In a systematic review and meta-analysis, Lynne Forrest and colleagues find that patients with lung cancer who are more socioeconomically deprived are less likely to receive surgical treatment, chemotherapy, or any type of treatment combined, compared with patients who are more socioeconomically well off, regardless of cancer stage or type of health care system.
Abstract
Background
Intervention-generated inequalities are unintended variations in outcome that result from the organisation and delivery of health interventions. Socioeconomic inequalities in treatment may occur for some common cancers. Although the incidence and outcome of lung cancer varies with socioeconomic position (SEP), it is not known whether socioeconomic inequalities in treatment occur and how these might affect mortality. We conducted a systematic review and meta-analysis of existing research on socioeconomic inequalities in receipt of treatment for lung cancer.
Methods and Findings
MEDLINE, EMBASE, and Scopus were searched up to September 2012 for cohort studies of participants with a primary diagnosis of lung cancer (ICD10 C33 or C34), where the outcome was receipt of treatment (rates or odds of receiving treatment) and where the outcome was reported by a measure of SEP. Forty-six papers met the inclusion criteria, and 23 of these papers were included in meta-analysis. Socioeconomic inequalities in receipt of lung cancer treatment were observed. Lower SEP was associated with a reduced likelihood of receiving any treatment (odds ratio [OR] = 0.79 [95% CI 0.73 to 0.86], p<0.001), surgery (OR = 0.68 [CI 0.63 to 0.75], p<0.001) and chemotherapy (OR = 0.82 [95% CI 0.72 to 0.93], p = 0.003), but not radiotherapy (OR = 0.99 [95% CI 0.86 to 1.14], p = 0.89), for lung cancer. The association remained when stage was taken into account for receipt of surgery, and was found in both universal and non-universal health care systems.
Conclusions
Patients with lung cancer living in more socioeconomically deprived circumstances are less likely to receive any type of treatment, surgery, and chemotherapy. These inequalities cannot be accounted for by socioeconomic differences in stage at presentation or by differences in health care system. Further investigation is required to determine the patient, tumour, clinician, and system factors that may contribute to socioeconomic inequalities in receipt of lung cancer treatment.
Please see later in the article for the Editors' Summary
Editors' Summary
Background
Lung cancer is the most commonly occurring cancer worldwide and the commonest cause of cancer-related death. Like all cancers, lung cancer occurs when cells begin to grow uncontrollably because of changes in their genes. The most common trigger for these changes in lung cancer is exposure to cigarette smoke. Most cases of lung cancer are non-small cell lung cancer, the treatment for which depends on the “stage” of the disease when it is detected. Stage I tumors, which are confined to the lung, can be removed surgically. Stage II tumors, which have spread to nearby lymph nodes, are usually treated with surgery plus chemotherapy or radiotherapy. For more advanced tumors, which have spread throughout the chest (stage III) or throughout the body (stage IV), surgery generally does not help to slow tumor growth and the cancer is treated with chemotherapy and radiotherapy. Small cell lung cancer, the other main type of lung cancer, is nearly always treated with chemotherapy and radiotherapy but sometimes with surgery as well. Overall, because most lung cancers are not detected until they are quite advanced, less than 10% of people diagnosed with lung cancer survive for 5 years.
Why Was This Study Done?
As with many other cancers, socioeconomic inequalities have been reported for both the incidence of and the survival from lung cancer in several countries. It is thought that the incidence of lung cancer is higher among people of lower socioeconomic position than among wealthier people, in part because smoking rates are higher in poorer populations. Similarly, it has been suggested that survival is worse among poorer people because they tend to present with more advanced disease, which has a worse prognosis (predicted outcome) than early disease. But do socioeconomic inequalities in treatment exist for lung cancer and, if they do, could these inequalities contribute to the poor survival rates among populations of lower socioeconomic position? In this systematic review and meta-analysis, the researchers investigate the first of these questions. A systematic review uses predefined criteria to identify all the research on a given topic; a meta-analysis is a statistical approach that combines the results of several studies.
What Did the Researchers Do and Find?
The researchers identified 46 published papers that studied people with lung cancer in whom receipt of treatment was reported in terms of an indicator of socioeconomic position, such as a measure of income or deprivation. Twenty-three of these papers were suitable for inclusion in a meta-analysis. Lower socioeconomic position was associated with a reduced likelihood of receiving any treatment. Specifically, the odds ratio (chance) of people in the lowest socioeconomic group receiving any treatment was 0.79 compared to people in the highest socioeconomic group. Lower socioeconomic position was also associated with a reduced chance of receiving surgery (OR = 0.68) and chemotherapy (OR = 0.82), but not radiotherapy. The association between socioeconomic position and surgery remained after taking cancer stage into account. That is, when receipt of surgery was examined in early-stage patients only, low socioeconomic position remained associated with reduced likelihood of surgery. Notably, the association between socioeconomic position and receipt of treatment was similar in studies undertaken in countries where health care is free at the point of service for everyone (for example, the UK) and in countries with primarily private insurance health care systems (for example, the US).
What Do These Findings Mean?
These findings suggest that patients in more socioeconomically deprived circumstances are less likely to receive any type of treatment, surgery, and chemotherapy (but not radiotherapy) for lung cancer than people who are less socioeconomically deprived. Importantly, these inequalities cannot be explained by socioeconomic differences in stage at presentation or by differences in health care system. The accuracy of these findings may be affected by several factors. For example, it is possible that only studies that found an association between socioeconomic position and receipt of treatment have been published (publication bias). Moreover, the studies identified did not include information regarding patient preferences, which could help explain at least some of the differences. Nevertheless, these results do suggest that socioeconomic inequalities in receipt of treatment may exacerbate socioeconomic inequalities in the incidence of lung cancer and may contribute to the observed poorer outcomes in lower socioeconomic position groups. Further research is needed to determine the system and patient factors that contribute to socioeconomic inequalities in lung cancer treatment before clear recommendations for changes to policy and practice can be made.
Additional Information
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.1001376.
The US National Cancer Institute provides information about all aspects of lung cancer for patients and health care professionals (in English and Spanish); a monograph entitled Area Socioeconomic Variations in U. S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 19751999 is available
Cancer Research UK also provides detailed information about lung cancer and links to other resources, such as a policy statement on socioeconomic inequalities in cancer and a monograph detailing cancer and health inequalities in the UK
The UK National Health Service Choices website has a page on lung cancer that includes personal stories about diagnosis and treatment
MedlinePlus provides links to other US sources of information about lung cancer (in English and Spanish)
Introduction
Lung cancer is the most commonly occurring cancer worldwide. In the USA and the UK it is the second most incident cancer [1],[2], as well as the most common cause of cancer mortality [2],[3]. Survival differs internationally. In the UK, fewer than 10% of those diagnosed with lung cancer survive for 5 years [3],[4], with higher survival rates found in Nordic countries [4],[5], the USA [2],[5], Australia, and Canada [4].
Lung cancers are classified into small cell (SCLC) and non-small cell (NSCLC) lung cancers. NSCLC is more common than SCLC and has a better survival rate [6]. National Institute for Health and Clinical Excellence (NICE) guidelines recommend radical surgery for stage I or II NSCLC [6]. Chemotherapy and radiotherapy are recommended for later-stage NSCLC patients and are the treatments of choice for SCLC [6]. Treatment intervention with surgery, chemotherapy, or radiotherapy has been shown to improve lung cancer survival [6].
Socioeconomic inequalities in incidence of, and survival from, the majority of cancers have been reported [1],[3],[7]. A recent non-systematic review revealed socioeconomic inequalities in receipt of treatment for colorectal cancer [8], and it has been suggested that socioeconomic differences in access to treatment might at least partially explain socioeconomic differences in survival [9]. Unintended variations in outcome that result from the way that health interventions are organised and delivered have been described as intervention-generated inequalities [10].
Incidence of lung cancer is higher [1],[11], and survival poorer [7], in the most deprived patient groups. However, it is not known whether socioeconomic inequalities in receipt of treatment exist for lung cancer and, if so, what contribution they make to overall socioeconomic inequalities in outcome. In order to explore the first of these questions, we undertook a systematic review and meta-analysis of cohort studies examining the association between socioeconomic position (SEP) and receipt of lung cancer treatment.
Methods
A protocol (see Text S2) was developed and systematic methods were used to identify relevant studies, assess study eligibility for inclusion, and evaluate study quality. The review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12] (see Text S1 for PRISMA checklist).
Literature Search
The online databases of MEDLINE and EMBASE were searched up to September 2012 (see Table S1 for full search strategies). No language restriction was applied. A search of Scopus uncovered no further papers. Additional studies were identified by reviewing the reference lists of all included studies and by using a forward citation search to identify more recent studies that had cited included studies. EndNote X5 software was used to manage the references.
Study Eligibility
Studies that met the following criteria were included in the review: primary, cohort studies of participants with a primary diagnosis of lung cancer (ICD10 C33 or C34) reported separately from other cancers; published in a peer-reviewed journal; where at least one reported outcome was receipt of treatment (measured by rates or odds of receiving treatment); and where receipt of this outcome was reported by a measure of SEP. Any curative or palliative treatment for lung cancer including surgery, chemotherapy, and radiotherapy was included.
Studies where SEP was included as a descriptive variable or confounder, but where outcomes for receipt of treatment by SEP were not presented, were not eligible for inclusion, but the authors were contacted to determine whether relevant data were available that might allow for inclusion in the review.
Studies where multivariable analysis was conducted (and included control for a minimum of age and sex as confounders); receipt of treatment was compared to not receiving treatment; odds ratios (ORs) and 95% confidence intervals (CIs) of receipt of treatment in low compared to high SEP were calculated; and SEP was not further stratified by another variable, were considered suitable for inclusion in meta-analysis.
Acceptable measures of SEP were: area-based indices of deprivation (e.g., Index of Multiple Deprivation [IMD], Townsend Score, Carstairs Index); and area or individual measures of income, poverty, or education level.
Multiple papers using the same or overlapping study data were included. Sensitivity analyses were conducted including all eligible papers and using different combinations of included papers, but only data from the better quality or more detailed paper in each overlapping study group were included in the final meta-analyses. Sensitivity meta-analyses are included in the supplemental material.
Study Selection and Data Extraction
Studies obtained from the database searches were independently assessed by two researchers (LFF and HW) in three phases: title, abstract, and full paper screening. Any disagreements at any of the screening stages were resolved by discussion between the two researchers in the first instance and with a third reviewer (JA) if agreement could not be reached. Data extraction was carried out by LFF using an Access database pro-forma developed for this purpose, and double-checked by HW.
There is evidence to suggest that health insurance status is an important factor relating to access to lung cancer care in countries such as the USA that rely on insurance-based health care systems [13]. Insurance status is less relevant and rarely measured in most other countries. Therefore, three analytical categories were developed a priori: studies conducted in a universal health care system (UHCS), free at the point of access (similar to the UK); studies conducted in countries with primarily private insurance health care systems (non-UHCS, similar to the USA) [14]; and studies conducted in countries with social insurance health care systems (similar to many European countries). No studies were identified that fell into the third category.
Study Quality
A study quality tool, adapted from existing quality tools [15],[16], was used to divide studies into six quality categories, with 1 being the lowest, and 6 the highest, quality (see Text S3). Quality assessment was carried out by LFF and checked by HW.
Cohort studies reporting only univariable analysis are of lower quality in terms of their ability to control for confounding. Only studies conducting multivariable analysis (quality scores 3–6) were included in the meta-analysis. All studies that met the inclusion criteria were analysed in the narrative synthesis.
Statistical Analysis
Trends in receipt of treatment across SEP groups were described in the narrative analysis of all studies that met the inclusion criteria.
Meta-analysis of eligible studies was undertaken using Cochrane Collaboration Review Manager 5.1. Natural logs of the ORs and their standard errors (SEs) were calculated for use in forest plots. Random-effects meta-analysis of the odds of treatment in the lowest compared to the highest SEP group was conducted. Where a study reported the most deprived class as the comparator, reverse ORs were calculated. Studies that presented a single OR as either an OR for a one unit increase in deprivation score or incremental quintile increase in income were not included.
Subgroup analyses by treatment type and health care system were conducted. In meta-analyses where a “substantial” percentage [17] of the variability appeared to be due to the heterogeneity of the studies rather than to chance, further subgroup analyses by stage, histology, and quality score were conducted, where appropriate, in order to examine potential sources of heterogeneity. A funnel plot was used to assess potential publication bias.
Results
Included Papers/Studies
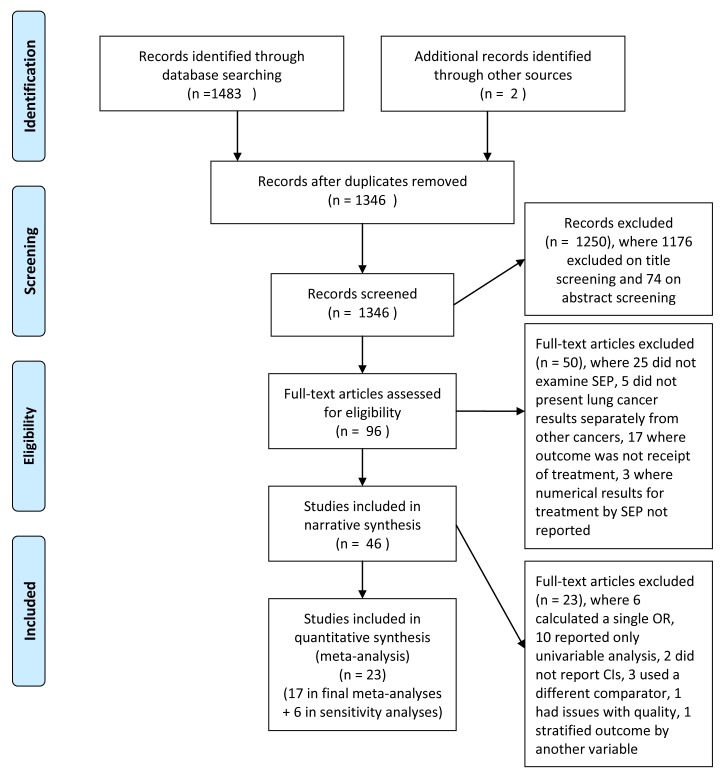
A total of 46 papers met the inclusion criteria and were included in the review (see the PRISMA flow diagram [Figure 1]). Twenty-eight papers were from UHCS countries (Tables 1 and 2). Of these, 19 UK papers examined 13 study populations, although as these included national and regional populations from different sources, there was some further population overlap. One UK paper also compared treatment in Scotland and Canada [18]. A further nine papers from Canada (2), Sweden (1), Australia (1), Italy (1), France (1), and New Zealand (3) were included. The three New Zealand papers all examined the same population.
Figure 1. Flow diagram of study selection and exclusion.
CI, confidence interval; SEP, socioeconomic position.
Table 1. Characteristics of included studies potentially suitable for meta-analysis (universal health care systems).
| Paper | Country of Study | Data Source (s) | Population Included | Years of Diagnosis | Measure of SEP | No. of SEP groups | Treatment given within | Age Range | Confounders Controlled For: | Quality Score | ||||
| Age | Sex | Stage | Histology | Other | ||||||||||
| Berglund et al, 2010 [19] | Sweden | Regional Lung Cancer Register (RLCR) - Sweden, Cause of Death Register and LISA (insurance and demographics) | Uppsala/Orebro region in central Sweden | 1996–2004 | Education levela | 3 | NR | 30+ | Yes | Yes | Yes | Yes | Performance status, year of diagnosis, smoking status | 6 |
| Berglund et al, 2012 [22] | England | Thames Cancer Registry, HES, LUCADA | South-east England | 2006–2008 | IMD 2007 income domain | 5 | NR | 0–80+ | Yes | Yes | Yes | Yes | Co-morbidity | 6 |
| Campbell et al, 2002 [35] | Scotland | Scottish Cancer Registry and hospital case notes | Random sample from North/NE Scotland (with hospital record) | 1995–1996 | Carstairs Index | 5 | 12 months | NR | Yes | Yes | Yes | Yes | Health board, distance to cancer centre, mode of admission | 5 |
| Crawford et al, 2009 [36] | England | Northern and Yorkshire Cancer Registry and Information Service (NYCRIS) | Northern and Yorkshire region | 1994–2002 | IMD 2004 (access to services domain removed) | 4 | 6 months | NR | Yes | Yes | No | Yes | Travel time (but overall results not stratified by travel time used here). Histology not included in receipt of any treatment analysis. | 4 |
| Erridge et al, 2002 [37] | Scotland | Scottish Cancer Registry and medical records | Scotland (with hospital record) | 1995 | Carstairs Index | 5 | 6 months | <60– 80+ | Yes | Yes | Yes | Yes | Health board (not inc in receipt of radiotherapy), diagnosis by specialist, management by oncologist | 6 |
| Erridge et al, 2009 [18] | Scotland/Canada | Scottish Cancer Registry and medical records; British Columbia Cancer Registry | Scotland/British Columbia | 1995 | Carstairs Index/average household income | 2 | 6 months | <60– 80+ | Yes | Yes | Yes | Yes | Travel time, CT scan | 4 |
| Gregor et al, 2001 [38] | Scotland | Scottish Cancer Registry and medical records | Scotland (with hospital record) | 1995 | Carstairs Index | 5 | 6 months | <60–80+ | Yes | Yes | Yes | Yes | Referral to specialist within 6 months of diagnosis | 6 |
| Jack et al, 2003 [39] | England | Thames Cancer Registry | South-east England | 1995–1999 | Townsend (median score per health authority) | Contin-uousb | NR | <35–85+ | Yes | Yes | Yes | Yes | First hospital visited is a radiotherapy centre, basis of diagnosis, incidence. Health authority/hospital used as 2nd level in multi-level model. | 4 |
| Jack et al, 2006 [40] | England | Thames Cancer Registry and medical records | South-east London (with hospital record) | 1998 | IMD 2000 | 5 | 6 months | <55–85+ | Yes | Yes | Yes | Yes | Consultant specialty, basis of diagnosis (hospital, number of symptoms in some analyses) | 6 |
| Jones et al,2008 [41] | England | Northern and Yorkshire Cancer Registry and Information Service (NYCRIS) | Northern and Yorkshire region | 1994–2002 | IMD 2004 (access to services domain removed) | Contin-uousc | NR | NR | Yes | Yes | No | Yes | Travel time to hospital | 4 |
| Mahmud et al, 2003 [42] | Ireland | National Cancer Registry of Ireland (NCRI) | Republic of Ireland | 1994–1998 | SAHRU area-based material deprivation index | 3 | 6 months | 15–80+ | Yes | Yes | No | Yes | Health board, year of diagnosis | 4/2d |
| McMahon et al, 2011 [43] | England | Eastern Cancer Registry and Information Centre (ECRIC) | East of England | 1995–2006 | IMD 2004 (access to services domain removed) | 5 | NR | <60–80+ | Yes | Yes | No | Yes | Year of diagnosis | 4 |
| Pollock &Vickers, 1998 [44] | England | HES FCEs | North/South Thames (admitted to hospital) | 1992–1995 | Townsend | 10 | NR | <100 | Yes | Yes | No | No | Hospital, mode of admission | 3 |
| Raine et al, 2010 [45] | England | HES FCEs | England (admitted to hospital) | 1999–2006 | IMD | 5 | NR | 50– 90+ | Yes | Yes | No | No | Trust, year of admission, mode of admission | 3 |
| Riaz et al, 2012 [34] | England | NCIN/UKACR cancer registries | England | 2004–2006 | IMD 2004 | 5 | NR | 0– 85+ | Yes | Yes | No | No | Government Office Region | 4 |
| Rich et al, 2011(1) [46] | England | LUCADA supplied by 157 NHS trusts | England | 2004–2007 | Townsend | 5 | NR | NR | Yes | Yes | Yes | Yes | Performance status. Adjusted for clustering by NHS trust | 5 |
| Rich et al, 2011(2) [21] | England | LUCADA and HES | England | 2004–2008 | Townsend | 5 | NR | 30–100 | Yes | Yes | Yes | Yes | Co-morbidity, ethnicity, surgery centre, radiotherapy centre, trial entry. Adjusted for clustering by NHS trust | 5 |
| Stevens et al, 2007 [23] | New Zealand | Regional hospital and oncology databases checked against NZ cancer registry | Auckland-Northland region patients managed in secondary care | 2004 | NZ Deprivation Index | 2 | NR | <60–80+ | Yes | Yes | Yes | Yes | Co-morbidity, private sector care, care discussed at MDM | 3 |
| Stevens et al, 2008 [47] | New Zealand | Regional hospital and oncology databases checked against NZ cancer registry | Auckland-Northland region patients managed in secondary care | 2004 | NZ Deprivation Index | 10 | NR | <60–80+ | Yes | Yes | Yes | Yes | Co-morbidity, private sector care, ethnicity | 5 |
Quality score ranges from 1 (lowest quality) to 6 (highest quality).
Socioeconomic index (SEI) and household income also measured but individual education level used in analyses as it contained least missing data.
Odds ratio for 1 unit increase in deprivation score, range unknown.
Odds ratio for 1 unit increase in deprivation score, range 1–80.
Quality score 4 where adjusted OR used and 2 where unadjusted rates used.
HES, Hospital Episode Statistics; HES FCE, Hospital Episode Statistics Finished Consultant Episode; IMD, Index of Multiple Deprivation; LUCADA, Lung Cancer Audit; MDM, multi-disciplinary meeting; NCIN/UKACR, National Cancer Information Network/UK Association of Cancer Registries; NR, not reported; OR, odds ratio; SEP, socioeconomic position; UHCS, universal health care system.
Table 2. Characteristics of included studies not suitable for meta-analysis (universal health care systems).
| Paper | Country of Study | Data Source (s) | Population Included | Years of Diagnosis | Measure of SEP | No of SEP Groups | Treatment Given Within | Age Range | Confounders Controlled For: | Reason for Exclusion | Quality score | ||||
| Age | Sex | Stage | Histology | Other | |||||||||||
| Battersby et al, 2004 [48] | England | HES and East Anglian Cancer Intelligence Unit | 17 PCTs in Norfolk, Suffolk and Cambridgeshire with HES record | 1997–2000 | IMD (weighted average for PCT) | NR | NR | NR | Yes | Yes | No | Yes | Incidence | Rate correlated against deprivation, by sex | 1 |
| Bendzsak et al, 2011 [49] | Canada | Ontario Cancer Registry linked to CIHI hospital data, Insurance data and RPD database | Ontario | 2003–2004 | Neighbourhood income | 5 | 12 months | 20–75+ | Yes | Yes | No | No | Univariable analysis | Univariable rate | 2 |
| Cartman et al, 2002 [50] | England | Northern and Yorkshire Cancer Registry and Information Service (NYCRIS) | Yorkshire region | 1986–1994 | NR | NR | NR | <65–75+ | Yes | Yes | No | Yes | Univariable analysis | Univariable rate | 1 |
| Hui et al, 2005 [51] | Australia | NSW Central Cancer Registry and hospital records | Residents of two area Health Services | 1996 | SEIFA-IRSD | 5 | NR | <50–70+ | Yes | Yes | Yes | Yes | Univariable analysis | Univariable rate | 2 |
| Madelaine et al, 2002 [52] | France | Manche Dept Cancer Registry | Manche | 1997–1999 | INSEE | 4 | NR | <54–75+ | Yes | Yes | Yes | Yes | Urban/rural | Unemployed used as low SEP group and SEP group 2 used as baseline | 2 |
| Pagano et al, 2010 [53] | Italy | Piedmont Cancer Registry of Turin | Turin | 2000–2003 | Education level | 3 | 12 months | <65–75+ | Yes | Yes | Yes | Yes | Marital status | Different comparator – other not no treatment | 2 |
| Patel et al, 2007 [54] | England | Thames Cancer Registry | Southeast England | 1994–2003 | IMD | 5 | 6 months | 0–100 | Yes | Yes | Yes | Yes | Cancer network, year of diagnosis | Adjusted rates with no CIs. Possible errors in numbers. | 2 |
| Stevens et al, 2009 [55] | New Zealand | Regional hospital and oncology databases checked against NZ cancer Registry listing | Auckland-Northland region patients managed in secondary care | 2004 | NZ Deprivation Index | 10 | NR | <60–80+ | Yes | Yes | Yes | Yes | Univariable analysis | Univariable OR. Multivariable SEP results not shown | 2 |
| Younis et al, 2008 [56] | Canada | Nova Scotia cancer registry and chart review | Nova Scotia | 2005 | Median household income | 2 | NR | 65–75+ | Yes | Yes | Yes | Yes | Co-morbidity, performance status, hospital, surgery type, post-op complications, surgeon, medical oncology, education level, distance to cancer centre, marital status, smoking history | Univariable rate. Multivariable OR only for referral by SEP | 2 |
Quality scores range from 1 (lowest quality) to 6 (highest quality).
CI, confidence interval; HES, Hospital Episode Statistics; IMD, Index of Multiple Deprivation; NR, not reported; NSW, New South Wales; OR, odds ratio; PCT, Primary Care Trust; SEIA-IRSD, Socioeconomic Indexes for Areas - Index of Relative Social Disadvantage; SEP, socioeconomic position; UHCS, universal health care system.
Eighteen papers were from non-UHCSs, all of which were from the USA (Tables 3 and 4). The majority of non-UHCS papers used sub-groups of the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) database population and, again, some population overlap was found.
Table 3. Characteristics of included studies potentially suitable for meta-analysis (non-universal health care systems).
| Paper | Country of Study | Data Source (s) | Population Included | Years of Diagnosis | Measure of SEP | No of SEP Groups | Treatment Given Within | Age Range | Confounders Controlled For: | Quality Score | ||||
| Age | Sex | Stage | Histology | Other | ||||||||||
| Bradley et al, 2008 [57] | USA | Michigan Cancer Registry and Michigan Medicare and Medicaid data | Medicare and Medicare/Medicaid patients in Michigan | 1997–2000 | Census tract median household income (high v low) | 2 | 6 months | 66–80+ | Yes | Yes | Yes | Yes | Co-morbidity, insurance type, ethnicity, urban/rural | 4 |
| Davidoff et al, 2010 [58] | USA | SEER cancer registry linked to Medicare data | Medicare patients from 16 SEER registries | 1997–2002 | Census tract median household income | 4 | 90 days | 66–85+ | Yes | Yes | Yes | Yes | Co-morbidity, performance status, ethnicity, marital status, rural/urban, prior Medicaid, tumour grade | 5 |
| Earle et al, 2000 [59] | USA | SEER cancer registry linked to Medicare data | Medicare patients from 11 SEER registries | 1991–1993 | Census tract median household income(increase in OR per quintile) | 5 | 4 months | 65–104 | Yes | Yes | Yes | Yes | Co-morbidity, year of diagnosis, ethnicity, rural/urban, teaching hospital, SEER area | 5 |
| Esnoala et al, 2008 [60] | USA | South Carolina central cancer Registry linked to inpatient and outpatient surgery files | South Carolina | 1996–2002 | Income, zip code level (poverty/not living in poverty) | 2 | NR | <50–80+ | Yes | Yes | Yes | Yes | Co-morbidity, year of diagnosis, insurance type, ethnicity, rural/urban, education, marital status, tumour location | 4 |
| Greenwald et al, 1998 [61] | USA | SEER cancer registry | 3 (Detroit, San Francisco, Seattle) out of 9 SEER registries | 1978–1982 | Census tract median household income (increase in OR per decile) | 10 | NR | < = 75 | Yes | Yes | Yes | Yes | Performance status, ethnicity | 6 |
| Hardy et al, 2009 [62] | USA | SEER cancer registry linked to Medicare data | Medicare patients from 17 SEER registries | 1991–2002 | % individuals below poverty line at census tract level | 4 | NR | 65–85+ | Yes | Yes | Yes | Yes | Co-morbidity, year of diagnosis, ethnicity, marital status, SEER area, other treatment | 5 |
| Hayman et al, 2007 [63] | USA | SEER cancer registry linked to Medicare data | Medicare patients from 11 SEER registries | 1991–1996 | Census tract median household income | 5 | 4 months/2 years | 65–85+ | Yes | Yes | Yes | Yes | Co-morbidity, year of diagnosis, ethnicity, SEER area, hospitalisation, teaching hospital, distance to nearest RT centre, receipt of chemotherapy | 5 |
| Lathan et al, 2008 [64] | USA | SEER cancer registry linked to Medicare data | Medicare patients from 11 SEER registries | 1991–1999 | Census tract median household income (inc in OR per quintile) | 5 | NR | 65+ | Yes | Yes | Yes | Yes | Co-morbidity, ethnicity, SEER registry, urban, non-profit hospital, patient volume, % of black patients in hospital | 5 |
| Polednak, 2001 [65] | USA | Connecticut Tumor Registry (SEER) and inpatient hospital discharge database (HDD) | Connecticut | 1992–1997 | Census tract poverty rate | 5 | NR | <55–80+ | Yes | Yes | Yes | No | Co-morbidity, ethnicity, marital status | 4 |
| Smith et al, 1995 [66] | USA | Virginia Cancer Registry and Medicare claims database | Medicare patients from Virginia cancer registry | 1985–1989 | Census tract: median household income by race and age | Contin-uousa | 6 months | 65–85+ | Yes | Yes | Yes | Yes | Co-morbidity, ethnicity, county of residence, distance to oncologist | 5 |
Quality scores range from 1 (lowest quality) to 6 (highest quality).
Odds ratio for increase per $10,000 income.
CI, confidence interval; non-UHCS, non-universal health care system; NR, not reported; OR, odds ratio; SEER, National Cancer Institute's Surveillance, Epidemiology and End Results database; SEP, socioeconomic position.
Table 4. Characteristics of included studies not suitable for meta-analysis (non-universal health care systems).
| Paper | Country | Data Source (s) | Population Included | Years of Diagnosis | Measure of SEP | No of SEP Groups | Treatment Given Within | Age Range | Confounders Controlled For: | Reasons for Exclusion | Quality Score | ||||
| Age | Sex | Stage | Histology | Other | |||||||||||
| Bach et al, 1999 [67] | USA | SEER cancer registry linked to Medicare data | Medicare patients from 10 SEER registries | 1985–1993 | Median income in zip code of residence (lowest quartile compared to highest 3) | 2 | NR | 65–75+ | Yes | Yes | Yes | Yes | Co-morbidity, ethnicity, SEER area | OR of surgery for black v white, univariable rates of surgery used here | 2 |
| Earle et al, 2002 [68] | USA | SEER cancer registry linked to Medicare data | Medicare patients from 11 SEER registries | 1991–1996 | Census tract median household income | 5 | any time | NR | Yes | Yes | Yes | Yes | Co-morbidity, ethnicity, year of diagnosis, teaching hospital, seen by oncologist, SEER area | SEP non sig in multivariable analysis but only univariable rate shown. | 2 |
| Lathan et al, 2006 [69] | USA | SEER cancer registry linked to Medicare data | Medicare patients from 11 SEER registries | 1991–1999 | Census tract median household income | 5 | NR | 65+ | Yes | Yes | Yes | Yes | Co-morbidity, ethnicity, SEER region, teaching hospital, rural/urban | Quality issues | 2 |
| Ou et al, 2008 [70] | USA | California Cancer Registry (part of SEER) | California | 1989–2003 | Composite measure (7 indicators of education, income and occupation) | 5 | NR | 0–89 | Yes | Yes | Yes | Yes | Ethnicity, tumour grade, tumour location, histologic grade, marital status | SEP not reported in multivariable analysis. Univariable rate shown. | 2 |
| Suga et al, 2010 [71] | USA | California Cancer Registry | Sacramento region in Northern California | 1994–2004 | Census tract composite variable income, education, employment, poverty, rent, housing value | 5 | NR | NR | Yes | Yes | Yes | Yes | Ethnicity, residence (urban/rural) | No CIs | 2 |
| Tammemagi et al, 2004 [72] | USA | Josephine Ford Cancer Center Tumor Registry | Detroit (receiving care at Henry Ford Health System) | 1995–1998 | Census tract median household income | Contin-uousa | NR | NR | Yes | Yes | Yes | Yes | Co-morbidity, ethnicity, marital status, smoking history, alcohol use, drug use | SEP not reported in multivariable analysis. Univariable OR shown. | 2 |
| Wang et al, 2008 [73] | USA | SEER cancer registry linked to Medicare data | Medicare patients 11 SEER registries | 1992–2002 | % below census tract poverty level | 4 | 4 months | 66–85 | Yes | Yes | Yes | Yes | Co-morbidity, ethnicity, year of diagnosis, grade, SEER region, census tract education, marital status, teaching hospital, radiation | SEP not reported in multivariable analysis.OR for consultation but not treatment shown. | 1 |
| Yang et al, 2010 [74] | USA | Florida Cancer registry linked to inpatient and outpatient medical records | Florida | 1998–2002 | Census tract poverty level | 4 | NR | <45–70+ | Yes | Yes | Yes | Yes | Univariable analysis only | Univariable rate | 2 |
Quality scores range from 1 (lowest quality) to 6 (highest quality).
Odds ratio for increase per $10,000 income.
CI, confidence interval; non-UHCS, non-universal health care system; NR, not reported; OR, odds ratio; SEER, National Cancer Institute's Surveillance, Epidemiology and End Results database; SEP, socioeconomic position.
An individual measure of SEP (education level) was used in one study [19]. All other studies used area-level measures of deprivation, income, poverty, or education level.
In terms of quality, the non-UHCS studies that carried out multivariable analyses had better control for confounding than did UHCS studies, as they tended to stratify by stage and histology. However, half of the non-UHCS papers used a Medicare-only population aged over 65, and so were more restrictive in population terms than the UHCS studies.
Twenty-nine papers met the criteria for meta-analysis—19 from UHCSs and 10 from non-UHCSs. However, six studies that examined receipt of treatment in low compared to high SEP presented the results as a single OR and so could not be included in the meta-analyses. Seventeen studies were included in the final meta-analyses and a further six in the sensitivity meta-analyses.
Surgery
Thirty-one papers (29 study populations) included receipt of surgery as an outcome—18 UHCS papers (15 study populations) and 13 non-UHCS papers (14 study populations) (Tables 5 and 6). Of the papers that reported measures of significance (CIs or p-values), 20 out of 27 (74%) reported that lower SEP was significantly associated with lower likelihood of surgery when comparing the lowest with the highest SEP group, although three of these 20 papers did not find a significant trend across groups.
Table 5. Likelihood of receipt of surgery by SEP group (universal health care systems).
| Study | No. Receiving Surgery | Cohort No./No. Eligible | Rate | Histology | OR/Rate in Q1 (95% CI) | OR/Rate in Q2 (95% CI) | OR/Rate in Q3 (95% CI) | OR/Rate in Q4 (95% CI) | OR/rate in Q5 (95% CI) | p-Value | Quality Score | Meta-Analysis | Further Information |
| Bendzsak et al, 2011 [49] | 1220 | 6499 | 18.77 | any | 21.1 | 18.3 | 19.7 | 18.8 | 16.8 | 0.02 | 2 | N | Univariable rate |
| Campbell et al, 2002 [35] | 85 | 653 | 13.02 | any | 1.00 | 0.76 (0.28 to 2.09) | 0.70 (0.27 to 1.84) | 0.88 (0.35 to 2.22) | 0.59 (0.23 to 1.53) | 0.423 | 5 | Y | P for trend |
| Hui et al, 2005 [51] | NR | 526 | any | 29 | 28 | 20 | 27 | 20 | 0.19 | 2 | N | Univariable rate | |
| Jack et al, 2003 [39] | NR | 32818 | any | 0.98 (0.95 to 1.01) | 0.7759 | 4 | N | ||||||
| Jack et al, 2006 [40] | 42 | 695 | 6.04 | any | 1.00 | 0.82 (0.33 to 2.07) | 0.89 (0.35 to 2.25) | 0.16 (0.03 to 0.73) | 0.75 (0.27 to 2.09) | 0.1326 | 6 | Y | Subset of Jack et al (2003) pop, p for trend |
| Jones et al,2008 [41] | 3552 | 34923 | 10.17 | any | 0.99 (0.99 to 1.00) | <0.01 | 4 | N | |||||
| Pollock &Vickers, 1998 [44] | 2869 | 38668 | 7.42 | any | 1.00 | 0.83 (0.69 to 1.00) | 0.73 (0.61 to 0.88) | 0.82 (0.68 to 0.98) | 0.58 (0.48 to 0.70) | <0.05 | 3 | Y | Hospital population, p for trend |
| Raine et al, 2010 [45] | 8790 | 36902 | 23.82 | any | 1.63 (1.49 to 1.77) | 1.58 (1.46 to 1.72) | 1.45 (1.35 to 1.57) | 1.34 (1.25 to 1.45) | 1.00 | <0.001 | 3 | Y | Elective admission population |
| Raine et al, 2010 [45] | 8923 | 186741 | 4.78 | any | 5.5 | 5.2 | 4.8 | 4.4 | 4.5 | NR | 2 | N | All admissions, univariable rate |
| Battersby et al, 2004 [48] | 387 | 4092 | 9.46 | NSCLC | −0.10 (−0.55 to 0.40) | NR | 1 | N | Rate by sex correlated with deprivation score (men), with overall treatment rate | ||||
| Battersby et al, 2004 [48] | NSCLC | −0.16 (−0.59 to 0.35) | NR | 1 | N | Rate by sex correlated with deprivation score (women) | |||||||
| Berglund et al, 2010 [19] | 626 | 3369 | 18.58 | NSCLC | 1.93 (1.25 to 3.00) | 1.33 (0.98 to 1.81) | 1.00 | NR | 6 | Y | |||
| Berglund et al, 2010 [19] | 534 | 932 | 57.30 | NSCLC | 2.84 (1.40 to 5.79) | 1.53 (1.01 to 2.31) | 1.00 | NR | 6 | Y(S) | Early stage only - stage IA-IIB | ||
| Berglund et al, 2012 [22] | 899 | 1826 | 49.18 | NSCLC | 1.00 | 0.74 (0.51 to 1.06) | 0.71 (0.49 to 1.02) | 0.73 (0.52 to 1.03) | 0.67 (0.48 to 0.95) | 0.29 | 6 | Y | Early stage only – stage IA-IIB, p for trend |
| Cartman et al, 2002 [50] | 2401 | 12570 | 19.10 | NSCLC | 19.1 | 18.6 | NR | 1 | N | Univariable rate | |||
| Crawford et al, 2009 [36] | 3335 | 18324 | 18.20 | NSCLC | 1.00 | 0.90 (0.81 to 1.00) | 0.82 (0.74 to 0.91) | 0.80 (0.72 to 0.89) | <0.05, <0.01, <0.01 | 4 | Y | Individual P values reported | |
| Mahmud et al, 2003 [42] | 866 | 4451 | 19.46 | NSCLC | 19.8 | 18.0 | 21.0 | NR | 2 | N | Univariable rate | ||
| McMahon et al, 2011 [43] | 2374 | 18813 | 12.62 | NSCLC | 1.00 | 0.95 (0.83 to 1.09) | 0.95 (0.83 to 1.08) | 0.90 (0.79 to 1.03) | 0.78 (0.65 to 0.94) | 0.018 | 4 | Y | P for trend |
| 0.96 (0.93 to 0.99) | 0.018 | N | Paper presents results in 2 different ways | ||||||||||
| Riaz et al, 2012 [34] | 6900 | 77349 | 8.92 | NSCLC | 1.00 | 0.88 (0.80 to 0.96) | 0.91 (0.83 to 0.99) | 0.82 (0.76 to 0.89) | 0.76 (0.70 to 0.83) | <0.01 | 4 | Y(S) | P for trend |
| Rich et al, 2011(1) [46] | 3427 | 24175 | 14.18 | NSCLC | 1.00 | 1.13 (0.98 to 1.32) | 1.18 (1.02 to 1.37) | 1.01 (0.87 to 1.16) | 1.11 (0.96 to 1.27) | 0.77 | 5 | Y(S) | Subset of Rich et al 2011 (2) pop, p for trend |
| Rich et al, 2011(2) [21] | 4481 | 34436 | 13.01 | NSCLC | 1.00 | 0.99 (0.88 to 1.11) | 1.04 (0.92 to 1.19) | 0.98 (0.84 to 1.13) | 0.86 (0.71 to 1.04) | 0.132 | 5 | Y(S) | P for trend |
Some studies reported SEP quintiles but others reported SEP in 2, 3, or 4 categories or as a continuous variable. Details of the number of SEP groups per study are given in Tables 1–4 in the column entitled “No. of SEP groups.” Quality scores range from 1 (lowest quality) to 6 (highest quality). Meta-analysis: Y, included in final meta-analysis; Y(S), included in sensitivity meta-analysis; N, not included in meta-analysis. Q1, high socioeconomic position, Q5, low socioeconomic position.
CI, confidence interval; NR, not reported; OR, odds ratio; pop, population; SEP, socioeconomic position; UHCS, universal health care system.
Table 6. Likelihood of receipt of surgery by SEP group (non-universal health care systems).
| Study | No. Receiving Surgery | Cohort No./No. Eligible | Rate | Stage(s) Included | Histology | OR/Rate in Q1 (95% CI) | OR/Rate in Q2 (95% CI) | OR/Rate in Q3 (95% CI) | OR/Rate in Q4 (95% CI) | OR/Rate in Q5 (95% CI) | p-Value | Quality Score | Meta-Analysis | Further Information |
| Bradley et al, 2008 [57] | 1336 | 2626 | 50.88 | I,II,IIIa | NSCLC | 1.00 | 0.80 (0.67 to 0.98) | <0.05 | 4 | Y | ||||
| Esnoala et al, 2008 [60] | NR | 2791 | local | NSCLC | 1.00 | 0.67 (0.51 to 0.88) | 0.005 | 4 | Y | |||||
| Greenwald et al, 1998 [61] | 3053 | 5157 | 59.20 | I | NSCLC | 1.076 | <0.0001 | 6 | N | SE = 0.011 (no CIs shown) | ||||
| Hardy et al, 2009 [62] | 11834 | 19658 | 60.20 | I,II | NSCLC | 1.00 | 0.92 (0.84 to 1.14) | 0.78 (0.75 to 1.03) | 0.68 (0.60 to 0.77) | >0.05, >0.05, <0.05 | 5 | Y | Individual p values reported corrected OR supplieda | |
| Lathan et al, 2008 [64] | 4563 | 9688 | 47.10 | I,II,III | NSCLC | 1.06 (1.02 to 1.11) | NR | 5 | N | Subset of Lathan et al (2006) pop | ||||
| Ou et al, 2008 [70] | 16185 | 19700 | 82.16 | I | NSCLC | 86.9 | 84.8 | 81.1 | 79.6 | 74.5 | <0.001 | 2 | N | |
| Smith et al, 1995 [66] | 801 | 2813 | 28.47 | local | NSCLC | 1.04 (0.90 to 1.19) | >0.001 | 5 | N | |||||
| Tammemagi et al, 2004 [72] | NR | 1155 | I,II | NSCLC | 1.19 (1.03 to 1.30) | 0.02 | 2 | N | Univariable OR | |||||
| Bach et al, 1999 [67] | 550 | 860 | 63.95 | I,II | NSCLC | 67.5 | 61.9 | NR | 2 | N | Surgery (blacks) | |||
| Bach et al, 1999 [67] | 7763 | 10124 | 76.68 | I,II | NSCLC | 78.0 | 70.7 | NR | 2 | N | Surgery (whites) | |||
| Polednak, 2001 [65] | 1385 | 1564 | 88.55 | I,II | NSCLC | 1.00 | 1.27 (0.74 to 2.18) | 1.15 (0.65 to 2.03) | 1.17 (0.67 to 2.04) | 1.78 (1.05 to 3.01) | >0.05, >0.05, >0.05, <0.05 | 4 | Y | Odds of not receiving surgery, individual p values reported |
| Smith et al, 1995 [66] | 57 | 2396 | 2.38 | distant | NSCLC | 1.27 (0.97 to 1.67) | >0.001 | 5 | N | |||||
| Suga et al, 2010 [71] | NR | 12395 | NSCLC | 1.17 | <0.001 | 2 | N | Surgery after invasive staging, no CIs | ||||||
| Suga et al, 2010 [71] | NR | 12395 | NSCLC | 1.18 | <0.001 | 2 | N | Surgery after non-invasive staging, no CIs | ||||||
| Lathan et al, 2006 [69] | NR | 14224 | NSCLC | 1.05 (1.02 to 1.08) | NR | 2 | N | |||||||
| Yang et al, 2010 [74] | NR | NR | all | all | 24.6 | 22.2 | 20.7 | 18.3 | <0.01 | 2 | N | Univariable analysis |
Some studies reported SEP quintiles but others reported SEP in 2, 3, or 4 categories or as a continuous variable. Details of the number of SEP groups per study are given in Tables 1–4 in the column entitled “No. of SEP groups.” Quality scores range from 1 (lowest quality) to 6 (highest quality). Meta-analysis: Y, included in final meta-analysis; Y(S), included in sensitivity meta-analysis; N, not included in meta-analysis. Q1, high socioeconomic position; Q5, low socioeconomic position.
We are grateful to the authors for supplying a corrected OR to allow inclusion of this study in the meta-analysis.
CI, confidence interval; non-UHCS, non-universal health care system; NR, not reported; OR, odds ratio; pop, population; SE, standard error; SEP, socioeconomic position.
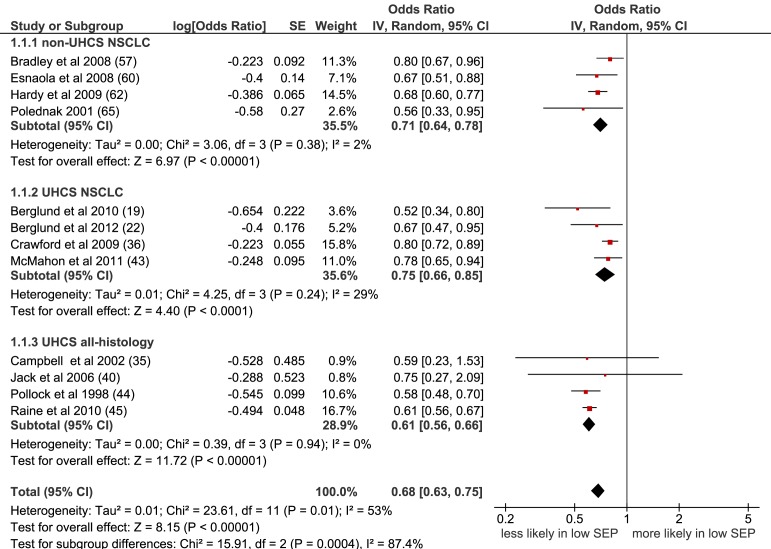
Meta-analysis of all 16 populations that were suitable for inclusion showed a significant negative effect of lower SEP on the likelihood of receiving surgery: OR = 0.72 (95% CI 0.65 to 0.80), p<0.001, I2 = 80% (Figure S1). Including only non-overlapping study populations (n = 12) gave a similar result: OR = 0.68 (95% CI 0.63 to 0.75), p<0.001, I2 = 53% (Figure 2). Similar results were also seen for the subgroup of eight papers including NSCLC patients only (OR = 0.73 [95% CI 0.68 to 0.80] p<0.001, I2 = 24%) (Figure S2) and with further stratification by health care system; NSCLC (UHCS): OR = 0.75 (95% CI 0.66 to 0.85), p<0.001, I2 = 29%; NSCLC (non-UHCS, early stage only, co-morbidity included): OR = 0.71 (95% CI 0.64 to 0.78) p<0.001; I2 = 2% (Figure 2).
Figure 2. Meta-analysis of odds of receipt of surgery in low versus high SEP.
CI, confidence interval; non-UHCS, non-universal health care system; NSCLC, non-small cell lung cancer; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
Lower SEP was associated with a lower likelihood of receiving lung cancer surgery, in both types of health care system, and in studies where histology and stage at diagnosis were taken into account.
Chemotherapy
Twenty-three papers included chemotherapy as an outcome—14 UHCS papers (12 populations) and nine non-UHCS papers (10 populations) (Tables 7 and 8). Of the 21 papers that reported measures of significance, 15 (71%) reported that lower SEP was significantly associated with lower likelihood of receipt of chemotherapy.
Table 7. Likelihood of receipt of chemotherapy by SEP group (universal health care systems).
| Study | No. Receiving Chemo | Cohort No./No. Eligible | Rate | Histology | OR/Rate in Q1 (95% CI) | OR/Rate in Q2 (95% CI) | OR/Rate in Q3 (95% CI) | OR/Rate in Q4 (95% CI) | OR/Rate in Q5 (95% CI) | p-Value | Quality Score | Meta-Analysis | Further Information |
| Berglund et al, 2012 [22] | 3661 | 10039 | 36.47 | any | 1.00 | 0.90 (0.77 to 1.06) | 0.78 (0.67 to 0.91) | 0.77 (0.66 to 0.89) | 0.75 (0.65 to 0.87) | <0.01 | 6 | Y | NSCLC stage IIIA-IV & all stage SCLC, p for trend |
| Campbell et al, 2002 [35] | 124 | 653 | 18.99 | any | 1.00 | 0.58 (0.21 to 1.57) | 0.72 (0.29 to 1.78) | 0.41 (0.16 to 1.05) | 0.39 (0.16 to 0.96) | 0.028 | 5 | Y | |
| Jack et al, 2003 [39] | NR | 32818 | any | 0.96 (0.94 to 0.98) | 0.0001 | 4 | N | Subset of Patel et al (2007) pop | |||||
| Jack et al, 2006 [40] | 108 | 695 | 15.54 | any | 1.00 | 1.04 (0.50 to 2.16) | 0.81 (0.38 to 1.70) | 0.89 (0.43 to 1.85) | 1.04 (0.48 to 2.25) | 0.9130 | 6 | Y | Subset of Patel et al (2007) pop, p for trend |
| Jones et al,2008 [41] | 5783 | 34923 | 16.56 | any | 0.99 (0.99 to 0.99) | <0.01 | 4 | N | |||||
| Patel et al, 2007 [54] | 11217 | 67312 | 16.66 | any | 18.3 | 15.7 | 14.5 | 12.8 | 12.8 | <0.001 | 2 | N | Adjusted rates, no CIs |
| Rich et al, 2011(1) [46] | 14168 | 59592 | 23.78 | any | 1.00 | 0.97 (0.90 to 1.04) | 0.89 (0.83 to 0.96) | 0.83 (0.77 to 0.89) | 0.85 (0.79 to 0.91) | <0.01 | 5 | Y(S) | |
| Hui et al, 2005 [51] | NR | 526 | any | 31 | 34 | 36 | 27 | 26 | 0.15 | 2 | N | Univariable rate | |
| Berglund et al, 2010 [19] | 1285 | 3369 | 38.14 | NSCLC | 1.35 (1.00 to 1.81) | 1.25 (1.03 to 1.52) | 1.00 | NR | 6 | Y | |||
| Pagano et al, 2010 [53] | 430 | 1231 | 34.93 | NSCLC | 1.00 | 0.98 (0.64 to 1.50) | 1.63 (1.08 to 2.44) | NR | 2 | N | Odds of receiving chemo +/or radio rather than surgery | ||
| Younis et al, 2008 [56] | 29 | 108 | 26.85 | NSCLC | 4.7 (1.3 to 17.8) | 1.0 | 0.015 | 2 | N | Odds of referral for adjuvant chemo after surgery, stage I, II, III | |||
| Cartman et al, 2002 [50] | 1349 | 2448 | 55.11 | SCLC | 52.1 | 56.8 | NR | 1 | N | Univariable rate | |||
| Crawford et al, 2009 [36] | 3619 | 5510 | 65.68 | SCLC | 1.00 | 1.10 (0.94 to 1.30) | 0.91 (0.78 to 1.08) | 0.94 (0.80 to 1.11) | >0.05 | 4 | Y | Individual p-values, all reported as >0.05 | |
| Mahmud et al, 2003 [42] | 425 | 1002 | 42.42 | SCLC | 37.8 | 40.5 | 50.2 | NR | 2 | N | Univariable rate |
Some studies reported SEP quintiles but others reported SEP in 2, 3, or 4 categories or as a continuous variable. Details of the number of SEP groups per study are given in Tables 1–4 in the column entitled “No. of SEP groups.” Quality scores range from 1 (lowest quality) to 6 (highest quality). Meta-analysis: Y, included in final meta-analysis; Y(S), included in sensitivity meta-analysis; N, not included in meta-analysis. Q1, high socioeconomic position; Q5, low socioeconomic position.
CI, confidence interval; NR, not reported; OR, odds ratio; pop, population; SEP, socioeconomic position; UHCS, universal health care system.
Table 8. Likelihood of receipt of chemotherapy by SEP group (non-universal health care systems).
| Study | No. Receiving Chemo | Cohort No./No. Eligible | Rate | Stage | Histology | OR/Rate in Q1 (95% CI) | OR/Rate in Q2 (95% CI) | OR/Rate in Q3 (95% CI) | OR/Rate in Q4 (95% CI) | OR/Rate in Q5 (95% CI) | p-Value | Quality Score | Meta-Analysis | Further Information |
| Bradley et al, 2008 [57] | 643 | 2348 | 27.39 | I,II, IIIa | NSCLC | 1.00 | 1.09 (0.87 to 1.37) | >0.05 | 4 | Y | ||||
| Hardy et al, 2009 [62] | 2951 | 19658 | 15.01 | I, II | NSCLC | 1.00 | 0.91 (0.81 to 1.02) | 0.96 (0.85 to 1.09) | 0.85 (0.74 to 0.98) | >0.05, >0.05, <0.05 | 5 | Y | Individual p-values reported | |
| Ou et al, 2008 [70] | 1175 | 19700 | 5.96 | I | NSCLC | 5.3 | 5.7 | 5.3 | 6.9 | 7.4 | 0.001 | 2 | N | Univariable analysis |
| Davidoff et al, 2010 [58] | 5499 | 21285 | 25.84 | IIIB, IV | NSCLC | 1.43 (1.28 to 1.60) | 1.17 (1.05 to 1.30) | 1.11 (1.00 to 1.22) | 1.00 | <0.01, <0.01, <0.05 | 5 | Y | Individual p-values reported | |
| Earle et al, 2000 [59] | 1356 | 6308 | 21.50 | IV | NSCLC | 1.07 (1.02 to 1.12) | 0.0077 | 5 | N | Subset of Earle (2002) | ||||
| Earle et al, 2002 [68] | 8813 | 12015 | 73.35 | IV | NSCLC | 41 | 41 | 36 | 31 | 27 | >0.05 | 2 | N | Univariable analysis only. SEP was included in multivariable analysis but non-sig (figs not reported) |
| Hardy et al, 2009 [62] | 26417 | 51243 | 51.55 | III, IV | NSCLC | 1.00 | 0.87 (0.78 to 0.96) | 0.76 (0.63 to 0.90) | 0.60 (0.45 to 0.79) | <0.05, <0.05, <0.05 | 5 | Y(S) | Individual p-values reported | |
| Tammemagi et al, 2004 [72] | NR | 1155 | III,IV | NSCLC | 1.09 (1.01 to 1.18) | 0.03 | 2 | N | Univariable OR | |||||
| Davidoff et al, 2010 [58] | 749 | 1946 | 38.49 | IIIB, IV | NSCLC | 0.86(0.69 to 1.08) | 0.96 (0.77 to 1.19) | 0.99 (0.81 to 1.22) | 1.00 | NR | 5 | N | Odds of single agent compared to two-agent chemo. | |
| Wang et al, 2008 [73] | 1521 | 3196 | 47.59 | II, IIIa | NSCLC | 1.00 | 1.08 (0.97 to 1.21) | 1.08 (0.97 to 1.21) | 0.97 (0.85 to 1.10) | NR | 1 | N | Odds of receiving oncology consultation. | |
| Yang et al, 2010 [74] | NR | NR | All | any | 32.2 | 30.7 | 29.9 | 30.1 | <0.01 | 2 | N | Univariable analysis |
Some studies reported SEP quintiles but others reported SEP in 2, 3, or 4 categories or as a continuous variable. Details of the number of SEP groups per study are given in Tables 1–4 in the column entitled “No. of SEP groups.” Quality scores range from 1 (lowest quality) to 6 (highest quality). Meta-analysis: Y, included in final meta-analysis; Y(S), included in sensitivity meta-analysis; N, not included in meta-analysis. Q1, high socioeconomic position; Q5, low socioeconomic position.
CI, confidence interval; non-UHCS, non-universal health care system; NR, not reported; OR, odds ratio; pop, population; SEP, socioeconomic position.
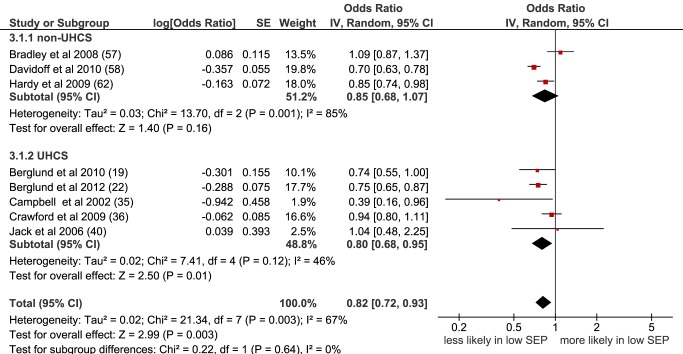
Meta-analysis of the ten populations that were suitable for inclusion found a significant negative effect of lower SEP on the likelihood of receiving chemotherapy: OR = 0.81 (95% CI 0.73 to 0.89), p<0.001, I2 = 68% (Figure S3). Similarly, in a meta-analysis of the eight papers containing non-overlapping populations that were selected for inclusion, the odds of receiving chemotherapy were significantly lower for those with low SEP compared to those with high SEP (OR = 0.82 [95% CI 0.72 to 0.93], p = 0.003, I2 = 67%), overall. A similar pattern was found in UHCS (OR = 0.80 [95% CI 0.68 to 0.95], p = 0.01, I2 = 46%); and in non-UHCS settings (OR = 0.85 [95% CI 0.68 to 1.07], p = 0.16, I2 = 85%), although this did not reach significance (Figure 3).
Figure 3. Meta-analysis of odds of receipt of chemotherapy in low versus high SEP.
CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
Radiotherapy
Eighteen papers (18 populations) examined receipt of radiotherapy for lung cancer—12 in UHCS settings (11 populations) and six in non-UHCS settings (seven populations) (Tables 9 and 10). Only one UHCS study found an association between SEP and receipt of radiotherapy. The non-UHCS studies had very heterogeneous outcomes.
Table 9. Likelihood of receipt of radiotherapy by SEP group (universal health care systems).
| Study | No. Receiving Radio | Cohort No./No. Eligible | Rate | Histology | OR/Rate in Q1 (95% CI) | OR/Rate in Q2 (95% CI) | OR/Rate in Q3 (95% CI) | OR/Rate in Q4 (95% CI) | OR/Rate in Q5 (95% CI) | p-Value | Quality Score | Meta-Analysis | Further Information |
| Berglund et al, 2012 [22] | 1054 | 2771 | 38.04 | any | 1.00 | 1.16 (0.88 to 1.54) | 1.17 (0.90 to 1.53) | 1.18 (0.91 to 1.53) | 0.99 (0.77 to 1.29) | 0.67 | 6 | Y | Stage III only, p for trend |
| Campbell et al, 2002 [35] | 412 | 653 | 63.09 | any | 1,00 | 2.08 (1.11 to 3.91) | 2.27 (1.24 to 4.16) | 1.47 (0.83 to 2.60) | 1.86 (1.05 to 3.28) | 0.378 | 5 | Y | P for trend |
| Jack et al, 2003 [39] | NR | 32818 | any | 1.00 (0.99 to 1.02) | 0.2048 | 4 | N | ||||||
| Jack et al, 2006 [40] | 338 | 695 | 48.63 | any | 1.00 | 1.24 (0.76 to 2.02) | 0.76 (0.46 to 1.26) | 0.98 (0.60 to 1.59) | 0.68 (0.41 to 1.14) | 0.0978 | 6 | Y | Subset of Jack et al (2003) pop, p for trend |
| Jones et al,2008 [41] | 13857 | 34923 | 39.68 | any | 0.99 (0.99 to 1.00) | <0.01 | 4 | N | |||||
| Rich et al, 2011(1) [46] | 12079 | 59592 | 20.27 | any | 1.00 | 1.08 (1.01 to 1.16) | 1.12 (1.04 to 1.20) | 1.12 (1.04 to 1.20) | 1.02 (0.95 to 1.09) | 0.80 | 5 | Y(S) | P for trend |
| Hui et al, 2005 [51] | NR | 526 | any | 52 | 62 | 51 | 55 | 55 | 0.84 | 2 | N | Univariable rate | |
| Stevens et al, 2009 [55] | 222 | 555 | 40.00 | any | 1.0 | 0.8 (0.4 to 1.5) | 0.6 (0.3 to 1.2) | 0.9 (0.5 to 1.6) | 0.7 (0.4 to 1.3) | >0.05 | 2 | N | Hosp pop, univariable OR |
| Berglund et al, 2010 [19] | 863 | 3369 | 25.62 | NSCLC | 0.91 (0.67 to 1.22) | 1.12 (0.93 to 1.36) | 1.00 | NR | 6 | Y | |||
| Erridge et al, 2002 [37] | 824 | 3177 | 25.94 | NSCLC/unknown | 1.00 | 0.94 (0.70 to 1.26) | 1.04 (0.79 to 1.38) | 1.33 (1.01 to 1.75) | 1.13 (0.84 to 1.51) | 0.10 | 6 | Y | |
| Mahmud et al, 2003 [42] | 1265 | 4451 | 28.42 | NSCLC | 26.1 | 29.0 | 29.9 | NR | 2 | N | Univariable rate | ||
| Cartman et al, 2002 [50] | 693 | 2448 | 28.31 | SCLC | 37.1 | 39.5 | NR | 1 | N | Univariable rate |
Some studies reported SEP quintiles but others reported SEP in 2, 3, or 4 categories or as a continuous variable. Details of the number of SEP groups per study are given in Tables 1–4 in the column entitled “No. of SEP groups.” Quality scores range from 1 (lowest quality) to 6 (highest quality). Meta-analysis: Y, included in final meta-analysis; Y(S), included in sensitivity meta-analysis; N, not included in meta-analysis. Q1, high socioeconomic position; Q5, low socioeconomic position.
CI, confidence interval; NR, not reported; OR, odds ratio; pop, population; SEP, socioeconomic position; UHCS, universal health care system.
Table 10. Likelihood of receipt of radiotherapy by SEP group (non-universal health care systems).
| Study | No. Receiving Radio | Cohort No./No. Eligible | Rate | Stage | Histology | OR/rate in Q1 (95% CI) | OR/rate in Q2 (95% CI) | OR/rate in Q3 (95% CI) | OR/rate in Q4 (95% CI) | OR/rate in Q5 (95% CI) | P value | Quality Score | Meta-analysis | Further information |
| Bradley et al, 2008 [57] | 950 | 2348 | 40.46 | I,II,IIIa | NSCLC | 1.00 | 0.97 (0.79 to 1.19) | >0.05 | 4 | Y | ||||
| Ou et al, 2008 [70] | 2779 | 19700 | 14.11 | I | NSCLC | 11.7 | 12.6 | 14.7 | 16.5 | 16.6 | <0.001 | 2 | N | Univariable analysis |
| Smith et al, 1995 [66] | 1323 | 2813 | 47.03 | local | NSCLC | 0.95 (0.83 to 1.09) | >0.001 | 5 | N | |||||
| Hardy et al, 2009 [62] | 43519 | 51243 | 84.93 | III,IV | NSCLC | 1.00 | 1.01 (0.96 to 1.07) | 0.93 (0.88 to 0.99) | 0.88 (0.82 to 0.93) | 0.05, <0.05, <0.05 | 5 | Y | Individual p-values reported | |
| Hayman et al, 2007 [63] | 6436 | 11084 | 58.07 | IV | NSCLC | 1.48 (1.17 to 1.87) | 1.50 (1.17 to 1.91) | 1.32 (1.01 to 1.72) | 1.25 (0.93 to 1.69) | 1.00 | <0.001 | 5 | Y(S) | |
| Smith et al, 1995 [66] | 1438 | 2396 | 60.02 | distant | NSCLC | 1.00 (0.90 to 1.12) | >0.001 | 5 | N | |||||
| Yang et al, 2010 [74] | NR | NR | ?? | any | 32.0 | 32.1 | 31.4 | 33.1 | 0.02 | 2 | N | Univariable analysis |
Some studies reported SEP quintiles but others reported SEP in 2, 3, or 4 categories or as a continuous variable. Details of the number of SEP groups per study are given in Tables 1–4 in the column entitled “No. of SEP groups.” Quality scores range from 1 (lowest quality) to 6 (highest quality). Meta-analysis: Y, included in final meta-analysis; Y(S), included in sensitivity meta-analysis; N, not included in meta-analysis. Q1, high socioeconomic position; Q5, low socioeconomic position.
CI, confidence interval; non-UHCS, non-universal health care system; NR, not reported; OR, odds ratio; pop, population; SE, standard error; SEP, socioeconomic position.
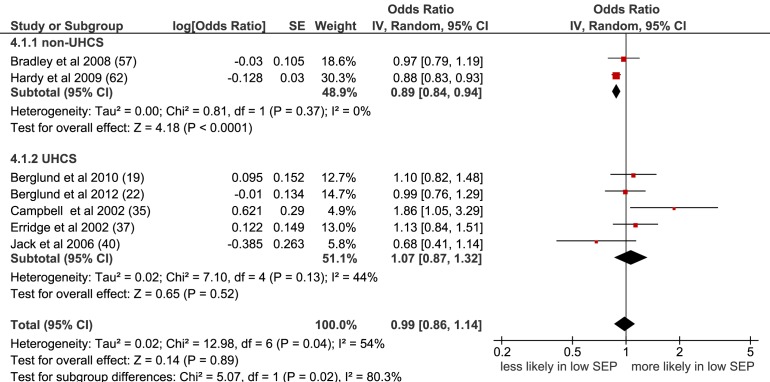
Overall, no association between SEP and receipt of radiotherapy was seen in the meta-analysis of the seven studies with non-overlapping populations selected for inclusion (OR = 0.99 [95% CI 0.86 to 1.14], p = 0.89, I2 = 54%) (Figure 4), or when all nine studies were included (OR = 0.95 [95% CI 0.85 to 1.06], p = 0.40, I2 = 71%) (Figure S4). A significant association was seen for non-UHCS studies but only two studies were included here, each looking at different stage patients.
Figure 4. Meta-analysis of odds of receipt of radiotherapy in low versus high SEP.
CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
Treatment Type not Specified
Seven papers (eight study populations) examined receipt of unspecified treatment, and three papers considered receipt of unspecified curative treatment in three populations (Tables 11–13). In the meta-analysis of five non-overlapping studies, low SEP was associated with a lower likelihood of receiving unspecified treatment (OR = 0.78 [95% CI 0.74 to 0.83], p<0.001, I2 = 0) (Figure 5). This was also seen when studies with overlapping populations were included (OR = 0.80 [95% CI 0.77 to 0.84], p<0.001, I2 = 17%) (Figure S5).
Table 11. Likelihood of receipt of any type of unspecified treatment by SEP group (universal health care systems).
| Study | No. Receiving Treatment | Cohort No./No. Eligible | Rate | Histology | OR/Rate in Q1 (95% CI) | OR/Rate in Q2 (95% CI) | OR/Rate in Q3 (95% CI) | OR/Rate in Q4 (95% CI) | OR/Rate in Q5 (95% CI) | p-Value | Quality Score | Meta-Analysis | Further Information |
| Crawford et al, 2009 [36] | 19667 | 34923 | 56.32 | any | 1.00 | 0.91 (0.86 to 0.97) | 0.82(0.77 to 0.88) | 0.79 (0.74 to 0.84) | <0.01 | 4 | Y | Individual p-values, all reported as <0.01 | |
| Erridge et al, 2009 [18] | 2186 | 3833 | 57.03 | any | 1.3 (1.1 to 1.5) | 1.00 | <0.05 | 4 | Y(S) | Scottish population | |||
| Erridge et al, 2009 [18] | 1372 | 2073 | 66.18 | any | 1.3 (1.1 to 1.7) | 1.00 | <0.05 | 4 | Y(S) | Canadian population | |||
| Jack et al, 2003 [39] | NR | 32818 | any | 0.98 (0.96 to 0.99) | 0.0091 | 4 | N | ||||||
| Jack et al, 2006 [40] | 414 | 695 | 59.57 | any | 1.00 | 0.91 (0.53 to 1.55) | 0.69 (0.40 to 1.19) | 0.57 (0.34 to 0.97) | 0.65 (0.37 to 1.13) | 0.03 | 6 | Y | Subset of Jack et al (2003) population, p for trend |
| Stevens et al, 2007 [23] | 285 | 565 | 50.44 | any | 1.0 | 0.9 (0.6 to 1.5) | 0.773 | 3 | Y(S) | Hospital population | |||
| Mahmud et al, 2003 [42] | 2678 | 4451 | 60.17 | NSCLC | 1.0 | 0.9 (0.8 to 1.1) | 1.0 (0.8 to 1.2) | 0.39, 0.958 | 4 | Y(S) | Odds of NOT receiving treatment—individual p-values reported | ||
| Mahmud et al, 2003 [42] | 694 | 1002 | 69.26 | SCLC | 1.0 | 1.0 (0.6 to 1.5) | 0.8 (0.5 to 1.3) | 0.888, 0.358 | 4 | Y(S) | Odds of NOT receiving treatment—individual p-values reported |
Some studies reported SEP quintiles but others reported SEP in 2, 3, or 4 categories or as a continuous variable. Details of the number of SEP groups per study are given in Tables 1–4 in the column entitled “No. of SEP groups.” Quality scores range from 1 (lowest quality) to 6 (highest quality). Meta-analysis: Y, included in final meta-analysis; Y(S), included in sensitivity meta-analysis; N, not included in meta-analysis. Q1, high socioeconomic position; Q5, low socioeconomic position.
CI, confidence interval; NR, not reported; OR, odds ratio; SEP, socioeconomic position; UHCS, universal health care system.
Table 13. Likelihood of receipt of any type of unspecified curative treatment by SEP group (universal health care systems).
| Study | No. Receiving Treatment | Cohort No. / No. Eligible | Rate/ Eligible Rate | Histology | OR/Rate in Q1 (95% CI) | OR/Rate in Q2 (95% CI) | OR/Rate in Q3 (95% CI) | OR/Rate in Q4 (95% CI) | OR/Rate in Q5 (95% CI) | p-Value | Quality Score | Meta-Analysis | Further Information |
| Erridge et al, 2009 [18] | 548 | 3833 | 14.30 | any | 1.1(0.9 to 1.4) | 1.00 | >0.05 | 4 | Y (S) | Scottish pop – subset of Gregor et al (2001) pop | |||
| Erridge et al, 2009 [18] | 546 | 2073 | 26.34 | any | 1.4(1.1 to 1.8) | 1.00 | <0.05 | 4 | Y | Canadian pop | |||
| Gregor et al, 2001 [38] | 627 | 3855/1423 | 16.26/44.06 | any | 1.00 | 1.14 (0.72 to 1.80) | 1.07 (0.69 to 1.66) | 0.95 (0.62 to 1.47) | 0.77 (0.51 to 1.16) | 0.25 | 6 | Y | Eligible = early stage |
| Stevens et al, 2008 [47] | 109 | 565 | 19.29 | any | 1.0 | 3.1 (1.0 to 9.7) | 1.4 (0.4 to 4.4) | 1.1 (0.4 to 0.3) | 0.6 (0.2 to 1.8) | 0.05, 0.60, 0.86, 0.40 | 5 | Y | Hospital pop - subset of Stevens et al (2007) pop, individual p-values reported |
Some studies reported SEP quintiles but others reported SEP in 2, 3, or 4 categories or as a continuous variable. Details of the number of SEP groups per study are given in Tables 1–4 in the column entitled “No. of SEP groups.” Quality scores range from 1 (lowest quality) to 6 (highest quality). Meta-analysis: Y, included in final meta-analysis; Y(S), included in sensitivity meta-analysis; N, not included in meta-analysis. Q1, high socioeconomic position, Q5, low socioeconomic position.
CI, confidence interval; NR, not reported; OR, odds ratio; pop, population; SEP, socioeconomic position; UHCS, universal health care system.
Figure 5. Meta-analysis of odds of receipt of unspecified treatment in low versus high SEP.
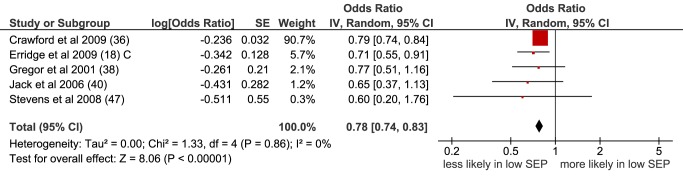
CI, confidence interval; OR, odds ratio; SE, standard error; SEP, socioeconomic position.
Table 12. Likelihood of receipt of any type of unspecified treatment by SEP group (non-universal health care systems).
| Study | No. Receiving Treatment | Cohort No./No. Eligible | Rate | Histology | OR/Rate in Q1 (95% CI) | OR/Rate in Q2 (95% CI) | OR/Rate in Q3 (95% CI) | OR/Rate in Q4 (95% CI) | OR/Rate in Q5 (95% CI) | p-Value | Quality Score | Meta-Analysis | Further Information |
| Ou et al, 2008 [70] | 18216 | 19700 | 92.47 | NSCLC | 94.7 | 94.1 | 92.2 | 91.9 | 87.2 | <0.001 | 2 | N | Stage I. Univariable analysis |
| Smith et al, 1995 [66] | 1697 | 2396 | 70.83 | NSCLC | 1.00 (0.91 to 1.11) | >0.001 | 5 | N | Distant stage | ||||
| Smith et al, 1995 [66] | 2343 | 2813 | 83.29 | NSCLC | 1.00 (0.88 to 1.13) | >0.001 | 5 | N | Local stage |
Some studies reported SEP quintiles but others reported SEP in 2, 3, or 4 categories or as a continuous variable. Details of the number of SEP groups per study are given in Tables 1–4 in the column entitled “No. of SEP groups.” Quality scores range from 1 (lowest quality) to 6 (highest quality). Meta-analysis: Y, included in final meta-analysis; Y(S), included in sensitivity meta-analysis; N, not included in meta-analysis. Q1, high socioeconomic position; Q5, low socioeconomic position.
CI, confidence interval; non-UHCS, non-universal health care system; NR, not reported; OR, odds ratio; pop, population; SE, standard error; SEP, socioeconomic position.
When the surgery, chemotherapy, and radiotherapy papers included in the separate treatment meta-analyses in this systematic review were analysed together to produce an overall summary effect meta-analysis OR, a similar result was seen, with low SEP associated with a lower likelihood of receiving any type of treatment. This was found when including only studies with non-overlapping populations (OR = 0.79 [95% CI 0.73 to 0.86], p<0.001, I2 = 77%) (Figure S6) and when including all eligible studies (OR = 0.80 [95% CI 0.75 to 0.86], p<0.001, I2 = 82%) (Figure S7).
Discussion
Principal Findings
To our knowledge, this is the first systematic review and meta-analysis examining socioeconomic inequalities in receipt of lung cancer treatment. It shows an association between low SEP and reduced likelihood of receipt of any type of treatment, surgery, and chemotherapy. The results were generally consistent across different health care systems.
Interpretation of Results
Surgery is suitable only for patients with early-stage NSCLC, and it has been suggested that patients with cancer in a lower SEP are more likely to present later and with later-stage disease [20]. This may help explain why socioeconomic inequalities in receipt of surgery are observed in some studies. However, presentation with later-stage cancer in lower SEP patients has not been consistently observed [19]. In this review, when receipt of treatment was examined in studies of early-stage patients only (from non-UHCS studies), low SEP remained associated with reduced likelihood of surgery. Thus, the association between SEP and receipt of surgery appears to be independent of stage. Similar results were seen for NSCLC studies in both health care systems.
Receipt of treatment may also be influenced by clinical suitability for treatment, and socioeconomic differences in the number of co-morbidities present may explain socioeconomic inequalities in treatment. In the three UHCS studies that took co-morbidity into account, SEP was not associated with receipt of surgery [21],[22] or of any treatment [23] when the trend across SEP groups was examined, suggesting that co-morbidity may be a potential mediator of socioeconomic inequalities in treatment in UHCSs. However, most of the non-UHCS studies did include co-morbidity as a confounder, and socioeconomic inequalities in treatment were still observed, suggesting that there may be differences between health care systems here.
Strengths and Weaknesses of the Review and of the Available Evidence
This is one of the first equity reviews published [24],[25], the first systematic review of the literature on intervention-generated inequalities in lung cancer treatment to our knowledge, and the first cancer equity review to include a meta-analysis. Extensive searches were carried out to identify studies. However, it is possible that not all relevant studies were obtained.
The included studies reported observational data only. The suitability of meta-analysis for observational studies has been questioned, as it may produce precise but spurious results [26]. Examining the possible sources of heterogeneity by conducting sensitivity analyses across different sub-groups may be less prone to bias than calculating an overall summary effect [26]. Here, although an overall summary effect OR was calculated, heterogeneity was taken into account. Separate analyses by type of treatment were carried out, with further stratification by stage and histology. Universal and non-UHCSs were examined separately and random effects rather than fixed effects meta-analyses were conducted. These precautions did not change the overall pattern of results seen.
Significant heterogeneity remained in some cases, which could be considered a limitation, although this is not surprising because of the characteristics of the studies included. For studies examining receipt of chemotherapy and radiotherapy it was generally not possible to differentiate between curative and palliative treatment and, if patterns of care differ for these by SEP, this might explain the high degree of heterogeneity seen. However, although there is some suggestion that heterogeneity can be considered high at >50% [17], when confidence intervals were calculated these were wide, so it was difficult to be confident about the degree of heterogeneity present [27].
Results for receipt of radiotherapy differed in the non-UHCS sub-group compared to overall but, as only two studies were included in this sub-group, it is difficult to be sure that different patterns of receipt of radiotherapy by SEP are due to differences in health care system.
Many of the non-UHCS studies used overlapping population sub-groups from the SEER database. There was also population overlap between some UHCS datasets. We attempted to include only substantially non-overlapping datasets within the final meta-analyses to ensure independence of results. A judgement had to be made as to which was the best-quality and most appropriate paper to include, but sensitivity analyses using different inclusion combinations (Figure S8) did not change the overall findings, nor did including all suitable studies regardless of population overlap (Figures S1, S3, S4, S5, S7).
Included papers contained data for patients diagnosed between 1978 and 2008. As treatment guidance has changed over time, older studies may be less applicable to current clinical practice. However, the majority of included studies were published within the last five years, and sensitivity analyses excluding studies published prior to 2000 did not change the overall findings.
Various measures of SEP were used, and these were categorised differently—an acknowledged problem in equity reviews [28]. All but one study measured SEP at the area level. This is a further limitation, as area-based measures of SEP are unlikely to be accurate markers of individual-level circumstances and access to resources [29]. Area-based measures of SEP can be calculated using address, making them easy to add to disease registers, such as those used in many of the studies synthesised here. However, the reliance on area-based markers of SEP may underestimate the strength of the true association between SEP and receipt of treatment.
Not all studies reported details of stage and histology—both of which influence treatment type—and very few UHCS studies took co-morbidity into account. Thus, the ORs used in the meta-analyses were not consistently adjusted for the same covariates. However, we attempted to take these factors into account in the quality scores and by conducting subgroup sensitivity analyses. Examining only high-quality studies did not alter our findings nor did sensitivity analyses, although consequent reduction in numbers did result in loss of significance in some analyses, potentially due to lack of power to detect differences.
In order to conduct meta-analysis it is necessary to compare the odds of treatment in the lowest-SEP group with the odds in the highest, which simplifies what may be a complex relationship across SEP groups. However, studies that reported a change in odds ratios across the SEP categories, and thus explored trends in receipt of treatment, generally supported the overall findings of the review.
A number of existing tools suitable for assessing cohort study quality were considered [15],[16]. However, none of these tools was entirely appropriate for the type of studies included and, as has been done in previous reviews [13],[30], we devised a unique tool, adapting and utilising aspects of other available tools. This approach has the benefit of producing a quality tool that is highly specific for the type of studies examined.
As with any systematic review, we are unable to exclude the possibility of publication bias. Studies reporting null findings are less likely to be published or, if they are published, not to report numerical outcomes [17]. A funnel plot to assess potential publication bias did not show obvious bias (Figure S9). However, a number of papers recovered in the search included SEP in the description of the study population but did not report receipt of treatment by SEP [31]–[34]. Study authors were contacted and asked to provide further information, but only one supplied the requested data [34]. It is likely that SEP was not significantly associated with receipt of treatment in the other studies, but this was not always clearly reported. However, publication bias is thought to be less important than other sources of bias, such as confounding, in meta-analyses of observational studies [26].
Implications for Policy and Practice
Socioeconomic inequalities in receipt of treatment may exacerbate socioeconomic inequalities in incidence of lung cancer, which is strongly associated with higher smoking rates in more deprived populations, so may further contribute to the poorer outcomes in lower SEP groups.
Socioeconomic inequalities in treatment may be due to differences in access to care. Within a non-UHCS it might be expected that socioeconomic differences in receipt of treatment would be observed due to income-related differences in health insurance status. Patients with lung cancer in the USA who do not have insurance have been shown to have more limited access to care [13]. However, as socioeconomic inequalities in receipt of lung cancer treatment were also observed in UHCSs that do not depend on ability to pay and in non-UHCS studies where insurance type was taken into account, this would suggest that other system factors may be contributing to this inequality. The extent to which receipt of treatment is influenced by factors such as patient choice is not known.
Variability at the patient, tumour, system, and individual clinician levels needs to be investigated before clear recommendations for changes to policy and practice can be made.
Future Research
This review has demonstrated a clear association between lower SEP and reduced likelihood of receiving surgery, chemotherapy, and any type of unspecified treatment for lung cancer. The reasons for these inequalities need to be more thoroughly investigated. Better-quality UHCS studies, including statistical control for co-morbidity and stratification by stage and histology—so that only those patients eligible for a particular treatment are included in the population-denominator—are required. It would also be useful to be able to distinguish between curative and palliative intent of treatment. In non-UHCS, studies in younger populations, examining a range of insurance providers, are required.
Further investigation into the system and patient factors that might contribute to socioeconomic inequalities in receipt of lung cancer care is necessary, to help develop interventions that ensure equitable receipt of appropriate treatment. This should include a quantitative exploration of inequalities at each stage of the care pathway as well as qualitative work exploring reasons for inequality. Inequalities in receipt of treatment may contribute to inequalities in cancer survival and so cohort survival analyses are warranted in order to investigate intervention-generated inequalities in lung cancer outcomes.
Supporting Information
Meta-analysis of odds of receipt of surgery in low versus high SEP (overlapping populations). CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
(TIF)
Meta-analysis of odds of receipt of surgery for NSCLC in low versus high SEP (non-overlapping populations). CI, confidence interval; OR, odds ratio; SE, standard error; SEP, socioeconomic position.
(TIF)
Meta-analysis of odds of receipt of chemotherapy in low versus high SEP (overlapping populations). CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
(TIF)
Meta-analysis of odds of receipt of radiotherapy in low versus high SEP (overlapping populations). CI = confidence interval, non-UHCS = non-universal health care system, OR = odds ratio, SE = standard error, SEP = socioeconomic position UHCS = universal health care system.
(TIF)
Sensitivity meta-analysis of odds of receipt unspecified treatment in low versus high SEP (overlapping populations). CI, confidence interval; OR, odds ratio; SE, standard error; SEP, socioeconomic position.
(TIF)
Meta-analysis of odds of receipt of any type of treatment in low versus high SEP. CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
(TIF)
Meta-analysis of odds of receipt of any type of treatment in low versus high SEP (overlapping populations). CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
(TIF)
Meta-analysis of odds of receipt of surgery in low versus high SEP (partially-overlapping populations). CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
(TIF)
Funnel plot to assess publication bias. CI, confidence interval; non-UHCS, non-universal health care system; NSCLC, non-small cell lung cancer; UHCS, universal health care system.
(TIF)
Full search strategies (MEDLINE and EMBASE).
(DOC)
PRISMA checklist.
(DOC)
Protocol.
(DOC)
Quality score checklist.
(DOC)
Abbreviations
- CI
confidence interval
- NSCLC
non-small cell lung cancer
- OR
odds ratio
- SCLC
small cell lung cancer
- SEER
National Cancer Institute's Surveillance, Epidemiology and End Results
- SEP
socioeconomic position
- UHCS
universal health care system
Funding Statement
LF (ESRC studentship ES/I020926/1) and HW are PhD students funded by ESRC as members of Fuse, the Centre for Translational Research in Public Health (www.fuse.ac.uk). JA, MW, and GR are funded in part as a staff member (JA), director (MW), and senior investigator (GR) of Fuse. Fuse is a UK Clinical Research Collaboration (UKCRC) Public Health Research Centre of Excellence. Funding for Fuse from the British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. The views expressed in this paper do not necessarily represent those of the funders or UKCRC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cancer Research UK Cancer incidence: UK statistics. Available: http://info.cancerresearchuk.org/cancerstats/incidence/. Accessed 09 January 2013.
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, et al .. (eds). SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations), National Cancer Institute. Bethesda, MD. Based on November 2011 SEER data submission, posted to the SEER web site, April 2012. Available: http://seer.cancer.gov/csr/1975_2009_pops09/. Accessed 09 January 2012.
- 3.Cancer Research UK Common cancers - UK mortality statistics. Available: http://info.cancerresearchuk.org/cancerstats/mortality/cancerdeaths/. Accessed 09 January 2013.
- 4. Coleman MP, Forman D, Bryant H, Butler J, Rachet B, et al. (2011) Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 377: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, et al. (2007) Recent cancer survival in Europe: a 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol 8: 784–796. [DOI] [PubMed] [Google Scholar]
- 6.NICE (2005) Clinical Guideline 24. Lung Cancer: the diagnosis and treatment of lung cancer. London: NICE.
- 7. Rachet B, Ellis L, Maringe C, Chu T, Nur U, et al. (2010) Socioeconomic inequalities in cancer survival in England after the NHS cancer plan. Br J Cancer 103: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aarts MJ, Lemmens VEPP, Louwman MWJ, Kunst AE, Coebergh JWW (2010) Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. Eur J Cancer 46: 2681–2695. [DOI] [PubMed] [Google Scholar]
- 9. Woods LM, Rachet B, Coleman MP (2006) Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol 17: 5–19. [DOI] [PubMed] [Google Scholar]
- 10.White M, Adams J, Heywood P (2009) How and why do interventions that increase health overall widen inequalities within populations? In: Babones S, editor. Health, inequality and society. Bristol: Policy Press.
- 11. Sidorchuk A, Agardh E, Aremu O, Hallqvist J, Allebeck P, et al. (2009) Socioeconomic differences in lung cancer incidence: a systematic review and meta-analysis. Cancer Causes Control 20: 459–471. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6 7: e1000097 doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slatore CG, Au DH, Gould MK (2010) on behalf of the American Thoracic Society Disparities in Healthcare G (2010) An Official American Thoracic Society Systematic Review: Insurance Status and Disparities in Lung Cancer Practices and Outcomes. Am J Respir Crit Care Med 182: 1195–1205. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson C, Gerard K (2005) Health care financing reforms: moving into the new millenium. Economics of Health Care Financing: The Visible Hand. Basingstoke: Palgrave Macmillan.
- 15.SIGN (2011) SIGN 50: A Guideline Developer's Handbook. Methodology Checklist 3: Cohort studies. Edinburgh: SIGN.
- 16. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, et al. (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med 4 10: e297 doi:10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Cochrane Collaboration (2008) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2. Higgins J, Green S, editors.
- 18. Erridge SC, Murray B, Williams L, Brewster D, Black R, et al. (2009) Improved survival from lung cancer in British Columbia compared to Scotland-Are different treatment rates the whole story? Lung Cancer 64: 358–366. [DOI] [PubMed] [Google Scholar]
- 19. Berglund A, Holmberg L, Tishelman C, Wagenius G, Eaker S, et al. (2010) Social inequalities in non-small cell lung cancer management and survival: a population-based study in central Sweden. Thorax 65: 327–333. [DOI] [PubMed] [Google Scholar]
- 20. Dalton SO, Frederiksen BL, Jacobsen E, Steding-Jessen M, Osterlind K, et al. (2011) Socioeconomic position, stage of lung cancer and time between referral and diagnosis in Denmark, 2001–2008. Br J Cancer 105: 1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rich AL, Tata LJ, Free CM, Stanley RA, Peake MD, et al. (2011) Inequalities in outcomes for non-small cell lung cancer: the influence of clinical characteristics and features of the local lung cancer service. Thorax 66: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 22. Berglund A, Lambe M, Lüchtenborg M, Linklater K, Peake MD, et al. (2012) Social differences in lung cancer management and survival in South East England: a cohort study. BMJ Open 2 doi:10.1136/bmjopen-2012-001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stevens W, Stevens G, Kolbe J, Cox B (2007) Lung cancer in New Zealand: Patterns of secondary care and implications for survival. J Thorac Oncol 2: 481–493. [DOI] [PubMed] [Google Scholar]
- 24. Welch V, Petticrew M, Ueffing E, Benkhalti Jandu M, Brand K, et al. (2012) Does Consideration and Assessment of Effects on Health Equity Affect the Conclusions of Systematic Reviews? A Methodology Study. PLoS ONE 7 3: e31360 doi:10.1371/journal.pone.0031360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas S, Fayter D, Misso K, Ogilvie D, Petticrew M, et al. (2008) Population tobacco control interventions and their effects on social inequalities in smoking: systematic review. Tob Control 17: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Egger M, Schneider M, Smith GD (1998) Meta-analysis Spurious precision? Meta-analysis of observational studies. BMJ 316: 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ioannidis J, Patsopoulos N, Evangelou E (2007) Uncertainty in heterogeneity estimates in meta-analyses. BMJ 335: 914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ogilvie D, Fayter D, Petticrew M, Sowden A, Thomas S, et al. (2008) The harvest plot: A method for synthesising evidence about the differential effects of interventions. BMC Med Res Methodol 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adams J, Ryan V, White M (2005) How accurate are Townsend Deprivation Scores as predictors of self-reported health? A comparison with individual level data. J Public Health 27: 101–106. [DOI] [PubMed] [Google Scholar]
- 30. Olsson JK, Schultz EM, Gould MK (2009) Timeliness of care in patients with lung cancer: a systematic review. Thorax 64: 749–756. [DOI] [PubMed] [Google Scholar]
- 31. Grose D, Devereux G, Brown L, Jones R, Sharma D, et al. (2011) Variation in comorbidity and clinical management in patients newly diagnosed with lung cancer in four Scottish centers. J Thorac Oncol 6: 500–509. [DOI] [PubMed] [Google Scholar]
- 32. Hall SE, Bulsara CE, Bulsara MK, Leahy TG, Culbong MR, et al. (2004) Treatment patterns for cancer in Western Australia: Does being indigenous make a difference? Med J Aust 181: 191–194. [DOI] [PubMed] [Google Scholar]
- 33. McCann J, Artinian V, Duhaime L, Lewis JW, Kvale PA, et al. (2005) Evaluation of the Causes for Racial Disparity in Surgical Treatment of Early Stage Lung Cancer. Chest 128: 3440–3446. [DOI] [PubMed] [Google Scholar]
- 34. Riaz SP, Lüchtenborg M, Jack RH, Coupland VH, Linklater KM, et al. (2012) Variation in surgical resection for lung cancer in relation to survival: Population-based study in England 2004–2006. Eur J Cancer 48: 54–60. [DOI] [PubMed] [Google Scholar]
- 35. Campbell NC, Elliott AM, Sharp L, Ritchie LD, Cassidy J, et al. (2002) Impact of deprivation and rural residence on treatment of colorectal and lung cancer. Br J Cancer 87: 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crawford SM, Sauerzapf V, Haynes R, Zhao H, Forman D, et al. (2009) Social and geographical factors affecting access to treatment of lung cancer. Br J Cancer 101: 897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erridge SC, Thomson CS, Davidson J, Jones RD, Price A (2002) Factors Influencing the Use of Thoracic Radiotherapy in Lung Cancer;an Analysis of the 1995 Scottish Lung Cancer Audit. Clin Oncol 14: 219–227. [DOI] [PubMed] [Google Scholar]
- 38. Gregor A, Thomson CS, Brewster DH, Stroner PL, Davidson J, et al. (2001) Management and survival of patients with lung cancer in Scotland diagnosed in 1995: results of a national population based study. Thorax 56: 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jack RH, Gulliford MC, Ferguson J, Moller H (2003) Geographical inequalities in lung cancer management and survival in South East England: evidence of variation in access to oncology services? Br J Cancer 88: 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jack RH, Gulliford MC, Ferguson J, Møller H (2006) Explaining inequalities in access to treatment in lung cancer. J Eval Clin Pract 12: 573–582. [DOI] [PubMed] [Google Scholar]
- 41. Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, et al. (2008) Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer 44: 992–999. [DOI] [PubMed] [Google Scholar]
- 42. Mahmud SM, Reilly M, Comber H (2003) Patterns of initial management of lung cancer in the Republic of Ireland: a population-based observational study. Lung Cancer 41: 57–64. [DOI] [PubMed] [Google Scholar]
- 43. McMahon M, Barbiere JM, Greenberg DC, Wright KA, Lyratzopoulos G (2011) Population-based trends in use of surgery for non-small cell lung cancer in a UK region, 1995–2006. Thorax 66: 453–455. [DOI] [PubMed] [Google Scholar]
- 44. Pollock AM, Vickers N (1998) Deprivation and emergency admissions for cancers of colorectum, lung, and breast in south east England: ecological study. BMJ 317: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raine R, Wong W, Scholes S, Ashton C, Obichere A, et al. (2010) Social variations in access to hospital care for patients with colorectal, breast, and lung cancer between 1999 and 2006: retrospective analysis of hospital episode statistics. BMJ 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rich AL, Tata LJ, Stanley RA, Free CM, Peake MD, et al. (2011) Lung cancer in England: Information from the National Lung Cancer Audit (LUCADA). Lung Cancer 72: 16–22. [DOI] [PubMed] [Google Scholar]
- 47. Stevens W, Stevens G, Kolbe J, Cox B (2008) Ethnic differences in the management of lung cancer in New Zealand. J Thorac Oncol 3 3: 237–244. [DOI] [PubMed] [Google Scholar]
- 48. Battersby J, Flowers J, Harvey I (2004) An alternative approach to quantifying and addressing inequity in healthcare provision: access to surgery for lung cancer in the east of England. J Epidemiol Community Health 58: 623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bendzsak A, Nenshi R, Darling G, Schultz SE, Gunraj N, et al. (2011) Overview of Lung Cancer Surgery in Ontario. Ann Thorac Surg 91: 361–366. [DOI] [PubMed] [Google Scholar]
- 50. Cartman ML, Hatfield AC, Muers MF, Peake MD, Haward RA, et al. (2002) Lung cancer: district active treatment rates affect survival. J Epidemiol Community Health 56: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hui AC, Vinod SK, Jalaludin BB, Yuile P, Delaney GP, et al. (2005) Socio-economic status and patterns of care in lung cancer. Aust N Z J Public Health 29: 372–377. [DOI] [PubMed] [Google Scholar]
- 52. Madelaine J, Guizard AV, Lefevre H, Lecarpentier MM, Launoy G (2002) [Diagnosis, treatment, and prognosis of lung cancer in the Manche (France) (1997–1999) according to patients socioeconomic characteristics]. Revue d Epidemiologie et de Sante Publique 50: 383–392. [PubMed] [Google Scholar]
- 53. Pagano E, Filippini C, Di Cuonzo D, Ruffini E, Zanetti R, et al. (2010) Factors affecting pattern of care and survival in a population-based cohort of non-small-cell lung cancer incident cases. Cancer Epidemiol 34: 483–489. [DOI] [PubMed] [Google Scholar]
- 54. Patel N, Adatia R, Mellemgaard A, Jack R, Moller H (2007) Variation in the use of chemotherapy in lung cancer. Br J Cancer 96: 886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stevens G, Stevens W, Purchuri S, Kolbe J, Cox B (2009) Radiotherapy utilisation in lung cancer in New Zealand: Disparities with optimal rates explained. N Z Med J 122 1306: 43–54. [PubMed] [Google Scholar]
- 56. Younis T, Al-Fayea T, Virik K, Morzycki W, Saint-Jacques N (2008) Adjuvant chemotherapy uptake in non-small cell lung cancer. J Thorac Oncol 3: 1272–1278. [DOI] [PubMed] [Google Scholar]
- 57. Bradley CJ, Dahman B, Given CW (2008) Treatment and Survival Differences in Older Medicare Patients With Lung Cancer as Compared With Those Who Are Dually Eligible for Medicare and Medicaid. J Clin Oncol 26: 5067–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Davidoff AJ, Tang M, Seal B, Edelman MJ (2010) Chemotherapy and Survival Benefit in Elderly Patients With Advanced Non–Small-Cell Lung Cancer. J Clin Oncol 28: 2191–2197. [DOI] [PubMed] [Google Scholar]
- 59. Earle CC, Venditti LN, Neumann PJ, Gelber RD, Weinstein MC, et al. (2000) Who Gets Chemotherapy for Metastatic Lung Cancer?. Chest 117: 1239–1246. [DOI] [PubMed] [Google Scholar]
- 60. Esnaola NF, Gebregziabher M, Knott K, Finney C, Silvestri GA, et al. (2008) Underuse of Surgical Resection for Localized, Non-Small Cell Lung Cancer Among Whites and African Americans in South Carolina. Ann Thorac Surg 86: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Greenwald HP, Polissar NL, Borgatta EF, McCorkle R, Goodman G (1998) Social Factors, Treatment, and Survival in Early-Stage Non-Small Cell Lung Cancer. Am J Public Health 88: 1681–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hardy D, Liu C-C, Xia R, Cormier JN, Chan W, et al. (2009) Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer 115: 2199–2211. [DOI] [PubMed] [Google Scholar]
- 63. Hayman JA, Abrahamse PH, Lakhani I, Earle CC, Katz SJ (2007) Use of Palliative Radiotherapy Among Patients With Metastatic Non-Small-Cell Lung Cancer. Int J Radiation Oncology Biol Phys 69: 1001–1007. [DOI] [PubMed] [Google Scholar]
- 64. Lathan CS, Neville BA, Earle CC (2008) Racial composition of hospitals: Effects on surgery for early-stage non-small-cell lung cancer. J Clin Oncol 26 26: 4347–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Polednak AP (2001) Disaparities in surgical treatment of early-stage non-small-cell lung cancer. Yale J Biol Med 74: 309–314. [PMC free article] [PubMed] [Google Scholar]
- 66. Smith TJ, Penberthy L, Desch CE, Whittemore M, Newschaffer C, et al. (1995) Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer 13: 235–252. [DOI] [PubMed] [Google Scholar]
- 67. Bach PB, Cramer LD, Warren JL, Begg CB (1999) Racial differences in the treatment of early-stage lung cancer. N Engl J Med 341: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 68. Earle CC, Neumann PJ, Gelber RD, Weinstein MC, Weeks JC (2002) Impact of Referral Patterns on the Use of Chemotherapy for Lung Cancer. J Clin Oncol 20: 1786–1792. [DOI] [PubMed] [Google Scholar]
- 69. Lathan CS, Neville BA, Earle CC (2006) The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol 24: 413–418. [DOI] [PubMed] [Google Scholar]
- 70. Ou SHI, Zell JA, Ziogas A, Anton-Culver H (2008) Low socioeconomic status is a poor prognostic factor for survival in stage I nonsmall cell lung cancer and is independent of surgical treatment, race, and marital status. Cancer 112: 2011–2020. [DOI] [PubMed] [Google Scholar]
- 71. Suga JM, Nguyen DV, Mohammed SM, Brown M, Calhoun R, et al. (2010) Racial disparities on the use of invasive and noninvasive staging in patients with non-small cell lung cancer. J Thorac Oncol 5: 1772–1778. [DOI] [PubMed] [Google Scholar]
- 72. Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P (2004) In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidemiol 57: 597–609. [DOI] [PubMed] [Google Scholar]
- 73. Wang J, Kuo Y, Freeman J, Goodwin J (2008) Increasing access to medical oncology consultation in older patients with stage II-IIIA non-small-cell lung cancer. Med Oncol 25: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang R, Cheung MC, Byrne MM, Huang Y, Nguyen D, et al. (2010) Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer 116: 2437–2447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Meta-analysis of odds of receipt of surgery in low versus high SEP (overlapping populations). CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
(TIF)
Meta-analysis of odds of receipt of surgery for NSCLC in low versus high SEP (non-overlapping populations). CI, confidence interval; OR, odds ratio; SE, standard error; SEP, socioeconomic position.
(TIF)
Meta-analysis of odds of receipt of chemotherapy in low versus high SEP (overlapping populations). CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
(TIF)
Meta-analysis of odds of receipt of radiotherapy in low versus high SEP (overlapping populations). CI = confidence interval, non-UHCS = non-universal health care system, OR = odds ratio, SE = standard error, SEP = socioeconomic position UHCS = universal health care system.
(TIF)
Sensitivity meta-analysis of odds of receipt unspecified treatment in low versus high SEP (overlapping populations). CI, confidence interval; OR, odds ratio; SE, standard error; SEP, socioeconomic position.
(TIF)
Meta-analysis of odds of receipt of any type of treatment in low versus high SEP. CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
(TIF)
Meta-analysis of odds of receipt of any type of treatment in low versus high SEP (overlapping populations). CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
(TIF)
Meta-analysis of odds of receipt of surgery in low versus high SEP (partially-overlapping populations). CI, confidence interval; non-UHCS, non-universal health care system; OR, odds ratio; SE, standard error; SEP, socioeconomic position; UHCS, universal health care system.
(TIF)
Funnel plot to assess publication bias. CI, confidence interval; non-UHCS, non-universal health care system; NSCLC, non-small cell lung cancer; UHCS, universal health care system.
(TIF)
Full search strategies (MEDLINE and EMBASE).
(DOC)
PRISMA checklist.
(DOC)
Protocol.
(DOC)
Quality score checklist.
(DOC)