Abstract
Introduction
Hypertonic saline (HS) can treat cerebral edema arising from a number of pathologic conditions. However, physicians are reluctant to use it during the first 24 h after stroke because of experimental evidence that it increases infarct volume when administered early after reperfusion. Here, we determined the effect of HS on infarct size in an embolic clot model without planned reperfusion.
Methods
A clot was injected into the internal carotid artery of male Wistar rats to reduce perfusion in the middle cerebral artery territory to less than 40 % of baseline, as monitored by laser-Doppler flowmetry. After 25 min, rats were randomized to receive 10 mL/kg of 7.5 % HS (50:50 chloride:acetate) or normal saline (NS) followed by a 0.5 mL/h infusion of the same solution for 22 h.
Results
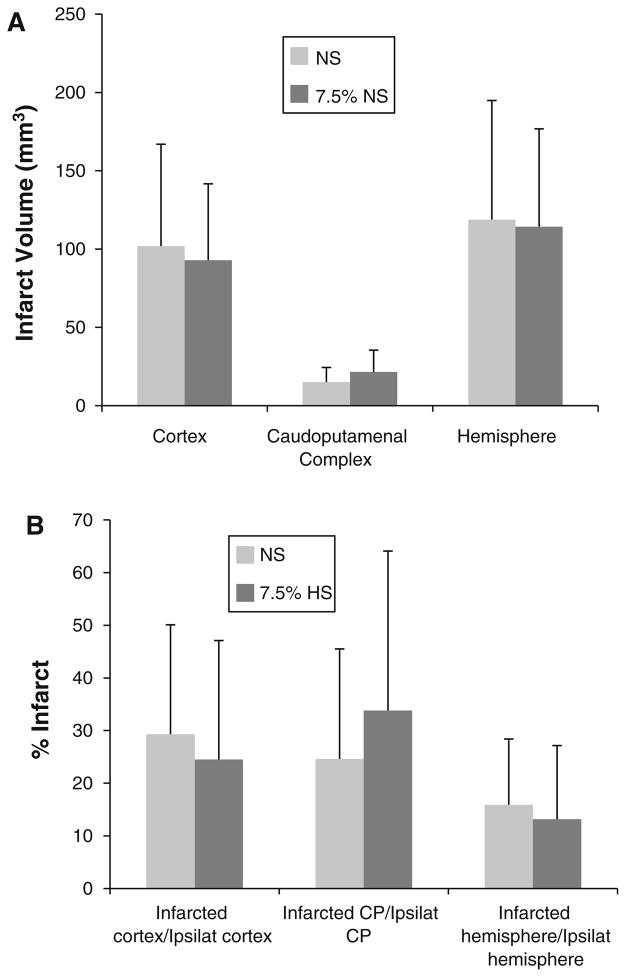
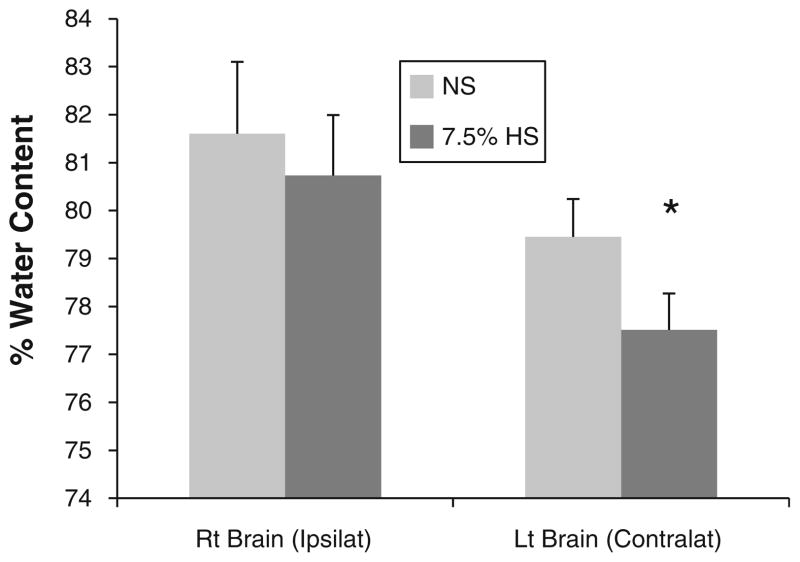
Infarct volume was similar between NS and HS groups (in mm3: cortex 102 ± 65 mm3 vs. 93 ± 49 mm3, p = 0.72; caudoputamenal complex 15 ± 9 mm3 vs. 21 ± 14, p = 0.22; total hemisphere 119 ± 76 mm3 vs. 114 ± 62, p = 0.88, respectively). Percent water content was unchanged in the infarcted hemisphere (NS 81.6 ± 1.5 %; HS 80.7 ± 1.3 %, p = 0.16), whereas the HS-treated contralateral hemisphere was significantly dehydrated (NS 79.4 ± 0.8 %; HS 77.5 ± 0.8 %, p < 0.01).
Conclusions
HS reduced contralateral hemispheric water content but did not affect ipsilateral brain water content when compared to NS. Infarct volume was unaffected by HS administration at all evaluated locations.
Keywords: Embolic stroke, Hypernatremia, Hypertonic saline, Normal saline, Tissue water content, Wistar rats
Introduction
Hypertonic saline (HS) has gained widespread use for the treatment of cerebral edema from multiple etiologies, including cerebral ischemia. In studies of both humans and animals, HS has been shown to effectively reduce brain swelling in the presence or the absence of elevated intracranial pressure (ICP) [1–16]. However, in 2000, Bhardwaj et al. reported that hypernatremia induced with 7.5 % saline infusion in the early reperfusion period substantially worsened cortical infarct volume. In their experiment, 2 h of middle cerebral artery (MCA) occlusion were followed by reperfusion, a 10 mL/kg bolus of 7.5 % HS (50:50 chloride: acetate) or normal saline (NS), and then 22 h of continuous infusion of the same study solution. Although brain water content in the ischemic hemisphere was equivalent between HS and NS controls, infarct volume remarkably doubled in rats treated with HS [17]. Additional studies showed that delaying administration of 7.5 % HS for 6 or 24 h after reperfusion following 2-h of MCA occlusion could reduce brain water content both ipsilateral and contralateral to the injury [12, 18]. Despite the beneficial findings after delayed administration, apprehension persists regarding the clinical utility of using high-tonicity HS in patients with hyperacute stroke and malignant swelling because of concerns over aggravating infarct volume.
Aggravation of infarction by 7.5 % HS may be specific for administration at the time of reperfusion. Ordinarily, reperfusion can accelerate the increase in blood–brain barrier permeability and sodium and chloride accumulation in the ischemic region [19]. Inducing hypernatremia during the first few hours of reperfusion may exacerbate sodium accumulation further. Clinically, most patients do not qualify to receive thrombolytics, and rapid reperfusion is not achieved. In these patients, the development of malignant brain swelling may force clinicians to consider early osmotherapy, including HS. In this experiment, we used a standard embolic clot model without defined, planned reperfusion. We tested the null hypothesis that administration of 7.5 % HS at 25 min after induction of focal ischemia does not increase infarct volume. Although tissue water content was measured, the primary objective was to assess HS’s effect on infarct size. At this early stage, the fate of neurons in the ischemic penumbra is largely undetermined and may be influenced by a number of factors, including potentially, exposure to a hypernatremic environment.
Methods
General Preparation and Animal Surgery
The investigational protocol was approved by the Institutional Animal Care and Use Committee, consistent with the National Institutes of Health guidelines for animal research. With exception of stroke mechanism, study parameters were set up to parallel the prior study by Bhardwaj et al. [17]. Adult male Wistar rats (250–450 g, non-fasting) were anesthetized with an isoflurane (1–2 % inhaled)-oxygen mixture and allowed to ventilate spontaneously. Under aseptic surgical technique, the right femoral vein and artery were cannulated to allow for vascular access for infusion, blood sampling, and continuous monitoring of arterial blood pressure. A heating lamp was used to maintain rectal temperature at 37 °C.
Thromboembolic Focal Ischemia
All experiments were performed by a single individual (TJKT). Cortical perfusion was measured by laser-Doppler flowmetry (LDF, Moor Instruments Ltd, model MBF3D) as previously described [20]. To allow continuous monitoring of LDF, the head piece of the stereotactic frame was modified to allow for free rotation around the longitudinal axis of the rat. Further modifications permitted placement of a snout mask allowing for spontaneous ventilation and a holder for the LDF probe. The probe was positioned over an area devoid of large cortical blood vessels using the coordinates 2 mm posterior and 6 mm lateral to the bregma. Its position was maintained for the duration of the experiment. The LDF signal was allowed to stabilize over a 30 min period before baseline measurements were obtained.
Focal ischemia was produced by middle cerebral artery occlusion after injecting a single preformed 25 mm long blood clot, as previously described [21]. In brief, through a lateral skin incision, the right common carotid artery (CCA), the right external carotid artery (ECA), and the internal carotid artery (ICA) were isolated. The right CCA was temporarily clamped. A 5.0 silk suture was loosely placed around the origin of the ECA, and the distal end of the ECA was ligated and severed. The ECA stump was manipulated so that it was positioned in line with the ICA. A modified PE-10 catheter with a 0.3 mm outer diameter filled with a 25-mm clot was attached to a 100-mcL Hamilton syringe and introduced into the ECA lumen through a small arteriotomy. 15 mm of the catheter’s length was gently advanced from the ECA into the lumen of the ICA. The clot along with 10–20 mcL of NS was injected into the ICA over 10 s. LDF was used to confirm arterial occlusion, which was defined as a decline in perfusion of the MCA territory to below 40 % of the baseline value. Rats that did not meet LDF criteria for arterial occlusion were excluded, resulting in a total of 2 exclusions for all described experiments. The decrease in flow was monitored for 25 min post-injection, allowing for clot stabilization, after which the catheter was withdrawn from the ECA which was ligated.
Rats were then randomized to receive a 10 mL/kg intravenous bolus of either 0.9 % NS (n = 11) or 7.5 % HS (50:50 acetate:chloride, n = 11), administered over 45 min. An acetate:chloride mixture was used to reduce the incidence of hyperchloremic metabolic acidosis. The loading dose was followed by continuous infusion of 0.5 mL/h of the same solution for 22 h. After the loading dose, animals were allowed to emerge from anesthesia and were provided free access to food but not water. Serum sodium, serum osmolarity, glucose, and arterial blood gases were measured at baseline, after completion of the bolus infusion, and after 22 h of continuous infusion. Osmolarity was measured via an automated freezing point depression micro-osmometer (Advanced Instruments, Norwood, MA). After 22 h of infusion, rats were deeply anesthetized and sacrificed. Brains were harvested and cut into seven coronal sections, which were stained with 1 % triphenyletrazolium chloride in saline at 37 °C for 30 min, as previously described [22]. Infarct volumes were calculated by an examiner blinded to group using image analysis software (SigmaScan Pro, Jandal). The area of infarction was calculated for each section and integrated across the whole hemisphere. The infarct volumes were derived for the cortex, caudoputamenal complex, and entire hemisphere and expressed as a percent of the contralateral uninjured structure, correcting for edema as described previously [23, 24].
In a separate experiment, a second set of rats were randomized to receive either 7.5 % HS (n = 11) or NS (n = 11) after embolization as described above. After 22 h of infusion, brains were harvested, bisected into left and right hemisphere, weighed, dried, and weighed again. The tissue water content of each hemisphere was expressed as a wet-to-dry weight ratio, as previously described [25]. Wet-to-dry ratio was calculated as % H2O = (1 − dry wt/wet wt) × 100 %.
To better characterize our model, in a separate experiment, ICP was monitored for a 24 h period after embolic stroke. Rats were anesthetized, and vascular access obtained as described above. The ICP transducer (Stamba Sensors, model TSD175A; Biopac Systems, Inc., Goleta, CA, USA) was inserted through a burr hole 1 mm rostral and lateral to the bregma on the right side, as previously described [26]. ICP was measured continuously using a digital ICP monitor (Model MPMS100A-1; Biopac Systems, Inc., Goleta, CA, USA). After embolization and reperfusion (n = 10), animals were allowed to emerge from anesthesia. A control group (n = 4) was also assessed to establish baseline ICP.
Statistical Analysis
The primary experiment was powered based on a previous investigation [17]. For a significance level of 0.05, a sample size of 10 per group would confer a power of 0.8 for the primary endpoint, hemispheric infarct size. An additional animal was added in case of tissue sample mishandling. Differences between groups were assessed using analysis of variance (ANOVA), except where repeated measures were performed. For example, weight difference with respect to baseline was analyzed with ANOVA. On the other hand with longitudinal repeated measures, after determining whether baseline values differed between groups, a general estimating equation model with baseline value as a covariate was used to assess group divergence. Differences were considered significant for p ≤ 0.05. Significance levels reported are those obtained from two-tailed tests. Statistical analysis was facilitated by Stata 12.1 (StataCorp; College Station, TX). Values are presented as mean ± standard deviation (SD) for all data.
Results
Physiological and laboratory values are listed in Table 1. Baseline values did not differ between groups. Mean arterial pressure, pH, paCO2, paO2, and glucose did not vary between groups over the experimental period. Body weight was stable with NS but rats treated with HS decreased from 425 ± 48 g at baseline to 387 ± 42 g after 22 h of infusion (p < 0.01; Table 1). The means for rectal temperature did not differ by greater than 0.1 °C between groups, but a statistical difference was detected. Serum sodium and osmolarity remained stable in the NS group but rose from baselines values of 138 ± 3 meq/L and 300 ± 5 mOsm/L to 158 ± 4 meq/L and 354 ± 8 mOsm/L, respectively, after HS administration (p < 0.01; Table 1).
Table 1.
Mean (SD) of physiological and laboratory data collected at baseline, after bolus infusion (45 min), and at experimental conclusion (22 h)
| Baseline | 45 min | 22 h post-ischemia | p (baseline)† | p (experiment)‡ | |
|---|---|---|---|---|---|
| Weight (g) | |||||
| 0.9 % NS | 416 (43) | NM | 423 (45) | ||
| 7.5 % HS | 425 (48) | NM | 387 (42) | 0.67 | <0.01 |
| MAP (mmHg) | |||||
| 0.9 % NS | 86 (5.5) | 89 (6.4) | 91 (6.3) | ||
| 7.5 % HS | 89 (6.2) | 92 (6.8) | 88 (7.3) | 0.32 | 0.97 |
| Rectal temp (LC) | |||||
| 0.9 % NS | 37.9 (0.2) | 38.1 (0.1) | 37.8 (0.2) | ||
| 7.5 % HS | 37.9 (0.1) | 38.0 (0.1) | 37.7 (0.1) | 1.0 | <0.01 |
| pH | |||||
| 0.9 % NS | 7.38 (0.04) | 7.38 (0.03) | 7.39 (0.02) | ||
| 7.5 % HS | 7.39 (0.03) | 7.39 (0.03) | 7.41 (0.03) | 0.36 | 0.19 |
| PaCO2 (mmHg) | |||||
| 0.9 % NS | 43.6 (3.2) | 45.6 (4.5) | 41.2 (3.5) | ||
| 7.5 % HS | 44.5 (3.8) | 47.6 (4.6) | 40.6 (4.3) | 0.57 | 0.70 |
| PaO2 (mmHg) | |||||
| 0.9 % NS | 154 (30) | 133 (25) | 135 (20) | ||
| 7.5 % HS | 145 (31) | 132 (28) | 130 (14) | 0.48 | 0.68 |
| Serum Na (mEq/L) | |||||
| 0.9 % NS | 138 (3) | 138 (3) | 138 (3) | ||
| 7.5 % HS | 138 (3) | 153 (3) | 158 (4) | 0.88 | <0.01 |
| Serum osmolarity (mOsm/L) | |||||
| 0.9 % NS | 300 (5) | 303 (4) | 305 (4) | ||
| 7.5 % HS | 300 (5) | 339 (7) | 354 (8) | 1.0 | <0.01 |
| Glucose (mg/dL) | |||||
| 0.9 % NS | 112 (17) | 110 (18) | 102 (20) | ||
| 7.5 % HS | 110 (19) | 98 (22) | 106 (22) | 0.76 | 0.62 |
The significance of group differences at baseline and over the course of the experiment is given on the right
NM not measured, HS hypertonic saline, NS normal saline, temp temperature
Significance of differences between NS and HS groups at baseline as determined by analysis of variance
Significance of differences between NS and HS groups during the experimental period were determined by longitudinal analysis, except for weight change (see text for details). Weight change was analyzed by computing the mean change in each group with respect to baseline, and significance of differences determined by analysis of variance
Brain infarct volumes were similar between NS and HS groups. Infarct volumes in the cortex (NS: 102 ± 65 mm3; HS: 93 ± 49 mm3, p = 0.72), caudoputamenal complex (NS: 15 ± 9 mm3; HS: 21 ± 14 mm3, p = 0.22), and total hemisphere (NS: 119 ± 76 mm3; HS: 114 ± 62 mm3, p = 0.88) did not differ between the two treatment groups (Fig. 1a). Infarct volumes were also expressed as a percentage of the intact contralateral structure (Fig. 1b).
Fig. 1.
Infarct volumes after 22 h of crystalloid infusion expressed as a uncorrected total volume and b percent infarct: ratio of infarct volume over volume of the intact contralateral structure, as calculated by Swanson et al. [24]. Comparisons were made between groups treated with normal saline (NS) and those treated with 7.5 % hypertonic saline (HS). Infarct volumes were calculated after 22 h of crystalloid infusion and are displayed as mean + SD. The total hemispheric infarct volume was divided into cortical and caudoputamenal (CP) regions. No statistical significance was reached in any comparison (p ≥ 0.22). N = 11 for both groups
The tissue water content in the ipsilateral hemisphere was not different between the NS group (81.6 ± 1.5 %) and HS group (80.7 ± 1.3 %, p = 0.16). However, water content in the contralateral hemisphere of the HS group (77.5 ± 0.8 %) was less than that in the NS group (79.4 ± 0.8 %, p < 0.01; Fig. 2).
Fig. 2.
Percent water content as measured by the wet-to-dry weight ratio for each hemisphere. The ratios are displayed as mean + SD. NS normal saline, HS hypertonic saline; *p < 0.01 versus the NS group; n = 11
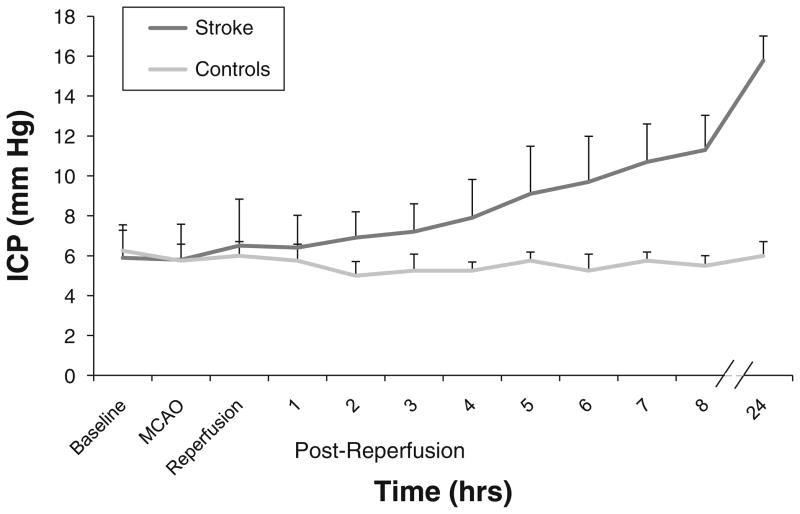
The results of ICP monitoring over a 24 h period are displayed in Fig. 3. Controls maintained an ICP of 5–6 mmHg throughout 24 h. The data shows a clear divergence of mean ICP by 2 h post-reperfusion in the stroke group. ICP averaged at 11.3 ± 1.7 mmHg at 8 h and peaked at 15.8 ± 1.2 mmHg at 24 h.
Fig. 3.
Mean ICP over time in control animals (n = 4) and in rats subjected to the embolic stroke protocol (n = 10). Values are displayed as mean + SD. Please note the discontinuity between 8 and 24 h
Discussion
Infusion of 7.5 % HS starting 25 min after embolic stroke and continuing for 22 h did not worsen the volume of infarction in this rat model. This result differs from the study by Bhardwaj et al. [17], where reperfusion with 7.5 % HS commencing immediately after 2 h of ischemia increased infarct volume. The major difference between the two studies is that Bhardwaj et al. used the intraluminal filament technique to produce 2 h of transient focal ischemia, whereas we induced large-artery embolization with a large clot. After embolization, it is possible for spontaneous recanalization to occur after clot injection, but the degree of reperfusion and the timing will be highly variable. In this regard, the model simulates large-vessel ischemic stroke without thrombolytic treatment, and our data suggests that inducing hypernatremia early in the first day after embolic stroke is not detrimental to infarct volume. The model also demonstrated a slow rise in ICP over 24 h, which did not reach pathological levels during the duration of this experiment.
It is possible that 7.5 % HS may adversely affect infarct volume only when reperfusion is established after vascular occlusion. Evidence in rats indicates that 3 h of MCA occlusion followed by 3 h of reperfusion increases blood–brain barrier permeability and brain sodium and chloride content compared to 6 h of permanent occlusion [19]. Infusing HS during this early period of reperfusion might adversely affect metabolically compromised cells in the penumbra and extend the region of infarction. Furthermore, delayed administration of tissue plasminogen activator can increase blood–brain barrier permeability through activation of matrix metalloproteinases [27], and administration of HS in this circumstance might amplify swelling. Therefore, the use of HS during clot removal or dissolution needs further evaluation.
Nevertheless, even during reperfusion, delayed use of HS after much of the area-at-risk has undergone cell death may be efficacious in controlling brain swelling and preventing secondary ischemia in less vulnerable tissue. After 2 h of MCA occlusion produced by the intraluminal filament model, 7.5 % HS was shown to significantly decrease water content in the ipsilateral and contralateral hemispheres when infusion was started 6 or 24 h after occlusion [13, 18]. Although blood–brain barrier permeability is increased at these later times points [18], some regions of the affected hemisphere still retain a significant reflection coefficient for sodium and chloride such that an osmotic effect can be generated.
In our study, where reperfusion may have only occurred spontaneously, early treatment with 7.5 % HS significantly reduced water content in the contralateral hemisphere alone. The lack of a significant decrease in the ipsilateral hemisphere may be attributable to the high variability in water content of the ipsilateral cortex in this model. It may also have resulted from variability in the volume of injured tissue within the entire hemisphere and to the blood–brain barrier permeability within the injured tissue. Most importantly, though, no trend was evident for a worsening of the mean water content in the injured hemisphere.
HS’s predominant effect on brain water is mediated through an intact BBB by the development of an osmotic gradient between the brain and the intravascular space. Water is mobilized out of the brain via perivascular aquaporin-4 channels [28]. Other effects include HS’s ability to change the rheological properties of blood. By improving viscosity, HS increases cerebral blood flow, which leads to vasoconstriction and secondary ICP reduction [29, 30]. Through an elevation in serum osmolarity, HS may also improve cerebral spinal fluid absorption [31]. Furthermore, HS has the potential to improve microvascular circulation and regional brain tissue perfusion, possibly by dehydration of cerebrovascular endothelium and erythrocytes, thereby facilitating capillary flow [32, 33]. Finally, HS seems to diminish the inflammatory response to brain injury [34].
The inflammatory cascade may play a substantial role in promoting secondary injury after cerebral ischemia and other brain injuries [35]. Since HS has been shown to reduce the inflammatory response, administration of HS has the potential to impact the natural history of a cerebral insult. In vitro studies on HS’s impact on the inflammatory cascade are mixed but favor a beneficial effect [36–44], with timing of exposure possibly altering this effect [36, 43]. To our knowledge, in vivo studies on HS’s impact on immunomodulation in the setting of cerebral ischemia are non-existent in the literature. There are experimental reports on beneficial immunomodulation of HS in the setting of shock [45–48]. In an experimental traumatic brain injury model, 7.2 % HS/10 % dextran-reduced adhesion of white blood cells by 90 % over controls and prevented the cerebral vasodilation observed in other groups [34]. Study of HS’s immunomodulatory effects in humans has been limited to healthy volunteers, healthy women undergoing hysterectomy, and pre-hospital administration for hypovolemic shock [49–52], with results again favoring a modest beneficial effect.
HS’s effectiveness for osmotherapy is related to the type of cerebral edema present and the amount of intact BBB/normal brain. HS works well for vasogenic edema (seen with brain tumors, abscess), but poorly for cytotoxic edema. In practice, pathologic states commonly have mixed edema types. Intracerebral hemorrhage and stroke have both cytotoxic and vasogenic edema [53, 54]. HS has recently gained some support for the prevention of perihematoma edema expansion in the setting of moderate-large intracerebral hemorrhage, through the use of a continuous 3 % HS infusion [55]. HS continuous infusion also demonstrated a trend toward preventing ICP crisis, and its use may also reduce in-hospital mortality in a mixed patient population with intracerebral hemorrhage, subarachnoid hemorrhage, and stroke [56].
The execution of clinical studies of human stroke with malignant swelling or elevated ICP is logistically difficult and rarely performed. Schwarz et al. revealed that 10 % HS was effective in treating stroke patients with ICP crises, with utility demonstrated in those patients who did not respond to 100 g of mannitol [7, 8]. They reported that, compared with 100 g of mannitol, 100 mL of 10 % HS hydroxyethyl starch solution provided a greater decrease in ICP, acted more quickly (25 vs. 45 min for mannitol), and was successful in 100 % of study patients (versus 10 of 14 patients who received mannitol) [8]. A successful treatment was defined as a reduction in ICP by 10 % or reversal of a dilated pupil, signifying reversal of herniation.
Clinically, elderly stroke patients typically present with multiple chronic co-morbidities, including heart disease and renal insufficiency and failure. Even without treatment, stable heart failure in the setting of acute stroke can be exacerbated due to the normal expected hypertensive blood pressure response. When HS is needed in patients with these co-morbidities, sodium retention and hypervolemia can occur, leading to pulmonary edema and acute congestive heart failure [57]. HS has been associated with acute nephrotoxicity [53], although this is poorly understood and rarely observed clinically [5, 55, 56, 58]. Independent from nephrotoxicity, sodium levels above 155 meq/L may be associated with elevations in serum BUN and creatinine [58]. In addition, a cerebral infarction may serve as an epileptic focus. Hypernatremia may produce neuronal depression and provoke irritability [59, 60], thereby promoting seizures. The presence of co-morbidities, however, should not preclude use of HS, but rather administration must be undertaken with caution and proper monitoring.
Electrolyte disturbances such as hyperchloremia introduce another set of potential clinical issues [61, 62], which may impact several organ systems. Utilizing balanced crystalloid solutions (such as a 50:50 acetate/chloride HS solution) reduces the risk of hyperchloremia [63]. Finally, central pontine myelinolysis (CPM), decreased platelet aggregation, and elevation of prothrombin times and partial thromboplastin times have either never been reported (in case of CPM) or reported rarely [64], although they are listed as potential dangers.
In this embolic model of large vessel stroke without planned reperfusion, early administration of HS did not affect infarct size and reduced contralateral hemispheric water content as compared to NS controls. Although the model was not constructed to create a pathological elevation in ICP, the observed elevation would likely be attenuated by HS, as evidenced by a reduction in water content of normal brain tissue. The results of this experiment only begin to address concerns about the early use of HS when osmotherapy is required following acute stroke. We would suggest avoiding any clinical extrapolation of these results, as this study only provided 24 h of observation and did not assess those that achieve planned reperfusion after cerebral infarction. Translation of any of these findings to clinical practice will ultimately require assessment of long-term neurobehavioral outcomes in combination with histopathological determination of cerebral injury.
Conclusion
In summary, in an embolic model of ischemic stroke without reperfusion, HS exposure in the first 24 h did not increase infarct volume beyond that observed with NS. HS did not increase edema in the peri-infarct region but did reduce water content in the non-infarcted hemisphere. Further experimental outcome data are required to better extrapolate to the clinical realm.
Acknowledgments
We would like to thank Adam Schiavi for manuscript review, Claire Levine for manuscript preparation, and Patricia Lamberti for figure preparation. This work was supported by National Institutes of Health Grant NS038684 (R.C.K.).
Footnotes
Conflict of interest None.
Contributor Information
Alexander Papangelou, Email: apapang1@jhmi.edu, Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Meyer 8-140, Baltimore, MD 21287-7840, USA. Department of Neurology, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Meyer 8-140, Baltimore, MD 21287-7840, USA.
Thomas J. K. Toung, Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Meyer 8-140, Baltimore, MD 21287-7840, USA
Allan Gottschalk, Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Meyer 8-140, Baltimore, MD 21287-7840, USA.
Marek A. Mirski, Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Meyer 8-140, Baltimore, MD 21287-7840, USA. Department of Neurology, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Meyer 8-140, Baltimore, MD 21287-7840, USA. Department of Neurosurgery, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Meyer 8-140, Baltimore, MD 21287-7840, USA
Raymond C. Koehler, Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins University School of Medicine, 600 North Wolfe Street, Blalock 1404, Baltimore, MD 21287-4961, USA
References
- 1.Ducey JP, Mozingo DW, Lamiell JM, Okerburg C, Gueller GE. A comparison of the cerebral and cardiovascular effects of complete resuscitation with isotonic and hypertonic saline, hetastarch, and whole blood following hemorrhage. J Trauma. 1989;29:1510–8. doi: 10.1097/00005373-198911000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Thomas D, Peterson B. Hypertonic saline lowers raised intracranial-pressure in children after head trauma. J Neurosurg Anesthesiol. 1992;4:4–10. doi: 10.1097/00008506-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36:795–800. doi: 10.1097/CCM.0B013E3181643B41. [DOI] [PubMed] [Google Scholar]
- 4.Gemma M, Cozzi S, Tommasino C, Mungo M, Calvi MR, Cipriani A, et al. 7.5 % hypertonic saline versus 20 % mannitol during elective neurosurgical supratentorial procedures. J Neurosurg Anesthesiol. 1997;9:329–34. doi: 10.1097/00008506-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Koenig MA, Bryan M, Lewin JL, 3rd, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70:1023–9. doi: 10.1212/01.wnl.0000304042.05557.60. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi AI, Wilson DA, Traystman RJ. Treatment of elevated intracranial pressure in experimental intracerebral hemorrhage: comparison between mannitol and hypertonic saline. Neurosurgery. 1999;44:1055–63. doi: 10.1097/00006123-199905000-00064. discussion 1063–4. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of hypertonic (10 %) saline in patients with raised intracranial pressure after stroke. Stroke. 2002;33:136–40. doi: 10.1161/hs0102.100877. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz S, Schwab S, Bertram M, Aschoff A, Hacke W. Effects of hypertonic saline hydroxyethyl starch solution and mannitol in patients with increased intracranial pressure after stroke. Stroke. 1998;29:1550–5. doi: 10.1161/01.str.29.8.1550. [DOI] [PubMed] [Google Scholar]
- 9.Simma B, Burger R, Falk M, Sacher P, Fanconi S. A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer’s solution versus hypertonic saline. Crit Care Med. 1998;26:1265–70. doi: 10.1097/00003246-199807000-00032. [DOI] [PubMed] [Google Scholar]
- 10.Suarez JI, Qureshi AI, Bhardwaj A, Williams MA, Schnitzer MS, Mirski M, et al. Treatment of refractory intracranial hypertension with 23.4 % saline. Crit Care Med. 1998;26:1118–22. doi: 10.1097/00003246-199806000-00038. [DOI] [PubMed] [Google Scholar]
- 11.Suarez JI, Qureshi AI, Parekh PD, Razumovsky A, Tamargo RJ, Bhardwaj A, et al. Administration of hypertonic (3 %) sodium chloride/acetate in hyponatremic patients with symptomatic vasospasm following subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1999;11:178–84. doi: 10.1097/00008506-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Toung TJ, Hurn PD, Traystman RJ, Bhardwaj A. Global brain water increases after experimental focal cerebral ischemia: effect of hypertonic saline. Crit Care Med. 2002;30:644–9. doi: 10.1097/00003246-200203000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Toung TJ, Tyler B, Brem H, Traystman RJ, Hurn PD, Bhardwaj A. Hypertonic saline ameliorates cerebral edema associated with experimental brain tumor. J Neurosurg Anesthesiol. 2002;14:187–93. doi: 10.1097/00008506-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Worthley LI, Cooper DJ, Jones N. Treatment of resistant intracranial hypertension with hypertonic saline. Report of two cases. J Neurosurg. 1988;68:478–81. doi: 10.3171/jns.1988.68.3.0478. [DOI] [PubMed] [Google Scholar]
- 15.Kerwin AJ, Schinco MA, Tepas JJ, 3rd, Renfro WH, Vitarbo EA, Muehlberger M. The use of 23.4 % hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. J Trauma. 2009;67:277–82. doi: 10.1097/TA.0b013e3181acc726. [DOI] [PubMed] [Google Scholar]
- 16.Raghavan M, Marik PE. Therapy of intracranial hypertension in patients with fulminant hepatic failure. Neurocrit Care. 2006;4:179–89. doi: 10.1385/NCC:4:2:179. [DOI] [PubMed] [Google Scholar]
- 17.Bhardwaj A, Harukuni I, Murphy SJ, Alkayed NJ, Crain BJ, Koehler RC, et al. Hypertonic saline worsens infarct volume after transient focal ischemia in rats. Stroke. 2000;31:1694–701. doi: 10.1161/01.str.31.7.1694. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Toung TJ, Sapirstein A, Bhardwaj A. Effect of duration of osmotherapy on blood–brain barrier disruption and regional cerebral edema after experimental stroke. J Cereb Blood Flow Metab. 2006;26:951–8. doi: 10.1038/sj.jcbfm.9600248. [DOI] [PubMed] [Google Scholar]
- 19.Yang GY, Betz AL. Reperfusion-induced injury to the blood–brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–64. doi: 10.1161/01.str.25.8.1658. discussion 1664–5. [DOI] [PubMed] [Google Scholar]
- 20.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–65. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 21.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Kirsch JR, Hashimoto K, London ED, Koehler RC, Traystman RJ. PPBP [4-phenyl-1-(4-phenylbutyl) piperidine] decreases brain injury after transient focal ischemia in rats. Stroke. 1996;27:2120–3. doi: 10.1161/01.str.27.11.2120. [DOI] [PubMed] [Google Scholar]
- 23.Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–21. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- 24.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 25.Toung TJ, Nyquist P, Mirski MA. Effect of hypertonic saline concentration on cerebral and visceral organ water in an uninjured rodent model. Crit Care Med. 2008;36:256–61. doi: 10.1097/01.CCM.0000295306.52783.1E. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Sagher O, Keep R, Hua Y, Xi G. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery. 2009;65:331–43. doi: 10.1227/01.NEU.0000345649.78556.26. discussion 343. [DOI] [PubMed] [Google Scholar]
- 27.Kahles T, Foerch C, Sitzer M, Schroeter M, Steinmetz H, Rami A, et al. Tissue plasminogen activator mediated blood-brain barrier damage in transient focal cerebral ischemia in rats: relevance of interactions between thrombotic material and thrombolytic agent. Vascul Pharmacol. 2005;43:254–9. doi: 10.1016/j.vph.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Zeynalov E, Chen CH, Froehner SC, Adams ME, Ottersen OP, Amiry-Moghaddam M, et al. The perivascular pool of aquaporin-4 mediates the effect of osmotherapy in postischemic cerebral edema. Crit Care Med. 2008;36:2634–40. doi: 10.1097/CCM.0b013e3181847853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng MY, Al-Rawi PG, Pickard JD, Rasulo FA, Kirkpatrick PJ. Effect of hypertonic saline on cerebral blood flow in poor-grade patients with subarachnoid hemorrhage. Stroke. 2003;34:1389–96. doi: 10.1161/01.STR.0000071526.45277.44. [DOI] [PubMed] [Google Scholar]
- 30.Muizelaar JP, Wei EP, Kontos HA, Becker DP. Cerebral blood flow is regulated by changes in blood pressure and in blood viscosity alike. Stroke. 1986;17:44–8. doi: 10.1161/01.str.17.1.44. [DOI] [PubMed] [Google Scholar]
- 31.Paczynski RP. Osmotherapy. Basic concepts and controversies. Crit Care Clin. 1997;13:105–29. doi: 10.1016/s0749-0704(05)70298-0. [DOI] [PubMed] [Google Scholar]
- 32.Heimann A, Takeshima T, Alessandri B, Noppens R, Kempski O. Effects of hypertonic/hyperoncotic treatment after rat cortical vein occlusion. Crit Care Med. 2003;31:2495–501. doi: 10.1097/01.CCM.0000084893.44650.CB. [DOI] [PubMed] [Google Scholar]
- 33.Shackford SR, Zhuang J, Schmoker J. Intravenous fluid tonicity: effect on intracranial pressure, cerebral blood flow, and cerebral oxygen delivery in focal brain injury. J Neurosurg. 1992;76:91–8. doi: 10.3171/jns.1992.76.1.0091. [DOI] [PubMed] [Google Scholar]
- 34.Hartl R, Medary MB, Ruge M, Arfors KE, Ghahremani F, Ghajar J. Hypertonic/hyperoncotic saline attenuates microcirculatory disturbances after traumatic brain injury. J Trauma. 1997;42:S41–7. doi: 10.1097/00005373-199705001-00008. [DOI] [PubMed] [Google Scholar]
- 35.Forsyth LL, Liu-DeRyke X, Parker D, Jr, Rhoney DH. Role of hypertonic saline for the management of intracranial hypertension after stroke and traumatic brain injury. Pharmacotherapy. 2008;28:469–84. doi: 10.1592/phco.28.4.469. [DOI] [PubMed] [Google Scholar]
- 36.Ciesla DJ, Moore EE, Zallen G, Biffl WL, Silliman CC. Hypertonic saline attenuation of polymorphonuclear neutrophil cytotoxicity: timing is everything. J Trauma. 2000;48:388–95. doi: 10.1097/00005373-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Cuschieri J, Gourlay D, Garcia I, Jelacic S, Maier RV. Hypertonic preconditioning inhibits macrophage responsiveness to endotoxin. J Immunol. 2002;168:1389–96. doi: 10.4049/jimmunol.168.3.1389. [DOI] [PubMed] [Google Scholar]
- 38.Loomis WH, Namiki S, Hoyt DB, Junger WG. Hypertonicity rescues T cells from suppression by trauma-induced anti-inflammatory mediators. Am J Physiol Cell Physiol. 2001;281:C840–8. doi: 10.1152/ajpcell.2001.281.3.C840. [DOI] [PubMed] [Google Scholar]
- 39.Rhee P, Wang D, Ruff P, Austin B, DeBraux S, Wolcott K, et al. Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit Care Med. 2000;28:74–8. doi: 10.1097/00003246-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Rizoli SB, Kapus A, Fan J, Li YH, Marshall JC, Rotstein OD. Immunomodulatory effects of hypertonic resuscitation on the development of lung inflammation following hemorrhagic shock. J Immunol. 1998;161:6288–96. [PubMed] [Google Scholar]
- 41.Coimbra R, Junger WG, Hoyt DB, Liu FC, Loomis WH, Evers MF. Hypertonic saline resuscitation restores hemorrhage-induced immunosuppression by decreasing prostaglandin E2 and interleukin-4 production. J Surg Res. 1996;64:203–9. doi: 10.1006/jsre.1996.0329. [DOI] [PubMed] [Google Scholar]
- 42.Coimbra R, Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic/hyperoncotic fluids reverse prostaglandin E2 (PGE2)-induced T-cell suppression. Shock. 1995;4:45–9. doi: 10.1097/00024382-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Gushchin V, Stegalkina S, Alam HB, Kirkpatrick JR, Rhee PM, Koustova E. Cytokine expression profiling in human leukocytes after exposure to hypertonic and isotonic fluids. J Trauma. 2002;52:867–71. doi: 10.1097/00005373-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Junger WG, Coimbra R, Liu FC, Herdon-Remelius C, Junger W, Junger H, et al. Hypertonic saline resuscitation: a tool to modulate immune function in trauma patients? Shock. 1997;8:235–41. [PubMed] [Google Scholar]
- 45.Attuwaybi B, Kozar RA, Gates KS, Moore-Olufemi S, Sato N, Weisbrodt NW, et al. Hypertonic saline prevents inflammation, injury, and impaired intestinal transit after gut ischemia/reperfusion by inducing heme oxygenase 1 enzyme. J Trauma. 2004;56:749–58. doi: 10.1097/01.ta.0000119686.33487.65. discussion 758–9. [DOI] [PubMed] [Google Scholar]
- 46.Gurfinkel V, Poggetti RS, Fontes B, da Costa Ferreira Novo F, Birolini D. Hypertonic saline improves tissue oxygenation and reduces systemic and pulmonary inflammatory response caused by hemorrhagic shock. J Trauma. 2003;54:1137–45. doi: 10.1097/01.TA.0000064452.37534.29. [DOI] [PubMed] [Google Scholar]
- 47.Powers KA, Zurawska J, Szaszi K, Khadaroo RG, Kapus A, Rotstein OD. Hypertonic resuscitation of hemorrhagic shock prevents alveolar macrophage activation by preventing systemic oxidative stress due to gut ischemia/reperfusion. Surgery. 2005;137:66–74. doi: 10.1016/j.surg.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 48.Shields CJ, Winter DC, Manning BJ, Wang JH, Kirwan WO, Redmond HP. Hypertonic saline infusion for pulmonary injury due to ischemia-reperfusion. Arch Surg. 2003;138:9–14. doi: 10.1001/archsurg.138.1.9. [DOI] [PubMed] [Google Scholar]
- 49.Angle N, Cabello-Passini R, Hoyt DB, Loomis WH, Shreve A, Namiki S, et al. Hypertonic saline infusion: can it regulate human neutrophil function? Shock. 2000;14:503–8. [PubMed] [Google Scholar]
- 50.Bulger EM, Cuschieri J, Warner K, Maier RV. Hypertonic resuscitation modulates the inflammatory response in patients with traumatic hemorrhagic shock. Ann Surg. 2007;245:635–41. doi: 10.1097/01.sla.0000251367.44890.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolsen-Petersen JA, Nielsen JO, Bendtzen K, Tonnesen E. Infusion of hypertonic saline (7.5 % NaCl) causes minor immunological changes in normovolaemic women. Acta Anaesthesiol Scand. 2004;48:224–33. doi: 10.1111/j.0001-5172.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- 52.Kolsen-Petersen JA, Nielsen JO, Tonnesen EM. Effect of hypertonic saline infusion on postoperative cellular immune function: a randomized controlled clinical trial. Anesthesiology. 2004;100:1108–18. doi: 10.1097/00000542-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Huang PP, Stucky FS, Dimick AR, Treat RC, Bessey PQ, Rue LW. Hypertonic sodium resuscitation is associated with renal failure and death. Ann Surg. 1995;221:543–54. doi: 10.1097/00000658-199505000-00012. discussion 554–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suarez JI. Hypertonic saline for cerebral edema and elevated intracranial pressure. Cleve Clin J Med. 2004;71(Suppl 1):S9–13. doi: 10.3949/ccjm.71.suppl_1.s9. [DOI] [PubMed] [Google Scholar]
- 55.Wagner I, Hauer EM, Staykov D, Volbers B, Dorfler A, Schwab S, et al. Effects of continuous hypertonic saline infusion on perihemorrhagic edema evolution. Stroke. 2011;42:1540–5. doi: 10.1161/STROKEAHA.110.609479. [DOI] [PubMed] [Google Scholar]
- 56.Hauer EM, Stark D, Staykov D, Steigleder T, Schwab S, Bardutzky J. Early continuous hypertonic saline infusion in patients with severe cerebrovascular disease. Crit Care Med. 2011;39:1766–72. doi: 10.1097/CCM.0b013e318218a390. [DOI] [PubMed] [Google Scholar]
- 57.Qureshi AI, Suarez JI, Bhardwaj A, Mirski M, Schnitzer MS, Hanley DF, et al. Use of hypertonic (3 %) saline/acetate infusion in the treatment of cerebral edema: effect on intracranial pressure and lateral displacement of the brain. Crit Care Med. 1998;26:440–6. doi: 10.1097/00003246-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Froelich M, Ni Q, Wess C, Ougorets I, Hartl R. Continuous hypertonic saline therapy and the occurrence of complications in neurocritically ill patients. Crit Care Med. 2009;37:1433–41. doi: 10.1097/CCM.0b013e31819c1933. [DOI] [PubMed] [Google Scholar]
- 59.Riggs JE. Neurologic manifestations of electrolyte disturbances. Neurol Clin. 2002;20:227–39. vii. doi: 10.1016/s0733-8619(03)00060-4. [DOI] [PubMed] [Google Scholar]
- 60.Castilla-Guerra L, del Carmen Fernandez-Moreno M, Lopez-Chozas JM, Fernandez-Bolanos R. Electrolytes disturbances and seizures. Epilepsia. 2006;47:1990–8. doi: 10.1111/j.1528-1167.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- 61.Handy JM, Soni N. Physiological effects of hyperchloraemia and acidosis. Br J Anaesth. 2008;101:141–50. doi: 10.1093/bja/aen148. [DOI] [PubMed] [Google Scholar]
- 62.Yunos NM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: chloride in critical illness. Crit Care. 2010;14:226. doi: 10.1186/cc9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McFarlane C, Lee A. A comparison of plasmalyte 148 and 0.9 % saline for intra-operative fluid replacement. Anaesthesia. 1994;49:779–81. doi: 10.1111/j.1365-2044.1994.tb04450.x. [DOI] [PubMed] [Google Scholar]
- 64.Reed RL, 2nd, Johnston TD, Chen Y, Fischer RP. Hypertonic saline alters plasma clotting times and platelet aggregation. J Trauma. 1991;31:8–14. doi: 10.1097/00005373-199101000-00002. [DOI] [PubMed] [Google Scholar]