Abstract
This review identifies a timeline to nanomedicine anticancer drug approval using the business model of inventors, innovators and imitators. By evaluating the publication record of nanomedicine cancer therapeutics we identified a trend of very few publications prior to FDA approval. We first enumerated the publications related to cancer involving polymers, liposomes or monoclonal antibodies and determined the number of citations per publication as well as the number of published clinical trials amongst the publications. Combining these data with the development of specific nanomedicines, we are able to identify an invention phase consisting of seminal papers in basic science necessary for the development of a specific nanomedicine. The innovation phase includes the first report, the development and the clinical trials involving that nanomedicine. Finally, the imitation phase begins after approval when others ride the wave of success by using the same formulation for new drugs or using the same drug to validate other nanomedicines. We then focused our analysis on nanomedicines containing camptothecin derivatives, which are not yet approved including two polymers considered innovations and one liposomal formulation in the imitation phase. The conclusion that may be drawn from the analysis of the camptothecins is that approved drugs reformulated in polymeric and liposomal cancer nanomedicines have a more difficult time navigating through the approval process than the parent molecule. This is probably due to the fact that for most currently approved drugs, reformulating them in a nanocarrier provides a small increase in performance that large pharmaceutical companies do not consider being worth the time, effort and expense of development. It also appears that drug carriers have a more difficult path through the clinic than monoclonal antibodies. The added complexity of nanocarriers also deters their use to deliver new molecular entities. Thus, the new drug candidates that might be most improved by drug delivery in nanocarriers are not formulated in this fashion.
Keywords: Camptothecin, Liposomes, Monoclonal Antibodies, Platinum Therapy, Polymers
1. Introduction
Richard Feynman proposed the concept of the nanosurgeons and nanodrug delivery devices in his talk “There’s Plenty of Room at the Bottom”, where he urged researchers to develop nanodevices capable of interacting with the body at the cellular level [1]. Today, over fifty years after Feynman’s talk, nanotechnology has become a significant focus of government spending leading to numerous publications and patents, but few cancer nanomedicines. Many of the cancer nanomedicines currently being investigated utilize small molecule cytotoxic agents discovered during the 1960’s including the platinates, camptothecins and adriamycins, which suffer from poor solubility, poor pharmacokinetics and various adverse side effects. Indeed, of the traditional low molecular weight drugs, only 5% that reach the clinical trial stage ever make it to the market [2]. Nanomedicines address these issues by improving the solubility and tailoring the release rates to improve the pharmacokinetics of the drug with the promise of reducing the adverse side effects [3]. However, from the vast compositional space of nanoparticles, liposomes, polymers and proteins developed to treat cancer, success is limited to just a few nanomedicines [4]. Each of these classes of nanotherapeutics have had varying success at each stage of the development process. Recent reviews on nanomedicine have identified the list of nanocarriers, which have made their way from the bench to FDA approved cancer therapies [4, 5].
Taking a bibliographic approach [6] we have evaluated a few of the first nanotherapeutics approved by the FDA for cancer therapy and identified the seminal papers that led to the development of these therapies. From these publications we generated a time line for each of the therapeutics to identify if there were similarities among various approaches that might be associated with ultimate FDA approval. These therapeutics might be classified using the business model of inventors, innovators and imitators (Economist.com/blogs/schumpeter (http://www.economist.com/blogs/schumpeter)). Inventors are those who create new technologies necessary for drug development. Innovators are those who use these invented technologies to develop a new drug. Once the first drug of the class has been approved, many imitators try to reproduce these results with other drugs or other drug delivery systems leading to a dramatic increase in publications. As pointed out elsewhere, imitation can be a good business strategy and by extension a good research strategy if the old is remixed with the new (Economist.com/blogs/schumpeter (http://www.economist.com/blogs/schumpeter). However, in the drug delivery field even imitation might not lead to a better formulation of a new drug.
Identifying a timeline from invention to innovation and finally imitation is our goal. By looking at the publication statistics for the field we hope to identify trends that resulted in FDA approved nanomedicines for the beneficial treatment of cancer patients.
2. Small molecule drugs vs. nanomedicines
The platinates, camptothecins and adriamycins along with many other cytotoxic agents suffer from poor water solubility, poor pharmacokinetics and numerous adverse side effects. These small molecule therapeutics achieve approval when the therapeutic benefits outweigh the adverse side effects. For example, camptothecin failed to achieve FDA approval due to the unpredictable side effects, however, chemical modification with a charged carboxylase-cleavable carbamate produced irinotecan (Camptosar©) [7]. This prodrug-forming chemical modification improved the water solubility and enabled more efficient dosing strategies with fewer adverse side effects as compared to camptothecin. The improved solubility and dosing facilitated accelerated FDA approval of irinotecan in 1996 and full approval in 1998. Although the approval process identified irinotecan as a beneficial therapeutic for patients, adverse reactions persist, such as serious diarrhea.
Nanocarriers, such as polymers and liposomes, also aim to improve the solubility and efficacy while decreasing the adverse side effects. Ideally, these aims are achieved by administering the active agent systemically in a protected fashion with tailored release at to the site of interest. Improved delivery also comes in the form of the enhanced permeation and retention (EPR) effect [8], which enables improved circulation of the nanocarrier with accumulation through the characteristic leaky vasculature at the site of the tumor. This strategy allows for a lower effective dose to be administered to the patient and a higher dose eventually arriving at the tumor. Potential nanocarriers capable of formulating nearly every small molecule therapeutic have infrequently achieved approval suggesting that the development process for small molecule drugs and nanocarriers are vastly different.
While small molecule and nanocarrier therapeutics are evaluated for efficacy and side effects, nanocarriers are also evaluated for each of the components within the formulation. Other concerns with nanocarriers that must be investigated prior to FDA approval include in vivo aggregation, recognition by the reticuloendothelial system leading to immune activation and controlled release of the therapeutic. This is further complicated when the therapeutic is covalently attached to the drug carrier as in the case of many polymers. The issues affecting drug delivery devices is underscored by the lack of nanocarriers approved by the FDA. The short list of polymers approved for cancer therapy in the U.S. includes Gliadel©, a poly(anhydride) implantable wafer approved for glioblastoma, [9] and a number of PEGylated proteins [10] with Zinostatin stimalmer© (SMANCS) approved in Japan [11]. The first two of these therapeutics are not considered nanomedicines because Gliadel© is not a nanocarrier and PEGylated proteins are nanosized prior to PEGylation. The absence of polymer nanomedicines receiving FDA approval is surprising due to the vast compositional space of polymer architectures and the burgeoning research within the field [12]. On the other hand, the list of liposomal drugs approved by the FDA for cancer therapy, with the active component and the company in parentheses, include Doxil© (Doxorubicin; Johnson & Johnson; recently reviewed by Barenholz [13]), DaunoXome© (Daunorubicin; Gilead), Mepact© (Muramyl tripeptide; IDM Pharma SAS) and Depocyt© (Cytarabine; Pacira Pharmaceuticals) with Myocet© (Doxorubicin; Enzon) approved in Europe and Canada [14]. Depocyt© is technically not a nanomedicine because the multi-lamellar vesicles are between 3–30 microns in size.
Monoclonal antibodies are nanomedicines that bridge the gap between small molecules and polymer or liposomal drug delivery vehicles. Some antibodies function as active agents, while others act as nanocarriers delivering drug conjugates to the tumor. Antibodies may then be considered ideal nanomedicines because they take advantage of the size and solubility of polymer and liposomal drug carriers and the unimolecular characteristics of small molecule therapeutics. This is evident by the success that monoclonal antibody therapy has had in recent years [15]. Currently, there are eleven antibodies approved for therapy listed here with the target and company in parentheses including Rituxan© (CD20; Genentech), Herceptin© (HER2; Genentech/Roche), Campath© (CD52; Genzyme), Zevalin© (CD20; labeled with Y-90; Biogen-Idec), Bexxar© (CD20; labeled with I-131; Coriza/GlaxoSmithKline), Erbitux© (EGFR; Imclone/Lilly), Avastin© (VEGF; Genentech/Roche), Vectibix© (EGFR; Amgen), Arzerra© (CD20; Genmab), Yervoy© (CTLA-4; Bristol-Myers Squibb), Adcetris© (CD30; Seattle Genetics) with Mylotarg© (CD33; labeled with calicheamicin; Wyeth/Pfizer) pulled from the market in 2010.
3. By the numbers
To first compare each of the fields we evaluated the publication record of cancer therapeutics over time. The publication data reported here reflects the citations available through SciFinder Scholar as of April 2012. Manuscripts published in 2012 were not included. The total number of publications annually, excluding reviews, was obtained for cancer as a whole, polymeric cancer therapy or liposomal cancer therapy. Clinical trials were then enumerated by limiting each of these searches accordingly using SciFinder Scholar delimiters (listed in Table 1). Publications of small molecule drugs were searched in the same manner and limited by polymeric or liposomal therapy to identify the subset of publications dealing with each of these areas. Average number of citations per publication was used to evaluate the publications in each area by dividing the number of citations by the total number of publications.
Table 1.
Comparison of publications, citations and percentage of clinical trials in cancer for polymer, liposome and monoclonal antibody therapeutics.‡
| Cancera | Platinatesa | Camptothecinsb | ||
|---|---|---|---|---|
| All | Publications | 2,850,000 | 19,000 | 10,000 |
| Clinical Trials | 86,000 (3%) | 2100 (11%) | 1500 (15%) | |
| Polymers | Publications | 9000 | 500 | 400 |
| Clinical Trials | 95(1%) | 4 (1%) | 5 (1%) | |
| Citations per Publicationa | 11.67 | 13.84 | 11.70b | |
| Liposomes | Publications | 12,000 | 600 | 300 |
| Clinical Trials | 500 (4%) | 90 (15%) | 14 (5%) | |
| Citations per Publicationa | 9.83 | 8.80 | 5.79b | |
| Monoclonal Antibodies | Publications | 35,000 | - | - |
| Clinical Trials | 2,800 (8%) | - | - | |
| Citations per Publicationa | 7.36 | - | - |
Citations per publication reported between the years 1985 and 2005.
Camptothecin citations per publication reported between the years 1995 and 2005.
Search terms - All Cancer: cancer NOT reviews; All Cancer Platinates: cancer AND platinum therapy NOT reviews; All Cancer Camptothecins: cancer AND camptothecin NOT review: Cancer Polymers: cancer AND polymer NOT review; Cancer Polymer Platinates: cancer AND polymer AND platinum therapy NOT review; Cancer Polymer Camptothecins: cancer AND polymer AND camptothecin NOT review; Cancer Liposomes: cancer AND liposome NOT review; Cancer Liposomal Platinates: cancer AND liposome AND platinum therapy; Cancer Liposomal Camptothecins: cancer AND liposome AND camptothecin; Cancer Monoclonal Antibodies: cancer AND monoclonal antibody therapy NOT review; Clinical Trials: Identified using the above search strings and the clinical trial selection in SciFinder Scholar.
In all, there have been nearly three million cancer publications dating back to the late 1800’s. Notably, there were only 10,000 publications in total prior to 1945, but over 140,000 in 2011 alone. The dramatic increase in the number of publications after World War II coincided with the National Cancer Institute Act of 1944 and the discovery that chemical warfare agents were leading to lymphoid and myeloid suppression and thus could be exploited for use in cancer therapy [16]. Of the three million total publications since 1945, 86,000 involve clinical trials, which amounts to about 3% of all publications. Certainly many more clinical trials have been performed that have not been published or were not identified by SciFinder Scholar as clinical trials, but we have decided to use this dataset to provide a representative but relative estimate of clinical trials to enable a general comparison between the modalities.
We evaluated the relative impact or influence of liposomes, polymers and monoclonal antibodies, by calculating the average number of citations per publication using data from the years between 1985 and 2005 (Table 1). We selected this time window because prior to 1985 there are fewer than 50 publications in each field providing, too few data points to accurately infer any information. Whereas, in the years after 2005, over 40% of papers do not have a citation compared to 30% between 1985 and 2005. During this period, the citations per publication for polymers and liposomes was 11.67 ± 0.60 and 9.83 ± 0.66, respectively. Although a trend exists, this difference is not statistically significant. Citation per publication for monoclonal antibodies, on the other hand, is significantly lower than both polymers (p < 0.0001) and liposomes (p < 0.05) with 7.36 ± 0.53 citations per publication as determined by a Kruskal-Wallis one-way ANOVA. Interestingly, this trend is inversely related to the number of FDA approved drugs on the market.
Taking a closer look at each of the fields, synthetic polymeric cancer therapeutics date back to the early 1960’s with fewer than 9000 publications and only 95 published clinical trials, which accounts for only 1% of publications in this field (Figure 1A). Liposomes have a total of over 12,000 publications dating back to the late 1960’s with over 500 clinical trials or more than 4% of publications (Figure 1B). Monoclonal antibodies on the other hand have over 35,000 papers and 2,800 clinical trials or 8% published since 1976. Interestingly, it took only 10 years (1986) for the total number of antibody publications to exceed 1000, whereas liposomes took 19 years (1991) and polymers took 36 years (1995). Enumerating clinical trials and the time that each field take to publish 1000 papers is simple and appears to track directly with the number of drugs approved by the FDA. It is also clear that there are very few drug delivery related publications in a field prior to drug approval.
Figure 1.

Nanomedicine publication profiles over time. A: Polymeric cancer therapeutics, B: liposomal cancer therapeutics and C: monoclonal antibody therapeutics. Solid line: Number of publications per year, Dashed line: number of citations per year and Dotted line: number of published clinical trials per year.
4. The timeline of drug approval
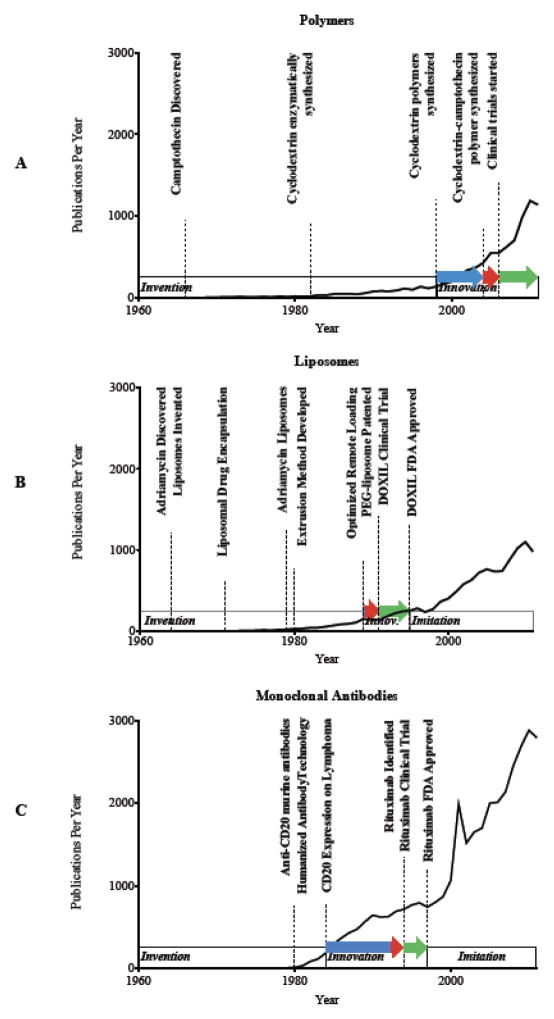
To more closely identify the publication history of the approved nanomedicines, we analyze the publications that came before and after drug approval. From these publications we are able to identify the inventors, innovators and imitators within a field. The inventors create the technology necessary for drug development including cell targeting markers, protein engineering technology, drug discovery, etc. The innovators remix the invented technology into a drug formulation and navigate the approval process. The imitators exploit the technology of the newly approved drug to ride the wave of success that preceded them. We identify the seminal publications during the invention phase critical for the development of each specific therapeutic. The innovation phase begins after the final invention was published and helps to determine the time that it took for those inventions to become a published therapeutic candidate. We also identify the number of years that each nanomedicine takes to enter clinical trials and the number of years from when clinical trials begin until drug approval. Imitation comes thereafter as demonstrated by the dramatic increase in publications, which include new potential therapeutics as well as basic research to identify mechanisms of action or new indications for the same drug. The invention, innovation and imitation phases are overlaid on the publication plot in Figure 2 for polymers, liposomes and monoclonal antibodies.
Figure 2.
Invention, innovation and imitation timeline overlapped on the plot of publications per year with seminal publications highlighted.
In 1997, Rituximab became the first monoclonal antibody approved for cancer therapy. Four publications involving rituximab were produced in 1994, one of which was a clinical trial, followed by twelve publications in 1997. Since 1997 there have been 11,500 publications involving rituximab. This is in comparison to the whole field of anticancer monoclonal antibody research, which achieved 7,400 publications prior to the approval of Rituximab and 27,000 publications since then. Certainly, many basic science publications other than the first twelve Rituximab publications led to the development and discovery of Rituximab, but it is clear that once this antibody was identified, there was a very focused route to deliver the antibody into the clinic.
The invention phase for rituximab is highlighted by the identification of murine monoclonal antibodies that target CD20 on B-cells [17, 18] followed by the technique of generating humanized antibodies [19, 20]. Finally, the identification of CD20 expression on B cell lymphomas [21] led Reff et al. to exploit these developments and generate a humanized anti-CD20 monoclonal antibody for treating B cell lymphoma [22]. This handful of seminal publications prior to the initial patent application for rituximab in 1993 [23] can be accurately attributed to the generation and approval of rituximab in 1997. Furthermore, based on these publication dates, the innovation phase lasted about nine years where the multiple inventions were combined to generate the final monoclonal antibody that was approved by the FDA for the treatment of cancer. Since then, many more antibody therapies have been approved, as the imitation phase has proven quite fruitful in this example.
To draw a comparison to liposomes the innovation phase started shortly after the final invention, due to the focus of the team to get a liposomal therapeutic into the clinic. The number of publications involving liposomal doxorubicin prior to 1995 was quite limited and the inventions that led to the formulation of Doxil© include the discovery of the adriamycins in 1964 and 1969 [24, 25] and the development of liposomes in the same year [26]. Gregoriadis first encapsulated enzymes in liposomes in the early 1970’s [27] and became a visionary in liposomal drug delivery by mapping out the landscape of the field and providing insight into the future success of the field [28; 29]. Tökes then first encapsulated doxorubicin in liposomes in 1979 [30]. The other inventions necessary for the development of Doxil© followed shortly thereafter including the development of an extrusion method to generate uniform vesicles [31], the development of PEG-DSPE [32] and the improved method of remote loading [33, 34]. Clinical trials on Doxil then started in 1991 and FDA approval came in 1995. While Rituximab© and Doxil© may follow similar invention and innovation phases, the comparison between a therapeutic antibody and a drug delivery vehicle is not appropriate due to the differing mechanisms of action as discussed previously. Two nanomedicines that bridge the gap between antibody-based therapy and liposomal drug delivery are Mylotarg© and Abraxane©. Mylotarg© is a calicheamicin conjugated anti-CD33 antibody that requires drug release to achieve a therapeutic effect. Abraxane©, on the other hand, is an albumin-based nanoparticle with paclitaxel encapsulated/associated with the protein aggregate.
The active agent of Mylotarg©, calicheamicin, was first discovered in 1989 from the broth of micromonospora echinospora [35]. Conjugation of this drug to an antibody was first invented by Hinman in 1993 using a hydrazone cleavable linker [36] based on data from 9 years prior showing the necessity for drug hydrolysis on antibodies to achieve therapeutic efficacy [37]. Just two years later, CD33 was identified on acute myoblastic leukemia cells along with the murine monoclonal antibody that recognizes CD33 [38]. Just two years after all of the pieces were in place for a therapeutic to be innovated, a patent was filed for Mylotarg© [39] followed by three publications in 1999, one of which was a clinical trial [40]. The clinical trials on Mylotarg© actually started in 1997 and approval came in 2000 under the accelerated drug approval program, but was pulled from the market in 2010 after questionable safety and efficacy data in post-approval studies.
Albumin’s ability to interact with hydrophobic molecules was first described in detail by Klotz in 1947 [41] and exploited in the development of Abraxane©. Twenty-one years later, albumin was administered to children with human growth hormone as a method for non-covalent protection of the hormone [42]. One year later, methotrexate was covalently attached to albumin for anti-cancer therapy [43]. Many researchers have investigated the effects of covalent drug attachment to albumin since the late 1960’s and paclitaxel was covalently attached to albumin in 1997 [44]. Abraxane©, however, was only made possible due to non-covalent association of paclitaxel with albumin using high-pressure jet technology patented in 1999 [45]. Clinical trials then started in 2001 and published a year later [46] with final approval for Abraxane© coming in 2005.
The short time between first report and clinical trials may be due to the proprietary nature of the research being conducted in that these products are designed and developed long before clinical trials start or before the therapeutics are reported in the literature. This, coupled with the estimated five-year time lag for papers to be cited, suggests that although we have identified the innovation phase as being less than ten years, there is likely a much shorter period from the invention phase to innovation. Indeed, rituximab was developed based on a report in 1989 that anti-idiotype antibodies react with tumor markers [47], suggesting only a five year innovation period to develop rituximab rather than a nine year period as the literature suggests. Furthermore, the difference between drugs is likely due to many factors leading to drug approval, including money, appropriate collaborations and the long-term focus of the research project. Indeed, large companies, such as IDEC Pharmaceuticals who developed rituximab, and Wyeth Ayerst Research who brought Mylotarg© to the clinic, generally benefit from having all of the required attributes to efficiently innovate new drugs and achieve FDA approval. On the other hand, a small US company named Vivorx Pharmaceuticals Inc., later named American Bioscience, developed and patented Abraxane© in 1999 [45]. This small drug company became viable after a $4 million dollar investment by Premeire Inc., which allowed them to launch the development of Abraxane©.
5. Nanomedicines in the pipeline
To determine if we can learn if the publication record can tell us anything about the prospects of nanomedicines, three nanomedicines containing camptothecin derivatives that are not yet approved are evaluated. Camptothecin was first identified in the mid-60’s by Wall and Wani [48] and showed promising anticancer effects, but the poor solubility and unpredictable side effects prevented the drug from being approved [7]. Since that time, the FDA has approved irinotecan and topotecan with another analogue, karenitecan, currently in clinical trials [49]. Studies involving the parent molecule and each of the analogues that followed have led to 10,000 publications, 15% of which are clinical trials. In contrast, polymer camptothecin publications exceed 350, five of which are clinical trials or 1.4% of publications (Figure 3), while liposomal formulations containing a camptothecin derivative have received just over 300 papers with 14 clinical trials or 4.7% (Figure 3). We also identified the publication statistics of platinates, which follow similar trends as summarized in Table 1 and represented graphically in Figure 3, but not discussed here.
Figure 3.
Polymer and liposomal cumulative publication profiles with small molecule drugs. A: Publication profile of polymer and liposomal therapeutics containing a camptothecin derivative and B: publication profile of polymer and liposomal therapeutics containing platinum. Black lines: Polymer publications, Grey lines: liposomal publications, Solid lines: all publications and Dotted lines: clinical trials.
There are many factors leading to the imbalance of clinical trials when comparing polymers to liposomes even though they have a similar number of publications. One reason is that polymer researchers focus on the appropriate drug attachment to optimize the pharmacokinetics, while many liposomal formulations may be readily generated without the requirement to devise new chemical linkages what are in essence new molecular entities. Thus liposomes allow for a wider range of release rates of the parent compound without generating new molecular entities. This enables efficacy studies to be directly correlated to the liposome properties and formulation characteristics.
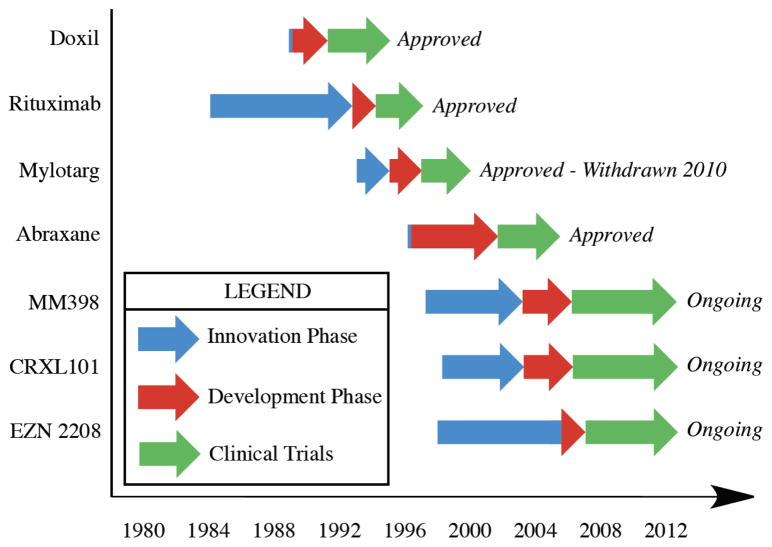
Evaluation of the publication record of two not yet approved polymeric drugs was then performed to compare to their trajectories of the approved drugs. These polymer nanocarriers are two of the more promising nanomedicines currently in clinical trials and are both based on the delivery of camptothecin. One nanocarrier (CRLX 101) is a cyclodextrin polymer containing camptothecin currently in Phase II clinical trials with Cerulean Pharma Inc. [50] and the other (EZN-2208) is a branched PEG construct by Enzon also in Phase II clinical trials. Due to the use of the same parent drug, the invention phase with these two nanomedicines overlap.
The inventions that lead to CRLX 101 include the patent for linear cyclodextrin copolymers in 1998 [51] which was published as a cationic transfection reagent in 2003 [52]. The polymer was then redesigned to prevent cytotoxicity while including a carboxylic acid for camptothecin attachment [53] and optimized for efficacy in mice a year later [54]. Phase I clinical trials with CRLX 101 started in 2006 and proceeded into phase II trials in 2008, which are still ongoing and to be completed in April 2013 [55]. The number of publications leading to phase two trials is limited to just six publications, one of which is a clinical trial. While CRLX 101 is being investigated by Cerulean Pharma Inc. the original polymer was developed in academia in collaboration with Insert Therapeutics Inc., now Calando. Calando then licensed the rights of CRLX 101 to Cerulean Pharma Inc., but it is clear that the focus of Davis et al. and the industrial collaborations have pushed cyclodextrin polymers into the clinic rapidly for camptothecin delivery as well as siRNA delivery.
Enzon’s PEG-camptothecin construct follows a more circuitous route due to poor phase II efficacy results received in 2005. PEG-camptothecin was first reported in 1998 by Greenwald [56] as a method to improve solubility of the drug and as a method to improve lactone stability. This version of pegylated camptothecin consisted of a linear 40kDa poly(ethylene glycol) with two camptothecin moieties at each end. Five more publications followed the original report in 1998, which led to a Phase II clinical trial in 2003, however, poor efficacy data led Enzon to pull the compound from clinical trials. After reevaluating the observed issues from the first construct they reformulated the compound with a branched 40kDa poly(ethylene glycol) containing a camptothecin derivative at each of four ends [57]. The camptothecin derivative employed is the metabolite of irinotecan and with four drug moieties improves the drug loading in this system. This construct entered the clinic in 2007 and is currently in phase II clinical trials with four publications thus far.
We then compare this to liposomal irinotecan, which is also in clinical development, but as part of the imitation phase following Doxil’s approval. Liposomal irinotecan was developed as a nanomedicine by Hermes Biosciences Inc. using a novel method of drug loading based on a patent filed in 1997 [58]. Thirty years after the development of liposomes [26] and the discovery of camptothecin [48], liposomal formulations containing camptothecin derivatives were first reported in the literature [59]. Drummond and coworkers then applied Kirpotin’s patent of intraliposomal drug stabilization [58] to a camptothecin derivative, irinotecan, as a basis for the MM-398 [60]. Furthermore, this formulation entered clinical trials in the same year and continues to be investigated in Phase III trials by Merrimack Pharmaceuticals. We have summarized the innovation phase in Figure 4 to emphasize the amount of time each of these nanomedicines have taken from invention to innovation to clinical trials and approval.
Figure 4.
Nanomedicine innovation and approval timeline. Blue arrows: Innovation phase, period from last invention to drug innovation; Red Arrows: Development phase, period from innovation to clinical trials; Green Arrows: Clinical trial phase, period from clinical trials to FDA decision. Lack of a blue arrowhead denotes concurrent innovation and clinical trials.
6. Lessons Learned Moving Forward
From these examples of approved drugs, and drugs in clinical trials during the innovation phase or the imitation phase all have similar publication trajectories. In the examples of approved nanomedicines, there are very few publications prior to FDA approval and in all cases even fewer publications prior to clinical trials. This is in stark contrast to the necessity for academicians to continue to publish to receive funding and the National Cancer Institute’s criteria of an extended publication list to receive grants. If in fact the NCI aims to bring anti-cancer drugs from bench to bedside, then the focus should be on funding nanocarrier drug formulations where there are few publications as a way to change the focus from publishing to drug approval as is the case with pharmaceutical companies. The more important issue is that inventors, innovators and imitators currently apply for the same funding from government sources. Perhaps a more appropriate solution that will result in more drugs in the clinic is to allow inventors to apply for basic science funding, innovators to apply for drug development funding and once a drug is approved specific funds are established for imitators.
This suggestion is like the child’s tale of a cat (drug development reality) who was very effective at keeping down the mouse population (drug approval). The mice decided the problem would be solved if they could put a bell (predictor of success) on the cat’s collar to alert them when the cat was coming. The problem is who can put the bell on the cat (identify potential solutions for nanocarrier drug delivery). Clearly we are unable to identify which basic science projects will translate into successful drugs. Our solution is to not try. Rather, we would replace peer review of basic science/new systems grants with an evolution inspired lottery system. Researchers with a documented laboratory to undertake the research would submit their CV and a 250-word abstract of their research plan. Program officials would validate the capability of each investigator to undertake the research and funding would be based on a lottery system. Some percentage of abstracts would be selected for two years of funding at a modest support level per year. An investigator would only be permitted to have one lottery grant.
At first, one might say ‘everyone would apply and poor ideas will be funded’. Our response is that ‘yes, everyone will apply and some poor ideas will be funded’ but this happens now. It has been documented that reviewers are unlikely to respond well to new ideas [61,62]. Rather reviewers promote established ideas. So seeding research by lottery will seed some new ideas and thus fund innovators. These new ideas could then receive additional funding by established channels. Like a favorable mutation, a new idea could evolve into a new drug treatment.
Furthermore, in 2011, the world’s governments spent about $10 billion to fund nanotechnology research with the US allocating nearly $2 billion to The National Nanotechnology Initiative and over $400 million of their budget directed to the NIH [63]. With increased funding directed toward nanotechnology, the structure of separated funding for inventors, innovators and imitators will enable more efficient development of guidelines for approval of each nanomedicine since those destined for the clinic will be identified from the onset. There are currently many nanomedicines in clinical trials [5] and with more nanomedicines being approved each year it is clear that the approval of these therapeutics lead to improved regulatory guidelines. This is truly what drives the imitation phase observed after the approval of a drug. Of great importance to the field of nanomedicines and drug delivery is the introduction of the Nanotechnology Regulatory Science Act of 2011 introduced by Sen. Mark Pryor, which will enable FDA spending to “…identify generalized principles and characteristics regarding the behavior of classes of nanomaterials with biological systems” [64]. This is a critical piece of legislation with the goal properly evaluating nanomedicines to generate clear guidelines for the approval of nanomedicines with the hope of delivering more drugs into the clinic.
One final aspect of drug approval that will facilitate more nanomedicines in the clinic is the surge in collaborative efforts between academia and the pharmaceutical companies [65]. While these programs are still in their infancy and issues related to intellectual property will continue to be addressed, the collaborations initialed through these partnerships will have lasting impacts on the field of drug development and nanomedicine approval.
Nanocarriers have a clear role to play in the future of therapeutics [66]. With a variety of nanocarriers entering the clinic and many more to come it is clear that the growing field has much more to offer to society. Inventors, innovators and imitators each hold an integral part to the progress of drug development and as we have shown here if we are truly interested in bringing more drugs into the clinic we should focus less on our publication record and more on devising scientific progress that translates into patient treatment.
Acknowledgments
This work was supported by NIH R01 GM061851, R21 AI093135. VJ Venditto was funded by an NIH Ruth L. Kirschstein National Research Service Award 1F32AI095062-01A1.
We thank Dr. Colin L. Walsh (UCSF) and Matthew R. Tiffany (UCSF) for helpful discussion and suggestions regarding the content of the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feynman RP. There’s plenty of room at the bottom. Eng Sci (CalTech) 1960;23:22–36. [Google Scholar]
- 2.Sarker D, Workman P. Pharmacodynamic biomarkers for molecular cancer therapeutics. Adv Cancer Res. 2007;96:213–268. doi: 10.1016/S0065-230X(06)96008-4. [DOI] [PubMed] [Google Scholar]
- 3.Blanco E, Hsiao A, Mann AP, Landry MG, Meric-Bernstam F, Ferrari M. Nanomedicine in cancer therapy: Innovative trends and prospects. Cancer Sci. 2011;102:1247–1252. doi: 10.1111/j.1349-7006.2011.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexis F, Pridgen EM, Langer R, Farokhzad OC. Nanoparticle technologies for cancer therapy. In: Schafer-Korting M, editor. Drug Delivery, Handbook of Experimental Pharmacology. Vol. 197. Springer-Verlag; Berlin: 2010. pp. 55–86. [DOI] [PubMed] [Google Scholar]
- 5.Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol Pharmaceut. 2011;8:2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 6.McNamee LM, Ledley FD. Patterns of technological innovation in biotechnology. Nature Biotechnol. 2012 doi: 10.1038/nbt.2389. In Press. [DOI] [PubMed] [Google Scholar]
- 7.Venditto VJ, Simanek EE. Cancer Therapies utilizing the camptothecins: A review of the in vivo literature. Mol Pharmaceut. 2010;7:307–349. doi: 10.1021/mp900243b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986;72:6387–6392. [PubMed] [Google Scholar]
- 9.Brem H, Lawson HC. The development of new brain tumor therapy utilizing the local and sustained delivery of chemotherapeutic agents form biodegradable polymers. Cancer. 1999;86:197–199. doi: 10.1002/(sici)1097-0142(19990715)86:2<197::aid-cncr2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Morpurgo M, Pasut G, Veronese FM. PEGylated proteins as cancer therapeutics. In: Torchilin VP, editor. Delivery of Protein and Peptide Drugs in Cancer. World Scientific Publishing Co; Singapore: 2006. pp. 85–110. [Google Scholar]
- 11.Maeda H. SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv Drug Deliv Rev. 2001;46:169–185. doi: 10.1016/s0169-409x(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 12.Fox ME, Szoka FC, Fréchet JM. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barenholz Y. Doxil – The first FDA-approved nano-drug: Lessons learned. J Control Release. 2012 doi: 10.1016/j.conrel.2012.03.020. In press. [DOI] [PubMed] [Google Scholar]
- 14.Marcato PD, Duran N. New aspects of nanopharmaceutical delivery systems. J Nanosci Nanotechnol. 2008;8:1–14. doi: 10.1166/jnn.2008.274. [DOI] [PubMed] [Google Scholar]
- 15.Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell. 2012;148:1081–1084. doi: 10.1016/j.cell.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman LS, Wintrobe MM. Nitrogen mustard therapy. J Am Med Assoc. 1946;132:126–32. doi: 10.1001/jama.1946.02870380008004. [DOI] [PubMed] [Google Scholar]
- 17.Stashenko P, Nadler LM, Hardy R, Schlosssman SF. Characterization of a human B lymphocyte- specific antigen. J Immunol. 1980;125:1678–1685. [PubMed] [Google Scholar]
- 18.Nadler LM, Ritz J, Hardy R, Pesando JM, Schlossman SF, Stashenko P. A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin Invest. 1981;67:134–140. doi: 10.1172/JCI110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison SL, Johnson MJ, Herzenberg SA, Oi VT. Chimeric human antibody molecules: Mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci USA. 1984;81:6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulianne GL, Hozumi N, Shulman MJ. Production of functional chimaeric mouse/human antibody. Nature. 1984;312:643–646. doi: 10.1038/312643a0. [DOI] [PubMed] [Google Scholar]
- 21.Horibe K, Nadler LM. Human B cell associated antigens defined by monoclonal antibodies. In: Heise EL, editor. Lymphocyte surface antigens. American Society for Histocompatability and Immunogenetics; New York: 1984. pp. 309–323. [Google Scholar]
- 22.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 23.Anderson DR, Hanna N, Leonard JE, Newman RA, Reff ME, Rastetter WH. United States Patent 5,736,137. Therapeutic application of chimeric and radiolabeled antibodes to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma. 1998
- 24.Arcamore F, Franceschi G, Orezzi P, Cassinelli G, Barbieri W, Mondelli R. Daunomycin. I. The structure of daunomycinone. J Am Chem Soc. 1964;86:15–19. [Google Scholar]
- 25.Arcamore F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C, Spalla C. Adriamycin, 14- hydroxydaimomycin, a new antitumor antibiotic from S. Peucetius var. caesius. Biotechnol Bioeng. 1969;11:1101–1110. doi: 10.1002/bit.260110607. [DOI] [PubMed] [Google Scholar]
- 26.Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Molec Biol. 1964;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 27.Gregoriadis G, Leathwood PD, Ryman BE. Enzyme entrapment in liposomes. FEBS Lett. 1971;14:95–99. doi: 10.1016/0014-5793(71)80109-6. [DOI] [PubMed] [Google Scholar]
- 28.Gregoriadis G. The carrier potential of liposomes in biology and medicine. New Engl J Med. 1976;295:704–710. doi: 10.1056/NEJM197609232951305. [DOI] [PubMed] [Google Scholar]
- 29.Gregoriadis G. The carrier potential of liposomes in biology and medicine. New Engl J Med. 1976;295:765–770. doi: 10.1056/NEJM197609302951406. [DOI] [PubMed] [Google Scholar]
- 30.Forssen EA, Tokes ZA. In vitro and in vivo studies with adriamycin liposomes. Biochim Biophys Res Commun. doi: 10.1016/0006-291x(79)91207-5. [DOI] [PubMed] [Google Scholar]
- 31.Szoka F, Olson F, Heath T, Vail W, Mayhew ED. Papahadjopoulos, Preparation of unilamellar liposomes of intermediate size (0.1–0.2 mumol) by a combination of reverse phase evaporation and extrusion through polycarbonate membranes. Biochim Biophys Acta. 1980;3:559–571. doi: 10.1016/0005-2736(80)90558-1. [DOI] [PubMed] [Google Scholar]
- 32.Woodle MC, Martin FJ, Yau-Yang A, Redmann CT. United States Patent 5,013,556. Liposomes with Enhanced Circulation Time. 1991
- 33.Barenholz Y. United States Patent 5,192,549. Loading and controlled release of amphiphatic molecules to and from liposomes. 1993
- 34.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphiphatic weak base. Biochim Biophys Acta. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
- 35.Lee MD, Manning JK, Williams DR, Kuck NA, Testa RT, Borders DB. Calicheamicins, a novel family of antitumor antibiotics. III. Isolation, purification and characterization of calicheamicins beta 1 Br, gamma 1 Br, alpha 2I, alpha 3I, beta 1I and delta 1I. J Antibiot. 1989;42:1070–1087. doi: 10.7164/antibiotics.42.1070. [DOI] [PubMed] [Google Scholar]
- 36.Hinman LM, Hamann PR, Wallace R, Menendez AT, Durr FE, Upeslacis J. Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer Res. 1993;53:3336–3342. [PubMed] [Google Scholar]
- 37.Trouet A, Masquelier M, Baurain R, Deprez-De Campeneere D. A covalent linkage between daunorubicin and proteins that is stable in serum and reversible by lysosomal hydrolases, as required for a lysosomotropic drug-carrier conjugate: in vitro and in vivo studies. Proc Natl Acad Sci USA. 1982;79:626–629. doi: 10.1073/pnas.79.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res. 1984;8:521–534. doi: 10.1016/0145-2126(84)90001-8. [DOI] [PubMed] [Google Scholar]
- 39.Hamann PR, Hinman L, Hollander I, Holycomb R, Hallett W, Tsou H-R, Weiss MJ. United States Patent 5,739,116. Enediyne derivatives useful for the synthesis of conjugates of methyltrithio antitumor agents. 1998
- 40.Sievers EL, Appelbaum FR, Spielberger RT, Forman SJ, Flowers D, Smith FO, Shannon-Dorcy K, Berger MS, Bernstein ID. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immuoconjugate. Blood. 1999;93:3678–3684. [PubMed] [Google Scholar]
- 41.Klotz IM. The effects of salts and proteins on the spectra of some dyes and indicators. Chem Rev. 1947;41:373–399. doi: 10.1021/cr60129a014. [DOI] [PubMed] [Google Scholar]
- 42.Collipp PJ, Snyder RD. Short Asthmatic children and human growth hormone: Evaluation of albumin- bound growth hormone. Clin Pediatr. 1968;7:659–664. doi: 10.1177/000992286800701106. [DOI] [PubMed] [Google Scholar]
- 43.Magnenat G, Schindler R, Isliker H. Transport of cytostatic agents by the plasma proteins. III. In vitro antitumor action of cytostatic-azoprotein conjugates. Eur J Cancer. 1969;5:33–40. doi: 10.1016/0014-2964(69)90086-3. [DOI] [PubMed] [Google Scholar]
- 44.Dosio F, Brusa P, Crosasso P, Arpicco S, Cattel L. Preparation, characterization and properties in vitro and in vivo of paclitaxel-albumin conjugate. J Control Res. 1997;47:293–304. [Google Scholar]
- 45.Desai NP, Tao C, Yang A, Louie L, Zheng T, Yao Z, Soon-Shiong P, Magdassi S. United States Patent 5,916,596. Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof. 1999
- 46.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, Esmaeli B, Ring SE, Bedikian A, Hortobagyi GN, Ellerhorst JA. Phase I and pharmacokinetic study of ABI–007, a cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–1044. [PubMed] [Google Scholar]
- 47.Miller RA, Hart S, Samoszuk M, Coulter C, Brown S, Czerwinski D, Kelkenberg J, Roystin I, Levy R. Shared idiotypes expressed by human B-cell lymphomas. New Engl J Med. 1989;321:851–857. doi: 10.1056/NEJM198909283211302. [DOI] [PubMed] [Google Scholar]
- 48.Wall ME, Wani MC, Cooke CE, Palmer KH, McPhail AT, Sim GA. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminate. J Am Chem Soc. 1966;88:3888–3890. [Google Scholar]
- 49.Daud A, Valkov N, Centeno B, Derderian J, Sullivan P, Munster P, Urbas P, DeConti RC, Berghorn E, Liu Z, Hausheer FH, Sullivan D. Phase II trial of karenitecin in patients with malignant melanoma: clinical and translational study. Clin Cancer Res. 2005;11:3009–3016. doi: 10.1158/1078-0432.CCR-04-1722. [DOI] [PubMed] [Google Scholar]
- 50.Yurkovetskiy AV, Fram RJ. XMT-1001, a novel polymeric camptothecin pro-drug in clinical development for patients with advanced cancer. Adv Drug Delivery Rev. 2009;61:1193–1202. doi: 10.1016/j.addr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Davis ME, Gonzalez H, Hwang S. United States Patent 6,509,323. Linear cyclodextrin copolymers. 2003
- 52.Gonzalez H, Hwang SJ, Davis ME. New class of polymers for the delivery of macromolecular therapeutics. Bioconj Chem. 1999;10:1068–1074. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- 53.Cheng J, Khin KT, Jensen GS, Liu A, Davis ME. Synthesis of linear beta-cyclodextrin-based polymers and their camptothecin conjugates. Bioconj Chem. 2003;14:1007–1017. doi: 10.1021/bc0340924. [DOI] [PubMed] [Google Scholar]
- 54.Cheng J, Khin KT, Davis ME. Antitumor activity of beta-cyclodextrin polymer-camptothecin conjugates. Molec Pharmaceut. 2004;1:183–193. doi: 10.1021/mp049966y. [DOI] [PubMed] [Google Scholar]
- 55.Davis ME. Design and development of IT-101, a cyclodextrin-containing polymer conjugate of camptothecin. Adv Drug Deliv Rev. 2009;61:1189–1192. doi: 10.1016/j.addr.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Greenwald RB, Pendri A, Conover CD, Lee C, Choe YH, Gilbert C, Martinez A, Xia J, Wu D, Hsue M. Camptothecin-20-PEG ester transport forms: the effect of spacer groups on antitumor activity. Bioorg Med Chem. 1998;6:551–562. doi: 10.1016/s0968-0896(98)00005-4. [DOI] [PubMed] [Google Scholar]
- 57.Sapra P, Kraft P, Mehlig M, Maltby J, Zhao H, Greenberger LM, Horak ID. Marked therapeutic efficacy of a novel polyethylene glycol-SN38 conjugate, EZN-2208, in xenograft models of B-cell non-Hodgkin’s lymphoma. Haematologica. 2009;94:1456–1459. doi: 10.3324/haematol.2009.008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirpotin D. United States Patent, 6,110,491. Compound-loaded liposomes and methods for their preparation. 2000
- 59.Sadzuka Y, Hirotsu S, Hirota S. Effect of liposomalization on the antitumor activity, side-effects and tissue distribution of CPT-11. Cancer Lett. 1998;127:99–106. doi: 10.1016/s0304-3835(98)00031-7. [DOI] [PubMed] [Google Scholar]
- 60.Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66:3271–3277. doi: 10.1158/0008-5472.CAN-05-4007. [DOI] [PubMed] [Google Scholar]
- 61.Eisenhart M. The paradox of peer review: Admitting too much or allowing too little? Res Sci Ed. 2002;32:241–255. [Google Scholar]
- 62.Thurner S, Hanel R. Peer-review in a world with rational scientists: Toward selection of the average. Eur Phys J. 2011;84:707–711. [Google Scholar]
- 63.National Science and Technology Council, Committee on Technology. The Nanotechnology Initiative – Supplement to the President’s 2012 Budget. 2011. [Google Scholar]
- 64.Nanotechnology Regulatory Science Act of 2011, S. 1662, 112th Cong. (2011).
- 65.Ratner M. Pfizer reaches out to academia – again. Nature Biotechnol. 2011;29:3–4. doi: 10.1038/nbt0111-3. [DOI] [PubMed] [Google Scholar]
- 66.National Institutes of Health. NIH News. May 3, 2012. NIH launches collaborative program with industry and researchers to spur therapeutic development. [Google Scholar]