Abstract
All cells exist within the context of a three-dimensional microenvironment in which they are exposed to mechanical and physical cues. These cues can be disrupted through perturbations to mechanotransduction, from the nanoscale-level to the tissue-level, which compromises tensional homeostasis to promote pathologies such as cardiovascular disease and cancer. The mechanisms of such perturbations suggest that a complex interplay exists between the extracellular microenvironment and cellular function. Furthermore, sustained disruptions in tensional homeostasis can be caused by alterations in the extracellular matrix, allowing it to serve as a mechanically based memory-storage device that can perpetuate a disease or restore normal tissue behaviour.
From simple bacteria and archaea to higher-level eukaryotes, survival is based upon the ability of the organism to respond to environmental pressures, including diverse sets of mechanical force. Virtually all organisms have evolved specific structures that are tailored to respond to physical force. For instance, single-celled organisms, such as bacteria, and complex multicellular eukaryotes, such as plants and animals, all express stretch-activated ion channels1,2. Cell surface receptors such as integrins, which transmit forces from the external environment across the cell membrane, are also evolutionarily conserved molecules. Indeed, integrin heterodimers span a diverse array of life forms, ranging from sponges to humans, and their complexity has increased with organism evolution owing to variants and functional redundancy3. Consistently, the complexity of the Tyr phosphorylation of adhesion plaque-associated proteins, an indicator of the adhesion complex’s capacity to engage in signal transduction in focal adhesions, has also developed concomitantly with integrin-mediated adhesion, suggesting a close evolutionary link between the diversity of signal transduction and mechanical interactions4.
Cells in multicellular tissues are subjected to a myriad of forces, including compressive, tensile, fluid shear stress and hydrostatic pressure, each of which plays an intricate part in the shaping, development and maintenance of the tissue. Importantly, the manner in which cells interact with these forces, and hence respond to them, is largely dictated by the physical properties of the cells, their adjacent cells and the extracellular matrix (ECM), which is the principal extracellular component of all tissues and organs (BOX 1). Biological materials, including cells, exhibit the characteristics of both solids and liquids, in that they are both elastic and viscous. They are thus viscoelastic, meaning that they will deform in a time-dependent manner upon applied force (that is, they partially flow) and can return towards their initial form following removal of the applied stress.
Box 1. Structure and composition of the extracellular matrix.
The extracellular matrix (ECM) is the principal extracellular component of all tissues and organs. It provides the scaffold that gives physical support to cells and regulates intercellular biochemical and biomechanical signalling. As a result, it has a role in a number of cellular processes, including adhesion, migration, apoptosis, proliferation and differentiation. The molecular components of the ECM include collagens, elastins, proteoglycans, fibronectin and laminin. At the molecular level, the ECM is capable of binding, integrating and controlling the presentation of growth factors and other ligands to cells104. The organization of the ECM is not static; it is a dynamic structure with varying composition and distribution between different tissues and also during the stages of development. Remodelling of the ECM occurs through an altered balance between matrix deposition and its degradation by matrix metalloproteinases, and also through enzymes, such as lysyl oxidase, that crosslink collagen and elastin16. The diversity of ECM composition and organization lends itself to a wide range of forms and functions, ranging from solid structures found in bones and teeth to the elastic and pliable matrix found in cartilage and tendons105. Disruptions and perturbations to this network result in a loss of cell and tissue homeostasis and lead to a number of diseases, including cancer.
Diseases such as atherosclerosis, arthritis, deafness, osteoporosis and cancer, and a number of developmental disorders, including Kartagener’s syndrome and Hutchinson–Gilford progeria syndrome, commonly result from an abnormal physiological response to extrinsic (applied) or intrinsic (cell-generated) cues5. Thus, the physical basis for disease can be a product of either altered tensional homeostasis, owing to, for example, altered cellular-level or tissue-level forces and material properties, or the perturbed cellular response to mechanical stimuli. Consequently, it is important to understand the functional link between the sensing of mechanical cues and the subsequent biochemical response, a process termed mechanotransduction, as this relationship is important for the maintenance of tensional homeostasis and for normal tissue structure and function. Despite the importance of this relationship, and although much is known about how biochemical signalling can direct cell behaviour, relatively little is known about how forces inherent to the cell and the cellular and non-cellular microenvironment contribute to the regulation of tissue fate and function (for reviews, see REFS 6–9).
Accordingly, the broad objective of this Review is to describe coordinated mechanoresponsiveness in the context of tissues and the cellular microenvironment. We begin with a discussion of how mechanical signals are sensed and interpreted through the molecular machinery that mediates mechanotransduction, such as integrins (which are present at focal adhesions) and cadherin complexes (which are present at adherens junctions). We next describe how mechanical cues are integrated into tissues and illustrate, using examples, the importance of mechanical context as a critical regulator of cell behaviour and a key determinant during tissue-specific development. We also describe how the ECM is able to direct the behaviour of cells by regulating tensional homeostasis over many length scales and timescales through a process termed mechanoreciprocity. Finally we discuss how, in certain instances, the ECM may act as a memory-storage device that is capable of orchestrating and perpetuating disease states that may regulate cancer relapse and the progression to metastasis.
Integrating mechanical cues in cells
The translation of local extrinsic mechanical events into global changes in cellular function is dependent upon cells possessing integrated machinery that is capable of sensing and responding to mechanical force. A number of sensory elements and mechanisms exist whereby cells are able to probe and detect external forces through a process termed mechanosensing. This force-sensing can occur through force-induced conformational or organizational changes in cellular molecules or structures, such as stretch-sensitive ion channels10, cadherin complexes in cell–cell adhesions11, G protein-coupled receptors, Tyr kinase receptors12–14 and integrins3. When the mechanical cue has been received, the signal is amplified and propagated through a series of force-dependent biochemical reactions, whereby intracellular signalling pathways become sequentially activated through mechanotransduction15. For example, in response to elevated tension within focal contacts, increases in integrin clustering and in the phosphorylation of focal adhesion kinase (FAK) ensue, and these molecular changes initiate a cascade of signalling events. This cascade includes the activation of Rho-family GTPases, such as RhoA, which stimulates actin remodelling, induces protein phosphorylations to promote cell survival, and alters the levels and activity of transcription factors to regulate gene expression. Another integrin-dependent signalling pathway that is activated in response to mechanical force is the mitogen-activated protein kinase–extracellular signal-regulated kinase (MAPK–ERK) pathway, which has been implicated in a number of cancers and regulates cell proliferation and differentiation to influence tissue development5,16.
Sensing and integrating force through focal adhesions
A rich diversity of mechanosensing mechanisms is present across virtually all biological systems. Among the most-studied mechanosensory complexes are focal adhesions (reviewed in REF. 17). These dynamic protein complexes consist of integrins and a multitude of adaptor and signalling proteins, including vinculin and talin, which, as an integrated unit, provide the mechanical link between the actomyosin cytoskeleton and the ECM. This bridging between cellular components and the ECM enables the focal adhesion to serve as the conduit through which signal transduction occurs in response to physical force. The application of external force can directly influence the shape, size and composition of focal adhesions, thereby demonstrating a direct correlation between force and biochemical signal generation18 (FIG. 1). Proteins within the focal adhesion, including the integrins themselves19 and plaque proteins such as talin20 and p130Cas (also known as BCAR1)21, undergo conformational changes in response to applied force. These force-dependent conformational changes can either stabilize protein–protein interactions, as is the case for the conformational change that occurs in an integrin when it binds to the ECM component fibronectin, or they can unravel the molecule to reveal cryptic binding sites, as in the case of talin. Talin undergoes a force-dependent unfolding in response to picoNewton forces, which results in the exposure of otherwise inaccessible vinculin-binding sites20,22. The net result of talin–vinculin binding in response to force is an increase in the clustering of integrins and the nucleation of adhesion plaque proteins, which facilitates the cell–substrate junction to activate signal transduction molecules at the intracellular face of the adhesion. Similarly to talin, ECM proteins such as fibronectin can also unfold in response to mechanical stress to reveal cryptic binding sites that reinforce integrin adhesions and promote focal adhesion complex assembly16.
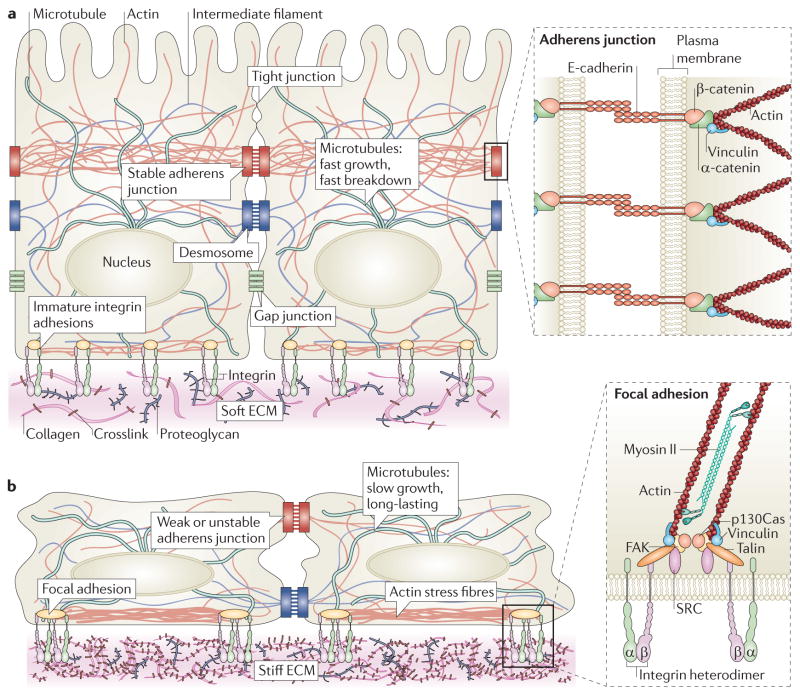
Figure 1. The mechanical network.
a | Tissues are mechanically integrated structures, the physical behaviour of which is defined by interconnected networks of cell–cell junctions, cell–matrix adhesions, intracellular filament networks (of actin, microtubules and intermediate filaments) and the extracellular matrix (ECM). Embedded throughout the network are mechanotransducing machines that convert mechanical stimuli into biochemical signals. This process, termed mechanotransduction, enables cells and tissues to sense and respond to their physical surroundings. The ECM controls network connectivity and tension on the network, thereby regulating sites of mechanotransduction. b | Cell–matrix adhesion complexes containing integrins can also directly sense the physical properties of the ECM. These complexes contain specialized protein sensors, including talin, p130Cas (also known as BCAR1), and integrins themselves, that undergo force-dependent conformational changes to elicit downstream signalling responses. The physical properties of the ECM are determined by its composition, the organization of its components, and their degree of intramolecular and intermolecular crosslinking. Interactions between the cell and ECM are dynamic, interwoven and reciprocal. Transcellular tension transmitted across adherens junctions affects ECM remodelling, which in turn regulates cell–matrix and cell–cell adhesions. Increased ECM stiffness owing to remodelling can result in changes in cell and nuclear shape, chromatin organization, assembly of cell–matrix adhesions (called focal adhesions), formation of actin stress fibres, destabilization of cell cell adhesions, and changes in microtubule dynamics. FAK, focal adhesion kinase.
To date, the most-studied mechanosensing mechanisms involve protein sensors that undergo conformational changes in response to a physical force. However, focal adhesions are theorized to have force-dependent behaviours through mechanisms that do not rely solely on protein switches23. In these strain-based mechanisms, forces on protein complexes may change intermolecular distances that ultimately lead to altered cellular function. Applying the concepts from this simple scenario to focal adhesions, there is a direct relationship between the intermolecular distances separating individual components within an adhesion and the rates at which signal-transducing adaptor proteins are recruited to the adhesion23. Strain on the complexes depends on the applied force and resistance provided by the ECM. In this model, focal adhesion assembly is naturally linked to ECM compliance and force24.
Sensing and integrating forces beyond focal adhesions
The majority of cell surface receptors that have been identified to date are characterized by their ability to respond to chemical factors. Despite the prevalence of these receptors, only a relatively small set (that is, the integrins and cadherins) appear to be capable of responding to mechanical cues. Given the importance of regulating and sensing forces across multiple length scales, from the molecular level to the tissue level, it stands to reason that a versatile set of force sensors must exist that have yet to be fully described. One explanation for the elusiveness of these types of force-sensors lies in the fact that many proteins fall into multiple receptor categories, such that they are capable of responding to mechanical strains and biochemical cues. Accordingly, a broadened view of the role of cell surface receptors is currently being developed that includes their ability to work across length scales and encompasses their generalized role in maintaining tissue structure and function.
There are multiple examples in which cell surface receptors serve to modify cell fate and tissue behaviour through the coupling of biochemical and mechanical cues. For instance, tumour cells can generate autologous gradients of chemokine (C-C motif) ligand 21 (CCL21) and CCL19 in response to interstitial flow. This force-mediated chemical gradient can promote lymph-node metastasis by increasing the frequency with which these ligands bind to chemokine (C-C motif) receptor 7 (CCR7), which is expressed on the tumour cell25,26. The implication of this mechanochemical coupling is that tumour cells may utilize low-magnitude shear forces to create and amplify autologous transcellular chemokine gradients that permit the tumour cell to move towards the draining lymphatic vessels by chemotaxis. It is plausible that, by using such an aggressive mechanochemical coupling mechanism, tumour cells would be able to exploit even a weak autocrine chemokine circuit to promote their directed migration and subsequent metastasis. Another illustration of the synergistic relationship between biochemical signalling and mechanical force is demonstrated by the mechanical-force model for Notch-signalling activation, which is referred to as the lift-and-cut model. The Notch pathway has a key role in regulating pre-existing developmental programmes by directing cell proliferation and cell death, promoting specific cell fates and activating distinct differentiation programmes. Fundamentally, Notch signals enable short-range communication between adjacent cells, and they are normally triggered by a ligand-induced activation mechanism. Prior to activation, Notch is in a metalloprotease-resistant conformation, which effectively blocks the site that must be cleaved by a metalloprotease for Notch activation. It seems reasonable to suggest that a mechanical force facilitates the substantial conformational changes deemed necessary to uncover the cleavage site required to activate Notch. Given that the ligand-binding domain of Notch is in close proximity to the protective Lin12–Notch repeat (LNR) modules, this coincides with data showing that ligand-binding confers sufficient force to peel away the LNR modules, providing the ‘lift’ that exposes the metalloprotease cleavage site where the ‘cut’ required for activation occurs27,28.
The local mechanical environment also plays a critical part in the organization and clustering of specific surface receptors, the functions of which are directly linked to their coordinated spatial organization29 (FIG. 2). Ephrin A receptor 2 (EPHA2) binds to membrane-bound ephrin A1 ligand that is presented on adjacent cells and, when stimulated, regulates cell growth, migration and adhesion, and is also associated with tumour angiogenesis and metastasis. Mechanically impeding the lateral movement of EPHA2s altered their spatial organization and clustering. Specific spatial assemblies elicit distinct signal responses associated with the ERBB, p53, integrin and MAPK pathways and an invasive phenotype, demonstrating that ephrin signalling is sensitive to spatio-mechanical cues from the local environment29.
Figure 2. Spatio-mechanical regulation of signalling pathways.
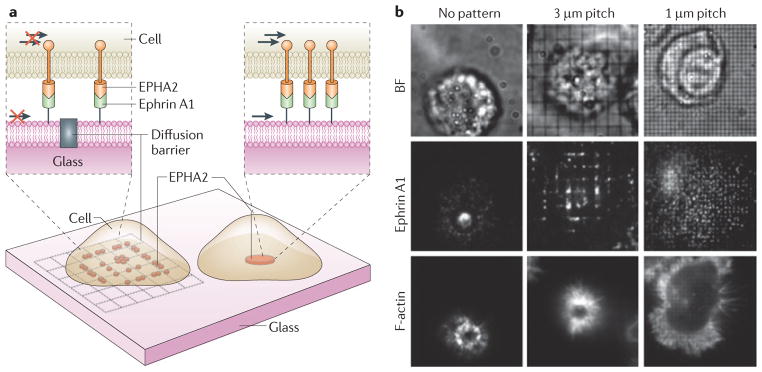
Evidence suggests that the mechanically induced spatial organization and clustering of cell surface receptors by the physical properties of materials, including the extracellular matrix (ECM), cell membrane, and cytoskeleton, can regulate a number of signal transduction pathways24,29,30. Juxtacrine signalling is one example in which physical contact between two cells is required for signalling, owing to the receptor (for example, ephrin A receptor 2 (EPHA2)) and ligand (for example, ephrin A1) pairs being presented on apposed cell membranes. a,b | To demonstrate how the mechanics of the microenvironment regulate EPHA2 ephrin A1 signalling in mammary epithelial cells, nanofabricated substrates, consisting of 10 nm high and 100 nm thick chromium lines arranged in a grid pattern (unrestricted (no pattern), 3 μm pitch and 1 μm pitch barriers), were used as a support for a membrane functionalized with laterally mobile (arrows) and fluorescently labelled ephrin A1 ligand, as schematically depicted (a). Representative bright field (BF) and epifluorescence images are also shown (b). When unimpeded, EPHA2 forms clusters with filamentous actin (F-actin) localized at the cell periphery, and is transported radially towards the centre of the cell upon ligand binding in an actomyosin-dependent mechanism29 (b, no pattern). However, the grid pattern creates diffusion barriers to receptor–ligand complexes, impeding their movement and resulting in their accumulation at barrier boundaries (b, ephrin A1: 3 μm and 1 μm pitch). Distinct cluster patterns yielded different signalling responses and changes in cytoskeletal morphology, which demonstrated a functional link between mechanical environment, receptor organization, signal activation and cell phenotype (b, F actin). Images are modified, with permission, from REF. 29 (2010) AAAS.
The strong correlation between spatial organization and function that has been demonstrated for EPHA2s is likely to be shared with other cell surface receptors, such as members of the ERBB family of receptors, which includes epidermal growth factor receptor (EGFR)30. In a recent study by Chung et al.31, EGFR dimers, which are formed before signalling, were found to be enriched in the cell periphery in an actin-dependent manner, resulting in a peripheral enhancement of EGF-induced signalling that theoretically enabled the cells to respond to growth factors in a polarized manner. The authors showed that receptor diffusion, and hence the spatial organization of receptors, was ultimately determined by actin organization at the membrane32,33. In this respect, it is important to note that nearly all forces, including shear stress, axial stress and cellular contractile forces, will induce actin reorganization when applied to the cell, and may affect receptor organization as a result34–37.
In general, distinct classes of biomolecules can reciprocally influence one another’s spatial organization. It therefore follows that changing the spatial organization of transmembrane receptors should ultimately influence the organization of lipids in the plasma membrane, and that modifying lipid domain formation will alter receptor distribution38. Force-induced reordering of biomolecules, including receptors, lipids and actin, can thus be propagated from one class of molecule to another, suggesting a global, interwoven regulation of cellular systems by intrinsic and extrinsic forces. Predictably, this global sensation of force can ultimately synergize with local mechanosensory mechanisms, such as protein unfolding, to dictate the cellular response to force.
Integrating mechanical cues in tissues
Tissue function arises from the coordinated behaviour of cells in both time and space. Consequently, a cell’s response to external stimuli is largely dictated by its interactions with the ECM, neighbouring cells and soluble cues from the microenvironment. As a result, cells in vivo do not function independently, but act in an integrated manner that depends strongly upon the mechanical and spatial context of their surroundings and their intrinsic mechanosensory mechanisms. Although the study of molecules and signalling out of context (that is, in a classic two-dimensional (2D) monolayer culture first) is useful for establishing the basic structure–function relationships of molecular interactions, these systems must ultimately be considered within the context of the multicellular tissues in which they reside.
The importance of mechanical context
Multiple examples demonstrate how the study of cells is highly context-dependent and that changing the local environment of the cell can have dramatic effects on cellular behaviour and phenotype. For example, mammary epithelial cells (MECs) incorporated into polarized, 3D tissue structures are resistant to myriad death cues, including chemotherapy drugs such as taxol and etoposide, as well as tumour necrosis factor (TNF) ligand and TNF-related apoptosis-inducing ligand (TRAIL). Conversely, MECs grown in 2D monolayers are exquisitely sensitive to cell death induced by these stimuli39. Furthermore, the position of epithelial cells in 3D engineered mammary epithelial tubules determines whether they undergo branching morphogenesis in response to EGF or hepatocyte growth factor (HGF), where branches are initiated exclusively from the ends of the tubules and not from the sides40. Differences between 2D and 3D have also been observed in cultured osteoblasts, in which changes in gene expression under static conditions and cellular responses to shear stress vary dramatically depending upon the extracellular environments41. The spatial location of epithelial–mesenchymal transitions induced in a 3D living tissue seems to coincide with, and may even be directed by, differences in cell shape and tissue geometry; this might be because these spatial cues reflect gradients of mechanical stress42. Indeed, experiments utilizing micromechanical devices to move cells into physical contact illustrate the critical role of cell–cell contacts as key regulators of the responsiveness of a cell to soluble factors and tissue signalling. Collectively, such studies emphasize how the nature of a cell’s response can be dramatically modified by its physical and chemical environment43.
Mechanical context is derived from the collective relationships between cells (mediated by cell–cell adhesions), between the cell and its ECM (mediated by cell–matrix adhesions) and between both types of adhesion and the intracellular-filament network (FIG. 1). The distribution of tension, and hence the sites of mechanotransduction, depends on the spatial connectivity and emergent material properties of the tissue network44. Tension develops on these networks owing to actomyosin contractility45,46. Analogously to the cellular effects of morphogen gradients, changing tissue geometry alters tension gradients, sites of mechanotransduction and the location of the proliferating, migrating and differentiating cells within a tissue47,48. Even small local changes in cell–cell or cell–ECM connectivity can have dramatic consequences for global tissue structure and function. In a recent demonstration of this principle, Martin et al.49 found that reducing the number of adherens junctions between cells, using a partial loss-of-function mutant or by laser-ablating small portions of the actomyosin network in tissues, resulted in tissue-wide epithelial tears during Drosophila melanogaster embryogenesis. On the single-cell level, the ablation of even a single actin fibre is able to induce a change in cell shape, alter cytoskeletal organization and modify focal adhesion assembly50,51. The connectivity in tissue networks is not static, but is dynamically rearranged as the need arises. For example, as a cell polarizes, the adaptor protein vinculin is recruited from sites of cell–matrix adhesion to sites of cell–cell adhesion, and stress fibres connected to focal adhesions reorganize into cortical bundles that run parallel with cell–cell contacts52 (FIG. 1). This adaptability in mechanical connectivity, coupled with mechanotransduction mechanisms, facilitates the coordinated spatial and temporal control of signal transduction, gene expression and cell behaviour, and delineates a hierarchy of communication in tissues that is formed by mechanical and biochemical connections.
The roles of mechanical cues in driving development
Although the genetic control of morphogenesis remains in vogue, a more comprehensive view of tissue development is beginning to be appreciated. This incorporates the large-scale coordination of cell shape and movement that is facilitated by mechanical force. Cell shape and motility are ultimately the products of a cell’s material properties, its contractile and protrusive forces, and the magnitude, direction and duration of the external forces that it is subjected to. Mechanical forces are thus the primary determinants in the organization of cells and embryos and, consequently, they are a key element in many of the fundamental processes that operate during development, including cell sorting, differentiation and compartmentalization53. Although several biochemical signals, including EGF, Notch and Hedgehog, are necessary for regulating myosin organization and activity during development, recent evidence demonstrates that mechanical feedback also has an essential role in regulating the contractile systems that drive embryogenesis. For instance, the deformation of cells by morphogenetic movement during D. melanogaster gastrulation induces the expression of the transcription factor Twist54 (FIG. 3). Twist and its transcriptional target Fog in turn stabilize apical myosin II, which promotes the formation of supracellular networks of contractile myosin fibres that are joined end-to-end at adherens junctions49,55. Twist, through regulation of G protein signalling and the transmembrane protein T48, controls the organization of, and tension on, the contractile network, and recruits adherens junctions to the sites of apical constriction, thereby facilitating the dynamic and precise control of cell shape56. In a positive feedback loop, force itself controls the recruitment of myosin to the apical cortex. Applying force to a cell within an embryo with a micropipette is sufficient to recruit myosin in 1–3 minutes to the apical cortex, whereas relieving tension with laser-ablation leads to a rapid loss of myosin from the cortex55,57. Remarkably, cutting a single cell–cell boundary in the amnioserosa of a D. melanogaster embryo with laser microsurgery rapidly terminates contractility in neighbouring cells, clearly demonstrating that the forces driving morphogenesis are sensitive to changes in the local mechanical environment58. Collectively, the rapid response of the embryo to force reflects robust and overlapping mechanotransduction mechanisms that are embedded throughout the tension network. The specific molecular details of these mechanisms remain unresolved, but an explosion of recent interest portends rapid progress.
Figure 3. The extracellular matrix and tensional homeostasis in development.
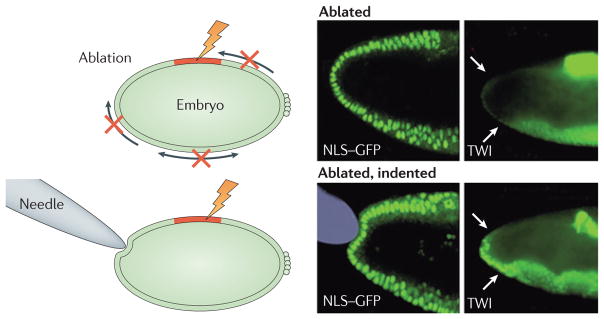
In tissue and organ morphogenesis, mechanical forces generated by morphogenetic movements play an important part in gene expression by activating developmental biochemical signalling cascades47. Embryogenesis provides an example of a multistage process that requires mechanical force, the subsequent localization of proteins, and biochemical signalling to work together. In this process, the cytoskeleton of the cells themselves has a key role in generating the contractile forces required for invagination, gastrulation, proliferation and differentiation44,106. During the onset of gastrulation, an early phase of embryonic development in Drosophila melanogaster, actin filaments are contracted by non-muscle myosins. Compressive forces result in the ectopic expression of the transcription factor Twist (TWI)54,107, which then directs significant changes in the shape of the developing embryo. When compressive forces (depicted by arrows in the schematic) are disrupted through the laser-ablation of dorsal cells (red region), there is a corresponding reduction in the level of mechanically induced TWI expression in the stomodeal primordium (white arrows) (ablated). Cell nuclei are visualized with a nuclear localization signal-tagged green fluorescent protein (NLS GFP) and immunofluorescence shows the distribution of TWI. Upon gentle compression of the stomodeal cells using a needle, TWI expression is restored in a mechanically induced mechanism (ablated, indented). Figure is modified, with permission, from REF. 54 (2008) Elsevier.
The roles of the ECM in driving development
The ECM is a crucial structural component that defines the mechanical behaviour of tissues. In gastrulation, for example, the assembly of fibronectin in the ECM into more complex fibrillar structures supports cell movement and shape changes that are essential for epiboly and radial intercalation59. The ECM also physically delineates tissue boundaries, enabling the compartmentalization of developing tissues60. Cells in the embryo also remodel the matrix as they pass over it, and this ECM remodelling commences coincidently with the initiation of morphogenetic movement. Indeed, the integrin-dependent assembly of the fibronectin matrix in the blastocoel roof of Xenopus laevis embryos probably requires cadherins, cortical actin fibres and myosin contractility61. Fibrillar assembly of the fibronectin matrix is also functionally linked to the forced unfolding of matrix protein domains by integrin receptors62,63. These results suggest that tension developed in the supracellular actomyosin network in the developing organism is transmitted through integrins to physically assemble the matrix, where biochemical–mechanical feedback again prevails. For example, in reconstituted ECMs the physical state and material rigidity of the ECM regulate the activation of Rho GTPases, increase myosin activity, enhance the formation of actin stress fibres and induce tissue tension concomitantly with ECM stiffening64. Furthermore, ephrin signalling controls the spatial organization of α5β1 integrin clusters along cell boundaries and thus also the spatiotemporal deposition of the fibronectin matrix65. As ephrin signalling is sensitive to the local mechanical environment, this system could allow cells to spatially coordinate matrix assembly using contextual information encoded in the pushes and pulls of neighbouring cells29.
The ability of the ECM to control cell fate and material properties is another dramatic example of the intricate, interwoven nature of mechanical regulation in developing tissues. The physical properties of the ECM direct the differentiation of stem cells down specific lineages66. As cells differentiate, they generally become stiffer in a cell-type specific manner and further tune their stiffness to the properties of the local materials that make up their ECM, progressively stiffening on more rigid material67. These changes in cell stiffness can in turn directly affect mechanotransduction. In response to applied stress, stiff differentiated cells deform less than compliant stem cells, resulting in attenuated levels of mechanotransduction67. This mechanotransduction mechanism seems ideally suited to allowing undifferentiated cells in the embryo to respond in a highly sensitive manner to coordinated mechanical cues, while ensuring that differentiated cells remain buffered against acute mechanical perturbations in homeostatic tissues. The material properties of cells are also used in other developmental processes. The level of cortical tension (stiffness, as measured by atomic force microscopy) is different in cells that make up the zebrafish ectoderm, mesoderm and endoderm68. Both experimental and theoretical models suggest that these differences in cell stiffness may support cell-sorting behaviours and could function in conjunction with differential cell adhesion to promote the formation of the three germ layers69.
The ECM in force homeostasis and disease
Mechanotransduction occurs on very fast timescales that can exceed the speed of signalling through soluble factors by orders of magnitude70–72. Forces can propagate through a wave-like mechanism across the cell body, along cytoskeletal filaments, in just 2 μs, and the subsequent activation of signal transduction networks occurs on the order of seconds73 (FIG. 4). These fast response times are crucial for highly dynamic cell processes. When the direction of motility is controlled by the physical stiffness of ECM, a process termed duro-taxis, cells migrate from softer to stiffer regions. This probably requires fast dynamic coordination between the mechanosensors (integrins) and response elements (actin cytoskeleton) that direct motility74. Dynamic processes such as these can contribute to pathology; for example, it has been proposed that metastatic cells disseminate from tumours by crawling along stiff fibres or following tension gradients. It is important to note, however, that most diseases linked with altered mechanotransduction, such as atherosclerosis and cancer, progress over months or even years, and therefore require sustained disruption to tensional homeostasis64,75. A prime candidate for achieving this type of sustained mechanoperturbation is the ECM.
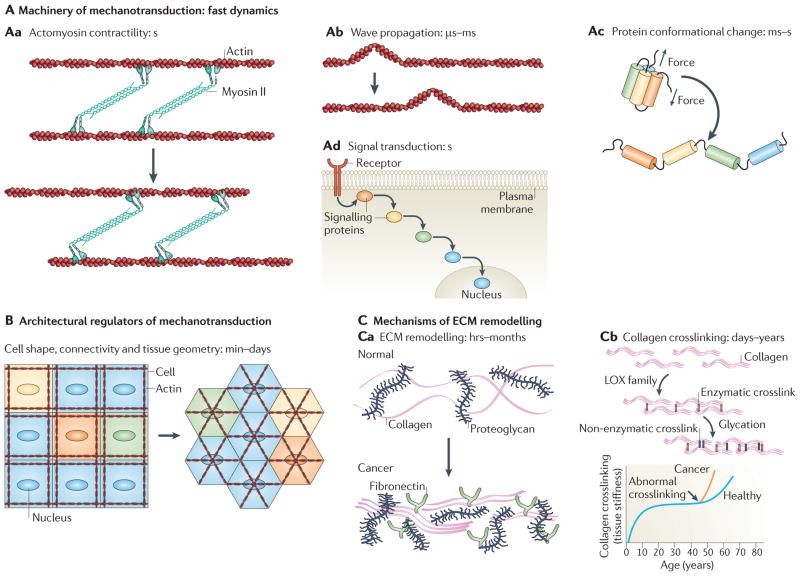
Figure 4. Sustaining mechanotransduction.
A | Mechanotransduction occurs on very fast timescales. Actomyosin cables contract at a rate of ~1 μm s–1 (Aa), and stress waves generated by contractility can rapidly propagate across cytoskeletal networks and structural materials (Ab). Stress on mechanically sensitive proteins (mechanosensors) can induce conformational changes, such as protein unfolding, on the order of milliseconds to seconds for pico Newton-level forces (Ac). During mechanotransduction, these changes in protein conformation activate biochemical signalling networks (Ad), through which signals originating at the cell membrane can travel to the nucleus in tens of seconds. Mechanical signalling thus occurs rapidly on timescales of seconds or less. B | Diseases that are linked to altered mechanical signalling require perturbations to be sustained. This can be achieved by genetically controlling the steady-state levels of mechanotransduction machinery and associated regulators. Alternatively, modifying the tissue architecture might sustain mechanical signalling perturbations. The distribution of forces throughout the tissue, and hence of the local sites of mechanotransduction, depend on cell shape, position and connectivity, as well as the intracellular organization of the cytoskeleton and other structural elements. C | The extracellular matrix (ECM), which largely determines tension in tissues, is remodelled during development and disease, which results in altered patterns of tension that can persist for hours, months or even longer (for example, in scar tissue) (Ca). Remodelling enzymes, including the crosslinking enzyme lysyl oxidase (LOX), alter the mechanical properties and spatial topology of the ECM (Cb). Enzymatic (LOX family; red) and non-enzymatic (glycation-mediated; blue) crosslinking in mature collagen filaments is particularly stable and can persist for decades in healthy adults. However, the enhanced activity of enzymes such as LOX in disease can enhance crosslinking, contribute to tissue stiffening, and alter mechanotransduction for prolonged durations.
The ECM directs cell behaviour on two length scales
As a structural material, the ECM controls spatial organization in the tissue across broad length scales, ranging from the nanoscale to the microscale and larger. On the nanoscale level, the ECM affects the organization of receptors on the cell surface and the sequestering of soluble factors. The nanoscale organization of the ECM can affect how growth factors are presented to their receptors (some can be tethered to the matrix) and how morphogens diffuse through tissue. Arrays of titanium dioxide (TiO2) nanotubes ranging in diameters from 22 nm to 300 nm, and in lengths of up to 1 mm, can affect cellular proliferation and motility while simultaneously changing the expression level of molecules associated with inflammation and coagulation76. These nanotubes are comparable in size to cell receptors and proteins and serve as a general example of how mechanical and topographical cues alone can affect cell behaviour.
On a more specific level, the spatial presentation of ECM ligands, such as fibronectin, vitronectin, laminin and collagen, and the nanotopography of the ECM, control integrin organization, adhesion assembly, and signal transduction to direct cell behaviour77,78. As shown through the use of functionalized gold dots on nanopatterned surfaces, differences in average ligand spacing of as little as ~10 nm seem to be capable of dictating whether integrins are able to assemble into focal adhesions79. This indicates that there is a critical threshold of ligand density that is required for integrin clustering and focal adhesion assembly. Cells are also capable of sensing and responding to gradual changes in the spacing of ECM ligands of as little as 1 nm over the entire length of a cell body80. Additionally, such relatively small gradients are able to direct migration and the alignment of cells and their cytoskeleton80. Because the cellular response to the spatial organization of matrix components also depends on the material properties of the matrix, this raises the important distinction that chemistry is not independent of mechanics. Matrix stiffness ultimately controls the cellular response to ligand presentation and matrix organization. Thus, when cells are cultured on ECM substrates of varying stiffness, focal adhesions fail to assemble below a critical stiffness even when matrix ligands are presented to the cell at saturated levels81.
At the microscale level and larger, the ECM controls cell shape and tissue boundaries. Matrix dimensionality is a dramatic example of how cell behaviour can be controlled at the microscale82. For instance, cells cultured in 1D, 2D and 3D show pronounced differences in their motility83,84, morphology and cytoskeletal organization85,86, as well as in the composition and function of their adhesions85, their viability86, and their response to soluble factors39. Spatial modifications in collagen organization at this length scale can additionally promote the metastatic behaviours of tissues, possibly by enhancing the viability and motility of disseminated cells. In this regard, multiphoton microscopy has been used to directly visualize cancer cells and macrophages exploiting collagen fibres as veritable ‘highways’ that seem to facilitate their invasion into the interstitial matrix and their rapid travel through the stroma87,88. As collagen fibres in the tumour are often tethered to blood vessels, these observations also raise the possibility of a direct route for tumour cell dissemination into the bloodstream89.
In summary, nanotopological features and larger-scale organization of the ECM control the motility and positioning of cells, their geometry, and their mechanical connectivity within the surrounding cellular and non-cellular microenvironment. Such physical rearrangements would be expected to occur on the order of hours and days, and are likely to persist at steady-states dictated by the ECM for considerably longer time durations of months and perhaps years. Such ECM topological reorganizing thereby provides for long-term patterning within the tissue that could elicit profound physiological changes through modifications of tissue-level mechanical forces and cellular mechanotransduction.
The ECM as a memory-storage device
Evidence linking ECM remodelling to disease progression is drawn from a compelling body of evidence implicating the status of the ECM (that is, its composition, organization and post-translational modification state) in cancer incidence and disease progression. Patients with cancer who present with high levels of fibrillar collagen show enhanced incidence of metastasis, and women with increased mammographic density, which is characterized by increased collagen and ductal and lobular epithelium, exhibit a greater risk of developing breast cancer75,90. Whether mammographic density predicts elevated ECM stiffness, and if and how fibrillar collagen could enhance tumour metastasis, awaits further clarification. Nevertheless, such provocative findings present an enticing argument that the status of the ECM could dictate tumour phenotype by modifying ECM tension. In this regard, one plausible molecular mechanism whereby modifications to the ECM could promote cancer progression is illustrated by recent data demonstrating strong associations among ECM stiffening, collagen crosslinking and expression, and the activity of the crosslinking enzyme lysyl oxidase (LOX) during breast tumour progression. These studies demonstrated that enhancing LOX-dependent collagen crosslinking stiffened the tissue and enhanced tumour progression, whereas inhibiting LOX-mediated collagen crosslinking not only prevented tissue stiffening but also delayed tumour progression and significantly reduced tumour incidence75 (FIG. 5). Indeed, many tumours show elevated expression of LOX and LOX-like enzymes, and data suggest that inhibiting LOX activity could reduce tumour metastasis by modifying tumour cell–ECM interactions or by altering the metastatic site (niche)74,75,91,92. This type of tissue and matrix remodelling is tissue-wide and systemic, and necessarily occurs over long timescales spanning many months and even years, and it will, by its very nature, result in non-uniform matrix remodelling and topological changes that could easily facilitate tumour invasion. In addition to directly fostering a disease state, the ability of the ECM to sustain perturbations over long periods of time may enable it to play a part in disease recurrence (FIG. 4c). For instance, it was demonstrated that tumours are self-seeded by circulating tumour cells and that tumours can recur at the site of removal of the primary tumour. Remodelling of the ECM could function as a type of retention mechanism, whereby the physical microenvironment of the diseased state is preserved so that circulating tumour cells in tissues adjacent to the site of the primary tumour promote disease recurrence93.
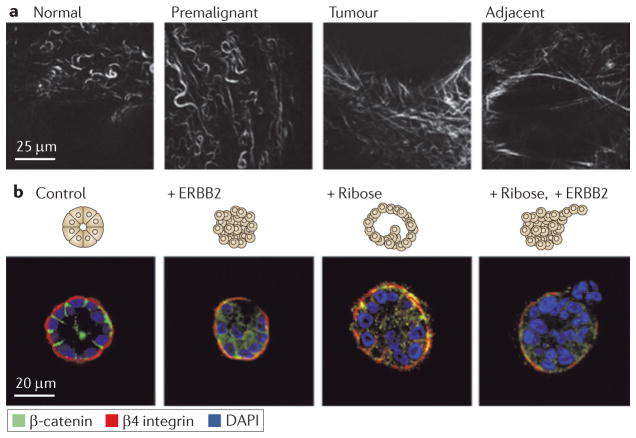
Figure 5. Tensional homeostasis in tumour progression.
Extracellular matrix (ECM) stiffness has been shown to be a potent regulator of cellular behaviour by affecting growth, survival, motility and differentiation. Tensional homeostasis requires a balance between the forces exerted on cells by the ECM and the reciprocal forces generated by cells themselves. When this balance is disrupted by stiffening the ECM, a number of signalling pathways can be adversely affected90. a | The collagen crosslinking enzyme lysyl oxidase (LOX) mediates collagen crosslinking and remodelling during breast tumour progression, which results in ECM stiffening. Second-harmonic generation imaging, a type of nonlinear microscopy, was used to show that the collagen in regions adjacent to epithelial lesions in mouse mammary glands undergoes significant morphological modifications, becoming progressively more linear during tumour progression. This remodelling of the ECM was also found to correlate with increased collagen crosslinking and the amount of the crosslinking enzyme LOX, suggesting an association with ECM stiffness, tissue fibrosis and breast tumorigenesis. b | However, matrix remodelling alone is insufficient to promote tumour invasion. Mammary epithelial cells cultured in three-dimensional gels assembled spherical acini, as shown with immunofluorescence of basally oriented β4 integrin and β catenin localized at cell cell junctions (control). Acini that only had the oncogene ERBB2 activated failed to produce an invasive phenotype (+ ERBB2), as did stiffening by nonspecific ribose crosslinking (+ Ribose). Invasion only occurred when stiffening was accompanied with oncogene activation (+ Ribose, + ERBB2). This suggests that matrix remodelling and oncogene activation synergistically induce metastatic progression and that inhibiting LOX and LOX-like enzymes could reduce tumour metastasis by modifying cell ECM interactions. DAPI, 4′,6-diamidino-2-phenylindole. Image is modified, with permission, from REF. 75 (2009) Elsevier.
Because of its capability to perpetuate either the healthy or diseased state, it is instructive to equate the ECM to a biological memory-storage device. On a basic level, memory devices store information for extended periods of time and allow systems to ‘write’ and ‘read’ this information. DNA is a classic example: it is an unusually stable molecule, the functional state of which is written by replication machinery and epi-genetic regulators and is read by the cell’s transcriptional machinery. The ECM, although not widely appreciated as a storage device, shares analogous features to DNA. Major components of the ECM are incredibly stable and are further subjected to covalent modifications that change their functional properties. An illustrative example is provided by collagen, the most abundant molecule in the ECM. The half-life for collagen before turnover through degradation by matrix metalloproteinases is 2–4 years in bone, 10–15 years in skin and ~100 years in tendon94. Even in tumours in which ECM remodelling is considered to be extremely fast, long-term intra-vital microscopy studies suggest that individual collagen fibres are remarkably stable and persist for at least 4 days, the maximum duration of these studies95. Ultimately, the topological and mechanical features of the ECM are read by cellular systems, including integrin adhesions. For collagen, these properties are imprinted by crosslinking enzymes, such as LOX, in a tissue-specific manner. Enzyme-catalysed, intermolecular and intrafibrillar crosslinks control the bundling of collagen molecules into fibres, dictate the mechanical properties of the fibres, and slow the turnover of collagen96. The crosslinks themselves are essentially irreversible and their quantity changes very little, from a steady-state perspective, over durations of decades in healthy adults94. Elastin, which provides elasticity to the ECM, is similarly stabilized by enzymatic crosslinking97.
Strong experimental evidence supports a link between tissue integrity and the cellular hardware that modifies, or writes, information to the ECM, including crosslinking enzymes, as discussed above, and matrix metalloproteinases. Altered proteolysis of the ECM by matrix metalloproteinases in cancer, a corruption of the information stored by the ECM, leads to unregulated tumour growth, inflammation and metastasis98. It also stands to reason that sustained physiological perturbation can be achieved by disruption of the primary cellular hardware that relays, or reads, information from the ECM. Indeed, cancer is frequently linked to altered integrin expression and activity, as well as to perturbations in key integrin-associated signalling proteins, such as SRC and FAK, and to changes in the levels and activity of actin-remodelling enzymes that are linked to mechanotransduction, including Rho and Rac GTPases. Appropriate matrix stiffness can support healthy tissue growth and homeostasis, but a cell’s perception of stiffness depends on the functional state of its adhesion machinery. For example, the β1 integrin-and actin-binding protein filamin A is necessary for cells to pull on collagen fibrils and remodel collagen matrices. Absence of filamin A impairs morphogenesis even in compliant matrices that normally support the process, whereas upregulation of filamin A allows cells to contract stiff collagen gels and undergo morphogenesis in an otherwise non-permissive microenvironment99.
How integrins perceive the ECM depends on their local micromechanical environment and, consequently, integrin–ECM interactions are precisely regulated by the physical and material properties of not only the ECM but also the glycocalyx, the cell membrane and the cell cortex24. This implies that any number of physical perturbations occurring at the cell surface could fundamentally change how cells read information from the ECM. In particular, physical perturbation of the cellular glycocalyx is likely to reflect a common but underappreciated disease mechanism. Drastic alterations to cell surface glycans are associated with many diseases, especially cancer, and rigorous theoretical models, some dating back more than 25 years, predict a strong dependence of integrin function on the physical properties of the glycocalyx24. The large, heavily glycosylated transmembrane protein mucin 1 (MUC1) is a major structural component of the tumour cell glyco-calyx, being overexpressed in 95% of breast cancers and 70% of solid tumours100. MUC1 can extend more than 100 nm from the cell surface, which is considerably farther than the ~20 nm that integrins can maximally extend, and consequently MUC1 can alter the interaction of integrins with the ECM100. Similarly, the proteoglycan hyaluronic acid forms thick coats on the exterior of tumour cells and is upregulated in a number of cancers, including pancreatic, colon and gastric cancers, and glioblastoma101–103. Abnormal levels of glycosylated molecules on the cell surface have been functionally linked to all stages of tumorigenesis and metastatic progression and are largely implicated in altering normal communication between cells and their tissue environment.
Multiple overlapping perturbations to the machinery that writes to and reads from the ECM are clearly demonstrated in diseases such as cancer. This raises the possibility that perturbations must accumulate in order to finally compromise the robustness of the storage system. The role of the ECM as a storage device that can promote and perpetuate disease states or even restore healthy tissue behaviour is underdeveloped. Yet, the accumulation of evidence implicating the ECM in sustained disruption of mechanical and chemical signalling argues for the importance of ongoing and future studies.
Concluding remarks and future directions
Sustained and coordinated tissue-level responses and reciprocal ECM-remodelling events that occur over long periods of time, and that span multiple length scales from the subcellular to the tissue level, are likely to contribute to the progression and initiation of diseases like cancer. Yet, although much effort has been exerted towards understanding the molecular details of pathobiology, more effort must be directed towards understanding how nano-, cell- and tissue-scale material properties are altered in disease and how these modifications impact signalling. Formulating an integrated mechanochemical perspective can lead to the effective development of tractable translational and clinical methods to control the way in which cells interact with their local physical and soluble microenvironment to effectively treat diseases, including cancer. To achieve this, there is an urgent need to extend our understanding of mechanotransduction beyond what is known for focal adhesions and ion channels, to discover new and alternate mechanoregulatory mechanisms that promote normal cell and tissue behaviour, and to study these networks in the correct tissue-like context. There is also a need to understand how biochemical alterations, such as the mutation of kinases, synergize with mechanical perturbations to drive disease progression. As is so dramatically presented in the case of development, there exists a clear role for tissue organization in mechanically dependent cell signalling. However, little is known about the specific mechanisms that operate to synergize and coordinate these integrated responses.
Any mutation or perturbation that causes defects in organization on the molecular, cellular, or whole-tissue level can alter cellular mechanosensing and will therefore conceivably contribute to disease. Much of the previous work in this field has focussed on soluble factors as being the mechanistic determinants that drive and coordinate tissue and matrix remodelling. However, it seems logical to predict that there is also a mechanism on the larger scale whereby mechanically altered cell and whole-tissue properties drive the upregulation of growth factors and chemokines, and that the perturbation of this might promote disease. As a more complete understanding and appreciation of the link between mechanics and biochemistry develops, the future challenge will be to take what is known from in vitro single-cell experiments and extend those principles to the tissue level in vivo. This is now becoming necessary because although much has been learned by studying the way in which single components interact with well-defined components of the ECM, it is now apparent that a host of long-range synergistic phenotypes exist that only manifest at the tissue and organ level, and these now urgently need our attention.
Acknowledgments
We apologize to all colleagues whose work cannot be cited owing to space limitations. This work was supported by the Breast Cancer Research Program of the US Department of Defense Era of Hope grant W81XWH-05-1-0330, US National Cancer Institute (NCI) grants U54CA143836-01, and US National Institutes of Health (NIH) NCI R01 CA138818-01A1 (to V.M.W.), as well as NIH NCI Breast Spore P50CA58207, which provided support for C.C.D.
Glossary
- Kartagener’s syndrome
A developmental disorder in which a disruption to mechanotransduction signalling by cilia-driven fluid flow results in the mirror-image reversal of internal organs
- Hutchinson–Gilford progeria syndrome
A disorder that is characterized by the rapid and dramatic appearance of ageing. The disease results from a genetic condition in which an abnormal version of the lamin A protein is produced, resulting in a highly unstable nuclear envelope. This is hypothesized to result in a disruption to mechanotransduction in vascular cells, contributing to arteriosclerosis, the leading cause of death for patients with this disease
- Mechanotransduction
The process through which cells sense and respond to their mechanical environment, such as the extracellular matrix, adjacent cells or external stresses. During mechanotransduction, mechanical signals are sensed and activate intracellular biochemical signalling pathways
- Actomyosin
Actin is one of the principal components of the cytoskeleton and forms a network of filaments with a class of molecular motors called myosins. The actomyosin network is best known for its role in contractility and force generation
- Interstitial flow
Present in all living systems, this type of fluid flow produces small currents through tissues and the extracellular matrix and is driven by dynamic stress
- Transcellular
Whereas ‘paracellular’ delineates processes occurring between cells, transcellular describes processes occurring through cells. One example is in transcellular transport, where molecules are moved through an epithelial cell layer
- Epithelial–mesenchymal transitions
Instances of a developmental programme that is hypothesized to be activated in metastasis and proliferation. This transition is characterized by an enhanced migratory capacity, loss of cell adhesion, the downregulation of E-cadherin, and a malignant phenotype
- Stomodeal primordium
In Drosophila melanogaster, the stomodeal primordium separates the anterior midgut from the middle germ layer, the mesoderm
- Gastrulation
A developmental change that is characterized by a large-scale movement of cells, from which the embryo first begins to take shape, transitioning from a spherical mass of cells into an organized multilayered structure establishing the three primary germ layers
- Amnioserosa
An extraembryonic epithelial tissue present in Drosophila melanogaster that is required for dorsal closure
- Epiboly
Formally defined as a growing of one part over another, epiboly is a coordinated movement occurring during gastrulation that is characterized by the thinning and spreading of a multilayered cell sheet
- Radial intercalation
A tissue-rearrangement process during development in which the cells in the deep germ layers of a developing embryo move towards the outer layers
- Blastocoel
A fluid-filled cavity that the embryo develops as it forms. It is the central region of a blastocyst
- Glycocalyx
The carbohydrate-enriched coating, consisting of proteoglycans and glycoproteins, of the plasma membrane of eukaryotic, bacterial and archaeal cells. It has a number of functions, including roles in cell adhesion, mechanotransduction, vascular physiology, pathology, and guiding cell movement during development
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Valerie M. Weaver’s homepage:http://weaverlab.surgery.ucsf.eduALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Sniadecki NJ. A tiny touch: activation of cell signaling pathways with magnetic nanoparticles. Endocrinol. 2010;151:451–457. doi: 10.1210/en.2009-0932. [DOI] [PubMed] [Google Scholar]
- 2.Monshausen GB, Gilroy S. Feeling green: mechanosensing in plants. Trends Cell Biol. 2009;19:228–235. doi: 10.1016/j.tcb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 3.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 4.Zaidel-Bar R. Evolution of complexity in the integrin adhesome. J Cell Biol. 2009;186:317–321. doi: 10.1083/jcb.200811067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nature Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 7.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 8.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 9.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nature Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 10.Lansman JB, Hallam TJ, Rink TJ. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? Nature. 1987;325:811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- 11.Tzima E, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 12.Felsenfeld DP, Schwartzberg PL, Venegas A, Tse R, Sheetz MP. Selective regulation of integrin-cytoskeleton interactions by the tyrosine kinase Src. Nature Cell Biol. 1999;1:200–206. doi: 10.1038/12021. [DOI] [PubMed] [Google Scholar]
- 13.Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Chen KD, et al. Mechanotransduction in response to shear stress - roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 15.Chen CS. Mechanotransduction — a field pulling together? J Cell Sci. 2008;121:3285–3292. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- 16.Vogel V, Sheetz MP. Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways. Curr Opin Cell Biol. 2009;21:38–46. doi: 10.1016/j.ceb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nature Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 18.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 20.del Rio A, et al. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. Presents a general role of force transduction, in which the mechanical stretching of single proteins can expose cryptic binding sites for other molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SE, Kamm RD, Mofrad MRK. Force-induced activation of Talin and its possible role in focal adhesion mechanotransduction. J Biomech. 2007;40:2096–2106. doi: 10.1016/j.jbiomech.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr Opin Cell Biol. 2006;18:472–481. doi: 10.1016/j.ceb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Paszek MJ, Boettiger D, Weaver VM, Hammer DA. Integrin clustering is driven by mechanical resistance from the Glycocalyx and the substrate. PLoS Comput Biol. 2009;5:e1000604. doi: 10.1371/journal.pcbi.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miteva DO, et al. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106:920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shields JD, et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. Demonstrates a mechanism for metastasis through which tumour cells are guided to the lymphatic vessels serving the tumour in a force-based process that is facilitated by interstitial flow. [DOI] [PubMed] [Google Scholar]
- 27.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon WR, et al. Structural basis for autoinhibition of Notch. Nature Struc Mol Biol. 2007;14:295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- 29.Salaita K, et al. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science. 2010;327:1380–1385. doi: 10.1126/science.1181729. Physical barriers, termed spatial mutations, were used to demonstrate a broadly applicable process through which EPHA2 signalling pathways could be regulated in a spatio-mechanical mechanism, underscoring the potential of the ECM and cell surface to modulate a broad range of mechanotransduction pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa MN, Radhakrishnan K, Wilson BS, Vlachos DG, Edwards JS. Coupled stochastic spatial and non-spatial simulations of ErbB1 signaling pathways demonstrate the importance of spatial organization in signal transduction. PLoS ONE. 2009;4:e6316. doi: 10.1371/journal.pone.0006316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung I, et al. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464:783–787. doi: 10.1038/nature08827. [DOI] [PubMed] [Google Scholar]
- 32.Sako Y, Kusumi A. Barriers for lateral diffusion of transferrin receptor in the plasma membrane as characterized by receptor dragging by laser tweezers: fence versus tether. J Cell Biol. 1995;129:1559–1574. doi: 10.1083/jcb.129.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusumi A, et al. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 34.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Girard PR, Nerem RM. Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins. J Cell Physiol. 1995;163:179–193. doi: 10.1002/jcp.1041630121. [DOI] [PubMed] [Google Scholar]
- 36.Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci USA. 2005;102:15895–15900. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Totsukawa G, et al. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaus K, Le Lay S, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol. 2006;174:725–734. doi: 10.1083/jcb.200603034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weaver VM, et al. β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci USA. 2003;100:14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez EW, Chen QK, Gjorevski N, Nelson CM. Tissue geometry patterns epithelial-mesenchymal transition via intercellular mechanotransduction. J Cell Biochem. 2010;110:44–51. doi: 10.1002/jcb.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peerani R, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paluch E, Heisenberg CP. Biology and physics of cell shape changes in development. Curr Biol. 2009;19:R790–R799. doi: 10.1016/j.cub.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 45.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Legant WR, et al. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc Natl Acad Sci USA. 2009;106:10097–10102. doi: 10.1073/pnas.0900174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson CM, et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci USA. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruiz SA, Chen CS. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells. 2008;26:2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF. Integration of contractile forces during tissue invagination. J Cell Biol. 2010;188:735–749. doi: 10.1083/jcb.200910099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar S, et al. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lele TP, et al. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol. 2006;207:187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- 52.Maddugoda MP, Crampton MS, Shewan AM, Yap AS. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells. J Cell Biol. 2007;178:529–540. doi: 10.1083/jcb.200612042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- 54.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates Twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. A dramatic demonstration of how mechanical deformations play a crucial and integral role in embryonic morphogenetic movements and have implications across virtually all stages of development. [DOI] [PubMed] [Google Scholar]
- 55.Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal. 2009;2:ra16. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- 56.Kolsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 2007;315:384–386. doi: 10.1126/science.1134833. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Gonzalez R, de Simoes SM, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–1342. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 59.Rozario T, Dzamba B, Weber GF, Davidson LA, DeSimone DW. The physical state of fibronectin matrix differentially regulates morphogenetic movements in vivo. Dev Biol. 2009;327:386–398. doi: 10.1016/j.ydbio.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW. Cadherin adhesion, tissue tension, and noncanonical Wnt signaling regulate fibronectin matrix organization. Dev Cell. 2009;16:421–432. doi: 10.1016/j.devcel.2009.01.008. Provides support for a matrix-assembly model in tissues, in which fibronectin fibril formation at cell surfaces is facilitated by cell–cell adhesions directing tension to the integrins that are required for assembly of the fibronectin matrix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Zhong C, et al. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Julich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- 66.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 67.Chowdhury F, et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nature Mater. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krieg M, et al. Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 69.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Poh YC, et al. Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS ONE. 2009;4:e7886. doi: 10.1371/journal.pone.0007886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Na S, et al. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, et al. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 73.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nature Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 74.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. Presents compelling evidence drawing a correlation between tissue stiffness and breast cancer malignancy. Stiffening through the collagen crosslinking enzyme LOX leads to forced integrin clustering and focal adhesion formation and results in enhanced growth-factor signalling and breast cancer malignancy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng L, et al. Whole genome expression analysis nanotubes on reveals differential effects of TiO2 vascular cells. Nano Lett. 2010;10:143–148. doi: 10.1021/nl903043z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geblinger D, Addadi L, Geiger B. Nano-topography sensing by osteoclasts. J Cell Sci. 2010;123:1503–1510. doi: 10.1242/jcs.060954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dalby MJ, Riehle MO, Johnstone H, Affrossman S, Curtis AS. In vitro reaction of endothelial cells to polymer demixed nanotopography. Biomaterials. 2002;23:2945–2954. doi: 10.1016/s0142-9612(01)00424-0. [DOI] [PubMed] [Google Scholar]
- 79.Cavalcanti-Adam EA, et al. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys J. 2007;92:2964–2974. doi: 10.1529/biophysj.106.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arnold M, et al. Induction of cell polarization and migration by a gradient of nanoscale variations in adhesive ligand spacing. Nano Lett. 2008;8:2063–2069. doi: 10.1021/nl801483w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan W, Oldenburg AL, Norman JJ, Desai TA, Boppart SA. Optical coherence tomography of cell dynamics in three-dimensional tissue models. Opt Express. 2006;14:7159–7171. doi: 10.1364/oe.14.007159. [DOI] [PubMed] [Google Scholar]
- 83.Friedl P, Brocker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 86.Ochsner M, Textor M, Vogel V, Smith ML. Dimensionality controls cytoskeleton assembly and metabolism of fibroblast cells in response to rigidity and shape. PLoS ONE. 2010;5:e9445. doi: 10.1371/journal.pone.0009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wyckoff JB, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 88.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 89.Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–2511. [PubMed] [Google Scholar]
- 90.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nature Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erler JT, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ng MR, Brugge JS. A stiff blow from the stroma: collagen crosslinking drives tumor progression. Cancer Cell. 2009;16:455–457. doi: 10.1016/j.ccr.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Leung CT, Brugge JS. Tumor self-seeding: bidirectional flow of tumor cells. Cell. 2009;139:1226–1228. doi: 10.1016/j.cell.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 94.Avery NC, Bailey AJ. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scand J Med Sci Sports. 2005;15:231–240. doi: 10.1111/j.1600-0838.2005.00464.x. [DOI] [PubMed] [Google Scholar]
- 95.Perentes JY, et al. In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nature Methods. 2009;6:143–145. doi: 10.1038/nmeth.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buehler MJ. Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies. J Mech Behav Biomed Mater. 2008;1:59–67. doi: 10.1016/j.jmbbm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Reiser K, McCormick RJ, Rucker RB. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. FASEB J. 1992;6:2439–2449. doi: 10.1096/fasebj.6.7.1348714. [DOI] [PubMed] [Google Scholar]
- 98.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gehler S, et al. Filamin A–β1 integrin complex tunes epithelial cell response to matrix tension. Mol Biol Cell. 2009;20:3224–3238. doi: 10.1091/mbc.E08-12-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Ann Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 101.Theocharis AD, Tsara ME, Papageorgacopoulou N, Karavias DD, Theocharis DA. Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim Biophys Acta. 2000;1502:201–206. doi: 10.1016/s0925-4439(00)00051-x. [DOI] [PubMed] [Google Scholar]
- 102.Toole BP. Hyaluronan promotes the malignant phenotype. Glycobiology. 2002;12:37R–42R. doi: 10.1093/glycob/12.3.37r. [DOI] [PubMed] [Google Scholar]
- 103.Varga I, et al. Expression of invasion-related extracellular matrix molecules in human glioblastoma versus intracerebral lung adenocarcinoma metastasis. Cen Eur Neurosurg. 2010;71:173–180. doi: 10.1055/s-0030-1249698. [DOI] [PubMed] [Google Scholar]
- 104.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lodish H, et al. Molecular Cell Biology. 4. W. H. Freeman; New York: 2000. [Google Scholar]
- 106.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nature Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. An excellent review highlighting the general role of force and mechanotransduction in biology, centring on how contractility plays a role in development through regulating tissue structure and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Farge E. Mechanical induction of twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]