Abstract
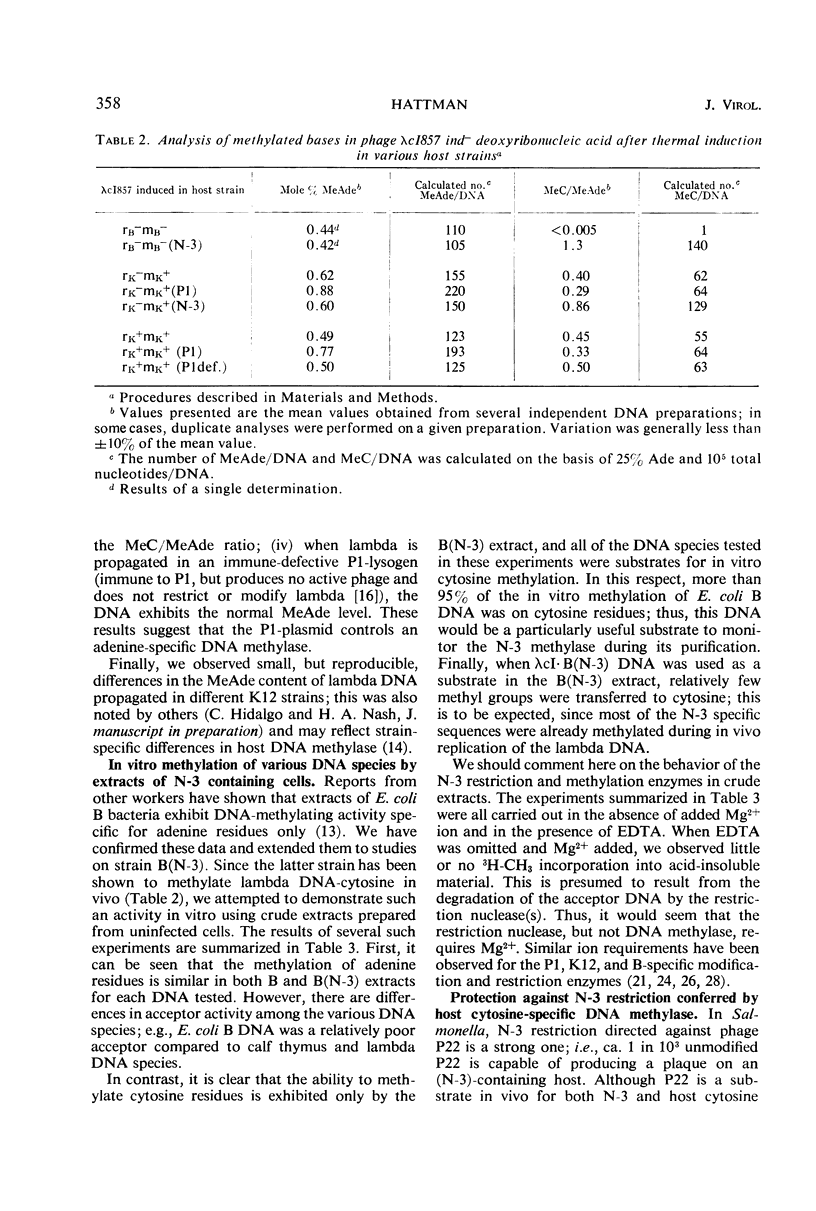
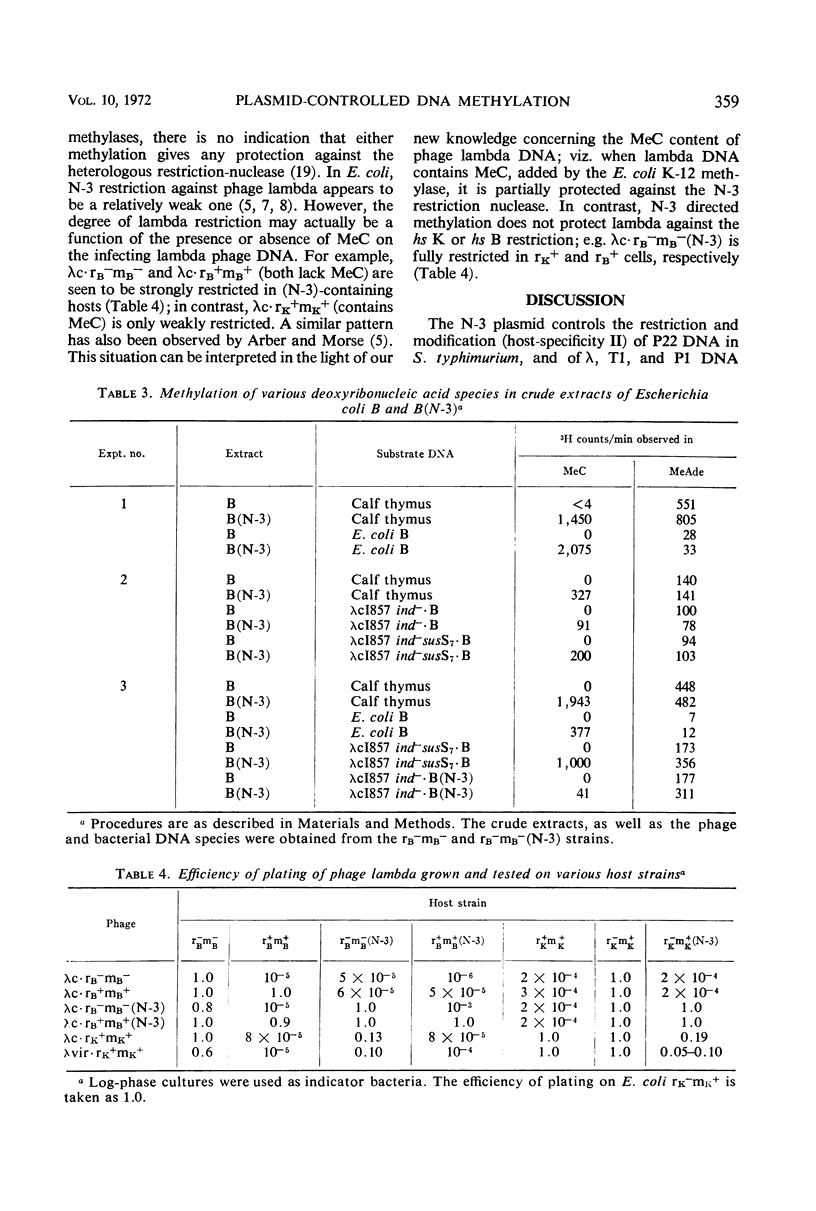
The N6-methyladenine (MeAde) and 5-methylcytosine (MeC) contents in deoxyribonucleic acid (DNA) of bacteriophage lambda has been analyzed as a function of host specificity. The following facts have emerged: (i) lambda grown on strains harboring the P1 prophage contain ca. 70 more MeAde residues/DNA molecule than lambda grown either in the P1-sensitive parent, or in a P1 immune-defective lysogen which does not confer P1 modification; (ii) lambda grown on strains harboring the N-3 drug-resistance factor contain ca. 60 more MeC residues/DNA molecule than lambda grown on the parental strain lacking the factor; (iii) lambda grown in Escherichia coli B strains is devoid of MeC, whereas lambda grown in a B (N-3) host contains a high level of MeC; (iv) the MeAde content in lambda DNA is not affected by the N-3 factor. These results suggest that P1 controls an adenine-specific DNA methylase, and that the N-3 plasmid controls a cytosine-specific DNA methylase. The N-3 factor has been observed previously to direct cytosine-specific methylation of phage P22 DNA and E. coli B DNA in vivo; in vitro studies presented here demonstrate this activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., DUSSOIX D. Host specificity of DNA produced by Escherichia coli. I. Host controlled modification of bacteriophage lambda. J Mol Biol. 1962 Jul;5:18–36. doi: 10.1016/s0022-2836(62)80058-8. [DOI] [PubMed] [Google Scholar]
- ARBER W., LATASTE-DOROLLE C. [Enlargement of the host area of bacteriophage lambda for Escherichia coli B]. Pathol Microbiol (Basel) 1961;24:1012–1018. [PubMed] [Google Scholar]
- ARBER W., MORSE M. L. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI. VI. EFFECTS ON BACTERIAL CONJUGATION. Genetics. 1965 Jan;51:137–148. doi: 10.1093/genetics/51.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber W., Kühnlein U. Mutationeller Verlust B-spezifischer Restriktion des Bakteriophagen fd. Pathol Microbiol (Basel) 1967;30(6):946–952. [PubMed] [Google Scholar]
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- Arber W., Rifat A., Wauters-Willems D., Kühnlein U. Host specificity of DNA produced by Escherichia coli. XVI. Phage lambda DNA carries a single site of affinity for A-specific restriction and modification. Mol Gen Genet. 1972;115(3):195–207. doi: 10.1007/BF00268883. [DOI] [PubMed] [Google Scholar]
- Bannister D., Glover S. W. Restriction and modification of bacteriophages by R+ strains of Escherichia coli K12. Biochem Biophys Res Commun. 1968 Mar 27;30(6):735–738. doi: 10.1016/0006-291x(68)90575-5. [DOI] [PubMed] [Google Scholar]
- Bannister D., Glover S. W. The isolation and properties of non-restricting mutants of two different host specificities associated with drug resistance factors. J Gen Microbiol. 1970 Apr;61(1):63–71. doi: 10.1099/00221287-61-1-63. [DOI] [PubMed] [Google Scholar]
- Boyer H. W. DNA restriction and modification mechanisms in bacteria. Annu Rev Microbiol. 1971;25:153–176. doi: 10.1146/annurev.mi.25.100171.001101. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Brown P. R., Murray K. The deoxyribonucleic acid modification enzyme of bacteriophage P1. Biochem J. 1972 Mar;127(1):1–10. doi: 10.1042/bj1270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOSKOCIL J., SORMO'VA Z. THE OCCURRENCE OF 5-METHYLCYTOSINE IN BACTERIAL DEOXYRIBONUCLEIC ACIDS. Biochim Biophys Acta. 1965 Mar 15;95:513–515. [PubMed] [Google Scholar]
- Doskocil J., Sormová Z. The sequences of 5-methylcytosine in the DNA of Escherichia coli. Biochem Biophys Res Commun. 1965 Jul 26;20(3):334–339. doi: 10.1016/0006-291x(65)90369-4. [DOI] [PubMed] [Google Scholar]
- Fujimoto D., Srinivasan P. R., Borek E. On the nature of the deoxyribonucleic acid methylases. Biological evidence for the multiple nature of the enzymes. Biochemistry. 1965 Dec;4(12):2849–2855. doi: 10.1021/bi00888a041. [DOI] [PubMed] [Google Scholar]
- Gough M., Lederberg S. Methylated bases in the host-modified deoxyribonucleic acid of Escherichia coli and bacteriophage lambda. J Bacteriol. 1966 Apr;91(4):1460–1468. doi: 10.1128/jb.91.4.1460-1468.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTMAN S. THE CONTROL OF HOST-INDUCED MODIFICATION BY PHAGE P1. Virology. 1964 Jun;23:270–271. doi: 10.1016/0042-6822(64)90291-0. [DOI] [PubMed] [Google Scholar]
- Hattman S. DNA methylation of T-even bacteriophages and of their nonglucosylated mutants: its role in P1-directed restriction. Virology. 1970 Oct;42(2):359–367. doi: 10.1016/0042-6822(70)90279-5. [DOI] [PubMed] [Google Scholar]
- Hattman S., Gold E., Plotnik A. Methylation of cytosine residues in DNA controlled by a drug resistance factor (host-induced modification-R factors-N 6 -methyladenine-5-methylcytosine). Proc Natl Acad Sci U S A. 1972 Jan;69(1):187–190. doi: 10.1073/pnas.69.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S. Variation of 6-methylaminopurine content in bacteriophage P22 deoxyribonucleic acid as a function of host specificity. J Virol. 1971 May;7(5):690–691. doi: 10.1128/jvi.7.5.690-691.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Smith H. O. A restriction enzyme from Hemophilus influenzae. II. J Mol Biol. 1970 Jul 28;51(2):393–409. doi: 10.1016/0022-2836(70)90150-6. [DOI] [PubMed] [Google Scholar]
- Kühnlein U., Arber W. Host specificity of DNA produced by Escherichia coli. XV. The role of nucleotide methylation in in vitro B-specific modification. J Mol Biol. 1972 Jan 14;63(1):9–19. doi: 10.1016/0022-2836(72)90518-9. [DOI] [PubMed] [Google Scholar]
- LEDERBERG S. Suppression of the multiplication of heterologous bacteriophages in lysogenic bacteria. Virology. 1957 Jun;3(3):496–513. doi: 10.1016/0042-6822(57)90006-5. [DOI] [PubMed] [Google Scholar]
- LEDINKO N. OCCURRENCE OF 5-METHYLDEOXYCYTIDYLATE IN THE DNA OF PHAGE LAMBDA. J Mol Biol. 1964 Sep;9:834–835. doi: 10.1016/s0022-2836(64)80191-1. [DOI] [PubMed] [Google Scholar]
- Linn S., Arber W. Host specificity of DNA produced by Escherichia coli, X. In vitro restriction of phage fd replicative form. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1300–1306. doi: 10.1073/pnas.59.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamelak L., Boyer H. W. Genetic control of the secondary modification of deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):57–62. doi: 10.1128/jb.104.1.57-62.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Revel H. R., Hattman S. M. Mutants of T2gt with altered DNA methylase activity: relation to restriction by prophage P1. Virology. 1971 Aug;45(2):484–495. doi: 10.1016/0042-6822(71)90348-5. [DOI] [PubMed] [Google Scholar]
- Roulland-Dussoix D., Boyer H. W. The Escherichia coli B restriction endonuclease. Biochim Biophys Acta. 1969 Nov 19;195(1):219–229. doi: 10.1016/0005-2787(69)90618-2. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Arber W., Kühnlein U. Host specificity of DNA produced by Escherichia coli. XIV. The role of nucleotide methylation in in vivo B-specific modification. J Mol Biol. 1972 Jan 14;63(1):1–8. doi: 10.1016/0022-2836(72)90517-7. [DOI] [PubMed] [Google Scholar]
- Takano T., Watanabe T., Fukasawa T. Mechanism of host-controlled restriction of bacteriophage lambda by R factors in Escherichia coli K12. Virology. 1968 Feb;34(2):290–302. doi: 10.1016/0042-6822(68)90239-0. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Buryanov Y. I., Belozersky A. N. Distribution of N6-methyladenine in DNA of T2 phage and its host Escherichia coli B. Nat New Biol. 1971 Mar 3;230(1):25–27. doi: 10.1038/newbio230025a0. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Takano T., Arai T., Nishida H., Sato S. Episome-mediated Transfer of Drug Resistance in Enterobacteriaceae X. Restriction and Modification of Phages by fi R Factors. J Bacteriol. 1966 Aug;92(2):477–486. doi: 10.1128/jb.92.2.477-486.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]



