Abstract
Recent studies have introduced the importance of Transient Receptor Potential Vanilloid Subtype 4 (TRPV4) channels in the regulation of vascular tone. TRPV4 channels are expressed in both endothelium and vascular smooth muscle cells and can be activated by numerous stimuli including mechanical (e.g. shear stress, cell swelling, and heat) and chemical (e.g. epoxyeicosatrienoic acids (EETs), endocanabinoids, 4α-phorbol esters). In the brain, TRPV4 channels are primarily localized to astrocytic endfeet processes which wrap around blood vessels. Thus, TRPV4 channels are strategically localized to sense hemodynamic changes and contribute to the regulation of vascular tone. TRPV4 channel activation leads to smooth muscle cell hyperpolarization and vasodilation. Here we review recent findings on the cellular mechanisms underlying TRPV4-mediated vasodilation, TRPV4 channel interaction with other proteins including Transient Receptor Potential Channel 1 (TRPC1), small conductance (KCa2.3) and large conductance (KCa1.1) calcium-activated, potassium-selective channels and the importance of caveolin-rich domains for these interactions to take place.
Introduction
Transient receptor potential (TRP) channels are non-selective cation channels expressed in almost all cells and permeable to Ca2+and Na+ions. The TRP channel superfamily is divided according to DNA and protein sequence homology 1; while this superfamily encompasses a large number of channels, the purpose of this review is to highlight recent findings on the role of TRPV4 channels in the regulation of vascular tone. TRPV4 channels are members of the vanilloid receptor subfamily and expressed in various tissues including lung, spleen, heart, endothelium, cochlear, liver, testes, fat and brain 2-5. TRPV4 channel currents carry Ca2+and Mg2+with permeability ratios of 6-10 PCa/PNaand 2-3 PMg/PNa, respectively 6-9 . The single channel conductance for TRPV4 channels is in the range of 90-100 pS (outward currents) and 50-60 pS (inward currents) 7, 9, 10.
At the structural level, the TRPV4 channel has six transmembrane spanning segments (TM1-6) with the pore region located between TM5 and TM6 10. The protein has 871 amino acids with intracellular C- and N-termini 10. Four TRPV4 subunits are needed to assemble a functional channel 10with part of its volume (~ 30%) in the plasma membrane and the rest (~70%) exposed intracellularly or extracellularly, allowing interactions with associated proteins 11. These include inositol trisphosphate (IP3) receptors 12, actin filaments 13, 14, microtubule-associated protein (MAP) 7 15, aquaporin (AQP) 5 16and AQP4 17, human osteosarcoma (OS) 9 18, transient receptor potential polycystic (TRPP) 2 19, caveolin 1 20, Cystic fibrosis transmembrane conductance regulator (CFTR) 21 and large conductance Ca2+activated K+channels (BK or K 1.1) 22, 23 Ca .
Functionally, TRPV4 channels stand out due to the broad range of stimuli that lead to their activation, including physical (cell swelling 5, heat 9, 24, mechanical 25) and chemical stimuli (endocannabinoids, arachidonic acid (AA), and 4-α-phorbol esters 26, 9). Table 1 and 2 provide a pharmacological overview of TRPV4 agonists and antagonists. Activation by swelling and endocanabinoids involves cytochrome P450 epoxygenase-dependent AA metabolism to epoxyeicosatrienoic acids (EETs) 26, 27, potent endogenous agonists for TRPV4 channels 2, 5, 22, 28-30. EETs cause vascular smooth muscle cell hyperpolarization, leading to vascular relaxation. The effect of EETs on smooth muscle cell hyperpolarization persists when the production of nitric oxide (NO) and prostacyclin are inhibited 31. Thus, EETs are commonly referred to as one of the endothelium-derived hyperpolarizing factors (EDHFs). The role of EETs is especially important in some key vascular beds, including the coronary circulation 31. EETs may directly bind to TRPV4 to exert their action. A putative arachidonate recognition site, where EETs could bind, is located at N-terminal cytoplasmic domain of TRPV4 4. However, there are some controversies regarding EET regioisomer selectivity for TRPV4. 11,12-EET and 14,15-EET are two predominant endogenous EET isoforms 32. However, one report showed that 5,6-EET and 8,9-EET but not 11,12-EET and 14,15-EET activate TRPV4 in TRPV4-overexpressing HEK293 cells 27. In contrast, several other studies demonstrated that 11,12-EET and 14,15-EET are able to activate TRPV4 in native smooth muscles 26, 30.
Table 1. Data summary for TRPV4 agonists (a).
| Agonist |
Potency
(IC50, μM) |
Species | Cross-reactivity |
|---|---|---|---|
| 4α-PDD | 0.16-0.92 0.16-0.93 4.4 |
human mouse rat |
--- |
| 5,6-EET, 8,9-EET,11,12- EET |
0.15 for 5,6-EET | human mouse Rat |
activates G protein coupled receptor |
| Bisandrographolide | 0.79-0.95 | mouse | -- |
| RN-1747 | 0.77 4.0 4.1 |
human mouse rat |
activates TRPV1 at 100 μM, inhibits TRPM8 (IC50=4μM) |
| GSK-1016790A | 0.003-0.005 0.0185 0.010 |
human mouse rat |
activates TRPV1 (EC50=5nM) |
Table 2. Data summary for TRPV4 antagonists (a).
| Antagonist |
Potency
(IC50, μM) |
Species | Cross-reactivity |
|---|---|---|---|
| RN-1734 | 2.3 5.9 3.2 |
human mouse rat |
--- |
| RN-9893 | <0.12 <0.06 <0.12 |
human mouse rat |
--- |
| HC-067047 | 0.048 0.017 0.133 |
human mouse rat |
inhibits TRPM8, HERG at submicromolar |
| Ruthenium Red | <0.086-1 <0.21-1 <0.2-0.33 |
human mouse rat |
inhibits all TRPVs, TRPM6, TRPM8, TRPA1, RyR1-3 |
| Capsazepine | 18.6 13.5 |
human rat |
Inhibits TRPV1, TRPM8 |
| Citral | 32 | mouse | Inhibits TRPA1, activates TRPV1, TRPV3, TRPM8 |
TRPV4 is unequivocally important for the regulation of vascular tone. However, the underlying molecular mechanisms remain unclear. Here, we describe recent advances on the role of TRPV4 channels in the peripheral circulation as well as the cerebral circulation, where TRPV4 channel expression is prominent in astrocytes.
TRPV4 is expressed in vascular smooth muscle cells
Immunostaining, Western blot and reverse transcription-polymerase chain reaction (RTPCR) showed the expression of TRPV4 in smooth muscle cells of rat cerebral arteries 33, smooth muscle of human and rat lung extraalveolar vessels 26, endothelium-denuded rat intralobar pulmonary arteries 34, 35, rat mesenteric artery smooth muscle cells 36, and rat and mouse aortic smooth muscle cells 37.
Dependent on the vascular bed and animal species, EET may act on TRPV4 channels expressed either in vascular smooth muscle cells, endothelial cells or both 31. In rat cerebral arteries, the endothelium-derived EETs diffuse to nearby smooth muscle cells, activating TRPV4 in smooth muscle cells 22. Resultant Ca2+entry stimulates Ca2+release from ryanodine receptors, causing an increased frequency of Ca2+sparks 22. The Ca2+sparks in turn activate KCa1.1 to hyperpolarize vascular smooth muscle cells, leading to vascular relaxation 22. A 11,12 EET- and 4α-PDD-activated TRPV4-like current was recorded in smooth muscle cells of mouse small mesenteric arteries. The current was absent in myocytes from TRPV4 knockout mice. EETs, via their action on smooth muscle TRPV4, were also found to induce smooth muscle hyperpolarization and vascular relaxation in mouse mesenteric arteries, the effect of which was absent in TRPV4 knockout mice 30. Endothelial disruption only caused a moderate reduction in 11,12-EET-induced smooth muscle hyperpolarization and vascular relaxation (by ~50%) in these arteries 30. Thus, the authors reasoned that the remaining 50% was endothelium-independent and could be attributed to direct EET action on smooth muscle TRPV4. In agreement, the authors also found that inhibiting KCa1.1 in smooth muscle cells could reduce the EET-induced responses by ~50%, further supporting the notion that the endothelium-independent component was ~50%. These data suggest a link between EET, smooth muscle TRPV4 and KCa1.1 in mouse mesenteric arteries. Because EETs can be produced by endothelial cells in response to physiological stimuli such as bradykinin, acetylcholine, pulsatile stretch and shear stress, the functional coupling of smooth muscle TRPV4 with KCa1.1 may play a major role in vascular tone control under different physiological conditions.
Up to the present, the function of smooth muscle TRPV4 has only been reported in rat cerebral arteries and mouse mesenteric arteries 22, 30. However, it is likely that a similar mechanism exists in other vascular beds. It is well documented that, in a great variety of artery types, EETs stimulate the activity of KCa1.1 channels in smooth muscle cells causing smooth muscle hyperpolarization and vascular relaxation 31. This mechanism has been documented in mouse skeletal arteries, human internal mammary arteries and coronary arteries from many species 31, 32. However, EETs do not directly act on KCa1.1 channels. Thus, the well-characterized EET-TRPV4-KCa1.1 axis may provide an attractive mechanistic explanation for smooth muscle relaxation in these arteries.
Recent studies found that TRPV4 may heteromerize with TRPC1 or TRPP2 to form heteromeric channels in vascular endothelial cells and renal cortical collecting duct cells 38, 39. TRPC1 is ubiquitously expressed in many cell types including vascular smooth muscle cells from many arteries 37. TRPP2 expression has also been identified in some artery types 37. In the future, it will be important to determine whether heteromeric TRPV4-C1 and/or TRPV4-P2 exist in vascular smooth muscle cells and whether EETs act on homomeric or heteromeric TRPV4 to initiate hyperpolarizing responses in vascular smooth muscle cells. Interestingly, studies have shown that TRPC1 and KCa1.1 can form a physical complex in vascular smooth muscle cells 40and that the complex plays an important role in smooth muscle hyperpolarization and the control of vascular tone 40. Based on this evidence, it is reasonable to propose the existence of a TRPV4-TRPC1-KCa1.1 complex in vascular smooth muscle cells. EETs may act on this complex to induce smooth muscle hyperpolarization and vascular relaxation.
TRPV4 is expressed in endothelial cells
In the endothelium, TRPV4 was first identified in mouse aorta by Bernd Nilius’s group, 7and since then, it has been shown to be ubiquitously expressed in endothelial cells of both large conductance vessels and small resistance vessels. Indeed, RT-PCR, western blot analysis and intracellular calcium measurements demonstrate that TRPV4 is functionally expressed in mouse aortic endothelial cells 29. Köhler’s group investigated the expression and function of TRPV4 in rat carotid artery and arteria gracilis endothelial cells by using in situ patch-clamp techniques, single-cell RT-PCR and pressure myography, 41whereas Alvarez and coworkers studied TRPV4 in rat pulmonary artery and microvascular endothelium 26. More recently, TRPV4 localization was examined by Willette et al.42in a variety of rat tissues, and a generalized pattern of immunoreactive TRPV4 staining was identified in the endothelium and epithelium 42.
From a functional perspective, as shown by Zhang et al. 43, acetylcholine-induced nitric oxide (NO) production was significantly reduced in vascular endothelial cells and EDHF-mediated relaxation was also attenuated in small mesenteric arteries of TRPV4 knockout mice. These results are in agreement with previous data from Köhler et al.44, showing that in large vessels, like carotid arteries, the inhibition of nitric oxide synthase almost completely abolished 4α-PDD induced vasodilation whereas in small vessels selective inhibition of calcium activated potassium channels (SKCa/KCa2.3 and IKCa/KCa3.1) inhibited the TRPV4-induced vasodilation. Very recently, Sonkusare et al. 45 demonstrated that even a small number of active TRPV4 channels were able to mediate local calcium signals that activated IK and SK channels and induced maximal dilation of resistance arteries, thereby contributing to the regulation of vascular function 45. Thus intracellular calcium increases mediated by TRPV4 channels trigger both NO-and/or EDHF-dependent vasodilatation, an effect that appears to be dependent on the vascular bed. Interestingly, in several cell types including endothelial and smooth muscle cells, calcium handling proteins are located in caveolae. KCa2.3 46and K 1.1 47 Ca channels have been shown to reside in caveolin-rich lipid domains. Direct measurement of calcium waves in endothelial cells have suggested that caveolae could be the sites that initiate calcium entry and calcium dependent signal transduction 48. Recent data demonstrate that, similar to TRPC1 49, TRPV4 may interact physically with the structural caveolar protein caveolin-1 and that the interaction is functionally important for 4α-PDD-evoked calcium increase 20. The fact that TRPV4 may heteromerize with TRPC1 39, as well as the work of Graziani and coworkers showing that caveolar integrity is essential for AA recruitment and EDHF signaling in porcine arteries 50, provides additional evidence in favor of a potential involvement of caveolar microdomains in TRP-mediated calcium signaling and subsequent vasodilation.
TRPV4 expression in astrocytes
In the brain, TRPV4 mRNA is expressed in both neuronal and non-neuronal cell types including astrocytes and microglia 51-54, endothelial cells and vascular smooth muscle cells 33. Importantly, and relevant to the control of vascular tone, Marrelli et al 33showed TRPV4 channel expression in endothelial cells of middle cerebral arteries and demonstrated its regulation by PLA2activation. Similar to the potential polarized expression of TRPV4 channels in the abluminal face of the endothelium 33, Benfenati et al. 55reported that expression of TRPV4 channels is localized mostly to astrocytic membranes at the interface between brain parenchyma and extracerebral liquid spaces and on astrocytic endfeet abutting pial and parenchymal blood vessels 55. The unique arrangement of TRPV4 channels on perivascular astrocyte processes was also reported by Butenko et al. 56 in the rat hippocampal CA1 region. Given that cell-cell communication by astrocytes is primarily mediated through dynamic intracellular Ca2+changes, information regarding the activity of TRPV4 channels in astrocytes is important to further our understanding of the physiological function of these cells in the CNS.
Moreover, the important observation that astrocytic endfeet processes possess parallel vasoactive mechanisms to those described in vascular cells, particularly endothelial cells, supports the notion that cerebral vascular smooth muscle cells are modulated from their luminal and abluminal sides by both endothelial cell and astrocyte signaling pathways, respectively. As with endothelial cells, astrocytes modulate vascular tone through K+signaling 57and via the release of AA metabolites such as EETs 58-60, 20-HETEs 61 and prostacyclin 62, 63. The resemblance of these vasoactive pathways to those described in vascular cells, in addition to the expression of TRPV4 channels in astrocytic endfeet processes at the gliovascular interface, points to the possibility that astrocytic TRPV4 channels are also involved in the regulation of vascular tone. In response to neuronal activity, glutamate released at the synapse activates metabotropic glutamate receptors (mGluR) in astrocytes leading to an increase in intracellular Ca2+which in turn activates PLA264. The resulting production of AA and its metabolism follows similar pathways to those described in endothelial cells (e.g. conversion to metabolites such EETs). As described above, EETs are endogenous activators of TRPV4 channels. Blanco et al. 65 showed that 11, 12 EET increased Ca2+oscillations in cortical astrocytes. The study, however, did not evaluate whether these Ca2+responses in astrocytes were indeed mediated via TRPV4 channel activation. mGluR-induced increases in Ca2+also has been shown to increase the single channel open probability of BK channels expressed in astrocytic endfeet processes 65, 57. Depending on the K+concentration released, efflux of K+from astrocytes results in vasodilation 57, 66 or vasoconstriction 66.
To date, TRPV4 channel function in astrocytes is associated with osmosensation, thus playing an important role in the maintenance of brain volume 6717 as achieved through the activity of AQP4 channels expressed in endfeet processes 17, 68, 69. It has been demonstrated that AQP4 channels colocalize with TRPV4 channels in astrocytic endfeet, providing evidence for their co-participation in regulatory volume decrease 17.
Although a role for astrocytic TRPV4 channels has yet to be demonstrated in the control of vascular tone, given their association with K+channels (also preferentially expressed in astrocytic endfeet processes 65, 70), it is tempting to speculate that the activation of TRPV4 channels in astrocytes contributes to K+channel signaling and neurovascular coupling. Along these lines, Higashimori et al.71showed that the synthetic EET analog 11-nonyloxy-undec-8(Z)-enoic acid or the mGluR agonist,t-ACPD significantly increased KCa1.1 channel currents in perivascular astrocytes. EETs-induced outward currents were also associated with an increase in the frequency of Ca2+oscillations in astrocytes, supporting the idea that EETs-induced intracellular Ca2+changes contribute to K+signaling in astrocytes 71and likely the control of vascular tone 57.
In addition to EETs, glutamate-mediated activation of mGluR in astrocytes results in the release a number of vasoactive signals (i.e. NO, ATP, adenosine), which could also contribute to astrocyte TRPV4 channel regulation. Among them, NO is of particular interest. TRPV4 channel activation is linked to NO production 43, 72, which in turn can lead to sustained increases in astrocyte Ca2+levels 73. A recent study showed that TRVP4 channel activation resulted in endothelial Ca2+increase and NO-mediated vasodilation 41. In addition, NO has been shown to induce sustained increases in astrocytic Ca2+in cultured astrocytes 73. Based on these studies and the fact that NO can readily cross the blood brain barrier and alter astrocyte 73and neuronal activity 74, another unresolved role for TRPV4 channels in the control of vascular tone may be linked to NO signaling. Thus, given their distinctive molecular and biophysical properties, their strategic expression within the neurovascular unit and their ability to respond to a variety of vascular- and glial-derived signals in the brain, TRPV4 channels expressed in astrocytes may be regarded as ideal candidates to sense and/or transduce hemodynamic information into a glial response (changes in intracellular calcium). Comparable to our current knowledge on TRPV4-channel induced activation in the endothelium and vascular smooth muscle cells, additional work is needed to determine whether astrocytic TRPV4 channels contribute to the regulation of vascular tone via similar mechanisms.
TRPV4 expression in perivascular nerves
TRPV4 channels have shown to be expressed in sensory nerves and to co-localize with calcitonin gene-related peptide (CGRP) as well as substance P 75, 76. Gao and Wang showed that the depressor effect of the TRPV4 channel, 4αPDD was attenuated following degeneration of capsaicin-sensitive sensory nerves or in the presence of CGRP8-37(an antagonist of CGRP). Moreover, they showed that intravenous administration of 4αPDD increased plasma CGRP; the hypotensive effect of TRPV4 channel activation was, at least in part, mediated by the activation of Ca2+-activated K+channels 36. Using a model of baroreflex impairment, McHugh et al. showed a role for TRPV4 channels as osmosensors in the portal region and their potential participation in the afferent input of the pressor response 77. The authors suggested that spinal afferents may relay information from the hepatic/portal environment to dorsal root ganglion neurons which express TRPV4 channels 78, 79resulting blood pressure regulation through sympathetic output77.
TRPV4 in disease
Although TRPV4 channels appear to have an important role in the regulation of vascular tone, TRPV4−/− mice do not show altered blood pressure at rest 30. Earley et al., suggested the participation of TRPV4 channels in a negative feedback mechanism which opposes hypertension in the presence of a hypertensive challenge 30, 43, 80. Using the synthetic TRPV4 activator, GSK1016790A, Willette et al provided evidence that circulatory collapse induced by exogenous TRPV4 activation is mediated by a NO-independent failure of the endothelial-epithelial permeability barrier in the lung and other tissues 42. Impaired pressure and stretch sensing has been reported in C-fibers of the dorsal root ganglia 15and retinal ganglion cells 81, respectively, in TRPV4−/− mice. Several reports suggest that TRPV4 channels are likely involved in hypoxia-induced pathogenesis. Following cerebral hypoxia/ischemia, TRPV4 channel expression is increased in hippocampal astrocytes resulting in augmented astrocytic Ca2+oscillatory frequency and possibly astroglial reactivity in the brain 56. In chronic hypoxic pulmonary hypertension, Yang and coworkers identified TRPV4 channels as an obligatory calcium entry pathway that is upregulated 35. In mouse mesenteric arteries, TRPV4 activity is favored by hypoxic insult that is associated with an increased Ca2+response in endothelial cells upon agonist stimulation, contributing to a potentiated EDH-mediated dilation.
As flow-activated channels in vascular endothelial cells, TRPV4 are good candidates for shear stress activation and, consequently, have been investigated in different models of arteriogenesis. In rats, after femoral artery ligation, TRPV4 participates in collateral remodeling and growth 82. The same group also provided evidence that pharmacological TRPV4 activation enhanced cerebral arteriogenesis83. TRPV4 channel activation has been associated with pulmonary hypertension 84, bone disorders 85, neurodegenerative skeletal muscle dysplasias 86 and hyponatremia 87, 88to name a few.
Summary and Perspectives
In summary, current studies suggest that activity of TRPV4 channels in endothelial and vascular smooth muscle cells contribute to the regulation of vascular tone. Importantly, TRPV4-induced Ca2+increases in endothelial and vascular smooth muscle cells contribute to vasodilation. The broad range of stimuli activating TRPV4 channels, along with their strategic location in the endothelium, favors flow and shear-stress mediated release of EDHF and vasodilation. Moreover, recent studies have shed light on the interaction between TRPV4 channels and SKCaand IKCa, suggesting a key cellular mechanism by which TRPV4-mediated Ca2+increases in endothelial cells induce vasodilatory responses 45. The structural arrangement of TRPV4 channels allows for interaction with a number of proteins including K+channels and other members of the TRP channel family (e.g TRPC1). Particular interest has also been placed on caveolin, as several calcium handling proteins from endothelial and smooth muscle cells reside in caveolae (e.g. KCa2.3 46and KCa1.1 47channels); the interaction between TRPV4 and caveolin-1 appear to be an important component of 4α-PDD-evoked calcium increase 20.
Studies have demonstrated the importance of TRPV4 channels expressed in vascular smooth muscle cells as mediators of vasodilation via EET. TRPV4-induced Ca2+increases lead to Ca2+sparks and subsequent activation of KCa1.1 channels, causing, in turn, smooth muscle hyperpolarization and vasodilation 22. In addition, TRPV4 channel interaction with other proteins such as the TRPV4-TRPC1-KCa1.1 complex in vascular smooth muscle cells may prove to be yet another mechanism for smooth muscle hyperpolarization and vascular relaxation.
Clearly, the wide expression of TRPV4 channels in various tissues along with the broad range of stimuli which can activate them, give rise to a multiplicity of mechanisms and pathologies associated with TRPV4 channel dysregulation. TRPV4 channels, thus, may represent a novel pharmacotherapeutic target in a wide range of diseases.
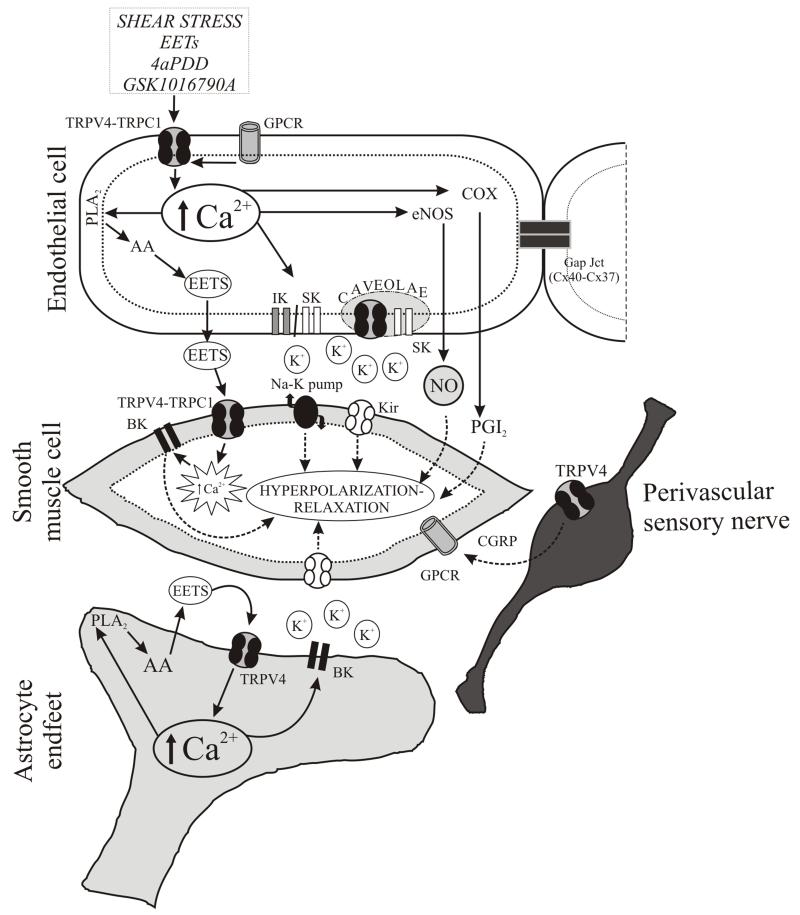
Figure 1. Contribution of TRPV4 channels to the regulation of vascular tone.
As shown, heteromeric TRPV4-TRPC1 channels expressed in endothelial cells can be activated by shear stress, agonists (4αPDD, GSK1016790A) and epoxyeicosatrienoic acids (EETs) resulting in an increase in intracellular Ca2+and the release of various vasoactive substances such as EETs, nitric oxide (NO) and prostaglandin (PGI2) leading to vasodilation. In addition, TRPV4 channels in caveolae interact with small conductance potassium channels (SK) contributing to the release of K+from endothelial cells. In smooth muscle cells, K+-induced hyperpolarization is mediated through the activation of the Na/K pump as well as inwardly rectifying potassium channels (Kir). TRPV4-TRPC1 channels in smooth muscle cells are activated by EETs which trigger Ca2+sparks from ryanodine receptors and the subsequent activation of large conductance calcium-activated potassium-selective channels (BK) resulting in smooth muscle cell hyperpolarization and vasodilation. In cerebral parenchymal arterioles, the abluminal side of the vessel is surrounded by astrocytic endfeet processes which also modulate vascular tone. Glutamate-mediated rise in intracellular Ca2+leads to vasodilation through activation of KCa1.1 channels, K+release and activation of Kir channels in smooth muscle cells. The rise in intracellular Ca2+stimulates phospholipase A2(PLA2) and mobilizes arachidonic acid (AA) which then is metabolized to form EETs (among other signals); EETs released at the gliovascular interface activates TRPV4 channels in astrocytic endfeet processes further contributing to the rise in intracellular Ca2+and K+channel signaling. In perivascular nerves, TRPV4 channel activation has been associated with the release of calcitonin gene-related peptide (CGRP) activation of G-protein coupled receptors (GPCR) in smooth muscle cells and vasodilation.
Acknowledgments
The authors thank Jennifer Iddings for comments on the manuscript. This paper was supported by grants from the National Heart, Lung and Blood Institute (R01 HL089067-02 to JAF), Hong Kong Research Grant Council (TBRS T13-706/11) and China National Science Foundation (31171100) to XY, and Action Recherche Concertée 06/11339, Fond de Recherche Scientifique Medicale 3.4547.03; 3.4.555.08F to GR.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 2.Plant TD, Strotmann R. Trpv4. Handb Exp Pharmacol. 2007;(179):189–205. doi: 10.1007/978-3-540-34891-7_11. [DOI] [PubMed] [Google Scholar]
- 3.Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286(2):C195–205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 5.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101(1):396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bodding M, Droogmans G, Nilius B. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002;277(37):33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277(16):13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H, Vriens J, Janssens A, Wondergem R, Droogmans G, Nilius B. Modulation of TRPV4 gating by intra- and extracellular Ca2+ Cell Calcium. 2003;33(5-6):489–495. doi: 10.1016/s0143-4160(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277(49):47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 10.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog Biophys Mol Biol. 2010;103(1):2–17. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Verma P, Kumar A, Goswami C. TRPV4-mediated channelopathies. Channels (Austin) 2010;4(4):319–328. doi: 10.4161/chan.4.4.12905. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Elias A, Lorenzo IM, Vicente R, Valverde MA. IP3 receptor binds to and sensitizes TRPV4 channel to osmotic stimuli via a calmodulin-binding site. J Biol Chem. 2008;283(46):31284–31288. doi: 10.1074/jbc.C800184200. [DOI] [PubMed] [Google Scholar]
- 13.Becker D, Muller M, Leuner K, Jendrach M. The C-terminal domain of TRPV4 is essential for plasma membrane localization. Mol Membr Biol. 2008;25(2):139–151. doi: 10.1080/09687680701635237. [DOI] [PubMed] [Google Scholar]
- 14.Ramadass R, Becker D, Jendrach M, Bereiter-Hahn J. Spectrally and spatially resolved fluorescence lifetime imaging in living cells: TRPV4-microfilament interactions. Arch Biochem Biophys. 2007;463(1):27–36. doi: 10.1016/j.abb.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278(25):22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Bandyopadhyay BC, Nakamoto T, Singh B, Liedtke W, Melvin JE, Ambudkar I. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem. 2006;281(22):15485–15495. doi: 10.1074/jbc.M600549200. [DOI] [PubMed] [Google Scholar]
- 17.Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP, Amiry-Moghaddam M. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci U S A. 2011;108(6):2563–2568. doi: 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Fu X, Gaiser S, Kottgen M, Kramer-Zucker A, Walz G, Wegierski T. OS-9 regulates the transit and polyubiquitination of TRPV4 in the endoplasmic reticulum. J Biol Chem. 2007;282(50):36561–36570. doi: 10.1074/jbc.M703903200. [DOI] [PubMed] [Google Scholar]
- 19.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182(3):437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation. 2008;117(8):1065–1074. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- 21.Arniges M, Vazquez E, Fernandez-Fernandez JM, Valverde MA. Swelling-activated Ca2+ entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J Biol Chem. 2004;279(52):54062–54068. doi: 10.1074/jbc.M409708200. [DOI] [PubMed] [Google Scholar]
- 22.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97(12):1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Fernandez JM, Andrade YN, Arniges M, Fernandes J, Plata C, Rubio-Moscardo F, Vazquez E, Valverde MA. Functional coupling of TRPV4 cationic channel and large conductance, calcium-dependent potassium channel in human bronchial epithelial cell lines. Pflugers Arch. 2008;457(1):149–159. doi: 10.1007/s00424-008-0516-3. [DOI] [PubMed] [Google Scholar]
- 24.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22(15):6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451(1):193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res. 2006;99(9):988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 28.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008;80(3):445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 29.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97(9):908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 30.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol. 2009;297(3):H1096–1102. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459(6):881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.eletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117(4):139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- 33.Marrelli SP, O’Neil RG, Brown RC, Bryan RM., Jr. PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;292(3):H1390–1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- 34.Martin E, Dahan D, Cardouat G, Gillibert-Duplantier J, Marthan R, Savineau JP, Ducret T. Involvement of TRPV1 and TRPV4 channels in migration of rat pulmonary arterial smooth muscle cells. Pflugers Arch. 2012;464(3):261–272. doi: 10.1007/s00424-012-1136-5. [DOI] [PubMed] [Google Scholar]
- 35.Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, Sham JS. Upregulation of osmomechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302(6):L555–568. doi: 10.1152/ajplung.00005.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao F, Wang DH. Hypotension induced by activation of the transient receptor potential vanilloid 4 channels: role of Ca2+-activated K+ channels and sensory nerves. J Hypertens. 2010;28(1):102–110. doi: 10.1097/HJH.0b013e328332b865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther. 2008;118(3):337–351. doi: 10.1016/j.pharmthera.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Du J, Wong WY, Sun L, Huang Y, Yao X. Protein Kinase G Inhibits Flow-Induced Ca2+ Entry into Collecting Duct Cells. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2011100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, Wong CO, Huang Y, Yao X. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol. 2010;30(4):851–858. doi: 10.1161/ATVBAHA.109.196584. [DOI] [PubMed] [Google Scholar]
- 40.Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, Yu S, Yao X. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104(5):670–678. doi: 10.1161/CIRCRESAHA.108.188748. [DOI] [PubMed] [Google Scholar]
- 41.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26(7):1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 42.Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther. 2008;326(2):443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- 43.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension. 2009;53(3):532–538. doi: 10.1161/HYPERTENSIONAHA.108.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohler R, Hoyer J. Role of TRPV4 in the Mechanotransduction of Shear Stress in Endothelial Cells. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. 2007. Chap 27. [PubMed] [Google Scholar]
- 45.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336(6081):597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SK(Ca) channels and caveolin-rich domains. Br J Pharmacol. 2007;151(3):332–340. doi: 10.1038/sj.bjp.0707222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riddle MA, Hughes JM, Walker BR. Role of caveolin-1 in endothelial BKCa channel regulation of vasoreactivity. Am J Physiol Cell Physiol. 2011;301(6):C1404–1414. doi: 10.1152/ajpcell.00013.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isshiki M, Anderson RG. Function of caveolae in Ca2+ entry and Ca2+-dependent signal transduction. Traffic. 2003;4(11):717–723. doi: 10.1034/j.1600-0854.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 49.Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278(29):27208–27215. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graziani A, Bricko V, Carmignani M, Graier WF, Groschner K. Cholesterol- and caveolin-rich membrane domains are essential for phospholipase A2-dependent EDHF formation. Cardiovasc Res. 2004;64(2):234–242. doi: 10.1016/j.cardiores.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 51.Everaerts W, Nilius B, Owsianik G. The vallinoid transient receptor potential channel Trpv4: From structure to disease. Prog Biophys Mol Biol. 2009 doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Cohen DM. The transient receptor potential vanilloid-responsive 1 and 4 cation channels: role in neuronal osmosensing and renal physiology. Curr Opin Nephrol Hypertens. 2007;16(5):451–458. doi: 10.1097/MNH.0b013e32821f6060. [DOI] [PubMed] [Google Scholar]
- 53.Konno M, Shirakawa H, Iida S, Sakimoto S, Matsutani I, Miyake T, Kageyama K, Nakagawa T, Shibasaki K, Kaneko S. Stimulation of transient receptor potential vanilloid 4 channel suppresses abnormal activation of microglia induced by lipopolysaccharide. Glia. 2012;60(5):761–770. doi: 10.1002/glia.22306. [DOI] [PubMed] [Google Scholar]
- 54.Shirakawa H, Nakagawa T, Kaneko S. Pathophysiological roles of transient receptor potential channels in glial cells. Yakugaku Zasshi. 2010;130(3):281–287. doi: 10.1248/yakushi.130.281. [DOI] [PubMed] [Google Scholar]
- 55.Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, Ferroni S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience. 2007;148(4):876–892. doi: 10.1016/j.neuroscience.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 56.Butenko O, Dzamba D, Benesova J, Honsa P, Benfenati V, Rusnakova V, Ferroni S, Anderova M. The Increased Activity of TRPV4 Channel in the Astrocytes of the Adult Rat Hippocampus after Cerebral Hypoxia/Ischemia. PLoS One. 2012;7(6):e39959. doi: 10.1371/journal.pone.0039959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 58.Amruthesh SC, Boerschel MF, McKinney JS, Willoughby KA, Ellis EF. Metabolism of arachidonic acid to epoxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and prostaglandins in cultured rat hippocampal astrocytes. J Neurochem. 1993;61(1):150–159. doi: 10.1111/j.1471-4159.1993.tb03550.x. [DOI] [PubMed] [Google Scholar]
- 59.Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27(5):971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- 60.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26(11):2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 62.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 63.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6(1):43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 64.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294(6):H2855–2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107(8):3811–6. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129(4):877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 68.Amiry-Moghaddam M, Frydenlund DS, Ottersen OP. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience. 2004;129(4):999–1010. doi: 10.1016/j.neuroscience.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 69.Amiry-Moghaddam M, Xue R, Haug FM, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004;18(3):542–544. doi: 10.1096/fj.03-0869fje. [DOI] [PubMed] [Google Scholar]
- 70.Price DL, Ludwig JW, Mi H, Schwarz TL, Ellisman MH. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res. 2002;956(2):183–193. doi: 10.1016/s0006-8993(02)03266-3. [DOI] [PubMed] [Google Scholar]
- 71.Higashimori H, Blanco VM, Tuniki VR, Falck JR, Filosa JA. Role of epoxyeicosatrienoic acids as autocrine metabolites in glutamate-mediated K+ signaling in perivascular astrocytes. Am J Physiol Cell Physiol. 2010;299(5):C1068–1078. doi: 10.1152/ajpcell.00225.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding XL, Wang YH, Ning LP, Zhang Y, Ge HY, Jiang H, Wang R, Yue SW. Involvement of TRPV4-NO-cGMP-PKG pathways in the development of thermal hyperalgesia following chronic compression of the dorsal root ganglion in rats. Behav Brain Res. 2010;208(1):194–201. doi: 10.1016/j.bbr.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 73.Bal-Price A, Moneer Z, Brown GC. Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia. 2002;40(3):312–323. doi: 10.1002/glia.10124. [DOI] [PubMed] [Google Scholar]
- 74.Ferraro G, Sardo P. Nitric oxide and brain hyperexcitability. In Vivo. 2004;18(3):357–366. [PubMed] [Google Scholar]
- 75.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578(Pt 3):715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koltzenburg M. The role of TRP channels in sensory neurons. Novartis Found Symp. 2004;260:206–213. discussion 213-220, 277-209. [PubMed] [Google Scholar]
- 77.McHugh J, Keller NR, Appalsamy M, Thomas SA, Raj SR, Diedrich A, Biaggioni I, Jordan J, Robertson D. Portal osmopressor mechanism linked to transient receptor potential vanilloid 4 and blood pressure control. Hypertension. 2010;55(6):1438–1443. doi: 10.1161/HYPERTENSIONAHA.110.151860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu TT, Bi HS, Lv SY, Wang XR, Yue SW. Neurol Res. 2010;32(5):466–471. doi: 10.1179/174313209X408945. [DOI] [PubMed] [Google Scholar]
- 79.Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135(3):937–946. doi: 10.1053/j.gastro.2008.05.024. 946 e931-932. [DOI] [PubMed] [Google Scholar]
- 80.Gao F, Sui D, Garavito RM, Worden RM, Wang DH. Salt intake augments hypotensive effects of transient receptor potential vanilloid 4: functional significance and implication. Hypertension. 2009;53(2):228–235. doi: 10.1161/HYPERTENSIONAHA.108.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ryskamp DA, Witkovsky P, Barabas P, Huang W, Koehler C, Akimov NP, Lee SH, Chauhan S, Xing W, Renteria RC, Liedtke W, Krizaj D. The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci. 2011;31(19):7089–7101. doi: 10.1523/JNEUROSCI.0359-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Troidl C, Troidl K, Schierling W, Cai WJ, Nef H, Mollmann H, Kostin S, Schimanski S, Hammer L, Elsasser A, Schmitz-Rixen T, Schaper W. Trpv4 induces collateral vessel growth during regeneration of the arterial circulation. J Cell Mol Med. 2009;13(8B):2613–2621. doi: 10.1111/j.1582-4934.2008.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schierling W, Troidl K, Apfelbeck H, Troidl C, Kasprzak PM, Schaper W, Schmitz-Rixen T. Cerebral arteriogenesis is enhanced by pharmacological as well as fluid-shear-stress activation of the Trpv4 calcium channel. Eur J Vasc Endovasc Surg. 2011;41(5):589–596. doi: 10.1016/j.ejvs.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 84.Ducret T, Guibert C, Marthan R, Savineau JP. Serotonin-induced activation of TRPV4-like current in rat intrapulmonary arterial smooth muscle cells. Cell Calcium. 2008;43(4):315–323. doi: 10.1016/j.ceca.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 85.Mizoguchi F, Mizuno A, Hayata T, Nakashima K, Heller S, Ushida T, Sokabe M, Miyasaka N, Suzuki M, Ezura Y, Noda M. Transient receptor potential vanilloid 4 deficiency suppresses unloading-induced bone loss. J Cell Physiol. 2008;216(1):47–53. doi: 10.1002/jcp.21374. [DOI] [PubMed] [Google Scholar]
- 86.Auer-Grumbach M, Olschewski A, Papic L, Kremer H, McEntagart ME, Uhrig S, Fischer C, Frohlich E, Balint Z, Tang B, Strohmaier H, Lochmuller H, Schlotter-Weigel B, Senderek J, Krebs A, Dick KJ, Petty R, Longman C, Anderson NE, Padberg GW, Schelhaas HJ, van Ravenswaaij-Arts CM, Pieber TR, Crosby AH, Guelly C. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet. 2010;42(2):160–164. doi: 10.1038/ng.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carreno FR, Ji LL, Cunningham JT. Altered central TRPV4 expression and lipid raft association related to inappropriate vasopressin secretion in cirrhotic rats. Am J Physiol Regul Integr Comp Physiol. 2009;296(2):R454–466. doi: 10.1152/ajpregu.90460.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tian W, Fu Y, Garcia-Elias A, Fernandez-Fernandez JM, Vicente R, Kramer PL, Klein RF, Hitzemann R, Orwoll ES, Wilmot B, McWeeney S, Valverde MA, Cohen DM. A loss-of-function nonsynonymous polymorphism in the osmoregulatory TRPV4 gene is associated with human hyponatremia. Proc Natl Acad Sci U S A. 2009;106(33):14034–14039. doi: 10.1073/pnas.0904084106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vincent F, Duncton MA. TRPV4 agonists and antagonists. Curr Top Med Chem. 2011;11(17):2216–2226. doi: 10.2174/156802611796904861. [DOI] [PubMed] [Google Scholar]
- 90.Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (gRAC) Br J Pharmacol. (5th edition) 2011;164(suppl 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]