Abstract
Stability in frontal brain electrical activity (i.e., electroencephalographic or EEG) asymmetry at 10 and 24 months was examined with respect to maternal ratings of internalizing and externalizing behaviors at 30 months in a sample of 48 children. Children with stable left frontal EEG asymmetry during infancy were rated higher in externalizing behaviors by their mothers, whereas children with stable right frontal EEG asymmetry were rated higher in internalizing behaviors. These findings highlight the need to focus on the early stability in physiological measures that may be implicated later in developing behavioral problems.
Keywords: EEG, frontal asymmetry, human infants, human toddlers, internalizing, externalizing
Recent research has found high levels of stability in the developmental trajectories of children with early identified behavior problems, both internalizing problems, such as fear and anxiety, and externalizing problems, such as aggression, hyperactivity, and impulsivity (e.g., Hill, Degnan, Calkins, & Keane, 2006; Kerr, Lunkenheimer, & Olson, 2007; Patterson, Shaw, Snyder, & Yoerger, 2005; Pihlakoski, Sourander, Aromaa, Rautava, Helenius, & Sillanpaa, 2006). Given the high level of developmental stability in these behaviors, the importance of early detection for children at-risk for these types of problems becomes crucial. Understanding early predictors of children’s behavior problems is important for identifying mechanisms underlying the development of these adjustment difficulties and thus allowing for intervention to occur as early as possible. One typical approach has been to examine early behaviors that may indicate potential risk for these problems; however, physiological indicators may be particularly useful in the identification of children at-risk for behavior problems. One physiological marker that may be important is the children’s underlying brain physiology, specifically asymmetries in the brain’s frontal scalp electrical activity. The goal of the current paper was to examine how frontal asymmetry measured across infancy is related to internalizing and externalizing behaviors during toddlerhood.
According to a model proposed by Fox (1991, 1994), frontal electroencephalogram (EEG) asymmetry is related to basic approach and withdrawal tendencies present from birth. Children with more left frontal EEG activation associated with approach tendencies are theorized to show more positive affect and exploratory behaviors, and children with more right frontal EEG activation associated with withdrawal tendencies are expected to show more negative affect and freezing or escape behaviors (Fox & Henderson, 1999). Differences in frontal EEG asymmetries are not only hypothesized to be related to early emotional reactivity but also are thought to reflect underlying capacities to regulate these emotional tendencies (Fox, 1994). Differences in children’s ability to regulate emotions has also been found to be related to their levels of internalizing and externalizing behaviors as early as toddlerhood (Calkins & Dedmon, 2000; Feng, Shaw, & Silk, 2008); thus, early patterns of frontal asymmetries may be important precursors to understanding individual differences in internalizing and externalizing behaviors. Following from Fox’s model (1991, 1994), greater right frontal asymmetry (i.e., greater relative right frontal activation) is hypothesized to be associated with behaviors indicative of internalizing disorders, such as active withdrawal, increased negative affect including fear and anxiety, and depressed mood. On the other hand, greater left frontal asymmetry (i.e., greater relative left frontal activation) has been hypothesized to be associated with a lack of ability to control approach behaviors and associated emotions, particularly anger, which might lead to externalizing behaviors such as impulsivity, hyperactivity, and aggression.
Often the approach in the developmental literature for examining continuity over time has been to examine continuity in behavioral patterns and then examine physiological factors, such as EEG asymmetry, related to this behavioral continuity. Given expected developmental changes over time in the expression of approach and withdrawal tendencies and the behaviors accompanying these tendencies (Achenbach & Rescorla, 2004), a better approach to identifying children potentially at-risk might be to examine stability in the early physiological precursors of these tendencies as a predictor of later behavior problems. This approach might produce better indicators of potential problems than approaches that establish stability in child behaviors over time and then examine the physiology associated with the behavioral stability. In the current study, we examined how stability in frontal EEG asymmetry from 10 to 24 months of age was related to maternal ratings of internalizing and externalizing behaviors later in toddlerhood.
An example of work that has highlighted physiological factors related to behavioral stability is the body of work that has demonstrated associations between patterns of behavioral inhibition, which include internalizing behaviors such as high anxiety and social withdrawal, and right frontal EEG asymmetry. For example, Fox, Henderson, Rubin, Calkins, and Schmidt (2001) found that children who were highly inhibited across the first four years of life displayed right frontal EEG asymmetry at 9 and 14 months. This finding is consistent with past findings where highly inhibited infants and children were found to have right frontal EEG asymmetries (Davidson & Rickman, 1999; Finman, Davidson, Colton, Straus, & Kagan, 1989; Fox, Bell, & Jones, 1992; Fox, Schmidt, Calkins, Rubin, & Coplan, 1996), and high levels of behavioral inhibition have been found to be related to later internalizing behaviors such as social wariness and social anxiety (Henderson, Fox, & Rubin, 2001; Schwartz, Snidman, & Kagan, 1999). Based on these associations, one would expect that right frontal EEG asymmetry would be directly related to ratings of internalizing behaviors; however, researchers have not examined direct links between frontal EEG asymmetry and internalizing behaviors. The current study extends past findings by examining stability in frontal asymmetry across infancy as it relates to maternal report of internalizing behaviors during toddlerhood, which can be a key developmental period for the emergence of behavior problems (Achenbach & Rescorla, 2004).
Although past work has examined physiological indicators of behavioral inhibition along with other factors thought to be related to internalizing behaviors, past research has typically not addressed physiological indicators of externalizing behaviors. Based on Fox’s model (Fox 1991, 1994), however, patterns of left frontal asymmetry would be expected to be related to behaviors that could be indicative of externalizing behaviors. The limited work in this area has not produced conclusive findings. Forbes, Shaw, Fox, Cohn, Silk, and Kovacs (2006) found that left frontal asymmetry in 3 to 9 year old boys was associated with higher aggressive behaviors, thus supporting the idea that left frontal asymmetry may be related to externalizing behaviors. Baving, Laucht, and Schmidt (2003) found that left frontal asymmetry was associated with externalizing behaviors in 11-year-old boys but right frontal asymmetry was associated with externalizing in girls of the same age. Henderson, Fox, and Rubin (2001) predicted that negative reactivity in children with left frontal asymmetry would not be associated with social inhibition and found that left frontal EEG asymmetry might be a protective factor for temperamentally reactive infants. Their hypothesis addressed what left frontal asymmetry would not be related to but did not take the prediction further to examine what left frontal asymmetry would be associated with.
One possible explanation is that left frontal asymmetry is associated with anger and approach behaviors, which in some cases could be associated with more serious externalizing behaviors like impulsivity and aggression. Fox et al. (2001) found that children who were continuously uninhibited from infancy across early childhood, a group labeled as exuberant, had left frontal asymmetry, and these children were rated by mothers to be higher in positive affect and sociability. The children were not higher in externalizing behaviors and thus did not appear to have dysregulated behavior patterns. Fox and colleagues (Fox et al., 2001) suggested that these children may be more inclined to use approach behaviors. However, one of the emotions theorized to be associated with approach behaviors is anger (Fox, 1991, 1994). Harmon-Jones, Harmon-Jones, Abramson, and Peterson (2008) found that anger was associated with approach tendencies in college-aged individuals, suggesting that approach tendencies may potentially be associated with both positive emotions (e.g., exuberance) and negative emotions (e.g., anger). Therefore, children high in approach behaviors may be more likely to develop problems with impulsivity and aggression because of an inability to control the negative emotions associated with their approach behaviors, specifically anger.
When looking at frontal EEG activity and conduct disorders, a typical approach has been to examine the brain activity in older children already diagnosed with conduct problems (e.g., Kusche, Cook, & Greenberg, 1993), which does not examine the potential role of physiology prospectively. Other research examining the relations between neuropsychological functioning and conduct disorder often examined behavioral indicators of frontal lobe activity, instead of directly assessing brain electrophysiology (e.g., Brennan, Hall, Bor, Najman, & Williams, 2003). As discussed earlier, the approach taken by Fox and colleagues (Fox et al., 2001) has been to examine children who display stability in temperamental traits and behavior. The pattern of EEG asymmetry typically at one time point is then examined in respect to the behavioral stability; however, stability in EEG asymmetry may be a key factor in understanding which children will develop stable behavioral trajectories of externalizing behaviors.
In the current study, we examined how stability in frontal asymmetry across infancy was associated with maternal ratings of internalizing and externalizing behaviors in toddlerhood. Toddlerhood is a particularly important time to examine internalizing and externalizing behaviors as this is the developmental period when problems in these areas can begin to emerge as toddlers should be developing skills in self-regulation (Kopp, 1982). Based on Fox’s model (1991, 1994) and past work examining relations between EEG frontal asymmetries and child behavior (e.g., Forbes et al., 2006; Henderson et al., 2001), we predicted that infants with stable right frontal EEG asymmetries would have higher internalizing scores in toddlerhood, whereas infants with stable left frontal EEG asymmetries would have higher externalizing scores in toddlerhood.
Method
Participants
The participants for this study included mothers and their children who participated in an ongoing longitudinal study on individual differences in cognitive and emotional development. Three time points were used in the current study: when children were 10 (T1), 24 (T2), and 30 (T3) months old. Only children with complete data (i.e., children with baseline EEG at 10 and 24 months and CBCL scores at 30 months) were used in the analyses for this paper, n = 48, 26 boys, 22 girls. Mothers and infants were recruited from birth announcements placed in the local newspaper and from a commercial mailing list of new parents. All infants were healthy and full-term with no prenatal, birth, or postnatal complications. Nineteen percent of the mothers had a high school education, 58 percent were college graduates, and 23 percent had advanced degrees. Twenty percent of fathers graduated from high school, 44 percent were college graduates, and 37 percent had advanced degrees. All of the mothers and the majority of fathers, 98%, were non-Hispanic ethnicity. Ninety-two percent of the mothers and 96% of fathers were Caucasian. The demographics of the sample reflect those of the population where the study was conducted.
Procedures
For the T1 assessment, infants visited the research lab on or within two weeks after their 10-month birthdays, so that two weeks separated the youngest and oldest children at the T1 lab visit. Baseline EEG was recorded for 1 minute while infants sat on their mothers’ laps. During the baseline recording, a research assistant manipulated a toy containing brightly colored balls on top of the testing table, 1.1 m in front of the infants. This procedure quieted the infants and yielded minimal eye movements and gross motor movements, thus allowing infants to tolerate the EEG cap for the recording. Mothers were instructed not to talk to infants during the EEG recording. At the end of the assessment, mothers were paid $20 for participating in the 10-month visit.
For the T2 assessment, infants visited the research lab on or within two months after their 2-year birthdays, so that two months separated the youngest and oldest children at the T2 lab visit. At this visit, baseline EEG was recorded for 1 minute while the infants sat in a high chair and watched a clip of the video Finding Nemo (sea turtles riding the East Australian Current). Mothers sat in a chair to the right of the infants. During the baseline recording, the TV monitor was 1.8 meters from the infants, and mothers were instructed not to talk during the EEG baseline recording. Mothers were paid $50 for participating in the 24-month visit. The children were given a packet of crayons to take home.
For both the 10 and 24 month assessments, the baseline EEG recordings were made from 16 left and right scalp sites: frontal pole (Fp1,Fp2), medial frontal (F3,F4), lateral frontal (F7,F8), central (C3,C4), anterior temporal (T3,T4), posterior temporal (T7,T8), parietal (P3,P4), and occipital (O1,O2), referenced to Cz. EEG was recorded using a stretch cap (Electro-Cap, Inc.) with electrodes in the 10/20 system pattern. After the cap was placed on the head, recommended procedures regarding EEG data collection with infants and young children were followed (Pivik, Broughton, Coppola, Davidson, Fox, & Nuwer, 1993). Specifically, a small amount of abrasive was placed into each recording site and the scalp gently rubbed. Following this, conductive gel provided by the cap manufacturer was placed in each site. Electrode impedances were measured and accepted if they were below 5K ohms. The electrical activity from each lead was amplified using separate SA Instrumentation Bioamps and bandpassed from 1 to 100 Hz. Activity for each lead was displayed on the monitor of the acquisition computer. The EEG signal was digitized on-line at 512 samples per second for each channel so that the data were not affected by aliasing. The acquisition software was Snapshot-Snapstream (HEM Data Corp.) and the raw data were stored for later analyses.
Mothers were contacted for the T3 assessment after the children were 30 months of age (mean age = 31.62 months, SD = 1.70). In this assessment, mothers completed the Child Behavior Checklist for Ages 1½ –5 (CBCL; Achenbach & Rescorla, 2000) as a measure of the toddlers’ internalizing and externalizing behaviors. The CBCLs were mailed to mothers. Mothers either brought the completed CBCL with them when they came in for their laboratory assessment or mailed them back in an envelope provided (mothers who had moved from the area were contacted about completing a CBCL). Mothers who completed the laboratory visit were given $35, and the children were given two small toys to take home with them.
Measures
EEG
Infant EEG data from T1 and T2 were examined and analyzed using EEG Analysis System software developed by James Long Company (Caroga Lake, NY). First, the data were re-referenced via software to an average reference configuration, with the 16 electrode sites evenly distributed across the head (Hagemann, Naumann, & Thayer, 2001). Then, the average reference EEG data were artifact scored for eye movements using a peak-to-peak criterion of 100 uV or greater. The criterion for scoring movement artifact was a potential greater than 200 uV peak-to-peak. These artifact-scored epochs were eliminated from all subsequent analyses. The data then were analyzed with a discrete Fourier transform (DFT) using a Hanning window of one-second width and 50% overlap. Power was computed for the 6 to 9 Hz frequency band. Infants and young children have a dominant frequency between 6 to 9 Hz (Bell & Fox, 1994; Marshall, Bar-Haim, & Fox, 2002), and this particular frequency band has been correlated with patterns of emotion reactivity and emotion regulation during infancy (Bell & Fox, 1994; Buss, Malmstadt, Dolski, Kalin, Goldsmith, & Davidson, 2003; Dawson, 1994) and early childhood (Fox et al., 2001). The power was expressed as mean square microvolts and the data transformed using the natural log (ln) to normalize the distribution.
Frontal EEG asymmetry values were computed by subtracting ln power at left frontal (F3) from ln power at right frontal (F4). In infants and young children, power in the 6–9 Hz band has been shown to be inversely related to cortical activation during emotion reactivity and regulation (Fox, 1994). Thus, a negative asymmetry score reflects greater right frontal activation, whereas a positive asymmetry score reflects greater left frontal activation. Because classifying children’s frontal asymmetry based on positive or negative asymmetry scores has been shown to be a reliable and valid measure of asymmetry (Allen, Coan, & Nazarian, 2004; Coan & Allen, 2004; Fox et al., 1992; Schmidt, 2008), groups were formed based on the stability of the signs of their asymmetry scores. Three frontal asymmetry groups were formed based on the stability of frontal EEG asymmetry scores: Infants who exhibited right frontal EEG asymmetry at both 10 and 24 months (stable right group; n = 9), infants who exhibited left frontal EEG asymmetry at both times (stable left group; n = 14), and infants who did not display stability in EEG asymmetry (change group; n = 25). See Table 1 for demographic information for each asymmetry group.
Table 1.
Demographic Information Presented Separately for Each Asymmetry Group
| Demographic | Left | Right | Change |
|---|---|---|---|
| n | 14 | 9 | 25 |
| Mean child age in months (SD) | 32.06 (2.51) | 31.40 (1.24) | 31.45 (1.25) |
| Child sex: % male | 35.71 | 66.67 | 60.00 |
| Maternal education: % college degree or higher | 64.29 | 77.78 | 68.00 |
| Paternal education: % college degree or higher | 76.92 | 87.50 | 68.00 |
| Maternal ethnicity: % non-Hispanic | 100.00 | 100.00 | 100.00 |
| Paternal ethnicity: % non-Hispanic | 100.00 | 88.89 | 100.00 |
| Maternal race: % Caucasian | 100.00 | 100.00 | 84.00 |
| Paternal race: % Caucasian | 92.86 | 100.00 | 96.00 |
Behavior problems
The Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000) consists of 99 child behaviors that mothers rated on a 3-point scale (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true). The broadband scales for internalizing behaviors (emotionally reactive, anxious/depressed, somatic complaints, and withdrawn scales) and externalizing behaviors (attention problems and aggressive behavior scales) were computed using CBCL software. The raw scores for internalizing and externalizing were used.
Results
Preliminary Analyses
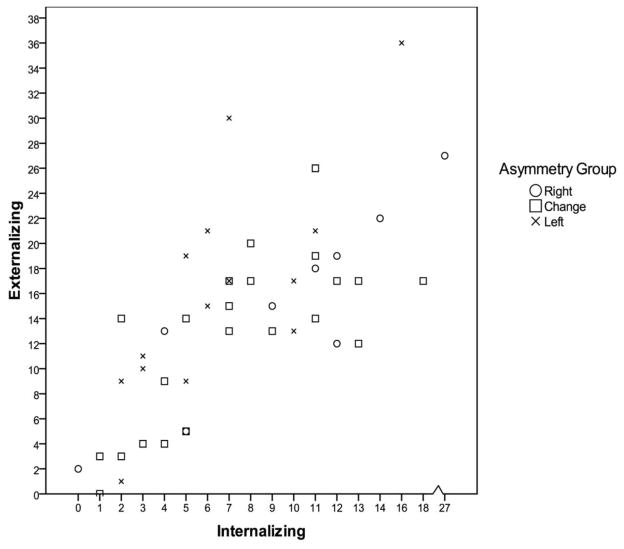
Child externalizing and internalizing scores were positively correlated, r(46) = .69, p < .01. As can be seen in the scatter plot of the data (see Figure 1), the correlation between internalizing and externalizing is not specific to one of the frontal EEG asymmetry groups. A high relation between internalizing and externalizing has been found in Achenbach’s work in the development of the scale (Achenbach & Recorla, 2000), and similar relations between internalizing and externalizing have been found in other samples of toddlers (e.g., Dietz, Jennings, Kelley, & Marshal, 2009; Shaw, Connell, Dishion, Wilson, & Gardner, 2009). According to Achenbach and Rescorla (2000), even though these behaviors may co-occur, some children have primarily internalizing problems whereas others have primarily externalizing problems. Because we were interested in examining which asymmetry group was primarily internalizing, child externalizing scores were controlled in the analyses examining differences in internalizing means for each of the frontal EEG asymmetry group. Similarly, to examine which group was rated as primarily externalizing, internalizing scores were controlled in analyses examining differences in externalizing scores for the frontal EEG asymmetry groups.
Figure 1.
Scatter plot of children’s internalizing and externalizing scores by frontal asymmetry group.
We examined associations between age from the 30-month assessment (T3) and other variables because there was a wider variability in age at that assessment than at the infant assessments. There were four non-significant trends in the data associated with child sex and child age at the 30-month visit (T3). First, there was a moderate negative association between internalizing scores and T3 child age, r(46) = −.26, p < .08. Second, baseline frontal EEG asymmetry scores at 24 months were moderately related to T3 child age, r(46) = .26, p < .08. Next, mothers rated girls lower in internalizing, M = 6.27, than boys, M = 8.88, t(1,46) = 1.80, p < .08. Similarly, mothers rated girls lower in externalizing, M = 12.05, than boys, M = 15.65, t(1,46) = 1.69, p < .10. No child sex differences were found in EEG baseline scores. Given these moderate associations, child age and sex were also controlled for in the analyses.
Stability of Asymmetry Groups
To examine the stability of asymmetry scores for each group, three paired sample t-tests were conducted. The asymmetry score means at 10 and 24 months were not significantly different from each other for the right asymmetry group, t(8) = .85, p = .42. For the left asymmetry group, the means were not significantly different from each other, t(13) = 2.07, p = .06, although the mean difference approached significance. For the change group, the means for 10 and 24 months were significantly different from each other, t(24) = 3.52, p = .002.
Asymmetry Group Differences in Internalizing Scores
Multiple regression analysis was used to examine group differences in the internalizing scores for the three asymmetry groups. Three control variables, child sex, child age, and externalizing scores, were entered on the first step of the regression. Child sex was dummy coded with males coded as 0 and females coded as 1. Child age and externalizing scores were centered to allow for proper interpretation of the group differences in internalizing scores (Cohen, Cohen, West, & Aiken, 2003). Contrast codes were formed according to procedures outlined in Cohen et al. (2003), and the three contrast codes of interest were those that compared the stable right asymmetry group to the stable left asymmetry group, the stable right asymmetry group to the change group, and the stable left asymmetry group to the change group. Separate regression analyses were conducted to test each of the contrasts of interest; however, the overall variance explained by the groups (i.e., the R2 change and F statistic for the step with the contrast codes) was the same in each regression analysis. Each contrast code represented a different way to partition the variance associated with the three groups (Cohen et al., 2003).
The results of the regression analyses are presented in Table 2, and the CBCL internalizing means for each frontal asymmetry group are presented in Figure 2. As seen in Table 2, group membership explained a significant amount of the variance in toddler internalizing behaviors, after controlling for the effects of child sex, child age, and externalizing behaviors. The stable left and stable right asymmetry groups had significantly different means for internalizing behaviors. The mean difference for the comparison of the stable left and change groups was moderately significant, but mean difference in internalizing scores for the comparison of the stable right and change groups was not significant. Infants who were in the stable right asymmetry group had significantly higher internalizing scores in toddlerhood than infants who were in the stable left asymmetry group.
Table 2.
Regression Analyses Examining Asymmetry Group Differences
| Beta | R2 | R2 change | F for step | |
|---|---|---|---|---|
| Predicting Internalizing: | ||||
| 1. Child sex | −.03 | .49 | .49** | 14.16** |
| Child age | −.11 | |||
| Externalizing | .49** | |||
| 2. Left - right contrast | −4.49** | .58 | .09* | 11.41** |
| Right - change contrast | 2.00 | |||
| Left - change contrast | −2.50+ | |||
| Predicting Externalizing: | ||||
| 1. Child sex | −1.88 | .48 | .48** | 13.72** |
| Child age | −.39 | |||
| Internalizing | 1.02** | |||
| 2. Left - right contrast | 6.29** | .59 | .11** | 12.17** |
| Right - change contrast | −.99 | |||
| Left - change contrast | 5.30** | |||
Notes. The betas reported are the unstandardized betas from the last step.
p < .10,
p < .05,
p < .01.
Figure 2.
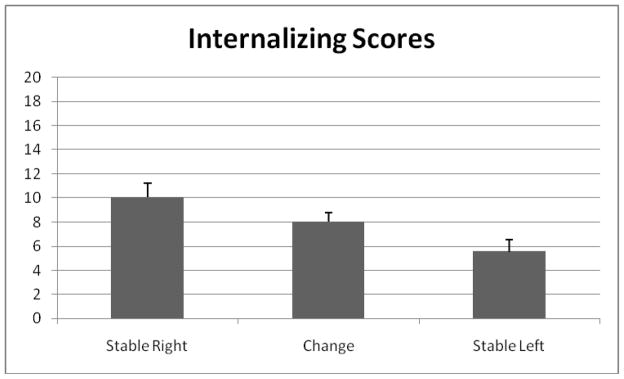
Mean differences in internalizing scores for the three frontal asymmetry groups, controlling for externalizing scores, child age, and child sex.
Asymmetry Group Differences in Externalizing Scores
A similar process was used to examine group differences in externalizing scores. The control variables, child sex, child age, and internalizing scores, were entered on the first step of the regression analysis. Again, child sex was dummy coded, and child age and internalizing scores were centered. The same three contrast codes were used to compare the stable right asymmetry group to the stable left asymmetry group, the stable right asymmetry group to the change group, and the stable left asymmetry group to the change group. Each contrast of interest was examined in a separate regression analysis.
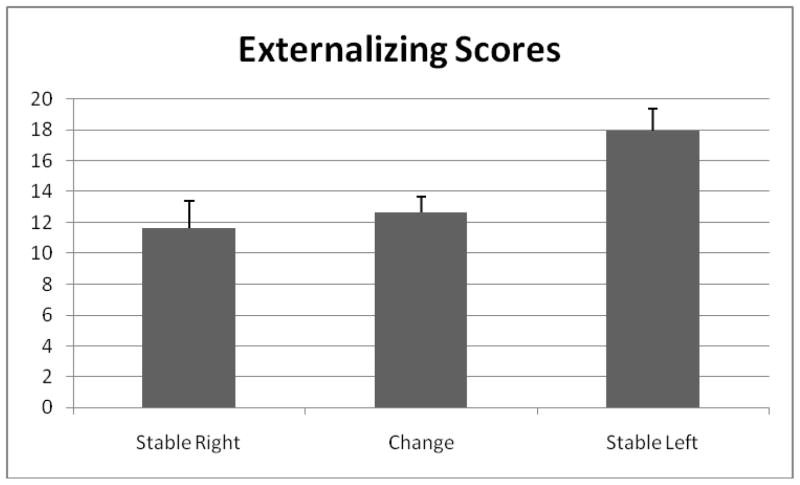
The results of the regression analyses are presented in Table 2, and the externalizing means for each frontal asymmetry group are depicted in Figure 3. Again, infant group membership explained a significant amount of the variance in toddler externalizing behaviors, after controlling for the variance explained by child sex, child age, and internalizing behaviors. The mean difference in externalizing scores was significant for the contrast comparing the means of the stable left versus the stable right asymmetry groups and for the contrast comparing the stable left versus the change asymmetry groups. The mean difference between the stable right and the change asymmetry groups was not significant. Infants in the stable left asymmetry group had significantly higher scores on externalizing behaviors during toddlerhood than infants in the stable right and change asymmetry groups.
Figure 3.
Mean differences in externalizing scores for the three frontal asymmetry groups, controlling for internalizing scores, child age, and child sex.
Discussion
The goal of the current study was to examine how stability in frontal EEG asymmetry across infancy related to maternal report of behavior problems in toddlerhood. The findings support our hypotheses based on Fox’s (1991, 1994) model because internalizing behaviors were associated with stable right frontal asymmetries and externalizing behaviors were associated with stable left frontal asymmetries. These findings indicate that brain physiology, particularly patterns of frontal asymmetry across infancy, may be a potential marker of children at-risk for the development of behavior problems at later ages.
As has been found in past research (e.g., Fox et al., 1992, 1996, 2001), right frontal asymmetry was associated with higher ratings of internalizing behaviors, such as higher anxiety and more withdrawal behaviors. Our findings extend previous work by examining direct links between frontal asymmetry and internalizing behaviors because the majority of the past research in this area has examined links between frontal asymmetry and measures of behavioral inhibition. Even though measures of behavior inhibition have been found to be related to internalizing behaviors (Henderson et al., 2001; Schwartz et al., 1999), the findings from the current study are some of the first to directly relate right frontal asymmetry to reports of internalizing behaviors. Additionally, past work in this area has typically focused on identifying patterns of behavioral continuity and then examining frontal asymmetry at one or two time points to examine if the physiology is associated with the behavioral continuity. One problem with this approach is that behaviors that are indicative of internalizing disorders are likely to change across childhood because of the rapid rate of development, including advances in language development and self-regulation (Achenbach & Rescorla, 2004). For example, the contexts that elicit behavioral inhibition are not the same in infancy as in toddlerhood. Therefore, it is often difficult to identify patterns of behavioral continuity as potential risk factors for internalizing disorders and has lead researchers to call for the examination of other risk factors besides behavior as early indicators of behavior problems (Bauer, Quas, & Boyce, 2002). Given the moderate stability in frontal asymmetry across early childhood (Bell & Fox, 1994; Fox, Calkins, & Bell, 1994), using stability in frontal asymmetry as a potential marker of risk for internalizing behaviors may prove to be more reliable than using stability in behavioral indicators.
We also found that toddlers rated higher in externalizing behaviors had stable left frontal EEG asymmetries from 10 to 24 months of age. These findings support those of Forbes et al. (2006) who found that left frontal EEG asymmetry was related to aggression in boys between 3 to 9 years of age. Fox et al. (2001), however, found that children with left frontal asymmetries were higher in exuberance, which included high levels of positive affect and sociability, but were not higher in externalizing behaviors. As with internalizing behaviors, perhaps the stability in asymmetry scores may be more important to understanding externalizing behaviors than asymmetry scores considered at one time point. Within the externalizing behavior problem literature, the children identified as being at the highest risk for stable trajectories are those showing a consistent constellation of problems early in life (Campbell, 2002; Campbell, Shaw, & Gilliom, 2000). Drawing from the current findings, another potential marker within the constellation of risk factors may be stable left frontal EEG asymmetries. According to Fox (1991, 1994; Calkins & Fox, 2002), left frontal asymmetry has been theorized to be related to active approach and self-regulation of the emotions and behaviors associated with active approach tendencies. Calkins and Dedmon (2000) found that toddlers high in externalizing behaviors were not able to regulate their emotions as well as toddlers who were low in externalizing behaviors. Findings from our study may indicate that toddlers with stable left frontal asymmetries may not be able to control their tendencies for active approach and the emotions associated with active approach, which includes anger (Harmon-Jones et al., 2009). Therefore, the children were rated higher on externalizing behaviors.
Results from both the internalizing and externalizing behaviors point to the potential value in using stability in frontal asymmetry as a means for identifying children potentially at-risk for these types of behaviors, especially given that we were able to identify approximately half of our sample as either stable right or stable left frontal asymmetries from 10 to 24 months of age. Although half of the infants did not have stable group classifications from 10 to 24 months, the right stable and left stable groups remained stable from 10 to 24 months, which can be seen in our analyses of the differences in the mean asymmetry scores for each group. The 10 month and 24 month mean asymmetry scores for the stable right and stable left asymmetry groups were not significantly different at the two times points. The change group did have significantly different asymmetry scores at 10 and 24 months. As has been found in previous work (Bell & Fox, 1994; Fox et al., 1994; Schmidt, 2008), the asymmetry scores in our study appeared to be stable across infancy for at least half of our sample. This stability points to the potential role that frontal asymmetry might play in early identification of children at-risk for dysregulated behaviors.
One of the strengths of our study is that we went beyond concurrent associations between physiology and behavior to longitudinally examine how infant physiology related to toddler behaviors. Furthermore, as is often the case, the broad band internalizing and externalizing scores for the toddlers were correlated with each other (Achenbach & Rescorla, 2000; Dietz et al., 2009; Shaw et al., 2009); however, we tested a more pure form of each broad band score by controlling for the other in the analyses (i.e., controlling for internalizing behavior when examining externalizing behavior and vice versa). Limitations of the current study include the use of a single reporter, in this case maternal report of child behavior. Future work should include multiple measures of child behavior problems including both observation and multiple reporters. Another limitation of the current study is the relatively homogenous sample used to examine these issues. However, there was variability in the internalizing and externalizing scores of the children in our sample, and some of the children were in the clinical and borderline clinical range for internalizing or externalizing behaviors. Additionally, our sample size did not allow us to examine possible moderating variables, such as child sex.
Taken together, our findings support the idea that lateralized brain activity may lay a foundation thought to be important to self-regulation (Calkins & Fox, 2002) and that ability to regulate approach and withdrawal tendencies may be a key piece in the development of maladaptive behaviors, both internalizing and externalizing. More work on how stability in physiology, such as stability in frontal asymmetry, is related to the development of internalizing and externalizing behaviors is needed to provide a means for detection of at-risk children as early as possible.
Acknowledgments
We thank all of the participants in this study and the graduate and undergraduate students affiliated with the project. We would especially like to thank Kimberly L. Day for her assistance in preparing this manuscript. This research was supported by funds awarded to Cynthia L. Smith from a Virginia Tech ASPIRES Award, a Virginia Tech College of Liberal Arts and Human Sciences Jerome Niles Faculty Research Award, and the Virginia Tech Institute for Society, Culture & Environment. This research also was supported by grants R03 HD043057 and R01 HD049878 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health
Contributor Information
Cynthia L. Smith, Department of Human Development, College of Liberal Arts and Human Sciences, Virginia Polytechnic Institute and State University
Martha Ann Bell, Department of Psychology, College of Science, Virginia Polytechnic Institute and State University.
References
- Achenbach TM, Rescorla LA. Manual for ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- Achenbach TM, Rescorla LA. Empirically based assessment and taxonomy: Applications to infants and toddlers. In: DelCarmen-Wiggins R, Carter A, editors. Handbook of infant, toddler, and preschool mental health assessment. Oxford: University Press; 2004. pp. 161–182. [Google Scholar]
- Allen J, Coan J, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Developmental and Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Baving L, Laucht M, Schmidt MH. Frontal EEG correlates of externalizing spectrum behaviors. European Child and Adolescent Psychiatry. 2003;12:36–42. doi: 10.1007/s00787-003-0307-5. [DOI] [PubMed] [Google Scholar]
- Bell MA, Fox NA. Brain development over the first year of life: Relations between EEG frequency and coherence and cognitive and affective behaviors. In: Dawson G, Fischer K, editors. Human behavior and the developing brain. New York: Guilford; 1994. pp. 314–345. [Google Scholar]
- Brennan PA, Hall J, Bor W, Najman JM, Williams G. Integrating biological and social processes in relation to early persistent aggression in boys and girls. Developmental Psychology. 2003;39:309–323. [PubMed] [Google Scholar]
- Buss KA, Malmstadt J, Dolski I, Kalin N, Goldsmith H, Davidson R. Right frontal brain activity, cortical, & withdrawal behavior in 6 months old infants. Behavioral Neuroscience. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28:103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA. Self-regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Development and Psychopathology. 2002;14:477–498. doi: 10.1017/s095457940200305x. [DOI] [PubMed] [Google Scholar]
- Campbell SB. Behavior problems in preschool children: Clinical and developmental issues. New York: Guilford Press; 2002. [Google Scholar]
- Campbell SB, Shaw DS, Gilliom M. Early externalizing behavior problems: Toddlers and preschoolers at risk for later maladjustment. Development and Psychopathology. 2000;12:467–488. doi: 10.1017/s0954579400003114. [DOI] [PubMed] [Google Scholar]
- Coan J, Allen J. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlational analysis for the behavioral sciences. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Davidson RJ, Rickman M. Behavioral inhibition and the emotional circuitry of the brain: Stability and plasticity during the early childhood years. In: Schmidt LA, Schulkin J, editors. Extreme fear, shyness, and social phobia: Origins, biological mechanisms, and clinical outcomes. New York, NY: Oxford University Press; 1999. pp. 67–87. [Google Scholar]
- Dawson G. Frontal electroencephalographic correlates of individual differences in emotion expression in infants: A brain systems perspective on emotion. In: Fox NA, editor. The development of emotion regulation: Biological and behavioral considerations. Vol. 59. 1994. pp. 2–3.pp. 135–151. Serial No. 240. [PubMed] [Google Scholar]
- Deitz LJ, Jennings KD, Kelley SA, Marshal M. Maternal depression, paternal psychopathology, and toddlers’ behavior problems. Journal of Clinical Child and Adolescent Psychology. 2009;38:48–61. doi: 10.1080/15374410802575362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Shaw DS, Silk JS. Developmental trajectories of anxiety symptoms among boys across early and middle childhood. Journal of Abnormal Psychology. 2008;117:32–47. doi: 10.1037/0021-843X.117.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finman R, Davidson RJ, Colton MB, Straus AM, Kagan J. Psychophysiological correlates of inhibition to the unfamiliar in children. Psychophysiology. 1989;26:S24. [Google Scholar]
- Forbes EE, Shaw DS, Fox NA, Cohn JF, Silk JS, Kovacs M. Maternal depression, child frontal asymmetry, and child affective behavior as factors in child behavior problems. Journal of Child Psychology and Psychiatry. 2006;47:79–87. doi: 10.1111/j.1469-7610.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Fox NA. If it’s not left, it’s right: Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processes underlying emotion regulation. In: Fox NA, editor. The development of emotion regulation: Biological and behavioral considerations. Monographs of the Society for Research in Child Development. Vol. 59. 1994. pp. 2–3.pp. 152–166. Serial No. 240. [PubMed] [Google Scholar]
- Fox NA, Bell MA, Jones NA. Individual differences in response to stress and cerebral asymmetry. Developmental Neuropsychology. 1992;8:161–184. [Google Scholar]
- Fox NA, Calkins SD, Bell MA. Neural plasticity and development in the first two years of life: Evidence from cognitive and socio-emotional domains of research. Development and Psychopathology. 1994;6:677–698. [Google Scholar]
- Fox NA, Henderson HA. Does infancy matter? Predicting social behavior from infant temperament. Infant Behavior and Development. 1999;22:445–455. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Calkins SD, Rubin KH, Copland RJ. The role of frontal activation in the regulation and dysregulation of social behavior during the preschool years. Development and Psychopathology. 1996;8:89–102. [Google Scholar]
- Hagemann D, Naumann E, Thayer JF. The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology. 2001;38:847–857. [PubMed] [Google Scholar]
- Harmon-Jones E, Harmon-Jones C, Abramson L, Peterson CK. PANAS positive activation is associated with anger. Emotion. 2009;9:183–196. doi: 10.1037/a0014959. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Fox NA, Rubin KH. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:68–74. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Hill AL, Degnan KA, Calkins SD, Keane SP. Profiles of externalizing behavior problems for boys and girls across preschool: The roles of emotion regulation and inattention. Developmental Psychology. 2006;42:913–928. doi: 10.1037/0012-1649.42.5.913. [DOI] [PubMed] [Google Scholar]
- Kerr DCR, Lunkenheimer ES, Olson SL. Assessment of child problem behaviors by multiple informants: A longitudinal study from preschool to school entry. Journal of Child Psychology and Psychiatry. 2007;48:967–975. doi: 10.1111/j.1469-7610.2007.01776.x. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Kusche CA, Cook ET, Greenberg MT. Neuropsychological and cognitive functioning in children with anxiety, externalizing, and comorbid psychopathology. Journal of Clinical Child Psychology. 1993;22:172–195. [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Patternson GR, Shaw DS, Snyder JJ, Yoerger K. Changes in maternal ratings of children’s overt and covert antisocial behavior. Aggressive Behavior. 2005;31:473–484. [Google Scholar]
- Pihlakoski L, Sourander A, Aromaa M, Rautava P, Helenius H, Sillanpaa M. The continuity of psychopathology from early childhood to preadolescence: A prospective cohort study of 3–12-year-old children. European Child and Adolescent Psychiatry. 2006;15:409–417. doi: 10.1007/s00787-006-0548-1. [DOI] [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox NA, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Connell A, Dishion TF, Wilson M, Gardner F. Improvements in maternal depression as a mediator of intervention effects on early childhood problem behavior. Development and Psychopathology. 2009;21:417–439. doi: 10.1017/S0954579409000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L. Patterns of second-by-second resting frontal brain (EEG) asymmetry and their relation to heart rate and temperament in 9-month-old human infants. Personality and Individual Differences. 2008;44:216–225. [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]