Abstract
Manipulating the immune system in order to induce clinically relevant responses against cancer is a longstanding goal. Interventions to enhance tumor specific immunity through vaccination, sustaining effector T cell activation, or increasing the numbers of tumor specific T cells using ex vivo expansion, have all resulted in clinical successes. Here we examine recent clinical advances and major ongoing studies in the field of cancer immunotherapy. Single agents have so far benefited a limited proportion of patients and future studies combining different types of immunotherapies and other therapeutic modalities, such as drugs against specific signaling pathways driving cancer cell growth, are needed to hopefully pave the way for the development of effective anti-cancer treatments causing durable responses.
The immune system and cancer
The rapid advancement of sequencing technologies has provided insight into the neoplastic process, which includes accumulated mutations of genes that are involved in crucial cellular signaling pathways. This has led to clinical successes of targeted therapies aiming to correct aberrant cellular signaling [1, 2]. However, clinical responses with targeted therapies are often short-lived due to the rapid development of resistance. Enhancing the cell-mediated immune response against tumor cells offers several advantages over targeted therapies, notably the generation of a long-term memory lymphocyte population patrolling the body to attack metastases before metastatic lesions are visible by traditional imaging modalities.
An effective immune response requires sufficient numbers of activated T cells capable of recognizing tumor antigens. It also requires appropriate engagement of positive co-stimulatory molecules on lymphocytes while limiting signaling through inhibitory “immune checkpoint” receptors. Here we summarize data from preclinical models and clinical trials using immunotherapy approaches, and highlight directions for the future.
Activation of the anti-tumor response through vaccination
Applying principles of vaccination to the development of cancer vaccines has proven challenging, probably because cancer cells have arisen from normal “self” tissues and do not trigger activation of the immune system as would microbial organisms. However, in the past two years several randomized clinical trials have shown benefits of cancer vaccines in prostate, lymphoma and melanoma patients.
A randomized trial of 512 metastatic prostate cancer patients reported a 4.1 month increase in median survival in patients receiving Sipuleucel-T, a vaccine consisting of autologous peripheral blood mononuclear cells pulsed with a fusion protein of GM-CSF and the prostate cancer antigen prostatic acid phosphatase [3]. Although this effect is modest, it demonstrates that the immune response can affect patient outcome and the therapy is now approved by the FDA for prostate cancer.
B cell lymphomas are monoclonal, originating from one cancerous B cell expressing a unique immunoglobulin, and the variable region of this antibody (termed idiotype) has been utilized as a unique patient specific tumor antigen. A vaccine consisting of an autologous idiotype protein conjugated to keyhole limpet hemocyanin (KLH) has been used in follicular lymphoma [4]. A cohort of 117 patients in complete response following chemotherapy (free of disease but at a high risk of recurrence), was randomized to receive the vaccine with GM-CSF or a KLH control with GM-CSF. Patients receiving the idiotype vaccine had an improved disease free survival of 44.2 months compared to 30.6 months for the control arm.
In metastatic melanoma, a randomized clinical trial in 185 patients comparing vaccination with gp100 peptide alone with or without high dose of the T cell growth factor Interleukin-2 reported that patients receiving the peptide vaccine and IL-2 combination experienced longer progression free survival and a higher response rate to the therapy (16% vs 6% for the group not receiving IL-2) [5]. Thus optimal vaccination may require rational combinations with other agents, such as cytokines.
Although these clinical trials represent an important milestone in the development of immune therapies, the overall benefits are modest. Responses to these vaccines might be improved through optimization of adjuvants, such as toll like receptor (TLR) agonists [6, 7], optimization of peptide length [8], and addition of cytokines [9] or potentially by combining vaccines use with other immune therapies, such as immune-modulating antibodies.
Promoting T cell function by modulating co-stimulation or co-inhibition
Immune activation is tightly regulated by co-receptors expressed on T cells (Figure 1). Co-stimulatory receptors include CD28 and ICOS (inducible T cell co-stimulator) of the Ig superfamily, as well as 4-1BB, OX40, CD27, CD30, CD40, GITR (glucocorticoid inducible TNF receptor-related protein), and HVEM (herpes-virus entry mediator) of the TNFR superfamily [10, 11]. These co-stimulatory signals are counterbalanced by co-inhibitory members of the Ig superfamily including CTLA-4, PD-1, BTLA (B and T lymphocyte attenuator), lymphocyte activation gene-3 (LAG-3), TIM3 (T cell immunoglobulin and mucin domain-containing protein 3), and VISTA (V-domain immunoglobulin suppressor of T cell activation) on T cells [10, 12–16]. The idea of blocking the immune co-inhibitors as a therapeutic anticancer strategy was suggested by James Allison over a decade ago [17]. Anti-CTLA-4 was used as a prototype but antibodies that either stimulate co-stimulatory T cell receptors or block other inhibitory immune-checkpoint molecules have been examined more recently.
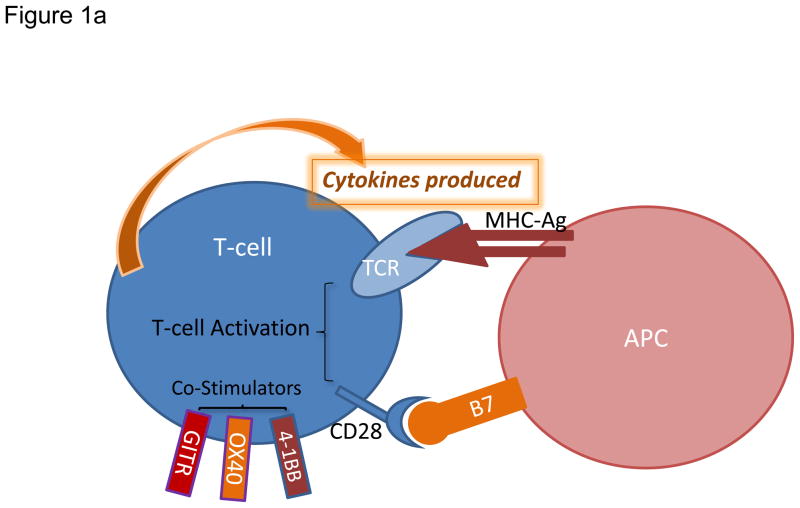
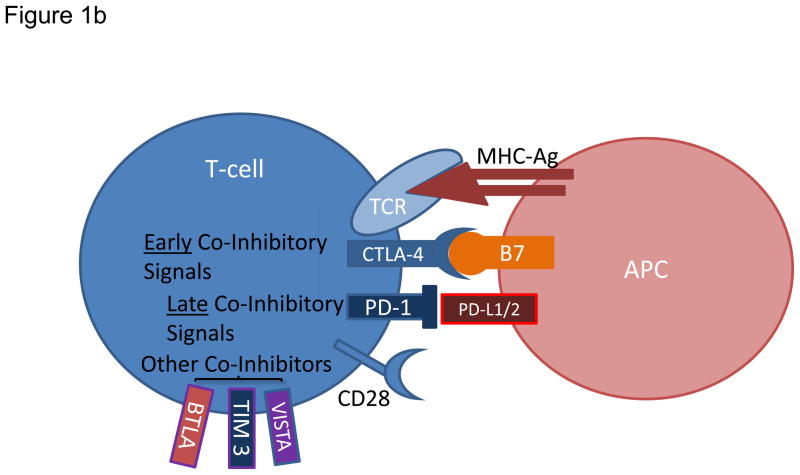
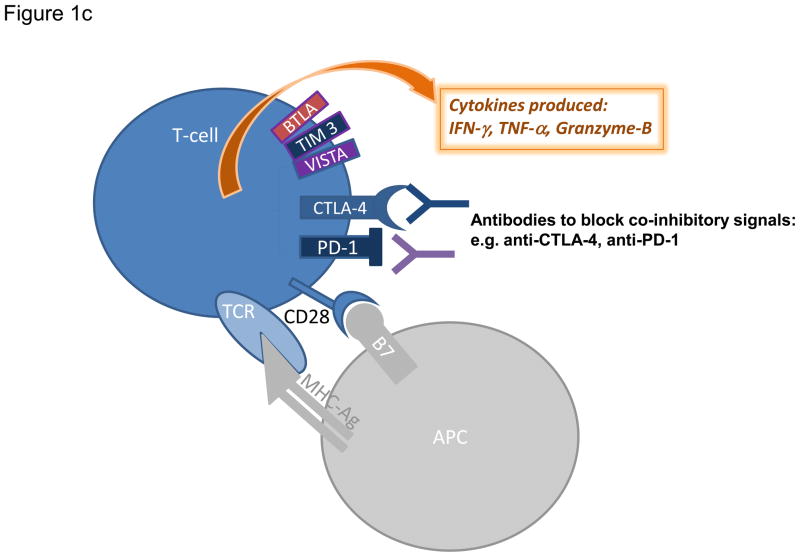
Figure 1. Modulation of T cell activation and current strategies promoting effector T cell functions.
a) Augmenting T cell activation by positive co-stimulation. Antigenic presentation triggers T cell activation and occurs when a peptide bound to major histocompatibility complex (MHC) molecule on an antigen presenting cell (APC) interacts with T cell receptor (TCR) on the surface of a T cell. In order to achieve optimal activation, additional co-stimulatory signals are required and primarily involve interaction between CD28 on T cells and B7 on APCs. Other T cell positive co-stimulators include 4-1BB, OX40, and GITR. b) Limiting T cell activation by negative co-stimulation. After T cell activation, cytotoxic T lymphocyte-associated protein 4 (CTLA-4) is mobilized to the cell surface and binds to B7 with greater affinity than CD28 therefore preventing signaling through CD28. Later inhibitory signals can be provided by co-inhibitors such as programmed cell death 1 (PD-1), which binds to PD-1 ligand 1 (PDL1). Other co-inhibitors of T cell activation include VISTA, TIM3, and BTLA. c) Sustaining T cell activation through blockade of negative co-stimulatory molecules. Blocking antibodies against CTLA-4 or PD-1 are currently employed to neutralize co-inhibitory receptors and prevent dampening of the T cell response. Blockade of these inhibitory immune checkpoints results in enhanced and sustained activation of tumor-specific T cells that produce cytokines including tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and granzyme B.
Turning on the stimulators: antibodies to 4-1BB (CD137), OX40 (134), GITR, and CD40
Animal models and clinical trials have focused on targeting the 4-1BB, OX40, GITR, and CD40 members of the TNFR superfamily. 4-1BB is expressed on activated T cells, activated NK cells, dendritic cells (DCs), and endothelial cells [18]. 4-1BB enhances T cell function by promoting T cell survival and memory generation [19], activating antigen-presenting cells (APCs) and endothelial cells, reducing T regulatory cell (Treg) suppressive capacity, and enhancing effector T cell resistance to Treg suppression [20, 21]. Agonistic anti-4-1BB monoclonal antibodies alone or in combination with vaccination can enable rejection of syngeneic tumors in pre-clinical models [22–25]. Phase I and II clinical trials with a fully humanized monoclonal anti-4-1BB antibody in patients with melanoma and renal cell carcinoma showed only very mild antitumor activity [26], suggesting that anti-41BB antibody may require combinations with other agents in order to demonstrate relevant antitumor activity.
OX40, expressed on activated CD4+ and CD8+ T cells, functions as a late co-stimulatory receptor [18, 27]. Ligation of OX40 enhances immune responses by prolonging CD4+ and CD8+ T cell survival and memory generation, preventing T cell tolerance [28–31], impairing the suppressor function of Treg [32, 33], and suppressing the generation of inducible Treg [34, 35]. An agonistic anti-OX40 antibody increases anti-tumor activity in a number of animal models [36]. Other reports indicate that anti-OX40 antibodies can work in combination with anti-4-1BB or vaccination to augment anti-tumor immune responses [37–40].
GITR is also expressed after T cell activation [41, 42]. It is constitutively expressed at high levels on Tregs, and can be further induced after Treg activation [43, 44]. GITR enhances proliferation and function of CD4+ and CD8+ T cells [45], inhibits Treg function and elicits effector T cell resistance to Treg-mediated suppression [46–48]. GITR may increase intra-tumor effector T cells to Tregs ratio [49–51]. Anti-GITR antibodies elicit anti-tumor responses in a number of animal models [52, 53]. In addition, anti-GITR antibodies can be combined with vaccination, chemotherapy, or anti-CTLA-4 antibody to augment anti-tumor activity [50, 54, 55].
CD40 is mainly expressed on APCs and endothelial cells [11]. Binding of CD40 to its ligand (CD40L/CD154) on activated T cells results in persistent T cell activation as well as expansion and activation of APCs. An agonistic anti-CD40 antibody has been shown to cause regression of pancreatic cancer in both human and mice via activation of macrophages, suggesting a T cell independent anti-tumor mechanism [56]. CD40 is also expressed on many types of tumor cells and its ligation may promote tumor cell apoptosis and growth arrest. An initial phase I clinical trial using an agonistic anti-CD40 antibody in patients with advanced solid tumors demonstrated modest antitumor activity [57]. However, a recent follow up phase I trial using the same anti-CD40 antibody showed less promising result [58].
Overall, agonistic antibodies against immune co-stimulators have demonstrated modest anti-tumor activities in early phases of clinical trials. The combination of agonistic antibodies with other treatment modalities may be needed to achieve optimal anti-tumor activity.
Turning off the brakes: antibodies against CTLA-4 (CD152), PD-1 (CD279), and PD-L1
CTLA-4 is expressed by activated CD4+ and CD8+ T cells. Upon T cell activation, CTLA-4 is rapidly mobilized from intracellular vesicles to the immune synapse where it outcompetes co-stimulator CD28 for binding to its ligands B7-1 and B7-2 and, as a result, ablates T cell activation (Figure 1b). In addition, CTLA-4 mediates cell cycle arrest and is essential for Treg suppression [10]. In human studies, anti-CTLA-4 enhances frequency of CD4+ and CD8+ T cells as well as the antibody response to tumor antigens [59–63]. In animal models, blocking monoclonal antibody against CTLA-4 promotes anti-tumor activity [10, 64, 65]. Phase I and Phase II clinical trials indicate that an anti-CTLA-4 antibody, ipilimumab, has significant antitumor activity in patients with advanced melanoma [66, 67]. A Phase III randomized, controlled trial in patients with metastatic melanoma showed that ipilimumab improved median overall survival by 3.7 months [68], leading to FDA approval of Ipilimumab for the treatment of patients with metastatic melanoma. A second randomized, phase III clinical trial recently showed that addition of ipilimumab to standard dacarbazine chemotherapy improved overall survival by 2.1 months in patients with metastatic melanoma [69].
PD-1 is expressed by activated CD4+ and CD8+ T cells, NK T cells, and APCs. It has two ligands, PD-L1 and PD-L2, with distinct expression profiles [70]. PD-L1, the main target for PD-1, is expressed broadly on T cells, B cells, APCs, and non-hematopoietic cells. PD-L2 is largely restricted to activated macrophages, myeloid dendritic cells and mast cells. PD-1−/− mice develop autoimmunity with high titers of autoantibodies, consistent with PD-1’s role as an inhibitor of T and B cells [71, 72]. Anti-PD-1 antibodies reduce tumor metastasis and growth in animal models [73, 74], whereas forced expression of PD-L1 in murine tumor cell lines enhances in vivo tumor growth [75]. In a Phase I trial in 39 patients with refractory metastatic melanoma, colorectal cancer, prostate cancer, non-small-cell lung cancer (NSCLC), or renal cell carcinoma (RCC), anti-PD-1 antibody (MDX-1106) resulted in complete or partial response in 3 patients and less significant tumor regression in 2 other patients [76]. A recent phase I clinical trial with an anti-PD1 antibody (BMS-936558) showed promising antitumor activity in patients with advanced NSCLC, RCC and melanoma [77]. Another phase I trial with anti-PD-L1 antibody showed mild to modest antitumor activity in patients with advanced NSCLC, melanoma and RCC [78]. Blocking antibodies against other T cell co-inhibitors including LAG-3 and TIM3 are still at early stages of development as potential anti-cancer agents [12, 16].
Amplifying existing tumor reactive T cells: Adoptive T cell therapy for the treatment of cancer
Cancer-reactive T cells recognizing antigens from solid tumors are found at low frequency in the peripheral blood of patients and can be isolated and cloned by limiting dilution for eventual amplification and re-infusion to the patient. However, this process requires 3–5 months to generate a product sufficient for infusion [79]. Clinical trials show modest clinical responses using cloned T cells recognizing MART-1 antigen for metastatic melanoma and there is poor persistence of the cells post transfer [80–82]. The limited clinical efficacy may be due to targeting a unique antigen that may not be uniformly expressed by all tumor cells leaving the growth of antigen negative cells unaffected [80]. In rare cases, therapy with T cell clones has been shown efficacious. For example, in a recent clinical trial with adoptively transferred NY-ESO-1-specific CD4 T cell clone one patient experienced complete tumor regression associated with persistence of the transferred cells for more than 80 days and with antigen spreading, or the appearance of specific immune responses to tumor antigens unrelated to NY-ESO-1 post transfer[83].
An alternative approach to find cancer fighting immune cells is to isolate and expand T cells found in the tumor itself, or Tumor Infiltrating Lymphocytes (TIL), naturally enriched in tumor-specific T cells [84, 85]. Besides recognizing shared tumor antigens melanoma TIL also recognize unique mutated antigens, such as mutated β-catenin [86]. The number and localization of TIL within the tumor has been correlated with clinical outcome for different malignancies [87–89]. Isolation and ex vivo rapid expansion of TILs from melanoma and other malignancies is now readily achievable [85, 90, 91]. Infusion of large numbers of expanded autologous TIL (up to 1011) to metastatic melanoma patients, followed by one course of high dose IL-2 as growth factor to sustain the persistence of TIL in vivo, resulted in 34% clinical response [92]. When pre-conditioning of the patients with cytoxan and fludarabine lymphodepleting regimen was introduced, half of the treated patients responded to the therapy, including10% of the patients that had complete disappearance of their tumors [93, 94]. Clinical response rate was even increased to 70% in a small cohort of patients undergoing an additional pre-conditioning with total body irradiation (TBI) of 12Gy [95]. Durable clinical responses have been observed and persistence of infused cells in responder patients have been measured for several years following therapy [96]. However, randomized clinical trials are needed to compare this therapy with standard approaches.
Lympho-depletion of the patient prior to TIL cell transfer extends the persistence and frequency of infused T cells in the blood and is crucial to the success of TIL adoptive cell therapy for melanoma [97, 98]. The mechanisms involved are unclear. In animal models, lympho-depletion induces compensatory homeostatic expansion that together with regulatory CD4+ T cell (Treg) depletion can induce strong anti-tumor activity [99]. In patients, cytoxan and fludarabine lympho-depleting regimen cause an increase in the levels of the homeostatic cytokines IL-7 and IL-15 while transiently ablating white blood cells, including Treg [95]. Addition of Total Body Irradiation to the regimen results in lymphoablation requiring autologous stem cell transfer to ensure the repopulation of the lymphoid compartment. The more profound lympho-depletion occurring with TBI may delay the reconstitution of the endogenous Treg compartment and favor better anti-tumor T-cell activity [100]. Cytoxan/cyclophosphamide and fludarabine also affect the myeloid compartment and cause rapid colonization of the spleen with a suppressive population of immature CD11b+ GR-1+ myeloid cells in mice (myeloid derived suppressor cells or MDSC), inhibiting T cells through nitric oxide production [101]. How TBI regimen impacts MDSC colonization of the spleen in patients is unclear, but potential clearance of MDSCs by TBI could contribute to the benefit of irradiation in the pre-conditioning regimen. Unraveling exactly how lympho-depletion potentiates TIL ACT requires further study.
Redirecting T cells utilizing TCR transduction
By cloning tumor-reactive T cells from peripheral blood, several T cell receptor (TCR) molecules recognizing known tumor antigens with great affinity/avidity have been identified. This has led to an application involving transfer of TCR specificity to a T cell population through genetic engineering. This offers the possibility of providing tumor-reactive T cells to a patient by modifying some of the patient’s own peripheral blood cells from a blood draw and re-infusing the modified cells. The modified cells acquire the ability to recognize the antigen defined by the transferred TCR, which will trigger cytokine expression as well as cytotoxicity towards targets expressing the antigen [102].
To facilitate the isolation of high-avidity TCR against human tumor antigens (restricted to HLA-A0201), isolation of TCR chains from CD8+ T cells obtained from immunized HLA-A0201 transgenic mice has been used. The transfer of high avidity murine TCR specific for human gp100 or human TCR recognizing human MART-1 to peripheral blood T cells of metastatic melanoma patients resulted in a 19% and 30% clinical response, respectively, although significant autoimmunity was noted [103]. Similarly, adoptive transfer of autologous PBMCs modified to express a high avidity carcinoembryonic antigen (CEA)-reactive TCR in 3 colorectal cancer patients led to tumor regression for one patient but dose limiting toxicity in the form of inflammatory colitis for all 3 patients [104]. More recently TCR transduction of a NY-ESO-1-reactive TCR, that was genetically altered to increase recognition of its antigen, in peripheral T cells of 6 patients with metastatic synovial cell carcinoma and 11 metastatic melanoma patients showed clinical response rates of 67% and 45%, respectively without significant toxicity [105, 106].
Overall, results from adoptive cell transfer of TCR-transduced peripheral blood T cells show positive clinical response if the TCR used has very high affinity. The choice of the tumor antigen to target is crucial for limiting potential autoimmune manifestations. Also, the presence of the endogenous TCR in the TCR-transduced cells, as well as mispairing of TCR chains between the recombinant and endogenous alpha and beta chains can reduce potency of the final product or lead to off-target toxicity. One approach to potentially increase potency and minimize side effects of this therapy is ablation of the endogenous TCR chains, using zinc finger nuclease technology, followed by insertion of the desired tumor antigen-specific TCR chain [107].
Chimeric antigen receptor (CAR) engineering
The repertoire of T cell receptors recognizing tumor-associated antigens (TAA) circulating in the peripheral blood is normally depleted of highly reactive cells through thymic selection or peripheral deletion and is composed of T cells bearing low to moderate affinity to TAA. Efforts to increase the affinity of T cells for tumor targets have focused on combining the high affinity recognition of an antibody to the efficient killing machinery of a T cell. This pairing was achieved through the engineering of a synthetic antigen receptor composed of the variable light and heavy chains of a particular antibody, fused into a single chain (scFv), giving the specificity for a defined cell surface tumor marker, coupled to an intracellular T cell signaling domain such as the CD3 zeta chain, which will trigger TCR-like signaling upon scFv engagement (Figure 2). This new receptor, or CAR, recognizes cell surface antigens with a very high affinity in a MHC unrestricted fashion. CAR-expressing T cells release cytokines and perform effector function upon antigen encounter [108]. The first CAR against cancer was developed against an ovarian cancer antigen[109]. CARs have since been made against numerous antigens and newer designs have incorporated additional costimulatory domains. Initial clinical studies showed a lack of persistence of CAR-modified T cells after adoptive cell transfer [110–112]. Second generation CARs were designed to give “signal 2” along with CD3 complex signaling by incorporating a signaling domain from the CD28 co-stimulatory molecule that resulted in increased proliferation and persistence of the T cells after activation[113–115]. Third generation CARs now include the endo-domains of TNF-R family members, such as 41BB, OX40, or CD27 [116–118].
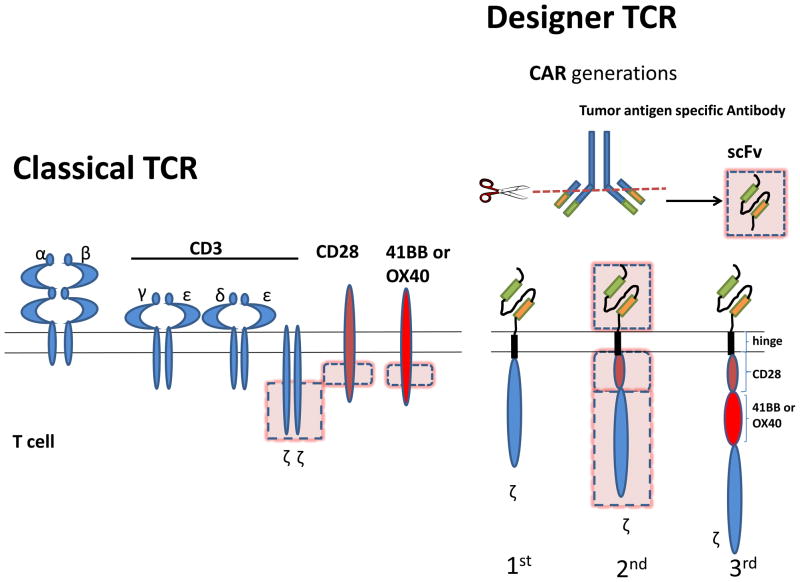
Figure 2. Structure of a classical TCR complex versus a Chimeric Antigen Receptor.
Classical TCR complex is composed of a TCR molecule, heterodimer formed by the association of α and β chain, each chain containing variable regions responsible for antigen binding. TCR is found in complex with CD3 γ, δ, ε and ζ chains which allow the transmission of intracellular signal. Full activation of a T cell requires additional signaling provided by co-stimulatory molecules such as CD28, 41BB and OX40. CARs borrow the hypervariable parts of a tumor specific antibody, combined into a single chain hypervariable fragment (scFv), which gives the CAR its specificity. This new scFv molecule is linked through a hinge region to intracellular signaling domain of CD3 zeta chain alone (first generation), or in combination with the intracellular signaling domain of a T cell costimulatory molecule, usually CD28 (second generation) and more recently involves the addition of another signaling domain from a second co-stimulatory molecule such as 41BB or OX40 (third generation).
In the clinic, the targeting of CD19 antigen for B cell malignancies with second and third generation CAR, utilizing either CD28 or 41BB as co-stimulatory signaling element, has shown clinical effectiveness associated with a complete disappearance of the normal B cell compartment and chronic hypogammaglobulinemia [118, 119]. CAR-modified T cells must be used with caution, however, as their greater affinity for antigen versus normal TCR recognition may result in destruction of normal cells expressing low amounts of antigen. This “on target” but “off tumor” effect may have serious consequences and may be difficult to foresee [120]. Two patients that were treated with CAR-modified T cells recognizing either HER2/neu tumor antigen on colon cancer cells or CD19 on chronic lymphocytic leukemia died of possible infusion-related toxicity, which at least in one case could be associated with low expression of targeted antigen (HER2) on normal epithelial cells of the lung [121, 122]. Dose escalation studies are needed to establish safe and effective therapeutic dose ranges for CAR-modified T cells. Another approach to insure safety is to build a switch in the infused T cells by inserting a gene that can cause the cells to die rapidly and whose expression can be triggered when needed by a soluble factor. An example is the recent development of a form of inducible caspase 9 expressing a cyclophilin-binding element that can be dimerized using a rapamycin analogue yielding the active form of the protein. 90% of infused T cells modified to express this construct die within 30 minutes of the patient taking the drug, allowing for a quick elimination of T cells causing unwanted side effects[123].
In summary, adoptive cell transfer of endogenous tumor infiltrating lymphocytes or TCR/CAR-engineered peripheral blood T cells in advanced cancer patients has resulted in objective clinical responses. New strategies now aim at enhancing the cell product by further engineering of the T cells, such as introduction of the expression of chemokine receptors for improved migration to the tumor or defined cytokines to help persistence or function of the transferred cells. Defining markers of clinical response to delineate patients that are more likely to benefit from the therapy is a future goal.
Concluding remarks
Optimally effective cancer immunotherapy may require combinatorial approaches. Synergistic combinations could include means of activating T cells, such as vaccination or the transfer of ex-vivo activated and expanded tumor reactive T cells, coupled with means of sustaining T cell activation, such as agonistic antibodies to co-stimulatory receptors or blocking antibodies to co-inhibitory receptors. As many clinical grade reagents are now available, potential combinations are abundant. Currently, there are over 20 registered clinical trials to study the combination of ipilimumab with vaccines, radiation, chemotherapy, hormonal therapy, cytokines, anti-PD1, and targeted therapy agents in patients with a broad range of cancers.
Immunotherapy approaches have so far targeted tumor antigens derived from over-expressed self-proteins. Recent work in cancer genomics, however, has highlighted the “foreign” part of the tumor tissue by unveiling the numerous genetic alterations found in tumors. Genomic sequencing of human breast and colon carcinomas indicates that each tumor contains almost 100 missense mutations, which potentially encode a large number of neo-antigens that can be recognized by the immune system [124, 125]. Releasing these antigens through use of tumoricidal agents without significant immune-suppressing activity could potentially prime T cell responses, which could then be enhanced and sustained by immunotherapy [126]. Radiation, certain chemotherapies, hormone ablation, and a new generation of targeted therapy agents can potentially be used as immunosupportive vaccines. For example, small molecule targeting of mutated BRAF kinase in advanced melanoma patients increased T cell tumor infiltration, suggesting use of this small molecule along with immunotherapeutic intervention might help activated T cells home to the tumor [127].
The specificity of melanoma TILs associated with clinical response during adoptive cell therapy is largely not accounted for by recognition of over-expressed self-antigens (e.g. Survivin), melanosomal differentiation antigens (e.g., MART-1 and gp100), or CT antigens (e.g., NY-ESO-1 and MAGE-A3) [128, 129]. Although the unknown reactivity may be accounted for undiscovered over-expressed melanoma self-antigens, it is also possible that TILs might recognize mutated self-antigens that constitute neo-epitopes driving more highly avid “non-self” T-cell responses. TIL reactivity to mutated self-antigen is best demonstrated in a melanoma patient who responded to TIL therapy. This patient’s tumor tissue harbored a β-catenin mutation that generated an HLA-A24-binding mutant peptide epitope strongly recognized by a TIL cell line isolated from the same patient [86].
Strong recognition of mutated self-antigens can also lead to “immunoediting” as a mechanism of tumor escape. This was demonstrated in a methylcholanthrene-induced mouse sarcoma model in which CD8+ T cell recognition of a mutant epitope from the spectrin-β2 gene was a potential tumor rejection antigen. However, the loss of expression of this immunogenic epitope, through immunoediting, became a driver of renewed tumor outgrowth [130]. Thus new approaches at attacking the cancer “mutanome” by immunotherapy need to target multiple mutant-epitopes simultaneously to prevent ultimate tumor escape.
Soluble factors secreted in the tumor microenvironment also contribute to create an immunosuppressive milieu acting as barrier to a functional T cell response. Reduced availability of certain amino acids within the tumor microenvironment, such as tryptophan or arginine, caused by macrophage expression of Indoleamine 2,3-dioxygenase (IDO) or myeloid cell expression of arginase, is detrimental to T cells and inhibits effector T cell activation, proliferation and survival [131, 132]. IDO expression is induced by prostaglandin E2 (PGE2) which in turn is synthesized by cyclooxygenase 2 (COX-2). Pharmacological inhibition of COX-2 has proven effective in lowering intra-tumor IDO levels in animal models and decreasing tumor burden [133, 134]. Adenosine accumulation in hypoxic tumors can directly inhibit TCR signaling and T cell function through binding to its receptors on T cell surface and inducing cytosolic cAMP elevation [135]. High intra tumor levels of the immunosuppressive molecule TGF-β have also been shown to dampen tumor immunity [136]. Future work needs to elucidate how these paracrine pathways affect immunotherapies.
Ultimately, the most effective cancer therapies may consist of combinations of diverse immunotherapy strategies, as well as rational combinations with other standard therapies such as targeted therapies and conventional chemotherapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caram MV, Schuetze SM. Advanced or metastatic gastrointestinal stromal tumors: systemic treatment options. Journal of surgical oncology. 2011;104:888–895. doi: 10.1002/jso.21930. [DOI] [PubMed] [Google Scholar]
- 2.Smalley KS, McArthur GA. The current state of targeted therapy in melanoma: this time it’s personal. Semin Oncol. 2012;39:204–214. doi: 10.1053/j.seminoncol.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 4.Schuster SJ, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–2794. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartzentruber DJ, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang BA, et al. Topical resiquimod promotes priming of CTL to parenteral antigens. Vaccine. 2009;27:5791–5799. doi: 10.1016/j.vaccine.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 7.Marshall NA, et al. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-gamma+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Res. 2012;72:581–591. doi: 10.1158/0008-5472.CAN-11-0307. [DOI] [PubMed] [Google Scholar]
- 8.Kenter GG, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 9.Sikora AG, et al. IFN-alpha enhances peptide vaccine-induced CD8+ T cell numbers, effector function, and antitumor activity. J Immunol. 2009;182:7398–7407. doi: 10.4049/jimmunol.0802982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peggs KS, et al. Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists. Clin Exp Immunol. 2009;157:9–19. doi: 10.1111/j.1365-2249.2009.03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonsatti E, et al. Biology and clinical applications of CD40 in cancer treatment. Semin Oncol. 2010;37:517–523. doi: 10.1053/j.seminoncol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Ngiow SF, et al. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedy JR, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierro S, et al. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin Ther Targets. 2011;15:91–101. doi: 10.1517/14712598.2011.540563. [DOI] [PubMed] [Google Scholar]
- 17.Leach DR, et al. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 18.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 19.Myers L, et al. Combined CD137 (4-1BB) and adjuvant therapy generates a developing pool of peptide-specific CD8 memory T cells. Int Immunol. 2006;18:325–333. doi: 10.1093/intimm/dxh371. [DOI] [PubMed] [Google Scholar]
- 20.Morris GP, et al. CD137 signaling interferes with activation and function of CD4+CD25+ regulatory T cells in induced tolerance to experimental autoimmune thyroiditis. Cell Immunol. 2003;226:20–29. doi: 10.1016/j.cellimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Robertson SJ, et al. CD137 costimulation of CD8+ T cells confers resistance to suppression by virus-induced regulatory T cells. J Immunol. 2008;180:5267–5274. doi: 10.4049/jimmunol.180.8.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melero I, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 23.Miller RE, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–1800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox RA, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito F, et al. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64:8411–8419. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- 26.Molckovsky A, Siu LL. First-in-class, first-in-human phase I results of targeted agents: highlights of the 2008 American society of clinical oncology meeting. J Hematol Oncol. 2008;1:20. doi: 10.1186/1756-8722-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 28.Bansal-Pakala P, et al. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7:907–912. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 29.Bansal-Pakala P, et al. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 30.Dawicki W, et al. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 31.Mousavi SF, et al. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181:5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda I, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 33.Valzasina B, et al. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 34.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 35.Vu MD, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinberg AD, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 37.Lee SJ, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–3012. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 38.Murata S, et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176:974–983. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 39.Lee SJ, et al. CD134 Costimulation Couples the CD137 Pathway to Induce Production of Supereffector CD8 T Cells That Become IL-7 Dependent. J Immunol. 2007;179:2203–2214. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]
- 40.Gri G, et al. OX40 ligand-transduced tumor cell vaccine synergizes with GM-CSF and requires CD40-Apc signaling to boost the host T cell antitumor response. J Immunol. 2003;170:99–106. doi: 10.4049/jimmunol.170.1.99. [DOI] [PubMed] [Google Scholar]
- 41.Nocentini G, et al. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci U S A. 1997;94:6216–6221. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu KY, et al. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J Biol Chem. 1999;274:13733–13736. doi: 10.1074/jbc.274.20.13733. [DOI] [PubMed] [Google Scholar]
- 43.McHugh RS, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 44.Kanamaru F, et al. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 45.Tone M, et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci U S A. 2003;100:15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu J, et al. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 47.Ji HB, et al. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol. 2004;172:5823–5827. doi: 10.4049/jimmunol.172.10.5823. [DOI] [PubMed] [Google Scholar]
- 48.Stephens GL, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 49.Cohen AD, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitsui J, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16:2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 51.Coe D, et al. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1367–1377. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ko K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou P, et al. Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced-immune activation and tumor immunity in CT26 tumors. J Immunol. 2007;179:7365–7375. doi: 10.4049/jimmunol.179.11.7365. [DOI] [PubMed] [Google Scholar]
- 54.Cohen AD, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ko HJ, et al. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 56.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vonderheide RH, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 58.Ruter J, et al. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther. 2010;10:983–993. doi: 10.4161/cbt.10.10.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liakou CI, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen H, et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci U S A. 2009;106:2729–2734. doi: 10.1073/pnas.0813175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carthon BC, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861–2871. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vonderheide RH, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 63.Yuan J, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korman AJ, et al. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peggs KS, et al. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 66.Callahan MK, et al. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37:473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolchok JD, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 68.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 70.Keir ME, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishimura H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 72.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 73.Iwai Y, et al. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 74.Nomi T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 75.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Topalian SL, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brahmer JR, et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khammari A, et al. Treatment of metastatic melanoma with autologous Melan-A/MART-1-specific cytotoxic T lymphocyte clones. J Invest Dermatol. 2009;129:2835–2842. doi: 10.1038/jid.2009.144. [DOI] [PubMed] [Google Scholar]
- 80.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dudley ME, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–251. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackensen A, et al. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 83.Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosenberg SA, et al. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 85.Dudley ME, et al. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robbins PF, et al. A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J Exp Med. 1996;183:1185–1192. doi: 10.1084/jem.183.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 88.Bogunovic D, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A. 2009;106:20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kilic A, et al. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J Surg Res. 2011;167:207–210. doi: 10.1016/j.jss.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 90.Topalian SL, et al. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods. 1987;102:127–141. doi: 10.1016/s0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- 91.Junker N, et al. Bimodal ex vivo expansion of T cells from patients with head and neck squamous cell carcinoma: a prerequisite for adoptive cell transfer. Cytotherapy. 2011;13:822–834. doi: 10.3109/14653249.2011.563291. [DOI] [PubMed] [Google Scholar]
- 92.Rosenberg SA, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. Journal of the National Cancer Institute. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 93.Dudley ME, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Besser MJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 95.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou J, et al. Selective growth, in vitro and in vivo, of individual T cell clones from tumor-infiltrating lymphocytes obtained from patients with melanoma. J Immunol. 2004;173:7622–7629. doi: 10.4049/jimmunol.173.12.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenberg SA, et al. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 99.Kline J, et al. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 100.Yao X, et al. Levels of peripheral CD4+FoxP3+ regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119:5688–5696. doi: 10.1182/blood-2011-10-386482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Angulo I, et al. Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood. 2000;95:212–220. [PubMed] [Google Scholar]
- 102.Johnson LA, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson LA, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parkhurst MR, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robbins PF, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180:6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robbins PF, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Provasi E, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med. 2012;18:807–815. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yun CO, et al. Targeting of T lymphocytes to melanoma cells through chimeric anti-GD3 immunoglobulin T-cell receptors. Neoplasia. 2000;2:449–459. doi: 10.1038/sj.neo.7900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hwu P, et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med. 1993;178:361–366. doi: 10.1084/jem.178.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Till BG, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kershaw MH, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jensen MC, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haynes NM, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood. 2002;100:3155–3163. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]
- 114.Maher J, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 115.Lo AS, et al. Anti-GD3 chimeric sFv-CD28/T-cell receptor zeta designer T cells for treatment of metastatic melanoma and other neuroectodermal tumors. Clin Cancer Res. 2010;16:2769–2780. doi: 10.1158/1078-0432.CCR-10-0043. [DOI] [PubMed] [Google Scholar]
- 116.Sadelain M, et al. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wilkie S, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:4901–4909. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 118.Porter DL, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kochenderfer JN, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ertl HC, et al. Considerations for the clinical application of chimeric antigen receptor T cells: observations from a recombinant DNA Advisory Committee Symposium held June 15, 2010. Cancer Res. 2011;71:3175–3181. doi: 10.1158/0008-5472.CAN-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brentjens R, et al. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morgan RA, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Di Stasi A, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sjoblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 125.Segal NH, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 126.Peggs KS, et al. Targeting immunosupportive cancer therapies: accentuate the positive, eliminate the negative. Cancer Cell. 2007;12:192–199. doi: 10.1016/j.ccr.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 127.Wilmott JS, et al. Selective BRAF inhibitors induce marked T cell infiltration into human metastatic melanoma. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 128.Kvistborg P, et al. TIL therapy broadens the tumor-reactive CD8+ T cell compartment in melanoma patients. Onco Immunology. 2012;1:409–418. doi: 10.4161/onci.18851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Andersen RS, et al. Dissection of T-cell antigen specificity in human melanoma. Cancer Res. 2012;72:1642–1650. doi: 10.1158/0008-5472.CAN-11-2614. [DOI] [PubMed] [Google Scholar]
- 130.Matsushita H, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bronte V, et al. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends in immunology. 2003;24:302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 132.Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Frontiers in bioscience. 2012;4:734–745. doi: 10.2741/e414. [DOI] [PubMed] [Google Scholar]
- 133.Lee SY, et al. The immune tolerance of cancer is mediated by IDO that is inhibited by COX-2 inhibitors through regulatory T cells. J Immunother. 2009;32:22–28. doi: 10.1097/CJI.0b013e31818ac2f7. [DOI] [PubMed] [Google Scholar]
- 134.Basu GD, et al. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol. 2006;177:2391–2402. doi: 10.4049/jimmunol.177.4.2391. [DOI] [PubMed] [Google Scholar]
- 135.Sitkovsky MV, et al. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 136.Yang L, et al. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends in immunology. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]