Abstract
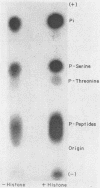
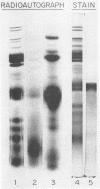
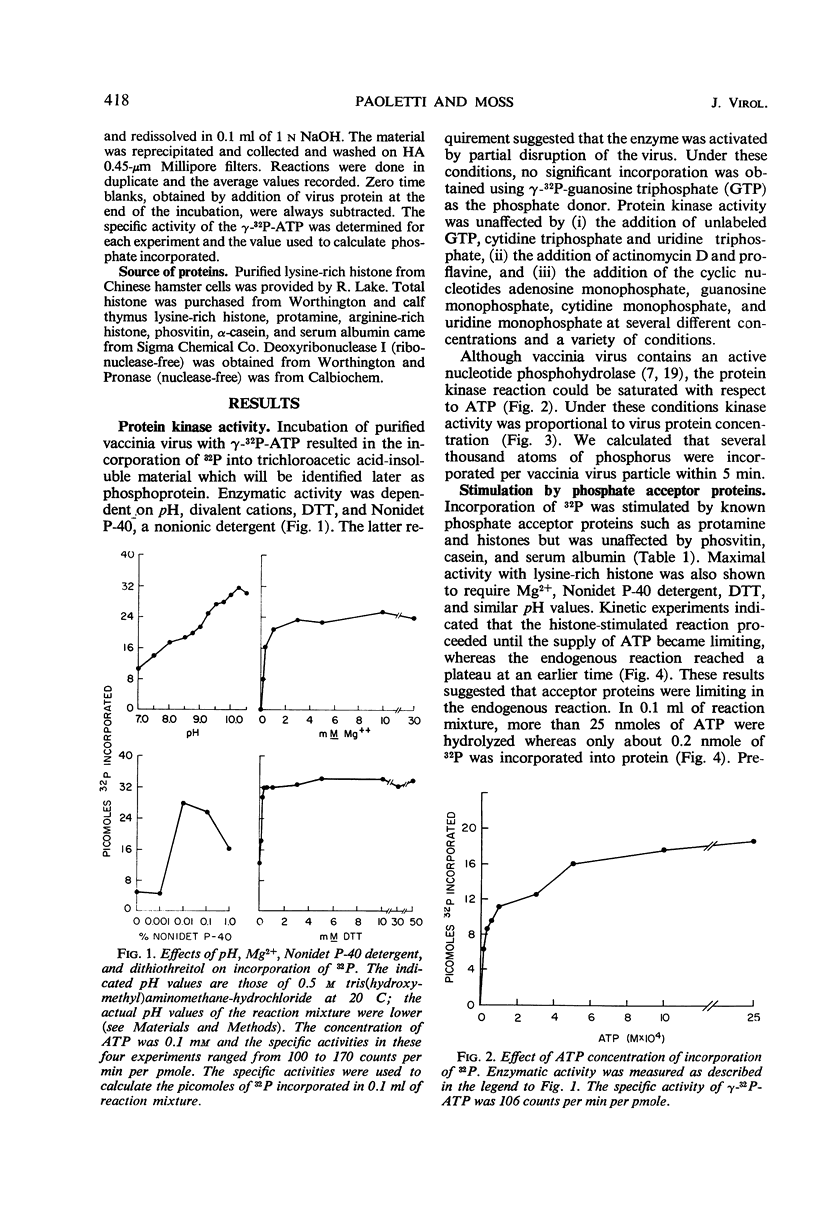
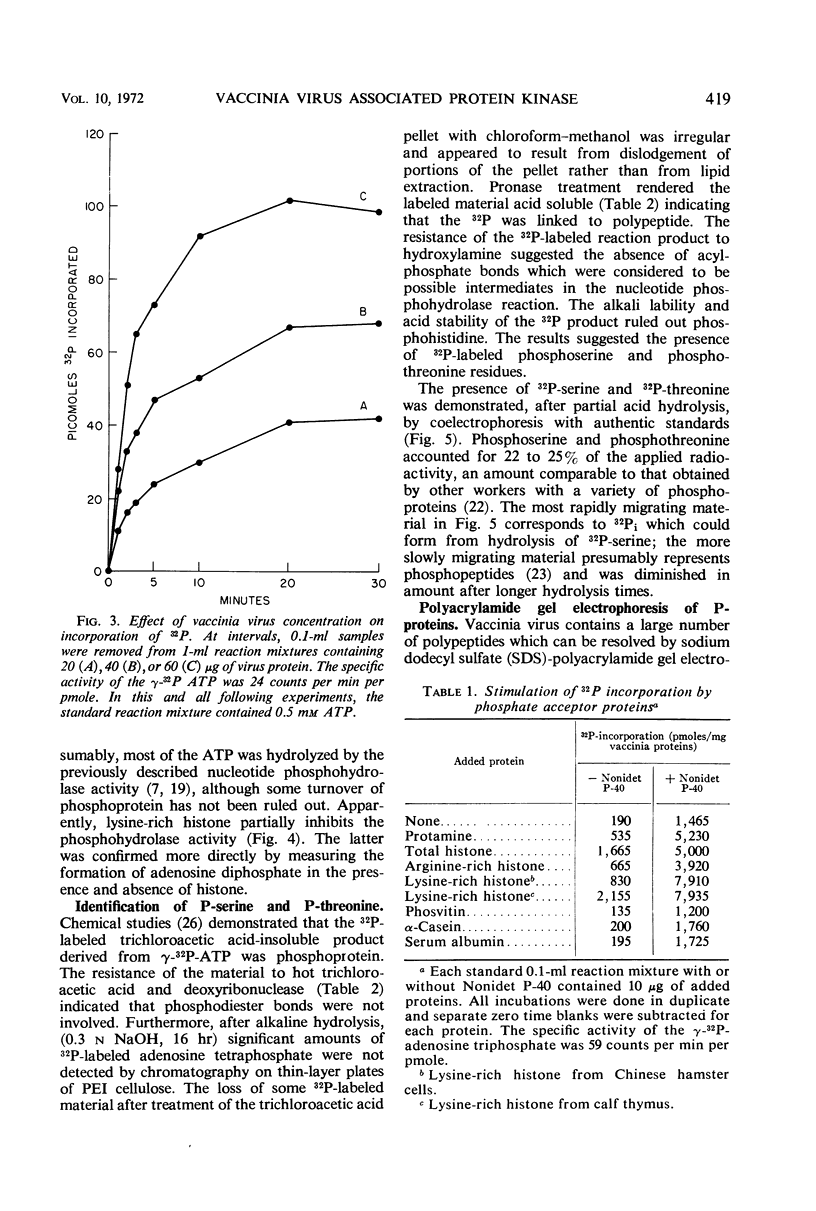
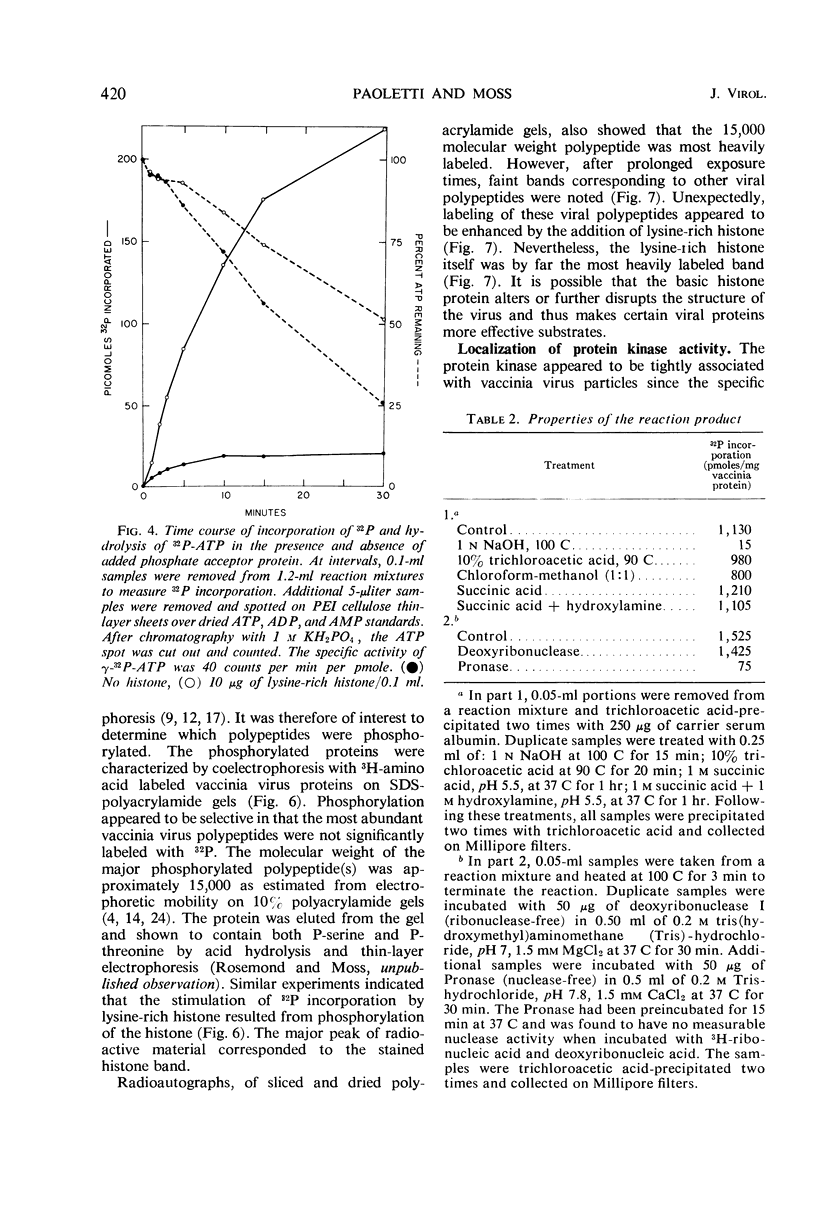
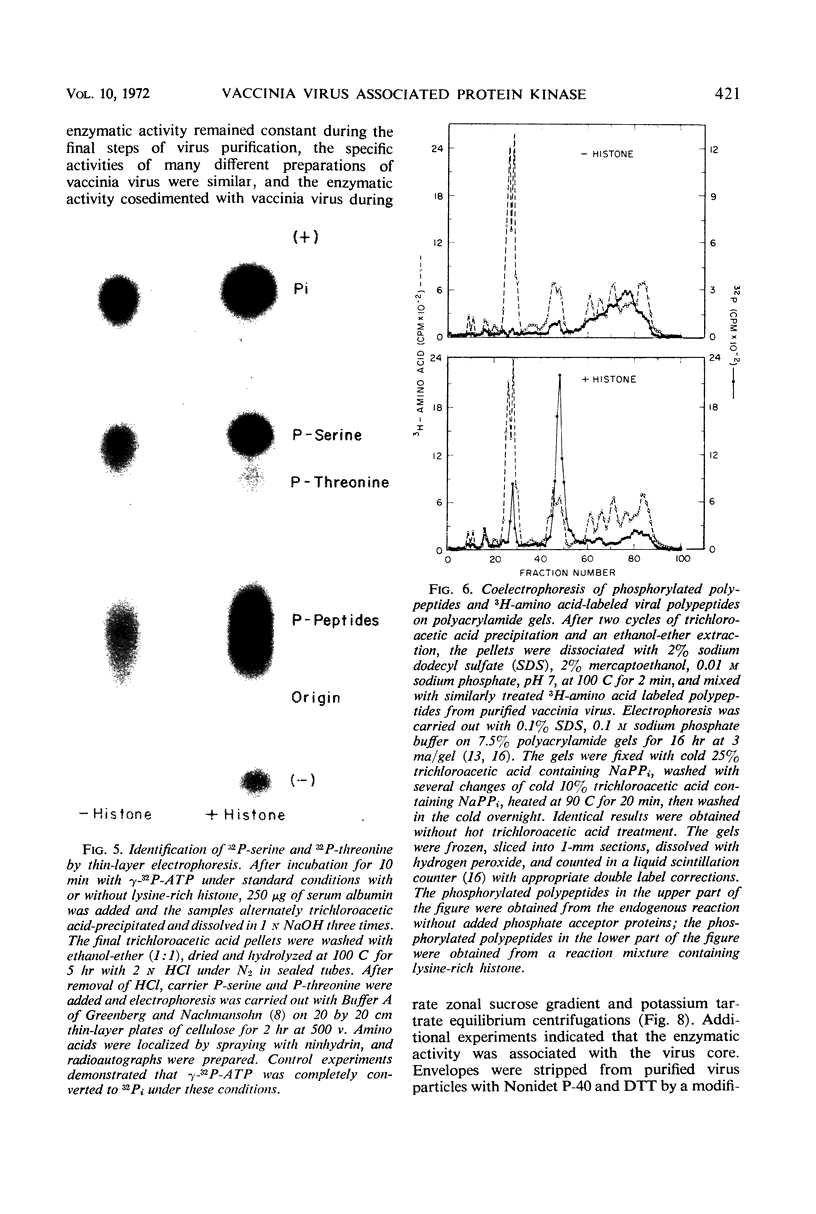
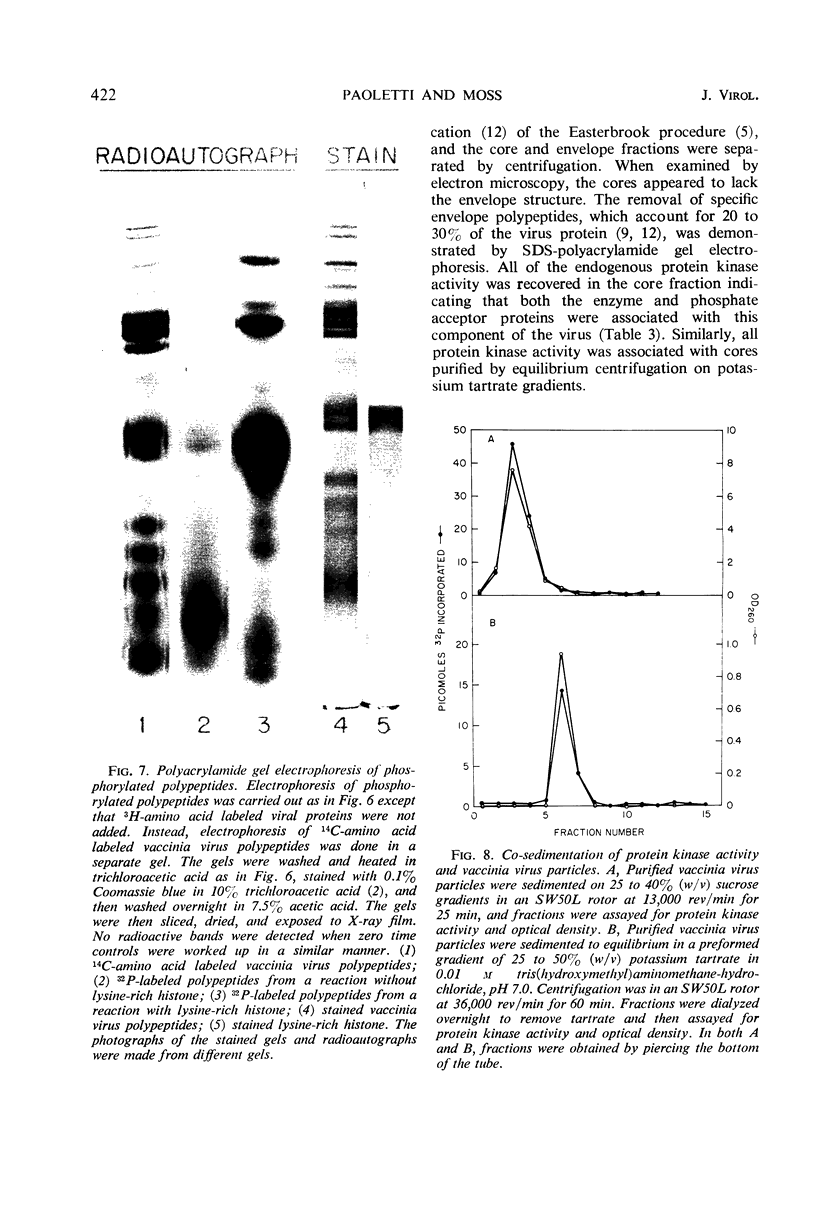
Incubation of purified vaccinia virus with γ-32P-adenosine triphosphate resulted in the incorporation of 32P into hot trichloroacetic acid-insoluble material. Enzymatic activity was completely dependent on the addition of divalent cations and was stimulated by nonionic detergents and dithiothreitol. Chemical studies demonstrated that serine and threonine residues of 15,000 molecular weight viral polypeptides were phosphorylated. In contrast, the major structural proteins were not phosphorylated or were phosphorylated to a much lesser extent. Added histones and protamine, but not serum albumin, casein, or phosvitin were phosphorylated by the partially disrupted vaccinia virus preparations. The protein kinase was tightly associated with vaccinia virus particles since the specific enzymatic activity remained constant during the final steps of virus purification, the specific activities of many different preparations of virus were similar, and the enzymatic activity cosedimented with vaccinia virus during rate zonal sucrose gradient and potassium tartrate gradient equilibrium centrifugations. Controlled degradation of vaccinia virus, with nonionic detergents and dithiothreitol, indicated that both the protein kinase and the specific phosphate acceptor proteins were located in the virus core.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubertin A. M., McAuslan B. R. Virus-associated nucleases: evidence for endonuclease and exonuclease activity in rabbitpox and vaccinia viruses. J Virol. 1972 Mar;9(3):554–556. doi: 10.1128/jvi.9.3.554-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Easterbrook K. B. Controlled degradation of vaccinia virions in vitro: an electron microscopic study. J Ultrastruct Res. 1966 Mar;14(5):484–496. doi: 10.1016/s0022-5320(66)80077-1. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- GREENBERG H., NACHMANSOHN D. STUDIES OF ACID PHOSPHOMONOESTERASES AND THEIR INHIBITION BY DIISOPROPYLPHOSPHOROFLUORIDATE. J Biol Chem. 1965 Apr;240:1639–1646. [PubMed] [Google Scholar]
- Gold P. H., Dales S. Localization of nucleotide phosphohydrolase activity within vaccinia. Proc Natl Acad Sci U S A. 1968 Jul;60(3):845–852. doi: 10.1073/pnas.60.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Moss B. Vaccinia virus structural polypeptide derived from a high-molecular-weight precursor: formation and integration into virus particles. J Virol. 1970 Dec;6(6):717–726. doi: 10.1128/jvi.6.6.717-726.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966 May;55(5):1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelo O. J., Woo S. L., Reimann E. M., Davie E. W. Effect of protein kinase on ribonucleic acid polymerase. Biochemistry. 1970 Nov 24;9(24):4807–4813. doi: 10.1021/bi00826a027. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Garon C. F. Glycoprotein synthesis in cells infected with vaccinia virus. I. Non-virion glycoproteins. Virology. 1971 Nov;46(2):221–232. doi: 10.1016/0042-6822(71)90025-0. [DOI] [PubMed] [Google Scholar]
- Moss B., Salzman N. P. Sequential protein synthesis following vaccinia virus infection. J Virol. 1968 Oct;2(10):1016–1027. doi: 10.1128/jvi.2.10.1016-1027.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Ospina J., Grace J. T., Jr Nucleotide phosphohydrolase in purified vaccinia virus. J Virol. 1968 Mar;2(3):167–172. doi: 10.1128/jvi.2.3.167-172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall C. C., Rogers H. W., Downer D. N., Gentry G. A. Protein kinase activity in equine herpesvirus. J Virol. 1972 Feb;9(2):216–222. doi: 10.1128/jvi.9.2.216-222.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ J. H., LIPMANN F. Phosphate incorporation into alkaline phosphatase of E. coli. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1996–2005. doi: 10.1073/pnas.47.12.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Stevely W. S., Stocken L. A. Phosphorylation of rat-thymus histone. Biochem J. 1966 Aug;100(2):20C–21C. doi: 10.1042/bj1000020c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]