Abstract
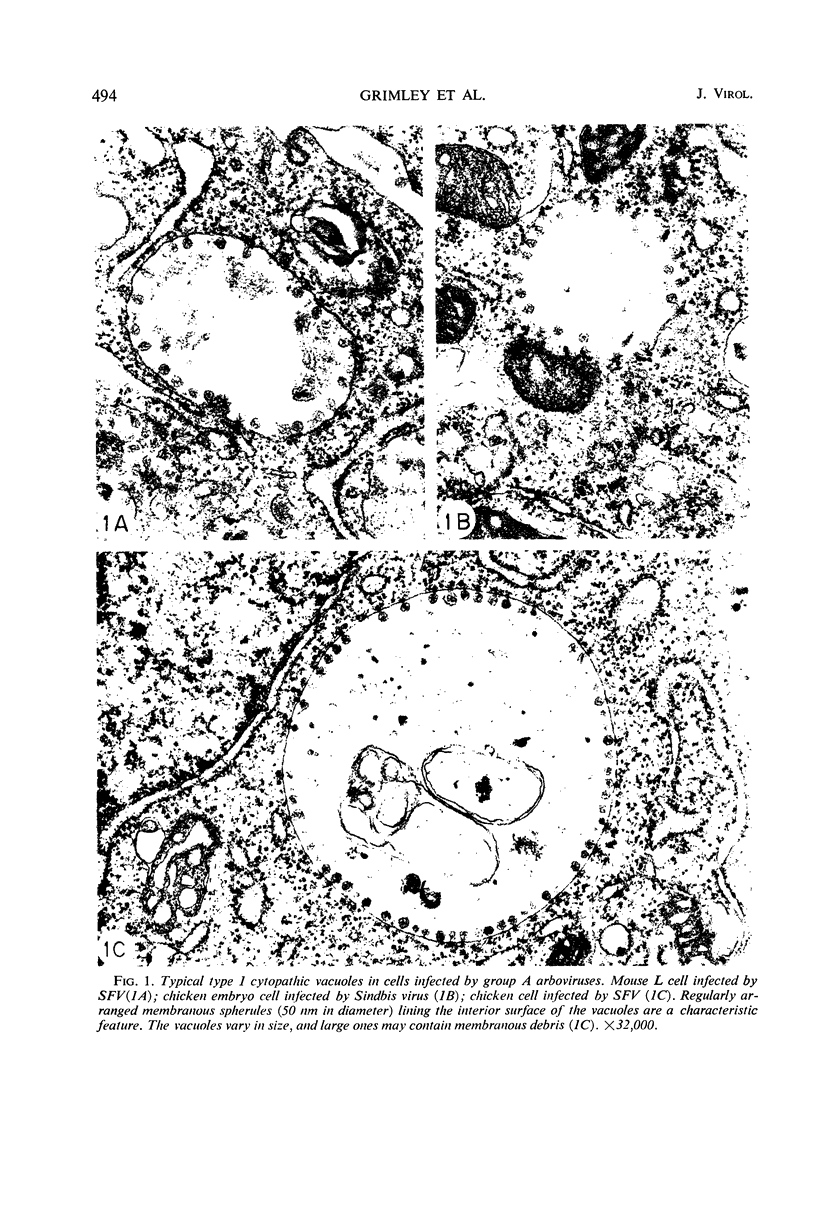
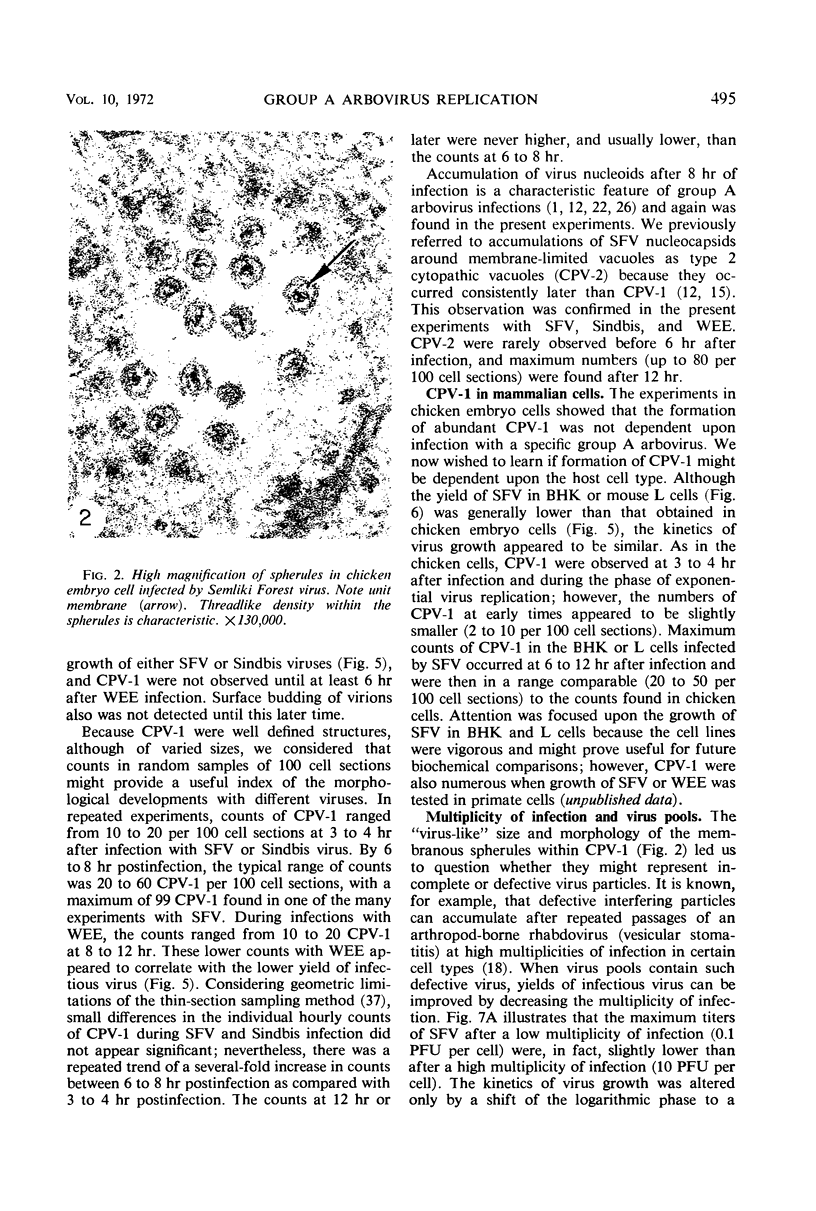
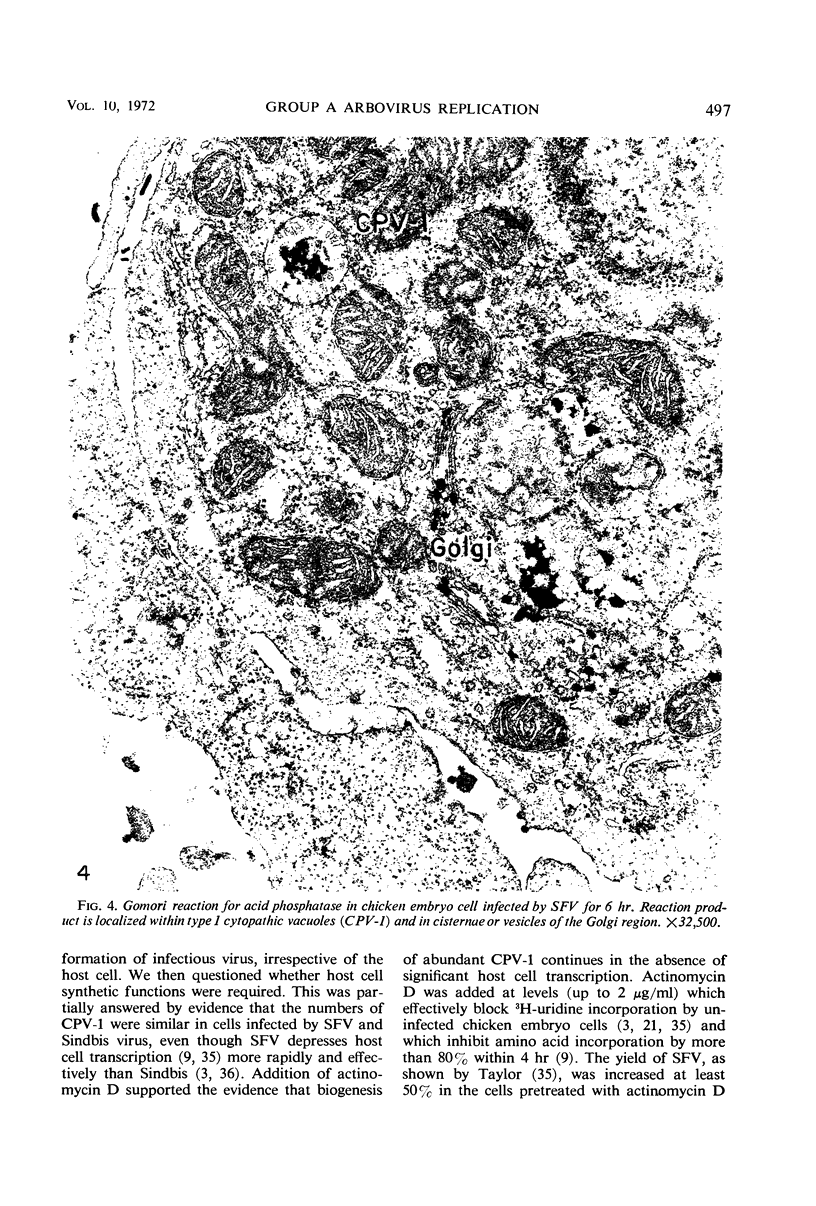
Intracytoplasmic membranous structures of a unique type were associated with the replication of three group A arboviruses: Semliki Forest virus (SFV), Sindbis virus, or Western equine encephalomyelitis virus. The structures, referred to as type 1 cytopathic vacuoles (CPV-1), were membrane-limited and characteristically lined by regular membranous spherules measuring 50 nm in diameter. The membranous spherules typically contained a fine central density, but were neither virus cores nor virions. Detection of CPV-1 by electron microscopy at 3 to 6 hr postinfection coincided with the time of rapid virus growth and preceded the accumulation of virus nucleocapsids. A range of 20 to 100 CPV-1 profiles were counted per 100 ultrathin cell sections at 6 to 9 hr postinfection when viruses were grown in chick embryo, baby hamster kidney, or mouse L cells. Maximum counts remained in the same range even when the multiplicity of infection was varied over 100-fold. Inhibition of cellular ribonucleic acid (RNA) and protein synthesis by actinomycin D during SFV infection did not decrease the counts of CPV-1; however, biogenesis of CPV-1 was decreased when viral replication was limited by inhibitors of viral RNA synthesis (guanidine) or of viral protein synthesis (cycloheximide). On the basis of present and earlier findings, we concluded that formation of CPV-1 must result from a virus-specified modification of pre-existing host cell macromolecules.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Ben-Ishai Z., Goldblum N., Becker Y. The intracellular site and sequence of Sindbis virus replication. J Gen Virol. 1968 May;2(3):365–375. doi: 10.1099/0022-1317-2-3-365. [DOI] [PubMed] [Google Scholar]
- Berezesky I. K., Grimley P. M., Tyrrell S. A., Rabson A. S. Ultrastructure of a rat cytomegalovirus. Exp Mol Pathol. 1971 Jun;14(3):337–349. doi: 10.1016/0014-4800(71)90005-0. [DOI] [PubMed] [Google Scholar]
- Bignami A., Parry H. B. Aggregations of 35-nanometer particles associated with neuronal cytopathic changes in natural scrapie. Science. 1971 Jan 29;171(3969):389–390. doi: 10.1126/science.171.3969.389. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology. 1970 Sep;42(1):112–122. doi: 10.1016/0042-6822(70)90243-6. [DOI] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson J. L., Trump B. F. Observations on the application to electron microscopy on the lead phosphate technique for the demonstration of acid phosphatase. Histochemie. 1965 Mar 5;4(6):470–487. doi: 10.1007/BF00281900. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Basis for variable response of arboviruses to guanidine treatment. J Virol. 1970 Nov;6(5):628–636. doi: 10.1128/jvi.6.5.628-636.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Berezesky I. K. Cytoplasmic fractions associated with Semliki Forest virus ribonucleic acid replication. J Virol. 1967 Apr;1(2):374–383. doi: 10.1128/jvi.1.2.374-383.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Grimley P. M. Inhibition of arbovirus assembly by cycloheximide. J Virol. 1969 Sep;4(3):292–299. doi: 10.1128/jvi.4.3.292-299.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Levy H. B., Carter W. B. Replication of semliki forest virus: three forms of viral RNA produced during infection. Proc Natl Acad Sci U S A. 1966 Aug;56(2):440–446. doi: 10.1073/pnas.56.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Protein synthesis directed by an arbovirus. J Virol. 1968 Jan;2(1):26–32. doi: 10.1128/jvi.2.1.26-32.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Sreevalsan T. Membrane binding of input arbovirus ribonucleic acid: effect of interferon or cycloheximide. J Virol. 1970 Aug;6(2):169–175. doi: 10.1128/jvi.6.2.169-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Berezesky I. K., Friedman R. M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Friedman R. M. Arboviral infection of voluntary striated muscles. J Infect Dis. 1970 Jul-Aug;122(1):45–52. doi: 10.1093/infdis/122.1-2.45. [DOI] [PubMed] [Google Scholar]
- Grimley P. M., Friedman R. M. Development of Semliki forest virus in mouse brain: an electron microscopic study. Exp Mol Pathol. 1970 Feb;12(1):1–13. doi: 10.1016/0014-4800(70)90070-5. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- KOVAC W., KUNZ C., STOCKINGER L. [Electron microscopic demonstration of the virus of early summer meningoencephalitis in HeLa cells]. Arch Gesamte Virusforsch. 1962;11:544–567. [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., HOWE C., ROSE H. M. Structure and development of viruses as observed in the electron microscope. V. Western equine encephalomyelitis virus. J Exp Med. 1961 Jan 1;113:219–234. doi: 10.1084/jem.113.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSSGAY M. STUDIES ON THE STRUCTURE OF A HEMAGGLUTINATING COMPONENT OF A GROUP A ARBO VIRUS (SINDBIS). Virology. 1964 Aug;23:573–581. doi: 10.1016/0042-6822(64)90241-7. [DOI] [PubMed] [Google Scholar]
- MUSSGAY M., WEIBEL J. Electron microscopic and biological studies on the growth of Venezuelan equine encephalitis virus in KB cells. Virology. 1962 Jan;16:52–62. doi: 10.1016/0042-6822(62)90201-5. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Whitfield S. G. Eastern equine encephalitis virus infection: electron microscopic studies of mouse central nervous system. Exp Mol Pathol. 1970 Oct;13(2):131–146. doi: 10.1016/0014-4800(70)90001-8. [DOI] [PubMed] [Google Scholar]
- Mécs E., Sonnabend J. A., Martin E. M., Fantes K. H. The effect of interferon on the synthesis of RNA in chick cells infected with Semliki forest virus. J Gen Virol. 1967 Jan;1(1):25–40. doi: 10.1099/0022-1317-1-1-25. [DOI] [PubMed] [Google Scholar]
- PENMAN S. STIMULATION OF THE INCORPORATION OF CHOLINE IN POLIOVIRUS-INFECTED CELLS. Virology. 1965 Jan;25:149–152. doi: 10.1016/0042-6822(65)90263-1. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. THE SOURCE OF THE RIBONUCLEIC ACID AND PHOSPHOLIPID OF SINDBIS VIRUS. Virology. 1963 Jul;20:446–456. doi: 10.1016/0042-6822(63)90093-x. [DOI] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Inhibition of interjacent ribonucleic acid (26S) synthesis in cells infected by Sindbis virus. J Virol. 1969 Aug;4(2):117–122. doi: 10.1128/jvi.4.2.117-122.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. S., Halperen S., Harkin J. C. Cytoplasmic membrane-bound vesicles in echovirus 12-infected cells. Virology. 1968 Oct;36(2):241–253. doi: 10.1016/0042-6822(68)90141-4. [DOI] [PubMed] [Google Scholar]
- Stollar B. D., Stollar V. Immunofluorescent demonstration of double-stranded RNA in the cytoplasm of Sindbis virus-infected cells. Virology. 1970 Sep;42(1):276–280. doi: 10.1016/0042-6822(70)90270-9. [DOI] [PubMed] [Google Scholar]
- TAYLOR J. STUDIES ON THE MECHANISM OF ACTION OF INTERFERON. I. INTERFERON ACTION AND RNA SYNTHESIS IN CHICK EMBRYO FIBROBLASTS INFECTED WITH SEMLIKI FOREST VIRUS. Virology. 1965 Mar;25:340–349. doi: 10.1016/0042-6822(65)90053-x. [DOI] [PubMed] [Google Scholar]
- Tan K. B. Electron microscopy of cells infected with Semliki forest virus temperature-sensitive mutants: correlation of ultrastructural and physiological observations. J Virol. 1970 May;5(5):632–638. doi: 10.1128/jvi.5.5.632-638.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Sambrook J. F., Bellett A. J. Semliki forest virus temperature-sensitive mutants: isolation and characterization. Virology. 1969 Jul;38(3):427–439. doi: 10.1016/0042-6822(69)90155-x. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R. Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963 Feb;12:131–155. [PubMed] [Google Scholar]
- Waite M. R., Pfefferkorn E. R. Phospholipid synthesis in Sindbis virus-infected cells. J Virol. 1970 Nov;6(5):637–643. doi: 10.1128/jvi.6.5.637-643.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]







