Abstract
Introduction
Co-infection with Hepatitis C (HCV) and HIV is common and HIV accelerates hepatic disease progression due to HCV. However, access to HCV treatment is limited and success rates are generally poor.
Methods
We conducted a systematic review and meta-analysis to assess HCV treatment outcomes in observational cohorts. Two databases (Medline and EMBASE) were searched using a compound search strategy for cohort studies reporting HCV treatment outcomes (as determined by a sustained virological response, SVR) in HIV-positive patients initiating HCV treatment for the first time.
Results
40 studies were included for review, providing outcomes on 5339 patients from 17 countries. The pooled proportion of patients achieving SVR was 38%. Significantly poorer outcomes were observed for patients infected with HCV genotypes 1 or 4 (pooled SVR 24.5%), compared to genotypes 2 or 3 (pooled SVR 59.8%). The pooled proportion of patients who discontinued treatment due to drug toxicities (reported by 33 studies) was low, at 4.3% (3.3–5.3%). Defaulting from treatment, reported by 33 studies, was also low (5.1%, 3.5–6.6%), as was on-treatment mortality (35 studies, 0.1% (0–0.2%)).
Conclusions
These results, reported under programmatic conditions, are comparable to those reported in randomised clinical trials, and show that although HCV treatment outcomes are generally poor in HIV co-infected patients, those infected with HCV genotypes 2 or 3 have outcomes comparable to HIV-negative patients.
Introduction
Co-infection with Hepatitis C (HCV) and HIV is common, and HIV accelerates hepatic disease progression due to HCV [1]. As a result, HCV has become a leading cause of death of people living with HIV in Western settings [2]. Successful treatment of HCV can improve hepatic fibrosis, reduce incidence of hepatocellular carcinoma, reduce mortality [3], [4], and has the potential to reduce disease transmission [5]. However, a number of factors contribute to the limited access to treatment for most of those infected globally: a long duration of therapy with a relatively complex system of treatment delivery, high drug costs, high toxicity of treatment and, perhaps most importantly, relatively poor success rates for HCV treatment in HIV/HCV co-infection.
A recent systematic review of clinical trials assessing HCV treatment outcomes in HIV co-infected patients reported that around 37% of patients achieve a sustained virological response (SVR) with pegylated interferon and ribavarin, with a lower success rate observed in patients infected with HCV genotypes 1 and 4 [6]. These outcomes are poorer than those seen in HIV negative patients [7]. Although clinical trials are appropriate for determining drug efficacy, outcomes may differ under programmatic conditions where adherence to treatment, patient and provider motivation and available resources may be limited [8]. We conducted a systematic review to assess the outcomes of HCV treatment in HIV co-infected patients in programmatic settings.
Methods
Search Strategy and Study Selection
Our systematic review was conducted in accordance with the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses group [9]. Using a pre-defined protocol (File S1) Medline and EMBASE were systematically searched from inception to 05 May, 2012 using a compound search strategy. The initial title screen was conducted by one of us (AD) with full text articles reviewed in duplicate (AD, NF). The bibliographies of relevant articles were also hand searched for potentially relevant articles. Agreement on inclusion of final articles was made through consensus by the same reviewers. No language or geographical restriction was applied during the search, but only English language publications were included in the final review.
All cohort studies that reported treatment outcomes for in HIV-positive patients chronically infected with HCV and initiating HCV treatment for the first time were reviewed. Studies were excluded if they reported outcomes among patients with selected co-morbidities other than HIV, such as haemophilic or transplant patients, and if treatment outcomes involved acute HCV infection. Randomised trials were excluded in keeping with the aim of assessing outcomes in programmatic settings (defined as cohort reports in health care settings where there was no randomisation or control group comparison). In cases of potential duplication of studies, the largest report covering the longest time period was included and authors were contacted for clarification.
Patient and study characteristics were extracted in duplicate (AD, KS), with third party arbitration in case of disagreement (NF). The primary outcome was the proportion of patients achieving a SVR, calculated on an ‘intent-to-treat’ basis with all patients starting treatment contributing to the denominator. Secondary outcomes included the proportion of patients achieving a rapid virological response (RVR), defined as an undetectable (<50 copies/mL) serum level of HCV RNA at week 4 of treatment; discontinuation of treatment due to adverse drug reactions; loss to care (default); and death.
Data Analysis
Point estimates and 95% confidence intervals (95% CI) were calculated for all primary and secondary outcomes. The variance of raw proportions was stabilised using a Freeman-Tukey type arcsine square-root transformation [10] and proportions were then pooled using a DerSimonian and Laird random effects model [11]. We calculated the τ2 statistic using DerSimonian and Laird’s method of moments estimator [11] to assess between-study heterogeneity [12]. Sources of heterogeneity were explored through univariate subgroup analyses to assess the potential influence of baseline liver damage, genotype, type of HCV treatment and co-treatment with highly-active antiretroviral therapy (HAART). All analyses were conducted using Stata version 12 (StataCorp LP, College Station, Texas, USA), with a P-value ≤0.05 considered as significant.
Results
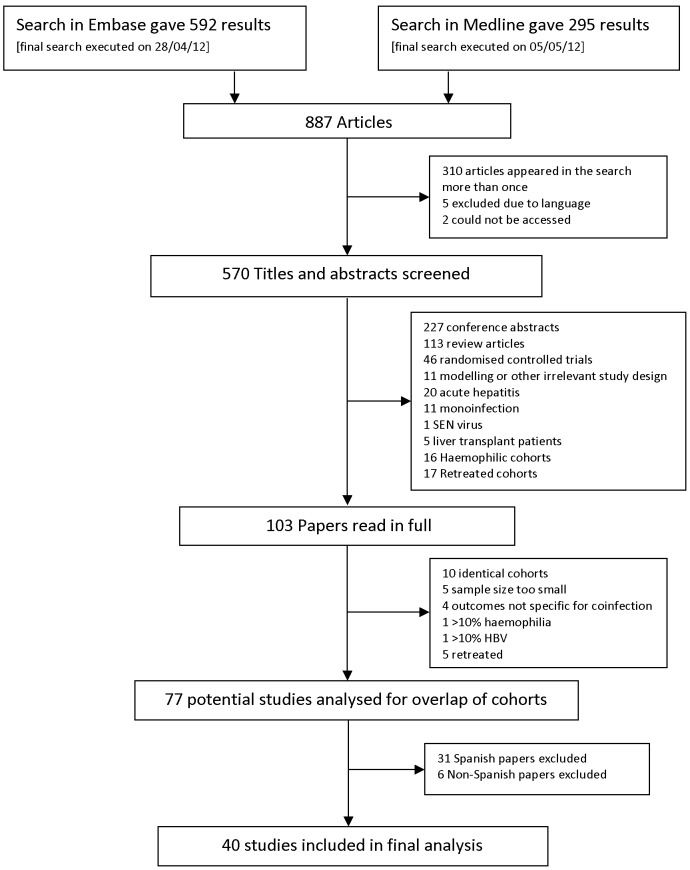
887 articles were screened, and 103 of these were reviewed in full (figure 1). After identification of further papers which did not meet the inclusion criteria (e.g. studies that included retreated patients or studies that did not report treatment outcomes in full), we retained 77 studies for detailed review. Over half of these studies (37) were from Spain, and after correspondence with authors, 37 studies were excluded as partial or complete duplicate cohorts [13]–[52]. The final analysis included data on 5339 patients from 40 studies in 17 countries (Table 1).
Figure 1. Identification of studies for inclusion.
Table 1. Characteristics of included studies.
| Study | Study Characteristics | Patient Characteristics | ||||||||||
| Study design | Study setting | Sample size | Age | Risk factor for HCV acquisition | Genotype | Advanced liver damage at baseline | CD4 count at baseline (cells/µL) | Concurrent HAART | HCV treatment: pegylated (PEG) or standard (STD) interferon (IFN) | HCV treatment: fixed-dose (FD) or weight-based (WB) Ribavarin (RBV) | Duration of HCV treatment | |
| Aguilar et al 2008 | Prospective cohort | Italy | 52 | 40 (37–42) Median (IQR) | NS | 1/4∶55.8%; 2/3∶44.2% | NS | 491 (411–620) Median (IQR) | 21.2% | PEG-IFN | WB RBV | All 48 weeks |
| Amorosa et al 2010 | Retrospective cohort | USA | 212 | 48 (43–52) Median (IQR) | 139 IVDU; 22 MSM; 87 WSM; 11 cocaine; 8 transfusion; 15 other | 1/4∶87.3%; 2/3∶13.6% | 32.5% | 487 (355–675) Mean (IQR) | 100% | PEG-IFN | WB RBV | All 48 weeks |
| Araujo et al 2011 | Prospective cohort | Brazil | 26 | 41 (32–56) Mean (range) | NS | 1/4∶57.7%; 2/3∶42.3% | 15.4% | 570 (327–956) Mean (range) | 69.2% | PEG-IFN | WB RBV | All 48 weeks |
| Avidan et al 2009 | Prospective cohort | USA Spain and Austria | 32 | NS | 14 IVDU; 18 MSM; 2 WSM | 1/4∶82.1%; 2/3∶17.9% | 17.9% | NS | 84.4% | PEG-IFN | WB RBV | All 48 weeks |
| Berenguer et al 2011 | Retrospective cohort | Spain | 1701 | 41 (37–44) Median (IQR) | 1382 IVDU; 75 excessive alcohol consumption | 1/4∶62.9%; 2/3∶34.3% | 38.2% | 514 (390–720) Median (IQR) | 88.3% | PEG-IFN | WB RBV | NS |
| Burbelo et al 2010 | Prospective cohort | USA | 29 | NS | 13 IVDU; 19 MSM | 1/4∶82.8%; 2/3∶17.2% | NS | All <100 | NS | PEG-IFN | WB RBV | All 48 weeks |
| Cesari et al 2009 | Retrospective cohort | Italy | 96 | 43 (41–46) Median (IQR) | NS | 1/4∶50%; 2/3∶50% | 17.7% | 556 (422–722) Median (IQR) | 90.6% | PEG-IFN | WB RBV | All 48 weeks |
| Cooper et al 2010 | Retrospective cohort | Canada | 41 | NS | NS | 1/4∶72.5%; 2/3∶27.5% | NS | 549 (±274); Mean (SD) | 82.9% | ‘All formulations of IFN and RBV included’ | NS | NS |
| Fleming et al 2005 | Retrospective cohort | USA | 21 | NS | NS | 1/4∶61.9%; 2/3∶38.1% | NS | NS | NS | NS | NS | NS |
| Gonvers et al 2010 | Prospective cohort | Switzerland | 47 | NS | 40 IVDU | 1/4∶48.9%; 2/3∶51.1% | 43.5% | NS | 76.6% | PEG-IFN | WB RBV | Gen 1/4 = 48 weeks; Gen 2/3 = 24 weeks |
| James et al 2012 | Retrospective cohort | Canada | 21 | 46.6 Mean | 9 IVDU; 15 MSM; 5 WSM; 9 blood products; 5 prisoners | 1/4∶52.4%; 2/3∶47.6% | 47.1% | 556 Mean | 71.4% | PEG-IFN | WB RBV | Gen 1 = 48 weeks; Gen 2/3 = 24, 32, 36 or 48 weeks according to viral response |
| Karlstrom et al 2008 | Prospective cohort | Sweden | 13 | 51 (38–62) Mean (range) | NS | 2/3∶100% | NS | 430 (250–800) Median (range) | 76.9% | PEG-IFN | WB RBV | All 24 weeks |
| Kieran et al 2011 | Retrospective cohort | Ireland | 107 | 40 (23–58) Median (range) | 67 IVDUs; 20 blood products; 14 sexual | 1/4∶51.4%; 2/3∶48.6% | 13.2% | 5 patients <200 | 71.9% | PEG-IFN | WB RBV | NS |
| Laufer et al 2011 | Prospective cohort | Argentina | 20 | 40.5 (±4.8) Mean (SD) | NS | 1/4∶100% | 50% | All >200; 521 (±218) Mean (SD) | 90% | PEG-IFN | WB RBV | All 48 weeks |
| Lerias de Almeida et al 2010 | Retrospective cohort | Brazil | 59 | 42 (±9) Mean (SD) | NS | NS | 67.3% | 432 Mean | NS | PEG-IFN | WB RBV | All 48 weeks |
| Lopez-Cortes et al 2012 | Prospective cohort | Spain | 58 | 44 (27–57) Median (range) | 47 IVDU | 2/3∶100% | 42.9% | 395 (92–1500) Median (range) | 87.9% | PEG-IFN | FD RBV | Continue 20 weeks after undetectable serum RNA-HCV |
| Macias et al 2010 | Prospective cohort | Spain | 97 | 42 (40–45) Median (range) | 87 IVDU | 1/4∶68.0%; 2/3∶32% | 78.5% | NS | 95.9% | PEG-IFN | WB RBV | Gen 1 or 4 = 48 or 72 weeks; Gen 2 or 3 = 24 or 48 weeks |
| Marchetti et al 2012 | Retrospective cohort | Italy | 98 | 44 (41–46) Median (IQR) | 87 IVDU; 3 MSM; 8 WSM | 1/4∶45.9%; 2/3∶54.1% | 74.5% | 430 (321.5–567); Median (IQR) | 98% | PEG-IFN plus RBV | WB RBV | 48 or 72 weeks, ‘according to genotype’ |
| Maru et al 2008 | Retrospective cohort | USA | 19 | NS | 19 prisoners | 1/4∶78.9%; 2/3∶21.1% | NS | 584 (490–696); Median (IQR) | 79% | PEG-IFN | Mix of WB and FD RBV | NS |
| Mehta et al 2006 | Retrospective cohort | USA | 29 | 39 (36–43) Median (IQR) | 19 IVDU | 1/4∶89.7%; 2/3∶10.3% | 52.9% | <200 = 2; 200–350 = 15; >350 = 12 | 58.6% | STD or PEG-IFN | ± RBV (dosing NS) | NS |
| Michielsen et al 2009 | Prospective cohort | Belgium | 37 | 34 (17–60) Median (range) | 15 IVDU; 7 blood products; 15 other or unknown | 1/4∶59.5%; 2/3∶40.5% | 55.9% | 481 (222–1169); Median (range) | 64.9% | PEG-IFN | WB RBV | All 52 weeks |
| Mira et al 2009 | Prospective cohort | Spain | 542 | NS | 462 IVDU | 1/4∶65%; 2/3∶35% | 68% | CD4≤250 = 39 patients; CD4>250 = 503 patients | 82.7% | PEG-IFN | WB RBV | Gen 1 or 4 = 48 weeks; Gen 2 or 3 = 24 or 48 weeks |
| Murray et al 2011 | Retrospective cohort | Canada | 64 | 44 (39–50) Median (IQR) | 33 IVDU; 27 MSM | 1/4∶51.5%; 2/3∶48.5% | 52% | 400 (270–510) Median (IQR) | 71.9% | PEG-IFN | Mix of WB and FD RBV | Gen 1 = 48 weeks; Gen 2/3 = 24 weeks (with potential to continue) |
| Nasti et al 2001 | Prospective cohort | Italy | 17 | 36 (27–47) Mean (range) | 17 IVDU | 1/4∶64.8%; 2/3∶35.2% | NS | 445 (144) Mean (SD) | 94.1% | STD-IFN | WB RBV | All 24 weeks |
| Neukam et al 2012 | Prospective cohort | Spain and Germany | 521 | 42 (39–46) Median (IQR) | 391 IVDU | 1/4∶70%; 2/3∶30% | 39.5% | 483 (355–665) Median (IQR) | – | PEG-IFN | WB RBV | Gen 1 or 4 = 48 or 72 weeks; Gen 2 or 3 = 24 weeks (when RVR achieved) |
| Nicot et al 2008 | Retrospective cohort | France | 35 | 41 (±8) Mean (SD) | NS | 1/4∶60%; 2/3∶40% | NS | 444 Mean | 68.6% | PEG-IFN | WB RBV | All 48 weeks |
| Nischalke et al 2010 | Prospective cohort | Germany | 109 | 45 (29–68) Mean (range) | NS | NS | NS | 524 (216–1902) Mean (range) | NS | PEG-IFN | RBV ‘according to current guidelines’ | 24 or 48 weeks ‘according to current guidelines’ |
| Poizot-Martin et al 2003 | Prospective cohort | France | 62 | 36 (34–40) Median (IQR) | 49 IVDU; 13 other | 1/4∶67.7%; 2/3∶32.3% | 76.7% | 494 (327–657) Median (IQR) | 88.7% | PEG or STD IFN | FD RBV | At least 24 weeks and up to 48 weeks |
| Reiberger et al 2011 | Retrospective cohort | Germany and Austria | 416 | 43 (±8) Mean (SD) | 201 IVDU; 83 MSM; 20 WSM; 21 blood products; 91 unknown | 1/4∶71.8%; 2/3∶28.2% | 35.1% | 530 (±242) Mean (SD) | 56.9% | PEG-IFN | FD RBV (adjusted for genotype but not weight) | All 48 weeks |
| Reiberger et al 2008 | Retrospective cohort | Austria | 30 | 37 (±8) Mean (SD) | NS | 1/4∶73.3%; 2/3∶26.7% | 50% | 568 (±276) Mean (SD) | 60% | PEG-IFN | FD RBV (adjusted for genotype but not weight) | All 48 weeks (with option to extend to 72 weeks) |
| Righi et al 2008 | Retrospective cohort | Italy | 43 | 41 (±6.7) Mean (SD) | 32 IVDU; 4 WSM | 1/4∶48.8%; 2/3∶51.2% | 40% | >500 in 22/43 patients; <350 in 6 patients | 37.2% | PEG-IFN | WB RBV | Gen 1 or 4 = 48 weeks; Gen 3a = 24 weeks |
| Sacchi et al 2011 | Prospective cohort | Italy | 19 | NS | NS | 1/4∶42.1%; 2/3∶57.9% | 18.2% | 458 (122–842); Median (range) | HAART suspended during HCV treatment | PEG-IFN | WB RBV | All 48 weeks |
| Santin et al 2006 | Prospective cohort | Spain | 60 | 38.1±5.3 Mean (SD) | 50 IVDU; 8 sexual | 1/4∶68.3%; 2/3∶31.7% | NS | 645 (±351) Mean (SD) | 90% | PEG-IFN | WB RBV | Gen 1 or 4 = 48 weeks; Gen 2 or 3 = 24 weeks |
| Sarmento-Castro et al 2007 | Prospective cohort | Portugal | 53 | 32.6 Mean | 45 IVDU; 8 sexual | 1/4∶52.8%; 2/3∶47.2% | 12.5% | 585 Mean | 69.8% | PEG-IFN | WB RBV | Gen 1 or 4 = 48 weeks; Gen 2 or 3 = 24 weeks |
| Taylor et al 2011 | Prospective cohort | USA | 11 | 46 (37–61) Mean (range) | All patients were recovering IVDU on methadone | 1/4∶100% | 54.5% | 498 (210–868) Mean (range) | 90.9% | PEG-IFN | WB RBV | All 48 weeks |
| Thein et al 2007 | Prospective cohort | Australia | 15 | 38.9 (±7.8) Mean (SD) | NS | 1/4∶40%; 2/3∶60% | 21.4% | 363 (328–612); Mean (IQR) | 33.3% | PEG-IFN | WB RBV | Gen 1 = 48 weeks; Gen 2 or 3 = 24 weeks (with option to extend) |
| Van den Eynde et al 2010 | Retrospective cohort | Spain | 278 | 39.8 (36.4–42.8) Median (range) | 236 IVDU | 1/4∶100% | 62.3% | 495 (357–692) | 88.8% | PEG-IFN | Mix of WB and FD RBV | All 48 weeks |
| Wagner et al 2011 | Retrospective cohort | USA | 72 | 48. 1 Mean | NS | 1/4∶70.8%; 2/3∶29.2% | NS | 534 (±234); Mean (SD) | 91.7% | PEG-IFN | RBV NS | 24–32, 48 or 72 weeks |
| Yotsuyanagi et al 2009 | Retrospective cohort | Japan | 60 | NS | High proportion blood products | 1/4∶36.7%; 2/3∶30% | NS | NS | NS | STD-IFN | RBV given in 35 patients, not in 25 (dose not stated) | Gen 1 or 4 or other = 48 weeks; Gen 2 or 3 = 24 weeks |
| Zinkernagel et al 2006 | Retrospective cohort | Switzerland | 160 | 41 (37–44) Median (IQR) | 122 IVDU; 21 WSM; 11 MSM; 6 blood products | 1/4∶44.1%; 2/3∶54.4% | 46.6% | 490 (334–662.5) Median (IQR) | 75.6% | PEG-IFN | FD RBV (dose adjusted for phenotype but not weight) | NS |
FD RBV, fixed-dose ribavarin; IQR, interquartile range; IVDU, intravenous drug use; MSM, men who have sex with men; NS, not stated; PEG-IFN, pegylated interferon; SD, standard deviation; STD-IFN, standard interferon; WB RBV, weight-based ribavarin; WSM, women who have sex with men.
The proportion of patients with liver damage at baseline ranged from 12.5% to 74%. The majority of studies (36) included a mix of HCV genotypes. Three studies (from Argentina, Spain and the USA) were exclusively comprised of patients infected with genotypes 1 and 4 and two studies (from Sweden and Spain) were exclusively comprised of patients infected with genotypes 2 and 3.
HCV treatment comprised pegylated interferon and weight-based ribavarin in most cases, and the majority of patients (84%) received concomitant antiretroviral therapy. Liver damage was assessed by biopsy in over half (25) of studies. One study used fibroscan to assess liver damage, and 3 studies used a combination of the 2 techniques. Nine studies did not assess liver damage while the remainder of the studies (3) did not state the method used.
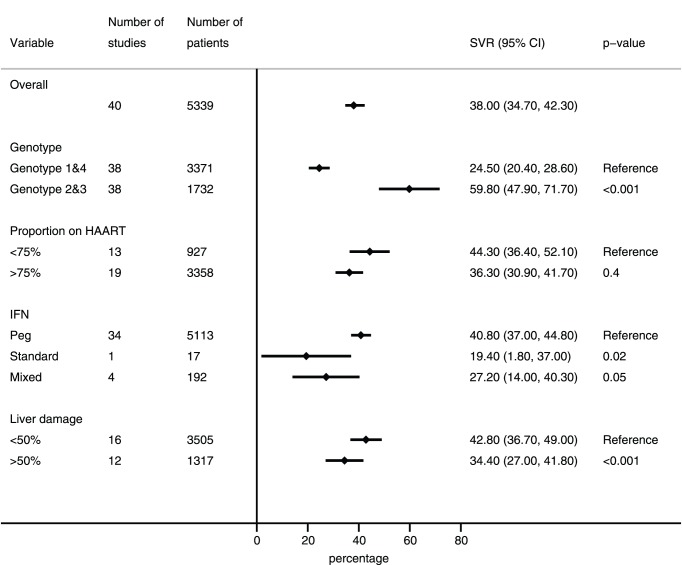
The proportion of patients achieving SVR ranged from 13.8% (2.2–32.9%) to 71.9% (48.2–90.5%), with a pooled proportion of 38% (34.7–42.3%) (τ2 0.037). Three studies were ‘adherent cohorts’ comprising only patients who completed treatment; removing these studies from the analysis did not affect the overall result. The result was also unaffected by a sensitivity analysis that included all studies from Spain regardless of potential overlap (pooled SVR 39%). The most important determinant of treatment success was HCV genotype, with significantly poorer outcomes for patients infected with HCV genotypes 1 or 4 (3371 patients, pooled SVR 24.5% (95%CI 20.4–28.6%), compared to genotypes 2 or 3 (1878 patients, pooled SVR 59.8% (95%CI 47.9–71.7%). Cohorts in which more than 50% of patients had advanced liver fibrosis at baseline (Metavir F3 or F4 or equivalent) [53] had poorer outcomes compared to cohorts where less than 50% of patients had advanced liver disease (42.8%[36.7–49%] versus 34.4%[27–41.8%]). Subgroup analyses are summarized in Figure 2.
Figure 2. Sustained virological response (SVR) in patients co-infected with HCV and HIV by disease, patient and treatment covariates.
Rapid virological response, reported by 5 studies, was achieved by 30.9% of patients (11.2–50.8%). The pooled proportion of patients who discontinued treatment due to drug toxicities (reported by 33 studies) was low, at 4.3% (3.3–5.3%). Defaulting from treatment, reported by 33 studies, was also low (5.1%, 3.5–6.6%), as was on-treatment mortality, (35 studies, 0.1% (0–0.2%)).
Discussion
Currently, access to effective HCV treatment is limited, particularly for those with HCV/HIV co-infection in resource-limited settings. This is reflected in this study by the paucity of data reoprted from such settings. Among the 40 studies assessed, only three were from resource-limited settings (two from Brazil and one from Argentina), and no reports were found from African countries, including Egypt where the burden of HCV is the highest in the world, or sub-Saharan Africa where the burden of HIV is the highest in the world. Limited access to treatment in resource-limited settings is in part due to the high cost of treatment, a perception of poorer outcomes of HCV treatment in HIV co-infected patients, and the potential difficulties associated with adherence and drug interactions under programmatic conditions.
Concern has recently been expressed that the relatively high efficacy of hepatitis treatment reported in clinical trials is not attained in subsequent effectiveness studies carried out in the general population under programmatic conditions [54]. In comparison to routine programmes, patients in clinical trials tend to be more adherent to treatment, and will usually receive treatment free of charge provided by highly motivated clinical staff working in optimal clinical settings [55]. Nevertheless, this review found that programmatic outcomes were in very close alignment to a systematic review of outcomes in clinical trials, which found that HCV treatment responses in HIV co-infected patients is lower than those observed in HIV-negative individuals [56]. Nevertheless, for HIV-positive patients infected with HCV genotypes 2 or 3, treatment outcomes are very similar (SVR 60%) to those reported for HIV-negative HCV patients infected with the same genotypes in programme settings (SVR 59%) [7].
Treatment completion was generally high, with few patients discontinuing treatment due to adverse events or defaulting from care. The use of HAART was not associated with better outcomes, which is consistent with other studies [57], [58].
We used a broad search strategy that allowed the inclusion of a large number of studies. We restricted studies to observational cohorts so that the expected outcomes would better reflect those observed in programmatic settings, but this can result in confounding. Concomitant use of medications, unreported mental or physical problems, or ancillary health service support could all influence treatment outcomes, but these factors were not reported and so could not be assessed. We attempted to use multivariate meta-regression to explore the potential influence of patient and programme level variables to explain differences in results between studies. However, this was restricted by inconsistent reporting between studies, so our exploration of associations was limited to univariate subgroup comparisons. In addition, bias may result from studies that pre-selected patients on the basis of characteristics that may influence treatment success, or excluded patients with risk factors for poor adherence. Furthermore, the final analysis only included studies published in English, which may lead to publication bias. Only five studies, however, were excluded on the basis of language and their influence would likely be small. Nevertheless, this review should be taken as an indication of outcomes and not as an exhaustive summary.
The treatment of HCV infection is likely to evolve rapidly as a result of a dynamic drug pipeline. For example, the first HCV protease inhibitors have just been recently approved. In the short to medium-term, however, the majority of HIV-positive patients living in resource-limited settings are unlikely to benefit from these newer treatments, just as they continue to lack access to many of the newer antiretroviral drugs for HIV that have been marketed in the West for many years. The results of this systematic review support the current practice of treatment in well resourced settings, whilst serving as a reminder for the need for better treatments. This review also highlights the need to encourage treatment of HCV/HIV co-infected patients in resource-limited settings to start programmes in parallel to efforts aimed at reducing costs of current treatment and gaining access to newer, interferon free regimens so that new advances in treatment can be rapidly accessed by all those that need them.
Supporting Information
Protocol.
(PDF)
PRISMA Checklist.
(DOC)
Acknowledgments
We thank the following people for taking the time to respond to requests for further information and clarification: Pablo Barreiro, Juan Berenguer, Luz Martin-Carbonero, Curtis Cooper, Salvador Resino Garcia, Susanna Naggie, Karin Neukam, Juan Antonio Pineda, Miguel Santin, and Norma Rallón.
Funding Statement
The authors have no support or funding to report.
References
- 1. Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, et al. (1999) Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 30: 1054–1058. [DOI] [PubMed] [Google Scholar]
- 2.Rockstroh J, Konopnicki D, Soriano V (2004) Hepatitis B and hepatitis C in the EuroSIDA cohort: prevalence and effect on mortality, AIDS, progression and response to HAART. 11th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, USA, Feb 8–11. Abstract 799.
- 3.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS (2010) A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 8: 280–288, 288 e281. [DOI] [PubMed]
- 4. Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, et al. (2012) Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA : the journal of the American Medical Association 308: 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durier N, Nguyen C, White LJ (2012) Treatment of hepatitis C as prevention: a modeling case study in Vietnam. PloS one 7: e34548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iorio A, Marchesini E, Awad T, Gluud LL (2010) Antiviral treatment for chronic hepatitis C in patients with human immunodeficiency virus. Cochrane database of systematic reviews: CD004888. [DOI] [PMC free article] [PubMed]
- 7. Ford N, Kirby C, Singh K, Mills EJ, Cooke G, et al. (2012) Chronic hepatitis C treatment outcomes in low- and middle-income countries: a systematic review and meta-analysis. Bulletin of the World Health Organization 90: 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vandenbroucke JP (2011) Why do the results of randomised and observational studies differ? BMJ 343: d7020. [DOI] [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freeman MF, Tukey J (1950) Transformations related to the angular and the square root. Ann Inst Stat Mathematics 21: 607–611. [Google Scholar]
- 11. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Controlled clinical trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aguilar Marucco D, Gonzalez de Requena D, Bonora S, Tettoni C, Bonasso M, et al. (2008) The use of trough ribavirin concentration to predict sustained virological response and haematological toxicity in HIV/HCV-co-infected patients treated with ribavirin and pegylated interferon. The Journal of antimicrobial chemotherapy 61: 919–924. [DOI] [PubMed] [Google Scholar]
- 14. Amorosa VK, Slim J, Mounzer K, Bruno C, Hoffman-Terry M, et al. (2010) The influence of abacavir and other antiretroviral agents on virological response to HCV therapy among antiretroviral-treated HIV-infected patients. Antiviral therapy 15: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Araujo ES, Dahari H, Neumann AU, de Paula Cavalheiro N, Melo CE, et al. (2011) Very early prediction of response to HCV treatment with PEG-IFN-alfa-2a and ribavirin in HIV/HCV-coinfected patients. Journal of viral hepatitis 18: e52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avidan NU, Goldstein D, Rozenberg L, McLaughlin M, Ferenci P, et al. (2009) Hepatitis C viral kinetics during treatment with peg IFN-alpha-2b in HIV/HCV coinfected patients as a function of baseline CD4+ T-cell counts. Journal of acquired immune deficiency syndromes 52: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berenguer J, von Wichmann MA, Quereda C, Miralles P, Mallolas J, et al. (2011) Effect of accompanying antiretroviral drugs on virological response to pegylated interferon and ribavirin in patients co-infected with HIV and hepatitis C virus. The Journal of antimicrobial chemotherapy 66: 2843–2849. [DOI] [PubMed] [Google Scholar]
- 18. Burbelo PD, Kovacs JA, Ching KH, Issa AT, Iadarola MJ, et al. (2010) Proteome-wide anti-hepatitis C virus (HCV) and anti-HIV antibody profiling for predicting and monitoring the response to HCV therapy in HIV-coinfected patients. The Journal of infectious diseases 202: 894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cesari M, Caramma I, Antinori S, Adorni F, Galli M, et al. (2009) Impact of hyperglycaemia and cholesterol levels on the outcome of hepatitis C virus (HCV) treatment in HIV/HCV-coinfected patients. HIV medicine 10: 580–585. [DOI] [PubMed] [Google Scholar]
- 20. Cooper CL, Giordano C, Mackie D, Mills EJ (2010) Equitable access to HCV care in HIV-HCV co-infection can be achieved despite barriers to health care provision. Therapeutics and clinical risk management 6: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fleming CA, Tumilty S, Murray JE, Nunes D (2005) Challenges in the treatment of patients coinfected with HIV and hepatitis C virus: need for team care. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 40 Suppl 5S349–354. [DOI] [PubMed] [Google Scholar]
- 22. Gonvers JJ, Heim MH, Cavassini M, Mullhaupt B, Genne D, et al. (2010) Treatment of hepatitis C in HCV mono-infected and in HIV-HCV co-infected patients: an open-labelled comparison study. Swiss medical weekly 140: w13055. [DOI] [PubMed] [Google Scholar]
- 23. James PD, Wong DKH (2008) Optimizing hepatitis C therapy in HIV/hepatitis C virus (HCV) coinfected patients: Analysis of HCV viral kinetics on treatment. Canadian Journal of Infectious Diseases and Medical Microbiology 23: 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karlstrom O, Sonnerborg A, Weiland O (2008) Similar hepatitis C virus RNA kinetics in HIV/hepatitis C virus monoinfected genotype 2 or 3 matched controls during hepatitis C virus combination therapy. AIDS 22: 899–901. [DOI] [PubMed] [Google Scholar]
- 25. Kieran J, Dillon A, Farrell G, Jackson A, Norris S, et al. (2011) High uptake of hepatitis C virus treatment in HIV/hepatitis C virus co-infected patients attending an integrated HIV/hepatitis C virus clinic. International journal of STD & AIDS 22: 571–576. [DOI] [PubMed] [Google Scholar]
- 26. Laufer N, Bolcic F, Rolon MJ, Martinez A, Reynoso R, et al. (2011) HCV RNA decline in the first 24 h exhibits high negative predictive value of sustained virologic response in HIV/HCV genotype 1 co-infected patients treated with peginterferon and ribavirin. Antiviral research 90: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lerias de Almeida PR, Alves de Mattos A, Valle Tovo C (2010) Sustained virological response according to the type of early virological response in HCV and HCV/HIV. Annals of hepatology 9: 150–155. [PubMed] [Google Scholar]
- 28. Lopez-Cortes LF, Ruiz-Valderas R, Jimenez-Jimenez L, Gonzalez-Escribano MF, Torres-Cornejo A, et al. (2012) Influence of IL28B polymorphisms on response to a lower-than-standard dose peg-IFN-alpha 2a for genotype 3 chronic hepatitis C in HIV-coinfected patients. PloS one 7: e28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Macias J, del Valle J, Rivero A, Mira JA, Camacho A, et al. (2010) Changes in liver stiffness in patients with chronic hepatitis C with and without HIV co-infection treated with pegylated interferon plus ribavirin. The Journal of antimicrobial chemotherapy 65: 2204–2211. [DOI] [PubMed] [Google Scholar]
- 30. Marchetti G, Nasta P, Bai F, Gatti F, Bellistri GM, et al. (2012) Circulating sCD14 is associated with virological response to pegylated-interferon-alpha/ribavirin treatment in HIV/HCV co-infected patients. PloS one 7: e32028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maru DS, Bruce RD, Basu S, Altice FL (2008) Clinical outcomes of hepatitis C treatment in a prison setting: feasibility and effectiveness for challenging treatment populations. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 47: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mehta SH, Lucas GM, Mirel LB, Torbenson M, Higgins Y, et al. (2006) Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS 20: 2361–2369. [DOI] [PubMed] [Google Scholar]
- 33. Michielsen P, Bottieau E, Van Vlierberghe H, Van Marck E, Vandemaele E, et al. (2009) Treatment of chronic hepatitis C in patients with human immunodeficiency virus (HIV) with weekly peginterferon alpha-2b plus ribavirin: a multi-centred Belgian study. Acta gastro-enterologica Belgica 72: 389–393. [PubMed] [Google Scholar]
- 34. Mira JA, Gutierrez-Valencia A, Gil Ide L, Merino D, Rivero A, et al. (2009) Efficacy and safety of pegylated interferon plus ribavirin in HIV and hepatitis C virus-coinfected patients with advanced immunosuppression. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 49: e84–91. [DOI] [PubMed] [Google Scholar]
- 35. Murray MC, Barrios R, Zhang W, Hull M, Montessori V, et al. (2011) Hepatitis C virus treatment rates and outcomes in HIV/hepatitis C virus co-infected individuals at an urban HIV clinic. European journal of gastroenterology & hepatology 23: 45–50. [DOI] [PubMed] [Google Scholar]
- 36. Nasti G, Di Gennaro G, Tavio M, Cadorin L, Tedeschi RM, et al. (2001) Chronic hepatitis C in HIV infection: feasibility and sustained efficacy of therapy with interferon alfa-2b and tribavirin. AIDS 15: 1783–1787. [DOI] [PubMed] [Google Scholar]
- 37. Neukam K, Camacho A, Caruz A, Rallon N, Torres-Cornejo A, et al. (2012) Prediction of response to pegylated interferon plus ribavirin in HIV/hepatitis C virus (HCV)-coinfected patients using HCV genotype, IL28B variations, and HCV-RNA load. Journal of hepatology 56: 788–794. [DOI] [PubMed] [Google Scholar]
- 38. Nicot F, Legrand-Abravanel F, Lafont T, Dubois M, Saune K, et al. (2008) Serum concentrations of ribavirin and pegylated interferon and viral responses in patients infected with HIV and HCV. Journal of medical virology 80: 1523–1529. [DOI] [PubMed] [Google Scholar]
- 39. Nischalke HD, Vogel M, Mauss S, Baumgarten A, Lutz T, et al. (2010) The cytotoxic lymphocyte antigen 4 polymorphisms affect response to hepatitis C virus-specific therapy in HIV(+) patients with acute and chronic hepatitis C virus co-infection. AIDS 24: 2001–2007. [DOI] [PubMed] [Google Scholar]
- 40. Poizot-Martin I, Marimoutou C, Benhaim S, Drogoul-Vey MP, Dinh T, et al. (2003) Efficacy and tolerance of HCV treatment in HIV-HCV coinfected patients: the potential interaction of PI treatment. HIV clinical trials 4: 262–268. [DOI] [PubMed] [Google Scholar]
- 41. Reiberger T, Aberle JH, Kundi M, Kohrgruber N, Rieger A, et al. (2008) IP-10 correlates with hepatitis C viral load, hepatic inflammation and fibrosis and predicts hepatitis C virus relapse or non-response in HIV-HCV coinfection. Antiviral therapy 13: 969–976. [PubMed] [Google Scholar]
- 42. Reiberger T, Obermeier M, Payer BA, Baumgarten A, Weitner L, et al. (2011) Considerable under-treatment of chronic HCV infection in HIV patients despite acceptable sustained virological response rates in a real-life setting. Antiviral therapy 16: 815–824. [DOI] [PubMed] [Google Scholar]
- 43. Righi E, Beltrame A, Bassetti M, Lindstrom V, Mazzarello G, et al. (2008) Therapeutical aspects and outcome of HIV/HCV coinfected patients treated with pegylated interferon plus ribavirin in an Italian cohort. Infection 36: 358–361. [DOI] [PubMed] [Google Scholar]
- 44. Sacchi A, Agrati C, D’Offizi G, Vlassi C, Rozera G, et al. (2011) The basal activation state of DC subsets correlates with anti-HCV treatment outcome in HCV/HIV co-infected patients. Clinical immunology 138: 178–186. [DOI] [PubMed] [Google Scholar]
- 45. Santin M, Shaw E, Garcia MJ, Delejido A, de Castro ER, et al. (2006) Efficacy and safety of pegylated interferon-alpha2b plus ribavirin for the treatment of chronic hepatitis C in HIV-infected patients. AIDS research and human retroviruses 22: 315–320. [DOI] [PubMed] [Google Scholar]
- 46. Sarmento-Castro R, Horta A, Vasconcelos O, Coelho H, Mendez J, et al. (2007) Impact of peginterferon alpha-2b and ribavirin treatment on liver tissue in patients with HCV or HCV-HIV co-infection. The Journal of infection 54: 609–616. [DOI] [PubMed] [Google Scholar]
- 47. Taylor LE, Bowman SE, Chapman S, Zaller N, Stein MD, et al. (2011) Treatment for hepatitis C virus genotype 1 infection in HIV-infected individuals on methadone maintenance therapy. Drug and alcohol dependence 116: 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thein HH, Maruff P, Krahn MD, Kaldor JM, Koorey DJ, et al. (2007) Improved cognitive function as a consequence of hepatitis C virus treatment. HIV medicine 8: 520–528. [DOI] [PubMed] [Google Scholar]
- 49. Van den Eynde E, Tiraboschi JM, Tural C, Sola R, Mira JA, et al. (2010) Ability of treatment week 12 viral response to predict long-term outcome in genotype 1 hepatitis C virus/HIV coinfected patients. AIDS 24: 975–982. [DOI] [PubMed] [Google Scholar]
- 50. Wagner G, Chan Osilla K, Garnett J, Ghosh-Dastidar B, Bhatti L, et al. (2011) Patient Characteristics Associated with HCV Treatment Adherence, Treatment Completion, and Sustained Virologic Response in HIV Coinfected Patients. AIDS research and treatment 2011: 903480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yotsuyanagi H, Kikuchi Y, Tsukada K, Nishida K, Kato M, et al. (2009) Chronic hepatitis C in patients co-infected with human immunodeficiency virus in Japan: a retrospective multicenter analysis. Hepatology research : the official journal of the Japan Society of Hepatology 39: 657–663. [DOI] [PubMed] [Google Scholar]
- 52. Zinkernagel AS, von Wyl V, Ledergerber B, Rickenbach M, Furrer H, et al. (2006) Eligibility for and outcome of hepatitis C treatment of HIV-coinfected individuals in clinical practice: the Swiss HIV cohort study. Antiviral therapy 11: 131–142. [PubMed] [Google Scholar]
- 53. Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24: 289–293. [DOI] [PubMed] [Google Scholar]
- 54. Mitchell AE, Colvin HM, Palmer Beasley R (2010) Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 51: 729–733. [DOI] [PubMed] [Google Scholar]
- 55.Scaglione SJ, Lok AS (2012) Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology 142: 1360–1368 e1361. [DOI] [PubMed]
- 56.Gluud LL, Marchesini E, Iorio A (2009) Peginterferon plus ribavirin for chronic hepatitis C in patients with human immunodeficiency virus. The American journal of gastroenterology 104: 2335–2341; quiz 2342. [DOI] [PubMed]
- 57. Chung RT, Evans SR, Yang Y, Theodore D, Valdez H, et al. (2002) Immune recovery is associated with persistent rise in hepatitis C virus RNA, infrequent liver test flares, and is not impaired by hepatitis C virus in co-infected subjects. AIDS 16: 1915–1923. [DOI] [PubMed] [Google Scholar]
- 58. Medrano J, Barreiro P, Resino S, Tuma P, Rodriguez V, et al. (2009) Rate and timing of hepatitis C virus relapse after a successful course of pegylated interferon plus ribavirin in HIV-infected and HIV-uninfected patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 49: 1397–1401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol.
(PDF)
PRISMA Checklist.
(DOC)