eudicots. For most double-corolla Clermontia species, the sepal-to-petal transformation is a complete one-for-one conversion. C. arborescens demonstrates the ancestral condition, displaying an outer perianth whorl of small green sepals and larger, showier petal organs in the inner whorl (Figure 1A). The identical first and second whorl organs present in C. parviflora, both of which are petaloid, reveal the double-corolla phenotype (Figure 1C). Two other Clermontia species are also figured to make the point about the double-corolla phenotype: C. fauriei (Figure 1B), with tiny sepals and one petal whorl, is otherwise very similar to C. oblongifolia (Figure 1D), which bears only petal-like organs.
Figure 1.
Groundplan diversity in representative Clermontia species. Floral morphology of the standard sepal-petal species (A) Clermontia arborescens and (B) C. fauriei and double-corolla species (C) C. parviflora and (D) C. oblongifolia are shown. C. arborescens and C. fauriei exhibit small green sepals (s) in the outer perianth whorl and five large showy petals (p5) in the inner perianth whorl. C. parviflora and C. oblongifolia bear 10 petal-like organs (p10) in both perianth whorls. Photos courtesy of Anne-Cathrine Scheen (A), John Game (B) and Jupiter Nielsen (C,D).
The genus Clermontia is endemic to the Hawaiian Islands, which allows for a system that can be studied on a recent and established evolutionary timescale. The Hawaiian Islands were formed by movement of the Pacific plate over a mantle plume; this resulted in islands that emerged from volcanic eruptions and erode in a southeast to northwest geographic progression. Further, unstable environments may allow for increased ecological viability of drastic groundplan differences that may not necessarily create any immediate increase in fitness. This may account for the success of a morphological radiation where a drastic groundplan difference is caused by small regulatory changes. The naturally occurring homeotic mutation in Clermontia offers an appropriate model for studying the potential role of altered B-function gene expression contributing to heterotopic petaloid organs within the core eudicots [14], and provides an example that may have general implications for the study of island radiations.
Methods
Scanning electron microscopy
RNAlater-preserved samples (Applied Biosystems, Carlsbad, CA, USA) were dissected to separate inner and outer perianth organs. Tissues were dehydrated in a graded ethanol series and chemically dried with hexamethyldisilazane as described in Costea et al. [15]. Samples were mounted on stubs, sputter coated with evaporated carbon, and micrographs were taken using a Hitachi-S-4000 scanning electron microscope (Hitachi, Ltd., Tokyo, Japan). Both adaxial and abaxial surfaces were analyzed.
Sequence generation and phylogenetic analysis
Genomic DNA was extracted from herbarium- or silica-preserved vegetative material using Qiagen DNeasy Plant Kits (Qiagen, Valencia, CA, USA). Isolation was performed with the use of universal 5S ribosomal DNA non-transcribed spacer (5S-NTS) primers previously described in Cox et al. [16]. Amplicons were cloned into the PDrive cloning vector (Qiagen) and five to eight colonies per sample were sequenced. 5S-NTS sequences were submitted to GenBank and have accession numbers [GenBank:JQ734775] to [GenBank:JQ734917]. B-function MADS box genes that were not obtained from 454 transcriptome sequencing (KAH and VAA, unpublished results) were isolated using RT-PCR with previously reported primers [17]. To gain insight into the relationships among the 5S-NTS sequences, a maximum likelihood phylogenetic tree was constructed. Similarly, a maximum likelihood tree was generated comprising the two Clermontia PI homolog duplicates as well as PI homologs from selected other taxa, including Campanulaceae, Asteraceae and outgroups. Sequences were aligned using MUSCLE with default settings [18]. The sequence alignments, with complete taxon names, voucher information (where available) and GenBank accession numbers are available in Additional files 1 and 2. A single most optimal tree for each data set was computed using the RaxML BlackBox web server (http://phylobench.vital-it.ch/raxml-bb/) running RaxML version 7.2.8 [19]. Default settings were used with the general time reversible plus gamma model of molecular evolution. One hundred bootstrap samples were generated to assess support for the inferred relationships. Local bootstrap values (in percentages) are indicated for branches with >50% support.
Quantitative polymerase chain reaction
RNAlater-preserved tissues (except for young leaves, these were floral organs from buds in various pre-anthesis stages) were dissected into organ groups as needed, total RNA was isolated using a Qiagen RNeasy Plant Kit, and mRNA was subsequently isolated using a Qiagen Oligotex mRNA Kit. Reverse transcription of mRNA with a Bio-Rad iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) was performed to synthesize cDNA. Each reaction comprised 20 ng of cDNA. Primers were designed from sequences of B-function and other MADS box genes discovered either by transcriptome sequencing (KAH and VAA, unpublished data) or isolated by RT-PCR and are listed in Additional file 3. Primers for each PI-like homolog yielded unique sequences when checked for specificity. Amplification and real-time detection was performed using Bio-Rad iQ SYBR Green Supermix on an MyiQ2 Real-Time Detection Thermal Cycler (Bio-Rad). Standardization was performed using β-actin to determine delta-delta critical threshold values. Bars represent standard deviations based on means of three independent experiments, each of which included two replicates. Gene expression is presented in as fold differences relative to expression in the highest expressing organ in each gene category, which was set to 10.
In situ hybridization
Clermontia flower buds were fixed with formalin-acetic acid-alcohol (FAA), embedded in paraffin and sectioned using standard methods. In situ hybridizations were performed essentially as described in Ruonala et al. [20]. For the post-hybridization washes, an InsituPro VsiVSi 3.0 (Intavis AG, Koeln, Germany) device was used with the protocol shown in Additional file 4. The color reaction to visualize hybridized probe was done for approximately 24 hours at room temperature. Sections were photographed using an AxioImagerZ1 (Zeiss, Munich, Germany) equipped with AxioCam MRc (Zeiss). The digoxigenin-labeled probe covered 430 bp of the Clermontia PI coding region, excluding the MADS domain. Primers used to amplify the Clermontia PI probe fragment were: forward, 5′ GCATGAGTACTGTAGCCCTTCC 3′; reverse 5′ GAAGAGTGGGGAAGGAAGATTT 3′.
Results
Perianth epidermal morphology
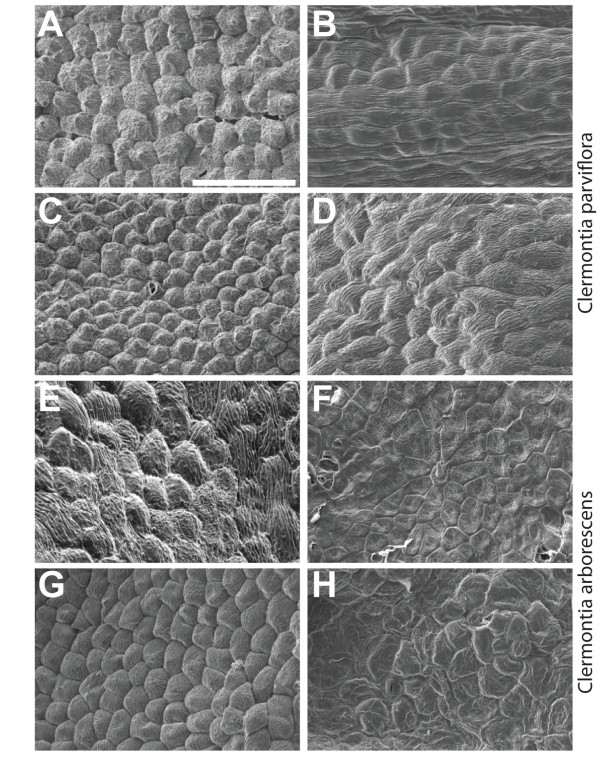
Scanning electron microscopy experiments were performed on the adaxial and abaxial surfaces of organs located in the outer two floral whorls for standard sepal-petal and double-corolla flowers. Cell surface morphologies of first and second whorl organs of the double-corolla species Clermontia parviflora revealed conical epidermal cells on the adaxial surfaces of both first and second whorl petaloid organs, strongly suggesting a homeotic conversion in the former (Figure 2A,C). While the inner whorl of the standard bipartite perianth species, C. arborescens, revealed conical epidermal cells on the adaxial surface, the outer whorl epidermal cells lacked the well-defined conical shape; such flattened, irregular cell morphologies are typical for sepal organs (Figure 2E,G).
Figure 2.
Epidermal micromorphology of perianth organs. Scanning electron microscope images from (A-D) C. parviflora and (E-H) C. arborescens on the adaxial (left panels) and abaxial (right panels) epidermal surfaces. Canonical Conical epidermal cell morphology, an indicator of petal identity, is discovered are apparent on the adaxial surface of the outer perianth organs of C. parviflora (A). Similar cone-shaped cells are observed on the adaxial surface of inner perianth organs in both C. parviflora (C) and C. arborescens (G). However, the outer perianth organs of C. arborescens display a flattened morphology more typical of sepal organs (E). Scale bar denotes 100 μm.
Phylogenetic analysis
Phylogenetic analysis of 5S-NTS sequences shows clear divergence of Clermontia from outgroup genera Brighamia and Delissea, and Clermontia’s sister genus Cyanea, and demonstrates extremely low sequence divergence among Clermontia species (Figure 3). The single-corolla species C. fauriei, the only species found on the oldest island, Kauai, forms a significantly distinct group from other Clermontia, which include both single- and double-corolla species (Figure 3 and Additional file 5). This indicates a probable single and geologically recent origin of the double-corolla trait within the genus, likely by the time of the emergence of Oahu (circa 3.5 million years ago), with numerous potential reversals to the standard sepal-petal format. Oahu holds two species with the double-corolla phenotype, C. persicifolia and C. oblongifolia, the former of which is endemic to that island. 5S-NTS sequence details and an alignment are presented in Additional files 1 and 6.
Figure 3.
Schematic representation of phylogenetic analysis of 5S-NTS ribosomal DNA sequences. Outgroups Brighamia and Delissea are displayed along with Clermontia’s sister genus Cyanea. C. fauriei (f) exhibits the standard sepal-petal groundplan and forms a distinct group separate from all other Clermontia species, including both sepal-petal and double-corolla species. Red indicates sequences derived from double-corolla DNA samples.
Expression analysis
qPCR analysis performed on homologs of the B-function genes PISTILLATA (PI), APETALA3 (AP3), and TOMATO MADS 6 (TM6) was used to elucidate expression patterns in outer perianth, inner perianth, stamen, carpal, vegetative apices and hypanthium (inferior ovary) tissues of the double-corolla species Clermontia parviflora and the standard sepal-petal species C. arborescens. This analysis indicated ectopic expression of the two PI paralogs in the first whorl of C. parviflora; no such drastic homeotic expression was observed for the other two genes, although AP3 expression appears to be slightly elevated relative to C. arborescens (Figure 4). Transcriptional analysis of other Clermontia MADS box gene homologs, SHORT VEGETATIVE PHASE (SVP), SUPPRESSOR OF OVEREXPRESSION OFCONSTANS (SOC1), AGAMOUS-LIKE 6 (AGL6) and SEPALLATA3 (SEP3), which are involved in various floral and non-floral functions [21-24], was also performed. No remarkable differences were found between expression patterns in these genes between sepal-petal and double-corolla floral organs (Additional file 7). In C. arborescens, detection of PI transcripts was restricted to the inner perianth organs and stamens, with no ectopic expression of PI homologs being observed in whorl 1, which is consistent with eudicot-standard lack of B-function genes in sepals (Figure 4). No expression was detected for either of the PI homologs in the carpel organs of C. parviflora (Figure 4), indicating that the up- versus downregulation of the PI homologs is precisely constrained to the perianth and stamen whorls, excluding a simple overexpression phenotype that would be expected to engender a pleiotropic phenotype.
Figure 4.
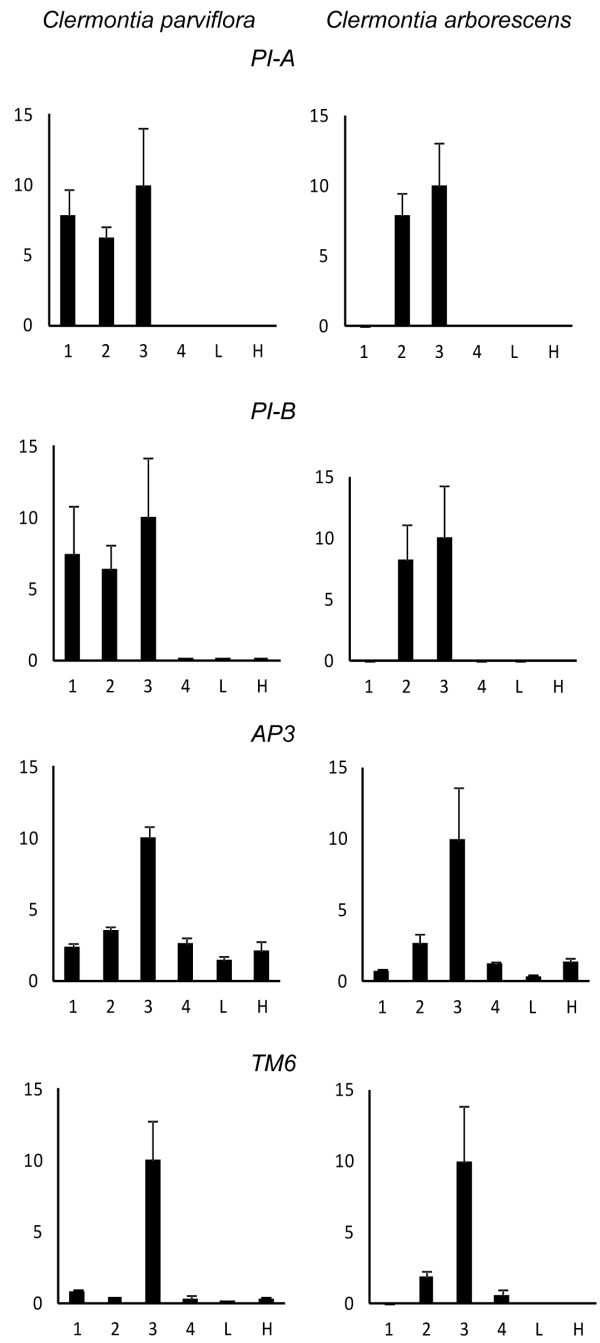
Quantitative gene expression of B-class genes in dissected floral organs. Expression of both PISTILLATA (PI) homologs is constrained to whorls 2 and 3 of Clermontia arborescens (top two right panels) but is extended to whorls 1, 2 and 3 in C. parviflora (top two left panels). No substantial difference in expression pattern is observed in other B-function genes, AP3 and TM6 homologs (bottom two panels). β-actin was used as an internal control. Bars indicate mean and standard deviation for three independent experiments each including two replicates. 1, outer perianth; 2, inner perianth; 3, stamen; 4, carpel; L, leaf; H, hypanthium.
In situ hybridization was used to study expression of both PI homologs in primordial stages of floral organ development of the double-corolla species Clermontia parviflora. Consistent with qPCR data, this analysis showed ectopic expression of PI homologs in the outer perianth whorl of developing double-corolla flower buds, as well as in the petal and stamen whorls, as expected (Figure 5).
Figure 5.

In situ analysis of PISTILLATA expression in flower primordia of Clermontia parviflora. In situ hybridization on flower primordia of C. parviflora displays expression of PI homologs in (A) early stage outer perianth organs and (B and C) in both perianth organs and whorl 3 in a late-stage bud. (D) Sense PI homolog controls show low background staining. Images are displayed at 20× (A), 10× (B and D) and 40× (C). Organs are numbered by whorl; b = bract.
Discussion
The petals of angiosperm flowers, laminar organs occupying the second floral whorl, often share characteristics such as expression of B-function MADS genes, showy morphology, pigmentation and production of aromatic compounds. However, petals exhibit substantial diversity and organs with petaloid characteristics can be found outside of the second floral whorl, making the definition and identification of these organs rather challenging. The adaxial epidermal surfaces of petals are frequently composed of conical cells whereas those of other floral and vegetative cells are typically not [25]. Identification of petaloid organs has been determined by observation of conical epidermal cells in cases of homeotic conversions involving petals [26,27]. Analysis of micromorphological characteristics on the adaxial epidermal surfaces of first-whorl organs in the double-corolla species Clermontia parviflora reveals conical cells, a marker of petal identity. This supports the hypothesis of homeotic transformation of sepals to petals.
Based on the phylogenetic analysis presented here and the geographic distribution of Clermontia, the origin of this homeotic transformation was likely established via a single and geologically recent occurrence. The Hawaiian Islands were formed by the northwestward movement of the Pacific tectonic plate over a fixed volcanic plume, resulting in a layout where the islands progress from oldest to youngest in a northwest to southeast manner [28]. C. fauriei is the only species found on Kauai, the oldest of the Hawaii Islands, and it displays the ancestral sepal-petal format. The neighboring island Oahu is home to five species, including C. fauriei and four double-corolla species [29]. 5S-NTS data support previous findings suggesting Clermontia’s substantial sequence divergence from its sister genus Cyanea but low sequence divergence within the genus [30]. Our data do indicate sequence divergence between C. fauriei and all remaining Clermontia species, including both standard sepal-petal and double-corolla species, perhaps reflective of a split age at least by the time of the origin of Kauai. The phylogenetic clustering of ancestral state and petal-petal format species observed suggests multiple potential reversals occurred during the radiation of Clermontia. Similar findings on Hawaiian lobelioid inter-relationships, including evolution of reversals in the Clermontia clade, have been reported by Givnish and collaborators [13,31,32].
The transformation of first-whorl sepal organs into organs bearing petal identity in double-corolla Clermontia species, which is often complete, led us to question whether this phenomenon may be regulated by ectopic expression of B-class genes. Our detection of ectopic expression of PI homologs in floral primordia indicates a likely role of PI in the differentiation and determination of outer whorl floral organs, resulting in the development of petal identity. Lacandonia schismatica (Triuridaceae) exhibits a homeotic transformation in which central stamens are surrounded by carpels. The simple displacement of the B-function has been shown to play a role in this morphological shift [33]. In dove tree (Davidia), early B-class gene expression in petaloid bracts has been suggested to lead to a partial petaloid phenotype; however, the lack of late-stage expression makes the mechanism unclear [34]. In Clermontia, not only is expression of PI homologs detected in floral primordia, but we have also shown continued expression in late-stage outer whorl floral organs, supporting the function of B-class genes in the maintenance of homeotic petal identity. Furthermore, we show that expression of a variety of other MADS-box gene homologs involved in various floral and non-floral functions, specifically AP3, TM6, SEP3, AGL6, SVP and SOC1, present expression patterns that are largely or completely consistent between standard sepal-petal and double-corolla species (Additional file 7).
Expression of a Clermontia AP3 homolog is detected in the outer whorl of both standard groundplan species and double-corolla species. Therefore, we hypothesize that ectopic expression of PI homologs would be sufficient to induce the obligate AP3-PI heterodimer autoregulatory feedback loop. In Arabidopsis, expression of PI alone is not able to induce petaloidy in vegetative organs; however, it is when co-expressed with AP3 in vegetative organs or expressed alone in outer whorl perianth [3]. The expression of an AP3 homolog in the outer whorl perianth in Clermontia indicates that the genetic background condition required for ectopic PI expression to induce petaloidy is present. SEP3 is not able to induce petaloidy on its own, but has been shown to increase petaloid characteristics of ectopic petaloid organs, and the heterodimer AP3-PI forms a ternary complex with SEP3 [35,36]. We hypothesize that in Clermontia, low-level expression of SEP3 and AP3 homologs in the ancestral condition would set up a context in which ectopic expression of PI would be enough to induce petal identity in the outer whorl perianth.
Gene duplications among B-class genes, resulting in the euAP3, TM6 and PI groups, have been shown to be of evolutionary significance in the production of floral diversity. It has been suggested that extension of the B-class gene model has played a diversity-generating role in cases with a history of gene duplication among PI homologs or AP3 homologs in taxonomic groups with otherwise undifferentiated first and second whorls [37]. Here, we demonstrate ectopic and sustained expression of both Clermontia PI homolog duplicates, which clearly derive from a lineage-specific duplication within Campanulaceae (Additional file 8). This apparent subfunctionalization event following gene duplication may have played a key role in the events following PI duplication in Clermontia. The two homologs may be largely functionally redundant, or one may be expressed first and induce the expression of the other, consistent with an autoregulatory feedback loop when obligatory dimerization with AP3 occurs.
The precise regulation that restricts ectopic expression of PI homologs to the first-whorl primordia in early and late-stage organs, as seen in Clermontia parviflora, indicates that small regulatory changes may be responsible for the dramatic change in the floral groundplan established in eudicots. This tight spatiotemporal regulation allows for the deployment of a drastic homeotic mutation without causing potentially deleterious pleiotropic effects, such as transformation of the carpel whorl into stamens. In Arabidopsis, ectopic expression of B-function genes has been shown to be sufficient to transform the carpel whorl into staminoid organs [3]. The specific regulation of this heritable mutation is necessary to produce viable organisms, since simple overexpression would be likely to cause infertility through the production of staminoid carpels. Other viable homeotic transformations within the angiosperm groundplan have occurred but have not led to radiations as seen in Clermontia.
The double-corolla phenotype may not have initially acted with adaptive advantage, its success perhaps relying instead on passive expansion of the mutation by random genetic drift in small populations in the unstable environments of the Hawaiian Islands. The sepal-petal ancestral status of Clermontia is supported by our phylogenetic analysis and the uniformly eudicot-standard floral morphology of its sister genus Cyanea and further closely related outgroup lobelioid species. While phylogenetic analysis demonstrates multiple possible reversals to the ancestral condition, the radiation of the double-corolla mutation indicates the long-term viability of the mutation. This gives insight into possible mechanisms that may have shaped diversity among floral groundplans in past unstable environments; small regulatory changes, as opposed to large coding-sequence differences, could account for morphological differentiation in other island groups such as the Hawaiian silverswords, Hawaiian mints and Canarian Crassulaceae.
Conclusions
Our morphological and gene expression data strongly suggest that a drastic and heritable phenotypic change, at the level of the floral groundplan, can originate from a homeotic mutation that is likely regulatory, being under precise spatiotemporal control as opposed to having pleiotropic characteristics. The uniqueness of this trait among core eudicots could be linked to increased ecological viability in an unstable island environment, a chance event that need not have posed any immediate adaptive benefit. We argue that the evolutionarily young morphological radiation of Clermontia may form a model system for general understanding of mechanisms of larger-scale angiosperm diversification in past, similarly unstable environments, in which small regulatory changes may have been responsible for modern-day groundplan differences.
Abbreviations
Bp: base pairs; qPCR: quantitative PCR; RT-PCR: reverse-transcriptase polymerase chain reaction; 5S-NTS: 5S ribosomal DNA non-transcribed spacer.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
VAA designed and led the study; RR performed in situ hybridization experiments; KAH executed all other experiments, analyzed data and drafted the manuscript. All authors read and approved the final version.
Supplementary Material
5S-NTS rDNA sequence alignment. Accession numbers and sequence data, as a MUSCLE alignment, are displayed in FASTA format.
Coding-sequence alignment of PISTILLATA-like genes from Clermontia and outgroups. Accession numbers and sequence data, as a MUSCLE alignment, are displayed in FASTA format.
Primers used for quantitative RT-PCR. Amplification of target and control genes was performed using the primer sequences listed in table format.
Command file for in situ hybridization. The method for in situ hybridization using the InsituPro Vsi 3.0 (Intavis AG) liquid handling robot is presented.
Phylogenetic analysis of 5S-NTS rDNA sequences. The single tree of maximum likelihood is shown with branches supported by bootstrap values >50% so indicated.
5S-NTS rDNA sequence data. Accession numbers and sequence data are displayed in table format.
Quantitative RT-PCR for MADS-box containing genes. Expression patterns of SVP, SOC1, AGL6, and SEP3 homologs in floral whorls 1,2,3,4, leaf and hypanthium for sepal-petal C. arborescens and double-corolla C. parviflora show no significant differences between species. Expression levels are shown as fold differences relative to β-actin and represent the mean and standard deviation of three independent experiments with two replicates each.
Phylogenetic analysis of PISTILLATA-like coding sequences from Clermontia and outgroups. Based on these data the two Clermontia duplicates clearly arose after common ancestry with Campanula, a close sister taxon in Campanulaceae, although it cannot be certain that additional copies remain undetected in this and other species, such as with Nyssa in Cornales. No other PI-like sequences from the same plant individuals represented here are available in GenBank. Clermontia parviflora PI-like copies are shown. The single tree of maximum likelihood is figured with bootstrap values indicated.
Contributor Information
Katherine A Hofer, Email: khofer@buffalo.edu.
Raili Ruonala, Email: raili.ruonala@gmail.com.
Victor A Albert, Email: vaalbert@buffalo.edu.
Acknowledgements
Funding for this research was provided by the University at Buffalo, State University of New York. We thank Charlotte Lindqvist, Teemu H Teeri, Paula Elomaa and Roosa Laitinen for generous assistance isolating B-function MADS box genes using RT-PCR, and The Bernice Pauahi Bishop Museum, New York Botanical Garden and National Tropical Botanical Garden for access to plant collections. A large number of people provided assistance with fieldwork, particularly Mika Bendiksby, Justin Bolton, Jack Fujii, Jennifer Johansen, Charlotte Lindqvist, Timothy J Motley, Johan Pillon and Anne-Cathrine Scheen. John Game, Jupiter Nielsen and Anne-Cathrine Scheen are thanked for sharing photographs of Clermontia species.
References
- Coen ES, Meyerowitz EM. The war of the whorls: genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- Goto K, Meyerowitz EM. Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev. 1994;8:1548–1560. doi: 10.1101/gad.8.13.1548. [DOI] [PubMed] [Google Scholar]
- Krizek BA, Meyerowitz EM. The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development. 1996;122:11–22. doi: 10.1242/dev.122.1.11. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Di Stilio VS, Schlüter PM. Complex patterns of gene duplication in the APETALA3 and PISTILLATA lineages of the Ranunculaceae. Int J Plant Sci. 2003;164:1–11. [Google Scholar]
- Rasmussen DA, Kramer EM, Zimmer EA. One size fits all? Molecular evidence for a commonly inherited petal identity program in Ranunculales. Am J Bot. 2009;96:96–109. doi: 10.3732/ajb.0800038. [DOI] [PubMed] [Google Scholar]
- Kanno A, Saeki H, Kameya T, Saedler H, Theissen G. Heterotopic expression of class B floral homeotic genes supports a modified ABC model for tulip (Tulipa gesneriana) Plant Mol Biol. 2003;52:831–841. doi: 10.1023/A:1025070827979. [DOI] [PubMed] [Google Scholar]
- Kalivas A, Pasentsis K, Tsaftaris AS, Polidoros AN. Heterotopic expression of B-class floral homeotic genes PISTILLATA/GLOBOSA supports a modified model for crocus (Crocus sativus L.) flower formation. DNA Seq. 2007;18:120–130. doi: 10.1080/10425170601060582. [DOI] [PubMed] [Google Scholar]
- Brockington SF, Rudall PJ, Frohlich MW, Oppenheimer DG, Soltis PS, Soltis DE. 'Living stones' reveal alternative petal identity programs within the core eudicots. Plant J. 2012;69:193–203. doi: 10.1111/j.1365-313X.2011.04797.x. [DOI] [PubMed] [Google Scholar]
- Geuten K, Becker A, Kaufmann K, Caris P, Janssens S, Viaene T, Theissen G, Smets E. Petaloidy and petal identity MADS-box genes in the balsaminoid genera Impatiens and Marcgravia. Plant J. 2006;47:501–518. doi: 10.1111/j.1365-313X.2006.02800.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xiang QY, Thomas DT, Wiegmann BM, Frohlich MW, Soltis DE. Molecular evolution of PISTILLATA-like genes in the dogwood genus Cornus (Cornaceae) Mol Phylogenet Evol. 2008;47:175–195. doi: 10.1016/j.ympev.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Litt A, Kramer EM. The ABC model and the diversification of floral organ identity. Semin Cell Dev Biol. 2010;21:129–137. doi: 10.1016/j.semcdb.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Park JH, Ishikawa Y, Ochiai T, Kanno A, Kameya T. Two GLOBOSA-like genes are expressed in second and third whorls of homochlamydeous flowers in Asparagus officinalis L. Plant Cell Physiol. 2004;45:325–332. doi: 10.1093/pcp/pch040. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Millam KC, Mast AR, Paterson TB, Theim TJ, Hipp AL, Henss JM, Smith JF, Wood KR, Sytsma KJ. Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae) Proc Biol Sci. 2009;276:407–416. doi: 10.1098/rspb.2008.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert VA, Gustafsson MHG, DiLaurenzio L. In: Molecular Systematics of Plants II. Soltis PS, Soltis DE, Doyle JJ, editor. The Netherlands: Kluwer Academic Publishers; 1998. Ontogenetics systematics, molecular developmental genetics and the angiosperm petal; pp. 349–374. [Google Scholar]
- Costea M, Spence I, Stefanovic S. Systematics of Cuscuta chinensis species complex (subgenus Grammica, Convolvulaceae): evidence for long-distance dispersal and one new species. Org Divers Evol. 2011;11:373–386. doi: 10.1007/s13127-011-0061-3. [DOI] [Google Scholar]
- Cox AV, Bennett MD, Dyer TA. Use of the polymerase chain reaction to detect spacer size heterogeneity in plant 5S-rRNA gene clusters and to locate such clusters in wheat (Triticum aestivum L.) TAG Theoretical and Applied Genetics. 1992;83:684–690. doi: 10.1007/BF00226685. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Dorit RL, Irish VF. Molecular evolution of genes controlling petal and stamen development: duplication and divergence within the APETALA3 and PISTILLATA MADS-box gene lineages. Genetics. 1998;149:765–783. doi: 10.1093/genetics/149.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Ruonala R, Rinne PL, Kangasjarvi J, van der Schoot C. CENL1 expression in the rib meristem affects stem elongation and the transition to dormancy in Populus. Plant Cell. 2008;20:59–74. doi: 10.1105/tpc.107.056721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregis V, Sessa A, Dorca-Fornell C, Kater MM. The Arabidopsis floral meristem identity genes AP1, AGL24 and SVP directly repress class B and C floral homeotic genes. Plant J. 2009;60:626–637. doi: 10.1111/j.1365-313X.2009.03985.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee I. Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot. 2010;61:2247–2254. doi: 10.1093/jxb/erq098. [DOI] [PubMed] [Google Scholar]
- Castillejo C, Romera-Branchat M, Pelaz S. A new role of the Arabidopsis SEPALLATA3 gene revealed by its constitutive expression. Plant J. 2005;43:586–596. doi: 10.1111/j.1365-313X.2005.02476.x. [DOI] [PubMed] [Google Scholar]
- Viaene T, Vekemans D, Becker A, Melzer S, Geuten K. Expression divergence of the AGL6 MADS domain transcription factor lineage after a core eudicot duplication suggests functional diversification. BMC Plant Biol. 2010;10:148. doi: 10.1186/1471-2229-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney HM, Bennett KMV, Dorling M, Sandbach L, Prince D, Chittka L, Glover BJ. Why do so many petals have conical epidermal cells? Ann Bot-London. 2011;108:609–616. doi: 10.1093/aob/mcr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish VF. Evolution of petal identity. J Exp Bot. 2009;60:2517–2527. doi: 10.1093/jxb/erp159. [DOI] [PubMed] [Google Scholar]
- Ojeda I, Francisco-Ortega J, Cronk QC. Evolution of petal epidermal micromorphology in Leguminosae and its use as a marker of petal identity. Ann Bot. 2009;104:1099–1110. doi: 10.1093/aob/mcp211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JP, Clague DA. How old is the Hawaiian biota? Geology and phylogeny suggest recent divergence. Proc Biol Sci. 2002;269:2429–2435. doi: 10.1098/rspb.2002.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers TG. Systematics of Clermontia (Campanulaceae-Lobelioideae) Systematic Botany Monographs. 1991;32:1–97. [Google Scholar]
- Givnish TJ, Sytsma KJ, Smith JF, Hahn WS. In: Hawaiian Biogeography: Evolution on a Hot Spot Archipelago. Wagner WL, Funk VA, editor. Washington, DC: Smithsonian Institution; 1995. Molecular evolution, adaptive radiation, and geographic speciation in Cyanea (Campanulaceae, Lobelioideae) pp. 288–337. [Google Scholar]
- Givnish TJ. In: Evolution on Islands. Grant P, editor. New York, USA: Oxford University Press; 1998. Adaptive radiation of plants on oceanic islands: classical patterns, molecular data, new insights; pp. 281–304. [Google Scholar]
- Givnish TJ. In: The Biology of Biodiversity. Kato M, editor. Tokyo, Japan: Springer; 1999. Adaptive radiation, dispersal, and diversification of the Hawaiian lobeliads; pp. 67–90. [Google Scholar]
- Álvarez-Buylla ER, Ambrose BA, Flores-Sandoval E, Englund M, Garay-Arroyo A, García-Ponce B, de la Torre-Bárcena E, Espinosa-Matías S, Martínez E, Piñeyro-Nelson A, Engström P, Meyerowitz EM. B-function expression in the flower center underlies the homeotic phenotype of Lacandonia schismatica (Triuridaceae) Plant Cell. 2010;22:3543–3559. doi: 10.1105/tpc.109.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekemans D, Viaene T, Caris P, Geuten K. Transference of function shapes organ identity in the dove tree inflorescence. New Phytol. 2012;193:216–228. doi: 10.1111/j.1469-8137.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- Pelaz S, Tapia-Lopez R, Alvarez-Buylla ER, Yanofsky MF. Conversion of leaves into petals in Arabidopsis. Curr Biol. 2001;11:182–184. doi: 10.1016/S0960-9822(01)00024-0. [DOI] [PubMed] [Google Scholar]
- Pelaz S, Gustafson-Brown C, Kohalmi SE, Crosby WL, Yanofsky MF. APETALA1 and SEPALLATA3 interact to promote flower development. Plant J. 2001;26:385–394. doi: 10.1046/j.1365-313x.2001.2641042.x. [DOI] [PubMed] [Google Scholar]
- Landis JB, Barnett LL, Hileman LC. Evolution of petaloid sepals independent of shifts in B-class MADS box gene expression. Dev Genes Evol. 2011;222:19–28. doi: 10.1007/s00427-011-0385-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
5S-NTS rDNA sequence alignment. Accession numbers and sequence data, as a MUSCLE alignment, are displayed in FASTA format.
Coding-sequence alignment of PISTILLATA-like genes from Clermontia and outgroups. Accession numbers and sequence data, as a MUSCLE alignment, are displayed in FASTA format.
Primers used for quantitative RT-PCR. Amplification of target and control genes was performed using the primer sequences listed in table format.
Command file for in situ hybridization. The method for in situ hybridization using the InsituPro Vsi 3.0 (Intavis AG) liquid handling robot is presented.
Phylogenetic analysis of 5S-NTS rDNA sequences. The single tree of maximum likelihood is shown with branches supported by bootstrap values >50% so indicated.
5S-NTS rDNA sequence data. Accession numbers and sequence data are displayed in table format.
Quantitative RT-PCR for MADS-box containing genes. Expression patterns of SVP, SOC1, AGL6, and SEP3 homologs in floral whorls 1,2,3,4, leaf and hypanthium for sepal-petal C. arborescens and double-corolla C. parviflora show no significant differences between species. Expression levels are shown as fold differences relative to β-actin and represent the mean and standard deviation of three independent experiments with two replicates each.
Phylogenetic analysis of PISTILLATA-like coding sequences from Clermontia and outgroups. Based on these data the two Clermontia duplicates clearly arose after common ancestry with Campanula, a close sister taxon in Campanulaceae, although it cannot be certain that additional copies remain undetected in this and other species, such as with Nyssa in Cornales. No other PI-like sequences from the same plant individuals represented here are available in GenBank. Clermontia parviflora PI-like copies are shown. The single tree of maximum likelihood is figured with bootstrap values indicated.