Abstract
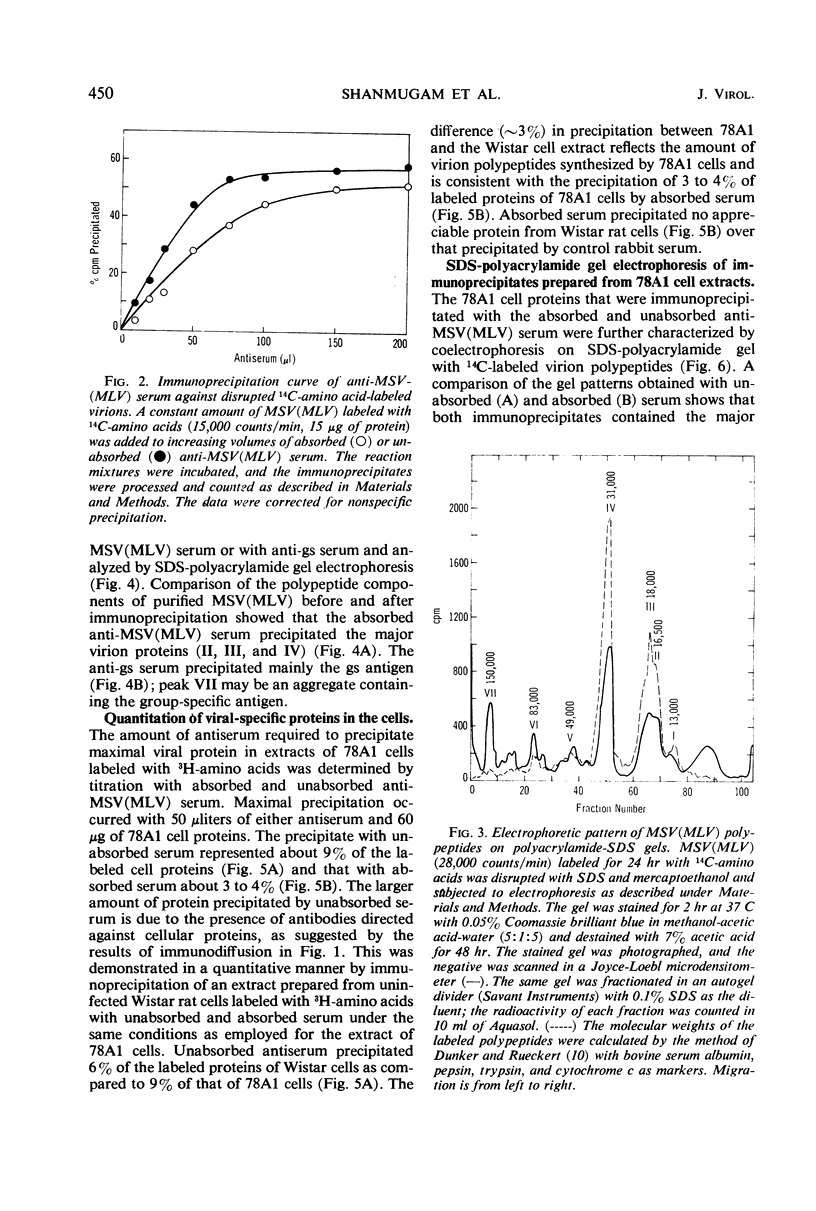
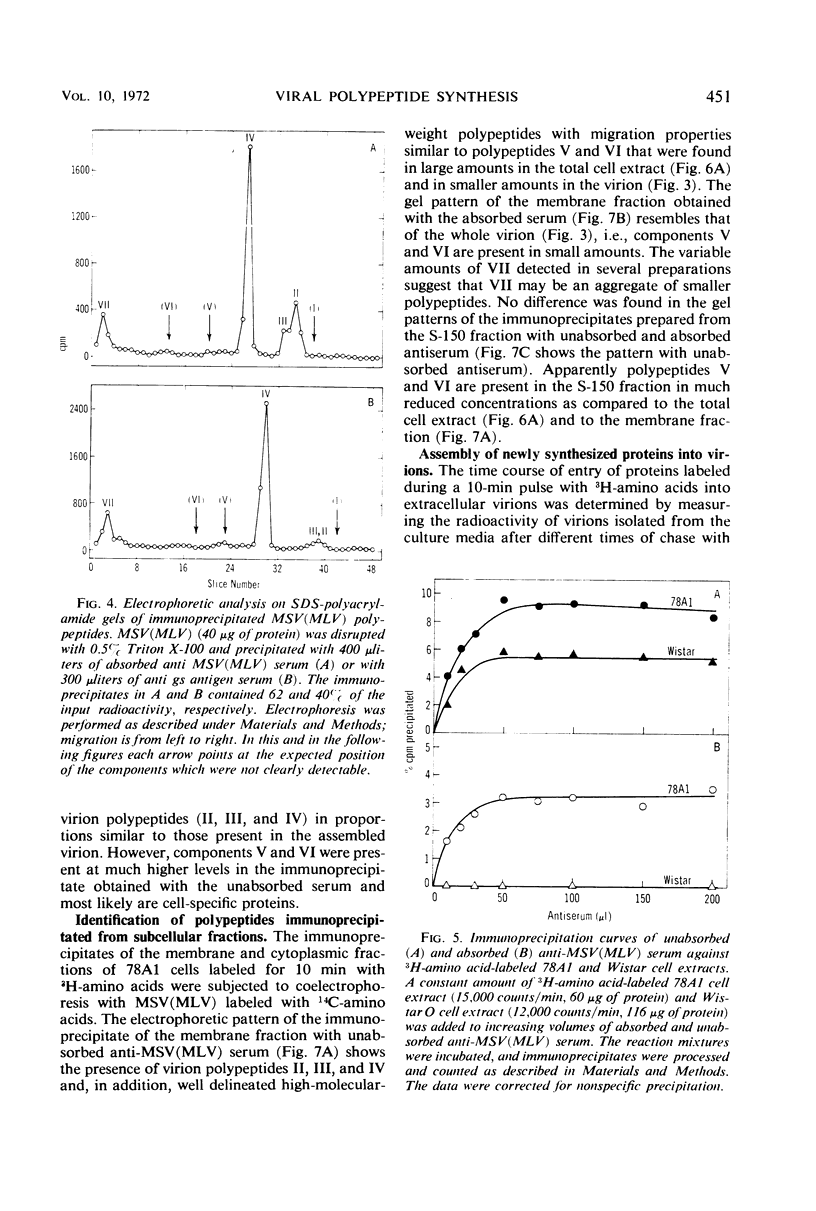
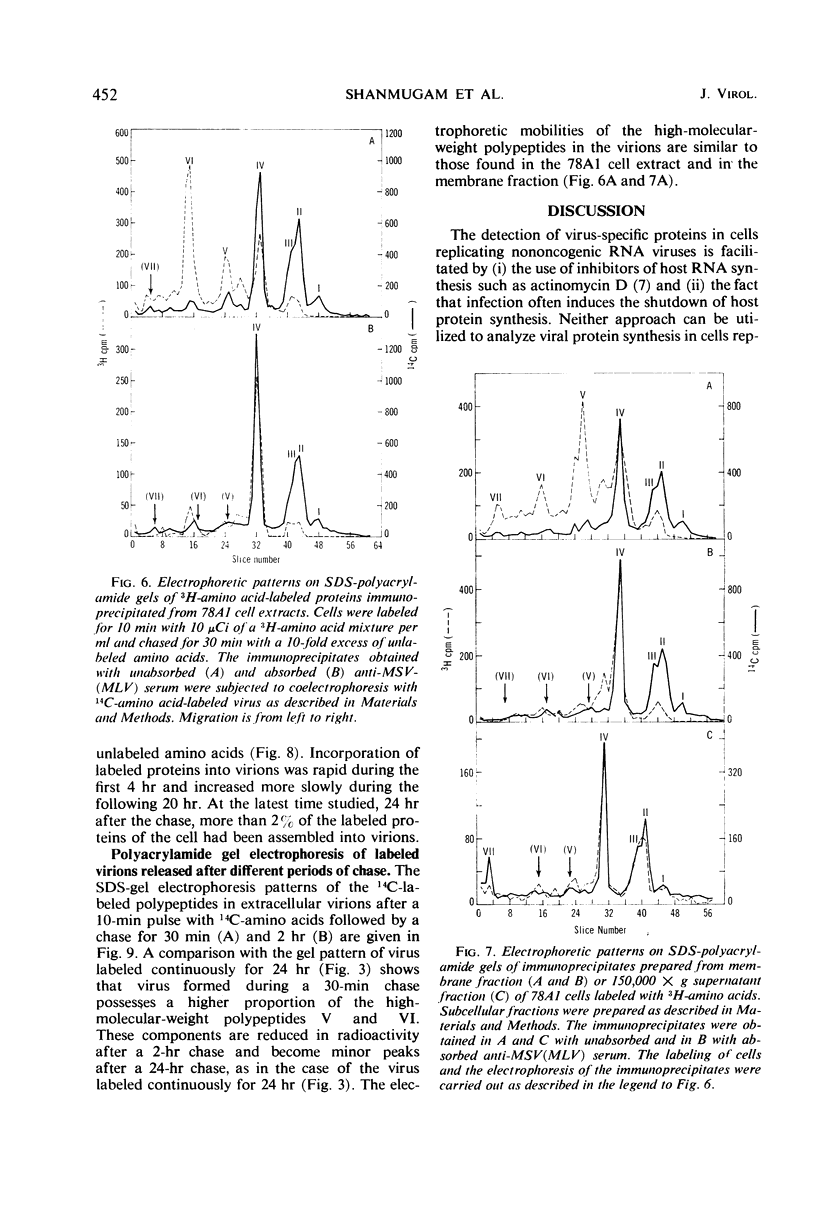
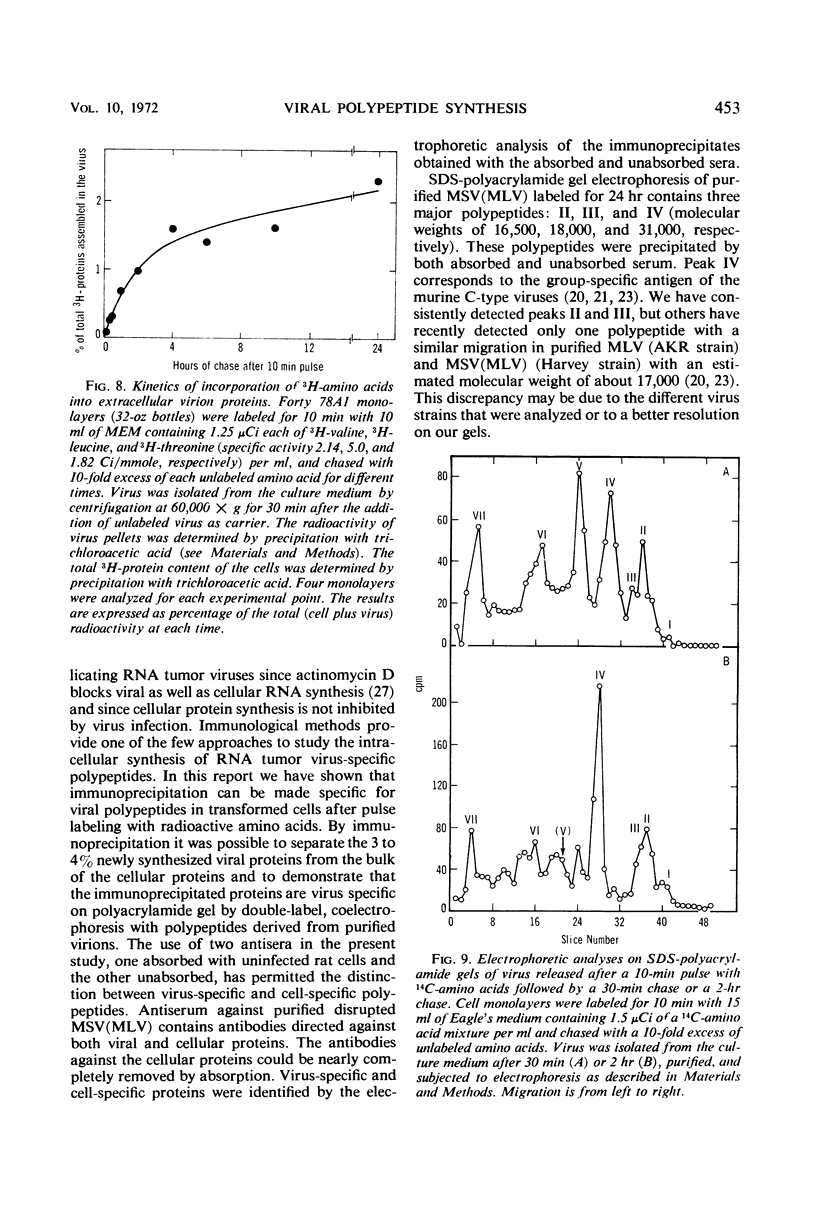
Antibodies to disrupted murine sarcoma-leukemia virus (MSV[MLV]) were used to study the synthesis of viral polypeptides in the transformed, virus-producing rat cell line 78A1. When cultures were labeled for 10 min with radioactive amino acids, about 9% of the total labeled proteins were precipitated with antiserum against purified MSV(MLV), and 3 to 4% were precipitated with the same antiserum after it had been absorbed with an extract from uninfected rat cells. The difference is due to the presence in the unabsorbed antiserum of antibodies to cellular proteins that are present in purified virus preparations. Intracellular viral proteins labeled with radioactive amino acids were isolated by immunoprecipitation and analyzed by electrophoresis in sodium dodecyl sulfate-polyacrylamide gels. The mobilities of intracellular viral polypeptides were identical to those of the purified virion. However, labeled polypeptides having electrophoretic mobilities lower than that of the major virion polypeptide, the group-specific antigen of molecular weight 31,000, were present in higher proportion in the total cell extract and in the membrane fraction than in the virion. These polypeptides appear to be of cellular origin for they were present only in minute amounts in the immunoprecipitates obtained with the absorbed serum. After a 10-min labeling period, radioactive proteins were assembled into extracellular virions rapidly for the first 4 hr followed by a slower rate. More than 2% of the total proteins of the cell labeled in a 10-min pulse were assembled into virions at the completion of a 24-hr chase. The high-molecular-weight polypeptides with the same mobilities as those detected in the immunoprecipitate of intracellular proteins were found in virions released from cells after a 10-min pulse. A larger proportion of these high-molecular-weight proteins was detected in virions released after short chase periods (30-120 min) than after longer chase periods (6-24 hr). Two possible interpretations of these data are that the high-molecular-weight cell-derived polypeptides (i) have a turnover rate higher than that of the major virion polypeptides or (ii) are cleaved proteolytically from the virions during long incubation in the culture media.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H., Bolognesi D. P. Polypeptides of avian RNA tumor viruses. II. Serological characterization. Virology. 1970 Dec;42(4):1113–1126. doi: 10.1016/0042-6822(70)90358-2. [DOI] [PubMed] [Google Scholar]
- Bernard C., Boiron M., Lasneret J. Transformation et infection chronique de cellules embryonnaires de rat par le virus du sarcome de Moloney. C R Acad Sci Hebd Seances Acad Sci D. 1967 Apr 24;264(17):2170–2173. [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967 Jun 14;26(2):279–292. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H. Polypeptides of avian RNA tumor viruses. 1. Isolation and physical and chemical analysis. Virology. 1970 Dec;42(4):1097–1112. doi: 10.1016/0042-6822(70)90357-0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Nucleic acid and proteins isolated from the Rauscher mouse leukemia virus (MLV). Proc Natl Acad Sci U S A. 1966 Jan;55(1):219–227. doi: 10.1073/pnas.55.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Fleissner E. Virus-specific antigens in hamster cells transformed by Rous sarcoma virus. J Virol. 1970 Jan;5(1):14–21. doi: 10.1128/jvi.5.1.14-21.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering G., Aoki T., Old L. J. Shared viral antigen of mammalian leukaemia viruses. Nature. 1970 Apr 18;226(5242):265–266. doi: 10.1038/226265a0. [DOI] [PubMed] [Google Scholar]
- Green M., Rokutanda M., Fujinaga K., Ray R. K., Rokutanda H., Gurgo C. Mechanism of carcinogenesis by RNA tumor viruses. I. An RNA-dependent DNA polymerase in murine sarcoma viruses. Proc Natl Acad Sci U S A. 1970 Sep;67(1):385–393. doi: 10.1073/pnas.67.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Kelloff G. J., Sarma P. S., Lane W. T., Turner H. C., Gilden R. V., Oroszlan S., Meier H., Myers D. D., Peters R. L. Group-specific antigen expression during embryogenesis of the genome of the C-type RNA tumor virus: implications for ontogenesis and oncogenesis. Proc Natl Acad Sci U S A. 1970 Sep;67(1):366–376. doi: 10.1073/pnas.67.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michelson-Fiske S., Haguenau F., Rabotti G. F. Cinétique du développement du virus du sarcoma de Rous (souche Schmidt-Ruppin). Estimation du temps d'incorporation d'arginine dans les protéines virales. Etude par autoradiographie au microscope électronique. C R Acad Sci Hebd Seances Acad Sci D. 1969 Dec 15;269(24):2475–2478. [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Enzymes and nucleotides in virions of Rous sarcoma virus. J Virol. 1971 Oct;8(4):409–416. doi: 10.1128/jvi.8.4.409-416.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni C. Structural proteins of Rauscher leukemia virus and Harvey sarcoma virus. Virology. 1972 Jan;47(1):1–7. doi: 10.1016/0042-6822(72)90232-2. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Fleissner E., Sarkar N. H., Aoki T. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. II. Mammalian leukemia-sarcoma viruses. J Virol. 1972 Feb;9(2):359–366. doi: 10.1128/jvi.9.2.359-366.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Fisher C. L., Stanley T. B., Gilden R. V. Proteins of the murine C-type RNA tumour viruses: isolation of a group-specific antigen by isoelectric focusing. J Gen Virol. 1970 Jul;8(1):1–10. doi: 10.1099/0022-1317-8-1-1. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Foreman C., Kelloff G., Gilden R. V. The group-specific antigen and other structural proteins of hamster and mouse C-type viruses. Virology. 1971 Mar;43(3):665–674. doi: 10.1016/0042-6822(71)90290-x. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]




