Abstract
Leishmania amazonensis parasites can cause diverse forms of leishmaniasis in humans and persistent lesions in most inbred strains of mice. In both cases, the infection is characterized by a marked immunosuppression of the host. We previously showed that amastigote forms of the parasite make use of surface-exposed phosphatidylserine (PS) molecules to infect host cells and promote alternative macrophage activation, leading to uncontrolled intracellular proliferation of the parasites. In this study, we demonstrated that treatment of infected mice with an PS-targeting monoclonal antibody ameliorated parasite loads and lesion development, which correlated with increased proliferative responses by lymphocytes. In addition, we observed an enhanced dendritic cell (DC) activation and antigen presentation in vitro. Our data imply that the recognition of PS exposed on the surface of amastigotes plays a role in down-modulating DC functions, in a matter similar to that of apoptotic cell clearance. This study provides new information regarding the mechanism of immune suppression in Leishmania infection.
INTRODUCTION
Leishmania amazonensis (L. amazonensis) infection in humans can cause a broad spectrum of diseases, ranging from self-healing skin lesions to very severe diffuse cutaneous leishmaniasis (1). In addition, in contrast to other species such as L. major or L. braziliensis, L. amazonensis produces a progressive disease in most mouse strains (2, 3). The mechanism responsible for this enhanced host susceptibility to L. amazonensis parasites remains unclear, but it seems not to be related to the differentiation of type-2 CD4+ T cells. Actually, IL-4-deficient mice remain susceptible to the infection, and the lack of this cytokine does not substantially change the overall cytokine response in infected mice (4, 5). In L. amazonensis-infected mice, CD4+ T cell activation is relatively weak and does not induce robust cytokine production (2, 6). Furthermore, no T cell clones are preferentially expanded, since there is no predominance of a single subset of T cell receptor-bearing CD4+ T cells in these infected mice (7, 8). There is as yet no comprehensive explanation for these findings, but some immunological aspects of the infection are being reported. For example, L. amazonensis amastigotes are known to be highly competent at infecting antigen-presenting cells, without proper up-regulation of their effector functions. Amastigote infection does not lead to an increased surface expression of MHC class II and co-stimulatory molecules by infected MΦs and DCs (9–11) or increased IL-12 production by these cells (12, 13). Rather, amastigote infection actively inhibits the induction of these molecules by LPS (14). The JAK/STAT signaling pathway, which is involved in DC maturation and differentiation, is inhibited by amastigote infection through a mechanism dependent on proteasome degradation (15). All of the above-described phenomena affect the proliferative and effector responses of CD4+ T cells.
We have previously reported that amastigotes of L. amazonensis employ a unique strategy to infect and regulate MΦ activity via the externalization of phosphatidylserine (PS) molecules (16, 17). PS is a phospholipid located in the inner leaflet of the plasma membrane that is translocated transiently by some cell types during cell activation and differentiation (18–20) and permanently during apoptotic cell death (21). Externalized PS molecules become targets for receptors involved in apoptotic cell clearance and for triggering anti-inflammatory responses by phagocytes, mainly characterized by the production of TGF-β1 (19) We found that lesion-derived amastigotes make use of PS molecules in a similar way, maintaining those molecules on their surface which serve as ligands for parasite endocytosis and MΦ modulation, in a mechanism that we termed apoptotic mimicry (16, 17). PS exposure on intracellular pathogens operates in several different infection models to facilitate infection and avoid the immune system. Apoptotic mimicry is relevant for the infection of organisms such as Trypanosoma cruzi and Toxoplasma gondii, in which their respective infective stages expose PS as a strategy to silently invade host cells (22, 23). Viral particles that carry enveloped membranes from their previous host cells also make use of exposed PS molecules to invade new cells (24–27). In addition, by inducing transient PS exposure on the surface of host cells, viral infections can spread signals derived from PS recognition, such as TGF-β1 and IL-10 production by neighboring phagocytes, to avoid full activation of the immune system (24). In fact, in viral infection models, administration of an PS-targeting monoclonal antibody can cure about 35% of guinea pigs infected with a lethal dose of Pichinde virus (a model for the human Lassa fever). The efficiency of cure can reach up to 65% of the animals when PS-targeting mAb is combined with standard anti-viral drugs. Furthermore, PS-targeting mAb treatment was also effective at rescuing BALB/c mice with lethal murine cytomegalovirus infections (24).
Now, we demonstrate that PS-targeting treatment of mice infected with L. amazonensis parasites decreases tissue parasite loads and lesion development. The in vivo effect of the antibody-based treatment correlates both with increased T cell proliferation and increased DC activation in vitro. DCs infected with lesion-derived amastigotes treated with PS-targeting mAb become more efficient APCs, both for exogenous antigens, such as OVA, or for parasite antigens. PS-targeting treatment could be employed, in co-administration with leishmanicidal drugs, as a therapeutic strategy for the most severe clinical forms associated with L. amazonensis infection. Our findings lead us to suggest that PS exposure by intracellular amastigotes of L. amazonensis acts as a novel mechanism to down-modulate host immune responses.
MATERIALS AND METHODS
Mice and parasites
Female C57BL/6 mice deficient in FcR (B6.129P2-Fcer1gtm1RavN12), OTII mice [B6.129S6-Rag2tm1Fwa Tg (TcraTcrb) 425Cbn], were purchased from Taconic Farms (Germantown, NY). Their corresponding wild-type controls, as well as BALB/c mice, were purchased from Harlan Sprague Dawley (Indianapolis, IN). All mice were kept under specific pathogen-free conditions and used at 6–8 weeks of age, according to protocols approved by the Animal Care and Use Committee of the University of Texas Medical Branch. Promastigotes of L. amazonensis (LV78) were cultured at 23°C in Schneider’s Drosophila medium (Invitrogen, Carlsbad, CA), pH 7.0, supplemented with 20% FBS (Sigma, St. Louis, MO) and 50 μg/ml of gentamicin. Parasite infectivity was maintained by in vivo passages in BALB/c mice, and cultures of less than six passages were used for infection.
Mouse infection and Ab treatment
Mice were infected i.d. in the right ear with 0.5–1 × 106 promastigotes. Starting one day prior to infection and every 2 days thereafter they were given i. p. injections of 100 μg of mch1N11 mAb, a humanized antibody consisting of the VH and VL of the human monoclonal antibody, 1N11, linked to mouse IgG2a constant region domains. mch1N11 recognizes complexes formed by PS molecules and murine/human β2 glycoprotein-1 with a similar affinity to bavituximAb (28). Other groups of mice received PBS or the isotype control antibody Aurexis® (tefibazumAb), a humanized antibody that targets ClfA, a protein located on the surface of virtually all strains of Staphylococcus aureus (29).
Amastigote purification
Infected tissues or infected MΦs were finely minced and homogenized with a tissue grinder (Thomas Scientific, Swedesberg, NJ). The cell suspension was centrifuged at 50 g for 10 min at 4°C. The supernatant was carefully removed and further centrifuged and washed 3 more times at 1,450 g for 17 min at 4°C. After 2 h incubation under rotation at 34°C to liberate endocytic membranes (30), amastigotes were further centrifuged and incubated for 16 h at 34°C. After this time, they were centrifuged and washed 3 times before use.
Generation of bone marrow-derived dendritic cells
Bone marrow-derived DCs (BMDCs) were generated from mice by cultivating fresh bone-marrow cells in complete IMDM (Invitrogen) containing 10% FBS, supplemented with 20 ng/ml of recombinant GM-CSF (eBioscience, San Diego, CA). To generate immature BMDCs we added fresh medium containing GM-CSF at days 3 and 6 of culture and carefully harvested floating cells at day 8.
Flow Cytometry
For staining of lymphocytes and DCs, the following specific mAbs and their corresponding isotype controls were purchased from eBioscience: anti-CD80 (16-10A1), anti-CD83 (Michel-17), anti-I-A/I-E (M5/114.15.2), anti-CD86 (GL1) as well as rat IgG1, IgG2a, and IgG2b; anti-CD4 (GK1.5), anti-CD11c (N418), and hamster IgG. Briefly, cells were washed, blocked with 2% of rat/mouse serum (Sigma) and 1 μg/ml FcRγ blocker (CD16/32, eBioscience) for 15 min and stained for specific surface molecules. All incubation procedures were performed on ice.
T cell proliferation
Stationary phase promastigotes suspended in PBS were submitted to 5 cycles of freeze and thawing (liquid nitrogen and 37°C water bath). The homogenate was centrifuged, and the soluble fraction (SLA-soluble leishmanial antigen) was aliquoted and stored at −80°C. For the determination of T cell proliferation by CFSE dilution, LN cells or purified lymphocyte populations were washed with PBS and suspended in PBS containing 5 μM of carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen) to achieve the concentration of 107 cells per ml and incubated 15 min at 37°C. Twice the total volume of complete medium was added, the cells were pelleted, suspended in 10 ml of complete medium, and incubated for 30 min at 37°C. Cells were washed once with complete medium, counted, and plated in U-bottomed, 96-well plates for stimulation. For thymidine incorporation, LN cells were stimulated for 3 days and 48 h post stimulation, and H3-thymidine (Amersham, Pittsburgh, PA) was added to the final concentration of 50 μCi/ml. Lymphocytes were stimulated with 25 μg/ml of SLA (total LN cells) or DC pulsed with SLA for 24 h at 1:10 DC/T cell ratio (purified T cells).
Cytokine production
Cytokine production was measured in the supernatant of LN cells or DC cultures by using the Bio-Plex Pro-Mouse Cytokine 23-plex Assay from Bio-Rad (Hercules, CA) following the manufacturer’s instructions. The exception was TGF-β, which was measured by using the ELISA Ready-SET-Go system purchased from eBioscience.
Parasite quantification by real-time PCR
Parasite loads were quantified by measuring the gene of L. amazonensis cysteine proteinase isoform 1 (Llacys1), which is a single-copy gene per haploid genome, and expressed in both developmental stages (31). Briefly, at 3 weeks post-infection, tissues from the inoculation site (~0.5 × 0.5 cm2) were collected for DNA extraction with a DNeasy kit (Qiagen, Valencia, CA). DNA (100 ng) was used for parasite detection by the UTMB Real-time PCR Core Facility (all reagents were purchased from Applied Biosystems, Foster City, CA). Each sample was run in duplicate and normalized by the amount of total DNA extracted. The number of parasites per sample was calculated based on a standard curve, as described in our previous studies (32).
Histochemical analysis
Infected ear tissues were collected at 3 weeks post infection, fixed in formalin solution for 2 days, dehydrated and embedded in paraffin. Tissues were sectioned at 6–8 μm, paraffin was removed, and the sections were stained by standard methods with hematoxylin and eosin. Infected BMMΦs attached to 13-mm2 coverslips were fixed in 100% methanol for 10 min and stained for 45 min with Giemsa (Sigma) diluted 1/5 in water. Slides were mounted by using Canadian balsam (Sigma). Micrographs were obtained by examining mounted slides under an AxioPlan II microscope (Zeiss, Thornwood, NY).
Statistical analysis
One- or two-way ANOVA was used for multiple group comparisons (GraphPad Software v5.0, San Diego, CA). Statistically significant values are referred to as follows: *, p<0.05; **, p<0.01; ***, p<0.001.
RESULTS
Attenuated disease following PS-targeting treatment in mice
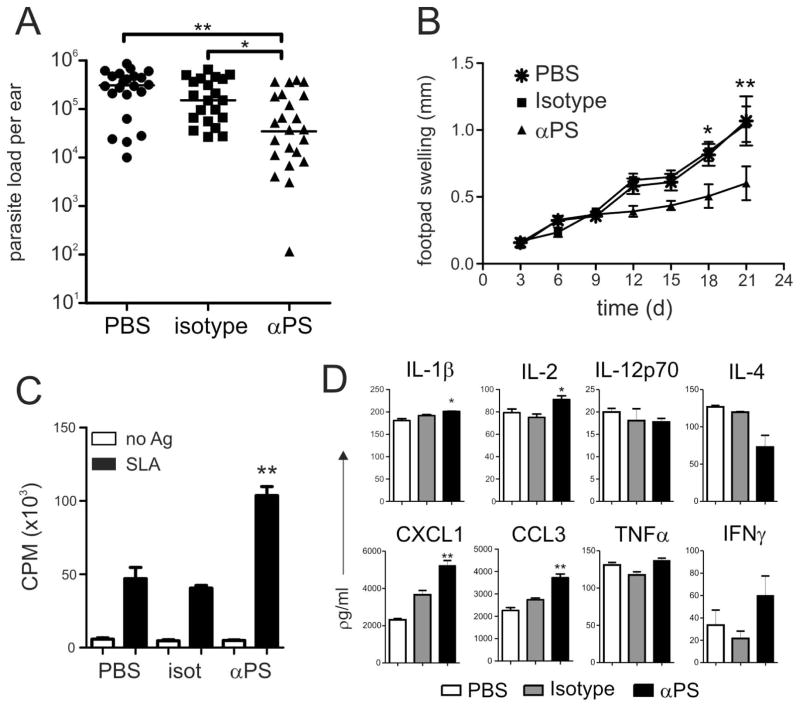
L. amazonensis amastigotes can utilize PS exposure as an important mechanism for macrophage infection and repressing host immune responses (19, 56). Enveloped viral particles employ PS exposure as a strategy to invade host cells. In some cases, treatment of infected mice with an PS-targeting monoclonal antibody led to the clinical cure of the infection (26, 27). To test whether PS-targeting antibody administration has a beneficial effect in L. amazonensis infection, we infected C57BL/6 mice in the ear dermis with 0.5–1×106 stationary-phase promastigotes and gave them intra-peritoneal injections of PS-targeting mAb (clone mch1N11, 100 μg) or an isotype control (Aurexis®, 100 μg) every 2 days, starting one day prior to infection. By the end of the third week of infection, we determined tissue parasite loads by real-time PCR. We found that treatment with PS-targeting, but not the isotype control mAb, significantly reduced tissue parasite loads compared to those in PBS-treated mice (Fig. 1A). PS-targeting-treated mice developed significantly smaller lesions at 18 and 21 days post-infection, although lesion development was still progressive in all group of mice (Fig. 1B). Histochemical analysis of infected ear tissue sections revealed that there were no overt alterations in the profile or the number of inflammatory cells that migrated to the lesions of the three groups of mice or accumulation of apoptotic cells in the tissue (Fig. S1). To understand the effect of PS-targeting treatment, we obtained draining lymph node (LN) cells at 3 weeks post-infection and evaluated cell proliferation and cytokine production after re-stimulation with soluble leishmanial antigen (SLA) for 3 days. Cells obtained from LNs of PS-targeting-treated mice had increased proliferative responses upon stimulation with SLA (Fig. 1C). While the cytokine profiles were generally comparable among all three groups of mice, significantly higher levels of IL-2, IL-1β, CXCL1 and CCL3 production by LN cells of PS-targeting-treated mice were observed (Fig. 1D). Our data indicate an attenuated disease development and enhanced T cell activation following PS-targeting mAb treatment.
Figure 1. PS-targeting treatment of infected mice ameliorates the disease.
C57BL/6 mice were infected with 0.5–1 × 106 parasites in the ear dermis or footpad and received i. p. injections of 100 μg of mAb mch1N11 (αPS) or isotype control every 2 days, starting one day prior to infection. (A) Parasite loads in the ear were determined by real-time PCR after 3 weeks of infection, and (B) footpad lesion sizes were determined by using a Vernon caliper. Draining LN cells from mAb-treated mice were obtained at 3 weeks post-infection and re-stimulated with SLA for 3 d. (C) A thymidine pulse was made, and, after 12 h, cell proliferation was evaluated or (D) SNs were collected and cytokine production measured by using a Bioplex kit. (A, C, D) Graph represents data from 3 pooled experiments. (B, C, D) Asterisks indicate statistical difference between αPS with isotype control (B) Graph represents one experiment with 5 mice per group. * p < 0.05, ** p < 0.01, *** p <0.001.
Enhanced DC and T cell activation following PS-targeting treatment of amastigotes in vitro
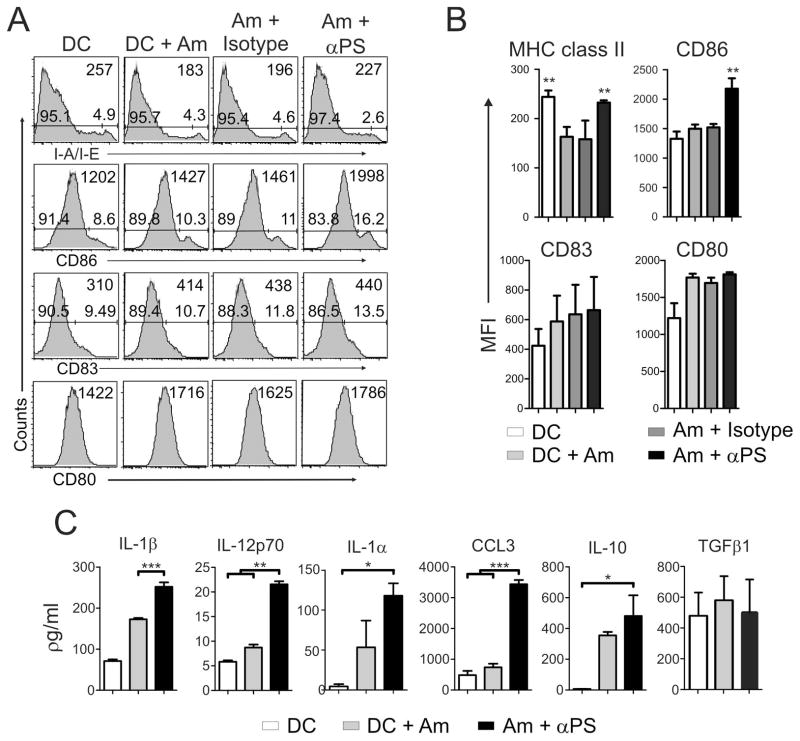
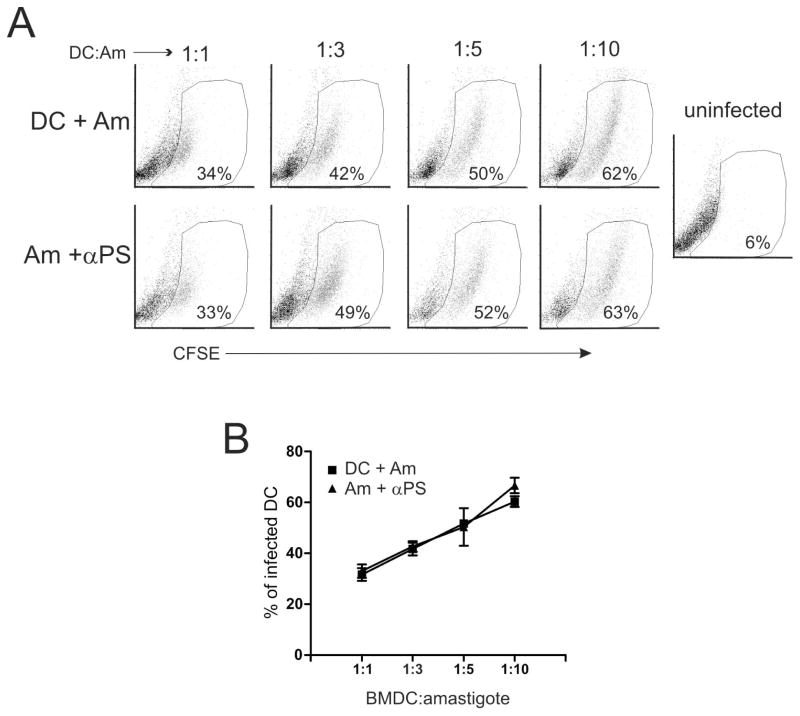
Recognition and uptake of apoptotic cells by antigen-presenting cells (APCs) are known to play a role in peripheral tolerance to self antigens (33). Upon recognition of non-self antigens derived from apoptotic cells or bodies, APCs can also be primed to induce T cell anergy (34–37). These effects are due to the stimulation of APCs through molecular patterns expressed by apoptotic cells, including complement factors, coupled serum proteins, and PS (34, 36, 38). Since PS-targeting treatment of infected mice decreased parasite tissue loads and enhanced the proliferative response of draining LN cells (Fig. 1), we investigated whether PS on amastigotes can modulate DC functions. We obtained amastigotes from lesions of BALB/c mice, which exposed high amounts of PS on their surface (Figure S2) (17), treated these parasites with PS-targeting or isotype control antibodies, and infected bone marrow-derived DCs. Then, we evaluated the expression of DC activation markers and cytokine production at 24 h post-infection. DCs infected with lesion-derived, PS-targeting-treated amastigotes presented no alterations regarding the expression of CD80 and CD83 molecules. However, expression of CD86 was up-regulated in the PS-targeting-treated group (Fig. 2A and B). As previously described (14), expression of MHC class II molecules was down-regulated on DCs infected with either untreated or isotype-treated amastigotes (Fig. 2A and B). This effect was abrogated by blocking PS molecules on the surface of the parasite (Fig. 2A and B). DCs infected with PS-targeting-treated amastigotes produced higher levels of IL-1α and β, IL-12p70, IL-10 and CCL3 as compared with DCs infected with untreated parasites (Fig. 2C). Unfortunately, we were not able to quantify cytokines produced by DCs infected with isotype control-treated parasites, due to kit limitations (Fig. 2C). These data suggest an increased inflammatory response of DCs infected with PS-targeting treated parasites, which may mean that PS exposure on the surface of the parasite prevented priming of DCs upon infection and contributed to the silent entry of the parasite. To access whether modulation of DC activation by blocking PS at the surface of the amastigotes is related to the number of internalized parasites, we infected DCs with lesion-derived, CFSE-labeled amastigotes that were pre-treated or not with mch1N11 PS-targeting antibodies and accessed internalization by flow cytometry. PS-targeting treated and untreated amastigotes infected DCs comparably (Fig. 3A and B), which differed from the findings from macrophage infection (16).
Figure 2. Blocking PS recognition boosts DC activation.
BMDCs were infected with lesion-derived amastigotes that were pre-treated with 9 μg/ml of mAb mch1N11 (αPS) or isotype control. After 24 h of infection, (A, B) DCs were harvested for the analysis of activation markers by flow cytometry, and (C) the production of cytokines in the SN was measured by a bioplex assay. (A) Graph representative of 3 independent experiments. Numbers at the upper-right of each plot represents the total population MFI of the indicated labeling. Numbers at the bottom of each plot indicate the percentage of positive/negative cells (B, C) Graph represents data from 3 pooled experiments. (B) Asterisks indicate statistical difference when compared to isotype control. Am – amastigotes. * p < 0.05, ** p < 0.01, *** p <0.001.
Figure 3. PS-targeting treatment does not alter amastigote uptake by DCs.
BMDCs were co-cultured with different amounts of lesion-derived, CFSE-labeled amastigotes that were pre-treated with 9 μg/ml of mAb mch1N11 (αPS). (A, B) After 24h of infection, BMDCs were harvested, stained for CD11c, and subjected to FACS analysis. Numbers in the dot plots indicate the percentage of CFSE+, CD11c+ BMDCs. (B) Graph represents data from 3 independent experiments.
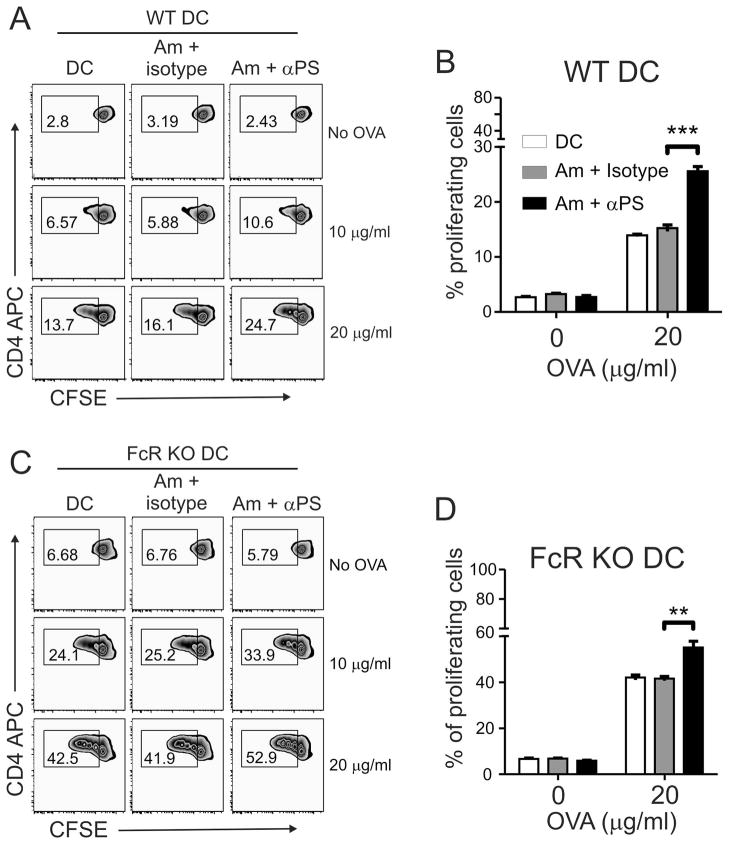
To understand the significance of these data, we tested the capacity of infected DCs for antigen presentation. We pulsed DCs with ovalbumin (OVA) and simultaneously infected them with lesion-derived amastigotes. After 24 h of infection, we harvested the DCs and cultivated them with CFSE-labeled CD4+ T cells purified from naïve OT-II transgenic mice to evaluate T cell proliferation after 3 days of co-culture. Fcγ Receptor (FcR) KO DCs (Fig. 4C and D) were used as a control for unspecific activation of DCs through FcR activation, since amastigotes treated with PS-targeting could be further opsonized (14, 17, 39). As shown in Fig. 4A and B, when wild-type DCs that had been infected with PS-targeting-treated amastigotes were used as APCs, the percentages of proliferative OT-II CD4+ T cells increased about 45% (comparing 5.8% in the isotype control vs. 10.6% in PS-targeting mAb, in the presence of 10 μg/ml OVA) or 35% (comparing 16.1% in the isotype control vs. 24.7% in PS-targeting mAb, in the presence of 20 μg/ml OVA). This effect was independent on FcRγII and FcRγIII since similar observations were made with FcR KO DCs, except that these KO DCs had higher baselines in presenting OVA to OT-II CD4+ T cells than did the wild-type counterparts, even in the absence of any parasites (Fig. 4C and D).
Figure 4. Blocking PS recognition enhances overall antigen presentation capacity of infected DCs.
(A, B) C57BL/6 WT and (C, D) FcR KO BMDCs were infected with lesion-derived amastigotes that were pre-treated with 9 μg/ml of mAb mch1N11 (PS-targeting) or isotype antibody in the presence or absence of different concentrations of OVA protein. After 24 h of infection, DCs were harvested and co-cultured with CFSE-labeled OTII CD4+ T cells for 3 days. The percentage of proliferating T cells was determined by CFSE dilution. (A, C) Graphs are representative of 3 independent experiments. (B, D) Graphs represent data from 3 pooled experiments. ** p <0.01, *** p <0.001.
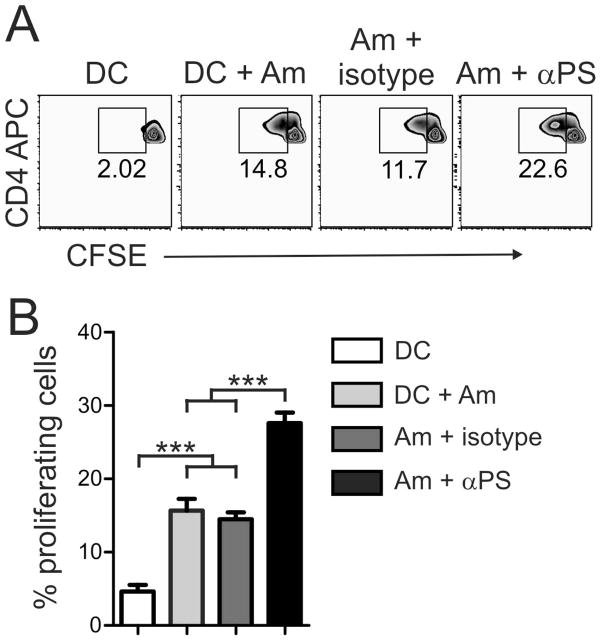
To find out whether PS-targeting treatment of amastigotes prior to infection could also improve the presentation of parasite antigens, we purified CD4+ T cells from draining LN of C57BL/6 mice at 5–7 weeks post-infection, labeled them with CFSE, and cultivated them with DCs that were infected with lesion-derived, PS-targeting-treated amastigotes. After 3 days of co-culture, we determined the percentage of proliferated CD4+ T cells. As shown in Fig. 5A, about 14.8% of CD4+ T cells re-stimulated with parasite antigens went through proliferation, whereas the percentage of proliferated T cells increased to 22.6% when PS-targeting-treated amastigotes were used to infect DCs. T cell proliferation levels were significantly increased in the PS-targeting-treated group (p < 0.01, Fig. 5B). Our data imply that DC antigen presentation was down-modulated by the recognition of PS molecules on the surface of lesion-derived amastigotes, leading to impaired proliferation of CD4+ T cells.
Figure 5. Blocking PS recognition enhances parasite antigen presentation by infected DCs.
(A) BMDCs were infected with lesion-derived amastigotes that were pre-treated with 9 μg/ml of mAb mch1N11 (PS-targeting) or isotype control. After 24 h of infection, DCs were harvested and co-cultured for 3 days with CFSE-labeled CD4+ T cells that were purified from draining LNs of infected mice. The percentage of proliferating cells was determined by CFSE dilution. (B) Graph represents data from 2 pooled experiments. *** p < 0.001.
DISCUSSION
The findings of surface-exposure of PS on intracellular pathogens and PS-targeting-based treatment are important for our understanding of immune regulation during host-pathogen interactions. For example, the presence of exposed PS on the viral membrane is essential for bleb formation, endocytosis, and infection of target cells (26, 27). Meanwhile, viruses that induce PS exposure in infected cells can also incorporate this phospholipid into their envelope during budding and egress (26), and HIV-1 is one of the examples (40, 41). More interesting, the administration of PS-targeting antibodies can promote the control of lethal infections with cytomegalovirus and Pichinde virus in the mouse and guinea pig models, respectively (24). The PS-targeting treatment may potentiate currently available anti-virus therapies (24).
In this cutaneous leishmaniasis model, we found decreased tissue parasite loads and attenuated lesions in mice receiving PS-targeting mAb, but not control mAb (Fig. 1). In contrast to the viral infection models, PS-targeting treatment was not sufficient to clear leishmanial infection. Our findings that PS-targeting treatment was able to restrain the kinetics of lesion progression (Fig. 1B) open a future research possibility for co-administering PS-targeting Ab and anti–leishmanial drugs (42) for better control of non-healing New World leishmaniasis associated with L. amazonensis infection. PS recognition either on the surface of the parasites or apoptotic host cells that migrate to the infection site is known to mediate anti-inflammatory responses, due to the induction of TGFβ1 and IL-10 production by macrophages and other surrounding cells (21, 43–46). Given the complex host-Leishmania infection and the low yield of lesion amastigotes from these mAb-treated mice, it was technically unfeasible for us to distinguish, at this stage, the direct effect of PS-targeting antibodies in our infection models. But, several possibilities may account for the attenuated infection in PS-targeting-treated mice. Firstly, PS-targeting mAb may act on some PS+ promastigotes (Fig. S2), because a small number of PS+ promastigotes is important for the PS− promastigotes to establish an infection (47). Secondly, PS-targeting mAb may act on PS+ host cells, even though our histological studies did not reveal overt changes in the profiles of infiltrated cells to the lesion sites (Fig. S1). Thirdly, PS-targeting mAb may act on amastigotes, which are virtually 100% PS+ cells (Fig S2) (17), a possibility that correlates with our observed lesion reduction on and after 12 days post-infection. This possibility also correlates with the blocking effects induced by annexin V and PS-targeting receptor antibodies observed from in vitro infection (16, 17). We feel that unspecific FcR activation due to further opsonization of PS-targeting-treated amastigotes does not play a major role in our infection system, as judged by our results observed using the in vitro infection of FcR KO dendritic cells (Fig. 4C and D) and in vivo treatment with humanized IgG PS-targeting antibodies (Fig. 1A and B), which are known to display lower affinity for mice FcγR (48). Regardless of the precise action of this PS-targeting mAb in vivo, it is clear that this treatment led to increased proliferative responses in lymphocytes purified from draining LN, as well as an increased production of cytokines and chemokines such as IL-1β, IL-2, CXCL1 and CCL3 (Fig 1C and D). Since several different lymphocyte populations are able to produce these cytokines and chemokines, we thought to further examine the impact of PS-targeting mAb by using in vitro infection systems. The description of the effect of apoptotic cells or PS liposomes on DCs function (33) led us to focus on the role of PS exposure by amastigotes on the antigen-presenting capacity of DCs. Recognition of PS on neutrophils or other apoptotic cells is capable of decreasing the expression of co-stimulatory molecules and blocking LPS-dependent DC activation (49). Antigens derived from apoptotic cells are known to be recognized and selected by DCs at the endosomal level, leading to a decreased formation of MHC class II-peptide complexes when compared to bacteria-derived antigens (50) and T cell tolerance induction (51–53). Apoptotic cell-dependent modulation of DCs activation and functions seems to be dependent on PS recognition in both human and mouse DCs (49). In this study, we observed that PS-targeting treatment of amastigotes prior to DC infection shifted DCs into an inflammatory state characterized by increased levels of IL-12p70, IL-1β/α, and CCL3 and a moderate increase in the expression of CD86 and MHC class II molecules (Fig. 2). IL-10 was also up-regulated in this system, a finding in agreement with reports showing the presence of this molecule when immune cells are prompted towards an inflammatory state, such as LPS stimulation (17, 54). This study confirms previous reports that infection with L. amazonensis amastigotes does not lead to DC activation (14, 15, 55). More importantly, it highlights the contribution of PS recognition to this immune suppression, because antigen presentation capacity of infected DCs was enhanced when amastigotes were pre-treated with PS-targeting antibodies. This conclusion was supported by the response to OVA, an exogenous antigen recognized by transgenic OT II cells, as well as by using CD4+ T cells primed for leishmanial antigens in vivo (Figs. 3 and 4). Of note, the enhanced proliferative response was not followed by the induction of a polarized T cell response (Fig. 1D). Since the induction of an efficient Th1 response is mandatory to achieve protection (56), PS-targeting treatment of amastigotes alone is not sufficient to induce a protective response. The effects of PS-targeting treatment were maintained when FcR KO DCs were used (Fig. 4C and D), suggesting that the increased amount of antibodies at the surface of the parasite, due to PS-targeting attachment, was not sufficient to induce FcR-dependent DC activation. This is possible that lesion-derived amastigotes are coated with host antibodies and that further parasite opsonization does not alter host cell activation or/and phagocytosis (57). The apparent reduction in the effect of PS-targeting treatment, observed when FcR KO DCs were used (Fig. 4C and D) is in accordance to the proposed “tethering and tickling” mechanism of PS recognition (58).
One of the challenges in L. amazonensis research is the stimulation of antigen-specific, high-quality T cell immune responses against the parasite. In the mouse model, immunization, vaccination and chemotherapeutic strategies were employed; however, none of them were sufficient to reach parasite clearance, and, as a consequence, the infection eventually progressed (59–61). This scenario is especially troubling in patients that develop diffuse cutaneous leishmaniasis, since they are refractory to most available treatments (1). Also, the recognition of PS-exposing amastigotes by receptors on the MΦ surface contributes to the production of TGF- β 1 and IL-10 and suppression of microbicidal effects of host cells (16, 17). Our in vivo and in vitro studies described in this report further strengthen the biological relevance of PS expression on L. amazonensis infection and the potential of PS-targeting antibodies in boosting host immune responses against the parasites. Since parasites replicate and persist within a specialized parasitophorous vacuole, a full understanding of PS in this infection will await genetic modification of the parasite to decrease or completely block PS exposure. One possible target is the enzyme phospholipid translocase, present in such organisms and successfully knocked-out in other Leishmania species (62). A better understanding of the pathogenic mechanism that involves PS exposure by amastigote forms and of a rational design of strategies to block its effect is fundamental for developing alternative strategies to treat clinical manifestations involving non-healing American cutaneous leishmaniasis.
Supplementary Material
Acknowledgments
We thank Linda Watkins for technical assistance and Mardelle Susman for assisting in manuscript preparation. This study was supported by NIH Grants AI043003 and AI076849 to L. S., a Brazilian National Research Council Scholarship (CNPq) to J. L. M. W. and the MCT/CNPq grant 471144/2008 to M. A. B.
References
- 1.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 2.Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect Immun. 2003;71:4278–4288. doi: 10.1128/IAI.71.8.4278-4288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vargas-Inchaustegui DA, Xin L, Soong L. Leishmania braziliensis infection induces dendritic cell activation, ISG15 transcription, and the generation of protective immune responses. J Immunol. 2008;180:7537–7545. doi: 10.4049/jimmunol.180.11.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones DE, Buxbaum LU, Scott P. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J Immunol. 2000;165:364–372. doi: 10.4049/jimmunol.165.1.364. [DOI] [PubMed] [Google Scholar]
- 5.Buxbaum LU, Uzonna JE, Goldschmidt MH, Scott P. Control of New World cutaneous leishmaniasis is IL-12 independent but STAT4 dependent. Eur J Immunol. 2002;32:3206–3215. doi: 10.1002/1521-4141(200211)32:11<3206::AID-IMMU3206>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Ji J, Sun J, Qi H, Soong L. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am J Trop Med Hyg. 2002;66:338–345. doi: 10.4269/ajtmh.2002.66.338. [DOI] [PubMed] [Google Scholar]
- 7.Ramer AE, Vanloubbeeck YF, Jones DE. Antigen-responsive CD4+ T cells from C3H mice chronically infected with Leishmania amazonensis are impaired in the transition to an effector phenotype. Infect Immun. 2006;74:1547–1554. doi: 10.1128/IAI.74.3.1547-1554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin L, Wanderley JL, Wang Y, Vargas-Inchaustegui DA, Soong L. The magnitude of CD4(+) T-cell activation rather than TCR diversity determines the outcome of Leishmania infection in mice. Parasite Immunol. 2011;33:170–180. doi: 10.1111/j.1365-3024.2010.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoine JC, Jouanne C, Lang T, Prina E, de Chastellier C, Frehel C. Localization of major histocompatibility complex class II molecules in phagolysosomes of murine macrophages infected with Leishmania amazonensis. Infect Immun. 1991;59:764–775. doi: 10.1128/iai.59.3.764-775.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoine JC, Lang T, Prina E, Courret N, Hellio R. H-2M molecules, like MHC class II molecules, are targeted to parasitophorous vacuoles of Leishmania-infected macrophages and internalized by amastigotes of L. amazonensis and L. mexicana. J Cell Sci. 1999;112 ( Pt 15):2559–2570. doi: 10.1242/jcs.112.15.2559. [DOI] [PubMed] [Google Scholar]
- 11.Prina E, Jouanne C, de Souza Lao S, Szabo A, Guillet JG, Antoine JC. Antigen presentation capacity of murine macrophages infected with Leishmania amazonensis amastigotes. J Immunol. 1993;151:2050–2061. [PubMed] [Google Scholar]
- 12.Cameron P, McGachy A, Anderson M, et al. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-kappaB signaling pathway. J Immunol. 2004;173:3297–3304. doi: 10.4049/jimmunol.173.5.3297. [DOI] [PubMed] [Google Scholar]
- 13.Ruhland A, Kima PE. Activation of PI3K/Akt signaling has a dominant negative effect on IL-12 production by macrophages infected with Leishmania amazonensis promastigotes. Exp Parasitol. 2009;122:28–36. doi: 10.1016/j.exppara.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prina E, Abdi SZ, Lebastard M, Perret E, Winter N, Antoine JC. Dendritic cells as host cells for the promastigote and amastigote stages of Leishmania amazonensis: the role of opsonins in parasite uptake and dendritic cell maturation. J Cell Sci. 2004;117:315–325. doi: 10.1242/jcs.00860. [DOI] [PubMed] [Google Scholar]
- 15.Xin L, Li K, Soong L. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol Immunol. 2008;45:3371–3382. doi: 10.1016/j.molimm.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Freitas Balanco JM, Moreira ME, Bonomo A, et al. Apoptotic mimicry by an obligate intracellular parasite downregulates macrophage microbicidal activity. Curr Biol. 2001;11 :1870–1873. doi: 10.1016/s0960-9822(01)00563-2. [DOI] [PubMed] [Google Scholar]
- 17.Wanderley JL, Moreira ME, Benjamin A, Bonomo AC, Barcinski MA. Mimicry of apoptotic cells by exposing phosphatidylserine participates in the establishment of amastigotes of Leishmania (L) amazonensis in mammalian hosts. J Immunol. 2006;176:1834–1839. doi: 10.4049/jimmunol.176.3.1834. [DOI] [PubMed] [Google Scholar]
- 18.Fischer K, Voelkl S, Berger J, Andreesen R, Pomorski T, Mackensen A. Antigen recognition induces phosphatidylserine exposure on the cell surface of human CD8+ T cells. Blood. 2006;108:4094–4101. doi: 10.1182/blood-2006-03-011742. [DOI] [PubMed] [Google Scholar]
- 19.Smrz D, Draberova L, Draber P. Non-apoptotic phosphatidylserine externalization induced by engagement of glycosylphosphatidylinositol-anchored proteins. J Biol Chem. 2007;282:10487–10497. doi: 10.1074/jbc.M611090200. [DOI] [PubMed] [Google Scholar]
- 20.Jeong J, Conboy IM. Phosphatidylserine directly and positively regulates fusion of myoblasts into myotubes. Biochem Biophys Res Commun. 2011;414:9–13. doi: 10.1016/j.bbrc.2011.08.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 22.Damatta RA, Seabra SH, Deolindo P, et al. Trypanosoma cruzi exposes phosphatidylserine as an evasion mechanism. FEMS Microbiol Lett. 2007;266:29–33. doi: 10.1111/j.1574-6968.2006.00495.x. [DOI] [PubMed] [Google Scholar]
- 23.Seabra SH, de Souza W, Damatta RA. Toxoplasma gondii exposes phosphatidylserine inducing a TGF-beta1 autocrine effect orchestrating macrophage evasion. Biochem Biophys Res Commun. 2004;324:744–752. doi: 10.1016/j.bbrc.2004.09.114. [DOI] [PubMed] [Google Scholar]
- 24.Soares MM, King SW, Thorpe PE. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat Med. 2008;14:1357–1362. doi: 10.1038/nm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laliberte JP, Moss B. Appraising the apoptotic mimicry model and the role of phospholipids for poxvirus entry. Proc Natl Acad Sci U S A. 2009;106:17517–17521. doi: 10.1073/pnas.0909376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 27.Mercer J, Helenius A. Apoptotic mimicry: phosphatidylserine-mediated macropinocytosis of vaccinia virus. Ann N Y Acad Sci. 2010;1209:49–55. doi: 10.1111/j.1749-6632.2010.05772.x. [DOI] [PubMed] [Google Scholar]
- 28.He J, Yin Y, Luster TA, Watkins L, Thorpe PE. Antiphosphatidylserine antibody combined with irradiation damages tumor blood vessels and induces tumor immunity in a rat model of glioblastoma. Clin Cancer Res. 2009;15:6871–6880. doi: 10.1158/1078-0432.CCR-09-1499. [DOI] [PubMed] [Google Scholar]
- 29.Domanski PJ, Patel PR, Bayer AS, et al. Characterization of a humanized monoclonal antibody recognizing clumping factor A expressed by Staphylococcus aureus. Infect Immun. 2005;73:5229–5232. doi: 10.1128/IAI.73.8.5229-5232.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saraiva EM, Pimenta PF, Pereira ME, de Souza W. Isolation and purification of amastigotes of Leishmania mexicana amazonensis by a gradient of Metrizamide. J Parasitol. 1983;69:627–629. [PubMed] [Google Scholar]
- 31.Lasakosvitsch F, Gentil LG, dos Santos MR, da Silveira JF, Barbieri CL. Cloning and characterisation of a cysteine proteinase gene expressed in amastigotes of Leishmania (L.) amazonensis. Int J Parasitol. 2003;33:445–454. doi: 10.1016/s0020-7519(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 32.Xin L, Vargas-Inchaustegui DA, Raimer SS, et al. Type I IFN receptor regulates neutrophil functions and innate immunity to Leishmania parasites. J Immunol. 184:7047–7056. doi: 10.4049/jimmunol.0903273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–231. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 34.Albacker LA, Karisola P, Chang YJ, et al. TIM-4, a Receptor for Phosphatidylserine, Controls Adaptive Immunity by Regulating the Removal of Antigen-Specific T Cells. J Immunol. 2010;185:6839–6849. doi: 10.4049/jimmunol.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia CQ, Campbell KA, Clare-Salzler MJ. Extracorporeal photopheresis-induced immune tolerance: a focus on modulation of antigen-presenting cells and induction of regulatory T cells by apoptotic cells. Curr Opin Organ Transplant. 2009;14:338–343. doi: 10.1097/MOT.0b013e32832ce943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurung P, Kucaba TA, Ferguson TA, Griffith TS. Activation-induced CD154 expression abrogates tolerance induced by apoptotic cells. J Immunol. 2009;183:6114–6123. doi: 10.4049/jimmunol.0901676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marin-Gallen S, Clemente-Casares X, Planas R, et al. Dendritic cells pulsed with antigen-specific apoptotic bodies prevent experimental type 1 diabetes. Clin Exp Immunol. 2010;160:207–214. doi: 10.1111/j.1365-2249.2009.04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann PR, Kench JA, Vondracek A, et al. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol. 2005;174:1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 39.Steplewski Z, Sun LK, Shearman CW, Ghrayeb J, Daddona P, Koprowski H. Biological activity of human-mouse IgG1, IgG2, IgG3, and IgG4 chimeric monoclonal antibodies with antitumor specificity. Proc Natl Acad Sci U S A. 1988;85:4852–4856. doi: 10.1073/pnas.85.13.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moody MA, Liao HX, Alam SM, et al. Anti-phospholipid human monoclonal antibodies inhibit CCR5-tropic HIV-1 and induce beta-chemokines. J Exp Med. 2010;207:763–776. doi: 10.1084/jem.20091281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lodge R, Ouellet M, Barat C, Andreani G, Kumar P, Tremblay MJ. HIV-1 promotes intake of Leishmania parasites by enhancing phosphatidylserine-mediated, CD91/LRP-1-dependent phagocytosis in human macrophages. PLoS One. 2012;7:e32761. doi: 10.1371/journal.pone.0032761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez U, Pinart M, Reveiz L, et al. Designing and reporting clinical trials on treatments for cutaneous leishmaniasis. Clin Infect Dis. 2010;51:409–419. doi: 10.1086/655134. [DOI] [PubMed] [Google Scholar]
- 43.Fadok VA, McDonald PP, Bratton DL, Henson PM. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. 1998;26:653–656. doi: 10.1042/bst0260653. [DOI] [PubMed] [Google Scholar]
- 44.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Boyanapalli R, McPhillips KA, Frasch SC, et al. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-gamma in a nitric oxide-dependent manner. J Immunol. 2010;185:4030–4041. doi: 10.4049/jimmunol.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wanderley JL, Pinto da Silva LH, Deolindo P, et al. Cooperation between apoptotic and viable metacyclics enhances the pathogenesis of Leishmaniasis. PLoS One. 2009;4:e5733. doi: 10.1371/journal.pone.0005733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Doffek K, Sugg SL, Shilyansky J. Phosphatidylserine regulates the maturation of human dendritic cells. J Immunol. 2004;173:2985–2994. doi: 10.4049/jimmunol.173.5.2985. [DOI] [PubMed] [Google Scholar]
- 50.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 51.Blachere NE, Darnell RB, Albert ML. Apoptotic cells deliver processed antigen to dendritic cells for cross-presentation. PLoS Biol. 2005;3:e185. doi: 10.1371/journal.pbio.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 54.O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin–10. Nat Rev Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 55.Soong L. Modulation of dendritic cell function by Leishmania parasites. J Immunol. 2008;180:4355–4360. doi: 10.4049/jimmunol.180.7.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 57.Guy RA, Belosevic M. Comparison of receptors required for entry of Leishmania major amastigotes into macrophages. Infect Immun. 1993;61:1553–1558. doi: 10.1128/iai.61.4.1553-1558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann PR, deCathelineau AM, Ogden CA, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velez ID, Gilchrist K, Arbelaez MP, et al. Failure of a killed Leishmania amazonensis vaccine against American cutaneous leishmaniasis in Colombia. Trans R Soc Trop Med Hyg. 2005;99:593–598. doi: 10.1016/j.trstmh.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Montalvo-Alvarez AM, Folgueira C, Carrion J, Monzote-Fidalgo L, Canavate C, Requena JM. The Leishmania HSP20 is antigenic during natural infections, but, as DNA vaccine, it does not protect BALB/c mice against experimental L. amazonensis infection. J Biomed Biotechnol. 2008;2008:695432. doi: 10.1155/2008/695432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell K, Diao H, Ji J, Soong L. DNA immunization with the gene encoding P4 nuclease of Leishmania amazonensis protects mice against cutaneous Leishmaniasis. Infect Immun. 2003;71:6270–6278. doi: 10.1128/IAI.71.11.6270-6278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weingartner A, Drobot B, Herrmann A, et al. Disruption of the lipid-transporting LdMT-LdRos3 complex in Leishmania donovani affects membrane lipid asymmetry but not host cell invasion. PLoS One. 2010;5:e12443. doi: 10.1371/journal.pone.0012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.