Abstract
Advances in hematopoietic cell transplantation (HCT) have broadened its indications for use and resulted in more long-term HCT survivors. Some survivors develop chronic kidney disease (CKD), however, the incidence and risk factors are unclear. We performed a systematic review of studies identified from databases (MEDLINE, EMBASE, Science Citation Index), conference abstracts, and reference lists from selected manuscripts. From 927 manuscripts, 28 patient cohorts were identified in which 9,317 adults and children underwent HCT and 7,317 (79%) survived to at least 100 days, permitting inclusion of 5,337 (73% of survivors) in quantitative analyses. Although definitions and measurements varied widely, approximately 16.6% of HCT patients developed CKD and estimated glomerular filtration rate (eGFR in ml/min/1.73m2) decreased by 24.5 after 24 months. This decrease was greater amongst patients undergoing allogeneic HCT (ΔeGFR = −40.0 versus −18.6 for autologous transplants). Several commonly reported risk factors for CKD were investigated, including acute renal failure, total body irradiation, graft versus host disease, and long-term cyclosporine use. In conclusion, CKD following HCT is likely to be common, however, prospective studies with uniform definitions of CKD and risk factors are needed to confirm these findings and better define the underlying mechanisms to promote therapies that prevent this complication.
Keywords: chronic kidney disease (CKD), hematopoietic stem cell transplantation, bone marrow transplantation, renal failure, meta-analysis, systematic review, risk factor, complications
Introduction
Each year, approximately 50–60,000 hematopoietic cell transplants (HCT) (1) occur worldwide for the treatment of a variety of hematologic and solid tumors, red blood cell dyscrasias, inborn errors of metabolism, and autoimmune disorders. Long-term survival rates have improved, leading to increasing numbers of HCT recipients who face long-term sequelae (2) associated with their underlying disease, the HCT preparative regimen, the HCT itself, or post-transplant diseases, infections, and treatments. Included in the list of complications after HCT is chronic kidney disease (CKD), with a reported incidence ranging from 3.6% (3) to 89% (4). The wide range may be related to variability in the definitions of CKD and populations that have been studied. Nonetheless, HCT survivors who develop CKD may be at greater risk for death and cardiovascular events because of the increased risk conferred by CKD (5–6). Although reviews describing CKD following HCT have been published (7–8), there have been no systematic reviews that have consolidated available data to define the burden of CKD and to identify associated risk factors. Therefore, this systematic review sought to address the following questions: 1) What is the incidence of CKD after HCT, and does it vary by transplant type (autologous versus allogeneic)? 2) Are there risk factors that are consistently reported and significantly associated with the development of CKD after HCT? 3) Do long-term survivors of HCT have an accelerated loss of kidney function compared to the general population? and 4) How common are other renal-related outcomes, including end stage renal disease (ESRD), hypertension (HTN), or proteinuria, that when present confer increased morbidity and mortality?
Materials and Methods
A MEDLINE search was conducted using the MeSH headings ‘kidney disease’ and ‘stem cell transplant’ through February 2007. Entry terms identified via the MEDLINE search were then used to search additional databases, including EMBASE and the Science Citation Index. In addition, abstracts from relevant scientific meetings (American Society of Nephrology and American Society of Hematology) between 2000 and 2006 were reviewed. All titles and abstracts were reviewed in duplicate (M.J.E and M.K) and were eligible for more detailed evaluation if they included the following a priori defined criteria: a cohort of ten or greater human patients who underwent HCT; patients whose survival exceeded 100 days after transplantation; and reporting of one or more parameters of renal function, including any of the following: serum creatinine (SCr), estimated or actual glomerular filtration rate (GFR), or creatinine clearance (CrCl). Manuscript authors were contacted to confirm data and to provide missing information, especially related to the presence of risk factors for CKD. Finally, references from excluded manuscripts (including case series and review articles) were reviewed for additional studies. Disagreements were resolved by consensus.
Manuscripts that met all inclusion and exclusion criteria were then retrieved for more detailed evaluation (M.J.E, M.K., and J.K.I), resulting in a small subset of the studies that were included for systematic review. Data from the manuscripts that met inclusion criteria were extracted (M.J.E., M.K., J.K.I.) in duplicate and disagreements were resolved by consensus after discussion. When data from the same cohort of HCT recipients were described in multiple publications, we cited the most representative publication with the greatest number of recipients and the longest period of follow-up (9–21). Data were extracted from each manuscript and analyzed over all of the papers and then according to type of transplant (autologous versus allogeneic and pediatric versus adult). If any or all of these data were not easily extracted from a study, attempts were made to contact one or more of the authors for clarification.
Studies that reported risk factors for CKD after HCT were compiled; those risk factors that were reported in two or more cohorts and that were associated with CKD after HCT in at least one of the reports were analyzed (acute renal failure [ARF], total body irradiation [TBI] exposure and dosage, development of chronic graft-versus-host disease [cGvHD], and long-term [greater than 60 days] cyclosporine [CsA] administration). ARF was generally defined as at least a doubling of the SCr or a fall of at least 25% in the eGFR. Chronic GVHD was exclusively described as present or absent; grades of cGVHD and descriptions of specific organ involvement were not presented in any study. Finally, because TBI dose was not consistently described, we divided the total administered TBI dose into greater than or less than 11 gray, irrespective of whether the dose was given in one or multiple doses. Finally long-term CsA use was universally described as CsA use for greater than 60 days.
The definitions of CKD varied widely throughout the manuscripts, ranging from a SCr greater than the institution’s normal range to eGFR less than 60 mL/min/1.73m2, to a drop in the eGFR by at least 25% compared to baseline. The incidence of CKD after HCT was determined by dividing the number of patients reported to have had CKD at any time after HCT by the number of patients who survived greater than 100 days and were included in the analysis. We compared proportions of patients with CKD using two-sample tests on the equality of proportions using large-sample statistics.
Next we examined changes in renal function by eGFR. Although CrCl is conceptually different from eGFR, it is commonly used as an estimate of glomerular filtration rate and, therefore, was used interchangeably to estimate this outcome. Some studies reported eGFR standardized to body surface area while others did not. To determine a weighted average of change in renal function, we combined all studies, irrespective of whether eGFR was standardized to body surface area, and reported these data as a change in milliliters per minute per 1.73m2 of body surface area (mL/min/1.73m2). Change in eGFR represents the eGFR post-transplant minus the eGFR pre-transplant (a negative value indicates a fall in eGFR). Estimates of variance around two or more means would be necessary to determine whether eGFR changes varied across groups. However, this data was not provided in the original studies, thus, comparisons of eGFR change by subgroups or study cohorts were not performed.
Analysis of risk factors was determined by the number of patients with and without CKD who were exposed to each specific risk factor. Odds ratios (ORs) were used as measures of association between each risk factor and CKD. We applied random-effects models to estimate the pooled ORs and 95% CI of CKD for each risk factor exposure compared with no exposure. DerSimonian and Laird’s method was used to account for between-study variability (22). Stratified meta-analysis of the association between risk factors and CKD were limited by small numbers of patients in each subgroup. Nonetheless, we examined potential sources of heterogeneity wherever possible by grouping exposures according to moderator variables including age group and type of HCT. Q-statistic was used to determine if between-study heterogeneity was present and the I2 statistic was used to quantify the magnitude of heterogeneity (23). We performed all analyses using Comprehensive Meta Analysis, version 2 (Biostat, Englewood, New Jersey), and Stata, version 9.2 (StataCorp, College Station, Texas). A P-value of < 0.05 was considered statistically significant.
Results
Study Identification
From screening 927 citations, 167 full text articles were retrieved for more detailed evaluation, 37 manuscripts were included for systematic review, and 26 studies met all criteria for data extraction and meta-analysis (two manuscripts reported two, distinct cohorts of patients each; the total number of cohorts, therefore, was 28) (Figure 1). The kappa coefficient for inter-rater agreement was 0.97. Although attempts were made to contact one or more of the authors associated with each of the original manuscripts, we successfully contacted representatives of 20 (3, 4, 11,13, 18, 20, 21, 24–36) of the 26 manuscripts for data clarification and/or supplementation.
Figure 1. Summary of manuscript inclusion and exclusion for systematic review and meta-analysis.
927 manuscripts were initially identified through data base searches, abstract and bibliographic review; 28 cohorts of patients met all inclusion criteria.
Description of Studies, Methods, Outcomes Assessment
Twenty eight (28) study cohorts met all inclusion and exclusion criteria for analysis (3, 4, 11, 13, 18, 20, 21, 24–42) (Table 1). These cohorts included a total of 9,317 HCT patients at 57 institutions from 9 countries (79.3% North America, 18.9% Europe, 1.2% Japan, and 0.6% Australia). Among these, a total of 7,317 (78.5%) survived to at least 100 days after HCT and 5,337 (73% of survivors) were evaluated for CKD after HCT. The patients in these cohorts were followed from 3 months to 9 years following transplantation.
Table 1.
Characteristics of studies reporting chronic kidney disease (CKD) following hematoptoietic cell transplantation (HCT).
| Author | Study Years | Type+ | Age++ | Definition of CKD | Method+++ | F/U Period (years)** | No. Transplanted | No. Survived^ | No. Assessed* | No. CKD $ | Incidence$$ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weiss4 | 1998–2002 | Allo | A | Decrease eGFR by > 25% | MDRD | 1 | 174 | 130 | 91 | 81 | 89.0% |
| Delgado^^28 | 1996–2004 | Allo | A | eGFR < 60mL/min/m2 for > 3 months | MDRD | 2 | 301 | 249 | 241 | 34 | 14.11% |
| Parikh36 | 1995–2000 | Allo | A | > 2x increase in SCr or > 25% decrease in CrCl | MDRD | 0.5 | 91 | 26 | 26 | … | … |
| Kist-van Holthe^^13 | 1998–2000 | Allo | P | eGFR < 30, 44, 70, 85mL/min/m2 at 3, 6, 12, 24 months, respectively | Schwartz | 5 | 66 | 43 | 40 | 4 | 10.00% |
| Kist-van Holthe^^13 | 1991–1998 | Allo | P | eGFR < 30, 44, 70, 85mL/min/m2 at 3, 6, 12, 24 months, respectively | Schwartz | 7.6 | 207 | 122 | 122 | 29 | 23.77% |
| Kumar32 | 1983–1992 | Allo | P | Elevated SCr, HTN, or abnormal urinalysis > 1 year after HCT | Schwartz | 6.6 | 55 | 23 | 17 | 5 | 29.41% |
| Bradley25 | 1985–1994 | Allo | P/A | Elevated renal function tests or requiring dialysis | SCr | 3.3 | 77 | 77 | 77 | 7 | 9.09% |
| Yee3 | 1980–1983 | Allo | P/A | Two fold increase in SCr | SCr | 1 | 123 | 93 | 82 | 3 | 3.66% |
| Dieterle^^29 | 1979–1988 | Allo | P/A | SCr > upper limit of institutional limit | CG | 3 | 169 | 113 | 113 | 62 | 54.87% |
| Giralt38 | 1998–2002 | Auto | A | SCr > 1.5x upper limit of institutional normal | SCr | 2.6 | 88 | 88 | 83 | 30 | 36.00% |
| Glynne39 | 1984–1994 | Auto | A | SCr > 1.36mg/dL or eGFR < 50mL/min/1.73m2 | Cr-EDTA | 2.9 | 186 | 112 | 58 | 3 | 5.20% |
| Lonnerholm21 | 1985–1990 | Auto | A | > 25% decrease in eGFR | CrCl & Cr-EDTA | 1 | 50 | 50 | 50 | 8 | 16.00% |
| Frisk20 | 1985–1997 | Auto | P | eGFR < 70mL/min/1.73m2 at > 6 months after transplant | CrCl & Cr-EDTA | 0.5 | 50 | 40 | 34 | 7 | 20.60% |
| Lonnerholm21 | 1985–1990 | Auto | P | > 25% decrease in eGFR | CrCl & Cr-EDTA | 3 | 22 | 22 | 22 | 4 | 18.18% |
| Carlson37 | 1987–1991 | Auto | P/A | Decrease eGFR by > 20% | Cr-EDTA | 0.5 – 1 | 24 | 19 | 19 | 15 | 79.00% |
| Miralbell^^35 | 1994–1997 | Both | A | Decrease eGFR by > 30% or SCr > 1.53mg/dL within 3 years | Cr-EDTA | 1.5 | 71 | 45 | 41 | 20 | 48.78% |
| Deconinck27 | 1990–1999 | Both | A | eGFR < 50mL/min/m2 for 12 months | CG | 4.3 | 181 | 79 | 79 | 10 | 12.66% |
| LeBlond34 | 1985–1991 | Both | A | Decrease eGFR by > 20% | CG & Inulin Clearance | 2 | 190 | 60 | 60 | 34 | 57.00% |
| Van Why41 | 1975–1988 | Both | P | Two fold increase in SCr or eGFR < 50mL/min/m2 | Schwartz | 1.5 | 92 | 64 | 63 | 9 | 14.29% |
| Leahey33 | 1970–1994 | Both | P | SCr level above normal | SCr | 5.6 | 53 | 34 | 26 | 1 | 3.85% |
| Patzer40 | 1992–1998 | Both | P/A | … | Inulin Clearance | 2 | 137 | 70 | 41 | … | … |
| Hingorani^^30 | 1991–2002 | Both | P/A | Two or more eGFR measurements (at least 30 days apart) < 60mL/min/m2 after day +100 | MDRD & Schwartz | 0.5 | 4528 | 3436 | 1635 | 376 | 23.00% |
| Borg24 | 1990–1997 | Both | P/A | Three consecutive SCr measurements > 1.36mg/dL | SCr | 2.7 | 64 | 47 | 47 | 4 | 9.30% |
| Igaki^^31 | 1987–2001 | Both | P/A | Continuous elevation of SCr > 1.10 mg/dL | SCr | 1.4 | 109 | 101 | 101 | 14 | 13.86% |
| Cohen12 | 1986–1996 | Both | P/A | Increase in SCr, azotemia, HTN, anemia | SCr | 1 – 9 | 505 | 505 | 505 | 36 | 7.10% |
| Miralbell^^42 | 1984–1994 | Both | P/A | SCr > 1.25mg/dL and/or abnormal urinary sediment and/or HTN | SCr | 1.5 | 99 | 84 | 79 | 18 | 22.78% |
| Tarbell9 | 1980–1988 | Both | P/A | 2x increase in SCr | SCr | 0.5 | 59 | 39 | 39 | 16 | 41.03% |
| Carter26 | … | Both | P/A | eGFR < 60mL/min/m2 for > 3 months | MDRD | 6.2 | 1546 | 1546 | 1546 | 56 | 3.60% |
|
| |||||||||||
| Totals | 9317 | 7317 | 5337 | 886 | 16.6% | ||||||
|
|
|||||||||||
Important characteristics from the 28 cohorts identified for systematic review, including years during which HCT occurred, types of HCT performed, age of recipients, definitions of CKD, method used to estimate renal function, follow up time, the numbers of patients transplanted, surviving to 100 days, used for assessment and experiencing CKD, and the incidence of CKD reported in each study. Weighted averages are included at bottom of the table. A great deal of variability was present with regard to definitions of CKD.
= Type of transplants performed in study: Allo = data from only allogeneic recipients; auto = data from only autologous recipients; both = data from both allogeneic and autologous transplant recipients.
= Age of transplant recipients: A = adults (greater than 18 years old); P = pediatrics (less than 18 years old); P/A: patients from all age groups included.
= Method used to determine renal function: MDRD = Modification of Diet in Renal Disease equation; SCr = serum creatinine; CG = Cockroft Gault equation; Cr-EDTA = urinary clearance of 51 creatinine-ethylenediaminetetracacetic acid; CrCl = 24 hour urinary clearance of creatinine.
= number of patients who survived greater than 100 days after HCT.
= number of patients who underwent HCT, survived greater than 100 days and had kidney function assessed.
= Median follow up time after HCT.
= number of patients who developed CKD (based on definition used in study).
= incidence of CKD in study cohort (number with CKD divided by number assessed).
= updated data came from personal communication with author of study. … = data not available.
Of the 28 cohorts, 5 (17.9%) prospectively followed patients for the development of CKD (13, 28, 35, 38, 40) while the remainder were retrospective. Seven (7) cohorts involved only children (13, 20, 21, 32, 33, 41), 9 involved only adults (4, 21, 27, 28, 34–36, 38, 39), and 12 contained both children and adults (3, 11, 12, 24–26, 29–31, 35, 37, 40). Nine (9) cohorts (3, 4, 13, 25, 28, 29, 32, 36) described renal outcomes after allogeneic transplants, 6 (20, 21, 37–39) involved autologous transplants only, and 13 (11, 12, 24, 26, 27, 30, 31, 33–35, 40–42) combined outcome data after both allogeneic and autologous transplants. One study (13) contained both a prospective and retrospective analysis on different cohorts of pediatric patients, all of whom underwent allogeneic HCTs. A different manuscript (21) retrospectively reported separate adult and pediatric cohorts following autologous HCT.
Kidney function was assessed in a variety of manners, including: formulaic estimation using the Schwartz formula (13, 30, 32, 41), the Cockcroft-Gault (CG) equation (27, 29, 34), and the Modification of Diet in Renal Disease (MDRD) equation (4,26,28,30,36); measurements of SCr (3,11,18,24,25,31,33,35,38), urinary creatinine clearance (CrCl) (20,21), or urinary clearance of 51creatinine-ethylenediaminetetracacetic acid (51Cr-EDTA) (20,21,35,37,39); and direct measurements using inulin clearance (34,40). Several manuscripts (20,21,30,34,37) used multiple methods to evaluate renal function. A variety of CKD definitions were used (Table 1); however, all studies specified that CKD occurred after at least 100 days following HCT.
A minority of the studies examined other long term, renal-related outcomes. These included 7 studies (25%) (13,27,29,34,35,41,43) that reported the incidence of ESRD requiring hemodialysis (HD), 8 (28.5%) (12,13,17,27,28,35,44,45) that described the incidence of HTN in the post-transplant period, and 3 (10.7%) (13,20,34) that reported on proteinuria. Similar to the definitions of CKD, the definitions for HTN and proteinuria varied widely. HTN was either not defined (27) or defined as greater than the 95th percentile for age (13,41), the need for blood pressure medications (43), diastolic blood pressure greater than 90mmHg (35), or a blood pressure measurement of 160/90 or greater (29,34). Proteinuria was defined as a positive dipstick result (20,34), the presence of microalbumin (20–200 mg/L) (13), or elevations in albuminuria compared with a group of healthy controls (40).
Incidence and Degree of CKD Following HCT
Of the 5,337 patients who survived greater than 100 days after HCT and who were available for assessment, 886 (16.6%) patients were reported to have developed CKD (range 3.6% [3] to 89% [4]). The estimated incidence of CKD after allogeneic HCT was 27.8% versus 25.2% for patients undergoing autologous HCT (difference 2.6%; p=0.40); however, studies that grouped allogeneic and autologous HCT patients had an estimated incidence of CKD that was 13.9% (difference approximately 11–14%; p<0.0001 for both comparisons to autologous and allogeneic alone). The estimated incidence of CKD after HCT was 12% higher (p<0.0001) in adult studies (30.2%) versus pediatric studies (18.2%). In studies that grouped pediatric and adult HCT recipients together, the estimated incidence of CKD after HCT was 14.3% (4% lower than pediatric studies alone, p=0.046; 16% lower than adult studies alone, p<0.0001). To explore a potential source of bias, the estimated incidence of CKD after HCT was calculated without data from the two largest studies (26,30): among the remaining studies, the estimated incidence was actually 4.4% higher at 21.1% (approximately 1 of 5 HCT recipients; p<0.0001). When comparing pooled totals of those who received allogeneic versus autologous HCT, allogeneic HCT was associated with a non-significant 31.4% increase in risk for CKD [OR (95% CI) 1.31 (0.93, 1.83); p =0.10].
The weighted pre-HCT eGFR for all studies in which eGFR was reported (13,20,21,28,34,35,37,40) was 101.9 mL/min/1.73m2. For all pre-specified groups, the weighted average pre-HCT eGFR was greater than 100 mL/min/1.73m2 (Table 3). The weighted-average change in eGFR over the time periods reported in all studies was −24.5 mL/min/1.73m2, most of which occurred during the first 12 months after HCT; at 12 and 24 months, eGFR was 78.7 and 77.4 mL/min/1.73m2, respectively. These changes were principally driven by patients undergoing allogeneic HCT, among whom the weighted eGFR values at baseline, 12, and 24 months after HCT were 103.3, 64.1, and 56.2 mL/min/1.73m2, respectively. In contrast, eGFR decreased more modestly after autologous HCT (107.4, 90.5, and 88.8 mL/min/1.73m2 at baseline, 12, and 24 months, respectively). Changes in eGFR among studies that reported both allogeneic and autologous transplants were between the values observed for allogeneic and autologous HCT with respective eGFR values of 101.0, 80.5, and 80.5 mL/min/1.73m2. Similar trends were encountered when the data were analyzed according to age groups (pediatric and adult). Similar to our findings among studies with adults, decreases in eGFR were principally driven by allogeneic cohorts among pediatric studies. To eliminate possible bias, eGFR values were calculated without measurement from the largest study that included these data (30). The respective eGFR values were 105.7, 76.5, and 71.9 with an overall change in eGFR of −32.9.
Table 3.
Estimated glomerular filtration rate data organized by transplant type.
| Author | Type+ | Age++ | No. Assessed* | Pretransplant eGFR | 1 Year eGFR | Final eGFR | ΔeGFR | |
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| All Studies | Delgado^^28 | Allo | A | 241 | 103.0 | 51.2 | 31.3 | −71.7 |
| Parikh36 | Allo | A | 26 | 106.0 | 52.0 | … | −54.0 | |
| Weiss^^4 | Allo | A | 91 | 80.0 | 81.9 | … | 1.9 | |
| Kist-van Holthe^^13 | Allo | P | 40 | 118.0 | 97.0 | 97.0 | −21.0 | |
| Kist-van Holthe^^13 | Allo | P | 122 | 116.0 | 98.0 | 104.0 | −12.0 | |
| Glynne39 | Auto | A | 58 | 111.0 | … | … | … | |
| Lonnerholm21 | Auto | A | 50 | 94.0 | 85.0 | 82.0 | −12.0 | |
| Frisk20 | Auto | P | 34 | 125.0 | … | 107.0 | −18.0 | |
| Lonnerholm21 | Auto | P | 22 | 114.0 | 103.0 | 103.0 | −11.0 | |
| Carlson37 | Auto | P/A | 19 | 92.7 | … | 58.0 | −34.7 | |
| Miralbell^^35 | Both | A | 41 | 102.0 | 73.0 | 77.0 | −25.0 | |
| LeBlond34 | Both | A | 60 | 107.0 | … | 92.0 | −15.0 | |
| Patzer40 | Both | P/A | 41 | 130.0 | 123.0 | 105.0 | −25.0 | |
| Hingorani^^30 | Both | P/A | 1635 | 100.0 | 79.6 | 79.6 | −20.4 | |
|
|
||||||||
| Weighted Averages | 2480 | 101.9 | 78.7 | 77.4 | −24.5 | |||
|
|
||||||||
| Allo Only | Delgado^^28 | Allo | A | 241 | 103.0 | 51.2 | 31.3 | −71.7 |
| Parikh36 | Allo | A | 26 | 106.0 | 52.0 | … | −54.0 | |
| Weiss^^4 | Allo | A | 91 | 80.0 | 81.9 | … | 1.9 | |
| Kist-van Holthe^^13 | Allo | P | 40 | 118.0 | 97.0 | 97.0 | −21.0 | |
| Kist-van Holthe^^13 | Allo | P | 122 | 116.0 | 98.0 | 104.0 | −12.0 | |
|
|
||||||||
| Weighted Averages | 520 | 103.3 | 64.1 | 56.2 | −40.0 | |||
|
|
||||||||
| Auto Only | Glynne39 | Auto | A | 58 | 111.0 | … | … | … |
| Lonnerholm21 | Auto | A | 50 | 94.0 | 85.0 | 82.0 | −12.0 | |
| Frisk20 | Auto | P | 34 | 125.0 | … | 107.0 | −18.0 | |
| Lonnerholm21 | Auto | P | 22 | 114.0 | 103.0 | 103.0 | −11.0 | |
| Carlson37 | Auto | P/A | 19 | 92.7 | … | 58.0 | −34.7 | |
|
|
||||||||
| Weighted Averages | 183 | 107.4 | 90.5 | 88.8 | −18.6 | |||
|
|
||||||||
| Allo Auto & | Miralbell^^35 | Both | A | 41 | 102.0 | 73.0 | 77.0 | −25.0 |
| LeBlond34 | Both | A | 60 | 107.0 | … | 92.0 | −15.0 | |
| Patzer40 | Both | P/A | 41 | 130.0 | 123.0 | 105.0 | −25.0 | |
| Hingorani^^30 | Both | P/A | 1635 | 100.0 | 79.6 | 79.6 | −20.4 | |
|
|
||||||||
| Weighted Averages | 1777 | 101.0 | 80.5 | 80.5 | −20.4 | |||
|
|
||||||||
Estimated GFR data organized by type of HCT performed. Data is organized by recipient age. Weighted averages are included at the bottom of each table.
= Type of transplants performed in study: Allo = data from only allogeneic recipients; Auto = data from only autologous recipients; Both = data from both allogeneic and autologous transplant recipients.
= Age of transplant recipients: A = adults (greater than 18 years old); P = pediatrics (less than 18 years old); P/A: patients from all age groups included.
= number of patients who underwent HCT, survived greater than 100 days and had kidney function assessed.
= updated data came from personal communication with author of study; … = data not available; Δ = post transplant minus pre transplant measurement: Final eGFR was a measurement taken between 18 and 36 months after HCT.
Risk Factors
A variety of risk factors were reported in many of the studies (3,4,10,13,14,16–18,20,26–30,34,35,37,39,41,42,44). These included ARF, GvHD (acute and chronic), type of transplant (allogeneic versus autologous), sex, age (pediatric versus adult), TBI (total dose and mode of exposure [single dose versus fractionated dose]), impaired baseline renal function (according to eGFR and/or SCr), long-term use of CsA, post transplant exposure to nephrotoxic medications (aminoglycosides, amphotericin B, and vancomycin), and the development of veno-occlusive disease (VOD). Among these, however, only ARF, cGvHD, long-term use of CsA, and TBI exposure were reported in two or more cohorts and were associated in at least one of those cohorts with the development of CKD after HCT. These risk factors were, therefore, consolidated and analyzed. ARF was assessed as a risk factor in 4 cohorts (including 1722 patients) (4,13,28,30), long term use of CsA in 5 cohorts (including 2031 patients) (26–28,31,41), cGvHD in 6 cohorts (including 1860 patients) (27,28,30,31,35,42), and the cumulative dose of TBI in 7 cohorts (including 1677 patients) (13,20,30,37,41,42).
In the presence of each of these risk factors, several studies demonstrated that the odds for developing CKD were increased (Figure 2). Furthermore, the pooled estimate for cGVHD as a risk factor appeared to be statistically significant (OR 1.75 [1.079, 2.836]). However, there was substantial heterogeneity (I2 statistic >50%) among studies for all of the risk factors, including the estimate for cGvHD, making the pooled estimates invalid.
Figure 2. Odds ratios (95% CI) for CKD following HCT, according to four commonly reported risk-factors (■).
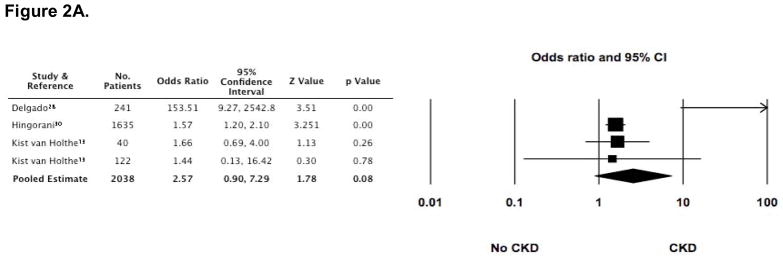
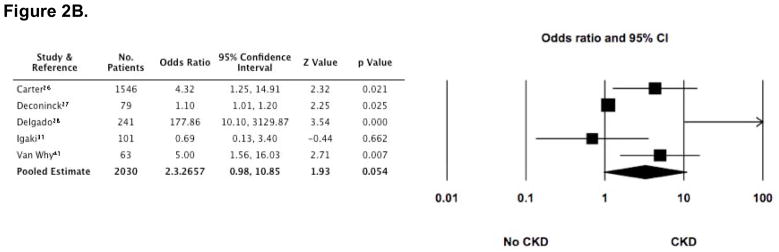
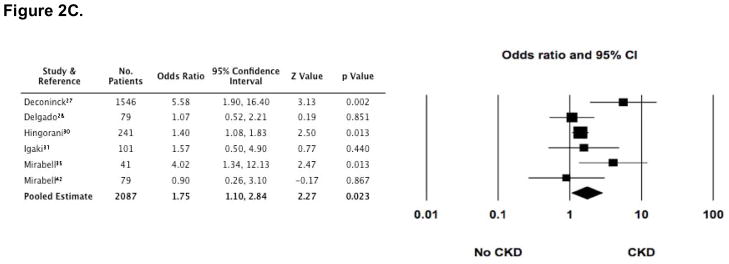
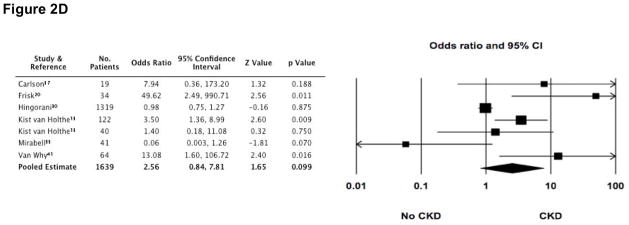
The size of each square is inversely proportion to variability of the study estimate. (A) Acute renal failure (ARF); I2 = 70.4. (B) Long-term (greater than 60 days) use of cyclosporine (CsA); I2 = 82.9. (C) Development of chronic graft versus host disease (cGvHD); although the pooled estimate appears statistically significant, the high degree of heterogeneity makes the pooled estimate invalid (I2 = 52.4). (D) Exposure to greater than 11 gray of total body irradiation (TBI); I2 = 74.1.
Other Long Term Outcomes
The incidence of other renal-related, long-term outcomes varied: ESRD occurred in 31 of 3794 (0.8%) patients, HTN was reported in 108 of 873 (12.4%) patients, and proteinuria occurred in 11 of 141 (7.8%) patients. Original manuscripts that reported these outcomes did not describe either the associated risk factors or the time course over which these outcomes occurred.
Discussion
As the indications for HCT continue to increase worldwide, more patients survive to experience a number of HCT-related toxicities (2,8). Although a variety of renal-related consequences of HCT are known to occur, the incidence of CKD has not been systematically reviewed and summarized. We, therefore, identified 26 manuscripts (28 cohorts) that documented the course of over 9,000 patients from around the world, some of whom developed CKD following HCT. One-hundred (100) day survival after HCT was around 80%, similar to the survival data cited elsewhere in the literature (1). Although definitions for CKD varied widely, we estimated the incidence was approximately 16% in this population of survivors (1 in 6). Overall, patients (adults and pediatric) who underwent HCT experienced a fall in their estimated glomerular filtration rate by at least 25mL/min/1.73m2, irrespective of age. For all groups of patients (HCT type and age of recipient), the majority of the eGFR decrement occurred during the first 12 months with less decline in renal function after year 1 (Table 3); this fall is more than expected in the general population where average yearly decline in eGFR is 0.75mL/min/1.73m2. (46) Furthermore, changes in kidney function were greater following allogeneic versus autologous transplantation. Although of a high degree of heterogeneity between studies precluded pooled analysis of risk factors, several common ones may be associated with the development of CKD following HCT.
Although there are limited data regarding the incidence of CKD, our estimate of CKD following HCT was very high. For comparison, in a Framingham Offspring Study of 2,585 community-dwelling adults, the incidence of CKD over a mean follow-up period of 18.5 years was 9.4% (47). Our estimate of CKD incidence was much higher at 16.6% over 2 years among a high risk group of patients undergoing HCT.
There are, however, several limitations to the precision of this estimate. On one hand, it may be an overestimate for several reasons. First, definitions of CKD were not consistent and the degree of kidney dysfunction was not stratified. As such, comparisons and consolidation of data across all studies were difficult. Additionally, our estimates of CKD incidence likely include a small number of prevalent patients, rather than only patients with normal renal function before undergoing HCT; this was not well-characterized in the studies reviewed.
On the other hand, our estimate of CKD incidence may be an underestimate for several reasons. First, the definitions for CKD were predominantly based on measurements of serum creatinine which may have overestimated eGFR in the setting of other comorbid conditions, including liver disease, malnutrition, and medication administration (48–52). Next, to be included in this analysis, patients were required to have survived to 100 days following HCT. During this time, patients with baseline kidney disease or those who developed kidney disease shortly after HCT would have been less likely to survive and therefore censored in the event of death. Next, CKD was generally defined in terms of the eGFR; other indicators of kidney damage (albuminuria or proteinuria) were not commonly and consistently considered in the definitions of CKD. Finally, the duration of follow-up and timing of the CKD assessment following HCT varied considerably between studies. Some studies with shorter follow-up may not have captured all patients who eventually developed CKD. With longer follow-up, many patients who undergo various types of HCT and survive past 100 days will continue to be exposed to additional risk factors for CKD (e.g., nephrotoxic medications or recurrence of their underlying disease) or they may acquire comorbid conditions that also increase their risk for CKD (e.g., hypertension, diabetes mellitus, or renovascular disease). This point is illustrated in a recent, retrospective Dutch study in which the incidence of CKD following HCT steadily rose over the course of 10 years of follow up (53).
The smaller decrement in eGFR among autologous HCT patients as compared to allogeneic patients is likely the result of several factors. First, autologous transplant patients receive milder conditioning regimens in the form of both chemo and radiation therapies. They are, therefore, much less likely to develop many complications related to these exposures. Second, autologous transplant recipients do not suffer from acute or chronic graft versus host disease, which expose the kidneys to both direct and indirect damage (54). Furthermore, they do not require calcineurin inhibitors to prevent or treat GvHD and therefore are not at risk for calcineurin inhibitor nephrotoxicty. Autologous HCT patients rarely develop complications like veno-occlusive disease, a condition caused by endothelial damage and dysfunction of the hepatic vasculature. Veno-occlusive disease causes renal disease secondary to a hepatorenal-type physiology that is mediated by direct renal tubular toxicity by bilirubin, as well as by the accumulation of endotoxins or nephrotoxic immunoglobulins. It is much more likely to occur following allogeneic versus autologous HCT (up to 54% versus 7%, respectively [55,56]) and has been shown to be a significant risk factor for ARF following HCT (55,56).
Small sample sizes and the heterogeneity of data prevented more definitive assessment of risk factors for CKD. Qualitatively, the heterogeneity largely stems from varying definitions of the risk factors. For instance, TBI has long been considered a significant risk factor for renal disease after HCT (11,17,35,42,57). The reported heterogeneity of TBI as a risk factor likely stems from the fact that TBI administration varied by total dose, the number of fractions administered, and by the amount of renal shielding used during its administration; these differences make data consolidation difficult. Additionally, drawing conclusions about risk factors can be difficult when the risk factors, themselves, are highly correlated. GvHD prevention and treatment are dependent on the administration of calcineurin inhibitors, which are known to be highly nephrotoxic. Furthermore, the higher number or longer duration of GvHD episodes requires longer duration of calcineurin inhibitor administration. Because these two risk factors are so intimately connected, it likely will be difficult to separate their independent effects on the risk of developing CKD after HCT. Furthermore, statistical heterogeneity demonstrated that the effects of these risk factors on the development of CKD in these studies were inconsistent, and more likely to be due to heterogeneity rather than chance.
In addition to CKD, a number of renal-related complications following HCT have been extensively reported in the literature as far back as the 1980s. A minority of studies evaluated here reported on other renal-related outcomes, including ESRD, HTN, and proteinuria. While decreased renal function occurs frequently, the requirement for dialysis is rare (less than 1%). Based on gender-adjusted expected incidence of ESRD in Caucasians between 40–49 years old in the US and a 30% rate of death or loss to follow up 1 year after HCT (and 50% at 2 years), Cohen and colleagues argue that a similar incidence of ESRD in HCT recipients in Wisconsin was 16 times the expected rate (58). Extrapolating this reasoning to our analysis suggests that the incidence of ESRD may be much higher than expected. The finding that most of the eGFR fall occurs during the first 12 months and then subsequently stabilizes between years 1 and 2 is reassuring and consistent with clinical experience that many risk factors for CKD exert their effects through early insults which are reduced over time (e.g., an ARF insult resolves or is withdrawn, TBI is given only once, and CsA use is terminated). Although some may conclude from these data that long-term renal dysfunction in this group is of minimal importance, this is not true. Patients undergoing HCT who survive and develop a drop in their eGFR or other evidence of chronic kidney damage will continue to be exposed to additional risk factors for further progression of CKD, including HTN, diabetes mellitus, recurrence of their underlying disease, nephrotoxic medications, and renovascular disease, to name only a few. In addition, HTN and proteinuria are also reported frequently in this population of patients (approximately 12% and 8%, respectively), thereby increasing the chance that mild decrements in renal function may result in continued and/or accelerated loss of kidney function over time. Finally, CKD, hypertension, and proteinuria independently increase the risk for all-cause and cardiovascular morbidity and mortality in a graded manner with higher risks associated with greater renal dysfunction (5,6).
The current analysis has several strengths and limitations. Strengths included the fact that this analysis rigorously examined the literature on CKD after HCT and extracted data on a large number of HCT patients from around the world. Only two manuscripts (both case reports) were excluded because they were in a language other than English. Authors were contacted to verify data and to provide additional data not described in their reports. Reasons for combining data in different manners were justified. Nonetheless, approximately 25% of survivors were not included in the analysis because of exclusion criteria or being lost to follow up. Because the majority of studies were retrospective, the quality of the predictor variables was limited. Many different definitions of CKD were employed throughout the studies, making consolidation and comparisons more challenging. In addition to varying definitions of CKD, variability in study cohorts may have also complicated interpretation of our results. For example, the estimated incidence of CKD among studies of patients undergoing allogeneic HCT was 27.8% and those undergoing autologous HCT was 25.2%, however, studies with both types were not in between, but much lower (13.9%). A similar pattern was found when examining adult and pediatric studies. Next, small study size made stratified meta-analysis of the association between risk factors and CKD difficult. The studies ranged over the past 20 years, during which time a number of changes have occurred: the number of HCTs has increased; the conditioning regimens have, in general become less toxic (“mini” allogeneic HCT, for example); the types of diseases for which patients receive HCT have expanded; and the ability to detect chronic kidney damage (i.e, proteinuria) have evolved. The vast majority of studies did not have a control group. Furthermore, accurately describing the incidence of CKD over time requires knowledge of the number of patients surviving at different time points and the proportion of these patients that have CKD; these data were not reported consistently. Importantly, our understanding of the implications of CKD and the definitions of CKD have evolved, resulting in definitions for CKD and methods to measure renal function that have varied widely. Our analyses of the incidence and risk factors for CKD were limited by the frequent grouping of patient populations by type and age (Table 1): 13 of the 28 cohorts grouped allogeneic and autologous transplants together, while 12 of the 28 cohorts grouped pediatric and adult patients together. The reporting of risk factors was also limited by various definitions, methods of assessment, and consistency of reporting. Additionally, specific types of transplants (autologous versus allogeneic) are used to treat different diseases in various patient types with different comorbid illnesses. In this setting, drawing conclusions about the risk of CKD following autologous versus allogeneic HCT or the risk of CKD following TBI administration might be misleading as the observed renal outcomes may be more reflective of the natural history of the disease as opposed the deleterious effects of the HCT and preparative regimens, per se. Finally, the majority of the studies presented renal function data no later than 24 months after HCT. A recent Dutch study (53) suggests that increasing numbers of patients develop CKD after longer follow up (at least 5 years). Evaluation of renal function in HCT patients over longer periods of time may prove that there is continued annual loss of eGFR at a rate that exceeds the decline seen in the general population.
In summary, CKD following HCT is likely to be a common problem that affects a significant proportion of patients who survive greater than 100 days after HCT. Commonly reported risk factors for CKD in this population include ARF, long-term CsA use, GvHD, and TBI; however, the contribution of these risk factors to the development of CKD remains unclear. Although early ESRD is rare among survivors, hypertension and proteinuria are also common comorbid conditions that may accelerate subsequent loss of kidney function and expose HCT survivors to increased morbidity and mortality. To better delineate the incidence of CKD and risk factors for developing CKD following HCT, prospective studies should include control groups, more consistent definitions of CKD, and standardized methods to measure renal function. With even greater numbers of patients undergoing HCT, a better understanding of the epidemiology and pathophysiology of kidney dysfunction may facilitate targeted therapies that minimize the detrimental impact of this common complication.
Table 2.
Reported estimated glomerular filtration rates (eGFR) before, 1 year after, and at the end of study following HCT.
| Author | Study Years | Type+ | Age++ | Pretransplant* eGFR | 1 Year eGFR | Final** eGFR | ΔeGFR |
|---|---|---|---|---|---|---|---|
| Weiss4 | 1998–2002 | Allo | A | 80.0 (29.2) | 81.9 (29.4) | 81.9 (29.4) | 1.9 |
| Delgado^^28 | 1996–2004 | Allo | A | 103.3 (38.0) | 51.2 (27.0) | 31.3 (17.1) | −72 |
| Parikh36 | 1995–2000 | Allo | A | 106 (29.5) | 52 (25) | 52 (25) | −54.0 |
| Kist-van Holthe^^13 | 1998–2000 | Allo | P | 116 (…) | 98 (…) | 104 (…) | −12 |
| Kist-van Holthe^^13 | 1991–1998 | Allo | P | 118 (…) | 97 (…) | 97 (…) | −21 |
| Lonnerholm21 | 1985–1990 | Auto | A | 94 (25) | 85 (21) | 82 (25) | −12 |
| Frisk20 | 1985–1997 | Auto | P | 125.8 (…) | … | 107 (…) | −18.8 |
| Lonnerholm21 | 1985–1990 | Auto | P | 114 (21) | 103 (29) | 103 (16) | −11 |
| Carlson37 | 1987–1991 | Auto | P/A | 92.7 (…) | … | 58.1 (…) | −34.6 |
| Miralbell^^35 | 1994–1997 | Both | A | 102 (24) | 73 | 77 (22) | −25 |
| LeBlond34 | 1985–1991 | Both | A | 107 (4) | … | 92 (3) | −15 |
| Patzer40 | 1992–1998 | Both | P/A | 130 (…) | 123 (…) | 105 (…) | −25 |
| Hingorani^^30 | 1991–2002 | Both | P/A | 100 (…) | 79.6 (…) | 79.6 (…) | −20.4 |
|
|
|||||||
| Weighted Averages | 101.9 | 78.7 | 77.4 | −24.5 | |||
|
|
|||||||
Studies that included data on eGFR are reported here. Many studies did not actually present eGFR data and are not shown here (3,9,12,24,25,26,27,29,31,32,33,38,41,42). Weighted averages appear at the bottom of the table. The table is organized by transplant type, then age of the HCT recipients. SCr = serum creatinine (mg/dL); ΔeGFR = glomerular filtration rate (mL/min/1.73m2); () = standard deviation;
= mean final study follow up measurement;
= mean pre transplant measurement;
= updated data came from personal communication with author of study;
= Type of transplants performed in study: Allo = data from only allogeneic recipients; auto = data from only autologous recipients; both = data from both allogeneic and autologous transplant recipients.
= Age of transplant recipients: A = adults (greater than 18 years old); P = pediatrics (less than 18 years old); P/A: patients from all age groups included; … = data not available; Δ = post transplant minus pre transplant measurement; Final eGFR was a measurement taken between 18 and 36 months after HCT.
Acknowledgments
This work was supported by grants from the National Institutes of Health (K23 DK064689 [C.R.P], K23 DK075929 [U.D.P], and KL2RR024123 [J.K.I]).
We thank Anne Brown for her help with our manuscript search strategy. We would like to thank several groups of authors who graciously reviewed their cohorts and provided us additional data: Drs. Thomas Lehmann and Alois Gratwohl; Dr. Joana Kist van Holthe; Drs. Baine Bieri and Raymond Miralbell; Drs. Julio Delgado and Stephen MacKinnon; Dr. Hiroshi Igaki; and Dr. Sangeeta Hingorani. Furthermore, we would like to thank the following individuals for providing additional insights, data, or contact information: Drs. Man-Fai Lam, Terje Forslund, Derwood Pamphilon, David Marks, Ken Bradstock, Dinna Cruz, Gad Kainer, Kenan Keven, Yoshinobu Kanda, Sylvia Stracke, Mutlu Arat, Yves Beguin, Eric Cohen, David Keeling, and Jean-Yves Cahn.
Footnotes
Authorship
Contribution: M.J.E., C.R.P, and U.D.P. conceived and designed the article; M.J.E. and U.D.P. analyzed and interpreted the data; M.J.E and U.D.P. drafted the article; M.J.E., C.R.P., J.K.I., and U.D.P. provided critical revision of the article for important intellectual content; M.J.E., C.R.P., and U.D.P. provided final approval of the article; and M.J.E, M.K., and J.K.I. collected and assembled the data.
Conflict-of-Interest disclosure: The authors declare no competing financial interests.
References
- 1.Current Use and Outcome of Hematopoietic Stem Cell Transplantation. CIBMTR; 2007. [Accessed January 14, 2008]. < http://campus.mcw.edu/AngelUploads/Content/CS_IBMTR2/_assoc/ECCBED0AF0A4492BB667FB6227DC7C06/summary05_Pt1_files/v3_document.htm>. [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 3.Yee GC, McGuire TR, St Pierre BA, et al. Minimal risk of chronic renal dysfunction in marrow transplant recipients treated with cyclosporine for 6 months. Bone Marrow Transplant. 1989;4:691–694. [PubMed] [Google Scholar]
- 4.Weiss AS, Sandmaier BM, Storer B, Storb R, McSweeney PA, Parikh CR. Chronic kidney disease following non-myeloablative hematopoietic cell transplantation. Am J Transplant. 2006;6:89–94. doi: 10.1111/j.1600-6143.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 6.Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048–1056. doi: 10.1093/ndt/gfh813. [DOI] [PubMed] [Google Scholar]
- 7.Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol. 2006;17:1995–2005. doi: 10.1681/ASN.2006020118. [DOI] [PubMed] [Google Scholar]
- 8.Cohen EP. Renal failure after bone-marrow transplantation. Lancet. 2001;357:6–7. doi: 10.1016/S0140-6736(00)03561-3. [DOI] [PubMed] [Google Scholar]
- 9.Tarbell NJ, Guinan EC, Niemeyer C, Mauch P, Sallan SE, Weinstein HJ. Late onset of renal dysfunction in survivors of bone marrow transplantation. Int J Radiat Oncol Biol Phys. 1988;15:99–104. doi: 10.1016/0360-3016(88)90352-5. [DOI] [PubMed] [Google Scholar]
- 10.Guinan EC, Tarbell NJ, Niemeyer CM, Sallan SE, Weinstein HJ. Intravascular hemolysis and renal insufficiency after bone marrow transplantation. Blood. 1988;72:451–455. [PubMed] [Google Scholar]
- 11.Tarbell NJ, Guinan EC, Chin L, Mauch P, Weinstein HJ. Renal insufficiency after total body irradiation for pediatric bone marrow transplantation. Radiother Oncol. 1990;18 (Suppl 1):139–142. doi: 10.1016/0167-8140(90)90195-3. [DOI] [PubMed] [Google Scholar]
- 12.Cohen EP, Piering WF, Kabler-Babbitt C, Moulder JE. End-stage renal disease (ESRD)after bone marrow transplantation: poor survival compared to other causes of ESRD. Nephron. 1998;79:408–412. doi: 10.1159/000045085. [DOI] [PubMed] [Google Scholar]
- 13.Kist-van Holthe JE, Bresters D, Ahmed-Ousenkova YM, et al. Long-term renal function after hemopoietic stem cell transplantation in children. Bone Marrow Transplant. 2005;36:605–610. doi: 10.1038/sj.bmt.1705110. [DOI] [PubMed] [Google Scholar]
- 14.Kist-van Holthe JE, Goedvolk CA, Brand R, et al. Prospective study of renal insufficiency after bone marrow transplantation. Pediatr Nephrol. 2002;17:1032–1037. doi: 10.1007/s00467-002-0989-9. [DOI] [PubMed] [Google Scholar]
- 15.Juckett MB, Cohen EP, Keever-Taylor CA, et al. Loss of renal function following bone marrow transplantation: an analysis of angiotensin converting enzyme D/I polymorphism and other clinical risk factors. Bone Marrow Transplant. 2001;27:451–456. doi: 10.1038/sj.bmt.1702797. [DOI] [PubMed] [Google Scholar]
- 16.Kist-van Holthe JE, van Zwet JM, Brand R, van Weel MH, Vossen JM, van der Heijden AJ. Bone marrow transplantation in children: consequences for renal function shortly after and 1 year post-BMT. Bone Marrow Transplant. 1998;22:559–564. doi: 10.1038/sj.bmt.1701388. [DOI] [PubMed] [Google Scholar]
- 17.Lawton CA, Cohen EP, Murray KJ, et al. Long-term results of selective renal shielding in patients undergoing total body irradiation in preparation for bone marrow transplantation. Bone Marrow Transplant. 1997;20:1069–1074. doi: 10.1038/sj.bmt.1701022. [DOI] [PubMed] [Google Scholar]
- 18.Cohen EP, Lawton CA, Moulder JE, Becker CG, Ash RC. Clinical course of late-onset bone marrow transplant nephropathy. Nephron. 1993;64:626–635. doi: 10.1159/000187412. [DOI] [PubMed] [Google Scholar]
- 19.Lawton CA, Cohen EP, Barber-Derus SW, et al. Late renal dysfunction in adult survivors of bone marrow transplantation. Cancer. 1991;67:2795–2800. doi: 10.1002/1097-0142(19910601)67:11<2795::aid-cncr2820671114>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Frisk P, Bratteby LE, Carlson K, Lonnerholm G. Renal function after autologous bone marrow transplantation in children: a long-term prospective study. Bone Marrow Transplant. 2002;29:129–136. doi: 10.1038/sj.bmt.1703312. [DOI] [PubMed] [Google Scholar]
- 21.Lonnerholm G, Carlson K, Bratteby LE, et al. Renal function after autologous bone marrow transplantation. Bone Marrow Transplant. 1991;8:129–134. [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Borg M, Hughes T, Horvath N, Rice M, Thomas AC. Renal toxicity after total body irradiation. Int J Radiat Oncol Biol Phys. 2002;54:1165–1173. doi: 10.1016/s0360-3016(02)03039-0. [DOI] [PubMed] [Google Scholar]
- 25.Bradley J, Reft C, Goldman S, et al. High-energy total body irradiation as preparation for bone marrow transplantation in leukemia patients: treatment technique and related complications. Int J Radiat Oncol Biol Phys. 1998;40:391–396. doi: 10.1016/s0360-3016(97)00578-6. [DOI] [PubMed] [Google Scholar]
- 26.Carter A, Choi M, Sun C, Francisco L, Forman S, Bhatia S. Delayed Chronic Kidney Disease (CKD) after Hematopoietic Cell Transplantation (HCT) Blood. 2006:108. doi: 10.1002/cncr.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deconinck E, Kribs M, Rebibou JM, Bulabois CE, Ducloux D, Cahn JY. Cytomegalovirus infection and chronic graft-versus-host disease are significant predictors of renal failure after allogeneic hematopoietic stem cell transplantation. Haematologica. 2005;90:569–570. [PubMed] [Google Scholar]
- 28.Delgado J, Cooper N, Thomson K, et al. The importance of age, fludarabine, and total body irradiation in the incidence and severity of chronic renal failure after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:75–83. doi: 10.1016/j.bbmt.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 29.Dieterle A, Gratwohl A, Nizze H, et al. Chronic cyclosporine-associated nephrotoxicity in bone marrow transplant patients. Transplantation. 1990;49:1093–1100. doi: 10.1097/00007890-199006000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Hingorani S, Guthrie KA, Schoch G, Weiss NS, McDonald GB. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 2007;39:223–229. doi: 10.1038/sj.bmt.1705573. [DOI] [PubMed] [Google Scholar]
- 31.Igaki H, Karasawa K, Sakamaki H, et al. Renal dysfunction after total-body irradiation. Significance of selective renal shielding blocks. Strahlenther Onkol. 2005;181:704–708. doi: 10.1007/s00066-005-1405-8. [DOI] [PubMed] [Google Scholar]
- 32.Kumar M, Kedar A, Neiberger RE. Kidney function in long-term pediatric survivors of acute lymphoblastic leukemia following allogeneic bone marrow transplantation. Pediatr Hematol Oncol. 1996;13:375–379. doi: 10.3109/08880019609030844. [DOI] [PubMed] [Google Scholar]
- 33.Leahey AM, Teunissen H, Friedman DL, Moshang T, Lange BJ, Meadows AT. Late effects of chemotherapy compared to bone marrow transplantation in the treatment of pediatric acute myeloid leukemia and myelodysplasia. Med Pediatr Oncol. 1999;32:163–169. doi: 10.1002/(sici)1096-911x(199903)32:3<163::aid-mpo1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Leblond V, Sutton L, Jacquiaud C, et al. Evaluation of renal function in 60 long-term survivors of bone marrow transplantation. J Am Soc Nephrol. 1995;6:1661–1665. doi: 10.1681/ASN.V661661. [DOI] [PubMed] [Google Scholar]
- 35.Miralbell R, Sancho G, Bieri S, et al. Renal insufficiency in patients with hematologic malignancies undergoing total body irradiation and bone marrow transplantation: a prospective assessment. Int J Radiat Oncol Biol Phys. 2004;58:809–816. doi: 10.1016/j.ijrobp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Parikh CR, McSweeney PA, Korular D, et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002;62:566–573. doi: 10.1046/j.1523-1755.2002.00455.x. [DOI] [PubMed] [Google Scholar]
- 37.Carlson K, Smedmyr B, Hagberg H, Oberg G, Simonsson B. Haemolytic uraemic syndrome and renal dysfunction following BEAC (BCNU, etoposide, ara-C, cyclophosphamide) +/− TBI and autologous BMT for malignant lymphomas. Bone Marrow Transplant. 1993;11:205–208. [PubMed] [Google Scholar]
- 38.Giralt S, Bensinger W, Goodman M, et al. 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase 1/2 trials. Blood. 2003;102:2684–2691. doi: 10.1182/blood-2002-10-3250. [DOI] [PubMed] [Google Scholar]
- 39.Glynne P, Powles R, Steele J, et al. Renal dysfunction following autologous bone marrow transplantation in adult patients with acute leukemia. Acta Oncol. 1996;35:709–712. doi: 10.3109/02841869609084003. [DOI] [PubMed] [Google Scholar]
- 40.Patzer L, Ringelmann F, Kentouche K, et al. Renal function in long-term survivors of stem cell transplantation in childhood. A prospective trial. Bone Marrow Transplant. 2001;27:319–327. doi: 10.1038/sj.bmt.1702763. [DOI] [PubMed] [Google Scholar]
- 41.Van Why SK, Friedman AL, Wei LJ, Hong R. Renal insufficiency after bone marrow transplantation in children. Bone marrow transplantation. 1991;7:383–388. [PubMed] [Google Scholar]
- 42.Miralbell R, Bieri S, Mermillod B, et al. Renal toxicity after allogeneic bone marrow transplantation: the combined effects of total-body irradiation and graft-versus-host disease. J Clin Oncol. 1996;14:579–585. doi: 10.1200/JCO.1996.14.2.579. [DOI] [PubMed] [Google Scholar]
- 43.Hale GA, Bowman LC, Rochester RJ, et al. Hemolytic uremic syndrome after bone marrow transplantation: clinical characteristics and outcome in children. Biol Blood Marrow Transplant. 2005;11:912–920. doi: 10.1016/j.bbmt.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Imai H, Oyama Y, Miura AB, Endoh M, Sakai H. Hematopoietic cell transplantation-related nephropathy in Japan. Am J Kidney Dis. 2000;36:474–480. doi: 10.1053/ajkd.2000.9787. [DOI] [PubMed] [Google Scholar]
- 45.San Miguel JF, Lahuerta JJ, Garcia-Sanz R, et al. Are myeloma patients with renal failure candidates for autologous stem cell transplantation? Hematol J. 2000;1:28–36. doi: 10.1038/sj.thj.6200003. [DOI] [PubMed] [Google Scholar]
- 46.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985 Apr;33(4):278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 47.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 48.Nix DE, Erstad BL, Nakazato PZ, Barletta JF, Matthias KR, Krueger TS. Estimation of creatinine clearance in end-stage liver disease. Ann Pharmacother. 2006;40:900–908. doi: 10.1345/aph.1G594. [DOI] [PubMed] [Google Scholar]
- 49.MacAulay J, Thompson K, Kiberd BA, Barnes DC, Peltekian KM. Serum creatinine in patients with advanced liver disease is of limited value for identification of moderate renal dysfunction: are the equations for estimating renal function better? Can J Gastroenterol. 2006;20:521–526. doi: 10.1155/2006/858053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Proulx NL, Akbari A, Garg AX, Rostom A, Jaffey J, Clark HD. Measured creatinine clearance from timed urine collections substantially overestimates glomerular filtration rate in patients with liver cirrhosis: a systematic review and individual patient meta-analysis. Nephrol Dial Transplant. 2005;20:1617–1622. doi: 10.1093/ndt/gfh839. [DOI] [PubMed] [Google Scholar]
- 51.Skluzacek PA, Szewc RG, Nolan CR, 3rd, Riley DJ, Lee S, Pergola PE. Prediction of GFR in liver transplant candidates. Am J Kidney Dis. 2003;42:1169–1176. doi: 10.1053/j.ajkd.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Ustundag Y, Samsar U, Acikgoz S, et al. Analysis of glomerular filtration rate, serum cystatin C levels, and renal resistive index values in cirrhosis patients. Clin Chem Lab Med. 2007;45:890–894. doi: 10.1515/CCLM.2007.130. [DOI] [PubMed] [Google Scholar]
- 53.Kersting S, Hene RJ, Koomans HA, Verdonck LF. Chronic kidney disease after myeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:1169–1175. doi: 10.1016/j.bbmt.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Panoskaltsis-Mortari A, Price A, Hermanson JR, et al. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103:3590–3598. doi: 10.1182/blood-2003-08-2827. [DOI] [PubMed] [Google Scholar]
- 55.Gruss E, Bernis C, Tomas JF, et al. Acute renal failure in patients following bone marrow transplantation: prevalence, risk factors and outcome. Am J Nephrol. 1995;15:473–479. doi: 10.1159/000168889. [DOI] [PubMed] [Google Scholar]
- 56.Hingorani SR, Guthrie K, Batchelder A, et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney Int. 2005;67:272–277. doi: 10.1111/j.1523-1755.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 57.Luxton RW. Radiation nephritis. Q J Med. 1953;22:215–242. [PubMed] [Google Scholar]
- 58.Cohen EP, Drobyski WR, Moulder JE. Significant increase in end-stage renal disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:571–572. doi: 10.1038/sj.bmt.1705643. [DOI] [PubMed] [Google Scholar]