Abstract
Apart from its principal role in bone metabolism and calcium homeostasis, vitamin D has been attributed additional effects including an immunomodulatory, anti-inflammatory, and possibly even neuroprotective capacity which implicates a possible role of vitamin D in autoimmune diseases like multiple sclerosis (MS). Indeed, several lines of evidence including epidemiologic, preclinical, and clinical data suggest that reduced vitamin D levels and/or dysregulation of vitamin D homeostasis is a risk factor for the development of multiple sclerosis on the one hand, and that vitamin D serum levels are inversely associated with disease activity and progression on the other hand. However, these data are not undisputable, and many questions regarding the preventive and therapeutic capacity of vitamin D in multiple sclerosis remain to be answered. In particular, available clinical data derived from interventional trials using vitamin D supplementation as a therapeutic approach in MS are inconclusive and partly contradictory. In this review, we summarise and critically evaluate the existing data on the possible link between vitamin D and multiple sclerosis in light of the crucial question whether optimization of vitamin D status may impact the risk and/or the course of multiple sclerosis.
Keywords: Multiple sclerosis, Vitamin D, Cholecalciferol, Prevention, Therapy, Risk factor, Supplementation, Personalised medicine, Targeted prevention, Tailored therapy
Review
Multiple sclerosis: background information
Multiple sclerosis (MS) is the most common chronic inflammatory disease of the central nervous system (CNS) in young adults in Western countries and often leads to early disability and retirement [1]. Typical clinical manifestations are optic neuritis, central paralysis, sensory disturbances, and difficulties in coordination and balance, as well as cognitive dysfunction, fatigue, and sleep disorders [1-3]. The initial course is usually relapsing-remitting, but after several years, the disease tends to convert into a secondary progressive form. A primary progressive course also exists but is much less common. It is estimated that 2.5 million people suffer from MS worldwide, and as in most autoimmune disorders, there is an obvious female preponderance of approximately 3 to 4:1 [1,4]. Importantly, most female patients are affected in their child-bearing age which may have fundamental consequences for family planning [5]. The cause of MS is not yet clear. Several genetic and environmental factors have been isolated to contribute to the risk of MS, among them vitamin D (VD) status, but the individual significance of each factor is not yet clear [6-10]. From the pathophysiological point of view, dysregulated encephalitogenic T cells are thought to initiate and to orchestrate in concert with abundant other immune cells an autoimmune multifocal CNS inflammation [11-13]. For decades, MS was considered to be a primarily demyelinating disorder predominantly affecting the CNS white matter. During recent years, however, it has become clear that MS also has a strong neurodegenerative component, which is most probably the underlying basis for the development of permanent disability [14-17]. Moreover, grey matter involvement has been increasingly recognised by means of histopathology and magnetic resonance imaging (MRI) [18-20]. Paraclinical tools for diagnosis, differential diagnosis, and monitoring of disease activity and progression in MS comprise cerebrospinal fluid examination, evoked potentials, MRI, and recently, optical coherence tomography [1,21-24].
Current treatment concepts comprise the application of immunomodulatory or immunosuppressive drugs such as interferon-beta, glatiramer acetate, fingolimod, natalizumab, or mitoxantrone [25,26]. Approval of additional new drugs is expected. However, most if not all of these drugs lack convincing neuroprotective capacity. Moreover, a substantial number of patients do not respond satisfyingly to these drugs or experience severe side effects [27-31]. Overall, there is still a need for improved therapeutic approaches, especially in neuroprotective substances [32].
Vitamin D: background information
Research on VD started around 1915, stimulated by the quest for an effective treatment of rickets. By the end of the 19th century, up to 90% of the children living in large cities throughout Northern Europe and the United States suffered from rickets, and the most common cause was the insufficient supply of VD due to low sun exposure as a side effect of increasing industrialisation. The transformation to an industrialised economy radically changed the living conditions for large parts of the population. Children often had to work many hours a day in factories or mines, being completely shielded from the sun. When VD deficiency was recognised as the main cause of rickets, a significant reduction of cases was achieved by preventive measures like radiation from ultraviolet lamps, greater amount of time spent outdoors, or fortification of food with VD [33].
The VD supply of the human organism is generally accomplished via two different routes: first, endogenous synthesis of VD3 (cholecalciferol) from its precursor 7-dehydrocholesterol in an ultraviolet (UV) B radiation-dependent process in the skin (wave length 290 to 315 nm); second, exogenous supply with VD3 or VD2 (ergocalciferol) by food, fortified food products, or supplements [34,35]. About 90% to 100% of the VD requirement of a human body is covered by sun exposure-dependent endogenous production [33,36]. The amount of UVB-radiation dependent VD production depends on numerous factors including individual factors like duration and frequency of sun exposure, the area of skin exposed to the sun, use of sun protection, skin pigmentation, age, sex, genetic factors, amounts of 7-dehydrocholesterol in the skin; geographic factors like latitude and altitude; as well as seasonal and meteorological factors like clouding and ozone levels [36]. The magnitude of endogenous VD synthesis is referenced to the minimum erythema dose (MED) which describes the minimum individual dose of UVB radiation needed for the development of a transient skin irritation. One MED of the entire body equals the release of 10,000 to 20,000 IU (250 to 500 μg) of VD3 [1]. Compared to the endogenous production of VD3, the food-related intake of VD2/3 is usually of inferior importance since only few food products contain significant amounts of VD (Table 1).
Table 1.
Dietary sources of vitamin D[37]
| Food product | Vitamin D content (μg/100g) | Required daily intake (in g) for 20 μg vitamin D |
|---|---|---|
| Cod liver oil |
330 |
6 |
| Smoked eel |
22 |
91 |
| Salmon |
3.8 |
526 |
| Avocado |
3.43 |
583 |
| Egg yolk |
2.9 |
690 |
| Liver (beef) |
1.7 |
1,176 |
| Butter |
1.2 |
1,667 |
| Pork |
0.11 |
18,182 |
| Milk (3.5%) | 0.088 | 22,727 |
Both VD2 and VD3 are biologically inactive. After intradermal synthesis or intestinal uptake, VD2 and VD3 are bound mainly to vitamin-D-binding-protein and transported to the liver, where they are enzymatically hydroxylated to 25(OH)VD (calcidiol). As this step is not tightly regulated and because of the relatively long half-life, serum levels of 25(OH)VD integrate both the endogenous and exogenous supply and provide a good estimate of an organism's VD status. The enzyme 1α-hydroxylase (CYP27B1), which is located mainly in the kidneys but also in other tissues, converts 25(OH)VD in a second hydroxylation step into the biologically active 1,25 dihydroxyvitamin D (1,25(OH)2VD; calcitriol) [35]. Unlike the first hydroxylation, this second step is tightly regulated, among others by parathormone and calcium/phosphate levels [38]. Calcitriol effects are mainly mediated via the intracellular VD receptor (VDR) which functions as a transcription factor and controls the expression of numerous genes. In its membrane-bound form, VDR mediates additional non-genomic functions including several signal transduction pathways [39,40].
An ongoing debate addresses the optimal serum levels of 25(OH)VD. Currently, most experts consider 25(OH)VD levels above 30 ng/ml (75 nmol/l) as adequate [41-43], which is supported by the observations that serum levels of parathormone start plateauing at serum 25(OH)VD of 30 to 40 ng/ml and that immunological effects need serum levels around 30 ng/ml [35,44]. Levels below 20 ng/ml (50 nmol/l) are considered deficient. Less clear are the upper limits since substantial variability occurs in naturally occurring 25(OH)VD levels. According to the literature, levels of up to 150 to 200 ng/ml (375 to 500 nmol/l) can be considered safe [41]. Against this background, a significant proportion of the human population worldwide shows an alarming VD inadequacy [35,44-46].
Since VD homeostasis is linked on multiple levels to the risk of not only various diseases such as cancer and autoimmune diseases, but also metabolic, cardiovascular, and psychiatric disorders [35,42,47,48], the question arises whether improvement of VD supply may prevent or even treat respective diseases. Indeed, recent estimations indicate that yearly, >110,000 deaths could be prevented by adequate VD supply [49].
Linking vitamin D and MS: immunoregulatory functions of vitamin D
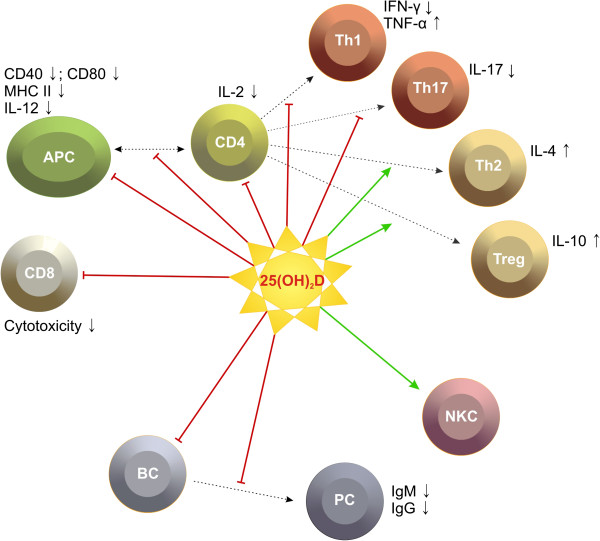
Apart from its fundamental role in calcium homeostasis and bone metabolism, increasing evidence suggests that VD has additional, particularly immunoregulatory functions which renders VD a promising candidate in both pathogenesis and treatment of autoimmune diseases such as MS. The capability of VD to modulate both innate and adaptive immune responses has been summarised in several comprehensive and excellent reviews [47,50-52]. With respect to the autoimmune MS pathophysiology [11,12], the following effects of VD on the immune system might be of particular interest: the ability to modulate the differentiation and function of antigen presenting cells which results in a reduced activation of potentially auto-aggressive T cells [53-55], the capacity to inhibit B cell and T cell proliferation and differentiation [56-58], the ability to shift the cytokine milieu from a pro-inflammatory, Th1/Th17-cell-mediated to a rather anti-inflammatory Th2-cell-mediated state [47,59], and finally, to facilitate the differentiation of regulatory T cells and function of natural killer cells [60,61]. Data on the VD effect on CD8 cells are still controversial. Figure 1 summarises the potential immunoregulatory effects of VD that might be pathophysiologically relevant in MS.
Figure 1.
Possible effects of vitamin D on immune cells. APC, antigen presenting cell; Th, T helper cell; Treg, regulatory T cell; BC, B cell; PC, plasma cell; NKC, natural killer cell. Figure was first published in [62] .
The presence of 1α-hydroxylase activity in neurons and microglia, and the presence of VD receptor in the CNS suggest local-, paracrine-, or autocrine-mediated effects of VD in the CNS [63,64]. Interestingly, data from in vitro or animal studies suggest that neurotrophic factors such as nerve growth factor, neurotrophin 3, and glial cell line-derived neurotrophic factor are regulated by VD which might indicate additional, possibly neuroprotective effects of VD [65]. Whether VD has clinically relevant neuroprotective properties still remains a subject of discussion.
Linking vitamin D and MS: how do genes contribute?
It is long known that genetic factors contribute to the risk of MS. In particular, an association with extended major histocompatibility complex haplotypes, especially those containing HLA-DRB1*1501, has been consistently shown in individuals of northern European ancestry [66,67]. The role of VD-related genes in determining MS risk or specific genetic interactions with VD is currently a hot focus of research and is not yet completely understood. So far, two interesting links merit mentioning: First, it was recently shown that the gene expression of allele HLA-DRB1*1501 is modulated by VD, and a highly conserved VD-responsive element has been identified in the promoter region of the HLA-DRB1*1501 haplotype, which may indicate a direct functional interaction between VD and the major locus determining genetic susceptibility to MS [68]. Second, loss of function variants in the CYP27B1 gene which encodes the enzyme that converts 25(OH)VD into its active form were shown to be associated with an increased MS risk [69]. In the same direction points a possible association between MS and VD-dependent rickets type I, which is a rare hereditary condition caused by a mutation in CYP27B1[70,71].
Linking vitamin D and MS: what do animal models tell us?
Further evidence for a causal relation between VD supply and both development and treatment of MS were derived from experimental autoimmune encephalomyelitis (EAE), the best established rodent animal model for MS. In murine EAE, prophylactic application of VD (starting at disease induction) resulted in a reduction of both disease incidence and severity. Likewise, the therapeutic VD application (starting at onset of symptoms) lead to a significant reduction of disease severity [72-74]. Interestingly, some studies suggested gender-specific efficacy of VD only in female mice [75]. In a recent study, continuous treatment of mice with UVR dramatically suppressed clinical signs of EAE. Interestingly, the therapeutic effect was paralleled by only a moderate and transient increase of serum 25(OH)VD levels, which suggests that directly UVR-mediated effects which were at least partly independent of VD contributed to this observation [76]. In another recent study using the cuprizone model, dietary VD could (partly) prevent chemically induced CNS demyelination in mice [77].
Linking vitamin D and MS: the clue to geographic and seasonal associations?
First hypotheses on a possible link between MS risk and VD deficiency were derived from the observation that the risk of MS is associated with latitude [78,79] which in turn shows a strong inverse correlation with UVB exposure. Furthermore, migrating from high to low latitude appears to reduce the MS risk [80]. This link was further corroborated by the observation of a MS risk lower than one would expect from the latitude in regions with a high consume of fatty VD-rich fish [81]. More recent investigations, however, suggest that this latitude gradient is fading which might be explained by several possible reasons, including better MS recognition, changes in lifestyle, and improvement of sanitary circumstances [34]. More indirect though not unambiguous support for a beneficial effect of VD comes from the observation that both MS risk and disease activity show a seasonal association. As shown in several studies including a very recent meta-analysis, humans born in spring have a significant higher risk to develop MS later in life than people born in autumn [82-85] which might be at least partially explained by longer in utero VD insufficiency due to lower motherly VD levels in winter/spring as compared to summer/autumn. Likewise, several methodically high quality studies showed an inverse association between sun exposure or outdoor activities during childhood and adolescence, and the risk of developing MS during adulthood [86-90]. In line with these reports is the recent observation that low sun exposure in fall/winter before disease onset was associated with a less favourable outcome [91]. Yet, all these studies have two major intrinsic limitations: first, despite a reported reasonable validity and reliability [92], the retrospective determination of sun exposure years or even decades in the past is inevitably subjected to recall bias [34], and prospective studies are hardly available. The determination of actinic damage as a surrogate parameter for cumulative sun exposition might be a viable loophole [34,86]. Second, sun exposure itself may have intrinsic immunomodulatory effects, independent of VD [76,93,94]. Also, not easy to harmonise with sun exposure or VD synthesis is the seasonal dependency of disease activity in already established MS. Several studies including a meta-analysis showed an excess of clinical exacerbations and MRI activity in spring/summer and a nadir in autumn/winter in the northern hemisphere [95-98]. Correspondingly, a reverse situation was observed in the southern hemisphere [99]. While a peak of disease activity in spring and a nadir in autumn in the northern hemisphere could be explained with a few-month lag in the course of serum VD levels, the situation in summer and winter does not easily fit with a protective role of VD. In conclusion, VD might contribute to some but cannot sufficiently explain all geographic and seasonal associations observed in MS.
Linking vitamin D and MS: the impact of vitamin D intake and serum level
The rather indirect impact of predictors of 25(OH)VD levels on MS has been discussed above. But, how does the 25(OH)VD serum level itself sway the risk and course of MS? Generally, if VD had a beneficial effect on MS risk, one would demand an inverse relation between VD intake or serum levels and MS incidence. Indeed, various studies demonstrated such a relation. Most data on this issue, however, are derived from epidemiologic or observational studies, meaning, that methodical limitations like selection bias, retrospective survey, and interference with various confounders should be kept in mind. One recent study suggests that already in utero levels of VD, which are completely dependent on the mother's VD status, impact the risk to develop MS later in life [100]. In a Canadian cohort study on children presenting with a first demyelinating event, the risk to develop definite MS within the following 3 years was inversely and independently correlated with the 25(OH)VD serum level [101]. Furthermore, data from a nested case–control study involving more than seven million individuals of the US military suggest that in healthy young white adults, higher 25(OH)VD levels are predictive of a significantly lower risk of developing MS (62% lower odds in the top quintile of 25(OH)VD serum levels compared to the bottom quintile), independent from latitude of residency in childhood [102]. Another study by the same group addressed the relation between VD intake and MS risk in a cohort of approximately 200,000 US women and reported a 33% reduction of MS incidence over a follow-up period of 30 years when comparing the top quintile and the bottom quintile of VD intake. Moreover, in women taking daily supplements containing at least 400 IU VD, a 41% lower MS incidence was observed when compared to women who did not take supplements [103]. Likewise, in another survey, intake of cod liver oil was associated with a 4-year delay of MS onset [90]. In summary, substantial evidence exists for an inverse association between VD and the risk of developing MS.
But, how does the situation look in already established MS? A number of studies consistently suggest that higher VD serum levels are associated with a more favourable disease course. In a small Finnish study, lower summer 25(OH)VD concentrations were measured in patients with a first MS relapse compared to healthy controls, and 25(OH)VD concentrations were significantly lower during relapses than in remission phases which may point to a regulative role of VD for MS activity [104]. Compelling support for this hypothesis comes from four independent recent reports, all showing a close relationship between clinical disease activity and 25(OH)VD concentrations: Two studies demonstrated that every 10 nmol/l increase of the VD serum level is correlated with a reduction of relapse occurrence of 11% and 13.7%, respectively [105,106]. A third study demonstrated a log linear association between serum VD concentration and MS relapse rate in that every doubling of serum levels reduced relapse rate by 27% [107]. The fourth study finally revealed a 34% reduction of relapse rate by every 10 ng/ml increase in paediatric onset MS [108]. In line with these clinical data, an inverse association between VD concentrations and disease activity on cranial MRI was recently demonstrated, but may possibly be restricted to patients without additional immunomodulatory treatment [109,110]. Of note, in studies addressing the relation between clinical disease activity and VD levels, a reverse association (low VD concentrations as a consequence rather than a cause of a relapse) cannot be completely ruled out. In contrast to the serum concentrations, a statistical difference in cerebrospinal fluid VD levels was neither observed between MS patients and healthy controls or in MS patients between phases of disease activity or in remission [111].
In conclusion, cumulating evidence quite consistently argues for a relationship between VD status and both risk and activity of MS. Of note, however, all these studies are methodically prone to bias and are therefore not suited to definitely proof such a relation.
Linking vitamin D and MS: what do interventional trials tell us?
The compelling evidence for the beneficial impact of higher VD serum concentrations on disease activity leads directly to two questions: (a) do patients with already established MS benefit from a therapeutic elevation of their VD levels and (b) if so, which 25(OH)VD serum levels should be strived for in MS patients? To reliably answer these crucial questions, high quality and sufficiently powered interventional trials are required. Moreover, important issues like optimal dosing schemes need further clarification. So far, there are only few prospective clinical studies on VD as a treatment for MS, some of them of rather questionable quality. An early uncontrolled study involving 16 MS patients (evidence level IIb) showed that regular intake of cod liver oil (equivalent to 5,000 IU VD/day) for a period of up to 2 years lead to a lower relapse rate as would have been expected from the participants' medical histories [112]. From today's point of view, design and sample size of this study are however not appropriate to address a therapeutic effect of VD. Another small and uncontrolled study with a primary focus on safety aspects (evidence level IIb) provided evidence that escalating VD doses up to 280,000 IU/week over a rather short period of 28 weeks are safe in MS patients. No significant effects on clinical parameters were observed, but there was a possible effect on MRI activity [113]. In a successive randomised controlled but open label study, the same group applied cholecalciferol (up to 40,000 or 4,000 IU/d) continuously for 52 weeks in 49 MS patients (evidence level Ib). Patients in the high dose arm showed a significant reduction of the annualised relapse rate [114]. In another randomised double blind and placebo-controlled study focusing on serological markers of disease activity (evidence level IIb), administration of 1,000 IE cholecalciferol for a period of 6 months lead to a significant increase of the anti-inflammatory cytokine transforming growth factor-β and to a partial reduction of the IL-2 level [115]. Another recent study (evidence level IIb) reported no significant differences between the high- or low-dose ergocalciferol on clinical and MRI parameters [116]. However, the design of this study (small number, inhomogeneous group of patients, short period of observation, relatively high dosages of “low-dose” ergocalciferol) has been criticised as not being suited to address the therapeutic capacity of VD in MS [117]. The capacity of low dose calcitriol to prevent disease progression in relapsing remitting multiple sclerosis (RRMS) when given in adjunction to undefined disease-modifying treatment strategies was investigated in a randomised and double blind but rather small study on 50 patients. After 12 months, no significant differences between verum and placebo arms were observed with respect to relapse rate and disability [118]. Two recently published studies from Finland and Norway, both applying 20,000 IU/week in a randomised, double blind and placebo-controlled design (evidence level Ib), yielded partly contradictory results with respect to clinical and MRI parameters. In the Finish study, mean 25(OH)VD serum levels in patients receiving VD over 1 year in addition to IFN-β increased to 110 nmol/l, and patients in the verum group showed significantly fewer gadolinium-enhancing lesions and a tendency to reduced disability accumulation and improved ambulation parameters. The annualised relapse rate was not different in both arms [119]. In an active subgroup of this study, an additional beneficial effect of VD on new/enlarging T2 hyperintense brain lesions was observed [120]. In the 96-week Norwegian trial, no significant differences were observed in annualised relapse rate, EDSS, MSFC, grip strength, or fatigue although the median 25(OH)VD serum concentration in the verum group raised to 121 nmol/l [121].
In summary, due to their ambiguous results, the so-far published interventional trials do not answer the question whether VD would be a treatment option in MS. The reasons for these heterogeneous results remain unclear. Given the substantial increase in serum concentration to greater 100 nmol/l in the two most recent trials [119,121], insufficient dosing is probably not a likely explanation. Further, well-designed interventional high-dose trials, which are at least partly better powered, are currently underway (Table 2) [122,123] and will hopefully contribute to elucidate the efficacy aspects.
Table 2.
Ongoing clinical trials on vitamin D in multiple sclerosis (registered at http://www.clinicaltrials.gov by January 2013)
| Trial title/registration number | Sponsor | Start date/estimated completion date | Intervention | Trial design | Number of participants | Main outcome parameters |
|---|---|---|---|---|---|---|
| Phase II study of efficacy of vitamin D supplementation in multiple sclerosis (EVIDIMS study) |
Charité-Universitätsmedizin Berlin, Germany |
December 2011/March 2015 |
Cholecalciferol 20,400 IU every other day (high dose) or cholecalciferol 400 IU/every other day (low dose) for 18 months |
Randomised double blind active controlled multicenter Phase II trial |
80 patients with RRMS or CIS |
Primary: cumulative number of new T2 lesions |
|
NCT01440062 |
|
|
Add-on to IFN β1b 250 μg every other day |
|
|
Secondary: annualised relapse rate, occurrence of disability progression, proportion of patients without disease activity, conversion rate into definite MS, cumulative number of T1 gadolinium-enhancing lesion, number and volume of new T1 hypointense lesions, number and volume of new T2 hyperintense lesions, changes in brain parenchymal volume, changes in magnet resonance spectroscopy, changes in retinal structure as determined by optical coherence tomography, changes in cognitive function and fatigue, change in health-related quality of life |
| Supplementation of VigantOL® oil versus placebo as add-on in patients with relapsing remitting multiple sclerosis receiving Rebif® treatment (SOLAR study) |
Merck-Serono GmbH |
February 2011/ March 2014 |
Cholecalciferol 14,000 IU/day or placebo for 96 weeks |
Randomised double blind placebo-controlled multicenter phase II trial |
348 patients with RRMS |
Primary: mean number of active lesions at week, proportion of relapse-free subjects |
|
NCT01285401 |
|
|
Add-on to IFN β1a 44 μg 3×/week |
|
|
Secondary: annualised relapse rate, proportion of subjects free from any EDSS progression, proportion of subjects free from disease activity, change in cognitive function, cumulative number of T1 gadolinium enhancing lesion, proportion of subjects free from new T1 hypointense lesions, change from baseline in the total volume of T2 lesions, percent brain volume change with respect to baseline |
| A multicentre, randomised, double-blind, placebo-controlled study of the efficacy of supplementary treatment with cholecalciferol in patients with relapsing multiple sclerosis treated with subcutaneous IFN Beta-1a 44 μg 3 times weekly |
Merck KGaA |
January 2010/July 2014 |
Cholecalciferol 2× 100,000 IU/month or placebo for 96 weeks |
Randomised double blind placebo-controlled multicenter phase II trial |
250 patients with RRMS |
Primary: reduction of relapse rate |
|
NCT01198132 |
|
|
Add-on to IFN β1a 44 μg 3×/week |
|
|
Secondary: number of relapse-free subjects, cumulative probability of progression of disability, number of new or extended lesions in T1- and T2-weighted MRI, changes in lesion load (T2), number of new lesions (T1 gadolinium activity and black holes), measurement and evaluation of cognitive ability, change in quality of life, safety of the treatment |
| A pilot study to assess the relative safety and immunology effects of low dose versus high dose cholecalciferol supplementation in patients with multiple sclerosis |
Johns Hopkins University, Baltimore, USA |
March 2010/December 2011 |
Cholecalciferol 10,000 IU/day (high dose) or cholecalciferol 400 IU/day (low dose) for 6 months |
Randomised double blind controlled multicenter phase II trial |
40 MS patients with or without immunomodulatory treatment and serum 25(OH)VD levels between 20–50 ng/ml |
Primary: safety of high-dose cholecalciferol, effects of cholecalciferol supplementation on serum immune markers |
|
NCT01024777 |
|
|
|
|
|
Secondary: clinical effects of cholecalciferol supplementation |
| A randomised controlled trial of vitamin D supplementation in multiple sclerosis |
Johns Hopkins University, Baltimore, USA |
March 2012/December 2014 |
Cholecalciferol 5,000 IU/day (high dose) or cholecalciferol 600 IU/day (low dose) for 24 months |
Randomised double blind controlled multicenter phase III trial |
172 RRMS patients with 25(OH)D-serum levels ≥ 15 ng/ml |
Primary: proportion of subjects that experience a relapse |
|
NCT01490502 |
|
|
Add-on to glatiramer acetate 20 mg/day |
|
|
Secondary: annualised relapse rate, occurrence of sustained disability progression, number of new T2 lesions, changes in brain parenchymal volume and cortical thickness, change in low-contrast visual acuity, change in health-related quality of life, development of hypercalcemia/related adverse effects |
| Pharmacodynamic and immunologic effects of vitamin D supplementation in patients with multiple sclerosis and healthy controls |
Johns Hopkins University, Baltimore, USA |
November 2010/June 2013 |
Cholecalciferol 5,000 IU/day for 90 days |
Non-randomised, open label single group assignment multicenter phase 1 trial |
60 patients with RRMS or healthy individuals |
Primary: change in mean serum level of 25(OH)VD |
|
NCT01667796 |
|
|
|
|
|
Secondary: cytokine levels and percentages of T and B cells, gene expression microarray |
| Role of vitamin D on the relapse rate of multiple sclerosis |
AlJohara M AlQuaiz, M.D., King Saud University, Saudi Arabia |
January 2013/October 2014 |
Cholecalciferol 50,000 IU/week or placebo for 12 months |
Randomised double blind controlled single centre phase II trial |
200 patients with RRMS |
Primary: relapse rate |
|
NCT01753375 |
|
|
|
|
|
Secondary: improvement in the EDSS score |
| Dose-related effects of vitamin D3 on immune responses in patients with clinically isolated syndrome and healthy control participants. An exploratory double blind placebo randomised controlled study |
University College Dublin, Ireland |
November 2012/May 2014 |
Cholecalciferol 5,000 IU/day or 10,000 IU/day or placebo for 24 weeks |
Randomised double blind placebo-controlled phase II trial |
45 patients with CIS without any immunomodulatory treatment and 39 healthy individuals |
Primary: change in the frequency of CD4 T cell subsets and cytokine responses of periphery blood mononuclear cells |
| NCT01728922 | Secondary: relapse occurrence, percentage of CIS patients in each treatment arm free from any evidence of disease activity, number of new T2 and gadolinium-enhancing lesions |
Abbreviations: CIS clinically isolated syndrome, EDSS expanded disability status scale, IFN interferon, IU international units; RRMS relapsing remitting multiple sclerosis.
With respect to safety, more clinical data already exist. Generally, (iatrogenic) VD excess can result in life-threatening hypercalcaemia and has been occasionally reported on the basis of single cases [124]. However, unlike supplementation with high dose calcitriol, which indeed seems to bear a significant risk of symptomatic hypercalcaemia [125], treatment of MS patients with even very high doses of cholecalciferol or ergocalciferol was repeatedly demonstrated to be safe [113,114,116,119,121]. While a Cochrane report published in 2010 concludes that available data are not yet sufficient to draw the right conclusions regarding safety of VD supplementation [126], another recent meta-analysis suggests that daily doses of 10,000 IE cholecalciferol can be considered safe [127].
Conclusions
In this review article, which follows the recommendations of the “EPMA White Paper” [128], we summarise and discuss available data on the role of VD for the development and disease course of MS. Many lines of evidence, in particular epidemiologic data, preclinical investigations, animal studies, and association studies on VD status and disease activity, suggest that higher serum concentrations of VD are beneficial in terms of the risk to develop MS as well as the further course of the disease in already-established MS. Moreover, VD supplementation is safe, cheap, and convenient to perform. Therefore, it is intriguing to hypothesise that boosting the VD serum levels would be an option to both prevent and treat MS. Despite the inherent methodological drawbacks of epidemiologic studies, existing data on the preventive capacity of higher VD levels are quite compelling. Final proof of this hypothesis would be reached by large-scale prospective epidemiological studies which will probably not be available in the near future, for obvious reasons. With respect to the therapeutic efficacy, an association between higher VD serum concentrations and a favourable disease course has been conclusively shown. Unfortunately, the so-far performed interventional trials, though not negotiating this hypothesis, also do not unambiguously support the idea that raising patients' VD levels would be favourably in terms of disease outcome. Hopefully, ongoing (Table 2) and future trials will shed more light on this aspect.
But, how are we going to deal with this issue in the meantime? From a pragmatical point of view and considering available data on efficacy, safety, tolerability, and last but not least costs, it seems to be reasonable to regularly control 25(OH)VD levels in MS patients, especially during winter months. In patients with inadequate VD, levels should be raised to at least 30–40 ng/ml (75–100 mmol/l), either by appropriate sun exposure and/or adequate VD supplementation. As a rule of thumb, supplementary 1 μg (40 IU) cholecalciferol will increase 25(OH)VD levels by 1 ng/ml (2.5 nmol/l).
Competing interests
JD received a limited grant for clinical research on vitamin D by Bayer Healthcare, speaker honoraria by Bayer Healthcare, Novartis, and Teva, consultancy honoraria by Bayer Healthcare, and travel support by Merck-Serono, Novartis, and Bayer Healthcare. FP received speaker honoraria and travel support by Bayer Healthcare, Teva, Sanofi-Aventis/Genzyme, Biogen Idec, Novartis, and Merck-Serono. FP is supported by the Artur-Arnstein Foundation Berlin, Germany, and has received travel reimbursement from the Guthy Jackson Charitable Foundation. AD declares no competing interests.
Authors’ contributions
AD and JD drafted the manuscript and did the literature search. FP critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Authors’ information
JD is a neurologist and a senior physician/scientist at the NeuroCure Clinical Research Center (NCRC), Charité-Universitätsmedizin Berlin, Germany, and has a research focus on the role of VD in MS. As head of the NCRC Neuroimmunological Study Outpatient Department, he is currently running a therapeutic phase II clinical trial on VD in MS. AD is a physician; she completed part of her neurological training at the NCRC. FP is a senior neurologist and head of the Neuroimmunological Group at the NCRC. His research focus is the translation of new diagnostic tools and therapeutic approaches in MS and related disorders into the clinical setting.
Contributor Information
Jan Dörr, Email: jan-markus.doerr@charite.de.
Andrea Döring, Email: andrea.doering@charite.de.
Friedemann Paul, Email: friedemann.paul@charite.de.
Acknowledgments
This work was supported by the DFG (Exc 257).
References
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Weinges-Evers N, Brandt AU, Bock M, Pfueller CF, Dörr J, Bellmann-Strobl J, Scherer P, Urbanek C, Boers C, Ohlraun S, Zipp F, Paul F. Correlation of self-assessed fatigue and alertness in multiple sclerosis. Mult Scler. 2010;16:1134–1140. doi: 10.1177/1352458510374202. [DOI] [PubMed] [Google Scholar]
- Veauthier C, Radbruch H, Gaede G, Pfueller C, Dörr J, Bellmann-Strobl J, Wernecke K-D, Zipp F, Paul F, Sieb J. Fatigue in multiple sclerosis is closely related to sleep disorders: a polysomnographic cross-sectional study. Mult Scler. 2011;17:613–622. doi: 10.1177/1352458510393772. [DOI] [PubMed] [Google Scholar]
- Handel AE, Jarvis L, McLaughlin R, Fries A, Ebers GC, Ramagopalan SV. The epidemiology of multiple sclerosis in Scotland: inferences from hospital admissions. PLoS One. 2011;6:e14606. doi: 10.1371/journal.pone.0014606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisow N, Döring A, Pfueller CF, Paul F, Dörr J, Hellwig K. Expert recommendations to personalization of medical approaches in treatment of multiple sclerosis: an overview of family planning and pregnancy. EPMA J. 2012;3:9. doi: 10.1186/1878-5085-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol. 2007;61:504–513. doi: 10.1002/ana.21141. [DOI] [PubMed] [Google Scholar]
- Handel AE, Giovannoni G, Ebers GC, Ramagopalan SV. Environmental factors and their timing in adult-onset multiple sclerosis. Nat Rev Neurol. 2010;6:156–166. doi: 10.1038/nrneurol.2010.1. [DOI] [PubMed] [Google Scholar]
- Goodin DS. The causal cascade to multiple sclerosis: a model for MS pathogenesis. PLoS One. 2009;4:e4565. doi: 10.1371/journal.pone.0004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010;9:727–739. doi: 10.1016/S1474-4422(10)70094-6. [DOI] [PubMed] [Google Scholar]
- McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122:1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegert DG. Multiple sclerosis: a disorder of altered T-cell homeostasis. Mult Scler Int. 2011;2011:461304. doi: 10.1155/2011/461304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick RA, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS, Antel JP, Arnold DL. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain. 1998;121(Pt 8):1469–1477. doi: 10.1093/brain/121.8.1469. [DOI] [PubMed] [Google Scholar]
- Vogt J, Paul F, Aktas O, Muller-Wielsch K, Dörr J, Dörr S, Bharathi BS, Glumm R, Schmitz C, Steinbusch H, Raine CS, Tsokos M, Nitsch R, Zipp F. Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Ann Neurol. 2009;66:310–322. doi: 10.1002/ana.21719. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Sinnecker T, Mittelstaedt P, Dörr J, Pfueller CF, Harms L, Niendorf T, Paul F, Wuerfel J. Multiple sclerosis lesions and irreversible brain tissue damage: a comparative ultrahigh-field strength magnetic resonance imaging study. Arch Neurol. 2012;69:739–745. doi: 10.1001/archneurol.2011.2450. [DOI] [PubMed] [Google Scholar]
- Sinnecker T, Dörr J, Pfueller CF, Harms L, Ruprecht K, Jarius S, Brück W, Niendorf T, Wuerfel J, Friedemann P. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708–714. doi: 10.1212/WNL.0b013e3182648bc8. [DOI] [PubMed] [Google Scholar]
- Bock M, Brandt AU, Dörr J, Kraft H, Weinges-Evers N, Gaede G, Pfueller CF, Herges K, Radbruch H, Ohlraun S, Bellmann-Strobl J, Kuchenbecker J, Zipp F, Paul F. Patterns of retinal nerve fiber layer loss in multiple sclerosis patients with or without optic neuritis and glaucoma patients. Clin Neurol Neurosurg. 2010;112:647–652. doi: 10.1016/j.clineuro.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Oberwahrenbrock T, Schippling S, Ringelstein M, Kaufhold F, Zimmermann H, Keser N, Young KL, Harmel J, Hartung H-P, Martin R, Paul F, Aktas O, Brandt AU. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int. 2012;2012:530305. doi: 10.1155/2012/530305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörr J, Wernecke KD, Bock M, Gaede G, Wuerfel JT, Pfueller CF, Bellmann-Strobl J, Freing A, Brandt AU, Friedemann P. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One. 2011;6:e18132. doi: 10.1371/journal.pone.0018132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Río J, Comabella M, Montalban X. Multiple sclerosis: current treatment algorithms. Curr Opin Neurol. 2011;24:230–237. doi: 10.1097/WCO.0b013e328346bf66. [DOI] [PubMed] [Google Scholar]
- Buck D, Hemmer B. Treatment of multiple sclerosis: current concepts and future perspectives. J Neurol. 2011;258:1747–1762. doi: 10.1007/s00415-011-6101-2. [DOI] [PubMed] [Google Scholar]
- Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence, detection, and management. Neurology. 2012;78:672–680. doi: 10.1212/WNL.0b013e318248deea. [DOI] [PubMed] [Google Scholar]
- Paul F, Dörr J, Wurfel J, Vogel HP, Zipp F. Early mitoxantrone-induced cardiotoxicity in secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2007;78:198–200. doi: 10.1136/jnnp.2006.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen PS, Bertolotto A, Edan G, Giovannoni G, Gold R, Havrdova E, Kappos L, Kieseier BC, Montalban X, Olsson T. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler. 2012;18:143–152. doi: 10.1177/1352458511435105. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler. 2012;18:932–946. doi: 10.1177/1352458511433302. [DOI] [PubMed] [Google Scholar]
- Limmroth V, Putzki N, Kachuck NJ. The interferon beta therapies for treatment of relapsing-remitting multiple sclerosis: are they equally efficacious? A comparative review of open-label studies evaluating the efficacy, safety, or dosing of different interferon beta formulations alone or in combination. Ther Adv Neurol Disord. 2011;4:281–296. doi: 10.1177/1756285611413825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RJ. Primary neuroprotection: the Holy Grail of multiple sclerosis therapy. Neurology. 2010;74:1018–1019. doi: 10.1212/WNL.0b013e3181d6b165. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol. 2010;9:599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, Vestergaard P. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int. 2009;20:133–140. doi: 10.1007/s00198-008-0626-y. [DOI] [PubMed] [Google Scholar]
- Database of the German Institut für Ernährungsinformation, Freudenstadt, Germany. [ http://www.ernaehrung.de/lebensmittel]
- White JH. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch Biochem Biophys. 2012;523:58–63. doi: 10.1016/j.abb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α, 25(OH)2 vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Carlberg C, Molnar F. Current status of vitamin D signaling and its therapeutic applications. Curr Top Med Chem. 2012;12:528–547. doi: 10.2174/156802612799436623. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souberbielle JC, Body JJ, Lappe JM, Plebani M, Shoenfeld Y, Wang TJ, Bischoff-Ferrari HA, Cavalier E, Ebeling PR, Fardellone P, Gandini S, Gruson D, Guérin AP, Heickendorff L, Hollis BW, Ish-Shalom S, Jean G, Von Landenberg P, Largura A, Olsson T, Pierrot-Deseilligny C, Pilz S, Tincani A, Valcour A, Zittermann A. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010;9:709–715. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. 2008;624:55–71. doi: 10.1007/978-0-387-77574-6_5. [DOI] [PubMed] [Google Scholar]
- Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- Hintzpeter B, Mensink GBM, Thierfelder W, Müller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- Peelen E, Knippenberg S, Muris AH, Thewissen M, Smolders J, Tervaert JWC, Hupperts R, Damoiseaux J. Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmun Rev. 2011;10:733–743. doi: 10.1016/j.autrev.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WB. An estimate of the global reduction in mortality rates through doubling vitamin D levels. Eur J Clin Nutr. 2011;65:1016–1026. doi: 10.1038/ejcn.2011.68. [DOI] [PubMed] [Google Scholar]
- Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- Correale J, Ysrraelit MC, Gaitán MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134:123–139. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45:190–197. doi: 10.1016/j.cyto.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire JM, Adams JS, Sakai R, Jordan SC. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74:657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- Bhalla AK, Amento EP, Serog B, Glimcher LH. 1,25-dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133:1748–1754. [PubMed] [Google Scholar]
- Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- Yu S, Cantorna MT. Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. J Immunol. 2011;186:1384–1390. doi: 10.4049/jimmunol.1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders J, Thewissen M, Peelen E, Menheere P, Tervaert JWC, Damoiseaux J, Hupperts R. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One. 2009;4:e6635. doi: 10.1371/journal.pone.0006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring A, Paul F, Dörr J. Vitamin D and multiple sclerosis: the role for risk of disease and treatment. Nervenarzt. 2012. [DOI] [PubMed]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/S1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- de Abreu DAF, Eyles D, Féron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34(Suppl 1):S265–S277. doi: 10.1016/j.psyneuen.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Dyment DA, Sadovnick AD, Ebers GC, Sadnovich AD. Genetics of multiple sclerosis. Hum Mol Genet. 1997;6:1693–1698. doi: 10.1093/hmg/6.10.1693. [DOI] [PubMed] [Google Scholar]
- Sadovnick AD. Genetic background of multiple sclerosis. Autoimmun Rev. 2012;11:163–166. doi: 10.1016/j.autrev.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Ramagopalan SV, Maugeri NJ, Handunnetthi L, Lincoln MR, Orton S-M, Dyment DA, Deluca GC, Herrera BM, Chao MJ, Sadovnick AD, Ebers GC, Knight JC. Expression of the multiple sclerosis-associated MHC class II Allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009;5:e1000369. doi: 10.1371/journal.pgen.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopalan SV, Dyment DA, Cader MZ, Morrison KM, Disanto G, Morahan JM, Berlanga-Taylor AJ, Handel A, De Luca GC, Sadovnick AD, Lepage P, Montpetit A, Ebers GC. Rare variants in the CYP27B1 gene are associated with multiple sclerosis. Ann Neurol. 2011;70:881–886. doi: 10.1002/ana.22678. [DOI] [PubMed] [Google Scholar]
- Torkildsen Ø, Knappskog PM, Nyland HI, Myhr KM. Vitamin D-dependent rickets as a possible risk factor for multiple sclerosis. Arch Neurol. 2008;65:809–811. doi: 10.1001/archneur.65.6.809. [DOI] [PubMed] [Google Scholar]
- Ramagopalan SV, Hanwell HEC, Giovannoni G, Knappskog PM, Nyland HI, Myhr K-M, Ebers GC, Torkildsen O. Vitamin D-dependent rickets, HLA-DRB1, and the risk of multiple sclerosis. Arch Neurol. 2010;67:1034–1035. doi: 10.1001/archneurol.2010.182. [DOI] [PubMed] [Google Scholar]
- Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Nashold FE, Spach KM, Hayes CE. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res. 2007;85:2480–2490. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175:4119–4126. doi: 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- Becklund BR, Severson KS, Vang SV, DeLuca HF. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci USA. 2010;107:6418–6423. doi: 10.1073/pnas.1001119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wergeland S, Torkildsen O, Myhr KM, Aksnes L, Mørk SJ, Bø L. Dietary vitamin D3 supplements reduce demyelination in the cuprizone model. PLoS One. 2011;6:e26262. doi: 10.1371/journal.pone.0026262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson ED, Bachrach CA, Wright FM. Some comments on the relationship of the distribution of multiple sclerosis to latitude, solar radiation, and other variables. Acta Psychiatr Scand Suppl. 1960;35:132–147. doi: 10.1111/j.1600-0447.1960.tb08674.x. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. An evaluation of the geographic distribution of multiple sclerosis. Acta Neurol Scand. 1966;42(Suppl 19):91. doi: 10.1111/j.1600-0404.1966.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Swank RL, Lerstad O, Strom A, Backer J. Multiple sclerosis in rural Norway its geographic and occupational incidence in relation to nutrition. N Engl J Med. 1952;246:722–728. doi: 10.1056/NEJM195205082461901. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF, Beebe GW, Norman JE Jr. Epidemiology of multiple sclerosis in US veterans: III. Migration and the risk of MS. Neurology. 1985;35:672–678. doi: 10.1212/WNL.35.5.672. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC. Timing of birth and risk of multiple sclerosis: population based study. BMJ. 2005;330:120. doi: 10.1136/bmj.38301.686030.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgiu S, Pugliatti M, Sotgiu MA, Fois ML, Arru G, Sanna A, Rosati G. Seasonal fluctuation of multiple sclerosis births in Sardinia. J Neurol. 2006;253:38–44. doi: 10.1007/s00415-005-0917-6. [DOI] [PubMed] [Google Scholar]
- Salzer J, Svenningsson A, Sundström P. Season of birth and multiple sclerosis in Sweden. Acta Neurol Scand. 2010;122:70–73. doi: 10.1111/j.1600-0404.2010.01396.x. [DOI] [PubMed] [Google Scholar]
- Dobson R, Giovannoni G, Ramagopalan S. The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude. J Neurol Neurosurg Psychiatr. 2012. [DOI] [PubMed]
- Van der Mei IAF, Ponsonby AL, Dwyer T, Blizzard L, Simmons R, Taylor BV, Butzkueven H, Kilpatrick T. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case–control study. BMJ. 2003;327:316. doi: 10.1136/bmj.327.7410.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam T, Gauderman WJ, Cozen W, Mack TM. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology. 2007;69:381–388. doi: 10.1212/01.wnl.0000268266.50850.48. [DOI] [PubMed] [Google Scholar]
- Dalmay F, Bhalla D, Nicoletti A, Cabrera-Gomez JA, Cabre P, Ruiz F, Druet-Cabanac M, Dumas M, Preux PM. Multiple sclerosis and solar exposure before the age of 15 years: case–control study in Cuba, Martinique and Sicily. Mult Scler. 2010;16:899–908. doi: 10.1177/1352458510366856. [DOI] [PubMed] [Google Scholar]
- Kampman MT, Wilsgaard T, Mellgren SI. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol. 2007;254:471–477. doi: 10.1007/s00415-006-0395-5. [DOI] [PubMed] [Google Scholar]
- McDowell TY, Amr S, Culpepper WJ, Langenberg P, Royal W, Bever C, Bradham DD. Sun exposure, vitamin D and age at disease onset in relapsing multiple sclerosis. Neuroepidemiology. 2011;36:39–45. doi: 10.1159/000322512. [DOI] [PubMed] [Google Scholar]
- McDowell TY, Amr S, Culpepper WJ, Langenberg P, Royal W, Bever C, Bradham DD. Sun exposure, vitamin D intake and progression to disability among veterans with progressive multiple sclerosis. Neuroepidemiology. 2011;37:52–57. doi: 10.1159/000329258. [DOI] [PubMed] [Google Scholar]
- Van der Mei IAF, Blizzard L, Ponsonby AL, Dwyer T. Validity and reliability of adult recall of past sun exposure in a case–control study of multiple sclerosis. Cancer Epidemiol Biomarkers Prev. 2006;15:1538–1544. doi: 10.1158/1055-9965.EPI-05-0969. [DOI] [PubMed] [Google Scholar]
- Lucas RM, Ponsonby AL, Dear K, Valery PC, Pender MP, Taylor BV, Kilpatrick TJ, Dwyer T, Coulthard A, Chapman C, Van der Mei I, Williams D, McMichael AJ. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology. 2011;76:540–548. doi: 10.1212/WNL.0b013e31820af93d. [DOI] [PubMed] [Google Scholar]
- Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol. 2011;11:584–596. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- Jin Y, De Pedro-Cuesta J, Söderström M, Stawiarz L, Link H. Seasonal patterns in optic neuritis and multiple sclerosis: a meta-analysis. J Neurol Sci. 2000;181:56–64. doi: 10.1016/S0022-510X(00)00408-1. [DOI] [PubMed] [Google Scholar]
- Handel AE, Disanto G, Jarvis L, McLaughlin R, Fries A, Ebers GC, Ramagopalan SV. Seasonality of admissions with multiple sclerosis in Scotland. Eur J Neurol. 2011;18:1109–1111. doi: 10.1111/j.1468-1331.2010.03318.x. [DOI] [PubMed] [Google Scholar]
- Meier DS, Balashov KE, Healy B, Weiner HL, Guttmann CRG. Seasonal prevalence of MS disease activity. Neurology. 2010;75:799–806. doi: 10.1212/WNL.0b013e3181f0734c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer DP, Schumann EM, Kümpfel T, Gössl C, Trenkwalder C. Seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000;47:276–277. doi: 10.1002/1531-8249(200002)47:2<276::AID-ANA28>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Tremlett H, Van der Mei IAF, Pittas F, Blizzard L, Paley G, Mesaros D, Woodbaker R, Nunez M, Dwyer T, Taylor BV, Ponsonby AL. Monthly ambient sunlight, infections and relapse rates in multiple sclerosis. Neuroepidemiology. 2008;31:271–279. doi: 10.1159/000166602. [DOI] [PubMed] [Google Scholar]
- Mirzaei F, Michels KB, Munger K, O'Reilly E, Chitnis T, Forman MR, Giovannucci E, Rosner B, Ascherio A. Gestational vitamin D and the risk of multiple sclerosis in offspring. Ann Neurol. 2011;70:30–40. doi: 10.1002/ana.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banwell B, Bar-Or A, Arnold DL, Sadovnick D, Narayanan S, McGowan M, O'Mahony J, Magalhaes S, Hanwell H, Vieth R, Tellier R, Vincent T, Disanto G, Ebers G, Wambera K, Connolly MB, Yager J, Mah JK, Booth F, Sebire G, Callen D, Meaney B, Dilenge ME, Lortie A, Pohl D, Doja A, Venketaswaran S, Levin S, Macdonald EA, Meek D. et al. Clinical, environmental, and genetic determinants of multiple sclerosis in children with acute demyelination: a prospective national cohort study. Lancet Neurol. 2011;10:436–445. doi: 10.1016/S1474-4422(11)70045-X. [DOI] [PubMed] [Google Scholar]
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- Munger KL, Zhang SM, O'Reilly E, Hernán MA, Olek MJ, Willett WC, Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.WNL.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- Soilu-Hänninen M, Airas L, Mononen I, Heikkilä A, Viljanen M, Hänninen A. 25-Hydroxyvitamin D levels in serum at the onset of multiple sclerosis. Mult Scler. 2005;11:266–271. doi: 10.1191/1352458505ms1157oa. [DOI] [PubMed] [Google Scholar]
- Simpson S Jr, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, Dwyer T, Gies P, Van der Mei I. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68:193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud-Péchoux S, Clerson P, De Paz R, Souberbielle JC. Relationship between 25-OH-D serum level and relapse rate in multiple sclerosis patients before and after vitamin D supplementation. Ther Adv Neurol Disord. 2012;5:187–198. doi: 10.1177/1756285612447090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runia TF, Hop WCJ, De Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79:261–266. doi: 10.1212/WNL.0b013e31825fdec7. [DOI] [PubMed] [Google Scholar]
- Mowry EM, Krupp LB, Milazzo M, Chabas D, Strober JB, Belman AL, McDonald JC, Oksenberg JR, Bacchetti P, Waubant E. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol. 2010;67:618–624. doi: 10.1002/ana.21972. [DOI] [PubMed] [Google Scholar]
- Mowry EM, Waubant E, McCulloch CE, Okuda DT, Evangelista AA, Lincoln RR, Gourraud P-A, Brenneman D, Owen MC, Qualley P, Bucci M, Hauser SL, Pelletier D. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72:234–240. doi: 10.1002/ana.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løken-Amsrud KI, Holmøy T, Bakke SJ, Beiske AG, Bjerve KS, Bjørnarå BT, Hovdal H, Lilleås F, Midgard R, Pedersen T, Benth JS, Sandvik L, Torkildsen O, Wergeland S, Myhr K-M. Vitamin D and disease activity in multiple sclerosis before and during interferon-β treatment. Neurology. 2012;79:267–273. doi: 10.1212/WNL.0b013e31825fdf01. [DOI] [PubMed] [Google Scholar]
- Holmøy T, Moen SM, Gundersen TA, Holick MF, Fainardi E, Castellazzi M, Casetta I. 25-hydroxyvitamin D in cerebrospinal fluid during relapse and remission of multiple sclerosis. Mult Scler. 2009;15:1280–1285. doi: 10.1177/1352458509107008. [DOI] [PubMed] [Google Scholar]
- Goldberg P, Fleming MC, Picard EH. Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses. 1986;21:193–200. doi: 10.1016/0306-9877(86)90010-1. [DOI] [PubMed] [Google Scholar]
- Kimball SM, Ursell MR, O'Connor P, Vieth R. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr. 2007;86:645–651. doi: 10.1093/ajcn/86.3.645. [DOI] [PubMed] [Google Scholar]
- Burton JM, Kimball S, Vieth R, Bar-Or A, Dosch H-M, Cheung R, Gagne D, D'Souza C, Ursell M, O'Connor P. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010;74:1852–1859. doi: 10.1212/WNL.0b013e3181e1cec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134:128–132. doi: 10.1016/S0165-5728(02)00396-X. [DOI] [PubMed] [Google Scholar]
- Stein MS, Liu Y, Gray OM, Baker JE, Kolbe SC, Ditchfield MR, Egan GF, Mitchell PJ, Harrison LC, Butzkueven H, Kilpatrick TJ. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology. 2011;77:1611–1618. doi: 10.1212/WNL.0b013e3182343274. [DOI] [PubMed] [Google Scholar]
- Grimaldi L, Barkhof F, Beelke M, Burton J, Holmoy T, Hupperts R, Killestein J, Rieckmann P, Schluep M, Smolders J. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology. 2012;78:841. doi: 10.1212/01.wnl.0000413180.13413.ce. [DOI] [PubMed] [Google Scholar]
- Shaygannejad V, Janghorbani M, Ashtari F, Dehghan H. Effects of adjunct low-dose vitamin d on relapsing-remitting multiple sclerosis progression: preliminary findings of a randomized placebo-controlled trial. Mult Scler Int. 2012;2012:452541. doi: 10.1155/2012/452541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soilu-Hänninen M, Aivo J, Lindström B-M, Elovaara I, Sumelahti M-L, Färkkilä M, Tienari P, Atula S, Sarasoja T, Herrala L, Keskinarkaus I, Kruger J, Kallio T, Rocca MA, Filippi M. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83:565–571. doi: 10.1136/jnnp-2011-301876. [DOI] [PubMed] [Google Scholar]
- Aivo J, Lindsröm B-M, Soilu-Hänninen M. A randomised, double-blind, placebo-controlled trial with vitamin D3 in MS: subgroup analysis of patients with baseline disease activity despite interferon treatment. Mult Scler Int. 2012;2012:802796. doi: 10.1155/2012/802796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman MT, Steffensen LH, Mellgren SI, Jørgensen L. Effect of vitamin D3 supplementation on relapses, disease progression and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler. 2012;18:1144–1151. doi: 10.1177/1352458511434607. [DOI] [PubMed] [Google Scholar]
- Smolders J, Hupperts R, Barkhof F, Grimaldi LME, Holmoy T, Killestein J, Rieckmann P, Schluep M, Vieth R, Hostalek U, Ghazi-Visser L, Beelke M. Efficacy of vitamin D(3) as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon beta-1a: a Phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci. 2011;311:44–49. doi: 10.1016/j.jns.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Dörr J, Ohlraun S, Skarabis H, Paul F. Efficacy of Vitamin D Supplementation in Multiple Sclerosis (EVIDIMS Trial): study protocol for a randomized controlled trial. Trials. 2012;13:15. doi: 10.1186/1745-6215-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JF, Shalev SM, Harris CA, Goodin DS, Josephson SA. Severe hypercalcemia following vitamin D supplementation in a patient with multiple sclerosis: a note of caution. Arch Neurol. 2012;69:129–132. doi: 10.1001/archneurol.2011.1199. [DOI] [PubMed] [Google Scholar]
- Wingerchuk DM, Lesaux J, Rice GPA, Kremenchutzky M, Ebers GC. A pilot study of oral calcitriol (1,25-dihydroxyvitamin D3) for relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatr. 2005;76:1294–1296. doi: 10.1136/jnnp.2004.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath VA, Fedorowicz Z, Asokan GV, Robak EW, Whamond L. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst Rev. 2010;12:CD008422. doi: 10.1002/14651858.CD008422.pub2. [DOI] [PubMed] [Google Scholar]
- Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- Golubnitschaja O, Costigliola V. EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3:14. doi: 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]