Abstract
We report the first study on the genetic control of flowering in Setaria, a panicoid grass closely related to switchgrass, and in the same subfamily as maize and sorghum. A recombinant inbred line mapping population derived from a cross between domesticated Setaria italica (foxtail millet) and its wild relative Setaria viridis (green millet), was grown in eight trials with varying environmental conditions to identify a small number of quantitative trait loci (QTL) that control differences in flowering time. Many of the QTL across trials colocalize, suggesting that the genetic control of flowering in Setaria is robust across a range of photoperiod and other environmental factors. A detailed comparison of QTL for flowering in Setaria, sorghum, and maize indicates that several of the major QTL regions identified in maize and sorghum are syntenic orthologs with Setaria QTL, although the maize large effect QTL on chromosome 10 is not. Several Setaria QTL intervals had multiple LOD peaks and were composed of multiple syntenic blocks, suggesting that observed QTL represent multiple tightly linked loci. Candidate genes from flowering time pathways identified in rice and Arabidopsis were identified in Setaria QTL intervals, including those involved in the CONSTANS photoperiod pathway. However, only three of the approximately seven genes cloned for flowering time in maize colocalized with Setaria QTL. This suggests that variation in flowering time in separate grass lineages is controlled by a combination of conserved and lineage specific genes.
Keywords: Setaria, foxtail millet, QTL mapping, flowering time, comparative genomics
Flowering is a major developmental transition in the life-history of plants, and the genetic manipulation of flowering time has been crucial in the domestication and spread of cereal grasses such as wheat, rice, and maize. However, much of our knowledge on the genetic control of flowering in grasses is derived from studies in rice, and it is unclear to what extent flowering time pathways are shared across grasses, or what the relative importance of the separate pathways are in the different grass subfamilies. Our understanding of the genetics of flowering time is particularly poor in the subfamily Panicoideae, containing sorghum and maize, where relatively few genes controlling flowering have been identified. In addition to sorghum and maize, in the tribe Andropogoneae, a third species of panicoid grass, foxtail millet (Setaria italica), in the tribe Paniceae, recently has been sequenced (Bennetzen et al. 2012). The availability of a recombinant inbred mapping population and a dense genetic map from a cross between foxtail millet and its wild relative green millet (Setaria viridis) gives an opportunity to examine the genetic control of flowering in Setaria. Furthermore, the availability of the genome sequence allows us to examine the extent to which there is conservation of genetic and genomic architecture for this trait across panicoid grasses.
Much of what is known about control of flowering is derived from studies in Arabidopsis, rice and the pooid grasses wheat, barley, and Brachypodium (Higgins et al. 2010). In Arabidopsis, both autonomous and environmentally mediated flowering time pathways have been identified (Amasino 2010). These pathways act in mature leaves and converge on a central integrating protein, FLOWERING LOCUS T (FT), which is transported to the apical meristem to promote transition of the vegetative meristem to an inflorescence meristem (Corbesier et al. 2007). Photoperiod and vernalization genetic pathways allow Arabidopsis to adjust flowering time responses across its geographic range. For example, in most temperate regions, Arabidopsis is a winter annual, germinating in the fall, overwintering, and then being stimulated to flower by lengthening days in the spring. Plants that germinate in the summer and fall are prevented from flowering by the repression of FT by FLOWERING LOCUS C (FLC), under regulation by FRIGIDA (FRI) (Shindo et al. 2005). Vernalization over the winter reduces the sensitivity of FLC to FRI, turning off FLC expression, and releasing the floral mobile signal FT from suppression (Amasino 2010). FT expression is amplified by up-regulation of CONSTANS (CO) in the photoperiod pathway as a result of the increasing day-length of spring (Yanovsky and Kay 2002).
The grasses also possess multiple pathways to control flowering time, only some of which are conserved with Arabidopsis. One conserved pathway is the CONSTANS (CO) photoperiod pathway, found in all terrestrial plants and in algae (Serrano et al. 2009; Valverde 2011). However, the regulation of the genes in this pathway has diverged over time. For instance, CO acts as a positive regulator of FT under long day conditions in winter annuals such as Arabidopsis, winter wheat, and barley (Greenup et al. 2009), whereas in the same conditions in rice (a short day plant) the ortholog of CO, HEADING DATE 1 (HD1), acts to suppress the FT ortholog HEADING DATE 3A (HD3A) (Izawa et al. 2002; Hayama et al. 2003; Song et al. 2010).
Rice also possesses a separate photoperiod regulated genetic pathway centered on EARLY HEADING DATE 1 (EHD1), which acts with HD1 to promote flowering via both HD3A and its co-ortholog RICE FLOWERING LOCUS T 1 (RFT1) in short day environments, but which acts alone on RFT1 to promote flowering under long day conditions (Komiya et al. 2009). EHD1 is negatively regulated by GRAIN HEADING DATE7 (GHD7) in long day conditions, and natural variation in GHD7 has been shown to regulate the local adaptation of rice cultivars to different latitudes (Xue et al. 2008).
The FLC-FRI vernalization pathway is not found in monocots, although winter annual species in the Pooid subfamily, such as wheat, barley, rye, and Brachypodium have an analogous genetic pathway involving VERNALIZATION1 (VRN1) and VERNALIZATION2 (VRN2) (Yan et al. 2003, 2004). Pooid grasses are long day plants, where vernalization up-regulates VRN1 expression, down-regulating VRN2, and removing the suppressive effect of VRN2 on the FT ortholog VERNALIZATION3 (VRN3) (Yan et al. 2006; Trevaskis et al. 2007). However, the vernalization pathway has not been described for rice, maize, sorghum, and the millets, which are either from tropical regions (rice, sorghum, maize) or are spring or summer annuals (foxtail millet).
Flowering time pathways in the grasses have been characterized in rice and the pooid grasses (Higgins et al. 2010) but are less well understood in the panicoid grasses. A few genes underlying variation in flowering time have been cloned in maize and sorghum, including INDETERMINATE SPIKELET1 (Colasanti et al. 2006), the noncoding control region of ZmRap2.7, VEGETATIVE TO GENERATIVE TRANSITION1 (VGT1) (Salvi et al. 2007), DWARF 8 (Thornsberry et al. 2001; Camus-Kulandaivelu et al. 2006), ZmCCT, a homolog of the rice photoperiod pathway gene GHD7 (Hung et al. 2012), CONZ1, a homolog of CO (Miller et al. 2008), ZFL1 and ZFL2, homologs of LEAFY in Arabidopsis (Bomblies et al. 2003), and DELAYED FLOWERING1 (DLF1), which is an ortholog of FLOWERING LOCUS D (FD) (Muszynski et al. 2006). In sorghum PSEUDO RESPONSE REGULATOR37 (PRR37) has been identified as the gene underlying Ma1, the locus that has the largest effect on flowering time and inflorescence maturation in sorghum (Murphy et al. 2011). In addition, quantitative genetic analyses have found four to six major quantitative trait loci (QTL) regions controlling flowering time variation in maize (Chardon et al. 2004; Salvi et al. 2009; Coles et al. 2010, 2011; Wang et al. 2010; Xu et al. 2012). There are also likely a large number of QTL of small effect that control flowering time, with evidence for allelic series at most loci (Buckler et al. 2009). In sorghum, a short day tropical species, meta-analysis of multiple QTL trials projected against a dense single-nucleotide polymorphism (SNP) map, suggests up to 17 loci affecting flowering time (Mace and Jordan 2011).
Sorghum and maize are panicoid crops that were domesticated in short-day environments, but foxtail millet (Setaria italica) was most likely domesticated from green millet (S. viridis) in the northern part of China, with more pronounced seasonal changes in photoperiod (Li and Wu 1996; Bettinger et al. 2010). Green millet is of interest in its own right, as it is a world-wide weed, adapted to multiple photoperiod regimes, including both short- and long-day cycles (Holm 1997; Dekker 2003), and a model for biofuels genetics, C4 photosynthesis research, and plant architectural modeling (Doust et al. 2009; Li and Brutnell 2011). A Sanger (Bennetzen et al. 2012) and Illumina (Zhang et al. 2012) genome sequence recently have been completed, along with several green millet accessions (Bennetzen et al. 2012). As part of the Sanger genome assembly effort an F7 recombinant inbred line (RIL) population of a cross between foxtail and green millet was genotyped using SNP markers, resulting in a 1000-loci genetic map (Bennetzen et al. 2012). We have used this population to investigate the genetic control of flowering time between foxtail and green millet in a variety of environments, to suggest candidate genes controlling flowering time variation in this population, and to compare QTL regions with those in the other domesticated panicoid grasses, maize and sorghum.
Materials and Methods
Plant materials, experimental design, and phenotyping
A total of 182 F7 RILs from an interspecific cross between S. italica accession B100 × S. viridis accession A10 (Wang et al. 1998; Bennetzen et al. 2012) were evaluated for flowering time in eight different trials. Two of the eight trials were conducted in greenhouses (GH) at Oklahoma State University (OK), four were conducted in the field (F) at Oklahoma State University and the University of Georgia (GA), and two were conducted in growth chambers (GC) at Oklahoma State University and the Boyce Thompson Institute, Ithaca, NY (BT). For the field trials, seed was germinated in greenhouses and transplanted into the field at the two- or three-leaf stage. The trials varied in photoperiod, temperature, plant spacing, and other environmental variables, with the two growth chamber experiments representing the shortest light period of 12 hr, the field trials and one greenhouse trial having light periods from 13 to 14.5 hr, and one greenhouse trial having a light period of 16 hr (Table 1). In addition, a growth chamber trial was performed in which the S. viridis A10 and S. italica B100 parents were grown under 12- and 16-hr light conditions, in the absence of other environmental variation, to examine the effect of photoperiod (see Supporting Information, File S1, for a comprehensive description of plant preparation and growing conditions).
Table 1. Summary of growth conditions.
| Trial | Mean Day Length, Hours | Mean Light Intensity, µmol.m-2.s-1 | Average Max. Temperature, ° | No. RILs Used | Comments |
|---|---|---|---|---|---|
| GH1-OK | 14 | 1400 | 26 | 182 | |
| GH2-OK | 16 | 1400 | 26.5 | 107 | |
| F1-OK | 14.2 | 2200 | 26.5 | 182 | |
| F2-OK | 14.2 | 2200 | 28 | 182 | |
| F1-GA | 14.3 | 2200 | 28 | 182 | Seeds vernalized after planting Seeds vernalized after planting |
| F2-GA | 14.3 | 2200 | 28 | 182 | |
| GC-BT | 12 | 750 | 31 | 182 | |
| GC-OK | 12 | 350 | 28 | 126 |
GH, greenhouse; OK, Oklahoma State University, Stillwater, OK; F, field; GA, University of Georgia, Athens, Georgia; GC, growth chamber; BT, Boyce Thompson Institute, Ithaca, New York.
Phenotypic measurement
Our primary interest is in understanding the genetic regulation of commitment to flowering, which is when the shoot apical meristem becomes an inflorescence meristem. We used the number of days until the inflorescence on the main culm was first visible in the sheath of the flag leaf (days to heading) as the measurement of time to flowering, for its reliability and ease of measurement. We did not measure time to anthesis or time to stigma exsertion (‘silking’) because Setaria species have inflorescences with multiple orders of branching, and flowers on the different branches and in different parts of the inflorescence open at different times (Doust and Kellogg 2002).
Molecular marker development
Most of the markers used in the QTL analysis map were SNP markers genotyped on an Illumina Golden Gate array, with SNP identification and probe design based on next-generation sequence data obtained in a random set of 48 of the RILs (Bennetzen et al. 2012). We added a number of simple sequence repeat (SSR), sequence-tagged-site (STS), and gene markers to the map, in order to compare the RIL map with previously published maps (Wang et al. 1998; Jia et al. 2009).
Almost 200 published SSR primer pairs (Jia et al. 2007, 2009; Gupta et al. 2012) were tested on the parents, and a total of 126 informative markers were chosen to complement the SNP markers. Polymerase chain reaction (PCR) fragment separation for SSR markers was done via agarose gels (1−3% depending on fragment sizes) or with an ABI PRISM 3730 Genetic Analyzer (Applied Biosystems). We also developed several STS markers for selected rice RFLP probes/sequences used in the F2 foxtail genetic map (Wang et al. 1998) and for some genes of interest (Table S1). Most of these were detected using tetra-prime ARMS-PCR, a method whereby two primer pairs can be used to amplify the two different alleles of a SNP in the same PCR (Ye et al. 2001) (see File S1 for details of molecular marker development and genotyping; see File S2 for genotypic and phenotypic scores). The rest were detected using enzymes that cut one or other allelic copy.
Genetic map development
Marker linkage analysis was done with the program JoinMap 4 (Van Ooijen 2006). Molecular markers were grouped using a maximum recombination frequency of 0.25 and a LOD (logarithm of odds ratio) threshold of 4, 5, or 6 (in one instance the use of a LOD threshold of 6 was necessary to separate linkage groups). Marker ordering calculation was done with the maximum likelihood mapping algorithm. The Kosambi function was used to convert recombination frequencies to cM distances. A single, most informative marker was selected from clusters of markers with the purpose to improve QTL mapping. Suspicious genotypic scores identified from the genotype probabilities table calculated by JoinMap, were verified and edited when necessary.
QTL analyses
SPSS version 19 (Armonk, NY) was used to calculate least square means, and QTL Cartographer Unix version 1.16 (Basten et al. 1994, 2002) and WinQTLCart version 2.5 (Wang et al. 2011) were used to perform the QTL analyses. QTL mapping was done with the composite interval mapping (CIM) method, using a genome scan interval of 1 cM, a window size of 10 and the forward and backward regression method (Jansen and Stam 1994; Zeng 1994). LOD threshold values were estimated via 1000 permutations (Churchill and Doerge 1994; Doerge and Churchill 1996).
Epistasis
Epistatic interactions were calculated with the program Epistacy (Holland 1998). Epistasis was measured for all pairs of markers, giving 233,586 tests for significance. Correcting for linked markers, using the eigenvalue variance method (Cheverud 2001), gives 222,826 tests, and a Bonferroni adjustment to the experiment-wide error rate of P < 0.05 gave an individual test P-value of P < 2.3 × 10−7 (Lynch and Walsh 1998).
Comparative genomic and candidate gene analysis
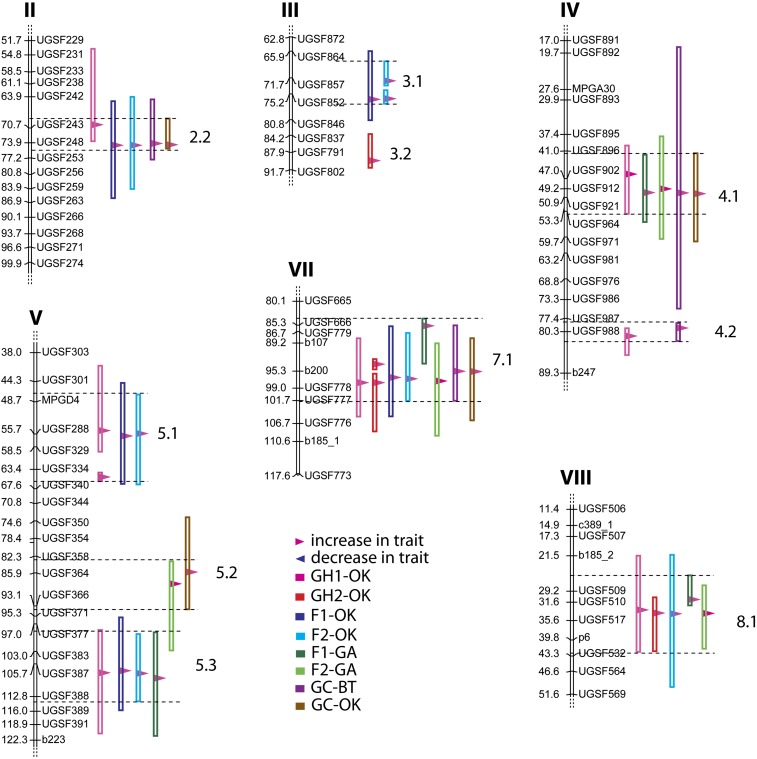
To compare QTL regions among the panicoid grasses, genomic coordinates of each Setaria QTL region were identified using colocalized markers from the genetic map (Bennetzen et al. 2012). Where QTL from multiple trials colocalized in the same map positions, common regions were defined as the region of overlap between individual QTL intervals (Figure 2). Syntenic dotplots were generated for comparisons between Setaria and sorghum and between Setaria and maize using the Synmap module in CoGe (Lyons and Freeling 2008; Tang and Lyons 2012). Setaria genome version 2.1 (id12240), sorghum genome version 1.4 (id93), and maize genome version 2 (id333) were used. We configured CoGe to assign gene pairs to classes based on their Ks values and thus to distinguish orthologous syntenic regions (resulting from speciation from a common ancestor) from paralogous syntenic regions that were the product of the much older pan-grass whole genome duplication (Paterson et al. 2004; Schnable and Lyons 2011). Only orthologous syntenic regions were used to compare QTL, and we use the term syntenic to refer to such regions in the rest of this paper. Syntenic depth was set as 1:1 between the diploid species sorghum and Setaria and 2:1 for maize and Setaria because of the additional whole genome duplication in maize (Blanc and Wolfe 2004; Swigonova et al. 2004). Syntenic regions in maize and sorghum were scanned for published maize and sorghum flowering time QTL (Buckler et al. 2009; Coles et al. 2010; Mace and Jordan 2011).
Figure 2 .
Major QTL regions found, showing QTL intervals from each trial and common regions searched for candidate genes. Dashed black lines delimit common QTL regions defined by overlapping QTL intervals. A full version of this figure is represented in Figure S1 and LOD curves for all trials in Figure S2.
Two approaches were used to identify candidate genes. First, more than 100 genes for flowering time were identified from the literature, using recent reviews (Greenup et al. 2009; Amasino 2010; Higgins et al. 2010; Song et al. 2010). Protein and nucleotide sequences of the flowering time genes were used in BLAST searches against S. italica genome sequence version 2.1 (www.Phytozome.net). The location of candidate genes on the foxtail millet genomic sequence was then compared to that of the QTL regions. Top hits in QTL regions were used in a reciprocal BLAST search to confirm identical query-hit pairs to flowering time genes. The second approach analyzed nucleotide and peptide sequences of all genes identified within each QTL interval. These were used as queries in BLASTP searches against the SwissProt database (Uniprot-Consortium 2012), and then Blast2GO (Conesa et al. 2005) was used to obtain gene ontology terms. These gene ontology terms were searched using “flower*” and related terms and candidate genes identified in this way were used as input to HMMer3.0 (Finn et al. 2011). HMMer 3.0 was used to annotate the genes in the Pfam database (Punta et al. 2012), and HMMer output was manually curated and traced back to the original publication to validate gene information. A gene was chosen as a real QTL candidate only if there was experimental evidence that it affected flowering. The presence of syntenic orthologs of candidate genes across the three genomes was also investigated.
Results
Phenotypic variation
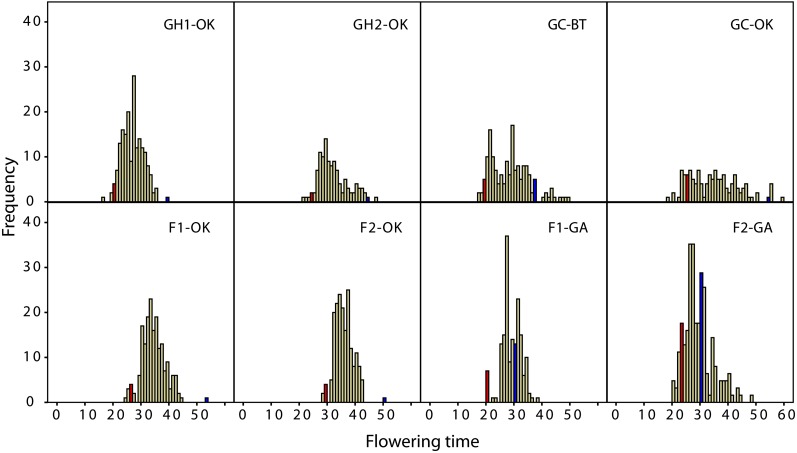
There was substantial variation in flowering time among the eight trials (Figure 1). The green millet parent always flowered earlier than the foxtail millet parent, and in all trials it was amongst the earliest accessions to flower (Figure 1). The foxtail millet parent was more variable in flowering time compared to the RILs, although in most trials it was among the latest to flower. Most trials showed at least some evidence of transgressive segregation, with RILs flowering both earlier than green millet and later than foxtail millet, although in only three trials was there a large amount of transgressive segregation. Growth trials of the parents under 12-hr and 16-hr photoperiod regimes in a constant growth chamber environment confirmed that S. viridis flowers earlier than S. italica, and that both species flower later and produce more leaves under the 16-hr than under the 12-hr light period (Table 2).
Figure 1 .
Histograms of the distribution of flowering times for each trial. The flowering time of the S. viridis parent is indicated by a red column and that of the S. italica parent by a blue column. GH, greenhouse; GC, growth chamber; BT, Boyce Thompson Institute, Ithaca, NY; OK, Oklahoma State University, Stillwater, OK; F, field; GA, University of Georgia, Athens, GA.
Table 2. Mean days to flowering and mean leaf number at flowering for the parental accessions of S. viridis A10 and S. italica B100 under 12- or 16-hr light conditions.
| S. viridis (12 hr) | S. italica (12 hr) | S. viridis (16 hr) | S. italica (16 hr) | |
|---|---|---|---|---|
| Days to flowering | 24.4 (0.39) | 52.5 (0.63) | 31.4 (0.27) | 74.2 (1.84) |
| Leaf number | 7.0 (0.0) | 17.9 (0.35) | 11.6 (0.17) | 24.9 (0.60) |
Values in parentheses represent the standard error of each estimate.
The distribution of flowering time differed between trials, with six of the eight trials showing a “bell”-shaped distribution and two showing either a flat or apparent bimodal distribution. The single trial with a relatively flat distribution (GC-OK) can be explained by the a priori selection of genotypes to represent the ends of the distribution, in order to have the most power possible in a trial where space limitations meant that only a restricted number of plants could be grown. The apparent bimodal distribution in GC-BT is mirrored to some extent in F1-GA and F2-GA, and suggests that there are substantial RIL by environment interactions. An analysis of variance (not shown) with trial and RIL as random factors showed significant differences in both main effects and the interaction between trial and RIL.
Genetic map
A total of 182 RILs were used to construct a genetic map, covering a total of 1125.4 cM. The map uses 684 uniquely positioned markers (only the most informative marker was selected from clusters of cosegregating markers), and includes 560 SNP markers, 101 SSR markers, six STS markers developed from specific genes, and 17 STS markers developed from RFLP markers used in the original F2 map (Wang et al. 1998). The six genes successfully genotyped were teosinte branched1 (tb1), barren stalk1 (ba1), a gene homologous to barren inflorescence2 (bif2-like), dwarf3 (d3), MORE AXILLARY BRANCHES 1 (MAX1), and MONOCULM 1 (MOC1). Genbank accessions for these markers are in File S1.
Marker order was well conserved for the SNP markers with the published foxtail millet map (Bennetzen et al. 2012). Sixty of the 70 SSR markers previously mapped in the F2 population mapped to similar positions in our map, and 10 mapped to a different position but to the correct linkage group (Jia et al. 2009). In addition, we had 17 shared markers with the original F2 map (Wang et al. 1998) and these were ordered in the same way in both maps. There were seven regions with gaps bigger than 10 cM, in linkage groups I, III (two gaps), V, VI, VII, and IX. Distance between markers ranged from 0.1 to 16.9 cM; the average distance between markers was 0.82 cM. Linkage group length ranged between 94.2 cM (VI) and 182.7 cM (IX). As previously reported (Bennetzen et al. 2012), significant segregation distortion was observed on most of linkage group II, and several sections of linkage groups III, IV, V, VII, and IX, because of an excess of the S. italica alleles in those regions. A 50-cM section on top of linkage group VI and a 62 cM section at the bottom of linkage group VII showed distorted segregation ratios too but in this case the S. viridis homozygotes were favored.
QTL analyses
A total of 16 flowering time QTL were identified in the eight trials, with nine identified in at least two trials (Figure 2, Figure S1, Figure S2, Table 3). The percentage of phenotypic variance explained by individual QTL ranged between 2.5% (for QTL 6.1 and 9.1) and 41.9% (QTL 4.1 in the GC-OK trial), and the total percentage of phenotypic variation explained by flowering time QTL on a per trial basis ranged from a low of 34.7% for the F1-GA trial to 88.1% for the GC-BT trial (Table 3). Of the nine QTL consistently identified in at least two trials, the one on linkage group IV (QTL 4.1) had the highest average percentage of phenotypic variation explained (23.84%), and was identified in five trials. Several QTL, such as QTL 4.1, 5.1, and 8.1, had multiple LOD peaks along the QTL interval, suggesting that several tightly linked loci may underlie a single QTL region.
Table 3. Additive effects in days and percentage of variation explained (in parentheses) for QTL identified in the eight trials.
| Linkage group | QTL | GH1-OK | GH2-OK | F1-OK | F2-OK | F1-GA | F2-GA | GC-BT | GC-OK |
|---|---|---|---|---|---|---|---|---|---|
| I | 1.1 | 0.9 (6.0) | |||||||
| II | 2.1 | −1.2 (4.0) | |||||||
| 2.2 | 1.6 (15.6) | 1.5 (11.3) | 1.2 (14.4) | 2.6 (12.0) | 2.1 (3.7) | ||||
| III | 3.1 | 1.0 (6.7) | 0.6 (3.3) | ||||||
| 3.2 | 1.7 (10.7) | ||||||||
| IV | 4.1 | 0.9 (5.9) | 1.0 (8.7) | 2.5 (22.9) | 4.6 (39.8) | 6.7 (41.9) | |||
| 4.2 | 1.1 (5.5) | 2.4 (7.0) | |||||||
| V | 5.1 | 0.9 (5.9) | 1.4 (10.9) | 1.1 (11.5) | |||||
| 5.2 | 1.3 (5.6) | 2.2 (4.8) | |||||||
| 5.3 | 1.9 (21.0) | 1.9 (18.2) | 1.0 (8.6) | 1.4 (12.9) | 1.7 (9.7) | ||||
| VI | 6.1 | −2.0 (2.5) | |||||||
| 6.2 | −2.2 (6.8) | ||||||||
| VII | 7.1 | 1.7 (10.1) | 2.7 (13.4) | 2.1 (14.1) | 1.3 (8.2) | 1.0 (6.7) | 2.6 (12.2) | 5.3 (22.5) | 4.5 (9.7) |
| VIII | 8.1 | 1.3 (13.1) | 2.1 (17.5) | 1.2 (15.4) | 0.8 (6.4) | 1.3 (6.1) | |||
| IX | 9.1 | 1.6 (2.5) | |||||||
| 9.2 | 1.0 (3.9) | ||||||||
| Total | 10.3 (83.1) | 6.5 (41.6) | 7.9 (61.2) | 6.4 (61.4) | 4.2 (34.7) | 11.6 (64.4) | 17.1 (88.1) | 19.1 (65.1) |
Values in parentheses are percentage variation explained for individual QTL from each trial and for the totals of each trial.
The additive effect on a per QTL basis across individual trials ranged between 0.6 days (QTL 3.1 in F2-OK) and 6.7 days (QTL 4.1 in the GC-OK trial; Table 3). The total additive effects on a per trial basis were 6.5−10.3 days in the greenhouse trials, 4.2−11.6 days in the field trials, and 17.1-19.1 days in the growth chamber trials. Total additive effects ranged from 29 to 188.8% of the difference in days to heading between the parents (Table S2).
The eight trials were grown under varying conditions in the greenhouse, field, and growth chamber. QTL7.1 was found in seven trials, QTL2.2, 4.1, and 8.1 in five trials, QTL5.3 in four trials, QTL5.1 in three trials, and QTL3.1, 4.2, and 5.2 in two trials. Four genomic regions with QTL identified from multiple trials (QTL3.1, 5.1, 5.3, and 8.1) lacked significant QTL from the two trials carried out under the shortest photoperiod in the growth chambers, but variation due to different photoperiods and other environmental effects in the trials may be confounded.
For most QTL, the domesticated S. italica alleles increased days to flowering, as expected given that S. italica flowered much later than S. viridis. However, for three of the 16 total flowering time QTL identified (QTL 2.1, 6.1, 6.2), S. viridis increased days to flowering, although each of these was only identified in a single trial.
Epistasis
A total of four significant epistatic interactions at the P < 2.3 × 10−7 level were detected in five of the eight trials conducted (Table 4). Two epistatic interactions were consistently identified in two trials and the other two were identified in a single trial. Epistatic interactions were detected between markers located in and outside of QTL regions as well as within and between linkage groups, and explained between 11.4 and 13.4% of the variance.
Table 4. Significant epistatic interactions between markers in the Setaria genome, and whether these markers colocalize with QTL regions.
| Marker 1 | Linkage Group and Position in cM | QTL Present? | Marker 2 | Linkage Group and Position in cM | QTL Present? | Interaction Was Observed in | % Variation Explained |
|---|---|---|---|---|---|---|---|
| UGSF827 | III-42.4 | No | UGSF867 | III-65.1 | No | F1-OK, F2-OK | 11.4/13.2 |
| UGSF436 | I-50.1 | QTL1.1 | UGSF578 | VIII-64.0 | No | F2-GA, GC-BT | 11.7/11.7 |
| b112 | I-51.4 | QTL1.1 | UGSF579 | VIII-65.2 | No | F2-GA | 13.4 |
| c562 | IV-40.3 | QTL4.1 | b185-1 | VII-110.6 | No | GC-OK | 11.8 |
Comparison of QTL regions
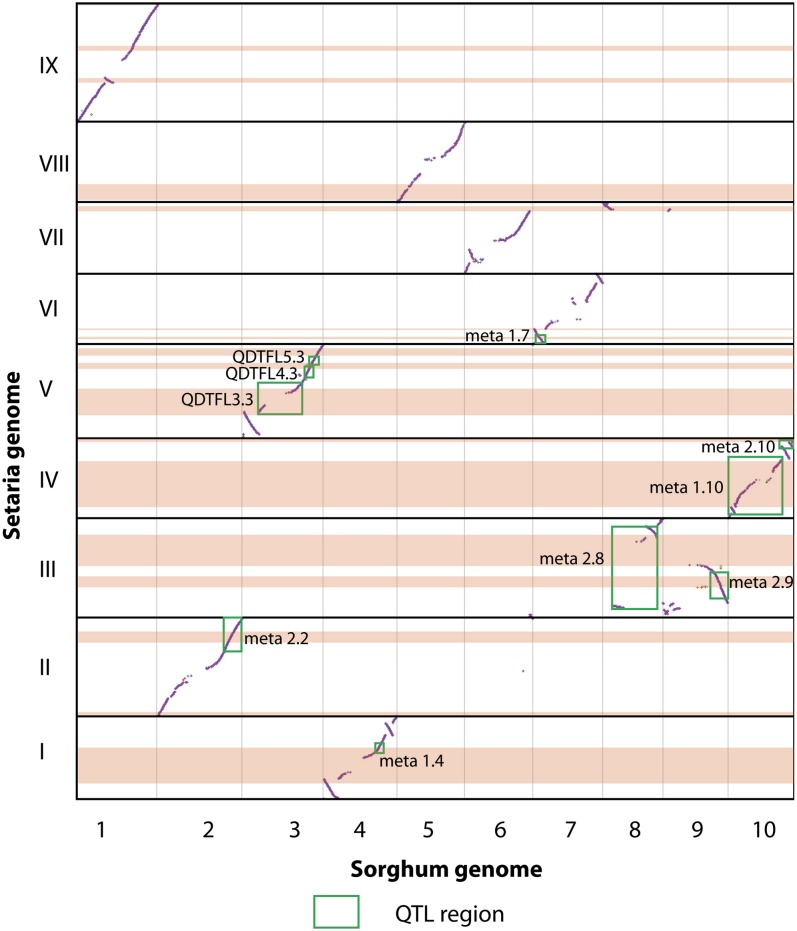
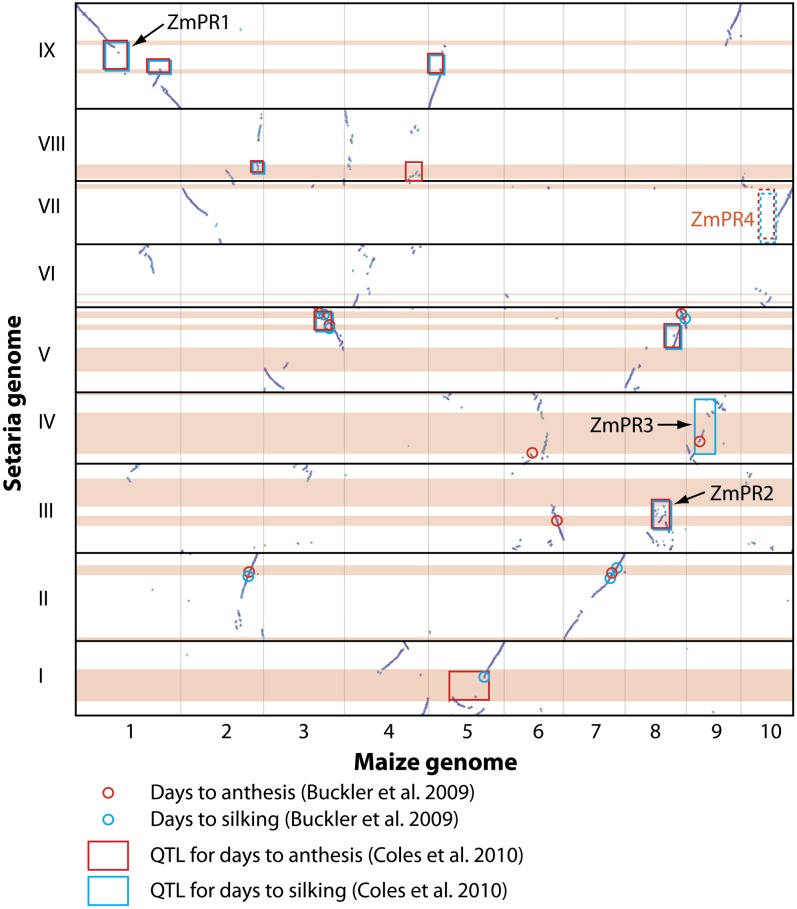
To compare QTL among the three species we used a meta-analysis of sorghum QTL trials (Mace and Jordan 2011), a joint multiple population analysis of photoperiod sensitivity in maize (Coles et al. 2010) and the NAM maize QTL study (Buckler et al. 2009). All three of these studies used SNP markers that could be unambiguously placed on the physical genome sequence, and in all three studies some of the identified QTL were syntenic with the QTL found in Setaria. Comparison of the syntenic dotplots shows that there is less genome rearrangement between sorghum and Setaria than between Setaria and maize (Figures 3 and 4).
Figure 3 .
Whole genome dotplot of Setaria vs. sorghum. Diagonal purple lines in each cell indicate regions of synteny between the two genomes. Horizontal pink bars indicate genomic extent of Setaria QTL, whereas green boxes indicate QTL and meta-QTL for flowering time from sorghum (Mace and Jordan 2011) that are syntenic with Setaria QTL. Labels for QTL identified from sorghum are the same as in the original paper (Mace and Jordan 2011).
Figure 4 .
Whole-genome dotplot of Setaria vs. maize. Diagonal purple lines in each cell indicate regions of synteny between the two genomes. Horizontal pink bars indicate genomic extent of Setaria QTL, while boxes (Coles et al. 2010) and circles (Buckler et al. 2009) indicate QTL from maize that are syntenic with Setaria QTL. Red boxes and circles indicate days to anthesis, blue boxes and circles indicate days to silking. ZmPR1, ZmPR2, ZmPR3, ZmPR4 represent the four most important QTL regions in maize meta-analyses (see text). ZmPR4 (in orange) is represented by a box made of dashed lines as it is the only one that is not syntenic with a Setaria QTL.
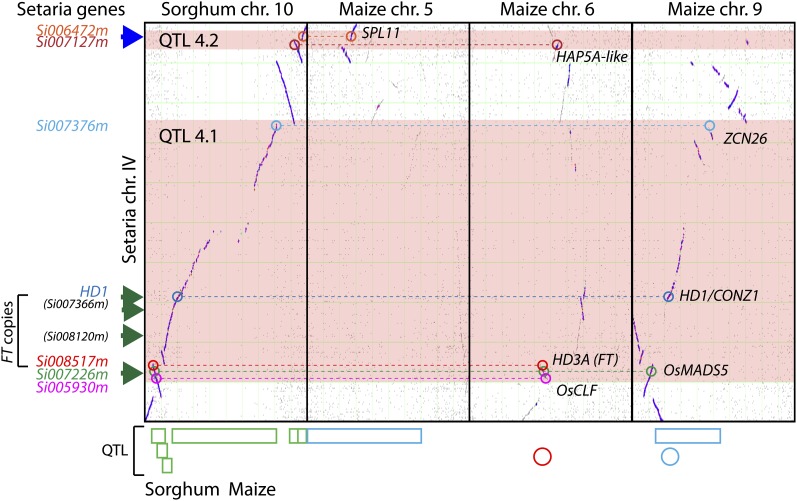
Genomic rearrangements were investigated in detail between Setaria chromosome IV and the corresponding sorghum and maize chromosomes (Figure 5). Four major syntenic blocks could be identified for the common region of QTL 4.1, three of which contain orthologous candidate genes. The genes have maintained, for the most part, their syntenic relationships, although there is evidence of partitioning of genes between the different maize chromosomes. QTL4.1 contains four LOD peaks, with two of these aligned with candidate genes (Figure 5). QTL4.2 is smaller, and has only a single LOD peak. Other QTL that show multiple LOD peaks include QTL2.2 and 8.1.
Figure 5 .
Analysis of Setaria chromosome IV and corresponding syntenic regions in sorghum and maize. Each panel represents a chromosome by chromosome dot-plot of Setaria chr. IV (vertical axis) vs., in turn, sorghum chr. 10, maize chr. 5, maize chr. 6, and maize chr. 9 (horizontal axis). Horizontal pink bars indicate genomic extent of Setaria QTL4.1 and 4.2. On the bottom axis are QTL regions identified in sorghum (Mace and Jordan 2011) and maize (Buckler et al. 2009; Coles et al. 2010). Sorghum QTL are in green boxes, maize QTL are in red or blue boxes (Coles et al. 2010) or circles (Buckler et al. 2009), with red indicating days to anthesis and blue indicating days to silking. Syntenic candidate genes identified in the Setaria sequence (SPL11, HAP5A-like, ZCN26, HD1, HD3A, OsMADS5) were mapped onto each of the syntenic regions in sorghum and maize using COGE. On the left of the graph are the Setaria proteins identified as orthologous to the candidate genes from other species. Three of the Setaria proteins are co-orthologs of HD3A/RFT1 (FT) in rice, but only Si008517 is in synteny with the four chromosomes from sorghum and maize. The other two co-orthologs, Si07366m and Si08120m, are not found in syntenic regions. HD1 is not annotated in the Setaria genome, thus there is no numbered Setaria protein associated with it. Four dark green arrows on the left hand side of the graph indicate the approximate position of the four LOD peaks in QTL 4.1 and one blue arrow the single LOD peak in QTL4.2.
Candidate genes
Several of the QTL regions contain candidate flowering pathway genes identified from rice, maize, sorghum, and Arabidopsis (Table 5). Identified candidate genes come from both autonomous and photoperiod-sensitive pathways, and span processes from initial light sensing (CRY2), coordination/regulation by the circadian clock (PRR95, PRR59, GI), the CONSTANS photoperiod pathway, the integration of flowering pathways (FT/HD3A/RFT1), and control of the transition of the shoot apical meristem from vegetative to flowering (FD/Dlf1, TFL1/RCN1). Phase change genes such as SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 9 (SPL9) and SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 11 (SPL11), and the microRNA miR156 were also identified as candidates for differences in flowering time. Some QTL regions identified in only a single environment lack obvious candidate genes involved in control of flowering time (i.e., QTL 2.1, 6.1, 6.2 and 9.1; Table 5). Interestingly, no QTL colocalize with genes involved in the vernalization pathway described from pooid grasses, suggesting that Setaria does not employ vernalization to cue flowering time.
Table 5. QTL number, interval (cM) for the region (entire interval for single QTL, overlapping common region for multiple QTL), flanking markers, marker at maximum LOD peak, and candidate genes within each QTL interval.
| QTL | Interval, cM | Flanking Markers | Marker at LOD Peak | Candidate Genes Colocalizing With Setaria QTL | Reference |
|---|---|---|---|---|---|
| 1.1 | 48.1−60.1 | MPGA8- UGSF467 | UGSF436 | OsARP6/ZmARP6 (Si017263m) | (Deal et al. 2005) |
| OsRCN2/ZCN2 (Si020516m) | (Nakagawa et al. 2002; Danilevskaya et al. 2008) | ||||
| ZmFPF1-like (Si018761m) | (Kania et al. 1997; Melzer et al. 1999; Smykal et al. 2004) | ||||
| LWD1(Si019593m) | (Wu et al. 2008) | ||||
| 2.1 | 0.01−1.29 | UGSF158- UGSF160 | UGSF160 | No candidate genes identified | |
| 2.2 | 65.9−75.1 | UGSF242- UGSF249 | UGSF248 | SPL9-like (Si030195m) | (Schwarz et al. 2008; Wang et al. 2009) |
| OsFTL4/ZCN18 (37,127,350-37,132,487) | (Danilevskaya et al. 2008) | ||||
| OsFPA (Si028909m) | (Schomburg et al. 2001; Baurle et al. 2007) | ||||
| OsPRR95 (Si029202m) | (Murakami et al. 2003, 2005) | ||||
| FD/DLF1 (Si031077m) | (Neuffer et al. 1997; Muszynski et al. 2006) | ||||
| OsMADS8/ZmMADS27 (Si030960m) | (Kang et al. 1997) | ||||
| 3.1 | 66.9−78.2 | UGSF864-UGSF848 | UGSF850 | HAP3B-like (Si023400m) | (Cai et al. 2007; Chen et al. 2007) |
| 3.2 | 84.2−91.4 | UGSF837-UGSF800 | UGSF799 | REF6-like (Si024583m) | (Noh et al. 2004) |
| 4.1 | 42−53.1 | UGSF896-UGSF961 | UGSF914 | OsCLF (Si005930m) | (Luo et al. 2009) |
| OsMADS5 (Si007226m) | (Kang and An 1997; Jeon et al. 2000) | ||||
| OsMADS55 (Si007357m) | (Lee et al. 2012) | ||||
| Hd3a(OsFTL2)/OsRFT1(OsFTL3)/ZCN15 (Si007366m/Si008517m/Si008120) | (Kojima et al. 2002; Tamaki et al. 2007; Komiya et al. 2008) | ||||
| HD1/CONSTANS (12,555,166-12,557,473) | |||||
| CONSTANS-like (Si006432m) | (Putterill et al. 1995; Yano et al. 2000) | ||||
| OsCRY2 (Si006039m) | (Hirose et al. 2006) | ||||
| OsFTL12/ZCN26 (Si007376m) | (Danilevskaya et al. 2008) | ||||
| 4.2 | 78.5−82.3 | UGSF987- UGSF988 | UGSF988 | HAP5-like (Si007127m) | (Ben-Naim et al. 2006; Wenkel et al. 2006) |
| Zm-SPL11 (Si006472m) | (Xie et al. 2006; Li et al. 2012) | ||||
| 5.1 | 44.7−65.1 | UGSF300- UGSF335 | UGSF289 | OsGI (Si000107m) | (Hayama et al. 2003) |
| miR156F (cg1) (11,531,587-11,532,171) | (Wu and Poethig 2006; Chuck et al. 2007) | ||||
| OsFTL1/ZCN14 (13,585,697-13,586,064) | (Izawa et al. 2002; Danilevskaya et al. 2008) | ||||
| Hd17/OsELF3 (Si000443m) | (Matsubara et al. 2008, 2012) | ||||
| 5.2 | 82.3−92.4 | UGSF358- UGSF365 | UGSF364 | FVE/OsFVE (Si001403m) | (Ausin et al. 2004; Baek et al. 2008) |
| OsLFL1 (Si004459m) | (Peng et al. 2007, 2008) | ||||
| OsSPA1 (Si000378m) | (Laubinger et al. 2006) | ||||
| OsFTL9/ZCN12 (Si005012m). | (Danilevskaya et al. 2008) | ||||
| 5.3 | 98.3−113.8 | UGSF379-UGSF388 | UGSF387 | REF6 (Si000062m). | (Noh et al. 2004) |
| 6.1 | 35.2 | UGSF690 | UGSF690 | No candidate genes identified | |
| 6.2 | 39.7−43.4 | UGSF686-p10 | p10 | No candidate genes identified | |
| 7.1 | 83.1−101 | UGSF665-UGSF778 | b200 | APETALA2 (Si012635m) | (Bowman et al. 1989; Kunst et al. 1989) |
| HAP5-like (Si012136m) | (Ben-Naim et al. 2006; Wenkel et al. 2006) | ||||
| 8.1 | 24.5−41.4 | b185_2- UGSF526 | UGSF513, UGSF525 | OsTFL1/OsRCN1/ZCN1/TFL1 (Si028168m) | (Shannon and Meekswagner 1991; Gustafsonbrown et al. 1994; Nakagawa et al. 2002) |
| OsPRR59(Si026170m) | (Murakami et al. 2003, 2005) | ||||
| 9.1 | 80.4−81.0 | UGSF77- UGSF79 | UGSF77 | No candidate genes identified | |
| 9.2 | 83.4−86.4 | UGSF93- p91 | p91 | REF6-like (Si034241m) | (Noh et al. 2004) |
Discussion
Phenotypic variation
The eight trials varied in a number of environmental variables, including photoperiod, temperature, light intensity, planting density, and water availability. Several of these, such as photoperiod and temperature, are known to have effects on flowering time (Garner and Allard 1920; Robertson 1968; Koornneef et al. 1991; Chew et al. 2012; Kim et al. 2012). In fact, early work on S. viridis found that earliest flowering was under a photoperiod of 8 hr light, and that a lower temperature of 22.5° led to faster flowering than at 30° (Schreiber and Oliver 1971). We too observed variation across the eight trials in flowering time of the parents (although S. viridis always flowered earlier than S. italica), and in the order in which the RILs flowered, but it is hard to assign these differences to the effect of any one environmental variable. Overall, increasing the day length delayed flowering of both the RILs and the parents of the cross. Furthermore, the growth chamber trial of the parents at 12 and 16 hr light demonstrated that both parents flowered earlier under a 12-hr regime. However, factors other than day length were likely responsible for the earlier heading of S. italica in the GA compared with the OK field trials, and for the delay in flowering observed in the OK compared with the BT growth chamber trial, as those trials were grown under similar photoperiod regimes. Much work needs to be done in order to understand how combinations of different environmental variables affect flowering time in these genotypes.
QTL analyses
Despite differences in management, experimental design, and environmental conditions of the eight trials, nine out 16 QTL were identified in at least two trials. In most cases the S. italica allele acted to increase days to flowering, as might be expected from the later flowering time of S. italica in all trials. However, for three of the 16 flowering time QTL, S. viridis alleles increased days to flowering. Very few epistatic interactions were detected, although our stringent Bonferroni-corrected false-discovery rate may have precluded real but less significant interactions. Of the four interactions discovered, one was between two markers outside of any QTL region, and three were between markers where one was in a QTL region and the other was not. No interaction was identified between two markers where both markers were in QTL regions (Table 4). In addition, epistasis was not seen in all trials, suggesting that such interactions are not a prominent feature of this data set. This is in agreement with earlier experiments on the F3 generation of this cross, which uncovered a similar pattern of interaction between the RFLP markers used to construct the map (Doust et al. 2004, 2005; Doust and Kellogg 2006).
In general, the analysis of syntenic blocks between Setaria and maize and sorghum reveals a more-or-less 1:1 relationship between Setaria and sorghum chromosomes but a more complicated set of relationships between Setaria and maize. This is no doubt due to the fractionation of the maize genome following the whole genome duplication 9−12 mya and its subsequent diploidization (Blanc and Wolfe 2004; Swigonova et al. 2004). Therefore, a single QTL region in Setaria may be dissected into syntenic blocks that occur on two maize chromosomes. In both of the comparisons, we found that single Setaria QTL will usually be comprised of multiple syntenic blocks when compared with either sorghum or maize. QTL4.1 (Figure 5) is a case in point, where at least two inversions have occurred in Setaria compared to the corresponding region on sorghum chromosome 10 (Figure 5, this article; Figure 11 in Bennetzen et al. 2012). The candidate genes that colocalize to this QTL region maintain local order on each syntenic block, but genomic inversions have resulted in an overall rearrangement compared to Setaria. The partitioning of syntenic regions between different chromosomes in maize has resulted in further dissociation of these genes, with most genes confined to one or other homeologous chromosome, although others such as OsMADS5 have copies on both maize homeologs.
The presence of multiple syntenic blocks within a single Setaria QTL region is correlated with the presence of multiple LOD peaks in a number of the QTL intervals (Figure 5, Figure S2), implying that these QTL cover regions where multiple genes may be involved in flowering time regulation. Candidate genes in these intervals are often colocated with one or other of these peaks, as can be seen for QTL4.1, a large QTL interval on Setaria chromosome IV (Figure 5).
Meta-analysis of QTL studies in maize has revealed a number of genomic regions where QTL for multiple traits are colocalized. Studies have recognized from four to six regions, on chromosomes 1, 8, 9, and 10 (Chardon et al. 2004; Coles et al. 2010; Xu et al. 2012). Four of these regions were shown to account for a high proportion of the variance in flowering time, with three (labeled ZmPR1, ZmPR2, and ZmPR3) accounting for approximately 10% each and the last (ZmPR4) accounting for approximately 40% (Coles et al. 2010). The genomic comparison of QTL in Setaria and maize reveals that ZmPR1, ZmPR2, and ZmPR3, on maize chromosomes 1, 8, and 9, are syntenic to QTL regions in Setaria, but that ZmPR4, the largest effect QTL region in maize on chromosome 10 (Coles et al. 2010), is not. However, there were also Setaria QTL without syntenic QTL in the other two species, suggesting that control of flowering time may involve both conserved and novel genetic loci in any one species. Such a pattern has been demonstrated for other traits such as shattering, where one gene, SHATTERING 1, appears to underlie QTL in multiple species (Lin et al. 2012), whereas most other genes are confined to individual species (Li and Gill 2006). Conservation of flowering time QTL across grasses for at least some loci was noted first several years ago, and our study demonstrates support for those earlier findings (Paterson et al. 1995).
Candidate genes
It is somewhat surprising that the high level of conservation in QTL position between Setaria and sorghum and maize is not reflected by conservation of the cloned flowering time genes from sorghum and maize as candidates for those QTL. Only three genes, DLF1 (FD), GI, and CONZ1 (CO), were identified in Setaria QTL intervals. Major effect genes such as ZmCCT, VGT1, and PRR37 did not colocalize with Setaria QTL. This lack of correspondence may either indicate real differences between the three species, or may simply reflect the relatively poor knowledge of genes underlying flowering variation in panicoid grasses. The latter seems likely because of the substantial number of candidate genes from rice and Arabidopsis that colocalize in the QTL intervals. In addition, most emphasis in flowering time studies in maize and sorghum has been on the effect of photoperiod, whereas the Setaria trials described here only sample a small range of photoperiods. The conservation of candidate genes across the three species in QTL such as QTL 4.1 likely indicates that genetic control of flowering is similar between the three species.
In contrast to maize, sorghum, and rice, Setaria appears to have been domesticated away from the tropics, in the northern temperate region of China (Bettinger et al. 2010). Furthermore, annual Setaria species in these regions germinate and grow in the spring and summer, and do not appear to need vernalization to successfully flower. This is supported by our failure to find genes such as VRN1 and VRN2 from the grass vernalization pathway colocalized with Setaria QTL. In contrast, a number of candidate genes were from the CONSTANS photoperiod pathway (SPA1, CO/HD1/CONZ1, HAP3-like, HAP5-like), as well as genes proposed to be involved in autonomous or endogenous pathways (FVE, FPA). Surprisingly, only two candidate genes (LFL1 and OsMADS5) from the alternative photoperiod pathway in rice (centered on EHD1) colocalized with Setaria QTL, perhaps due to the limited range of photoperiods sampled. The presence of this pathway in maize has already been established with the discovery of a GHD7 ortholog (ZmCCT) (Hung et al. 2012), and we predict that this pathway will prove to be present in Setaria as well.
A number of circadian clock elements colocalized to Setaria QTL, including PRR59, PRR95, and GI, as well as genes that closely interact with these, such as ELF3-like and ELF4-like genes (Higgins et al. 2010; Yang et al. 2012). GI has multiple roles, including directly interacting with miR172 to control phase change independent of photoperiod (Jung et al. 2007) and with CONSTANS (CO) in the main photoperiodic flowering time pathway (Hayama et al. 2003). The two pseudoresponse regulators PRR59 and PRR95 are related to PRR37, which underlies the major flowering time locus Ma1 in sorghum (Murphy et al. 2011), but PRR37 was not located in any of the identified QTL. It is possible that modifications to any of these circadian clock elements can be used to produce differences in flowering time. Interestingly, the PRR37 ortholog in maize does colocalize to a QTL for flowering time, potentially creating a difference in control of flowering time between Setaria and the other two species. Studies in switchgrass (Panicum virgatum), a closely related species to Setaria, may reveal whether these differences are diagnostic for the different tribes to which maize and sorghum (Andropogoneae) and Setaria and switchgrass (Paniceae) belong.
Setaria has three copies of FT in QTL4.1 that are co-orthologous to HD3A and RFT1 in rice, but only HD3A and one of the Setaria copies (Si08517m) are in synteny. FT is part of the large PEBP protein family, which has undergone several rounds of duplication in grasses as compared to Arabidopsis (Chardon and Damerval 2005), and Setaria has 22 PEBP proteins (Bennetzen et al. 2012). In maize, ZCN8 has been described as the functional FT homolog, identified through expression analysis (Danilevskaya et al. 2008), but the Setaria ortholog of ZCN8 did not colocalize to any of the QTL intervals. Another subclade of the PEPB protein subfamily contains TERMINAL FLOWER-like (TFL) genes, which act antagonistically to FT in Arabidopsis to prolong the vegetative phase of the apical meristem (Kardailsky et al. 1999; Kobayashi et al. 1999). A TFL1 homolog was found in Setaria QTL8.1.
Lastly, a number of genes involved in phase change and the regulation of the length of the vegetative growth phase were also identified (SPL9, SPL11, miR156), suggesting that phase change and flowering time are intimately connected. Recent work on flowering time regulators such as GI and APETALA1 have uncovered an intricate coupling of phase change and flowering time pathways (Yant et al. 2010; Huijser and Schmid 2011), indicating that a more holistic view of gene regulation is necessary to understand flowering time in plants.
The eight trials in this study showed remarkable similarities in the position of QTL for flowering, given the diversity of the growing conditions. This finding suggests that the integration of flowering pathways performed by the plants provides considerable buffering against environmental change. However, the presence of a number of QTL regions found only in the longer-day environments suggests that, in common with other grasses, long days likely suppress flowering. This was corroborated by the later flowering time of the parents under 16-hr light than under 12-hr light, when both were grown in a controlled environment. The candidate genes identified are mainly from the CONSTANS photoperiod pathway but also include members of the EHD1 photoperiod and autonomous pathways. We did not explore the influence of phase change on flowering time, but the presence of several candidate genes for phase change suggests that this will be a fruitful area of investigation. The presence of genes conserved across grasses and dicots in the syntenic QTL regions between Setaria, maize, and sorghum suggests that an ancestral flowering time pathway involving CONSTANS is conserved across grasses, while the absence of many of the cloned genes of major effect from maize in these same QTL suggests that each species has undergone further lineage specific evolution in genetic control of flowering time. We have identified several genome regions based on the QTL analyses and have embarked on fine-mapping loci using a combination of hybrid inbred families and gene expression surveys.
Supplementary Material
Acknowledgments
We thank Jerry Moore and Josh Massey of the Cimarron Valley Research Station for their advice and help in the Oklahoma field trials and Jessica Stromski for invaluable help in database management and field assistance. Funding sources to A.N.D. from Department of Energy (DE-FG02-08ER64636) and the Oklahoma Center for Science and Technology (PSB08-007, PS11-035B) and to K.M.D. from the National Institute of Food and Agriculture Plant Feedstock Genomics for Bioenergy Program (#2008-35504-04851).
Footnotes
Communicating editor: D. J. de Koning
Literature Cited
- Amasino R., 2010. Seasonal and developmental timing of flowering. Plant J. 61: 1001–1013 [DOI] [PubMed] [Google Scholar]
- Ausin I., Alonso-Blanco C., Jarillo J. A., Ruiz-Garcia L., Martinez-Zapater J. M., 2004. Regulation of flowering time by FVE, a retinoblastoma-associated protein. Nat. Genet. 36: 162–166 [DOI] [PubMed] [Google Scholar]
- Baek I. S., Park H. Y., You M. K., Lee J. H., Kim J. K., 2008. Functional conservation and divergence of FVE genes that control flowering time and cold response in rice and Arabidopsis. Mol. Cells 26: 368–372 [PubMed] [Google Scholar]
- Basten C. J., Weir B. S., Zeng Z. B., 1994. Zmap-a QTL cartographer, pp. 65–66 in 5th World Congress on Genetics Applied to Livestock Production: Computing Strategies and Software, edited by Smith C., Gavora J. S., Benkel B., Chesnais J., Fairfull W. et al. Organizing Committee, Guelph, Ontario, Canada [Google Scholar]
- Basten C. J., Weir B. S., Zeng Z. B., 2002. QTL Cartographer Version 1.16. North Carolina State University, Raleigh, NC [Google Scholar]
- Baurle I., Smith L., Baulcombe D. C., Dean C., 2007. Widespread role for the flowering-time regulators FCA and FPA in RNA-mediated chromatin silencing. Science 318: 109–112 [DOI] [PubMed] [Google Scholar]
- Ben-Naim O., Eshed R., Parnis A., Teper-Bamnolker P., Shalit A., et al. , 2006. The CCAAT binding factor can mediate interactions between CONSTANS-like proteins and DNA. Plant J. 46: 462–476 [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Schmutz J., Wang H., Percifield R., Hawkins J., et al. , 2012. Reference genome sequence of the model plant Setaria. Nat. Biotechnol. 30: 555. [DOI] [PubMed] [Google Scholar]
- Bettinger R. L., Barton L., Morgan C., Chen F. H., Wang H., et al. , 2010. The transition to agriculture at Dadiwan, People’s Republic of China. Curr. Anthropol. 51: 703–714 [Google Scholar]
- Blanc G., Wolfe K. H., 2004. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 16: 1667–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies K., Wang R. L., Ambrose B. A., Schmidt R. J., Meeley R. B., et al. , 2003. Duplicate FLORICAULA/LEAFY homologs zfl1 and zfl2 control inflorescence architecture and flower patterning in maize. Development 130: 2385–2395 [DOI] [PubMed] [Google Scholar]
- Bowman J. L., Smyth D. R., Meyerowitz E. M., 1989. Genes directing flower development in Arabidopsis. Plant Cell 1: 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler E. S., Holland J. B., Bradbury P. J., Acharya C. B., Brown P. J., et al. , 2009. The genetic architecture of maize flowering time. Science 325: 714–718 [DOI] [PubMed] [Google Scholar]
- Cai X. N., Ballif J., Endo S., Davis E., Liang M. X., et al. , 2007. A putative CCAAT-binding transcription factor is a regulator of flowering timing in Arabidopsis. Plant Physiol. 145: 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus-Kulandaivelu L., Veyrieras J. B., Madur D., Combes V., Fourmann M., et al. , 2006. Maize adaptation to temperate climate: relationship between population structure and polymorphism in the Dwarf8 gene. Genetics 172: 2449–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardon F., Damerval C., 2005. Phylogenomic analysis of the PEBP gene family in cereals. J. Mol. Evol. 61: 579–590 [DOI] [PubMed] [Google Scholar]
- Chardon F., Virlon B., Moreau L., Falque M., Joets J., et al. , 2004. Genetic architecture of flowering time in maize as inferred from quantitative trait loci meta-analysis and synteny conservation with the rice genome. Genetics 168: 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. Z., Zhang X. Q., Wei P. C., Chen Q. J., Ren F., et al. , 2007. AtHAP3b plays a crucial role in the regulation of flowering time in Arabidopsis during osmotic stress. J. Biochem. Mol. Biol. 40: 1083–1089 [DOI] [PubMed] [Google Scholar]
- Cheverud J. M., 2001. A simple correction for multiple comparisons in interval mapping genome scans. Heredity 87: 52–58 [DOI] [PubMed] [Google Scholar]
- Chew Y. H., Wilczek A. M., Williams M., Welch S. M., Schmitt J., et al. , 2012. An augmented Arabidopsis phenology model reveals seasonal temperature control of flowering time. New Phytol. 194: 654–665 [DOI] [PubMed] [Google Scholar]
- Chuck G., Cigan A. M., Saeteurn K., Hake S., 2007. The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat. Genet. 39: 544–549 [DOI] [PubMed] [Google Scholar]
- Churchill G. A., Doerge R. W., 1994. Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti J., Tremblay R., Wong A. Y. M., Coneva V., Kozaki A., et al. , 2006. The maize INDETERMINATEI flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genomics 7: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles N. D., McMullen M. D., Balint-Kurti P. J., Pratt R. C., Holland J. B., 2010. Genetic control of photoperiod sensitivity in maize revealed by joint multiple population analysis. Genetics 184: 799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles N. D., Zila C. T., Holland J. B., 2011. Allelic effect variation at key photoperiod response quantitative trait loci in maize. Crop Sci. 51: 1036–1049 [Google Scholar]
- Conesa A., Gotz S., Garcia-Gomez J. M., Terol J., Talon M., et al. , 2005. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676 [DOI] [PubMed] [Google Scholar]
- Corbesier L., Vincent C., Jang S. H., Fornara F., Fan Q. Z., et al. , 2007. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Danilevskaya O. N., Meng X., Hou Z. L., Ananiev E. V., Simmons C. R., 2008. A genomic and expression compendium of the expanded PEBP gene family from maize. Plant Physiol. 146: 250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal R. B., Kandasamy M. K., McKinney E. C., Meagher R. B., 2005. The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis. Plant Cell 17: 2633–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J., 2003. The foxtail (Setaria) species-group. Weed Sci. 51: 641–656 [Google Scholar]
- Doerge R. W., Churchill G. A., 1996. Permutation tests for multiple loci affecting a quantitative character. Genetics 142: 285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doust A. N., Kellogg E. A., 2002. Inflorescence diversification in the panicoid “bristle grass” clade (Paniceae, Poaceae): Evidence from molecular phylogenies and developmental morphology. Am. J. Bot. 89: 1203–1222 [DOI] [PubMed] [Google Scholar]
- Doust A. N., Kellogg E. A., 2006. Effect of genotype and environment on branching in weedy green millet (Setaria viridis) and domesticated foxtail millet (Setaria italica) (Poaceae). Mol. Ecol. 15: 1335–1349 [DOI] [PubMed] [Google Scholar]
- Doust A. N., Devos K. M., Gadberry M. D., Gale M. D., Kellogg E. A., 2004. Genetic control of branching in foxtail millet. Proc. Natl. Acad. Sci. USA 101: 9045–9050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doust A. N., Devos K. M., Gadberry M. D., Gale M. D., Kellogg E. A., 2005. The genetic basis for inflorescence variation between foxtail and green millet (Poaceae). Genetics 169: 1659–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doust A. N., Kellogg E. A., Devos K. M., Bennetzen J. L., 2009. Foxtail millet: a sequence-driven grass model system. Plant Physiol. 149: 137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R. D., Clements J., Eddy S. R., 2011. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 39: W29–W37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner W. W., Allard H. A., 1920. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. Agricultural Research 18: 553–606 [Google Scholar]
- Greenup A., Peacock W. J., Dennis E. S., Trevaskis B., 2009. The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann. Bot. (Lond.) 103: 1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Kumari K., Sahu P. P., Vidapu S., Prasad M., 2012. Sequence-based novel genomic microsatellite markers for robust genotyping purposes in foxtail millet Setaria italica (L.) P. Beauv. Plant Cell Rep. 31: 323–337 [DOI] [PubMed] [Google Scholar]
- Gustafsonbrown C., Savidge B., Yanofsky M. F., 1994. Regulation of the arabidopsis floral homeotic gene APETALA1. Cell 76: 131–143 [DOI] [PubMed] [Google Scholar]
- Hayama R., Yokoi S., Tamaki S., Yano M., Shimamoto K., 2003. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422: 719–722 [DOI] [PubMed] [Google Scholar]
- Higgins J. A., Bailey P. C., Laurie D. A., 2010. Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS ONE 5: e10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose F., Shinomura T., Tanabata T., Shimada H., Takano M., 2006. Involvement of rice cryptochromes in de-etiolation responses and flowering. Plant Cell Physiol. 47: 915–925 [DOI] [PubMed] [Google Scholar]
- Holland J. B., 1998. EPISTACY: A SAS program for detecting two-locus epistatic interactions using genetic marker information. J. Hered. 89: 374–375 [Google Scholar]
- Holm L. G., 1997. World Weeds: Natural Histories and Distribution. Wiley, New York, Chichester [Google Scholar]
- Huijser P., Schmid M., 2011. The control of developmental phase transitions in plants. Development 138: 4117–4129 [DOI] [PubMed] [Google Scholar]
- Hung H. Y., Shannon L. M., Tian F., Bradbury P. J., Chen C., et al. , 2012. ZmCCT and the genetic basis of day-length adaptation underlying the postdomestication spread of maize. Proc. Natl. Acad. Sci. USA 109: E1913–E1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa T., Oikawa T., Sugiyama N., Tanisaka T., Yano M., et al. , 2002. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev. 16: 2006–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. C., Stam P., 1994. High-resolution of quantitative traits into multiple loci via interval mapping. Genetics 136: 1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J. S., Lee S., Jung K. H., Yang W. S., Yi G. H., et al. , 2000. Production of transgenic rice plants showing reduced heading date and plant height by ectopic expression of rice MADS-box genes. Mol. Breed. 6: 581–592 [Google Scholar]
- Jia X. P., Shi Y. S., Song Y. C., Wang G. Y., Wang T. Y., et al. , 2007. Development of EST-SSR in foxtail millet (Setaria italica). Genet. Resour. Crop Evol. 54: 233–236 [Google Scholar]
- Jia X. P., Zhang Z. H., Liu Y. H., Zhang C. W., Shi Y. S., et al. , 2009. Development and genetic mapping of SSR markers in foxtail millet Setaria italica (L.) P. Beauv. Theor. Appl. Genet. 118: 821–829 [DOI] [PubMed] [Google Scholar]
- Jung J. H., Seo Y. H., Seo P. J., Reyes J. L., Yun J., et al. , 2007. The GIGANTEA-regulated MicroRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 19: 2736–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. G., An G. H., 1997. Isolation and characterization of a rice MADS box gene belonging to the AGL2 gene family. Mol. Cells 7: 45–51 [PubMed] [Google Scholar]
- Kang H. G., Jang S., Chung J. E., Cho Y. G., An G., 1997. Characterization of two rice MADS box genes that control flowering time. Mol. Cells 7: 559–566 [PubMed] [Google Scholar]
- Kania T., Russenberger D., Peng S., Apel K., Melzer S., 1997. FPF1 promotes flowering in Arabidopsis. Plant Cell 9: 1327–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I., Shukla V. K., Ahn J. H., Dagenais N., Christensen S. K., et al. , 1999. Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kim J. J., Lee J. H., Kim W., Jung H. S., Huijser P., et al. , 2012. The microRNA156-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3 Module Regulates Ambient Temperature-Responsive Flowering via FLOWERING LOCUS T in Arabidopsis. Plant Physiol. 159: 461–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Kaya H., Goto K., Iwabuchi M., Araki T., 1999. A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Kojima S., Takahashi Y., Kobayashi Y., Monna L., Sasaki T., et al. , 2002. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 43: 1096–1105 [DOI] [PubMed] [Google Scholar]
- Komiya R., Ikegami A., Tamaki S., Yokoi S., Shimamoto K., 2008. Hd3a and RFT1 are essential for flowering in rice. Development 135: 767–774 [DOI] [PubMed] [Google Scholar]
- Komiya R., Yokoi S., Shimamoto K., 2009. A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136: 3443–3450 [DOI] [PubMed] [Google Scholar]
- Koornneef M., Hanhart C. J., Vanderveen J. H., 1991. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 229: 57–66 [DOI] [PubMed] [Google Scholar]
- Kunst L., Klenz J. E., Martinezzapater J., Haughn G. W., 1989. Ap2 gene determines the identity of perianth organs in flowers of Arabidopsis thaliana. Plant Cell 1: 1195–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubinger S., Marchal V., Gentilhomme J., Wenkel S., Adrian J., et al. , 2006. Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 133: 3213–3222 [DOI] [PubMed] [Google Scholar]
- Lee J. H., Park S. H., Ahn J. H., 2012. Functional conservation and diversification between rice OsMADS22/OsMADS55 and Arabidopsis SVP proteins. Plant Sci. 185: 97–104 [DOI] [PubMed] [Google Scholar]
- Li P. H., Brutnell T. P., 2011. Setaria viridis and Setaria italica, model genetic systems for the Panicoid grasses. J. Exp. Bot. 62: 3031–3037 [DOI] [PubMed] [Google Scholar]
- Li W., Gill B. S., 2006. Multiple genetic pathways for seed shattering in the grasses. Funct. Integr. Genomics 6: 300–309 [DOI] [PubMed] [Google Scholar]
- Li W., Ahn I. P., Ning Y. S., Park C. H., Zeng L. R., et al. , 2012. The U-Box/ARM E3 ligase PUB13 regulates cell death, defense, and flowering time in Arabidopsis. Plant Physiol. 159: 239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wu S. Z., 1996. Traditional maintenance and multiplication of foxtail millet (Setaria italica (L) P. Beanv) landraces in China. Euphytica 87: 33–38 [Google Scholar]
- Lin Z. W., Li X. R., Shannon L. M., Yeh C. T., Wang M. L., et al. , 2012. Parallel domestication of the Shattering1 genes in cereals. Nat. Genet. 44: 720–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Platten D., Chaudhury A., Peacock W. J., Dennis E. S., 2009. Expression, imprinting, and evolution of rice homologs of the polycomb group genes. Mol Plant 2: 711–723 [DOI] [PubMed] [Google Scholar]
- Lynch M., Walsh B., 1998. Genetics and Analysis of Quantitative Traits. Sinauer, Sunderland, MA [Google Scholar]
- Lyons E., Freeling M., 2008. How to usefully compare homologous plant genes and chromosomes as DNA sequences. Plant J. 53: 661–673 [DOI] [PubMed] [Google Scholar]
- Mace E. S., Jordan D. R., 2011. Integrating sorghum whole genome sequence information with a compendium of sorghum QTL studies reveals uneven distribution of QTL and of gene-rich regions with significant implications for crop improvement. Theor. Appl. Genet. 123: 169–191 [DOI] [PubMed] [Google Scholar]
- Matsubara K., Kono I., Hori K., Nonoue Y., Ono N., et al. , 2008. Novel QTLs for photoperiodic flowering revealed by using reciprocal backcross inbred lines from crosses between japonica rice cultivars. Theor. Appl. Genet. 117: 935–945 [DOI] [PubMed] [Google Scholar]
- Matsubara K., Ogiso-Tanaka E., Hori K., Ebana K., Ando T., et al. , 2012. Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol. 53: 709–716 [DOI] [PubMed] [Google Scholar]
- Melzer S., Kampmann G., Chandler J., Apel K., 1999. FPF1 modulates the competence to flowering in Arabidopsis. Plant J. 18: 395–405 [DOI] [PubMed] [Google Scholar]
- Miller T. A., Muslin E. H., Dorweiler J. E., 2008. A maize CONSTANS-like gene, conz1, exhibits distinct diurnal expression patterns in varied photoperiods. Planta 227: 1377–1388 [DOI] [PubMed] [Google Scholar]
- Murakami M., Ashikari M., Miura K., Yamashino T., Mizuno T., 2003. The evolutionarily conserved OsPRR quintet: rice pseudo-response regulators implicated in circadian rhythm. Plant Cell Physiol. 44: 1229–1236 [DOI] [PubMed] [Google Scholar]
- Murakami M., Matsushika A., Ashikari M., Yamashino T., Mizuno T., 2005. Circadian-associated rice pseudo response regulators (OsPRRs): Insight into the control of flowering time. Biosci. Biotechnol. Biochem. 69: 410–414 [DOI] [PubMed] [Google Scholar]
- Murphy R. L., Klein R. R., Morishige D. T., Brady J. A., Rooney W. L., et al. , 2011. Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum. Proc. Natl. Acad. Sci. USA 108: 16469–16474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszynski M. G., Dam T., Li B., Shirbroun D. M., Hou Z. L., et al. , 2006. Delayed flowering1 encodes a basic leucine zipper protein that mediates floral inductive signals at the shoot apex in maize. Plant Physiol. 142: 1523–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Shimamoto K., Kyozuka J., 2002. Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER 1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. Plant J. 29: 743–750 [DOI] [PubMed] [Google Scholar]
- Neuffer M. G., Coe E. H., Wessler S. R., 1997. Mutants of Maize, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Noh B., Lee S. H., Kim H. J., Yi G., Shin E. A., et al. , 2004. Divergent roles of a pair of homologous jumonji/zinc-finger-class transcription factor proteins in the regulation of Arabidopsis flowering time. Plant Cell 16: 2601–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A. H., Lin Y. R., Li Z. K., Schertz K. F., Doebley J. F., et al. , 1995. Convergent domestication of cereal crops by independent mutations at corresponding genetic loci. Science 269: 1714–1718 [DOI] [PubMed] [Google Scholar]
- Paterson A. H., Bowers J. E., Chapman B. A., 2004. Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proc. Natl. Acad. Sci. USA 101: 9903–9908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L. T., Shi Z. Y., Li L., Shen G. Z., Zhang J. L., 2007. Ectopic expression of OsLFL1 in rice represses Ehdl by binding on its promoter. Biochem. Biophys. Res. Commun. 360: 251–256 [DOI] [PubMed] [Google Scholar]
- Peng L. T., Shi Z. Y., Li L., Shen G. Z., Zhang J. L., 2008. Overexpression of transcription factor OsLFL1 delays flowering time in Oryza sativa. J. Plant Physiol. 165: 876–885 [DOI] [PubMed] [Google Scholar]
- Punta M., Coggill P. C., Eberhardt R. Y., Mistry J., Tate J., et al. , 2012. The Pfam protein families database. Nucleic Acids Res. 40: D290–D301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterill J., Robson F., Lee K., Simon R., Coupland G., 1995. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc-finger transcription factors. Cell 80: 847–857 [DOI] [PubMed] [Google Scholar]
- Robertson G. W., 1968. A biometeorological time scale for a cereal crop involving day and night temperatures and photoperiod. Int. J. Biometeorol. 12: 191–223 [Google Scholar]
- Salvi S., Sponza G., Morgante M., Tomes D., Niu X., et al. , 2007. Conserved noncoding genomic sequences associated with a flowering-time quantitative trait locus m maize. Proc. Natl. Acad. Sci. USA 104: 11376–11381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S., Castelletti S., Tuberosa R., 2009. An updated consensus map for flowering time QTLs in maize. Maydica 54: 501–512 [Google Scholar]
- Schnable J. C., Lyons E., 2011. Comparative genomics with maize and other grasses: from genes to genomes! Maydica 56: 183–199 [Google Scholar]
- Schomburg F. M., Patton D. A., Meinke D. W., Amasino R. M., 2001. FPA, a gene involved in floral induction in Arabidopsis, encodes a protein containing RNA-recognition motifs. Plant Cell 13: 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M. M., Oliver L. R., 1971. 2 new varieties of Setaria viridis. Weed Sci. 19: 424 [Google Scholar]
- Schwarz S., Grande A. V., Bujdoso N., Saedler H., Huijser P., 2008. The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Mol. Biol. 67: 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano G., Herrera-Palau R., Romero J. M., Serrano A., Coupland G., et al. , 2009. Chlamydomonas CONSTANS and the evolution of plant photoperiodic signaling. Curr. Biol. 19: 359–368 [DOI] [PubMed] [Google Scholar]
- Shannon S., Meekswagner D. R., 1991. A mutation in the Arabidopsis Tfl1 gene affects inflorescence meristem development. Plant Cell 3: 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo C., Aranzana M. J., Lister C., Baxter C., Nicholls C., et al. , 2005. Role of FRIGIDA and FLOWERING LOCUS C in determining variation in flowering time of Arabidopsis. Plant Physiol. 138: 1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smykal P., Gleissner R., Corbesier L., Apel K., Melzer S., 2004. Modulation of flowering responses in different Nicotiana varieties. Plant Mol. Biol. 55: 253–262 [DOI] [PubMed] [Google Scholar]
- Song Y. H., Ito S., Imaizumi T., 2010. Similarities in the circadian clock and photoperiodism in plants. Curr. Opin. Plant Biol. 13: 594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigonova Z., Lai J. S., Ma J. X., Ramakrishna W., Llaca V., et al. , 2004. Close split of sorghum and maize genome progenitors. Genome Res. 14: 1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Matsuo S., Wong H. L., Yokoi S., Shimamoto K., 2007. Hd3a protein is a mobile flowering signal in rice. Science 316: 1033–1036 [DOI] [PubMed] [Google Scholar]
- Tang H., Lyons E., 2012. Unleashing the genome of Brassica rapa. Front Plant Sci 3: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry J. M., Goodman M. M., Doebley J., Kresovich S., Nielsen D., et al. , 2001. Dwarf8 polymorphisms associate with variation in flowering time. Nat. Genet. 28: 286–289 [DOI] [PubMed] [Google Scholar]
- Trevaskis B., Hemming M. N., Dennis E. S., Peacock W. J., 2007. The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci. 12: 352–357 [DOI] [PubMed] [Google Scholar]
- UniProt-Consortium , 2012. Reorganizing the protein space at the Universal Protein Resource (UniProt). Nucleic Acids Res. 40: D71–D75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F., 2011. CONSTANS and the evolutionary origin of photoperiodic timing of flowering. J. Exp. Bot. 62: 2453–2463 [DOI] [PubMed] [Google Scholar]
- Van Ooijen J. W., 2006. JoinMap 4, Software for the Calculation Of Genetic Linkage Maps In Experimental Populations, edited by van Ooijen J. W. B. V. Kyazma, Wageningen, Netherlands [Google Scholar]
- Wang Z. M., Devos K. M., Liu C. J., Wang R. Q., Gale M. D., 1998. Construction of RFLP-based maps of foxtail millet, Setaria italica (L.) P. Beauv. Theor. Appl. Genet. 96: 31–36 [Google Scholar]
- Wang J. W., Czech B., Weigel D., 2009. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138: 738–749 [DOI] [PubMed] [Google Scholar]
- Wang C. L., Chen Y. H., Ku L. X., Wang T. G., Sun Z. H., et al. , 2010. Mapping QTL associated with photoperiod sensitivity and assessing the importance of QTL × environment interaction for flowering time in maize. PLoS ONE 5: e14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Basten C. J., Zeng Z. B., 2011. Windows QTL Cartographer 2.5. North Carolina State University, Raleigh, NC [Google Scholar]
- Wenkel S., Turck F., Singer K., Gissot L., Le Gourrierec J., et al. , 2006. CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 18: 2971–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Poethig R. S., 2006. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133: 3539–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. F., Wang Y., Wu S. H., 2008. Two new clock proteins, LWD1 and LWD2, regulate Arabidopsis photoperiodic flowering. Plant Physiol. 148: 948–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K. B., Wu C. Q., Xiong L. Z., 2006. Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol. 142: 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Liu Y. X., Liu J., Cao M. J., Wang J., et al. , 2012. The genetic architecture of flowering time and photoperiod sensitivity in maize as revealed by QTL review and meta analysis. J. Integr. Plant Biol. 54: 358–373 [DOI] [PubMed] [Google Scholar]
- Xue W. Y., Xing Y. Z., Weng X. Y., Zhao Y., Tang W. J., et al. , 2008. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 40: 761–767 [DOI] [PubMed] [Google Scholar]
- Yan L., Loukoianov A., Tranquilli G., Helguera M., Fahima T., et al. , 2003. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 100: 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L. L., Loukoianov A., Blechl A., Tranquilli G., Ramakrishna W., et al. , 2004. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Fu D., Li C., Blechl A., Tranquilli G., et al. , 2006. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 103: 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Peng Q., Chen G. X., Li X. H., Wu C. Y., 2012. OsELF3 is involved in circadian clock regulation for promoting flowering under long-day conditions in rice. Mol. Plant. doi:10.1093/mp/sss062 [DOI] [PubMed] [Google Scholar]
- Yano M., Katayose Y., Ashikari M., Yamanouchi U., Monna L., et al. , 2000. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the arabidopsis flowering time gene CONSTANS. Plant Cell 12: 2473–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky M. J., Kay S. A., 2002. Molecular basis of seasonal time measurement in Arabidopsis. Nature 419: 308–312 [DOI] [PubMed] [Google Scholar]
- Yant L., Mathieu J., Dinh T. T., Ott F., Lanz C., et al. , 2010. Orchestration of the floral transition and floral development in arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22: 2156–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Dhillon S., Ke X. Y., Collins A. R., Day I. N. M., 2001. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 29: e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z. B., 1994. Precision mapping of quantitative trait loci. Genetics 136: 1457–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. Y., Liu X., Quan Z. W., Cheng S. F., Xu X., et al. , 2012. Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat. Biotechnol. 30: 549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.