Abstract
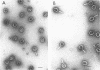
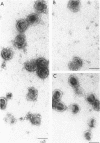
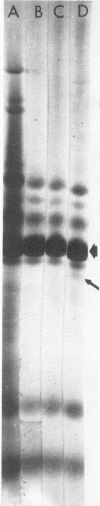
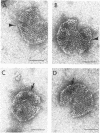
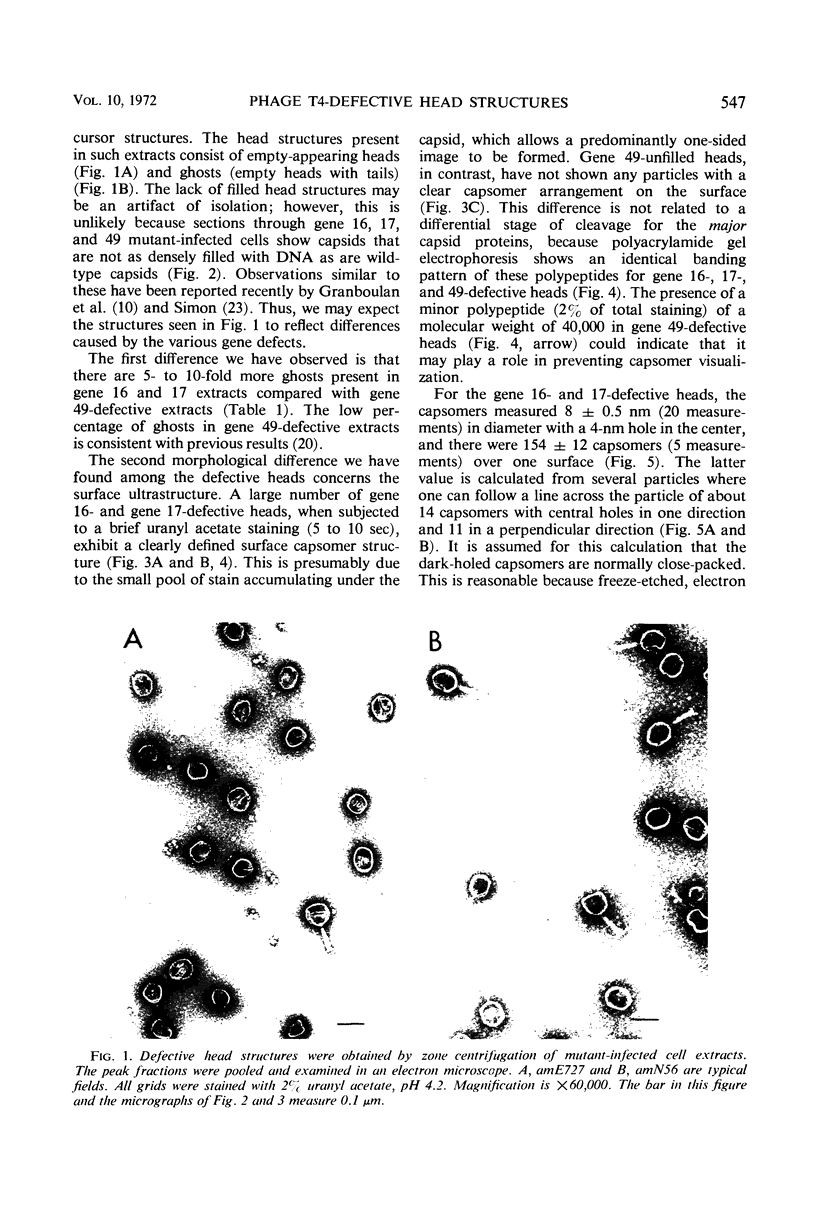
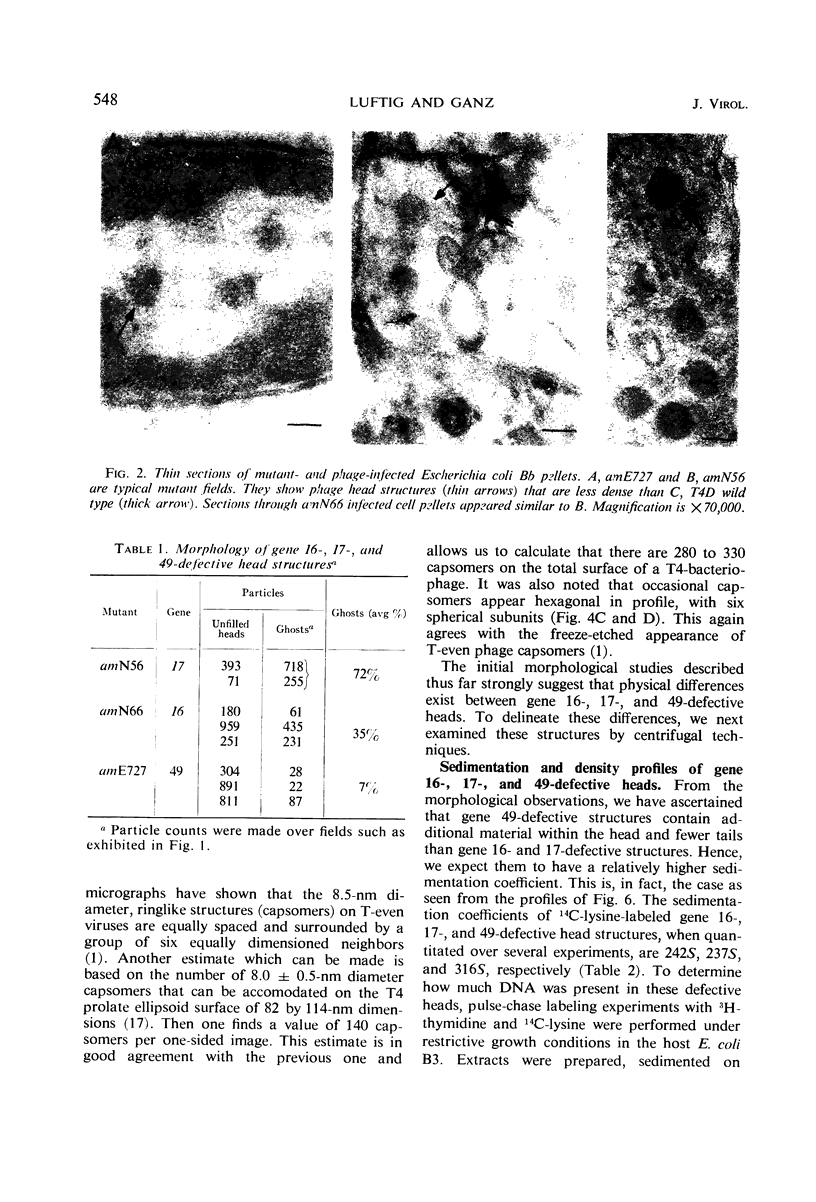
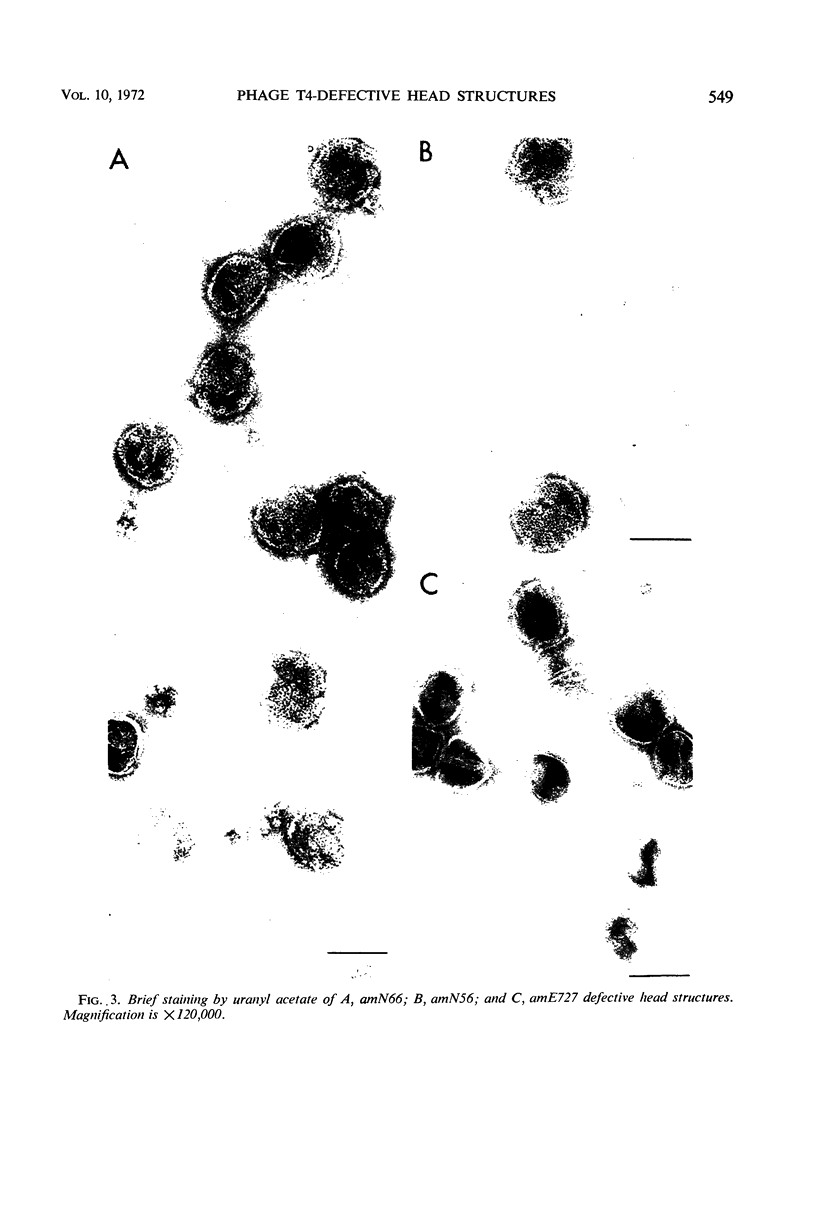
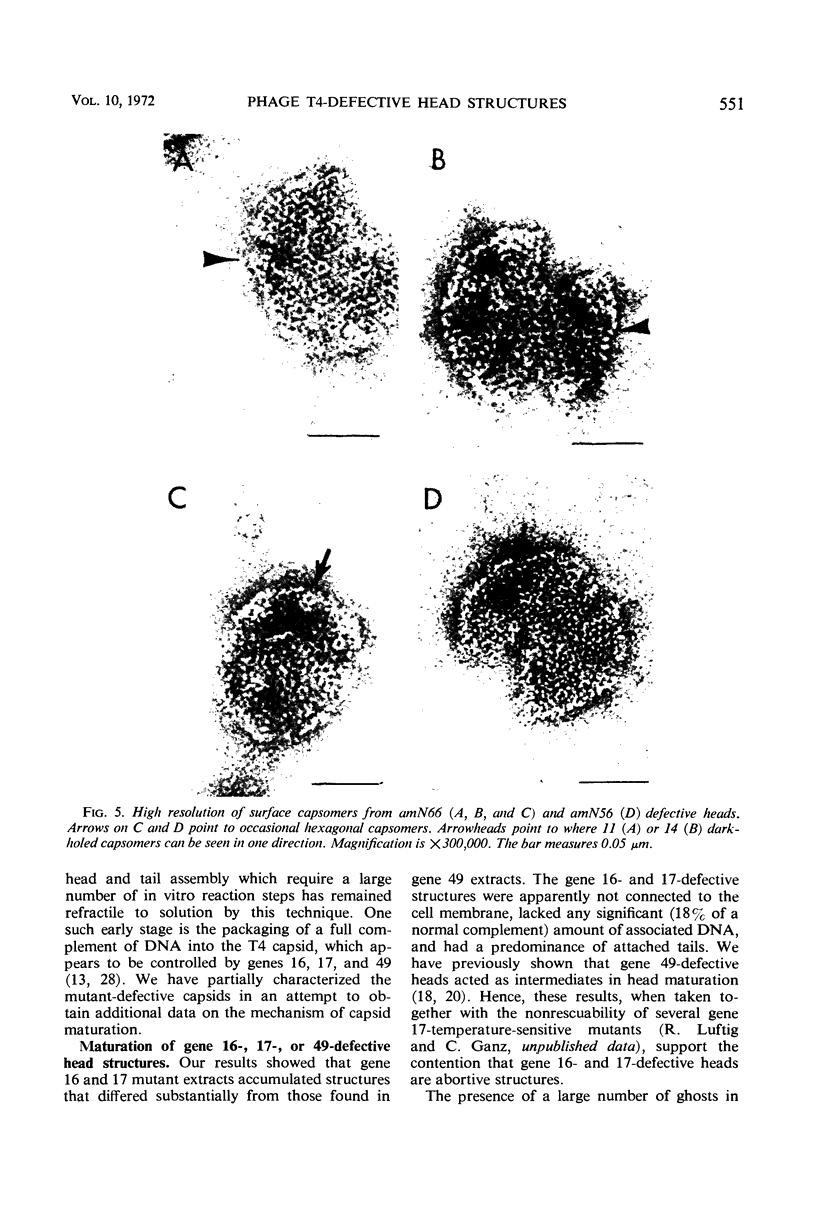
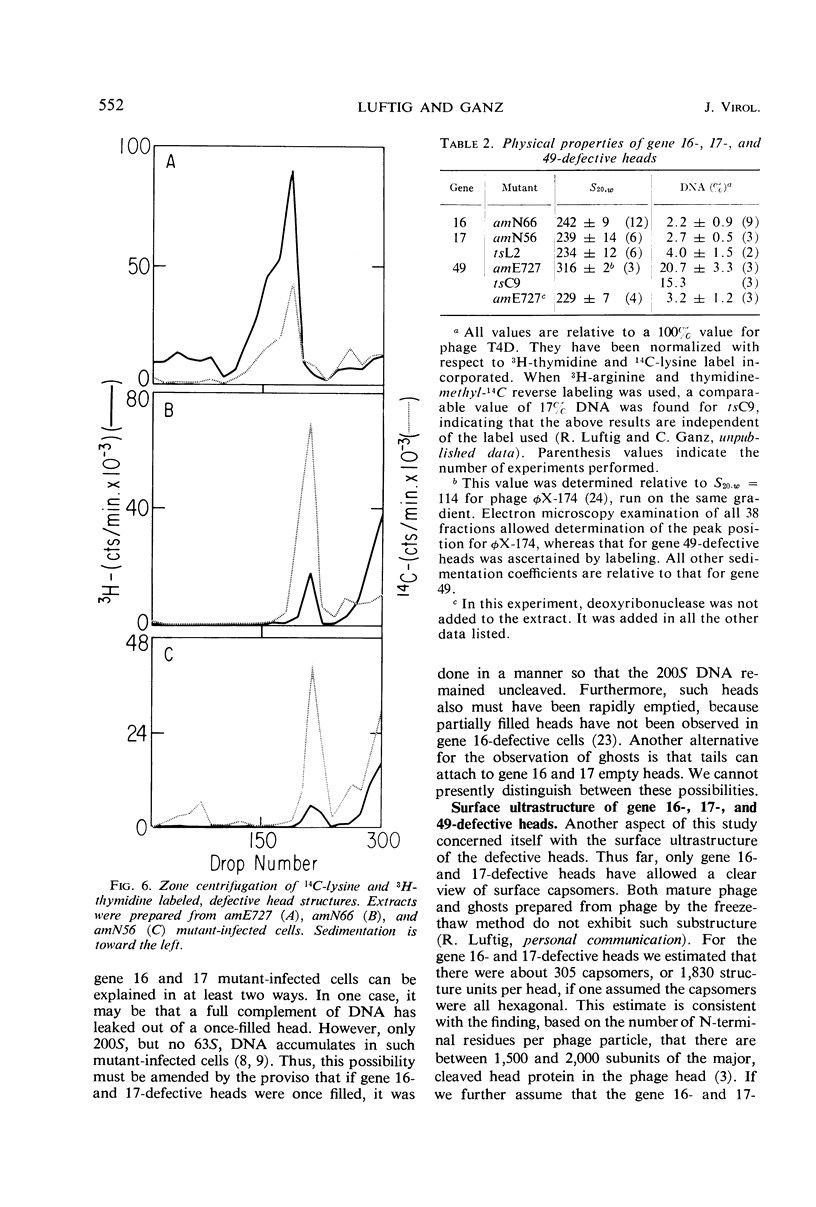
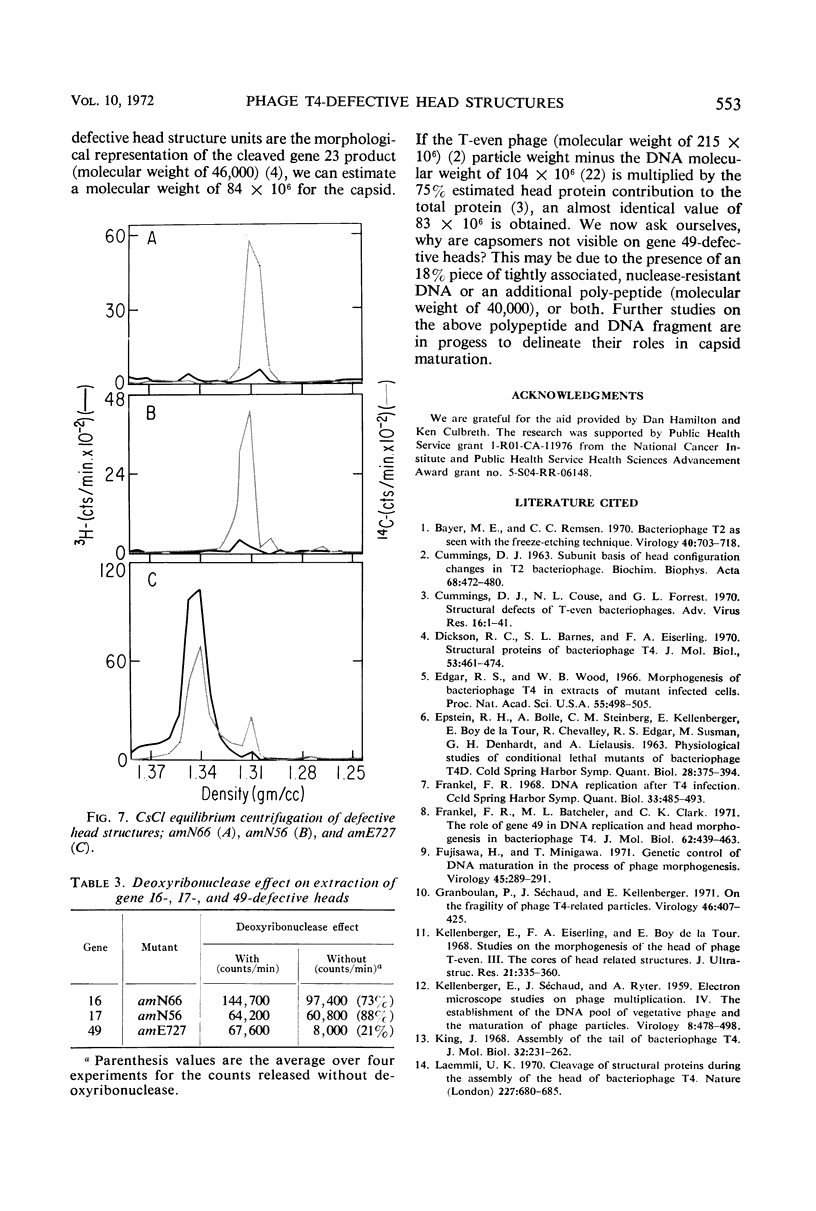
Defective heads present in extracts of bacteriophage T4 gene 16, 17, or 49 mutant-infected cells have been characterized. All appeared as empty shells when examined by negative-stain electron microscopy and showed essentially the same polypeptide pattern on sodium dodecyl sulfate-acrylamide gels. However, when analyzed by several other methods, gene 16- and 17-defective heads were shown to differ markedly from phage heads present in gene 49-defective extracts. First, the gene 16- and 17-defective structures were found to possess a large number of attached tails (50%, rather than about 5%). Second, they contained less nuclease-resistant deoxyribonucleic acid (DNA) (3 versus 18% of a phage equivalent), had a smaller sedimentation coefficient (240 versus 315S), and a lighter density (1.31 vs. 1.34 g/ml) than gene 49-defective heads. Third, they were not attached to the intracellular DNA pool through a deoxyribonuclease-sensitive linkage. Finally, 8-nm diameter capsomers were clearly revealed on the surface of many gene 16- and 17-defective structures. There was a total of 305 ± 25 capsomers per particle, which yielded an approximate molecular weight of 84 × 106 for these heads. The capsomers were presumably not seen on gene 49-defective heads because of the large amount (18%) of associated DNA.
These results support the contention that gene 16- and 17-defective heads, in contrast to gene 49-defective structures, represent abortive rather than intermediate structures in T4 head morphogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Remsen C. C. Bacteriophage T2 as seen with the freeze-etching technique. Virology. 1970 Mar;40(3):703–718. doi: 10.1016/0042-6822(70)90215-1. [DOI] [PubMed] [Google Scholar]
- CUMMINGS D. J. Subunit basis of head configurational changes in T2 bacteriophage. Biochim Biophys Acta. 1963 Mar 26;68:472–480. doi: 10.1016/0006-3002(63)90169-0. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Couse N. L., Forrest G. L. Structural defects of T-even bacteriophages. Adv Virus Res. 1970;16:1–41. doi: 10.1016/s0065-3527(08)60020-2. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. DNA replication after T4 infection. Cold Spring Harb Symp Quant Biol. 1968;33:485–493. doi: 10.1101/sqb.1968.033.01.056. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Minagawa T. Genetic control of the DNA maturation in the process of phage morphogenesis. Virology. 1971 Jul;45(1):280–291. [PubMed] [Google Scholar]
- Granboulan P., S echaud J., Kellenberger E. On the fragility of phage T4-related particles. Virology. 1971 Nov;46(2):407–425. doi: 10.1016/0042-6822(71)90042-0. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J., RYTER A. Electron microscopical studies of phage multiplication. IV. The establishment of the DNA pool of vegetative phage and the maturation of phage particles. Virology. 1959 Aug;8:478–498. doi: 10.1016/0042-6822(59)90050-9. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Eiserling F. A., Boy de la Tour E. Studies on the morphopoiesis of the head of phage T-even. 3. The cores of head-related structures. J Ultrastruct Res. 1967 Dec 12;21(3):335–360. doi: 10.1016/s0022-5320(67)80099-6. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Luftig R. B. Further studies on the dimensions of viral and protein structures using the catalase crystal internal marker technique. J Ultrastruct Res. 1968 Apr;23(1):178–181. doi: 10.1016/s0022-5320(68)80041-3. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. II. Studies on the maturation of gene 49-defective head intermediates. J Virol. 1972 Feb;9(2):377–389. doi: 10.1128/jvi.9.2.377-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- Mosig G. Distances separating genetic markers in T4 DNA. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1177–1183. doi: 10.1073/pnas.56.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Hearst J. E. Molecular weights of homogeneous coliphage DNA's from density-gradient sedimentation equilibrium. J Mol Biol. 1969 Aug 28;44(1):143–160. doi: 10.1016/0022-2836(69)90410-0. [DOI] [PubMed] [Google Scholar]
- Simon L. D. Infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope: T4 head morphogenesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):907–911. doi: 10.1073/pnas.69.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wood W. B., Edgar R. S., King J., Lielausis I., Henninger M. Bacteriophage assembly. Fed Proc. 1968 Sep-Oct;27(5):1160–1166. [PubMed] [Google Scholar]