Abstract
Women who experience hot flashes as a side effect of tamoxifen therapy often try botanical remedies such as black cohosh to alleviate these symptoms. Since pharmacological activity of tamoxifen is dependent on the metabolic conversion into active metabolites by the action of cytochromes P450 2D6 and 3A4, the objective of this study was to evaluate whether black cohosh extracts can inhibit formation of active tamoxifen metabolites and possibly reduce its clinical efficacy.
At 50 µg/ml, a 75% ethanolic extract of black cohosh inhibited formation of 4-hydroxy-tamoxifen by 66.3%, N-desmethyl tamoxifen by 74.6% and α-hydroxy tamoxifen by 80.3%. In addition, using midazolam and dextromethorphan as probe substrates, this extract inhibited CYP3A4 and CYP2D6 with IC50 values of 16.5 and 50.1 µg/ml, respectively.
Eight triterpene glycosides were identified as competitive CYP3A4 inhibitors with IC50 values ranging from 2.3–5.1 µM, while the alkaloids protopine and allocryptopine were identified as competitive CYP2D6 inhibitors with Ki values of 78 and 122 nM, respectively.
The results of this study suggests that co-administration of black cohosh with tamoxifen might interfere with the clinical efficacy of this drug. However, additional clinical studies are needed to determine the clinical significance of these in vitro results.
Keywords: triterpenes, alkaloids, P450 inhibition, drug-herb interaction
Introduction
The roots/rhizomes of black cohosh (Cimicifuga racemosa L. (Nutt.) (syn. Actaea racemosa L.) have been used traditionally by Native Americans to treat colds, rheumatism as well as for alleviating menopausal symptoms such as hot flashes (McKenna et al., 2001). Because of the risks of hormone replacement therapy (Rossouw et al., 2002), black cohosh preparations are popular among women seeking alternative treatments for menopausal complaints (Mahady et al., 2003). Extensive preclinical and clinical investigations have provided conflicting evidence regarding effectiveness of black cohosh (Borrelli and Ernst, 2008). Early studies suggested that black cohosh extracts were effective in reducing the frequency and intensity of hot flashes among perimenopausal and postmenopausal women (Frei-Kleiner et al., 2005; Kronenberg and Fugh-Berman, 2002; Osmers et al., 2005; Wuttke et al., 2003), while several trials including recent double-blind placebo-controlled studies demonstrated no vasomotor symptom benefits (Geller et al., 2009; Jacobson et al., 2001; Liske et al., 2002; Newton et al., 2006). However, the impact of the recent negative results on consumer choices is hard to predict. Given the risks of hormone replacement therapy, many women will probably continue to use black cohosh preparations. Thus, the issue of potential interactions of black cohosh supplements with prescription medications remains clinically relevant.
We are studying potential interactions of black cohosh with tamoxifen (TAM) which is being used for the treatment or prevention of estrogen receptor-positive breast cancer. TAM reduces the risk of breast cancer in women at high risk by almost 50% and the rate of mortality by approximately 25% (EBCTCG; Fisher et al., 2005). Pharmacological effects of TAM depend on its metabolic conversion into two active metabolites, 4-hydroxytamoxifen (4-OH TAM) and N-desmethyl-4-hydroxytamoxifen (endoxifen), with endoxifen being more abundant in vivo (Lien et al., 1989; Stearns et al., 2003). In vitro studies have identified cytochromes P450 2D6 and CYP3A4 as the dominant, although not exclusive, isoforms responsible for the production of active metabolites (Crewe et al., 1997; Desta et al., 2004). In addition to active metabolites, TAM is converted into a number of other metabolites most notably α-hydroxytamoxifen (α-OH TAM), which has been implicated as a genotoxic metabolite of TAM (Phillips, 2001) (see Figure 1).
Figure 1.
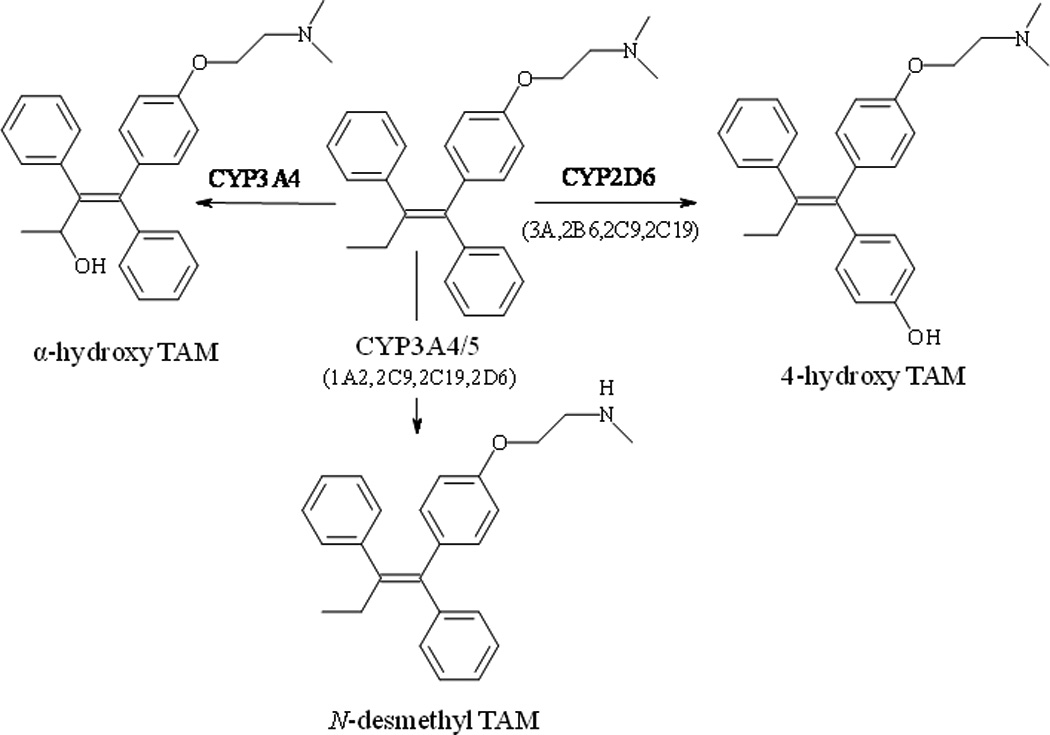
Metabolic pathways of tamoxifen. The contribution of isoforms to the formation of metabolites is labeled according to Desta et al. (Desta et al., 2004)
Based on these considerations, one can expect that co-administration of drugs or herbs that inhibit CYP2D6 and/or CYP3A4 can negatively impact clinical efficacy of TAM. This issue has received significant interest after Stearns and coworkers found that co-administration of selective serotonin reuptake inhibitors (SSRIs) with TAM significantly reduced in vivo concentrations of active TAM metabolites (Stearns et al., 2003). SSRIs are commonly prescribed to treat depression and hot flashes, which are serious side-effects of TAM therapy (Mortimer et al., 2008). However, some SSRIs, such as fluoxetine, are also potent CYP2D6 inhibitors, and there is a concern that these drugs may reduce effectiveness of TAM therapy. This work has spurred debate as to whether drugs that inhibit CYP2D6 should be prescribed together with TAM (Dezentje et al., 2010; Sideras et al., 2010).
Since hot flashes are the main indication for black cohosh use, women who experience this side effect of TAM may choose to use black cohosh as a presumably safe remedy. Even though the recent evidence suggests that black cohosh is ineffective for alleviation of menopausal hot flashes, this per se may not prevent women from taking these supplements to alleviate hot flashes associated with TAM therapy. Thus, it is of interest to understand whether black cohosh supplements can inhibit CYP450 enzymes involved in the metabolism of TAM. In this study, the potential of a 75% ethanolic extract of black cohosh to affect metabolism of TAM was evaluated in vitro using human liver microsomes pooled from multiple women donors as a model system.
Materials and methods
Chemicals and reagents
All chemicals except as noted below were obtained from Sigma-Aldrich (St. Louis, MO). α-OH TAM and the stable isotopically labeled internal standards, 4-OH TAM-ethyl-d5 and N-desmethyl-TAM-ethyl-d5, were obtained from Toronto Research Chemicals Inc., (North York, Ontario, Canada). Dextrorphan-d3, midazolam, 1’-hydroxy-midazolam and flurazepam were purchased from Cerilliant (Round Rock, TX). Allocryptopine was purchased from MP Biosciences (San Diego, CA) and protopine was purchased from ChemDiv (San Diego, CA). The investigated triterpene glycosides were isolated from roots/rhizomes and aerial parts of black cohosh using procedures described previously (Chen et al., 2002a; Chen et al., 2002b; Fabricant, 2006). Human liver microsomes pooled from ten women donors (protein content 20 mg/ml; cytochrome P450 total activity: 370 pmol/min × mg protein) were obtained from Gentest (Woburn, MA). All organic solvents were HPLC-grade and were purchased from Fisher Scientific (Fair Lawn, NY).
Plant materials
The raw plant material and the corresponding 75% ethanolic extract used in this study were identical to that used in a recent Phase II clinical trial and were previously described in detail (Chen et al., 2002a; Chen et al., 2002b; Fabricant, 2006; Geller et al., 2009; Powell et al., 2008; van Breemen et al., 2010). In brief, the plant material was acquired from Naturex (previously Pure World, South Hackensack, NJ), botanically authenticated by the UIC/NIH Center for Botanical Dietary Supplements Research, and characterized by PCR, vouchers and microscopy (Xu et al., 2002). Milled roots/rhizomes were extracted with 75% ethanol by large-scale percolation, vacuum-dried at 45 °C and milled though a 60-mesh screen to yield a powdered extract. The extract was standardized to 5.6% of four triterpene glycosides (Fabricant, 2006).
Bioassay-guided fractionation
Using the fractionation scheme outlined in Figure 2 (Powell et al., 2008), 75% ethanolic extract was first partitioned with between water and ethyl acetate. The water partition was fractionated on Amberlite XAD-2 resin to yield water and a methanol-soluble fractions. The methanol fraction was then subjected to pH-zone refinement fast centrifugal partition chromatography (FCPC) using water/butanol/ethyl acetate (5:4:1 v/v/v) as the solvent system to produce six chemically distinct fractions (FCPC 1–6). More detailed description of the fractionation procedure has been published elsewhere (Godecke et al., 2009b; Powell et al., 2008).
Figure 2.
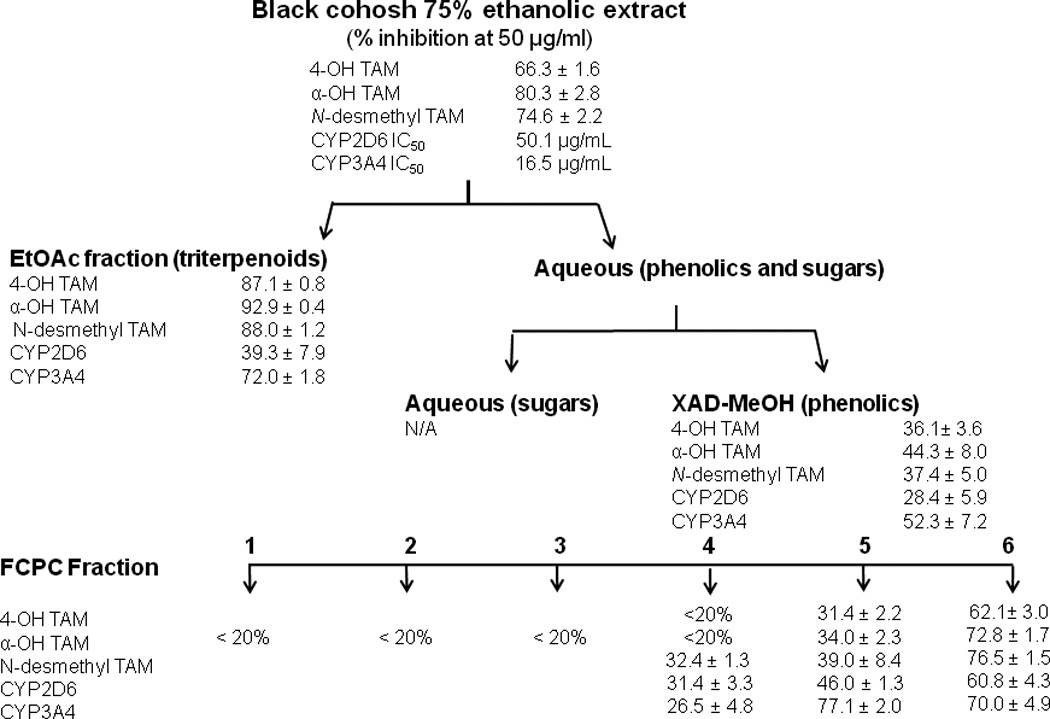
Results of the bioassay-guided fractionation. Crude extract and all fractions were tested at a final concentration of 50 µg/ml. Data represent average of triplicates ± S.D. CYP2D6 and CYP3A4 represent activities in standard assays for dextromethorphan demethylation and midazolam hydroxylation, respectively.
Tamoxifen metabolism by liver microsomes
Prior to conducting enzyme inhibition studies, experimental conditions such as incubation time and protein concentration were optimized to ensure that initial reaction rates could be measured and that no secondary metabolism occurred. In the final method, incubation mixtures (200 µL) consisting of 0.1 mg/ml liver microsomes, 2 µM TAM (diluted from 250 µM stock solution in 10 mM HCl) and black cohosh fractions (50 µg/ml) or isolated compounds (10 µM) in potassium phosphate buffer (0.1 M, pH 7.4) were preincubated for 10 min at 37°C before enzymatic reactions were initiated by addition of NADPH (1 mM). After a 20-min incubation period, the reactions were stopped by addition of ice-cold stop solution (CH3CN/H2O/HCOOH; 90:10:4, v/v/v) containing internal standards (4-OH TAM ethyl-d5 and N-desmethyl-TAM ethyl-d5). Positive controls were carried out with the selective inhibitors ketoconazole (1 µM; CYP3A4 inhibitor) and quinidine (1 µM; CYP2D6 inhibitor). Test compounds were dissolved in DMSO, and the final DMSO concentration did not exceed 0.1%, which was significant since CYP3A4 and some other isoforms are strongly inhibited by DMSO (Chauret et al., 1998).
LC-MS analysis of TAM metabolites
Reverse phase HPLC separations of TAM metabolites were carried out using an Atlantis® T3 (Waters, Milford, MA) 2.1 × 100 mm (5 µm particle size) C18 column with an Agilent (Palo Alto, CA) 1100 solvent delivery system. Metabolites were separated using a 30-min linear gradient from 26–60% MeCN in 0.1% aqueous formic acid at a flow rate of 0.21 ml/min. The column was thermostated at 30°C. The eluent from the column was introduced into an Agilent (Palo Alto, CA) single quadrupole mass spectrometer operated in positive ion electrospray mode. The data were acquired using selected ion monitoring (SIM) of protonated molecules α-OH TAM and 4-OH TAM at m/z 388.2, N-desmethyl TAM at m/z 358.2 and internal standards 4-OH TAM ethyl-d5 at m/z 393.2 and N-desmethyl TAM ethyl-d5 at m/z 363.2. Identification of metabolites was accomplished by comparison of retention times with authentic standards. The accuracies of the measurement of the investigated metabolites were >90%. The intra-assay coefficient of variation, which includes variability in incubation conditions as well as analytical method variability, was 10% for α-OH TAM, 12% for 4-OH TAM and 4% for N-desmethyl TAM (n=5).
CYP2D6 and CYP3A4 probe metabolism inhibition assay
Microsomal dextromethorphan N-demethylation (CYP2D6) and midazolam 1’-hydroxylation (CYP3A4) assays were carried out as previously described (Walsky and Obach, 2004). Metabolites were separated using a Zorbax Eclipse XDB (2.1 × 50 mm 1.8 µm) C18 column (Agilent, Santa Clara, CA) and a mobile phase consisting of 0.1 % formic acid in water (mobile phase A) and methanol (mobile phase B). Dextromethorphan metabolites were separated using the following linear gradient program: 19 % B for 2 min, 19 to 80 % B in 0.1 min, 80% B for 9 min, and finally returning to 19% B. Midazolam metabolites were separated using the gradient program that consisted of 40% B for 2 min, 40 to 80% B in 1 min, 80% B for 4 min, and finally returning to 40% B. The flow rate was 0.2 ml/min, and the column temperature was 33°C for all analyses. Positive ion electrospray was used with SIM to measure protonated molecules of dextrorphan at m/z 258.1 and internal standard dextrorphan-d3 at m/z 261.1 or protonated molecules of 1’-hydroxy-midazolam at m/z 342.2 and internal standard flurazepam at m/z 388.2.
LC-MS dereplication
Active fractions were analyzed by LC-MS using positive ion electrospray on a Waters Synapt© hybrid quadrupole/time-of-flight mass spectrometer. Separations were carried out on a Hypersil GOLD 2.1 × 150 mm 5 µm column using a gradient from 6–65% MeCN/0.1% formic acid over 35 min at a flow rate of 0.2 ml/min. Product ion tandem mass spectra were recorded at a collision energy of 25 eV. Elemental compositions and fragmentation data were searched against Beilstein CrossFire Commander database of known natural products. Final confirmation of proposed structures was carried out by comparison with authentic standards.
Results
Metabolites of TAM formed by human liver microsomes are shown in the LC-MS chromatogram in Figure 3. As expected, the most abundant metabolite was N-desmethyl TAM eluting at 22.6 min, while active metabolite 4-OH TAM (retention time 17.4 min) was a minor metabolite. The most abundant oxidation product was tamoxifen N-oxide eluting at 24 min. However, since tamoxifen N-oxide has not been implicated in the biological activity of TAM, it was not investigated further After the analytical method had been optimized, a 75% ethanolic extract of black cohosh was tested for its ability to inhibit formation of TAM metabolites. As shown in Figure 2, crude 75% ethanolic extract showed strong inhibition of formation of α-OH TAM, 4-OH TAM, and N-desmethyl-TAM. Results of the bioassay-guided fractionation indicate that most of the activity was concentrated in the ethylacetate partition and FCPC fractions 5 and 6 (Figure 2) and these fractions were further investigated in order to identify active components.
Figure 3.
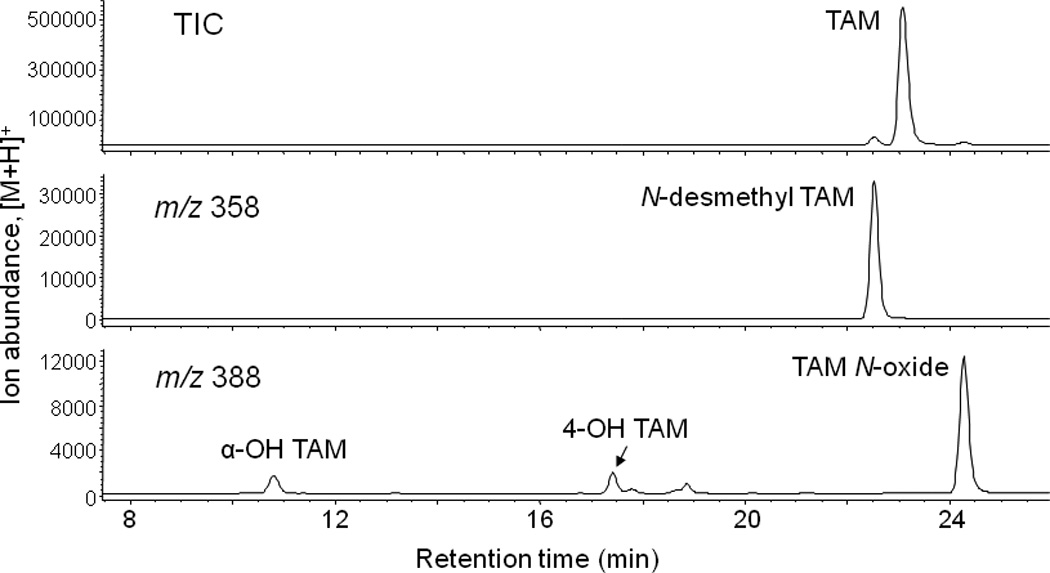
LC-MS chromatogram of tamoxifen metabolites formed during incubation of 2 µM tamoxifen with human liver microsomes pooled from women donors.
Identification and characterization of CYP3A4 inhibitors
Crude 75% ethanolic extract of black cohosh showed strong activity against CYP3A4 in the midazolam hydroxylation assay with an IC50 value of 16.5 µg/ml. Since LC-MS analysis revealed that the ethylacetate partition contained primarily triterpene glycosides (data not shown), we tested several purified compounds that had been previously isolated from black cohosh.. Among the 22 triterpenes tested, eight compounds showed more than 50% inhibition of CYP3A4 at the test concentration of 10 µM (Table 1 and Figure 4). All active compounds were moderately potent CYP3A4 inhibitors with IC50 values ranging from 2.3–5.1 µM. None of the tested compounds had activity against CYP2D6.
Table 1.
CYP3A4 inhibition by black cohosh triterpenes
| Compound | % inhibition (10 µM) | IC50(µM) | |
|---|---|---|---|
| 1 | 23-epi-26-deoxyactein | N/Aa | |
| 2 | 23-O-acetylshengmanol-3-α-L-arabinopyranoside (23R) | 81.6±0.9b | 2.3±0.2 |
| 3 | 25-anhydrocimigenol-3-O-β-xylopyranoside | N/A | |
| 4 | 26-deoxyactein | N/A | |
| 5 | Cimiracemoside P | N/A | |
| 6 | Cimiracemoside J | N/A | |
| 7 | Cimiracemoside K | 85.2±0.3 | 3.8±0.5 |
| 8 | 25-O-acetylcimigenol-3-O-β-xylopyranoside | N/A | |
| 9 | 25-O-acetylcimigenol-3-O-α-arabinopyranoside | N/A | |
| 10 | 25-anhydrocimigenol-3-O-β-arabinopyranoside | N/A | |
| 11 | Actein (R,S) | N/A | |
| 12 | 23-O-acetylshengmanol-3-β-D-xylopyranoside | 80.7±0.6 | 2.7±0.2 |
| 13 | Cimiracemoside N | N/A | |
| 14 | 2’-O-acetyl-Actein | N/A | |
| 15 | Cimiracemoside O | 74.8±0.1 | 5.1±0.4 |
| 16 | Cimiracemoside L | 94.2±0.3 | 2.4±0.2 |
| 17 | Cimicifugoside H-1 | N/A | |
| 18 | Cimicifugoside M | 65.8±0.5 | 4.3±0.3 |
| 19 | 1-α-hydroxycimigenol-3-O- α-L-arabinopyranoside | N/A | |
| 20 | 7-β-hydroxycimigenol aglycone | 64.9±1.1 | N/Dc |
| 21 | 1-α-hydroxycimigenol-3-O-β-D-xylopyranoside | N/A | |
| 22 | 25-O-acetyl-7-β-hydroxycimigenol-3-O-β-xylopyranoside | 61.8±0.2 | N/D |
N/A, Not active = < 50% inhibition at 10 µM
Data represent average of triplicates ± S.D
N/D Not determined due to small quantities of available compound
Figure 4.
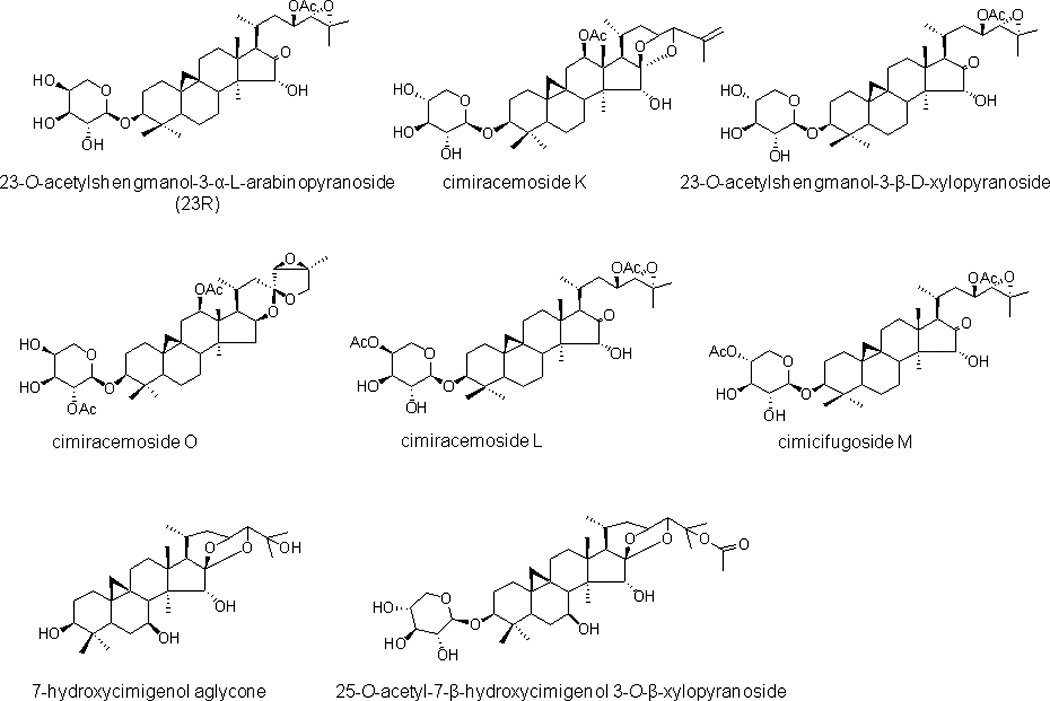
Structures of CYP3A4 inhibitors from black cohosh.
Next, we sought to determine the mechanism of inhibition by carrying out classical kinetics studies using several substrate and inhibitor concentrations. Due to sample limitations, only two active compounds, 16 and 12, were evaluated. The Lineweaver-Burk plots (Figure 5) indicate that both compounds are competitive inhibitors with Ki values of 1.1 µM and 1.7 µM for 16 and 12, respectively.
Figure 5.
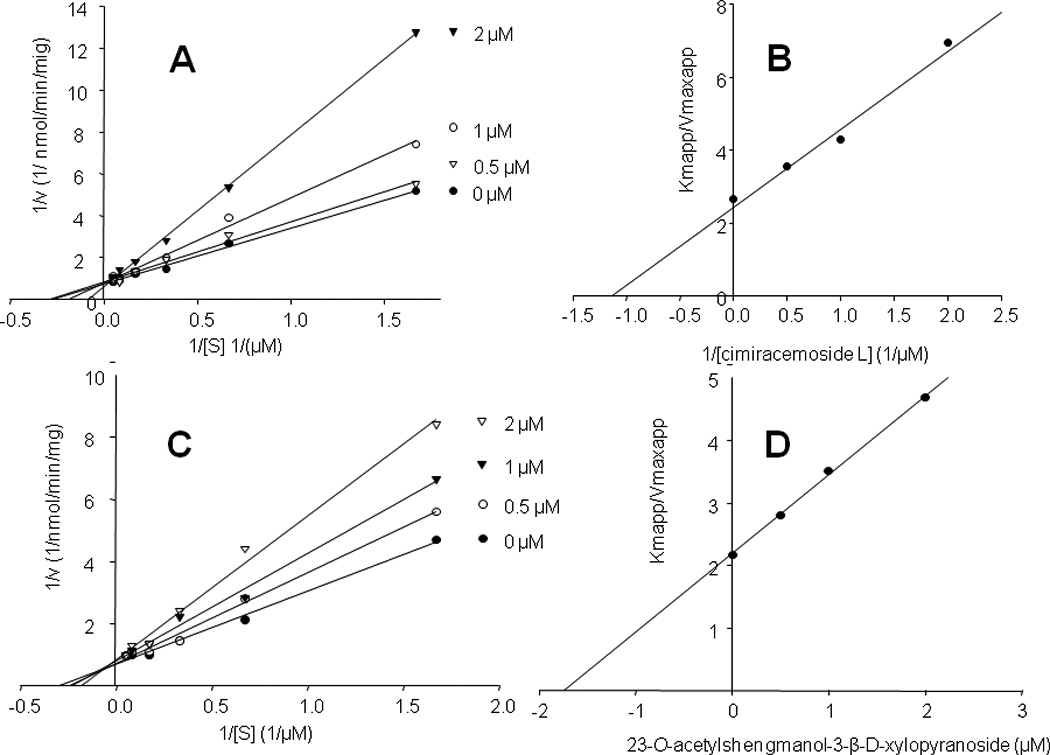
Lineweaver-Burk plots for cimiracemoside L (16) (panel A) and 23-O-acetylshengmanol-3-β-D-xylopyranoside (12) (panel C) indicate that they are competitive inhibitors. Inhibitions constants calculated from secondary plots of slopes (Kmapp/Vmaxapp) versus ligand concentration (panels B and D) were 1.1 µM and 1.7 µM for 16 and 12, respectively. Kinetic analyses were carried out using midazolam as a standard CYP3A4 substrate
As shown in Table 2, triterpenes primarily inhibited formation of N-desmethyl TAM and α-OH TAM, which is expected based on the known contribution of CYP3A4 to the formation of these metabolites. Interestingly, two compounds, 12 and 2 also showed moderate inhibition of 4-OH TAM formation. It is possible that these triterpenes inhibit other isoforms that may contribute to the formation of this metabolite.
Table 2.
Effects of triterpenes on TAM metabolism
| % Inhibition | |||
|---|---|---|---|
| Compound | 4-OH TAM | N-desmethyl TAM | α-OH TAM |
| 23-O-acetylshengmanola-3-α-L-arabinopyranoside (23R) | 58.8±3.4b | 82.1±1.5 | 89.6±1.7 |
| Cimiracemoside K | 16.1±8.0 | 63.0±1.8 | 67.2±2.4 |
| 23-O-acetylshengmanol-3-β-D-xylopyranoside | 55.2±5.3 | 76.7±1.6 | 83.7±2.9 |
| Cimiracemoside O | 36.7±6.7 | 53.3±5.0 | 58.3±5.7 |
| Cimiracemoside L | 39.1±3.6 | 72.6±2.5 | 75.0±2.8 |
| Cimicifugoside M | < 10 | 44.2±3.9 | 42.7±6.0 |
| Ketoconazole (1µM) | < 10 | 93.9 | 96.4 |
All compounds were tested at 10 µM
Data represent average of triplicate measurements ± S.D.
Identification of CYP2D6 inhibitors
Crude 75% ethanolic extract showed moderate activity against CYP2D6 in the dextromethorphan demethylation assay with an IC50 value of 50.1 µg/ml. Since most known CYP2D6 inhibitors contain nitrogen in their molecules, we first tested pure commercially available nitrogen-containing compounds that had been previously identified in black cohosh (Godecke et al., 2009a) (see Table S1 in the Supplement). None of these compounds showed activity against either CYP2D6 or CYP3A4. Thus, it was clear that heretofore unknown compound(s) were responsible for the observed inhibition.
Of particular interest were two compounds with protonated molecules of m/z 354 and m/z 370 present in FCPC Fraction 6 and whose product ion spectra are shown in Figure 6. High resolution accurate mass measurements provided elemental compositions of C19H19NO5 and C20H23NO5, respectively. By combining database searching and interpretation of tandem mass spectra, these two compounds were identified as protopine and allocryptopine, two members of the protopine class of alkaloids. Kinetic studies using dextromethorphan as the probe substrate indicated that both compounds are competitive inhibitors of CYP2D6 with the corresponding Ki values of 78 nM and 122 nM for protopine and allocryptopine, respectively (Figure 7).
Figure 6.
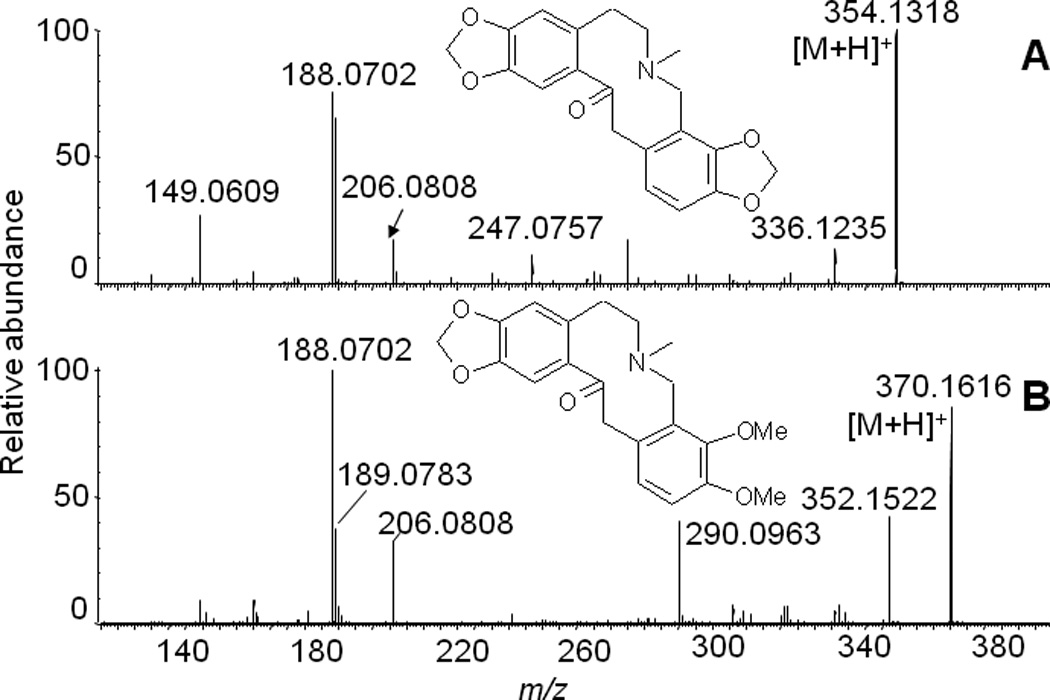
Positive ion electrospray product ion tandem mass spectra of CYP2D6 inhibitors protopine (A) and allocryptopine (B). The identity of each compound was confirmed by comparison with an authentic standard.
Figure 7.
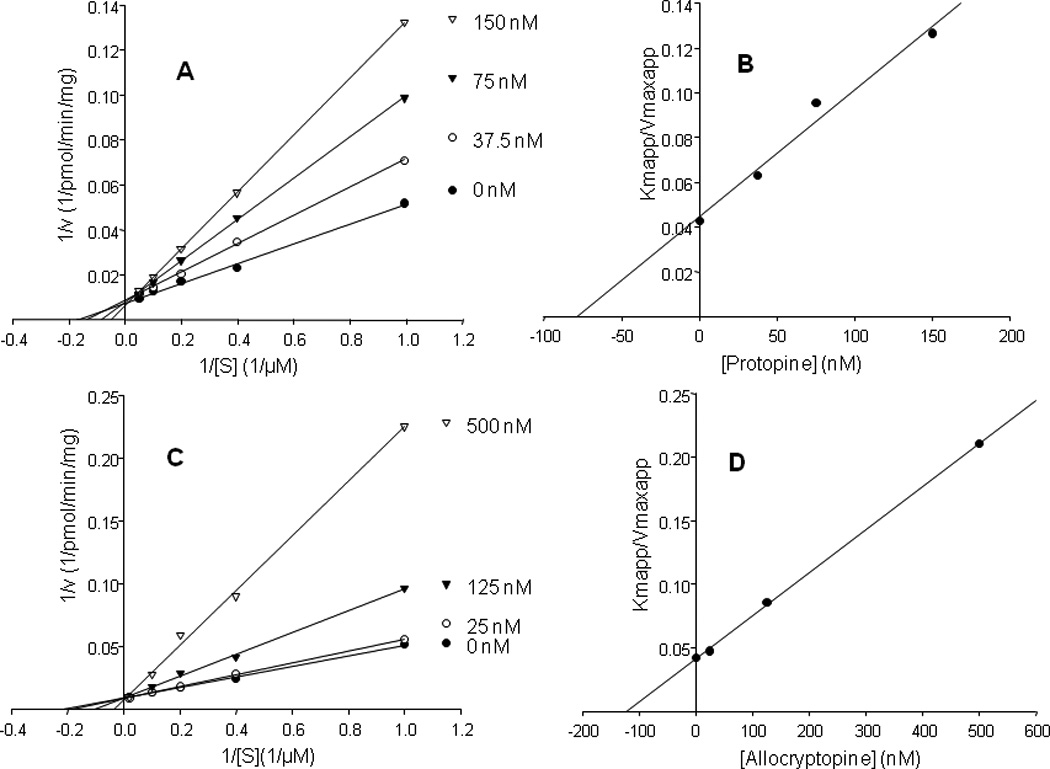
Lineweaver-Burk and secondary plots of slopes (Kmapp/Vmaxapp) versus ligand concentrations for protopine (panels A and C) and allocryptopine (panels B and D) indicating that they are competitive inhibitors with Ki values of 78 nM and 122 nM, respectively. Kinetics analyses were carried out using dextromethorphan as a standard CYP2D6 substrate.
Using reference standards, the amounts of protopine and allocryptopine in the ethanolic extract of black cohosh were determined to be 0.0063% and 0.0088%, respectively, while their levels in the active FCPC fraction 6 were 0.038 and 0.045%, respectively. Since both compounds are present in the same active fraction, we next determined whether there is any interaction between them by measuring inhibition by various combinations of the inhibitors. The results of these experiments indicated that protopine and allocryptopine are mutually exclusive inhibitors (see Yonetani and Theorell, 1964). As such, the total activity of the mixture of these two alkaloids is a simple sum of activities of each compound.
The effects of protopine and allocryptopine on TAM metabolism are shown in Table 3. The results show that these two alkaloids significantly reduced formation of 4-OH TAM while they had marginal effects on the production of the other two TAM metabolites. This indicates that they are quite selective for the CYP2D6 isoform. The data also indicate that protopine and allocryptopine can account for approximately half of the observed activity of FCPC Fraction 6. It is interesting to note that quinidine did not completely abolish formation of 4-OH TAM. This finding is in agreement with previous studies that found that quinidine does not completely block 4-OH TAM formation, indicating that CYP2D6 is not the exclusive isoform responsible for its formation (Crewe et al., 1997; Dehal and Kupfer, 1997; Desta et al., 2004).
Table 3.
Effects of protopine and allocryptopine on TAM metabolism
| % Inhibition | |||
|---|---|---|---|
| Compound | 4-OH TAM | N-desmethyl TAM | α-OH TAM |
| Protopine (10 µM) | 63.4±7.7a | 15.8±1.3 | 12.4±6.3 |
| Allocryptopine (10 µM) | 57.9±4.3 | 16.8±2.8 | < 10 |
| Protopine+Allocryptopineb | 32.1±6.3 | < 10 | < 10 |
| FCPC Fraction 6 (50 µg/ml) | 62.1±3.0 | 76.5±3.0 | 72.8±1.1 |
| Quinidine (1 µM) | 57.6±1.9 | < 10 | < 10 |
Data represent average of triplicates ±S.D.
Alkaloids were mixed in concentrations corresponding to those in FCPC Fraction 6. At 50 µg/ml of Fraction 6, the concentrations of protopine and allocryptopine were 53 and 61 nM, respectively.
Discussion
Treatment of hot flashes is the most common indication for black cohosh use, and occurrence of hot flashes is a serious clinical issue with TAM therapy, which often leads to poor compliance (Fisher et al., 1998). At least two clinical trials examined effects of black cohosh extracts on TAM induced hot flashes with one finding no effect (Jacobson et al., 2001) and another reporting significant efficacy of black cohosh (Hernandez Munoz and Pluchino, 2003). Since women are continuing to use black cohosh, it is of interest to understand whether black cohosh supplements have any effect on TAM efficacy.
In this study we found that a 75% ethanolic extract of black cohosh has the potential to interfere with the metabolic conversion of TAM into active metabolite 4-OH TAM and N-desmethyl-4-OH TAM (endoxifen) via inhibition of formation of N-desmethyl TAM. Because of the dominant role of CYP3A4 and CYP2D6 in the metabolism of TAM, this study primarily focused on the identification of inhibitors of these two isoforms. Specific triterpene glycosides were found to inhibit CYP3A4 mediated metabolism of TAM. This class of compounds has been the major focus of phytochemical investigations of black cohosh for several decades, and numerous congeners have been isolated and characterized. Two recent in vitro studies described weak inhibition of CYP3A4 by triterpenes (Huang et al., 2010; Tsukamoto et al., 2005), and our data for the major triterpenes are in agreement with these studies. Interestingly, 12, which was found moderately active in this study, was determined to be only a weak inhibitor by Tsukamoto et al. (Tsukamoto et al., 2005). One possible explanation of this discrepancy lies in different experimental conditions used in the two studies. For example, Tsukamoto and coworkers used substrate concentration well above Km, which effectively prevented competitive inhibitors from binding to the enzyme. In addition, they used 0.5% DMSO which is known to significantly inhibit CYP3A4, thereby masking inhibition by the triterpenes. Similarly, the study by Huang et al. only mentions that organic solvent content was less than 1%, but did not specify the exact amount.
Our data indicate that triterpene glycosides are moderately potent competitive inhibitors, but with an apparent lack of structure-activity relationship. It is important to note that abundant triterpenes, such as the standardization markers 1 and 11 as well as the cogeneric 4, were inactive against CYP3A4 and CYP2D6. This suggests that chemical standardization based on these abundant triterpene markers does not establish a quantitative measure for the extract’s potential to inhibit CYP3A4 or CYP2D6. Because all of the active triterpenoids identified in this study were minor constituents, one or a few individual compounds are unlikely to fully account for all the observed activity of the crude extract, suggesting the presence of additional active compounds. For example, the most abundant active compound was 12 which was present at a level of 1.6 mg/g of crude extract (Fabricant, 2006). In the reaction mixture, this would amount to a concentration of 22 nM, which is below the observed Ki.
The data from this study are in contrast with two clinical studies that reported no effect of black cohosh on the activity of CYP3A4 in vivo (Gurley et al., 2006; Gurley et al., 2005), although one of the studies noted a small trend toward CYP3A4 inhibition by black cohosh (Gurley et al., 2005). One obvious explanation for this discrepancy is differences in the types of extracts used. Although the in vivo studies reported the content of major (inactive) marker triterpenoids such as 1 and 11, the content of active CYP3A4 inhibitors in those extracts was not known, nor was activity of the crude extract tested in an in vitro assay to establish possible in vitro-in vivo correlation. A recent pharmacokinetic study on 1 (van Breemen et al., 2010) found that black cohosh triterpenes are orally bioavailable; however, the pharmacokinetics of the CYP3A4 active triterpenes has not been investigated to date. Thus, whether the extract investigated in the present study possesses in vivo activity needs to be determined in a carefully designed clinical study.
Several in vitro and in vivo studies have addressed the potential of black cohosh to inhibit CYP2D6. An in vivo study by Gurley et al. found a small, but statistically significant inhibition of CYP2D6 (Gurley et al., 2005). In a follow-up study by the same group, black cohosh was found to have no effect on CYP2D6 in vivo (Gurley et al., 2008). Recent in vitro studies have provided conflicting reports with one reporting high (Ho et al., 2011), one moderate (Huang et al., 2010), and one no activity against CYP2D6 (Sevior et al., 2010). The identification of protopine and allocryptopine as potent CYP2D6 inhibitors is an important new discovery in this study that may provide a possible explanation for apparent discrepancies among these previous studies. Many alkaloids, particularly those that possess methylenedioxyphenyl moiety, are potent (often mechanism-based) inhibitors of CYP2D6, yet the presence of this class of compounds in black cohosh has largely been overlooked,. Consequently, small differences in their quantities whether resulting from extraction procedures or growing conditions, can have profound effects on the observed biological activities of black cohosh extracts. It is also important to note that protopine and allocryptopine cannot fully account for the observed CYP2D6 activity and that other unidentified active compounds are still present.
When it comes to potential clinical significance of inhibition of TAM metabolism by black cohosh there are a number of issues to consider. First, it is important to note that in vitro inhibition observed in this study may or may not translate into measurable in vivo activity. Second, it has been shown that serum concentrations of TAM metabolites do not always predict clinical outcome (Bratherton et al., 1984; Langan-Fahey et al., 1990). The concentrations of active TAM metabolites that trigger hot flashes and those necessary for activity may or may not be the same or even linked. Thus, even if black cohosh inhibits formation of TAM metabolites in vivo, the final clinical outcome of such interaction can be determined only in a carefully designed clinical study. Unfortunately, the two studies that examined effects of black cohosh on TAM induced hot flashes (Hernandez Munoz and Pluchino, 2003; Jacobson et al., 2001) did not measure the concentrations of active metabolites, thus it is not clear what effects, if any, black cohosh had on TAM metabolism. Finally, due to the polymorphic nature of CYP2D6, co-administration of CYP2D6 inhibitors with TAM may be more relevant for extensive and intermediate metabolizers than for poor metabolizers (Sideras et al., 2010). Reduction in formation of α-OH TAM via inhibition of CYP3A4 by black cohosh may be a potentially beneficial aspect of this interaction. This metabolite has been implicated as a causative factor in increasing the risk of endometrial cancer during TAM therapy. Thus, black cohosh supplements might protect women from this side effect of TAM therapy.
Conclusions
In this study, a 75% ethanolic extract of black cohosh was found to interfere with TAM metabolism by inhibition of cytochrome P450 2D6 and CYP3A4. Eight triterpene glycosides were found to inhibit CYP3A4 mediated metabolism, while two alkaloids, protopine and allocryptopine were identified as potent inhibitors of CYP2D6. Based on these in vitro results, black cohosh supplements have potential to interfere with clinical efficacy of TAM. However, whether these findings will have any clinical significance needs to be determined in a well-designed clinical trial. The results of this study do not provide sufficient evidence for warning against concomitant use of black cohosh and TAM, but that the patients consuming black cohosh supplements during TAM therapy should inform their physicians so that they may be properly monitored for potential interactions.
Supplementary Material
Acknowledgement
This work was supported by grant P50AT00155 from the Office of Dietary Supplements, the National Institute of General Medical Sciences, the Office for Research on Women’s Health, and the National Center for Complementary and Alternative Medicine.
Footnotes
Declaration of interest
The authors declare no conflict of interest.
REFERENCES
- Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms: a systematic review of its efficacy. Pharmacol Res. 2008;58:8–14. doi: 10.1016/j.phrs.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Bratherton DG, Brown CH, Buchanan R, Hall V, Kingsley Pillers EM, Wheeler TK, Williams CJ. A comparison of two doses of tamoxifen (Nolvadex) in postmenopausal women with advanced breast cancer: 10 mg bd versus 20 mg bd. Br J Cancer. 1984;50:199–205. doi: 10.1038/bjc.1984.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauret N, Gauthier A, Nicoll-Griffith DA. Effect of common organic solvents on in vitro cytochrome P450-mediated metabolic activities in human liver microsomes. Drug Metab Dispos. 1998;26:1–4. [PubMed] [Google Scholar]
- Chen SN, Fabricant DS, Lu ZZ, Fong HH, Farnsworth NR. Cimiracemosides I-P, new 9,19-cyclolanostane triterpene glycosides from Cimicifuga racemosa. J Nat Prod. 2002a;65:1391–1397. doi: 10.1021/np0200818. [DOI] [PubMed] [Google Scholar]
- Chen SN, Li W, Fabricant DS, Santarsiero BD, Mesecar A, Fitzloff JF, Fong HH, Farnsworth NR. Isolation, structure elucidation, and absolute configuration of 26-deoxyactein from Cimicifuga racemosa and clarification of nomenclature associated with 27-deoxyactein. J Nat Prod. 2002b;65:601–605. doi: 10.1021/np010494t. [DOI] [PubMed] [Google Scholar]
- Crewe HK, Ellis SW, Lennard MS, Tucker GT. Variable contribution of cytochromes P450 2D6, 2C9 and 3A4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochem Pharmacol. 1997;53:171–178. doi: 10.1016/s0006-2952(96)00650-8. [DOI] [PubMed] [Google Scholar]
- Dehal SS, Kupfer D. CYP2D6 catalyzes tamoxifen 4-hydroxylation in human liver. Cancer Res. 1997;57:3402–3406. [PubMed] [Google Scholar]
- Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- Dezentje VO, van Blijderveen NJ, Gelderblom H, Putter H, van Herk-Sukel MP, Casparie MK, Egberts AC, Nortier JW, Guchelaar HJ. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast c. J Clin Oncol. 2010;28:2423–2429. doi: 10.1200/JCO.2009.25.0894. [DOI] [PubMed] [Google Scholar]
- EBCTCG. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Fabricant DS. Pharmacognostic investigation of black cohosh (Cimicifuga racemosa, L. Nutt.) University of Illinois at Chicago; 2006. [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Frei-Kleiner S, Schaffner W, Rahlfs VW, Bodmer C, Birkhäuser M. Cimicifuga racemosa dried ethanolic extract in menopausal disorders: a double-blind placebo-controlled clinical trial. Maturitas. 2005;51:397–404. doi: 10.1016/j.maturitas.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Geller SE, Shulman LP, van Breemen RB, Banuvar S, Zhou Y, Epstein G, Hedayat S, Nikolic D, Krause EC, Piersen CE, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009;16:1156–1166. doi: 10.1097/gme.0b013e3181ace49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godecke T, Lankin DC, Nikolic D, Chen SN, van Breemen RB, Farnsworth NR, Pauli GF. Guanidine alkaloids and Pictet-Spengler adducts from black cohosh (Cimicifuga racemosa) J Nat Prod. 2009a;72:433–437. doi: 10.1021/np8006952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godecke T, Nikolic D, Lankin DC, Chen SN, Powell SL, Dietz B, Bolton JL, van Breemen RB, Farnsworth NR, Pauli GF. Phytochemistry of cimicifugic acids and associated bases in Cimicifuga racemosa root extracts. Phytochem Anal. 2009b;20:120–133. doi: 10.1002/pca.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley B, Hubbard MA, Williams DK, Thaden J, Tong Y, Gentry WB, Breen P, Carrier DJ, Cheboyina S. Assessing the clinical significance of botanical supplementation on human cytochrome P450 3A activity: comparison of a milk thistle and black cohosh product to rifampin and clarithromycin. J Clin Pharmacol. 2006;46:201–213. doi: 10.1177/0091270005284854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Khan IA, Shah A. In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clin Pharmacol Ther. 2005;77:415–426. doi: 10.1016/j.clpt.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Swain A, Hubbard MA, Williams DK, Barone G, Hartsfield F, Tong Y, Carrier DJ, Cheboyina S, Battu SK. Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: effects of milk thistle, black cohosh, goldenseal, kava kava, St. John's wort, and Echinacea. Mol Nutr Food Res. 2008;52:755–763. doi: 10.1002/mnfr.200600300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Munoz G, Pluchino S. Cimicifuga racemosa for the treatment of hot flushes in women surviving breast cancer. Maturitas. 2003;44(Suppl 1):S59–S65. doi: 10.1016/s0378-5122(02)00349-3. [DOI] [PubMed] [Google Scholar]
- Ho SH, Singh M, Holloway AC, Crankshaw DJ. The effects of commercial preparations of herbal supplements commonly used by women on the biotransformation of fluorogenic substrates by human cytochromes P450. Phytother Res. 2011;25:983–989. doi: 10.1002/ptr.3371. [DOI] [PubMed] [Google Scholar]
- Huang Y, Jiang B, Nuntanakorn P, Kennelly EJ, Shord S, Lawal TO, Mahady GB. Fukinolic acid derivatives and triterpene glycosides from black cohosh inhibit CYP isozymes, but are not cytotoxic to Hep-G2 cells in vitro. Curr Drug Saf. 2010;5:118–124. doi: 10.2174/157488610790936150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JS, Troxel AB, Evans J, Klaus L, Vahdat L, Kinne D, Lo KM, Moore A, Rosenman PJ, Kaufman EL, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol. 2001;19:2739–2745. doi: 10.1200/JCO.2001.19.10.2739. [DOI] [PubMed] [Google Scholar]
- Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med. 2002;137:805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- Langan-Fahey SM, Tormey DC, Jordan VC. Tamoxifen metabolites in patients on long-term adjuvant therapy for breast cancer. Eur J Cancer. 1990;26:883–888. doi: 10.1016/0277-5379(90)90191-u. [DOI] [PubMed] [Google Scholar]
- Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989;49:2175–2183. [PubMed] [Google Scholar]
- Liske E, Hanggi W, Henneicke-von Zepelin HH, Boblitz N, Wustenberg P, Rahlfs VW. Physiological investigation of a unique extract of black cohosh (Cimicifugae racemosae rhizoma): a 6-month clinical study demonstrates no systemic estrogenic effect. J Womens Health Gend Based Med. 2002;11:163–174. doi: 10.1089/152460902753645308. [DOI] [PubMed] [Google Scholar]
- Mahady GB, Parrot J, Lee C, Yun GS, Dan A. Botanical dietary supplement use in peri- and postmenopausal women. Menopause. 2003;10:65–72. doi: 10.1097/00042192-200310010-00011. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Jones K, Humphrey S, Hughes K. Black cohosh: efficacy, safety, and use in clinical and preclinical applications. Altern Ther Health Med. 2001;7:93–100. [PubMed] [Google Scholar]
- Mortimer JE, Flatt SW, Parker BA, Gold EB, Wasserman L, Natarajan L, Pierce JP. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat. 2008;108:421–426. doi: 10.1007/s10549-007-9612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton KM, Reed SD, LaCroix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy, or placebo: a randomized trial. Ann Intern Med. 2006;145:869–879. doi: 10.7326/0003-4819-145-12-200612190-00003. [DOI] [PubMed] [Google Scholar]
- Osmers R, Friede M, Liske E, Schnitker J, Freudenstein J, Henneicke-von Zepelin HH. Efficacy and safety of isopropanolic black cohosh extract for climacteric symptoms. Obstet Gynecol. 2005;105:1074–1083. doi: 10.1097/01.AOG.0000158865.98070.89. [DOI] [PubMed] [Google Scholar]
- Phillips DH. Understanding the genotoxicity of tamoxifen? Carcinogenesis. 2001;22:839–849. doi: 10.1093/carcin/22.6.839. [DOI] [PubMed] [Google Scholar]
- Powell SL, Godecke T, Nikolic D, Chen SN, Ahn S, Dietz B, Farnsworth NR, van Breemen RB, Lankin DC, Pauli GF, et al. In vitro serotonergic activity of black cohosh and identification of N(omega)-methylserotonin as a potential active constituent. J Agric Food Chem. 2008;56:11718–11726. doi: 10.1021/jf803298z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Sevior DK, Hokkanen J, Tolonen A, Abass K, Tursas L, Pelkonen O, Ahokas JT. Rapid screening of commercially available herbal products for the inhibition of major human hepatic cytochrome P450 enzymes using the N-in-one cocktail. Xenobiotica. 2010;40:245–254. doi: 10.3109/00498251003592683. [DOI] [PubMed] [Google Scholar]
- Sideras K, Ingle JN, Ames MM, Loprinzi CL, Mrazek DP, Black JL, Weinshilboum RM, Hawse JR, Spelsberg TC, Goetz MP. Coprescription of tamoxifen and medications that inhibit CYP2D6. J Clin Oncol. 2010;16:2768–2776. doi: 10.1200/JCO.2009.23.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- Tsukamoto S, Aburatani M, Ohta T. Isolation of CYP3A4 Inhibitors from the Black Cohosh (Cimicifuga racemosa) Evid Based Complement Alternat Med. 2005;2:223–226. doi: 10.1093/ecam/neh086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen RB, Liang W, Banuvar S, Shulman LP, Pang Y, Tao Y, Nikolic D, Krock KM, Fabricant DS, Chen SN, et al. Pharmacokinetics of 23-epi-26-deoxyactein in women after oral administration of a standardized extract of black cohosh. Clin Pharmacol Ther. 2010;87:219–225. doi: 10.1038/clpt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsky RL, Obach RS. Validated assays for human cytochrome P450 activities. Drug metabolism and disposition: the biological fate of chemicals. 2004;32:647–660. doi: 10.1124/dmd.32.6.647. [DOI] [PubMed] [Google Scholar]
- Wuttke W, Seidlova-Wuttke D, Gorkow C. The Cimicifuga preparation BNO 1055 vs. conjugated estrogens in a double-blind placebo-controlled study: effects on menopause symptoms and bone markers. Maturitas. 2003;44(Suppl 1):S67–S77. doi: 10.1016/s0378-5122(02)00350-x. [DOI] [PubMed] [Google Scholar]
- Xu H, Fabricant DS, Piersen CE, Bolton JL, Pezzuto JM, Fong H, Totura S, Farnsworth NR, Constantinou AI. A preliminary RAPD-PCR analysis of Cimicifuga species and other botanicals used for women's health. Phytomedicine. 2002;9:757–762. doi: 10.1078/094471102321621403. [DOI] [PubMed] [Google Scholar]
- Yonetani T, Theorell H. Studies on Liver Alcohol Hydrogenase Complexes. 3. Multiple inhibition kinetics in the presence of two competitive inhibitors. Arch Biochem Biophys. 1964;106:243–251. doi: 10.1016/0003-9861(64)90184-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


